Key points
High altitude developmental hypoxia causes intrauterine growth restriction and cardiovascular programming. However, some mammals exposed chronically to high‐altitude hypoxia have less growth restriction suggesting certain protection.
Cardiovascular defence mechanisms during acute fetal hypoxia divert blood flow from the periphery towards the brain, heart and adrenals. In contrast, little is known about the cardiovascular defence mechanisms during chronic fetal hypoxia.
Here, we established the cardiovascular responses in fetal sheep that were conceived, gestated, born and studied at 3600 m. The data suggest that chronically hypoxic pregnant ewes and their fetuses have evolved different mechanisms from sea level pregnancies to withstand chronic hypoxia.
The cardiovascular responses to acute hypoxia are blunted in the chronically hypoxic fetus. These findings points towards compensatory mechanisms in the highland fetus at the level of the cells and molecules rather than mounting major cardiovascular responses, saving oxygen not easily available in the Alto Andino.
Abstract
High‐altitude hypoxia causes intrauterine growth restriction and cardiovascular programming. However, adult humans and animals that have evolved at altitude show certain protection against the effects of chronic hypoxia. Whether the highland fetus shows similar protection against high altitude gestation is unclear. We tested the hypothesis that high‐altitude fetal sheep have evolved cardiovascular compensatory mechanisms to withstand chronic hypoxia that are different from lowland sheep. We studied seven high‐altitude (HA; 3600 m) and eight low‐altitude (LA; 520 m) pregnant sheep at ∼90% gestation. Pregnant ewes and fetuses were instrumented for cardiovascular investigation. A three‐period experimental protocol was performed in vivo: 30 min of basal, 1 h of acute superimposed hypoxia (∼10% O2) and 30 min of recovery. Further, we determined ex vivo fetal cerebral and femoral arterial function. HA pregnancy led to chronic fetal hypoxia, growth restriction and altered cardiovascular function. During acute superimposed hypoxia, LA fetuses redistributed blood flow favouring the brain, heart and adrenals, whereas HA fetuses showed a blunted cardiovascular response. Importantly, HA fetuses have a marked reduction in umbilical blood flow versus LA. Isolated cerebral arteries from HA fetuses showed a higher contractile capacity but a diminished response to catecholamines. In contrast, femoral arteries from HA fetuses showed decreased contractile capacity and increased adrenergic contractility. The blunting of the cardiovascular responses to hypoxia in fetuses raised in the Alto Andino may indicate a change in control strategy triggered by chronic hypoxia, switching towards compensatory mechanisms that are more cost‐effective in terms of oxygen uptake.
Key points
High altitude developmental hypoxia causes intrauterine growth restriction and cardiovascular programming. However, some mammals exposed chronically to high‐altitude hypoxia have less growth restriction suggesting certain protection.
Cardiovascular defence mechanisms during acute fetal hypoxia divert blood flow from the periphery towards the brain, heart and adrenals. In contrast, little is known about the cardiovascular defence mechanisms during chronic fetal hypoxia.
Here, we established the cardiovascular responses in fetal sheep that were conceived, gestated, born and studied at 3600 m. The data suggest that chronically hypoxic pregnant ewes and their fetuses have evolved different mechanisms from sea level pregnancies to withstand chronic hypoxia.
The cardiovascular responses to acute hypoxia are blunted in the chronically hypoxic fetus. These findings points towards compensatory mechanisms in the highland fetus at the level of the cells and molecules rather than mounting major cardiovascular responses, saving oxygen not easily available in the Alto Andino.
Abbreviations
- ABF
adrenal blood flow
- BBF
brain blood flow
- CBF
carotid blood flow
- CVR
carotid vascular resistance
- FBF
femoral blood flow
- FVR
femoral vascular resistance
- HA
high‐altitude
- HBF
heart blood flow
- HR
heart rate
- IUGR
intrauterine growth restriction
- LA
low‐altitude
- MCA
middle cerebral artery
- MSAP
mean systemic arterial pressure
- NA
noradrenaline
- NO
nitric oxide
- NOS
nitric oxide synthases
- pD2
−logEC50
- Phe
phenylephrine
- UBF
umbilical blood flow
Introduction
Pregnant women and their unborn babies from several regions of the world are chronically exposed to the low oxygen milieu of high altitude mountains or plateaus. Such women come from different ethnicities, and some have resided in highlands for hundreds of generations (Beall, 2007; Simonson et al. 2010; Wang et al. 2011; Browne et al. 2015). Despite acclimatization, pregnancy at high altitude is clearly a potential burden for both mother and fetus. In fact, the incidence of pregnancy complications and neonatal morbidity, such as intrauterine growth restriction (IUGR), fetal hypoxia, stillbirth and respiratory distress in neonates is significantly increased in high altitude populations (Giussani et al. 2001; Keyes et al. 2003; Gonzales et al. 2008; Soria et al. 2013). Thus, in Andean high‐altitude locations (> 3200 m) there is an increased infant mortality rate (Keyes et al. 2003; Gonzales et al. 2008) relative to lowland populations. Interestingly, human populations that have resided for the longest time period at high altitude in Tibet and Bolivia have the greatest reduction in the incidence of growth restriction, suggesting graded adaptation to this milieu (Moore, 2001; Giussani et al. 2001; Soria et al. 2013). Similarly, sheep brought by the Spanish Conquistadores 500 years ago and now permanently resident for many generations in the Andean altiplano (or Alto Andino, the high‐altitude plateau) have substantially smaller fetuses and newborn lambs compared to those residing at low altitude (Parraguez et al. 2005; Herrera et al. 2007). However, these animals show a reduced neonatal morbidity and mortality when compared with sheep newly arrived in the highlands (Herrera et al. 2010, 2011).
In normal pregnancy, acute hypoxia is a common challenge to the fetus, for instance during transient compression of the umbilical cord. In the last decades, the fetal cardiovascular responses to acute hypoxia have been extensively studied and described (Cohn et al. 1974; Parer, 1980; Alonso et al. 1989; Pérez et al. 1989; Giussani et al. 1993; Fletcher et al. 2006; Bennet & Gunn, 2009; Thakor et al. 2010; Kane et al. 2012; Herrera et al. 2012). These responses are coordinated by the autonomic nervous system, unleashing an initial peripheral vasoconstriction triggered by a carotid chemoreflex (Giussani et al. 1993) and mediated by increased sympathetic activity (Giussani et al. 1993; Bennet & Gunn, 2009). Fetal hormones liberated into the blood stream maintain the peripheral constriction (Pérez et al. 1989; Fletcher et al. 2006) and there is now evidence of a local oxidant tone acting directly at the level of the fetal vasculature (Thakor et al. 2010, 2015; Kane et al. 2012; Herrera et al. 2012). However, the effect of chronic hypoxia on the fetal cardiovascular responses to superimposed acute hypoxia is less well understood and very much understudied. Until now, the few attempts have involved mostly sea level animal models made chronically hypoxic during pregnancy via several techniques (see Giussani & Davidge, 2013) and an elegant series of studies investigating the effects on fetal physiology of sea level sheep exposed to high altitude (Kamitomo et al. 1993; Longo et al. 1993; Gilbert et al. 2003; Pereyra et al. 2007). To the best of our knowledge, the basal cardiovascular effects and the responses to acute hypoxia on fetuses conceived, gestated and studied at actual high altitude have never been investigated. The present study used an established cohort of sheep exposed to the high altitude of the Alto Andino for several generations (Llanos et al. 2003; Herrera et al. 2007) to test the hypothesis that highland fetal sheep have evolved cardiovascular compensatory mechanisms to withstand basal and superimposed acute hypoxia that are different from lowland fetal sheep.
Methods
The Faculty of Medicine Bioethics Committee of the University of Chile approved all experimental procedures (Protocol CBA (Animal Bioethics Committee) No. 097, Faculty of Medicine, University of Chile (FMUCH)). The studies on animals were performed according to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1996) and adhered to the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Animals
Seven time‐mated pregnant sheep (Ovis aries) from several generations at high altitude (HA; Putre Research Station, University of Chile, 3600 m above sea level) and eight pregnant sheep from low altitude (LA; Santiago, Faculty of Medicine, University of Chile, 520 m above sea level) were bred and maintained under standard housing conditions. The HA fetuses were conceived, gestated, instrumented and studied at the Andean altiplano (Putre Research Station), while the low altitude animals were bred and studied at near sea‐level. Both groups of fetuses were studied at the gestational age of 133 ± 3 days for the in vivo protocols (term ∼148 days). Similar in gestational age but uninstrumented fetuses (HA: n = 5; LA: n = 5; 134 ± 2 days) were used for the collection of small resistance arteries for the ex vivo myography studies.
Surgical preparation and in vivo experiments
All surgical procedures were made under aseptic conditions following food and water deprivation for 24 h. Between 125 and 130 days of gestation, pregnant sheep were premedicated with atropine (0.04 mg kg−1 i.m., Atropina Sulfato, Laboratorio Chile, Santiago, Chile), induced for intubation (5–7 mg kg−1 i.v. sodium thiopentone, Tiopental Sódico, Laboratorio Biosano SA, Santiago, Chile), and anaesthetized with 0.5–2% isofluorane in 50:50 oxygen and nitrous oxide. A midline laparotomy and hysterotomy was performed, and polyvinyl catheters (0.8 mm ID) were inserted into the fetal carotid artery and in the abdominal aorta and cava vein via hind limb fetal vessels. Ultrasonic flow probes (2R, Transonic Systems, Ithaca, NY, USA) were implanted around fetal femoral and carotid arteries. Additionally, polyvinyl catheters were placed into the femoral artery and amniotic cavity of the ewes (Giussani et al. 1993, 1994). After surgery, animals were returned to the yard and received a prophylactic treatment of ampicillin i.m. (500 mg; Ampicilina, Laboratorio Best Pharma, Santiago, Chile), and gentamicin (80 mg; Gentamicina Sulfato, Laboratorio Biosano SA, Santiago, Chile) into the amniotic cavity. Then catheters were filled with heparinized saline (250 IU heparin per ml 0.9% NaCl) and maintained patent by daily flushing.
Experimental procedure
All the in vivo experiments started at least 5 days after surgery and were based on a three‐period protocol: 30 min of basal, 1 h of hypoxia and 30 min of recovery. Hypoxia was induced by a transparent respiratory hood placed over the maternal head supplied with a controlled mixture of gases (50 l min−1, ∼12–18% O2 and 2–3% CO2 in N2) designed to reduce the fetal to 12–14 Torr in the ascending aorta. During basal and recovery periods the animals breathed atmospheric air.
Measurements and calculations
Maternal and fetal arterial blood samples (0.5 ml) were taken in heparinized syringes at 0, 15 and 30 min of basal, at 15 min intervals during hypoxia, and at 15 and 30 min of recovery. These samples were analysed for pH, , (BMS 3Mk2 Blood Microsystem and PHM 73 pH/Blood Gas Monitor, Radiometer, Copenhagen, Denmark), percentage of oxygen saturation of haemoglobin () and haemoglobin concentration [Hb] (OSM2 Haemoximeter, Radiometer, Copenhagen, Denmark).
Systemic arterial pressure was obtained continuously using a pressure transducer. In addition, fetal carotid (CBF) and femoral (FBF) blood flows were measured with the Transonic flow probes. All cardiovascular variables were recorded with a data acquisition system (Powerlab/8SP System and Chart v5.0 Software; ADInstruments, Bella Vista, NSW, Australia) connected to a personal computer. From these data, mean systemic arterial pressure (MSAP), heart rate (HR), and carotid and femoral vascular resistance (CVR and FVR respectively) were calculated.
In addition, the fetal organ blood flows to brain, heart, adrenals and umbilico‐placental vascular bed were determined by the fluorescent microspheres method. Different coloured microspheres (106) were injected into inferior vena cava for each experimental period (Fluospheres, Molecular Probes, USA). During the injection, reference blood samples were drawn at 3.2 ml min−1 for 90 s from the ascending aorta for brain and heart blood flow determinations and from descending aorta for adrenal and umbilico‐placental determinations (Heymann et al. 1977; Sanhueza et al. 2005). A crimson colour was used as an internal standard for all the experiments (Prinzen & Bassingthwaighte, 2000). On completion of experiments, ewes and fetuses were killed with a sodium thiopentone overdose (200 mg kg−1 slow i.v.; Tiopental Sódico, Laboratorio Biosano SA, Santiago, Chile). Fetuses and their organs were weighed and dissected. The organs in which we measured blood flow were digested in 3 m ethanolic KOH with 0.5% Tween 80, filtered with 10 μm pore (Gaiser, Kappel‐Grafenhausen, Germany) and rinsed with phosphate‐buffered saline (PBS) and demineralised water. Dye fluorescence retained in the filtered microspheres was extracted dissolving the microspheres for 2 h with 10 ml of diethylene glycol monoethyl ether (Aldrich) and measured with a luminescence spectrophotometer (Perkin‐Elmer L55, Waltham, MA, USA), using the specific excitation/emission wavelength and slit widths for each coloured sphere as follows:
where, is blood flow in millilitres per minute and F the measured fluorescence intensity (Tan et al. 1997). The organ vascular resistance was calculated by dividing perfusion pressure by the blood flow. To assess relative weight reduction we calculated the fetal organs to body weight ratio, and uteroplacental efficiency was calculated as the ratio of fetal body weight to uterus plus placental weight. Further, to determine growth symmetry, we calculated the brain to liver weight ratio.
Ex vivo studies
Uninstrumented pregnant sheep (gestational age 134 ± 2 days) were killed with a sodium thiopentone overdose (200 mg kg−1 slow i.v. bolus; Tiopental Sódico, Laboratorio Biosano SA, Santiago, Chile), and the fetal brain and hind limb were removed and immediately immersed in cold saline. Middle cerebral arteries (MCAs) and third generation femoral arteries were dissected and mounted in an isometric force transducer (model DSC 6; Kistler Morce, Seattle, WA, USA) of a wire myograph (dual‐wire myograph; Danish Myo Technologies, Aarhus, Denmark). During experimentation the myograph bath was filled with Krebs buffer maintained at 39°C and aerated with 95% O2 and 5% CO2. Each artery was stretched to its individual optimal lumen diameter, considered as the diameter at which it developed the strongest contractile response to 125 mm K+, as previously described (Herrera et al. 2007). Contractile agonists were evaluated under basal tone. A concentration–response curve was constructed for potassium chloride (KCl, 4.75–125 mm) with Krebs buffer washes between the different concentrations.
Cumulative concentration–response curve for noradrenaline (NA; 10−10 to 10−3 m) and phenylephrine (Phe; 10−10 to 10−3 m) were constructed for femoral and middle cerebral arteries. Each concentration–response was determined 2 min after adding each dose.
Solutions and drugs
Krebs buffer contained (mm): 118.5 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, and 5.5 glucose with a pH of 7.4. In 125 mm K+ buffer, all of the NaCl was replaced by an equimolar amount of KCl. All reagents and drugs were obtained from Sigma‐Aldrich (St Louis, MO, USA).
Statistical analysis
Data are expressed as means ± SEM and significance was accepted when P < 0.05. For some in vivo experiments (MSAP, HR, CBF, CVR, FBF and FVR), we calculated the average of 15 min intervals, basal period (0–15; 16–30 min); early hypoxia (31–45; 46–60 min); late hypoxia (61–75; 76–90 min); and recovery (91–105; 106–120 min). Next, we utilized a two‐way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test to determine statistical differences. These two tests were also used to determine statistical comparisons between experimental time and groups in organ blood flows. For ex vivo experiments, the dose–response curves were analysed in terms of sensitivity (EC50 or pD 2) and maximal response (K max) by fitting the experimental data to a sigmoidal equation (Prism 5.0; GraphPad Software, La Jolla, CA, USA). Contractile responses were expressed in absolute tension (mN mm−1) or as a percentage of maximal response to KCl (%K max). Sensitivity was calculated as pD 2, where pD 2 = −log [EC50], EC50 being the concentration at which 50% of the maximal response was obtained. Differences between mean values were compared by Student's t test for unpaired data (Glantz & Slinker, 2001). For all comparisons, statistical significance was accepted when P < 0.05.
Results
Fetal and organ weights
The HA fetal lambs showed a significant reduction of 26% in fetal weight relative to LA at the same gestational age (133 ± 3 days). In addition, absolute brain and liver weights were lower and the brain to liver weight ratio was increased in HA relative to LA fetuses. Further, the adrenals relative to body weight, and the placental absolute and relative weight were increased in the HA group (Table 1). Accordingly, the calculated uteroplacental efficiency was markedly diminished in HA pregnancies (Table 1).
Table 1.
Fetal body and organ weights
| LA | HA | % change | ||
|---|---|---|---|---|
| Body | Absolute | 3613 ± 176 | 2680 ± 168a | 74.2a |
| Brain | Absolute | 55.0 ± 1.9 | 44.5 ± 1.0a | 80.9a |
| Relative | 1.549 ± 0.058 | 1.683 ± 0.114 | 108.7 | |
| Heart | Absolute | 27.12 ± 1.69 | 22.75 ± 2.28 | 83.9 |
| Relative | 0.736 ± 0.026 | 0.853 ± 0.082 | 115.9 | |
| Liver | Absolute | 125.7 ± 6.5 | 81.8 ± 8.6a | 65.1a |
| Relative | 3.839 ± 0.329 | 3.030 ± 0.168 | 78.9 | |
| Adrenals | Absolute | 0.377 ± 0.018 | 0.410 ± 0.038 | 108.8 |
| Relative | 0.0105 ± 0.0008 | 0.0156 ± 0.0020a | 148.6a | |
| Placenta+uterus | Absolute | 1812 ± 211 | 2840 ± 83a | 156.7a |
| Relative | 52 ± 6 | 107 ± 8a | 205.8a | |
| Uteroplacental efficiency | Relative | 205 ± 16.4 | 96 ± 6.1a | 46.8a |
| Brain/liver weight | Relative | 0.445 ± 0.024 | 0.592 ± 0.060a | 133.3a |
Fetal body weight (g) and organ absolute weight (g) and relative to body weight ratio. Uteroplacental efficiency was calculated and fetal body weight to uteroplacental weight. Growth symmetry was calculated as brain/liver weight. Values are means ± SEM. Significant difference (P ≤ 0.05): a vs. LA; Student's t test for all variables.
Maternal arterial blood gases and cardiovascular variables
The LA pregnant ewes showed blood gas and cardiovascular values within the normal range for this gestational age during basal conditions (Pérez et al. 1989). HA ewes had lower , and , higher pH and [Hb] compared to LA ewes. However, HA ewes had similar O2 content relative to LA ewes (Table 2). During the acute hypoxic episode, the , and O2 content decreased only in the LA ewes relative to their basal period. In addition, (isocapnic hypoxia), MSAP and HR were maintained unchanged from baseline in both groups during the experimental protocol (Table 2).
Table 2.
Arterial blood gases and cardiovascular variables in pregnant ewe
| Basal | Hypoxia | Recovery | ||
|---|---|---|---|---|
| pH | LA | 7.484 ± 0.018 | 7.485 ± 0.019 | 7.504 ± 0.015 |
| HA | 7.520 ± 0.01a | 7.511 ± 0.014a | 7.501 ± 0.016 | |
| (Torr) | LA | 100 ± 3 | 52 ± 5c | 105 ± 5 |
| HA | 48 ± 2a | 44 ± 3a | 49 ± 1a | |
| (Torr) | LA | 32.2 ± 1.0 | 31.1 ± 1.2 | 30.7 ± 1.5 |
| HA | 25.5 ± 1.4a | 23.9 ± 1.1a | 23.8 ± 0.5a | |
| [Hb] (g dl−1) | LA | 9.1 ± 0.5 | 8.9 ± 0.5 | 9.1 ± 0.6 |
| HA | 11.7 ± 0.4a | 11.3 ± 0.3a | 11.4 ± 0.3a | |
| (%) | LA | 100 ± 1 | 77 ± 3c | 100 ± 1 |
| HA | 80 ± 3a | 75 ± 3 | 82 ± 3a | |
| O2 cont (ml O2 dl−1) | LA | 12.2 ± 0.7 | 10.1 ± 0.9c | 12.3 ± 0.9 |
| HA | 12.3 ± 0.4 | 11.1 ± 0.5 | 12.0 ± 0.6 | |
| MSAP (mmHg) | LA | 89 ± 6 | 94 ± 7 | 91 ± 7 |
| HA | 87 ± 8 | 90 ± 9 | 89 ± 9 | |
| HR (min−1) | LA | 119 ± 9 | 121 ± 7 | 121 ± 7 |
| HA | 117 ± 8 | 122 ± 10 | 116 ± 9 |
Arterial pH, blood gases, haemoglobin concentration ([Hb]), percentage of oxyhaemoglobin (), arterial oxygen content (O2 cont), mean systemic arterial pressure (MSAP) and heart rate (HR), in low altitude (LA) and high altitude (HA) pregnant sheep during basal, hypoxia and recovery. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA; b vs. basal and c vs. all in the same group; ANOVA + Newman–Keuls test for all variables.
Fetal arterial blood gases
During basal conditions, LA fetal sheep showed blood gas values within the normal range for this gestational age (Pérez et al. 1989). HA fetal sheep showed lower , and , and higher [Hb] relative to LA fetal sheep. In contrast, HA and LA fetuses had similar values for pH and O2 content (Table 3). During the acute hypoxic episode the , and O2 content were reduced relative to basal values in both groups. In addition, pH was reduced only in the LA fetal sheep. During recovery, all variables returned towards basal values in both groups, with the exception of pH, which was maintained at a reduced level during recovery in the LA fetuses (Table 3).
Table 3.
Ascending aorta blood gases in fetal sheep
| Basal | Hypoxia | Recovery | ||
|---|---|---|---|---|
| pH | LA | 7.416 ± 0.007 | 7.354 ± 0.022b | 7.346 ± 0.019b |
| HA | 7.436 ± 0.007 | 7.413 ± 0.008a | 7.414 ± 0.007a | |
| (Torr) | LA | 23.4 ± 1.0 | 14.6 ± 0.6c | 23.9 ± 1.1 |
| HA | 14.8 ± 1.0a | 11.7 ± 0.3c | 14.9 ± 0.9a | |
| (Torr) | LA | 46.5 ± 1.3 | 44.2 ± 1.5 | 43.8 ± 1.6 |
| HA | 34.3 ± 1.4a | 32.4 ± 1.4a | 32.4 ± 1.6a | |
| [Hb] (g dl−1) | LA | 9.3 ± 0.3 | 9.4 ± 0.2 | 9.0 ± 0.3 |
| HA | 11.1 ± 0.4a | 10.6 ± 0.3a | 10.7 ± 0.3a | |
| (%) | LA | 59.2 ± 2.0 | 25.5 ± 2.6c | 53.0 ± 2.8 |
| HA | 42.3 ± 4.5a | 28.4 ± 1.3c | 42.2 ± 4.3a | |
| O2 cont (ml O2 dl−1) | LA | 7.3 ± 0.1 | 3.2 ± 0.3c | 6.4 ± 0.4 |
| HA | 6.2 ± 0.7 | 4.1 ± 0.3c | 6.0 ± 0.6 |
Arterial pH, blood gases, haemoglobin concentration ([Hb]), percentage of oxyhaemoglobin () and arterial oxygen content (O2 cont) in low altitude (LA) and high altitude (HA) fetal sheep during basal, hypoxia and recovery. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA; b vs. basal and c vs. all in the same group; ANOVA + Newman–Keuls test for all variables.
Fetal systemic circulation
During basal conditions, HA fetal sheep showed a slightly but significantly lower mean systemic arterial pressure (MSAP) and an elevated fetal heart rate relative to LA fetal sheep. While MSAP increased in LA fetal sheep during acute hypoxia and recovery, HA fetal sheep did not exhibit the classic increase in MSAP during an episode of superimposed acute hypoxia and showed no changes in the MSAP throughout the experimental protocol (Fig. 1). Similarly, the classic fall in fetal heart rate at the onset of acute hypoxia seen in LA animals was not present in HA fetuses (Fig. 1). During recovery, both groups returned to basal HR values.
Figure 1. Cardiovascular function in fetal sheep .
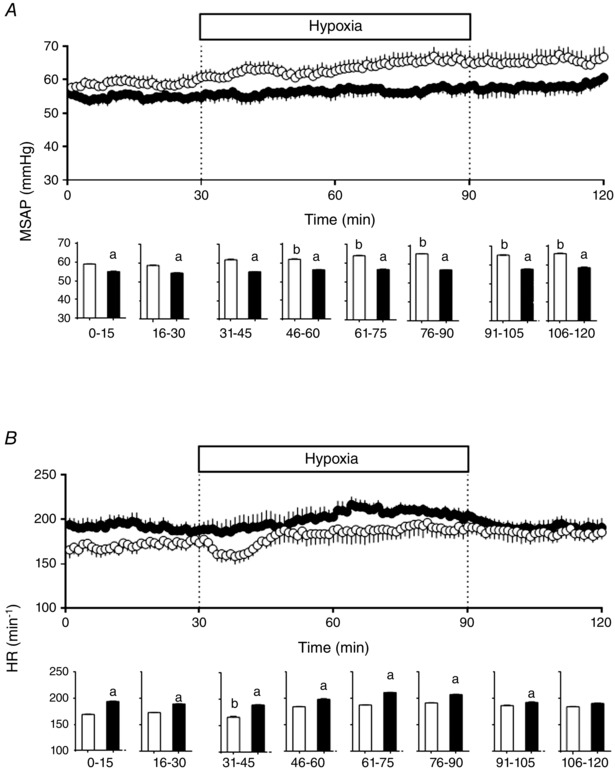
Mean systemic arterial pressure (MSAP, A) and heart rate (HR, B) and in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Histograms represent 15 min average of the experimental periods (basal, early hypoxia, late hypoxia and recovery). Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA and b vs. basal.
Fetal carotid circulation
During basal conditions, the CBF was higher in the HA fetal sheep compared to LA fetal sheep. Consequently, the CVR was decreased in HA compared to LA fetuses (Fig. 2). During the superimposed hypoxic episode, the CBF and CVR did not change in the HA fetal lambs, whereas in the LA group the CBF increased and CVR decreased, significantly compared to baseline. During the recovery period, both variables returned to basal levels in the LA group (Fig. 2).
Figure 2. Carotid function in fetal sheep .
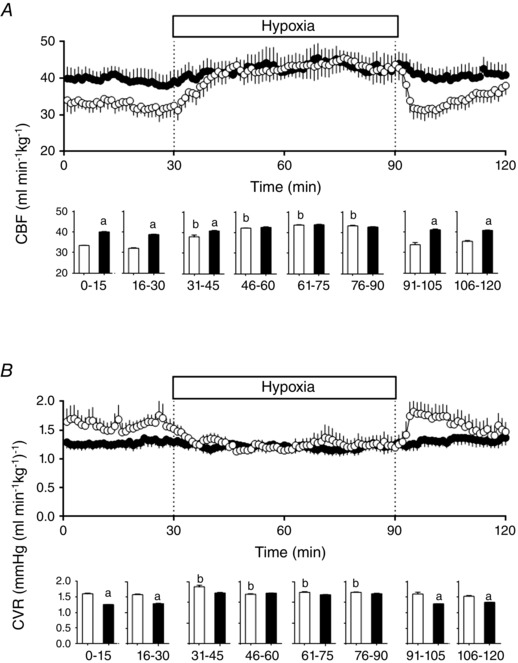
Carotid blood flow (CBF, A) and carotid vascular resistance (CVR, B) in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Histograms represent 15 min average of the experimental periods (basal, early hypoxia, late hypoxia and recovery). Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA and b vs. basal.
Fetal femoral circulation
During basal conditions, HA fetal sheep showed a higher FBF compared to the LA group. However, FVR was similar between the two groups (Fig. 3). While FBF decreased and FVR increased significantly during acute hypoxia in LA fetuses, these changes were markedly diminished or absent in HA fetuses. These variables returned to basal levels in the recovery period in both groups (Fig. 3).
Figure 3. Femoral function in fetal sheep .
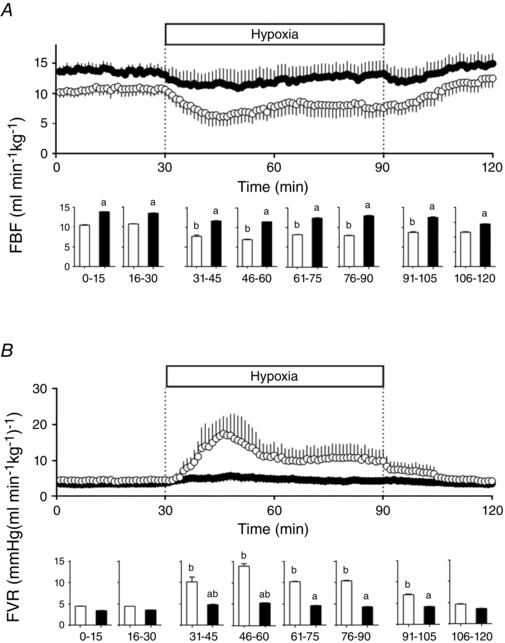
Femoral blood flow (FBF, A) and femoral vascular resistance (FVR, B) in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Histograms represent 15 min average of the experimental periods (basal, early hypoxia, late hypoxia and recovery). Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA and b vs. basal.
Fetal organ blood flow, vascular resistance and oxygen delivery
During basal conditions, the fetal sheep showed no differences in the blood flow (Fig. 4), vascular resistance (Table 4) and oxygen delivery (Table 5) to the brain between the two groups. During the acute hypoxic episode, both groups of fetal sheep increased the cerebral blood flow (Fig. 4), decreased the cerebral vascular resistance (Table 4) and maintained the O2 delivery to the brain (Table 5), relative to baseline. During recovery, values for brain blood flow and vascular resistance returned to baseline in both groups (Fig. 4; Table 4).
Figure 4. Organ blood flow in fetal sheep .
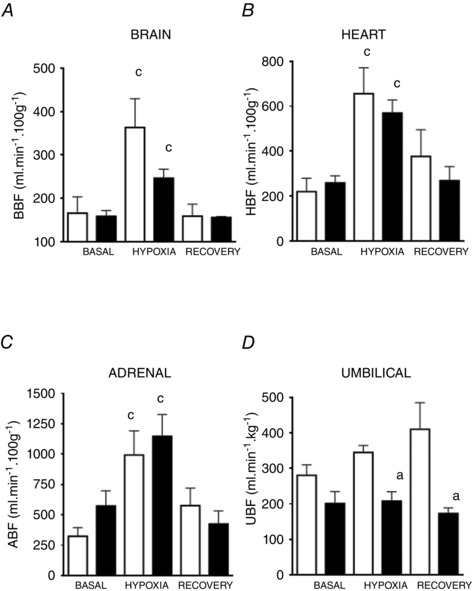
Blood flow to brain (BBF, A), heart (HBF, B), adrenal (ABF, C) and umbilical (UBF, D) vascular beds in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA and c vs. all periods in the group.
Table 4.
Organ vascular resistance in fetal sheep ((ml min−1)−1)
| Basal | Hypoxia | Recovery | ||
|---|---|---|---|---|
| Brain | LA | 0.419 ± 0.078 | 0.191 ± 0.021c | 0.445 ± 0.070 |
| HA | 0.359 ± 0.038 | 0.222 ± 0.017c | 0.365 ± 0.031 | |
| Heart | LA | 0.293 ± 0.049 | 0.156 ± 0.060b | 0.209 ± 0.078 |
| HA | 0.222 ± 0.025 | 0.102 ± 0.003c | 0.225 ± 0.040 | |
| Adrenals | LA | 0.220 ± 0.034 | 0.070 ± 0.010c | 0.178 ± 0.040 |
| HA | 0.116 ± 0.022a | 0.053 ± 0.008c | 0.165 ± 0.022 | |
| Umbilical | LA | 0.226 ± 0.032 | 0.195 ± 0.007 | 0.166 ± 0.040 |
| HA | 0.286 ± 0.046 | 0.278 ± 0.026a | 0.351 ± 0.035a |
Fetal organs vascular resistance in low altitude (LA) and high altitude (HA) fetal sheep during basal, hypoxia and recovery. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA; b vs. basal and c vs. all in the same group; ANOVA + Newman–Keuls test for all variables.
Table 5.
Organ oxygen delivery in fetal sheep (ml O2 min−1)
| Basal | Hypoxia | Recovery | ||
|---|---|---|---|---|
| Brain | LA | 14.97 ± 3.87 | 9.37 ± 1.55 | 9.80 ± 3.57 |
| HA | 11.78 ± 0.78 | 9.83 ± 1.23 | 10.13 ± 1.35 | |
| Heart | LA | 17.05 ± 5.67 | 21.40 ± 4.51 | 21.48 ± 7.60 |
| HA | 19.18 ± 2.08 | 22.55 ± 2.22 | 16.20 ± 2.34 | |
| Adrenals | LA | 24.95 ± 6.84 | 30.47 ± 8.80 | 28.63 ± 8.89 |
| HA | 36.48 ± 7.14 | 34.68 ± 2.95 | 25.53 ± 4.63c | |
| Umbilical | LA | 16.10 ± 1.43 | 8.97 ± 0.69c | 16.18 ± 3.65 |
| HA | 12.75 ± 0.85 | 6.38 ± 0.37b | 9.80 ± 1.30a |
Fetal organs O2del in low altitude (LA) and high altitude (HA) fetal sheep during basal, hypoxia and recovery. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA; b vs. basal and c vs. all in the same group; ANOVA + Newman–Keuls test for all variables.
Similarly, myocardial blood flow (Fig. 4), vascular resistance (Table 4) and O2 delivery (Table 5) followed a comparable basal and response pattern throughout the experiment, without differences between groups. In contrast, adrenal vascular resistance (Table 4) was diminished in HA relative to LA during baseline. However, fetal adrenal blood flow (Fig. 4), vascular resistance (Table 4) and O2 delivery (Table 5) showed the same response pattern to superimposed hypoxia observed for the brain and heart, in both groups of fetuses. All of the adrenal variables returned towards basal values during recovery.
Values for the umbilical blood flow (Fig. 4), vascular resistance (Table 4) and O2 delivery (Table 5) were similar between the two groups during basal conditions. However, during acute hypoxia and recovery, the umbilical blood flow increased (Fig. 4) in LA relative to HA fetuses. In marked contrast, the umbilical blood flow and resistance were maintained during the entire protocol in the HA group. Consequently, the umbilical O2 delivery during hypoxia was significantly decreased in HA fetuses (Fig. 4; Table 4, 5).
Contractile function of middle cerebral arteries
The contractile responses of MCAs to potassium chloride showed a higher maximal response in HA fetuses relative to the LA fetal sheep (2.06 ± 0.15 vs. 4.16 ± 0.46 mm mm−1, P < 0.05, respectively), while no differences in sensitivities were observed between groups (EC50, 28.41 ± 1.71 vs. 25.64 ± 4.09, respectively; Fig. 5 A). The adrenergic contractile response to noradrenaline and phenylephrine was assessed in MCAs, where the HA fetal group showed a marked decrease in the maximal response to both drugs relative to LA animals (Fig. 5 B and C). The sensitivity to phenylephrine was enhanced in HA relative to LA fetuses (Fig. 5 C).
Figure 5. Contractile function in middle cerebral arteries from fetal sheep .
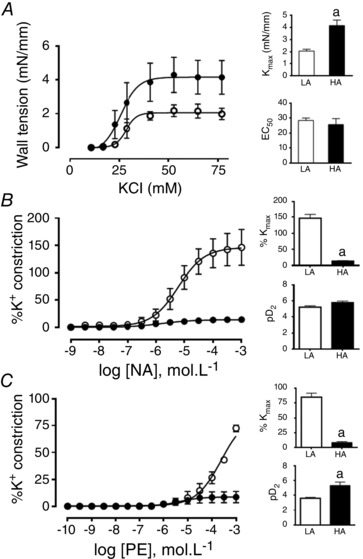
Vasoconstrictor response of middle cerebral arteries to potassium chloride (KCl, A), noradrenaline (NA, B), and phenylephrine (Phe, C) in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs. LA.
Contractile function of femoral arteries
Small resistance femoral arteries from HA fetuses showed a diminished contractile response and sensitivity to K+ compared to the LA fetuses (Fig. 6 A). However, in contrast to cerebral arteries, femoral arteries from HA fetuses showed a marked increase in the maximal response (214.1 ± 15.9 vs. 47.8 ± 3.3 %, P < 0.05, respectively) and sensitivity (pD 2: 5.11 ± 0.14 vs. 4.58 ± 0.10, P < 0.05, respectively) to noradrenaline relative to the LA fetuses (Fig. 6 B). Further, the maximal response to phenylephrine was enhanced while the sensitivity was decreased in femoral arteries isolated from HA compared to LA fetuses (Fig. 6 C).
Figure 6. Contractile function in femoral arteries from fetal sheep .
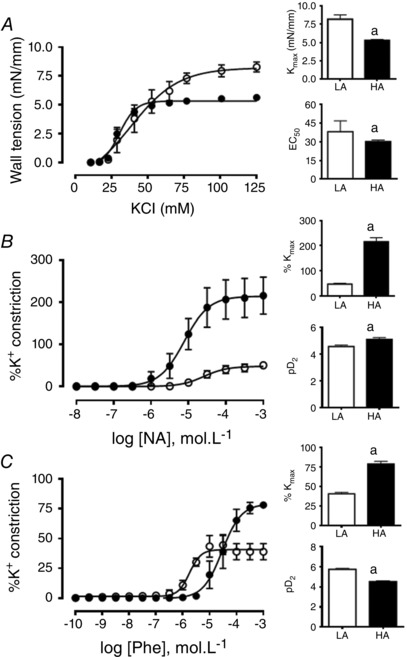
Vasoconstrictor response of femoral arteries to potassium chloride (KCl, A), noradrenaline (NA, B), and phenylephrine (Phe, C) in low altitude (LA, open circles/bars) and high altitude (HA, filled circles/bars) fetal lambs. Values are means ± SEM. Significant differences (P ≤ 0.05): a vs LA.
Discussion
To the best of our knowledge, this is the first study to investigate fetal cardiovascular function in vivo and ex vivo in high altitude adapted sheep at the Alto Andino. The data support the hypothesis tested that high‐altitude fetal sheep have evolved cardiovascular compensatory mechanisms to withstand basal and superimposed acute hypoxia that are different from lowland sheep, demonstrating markedly blunted cardiovascular responses to superimposed acute hypoxia.
The HA fetal sheep showed a reduced body weight for the gestational age relative to lowlanders. This reduction signifies intrauterine growth restriction (IUGR) and it has been described previously as an effect of altitude independent of nutrition (Lichty et al. 1957; Giussani et al. 2001; Moore, 2003; Herrera et al. 2007, 2010; Soria et al. 2013). The reduction in the HA fetal weight may be attributed to a reduction in the O2 supply (Soria et al. 2013), which involves adaptations like reductions in metabolic processes such as ATP production and demand (Wheaton & Chandel, 2011), reducing the Na+–K+‐ATPase activity and protein synthesis, consistent with a total decrease of fetal oxygen uptake (Richardson & Bocking, 1998; Wheaton & Chandel, 2011). The brain‐to‐liver weight ratio, an established index of asymmetric growth restriction, is significantly greater in HA compared to LA fetuses, as previously established in chronic fetal hypoxia at sea level (Llanos et al. 1980). Additionally, the absolute and relative placenta plus uterine weight was increased in HA pregnancies. Nevertheless, this increase in uteroplacental weight was insufficient to maintain fetal growth, yielding fetal growth restriction and thereby a fall in uteroplacental efficiency (see Fowden et al. 2009 for review; Londero et al. 2013).
There is disagreement about the effects of chronic hypoxia on placental phenotype, which may be due to varying duration, and/or magnitude and/or onset of hypoxia exposure as well as due to species differences. Some studies suggest decreased placental weight (Huang et al. 2004) while others reported increased weight and decreased efficiency under the influence of chronic hypoxia (Parraguez et al. 2006; Richter et al. 2012; Herrera et al. 2014). However, there is a common agreement that the failure of the placenta to meet the increasing demand for oxygen and substrate of the developing fetus ultimately ends in fetal growth restriction (Herrera et al. 2014). Under chronic hypoxia there is an increased oxidative stress that may be inducing damage to the developing placenta and fetus (Tissot van Patot et al. 2012; Richter et al. 2012; Giussani et al. 2012; Herrera et al. 2014). However, placentas from women of high altitude populations developed under hypoxic conditions may have less oxidative damage, providing a greater cushion to protect adverse effects on the fetus (Tissot van Patot et al. 2012; Herrera et al. 2014).
The hypoxic environment of high altitude triggers cardiorespiratory compensatory responses that tend to adapt the organism to harness the oxygen bioavailability. For instance, an increased haemoglobin concentration in the fetal and maternal blood increases the oxygen transport capacity (Kitanaka et al. 1989) and leads to maintained arterial O2 content at similar levels to those calculated for LA fetal sheep and mothers. Additionally, the ventilatory response in the HA pregnant we leads to a better , with a mild alkalaemia and a decreased arterial , which may help the haemoglobin loading by O2 in the lung, by shifting to the left the O2 dissociation curve. All of the above are well‐known compensatory responses to high altitude hypoxia. Regarding the systemic blood pressure, HA pregnant sheep showed no differences relative to LA pregnant ewes in both diastolic and systolic pressures in agreement with no blood pressure changes in acclimatized highlanders compared to lowlanders (León‐Velarde et al. 2010). In contrast to their mothers, the HA fetal sheep showed lower MSAP than LA fetuses during basal conditions. Furthermore, during acute hypoxia LA fetal sheep showed an increase in MSAP, a response attributed to increases in the sympathetic tone and in circulating catecholamine levels, among others factors (Cohn et al. 1974; Pérez et al. 1989; Giussani et al. 1993, 1994). This slight and transient hypertension in the LA fetal sheep during acute hypoxia (Cohn et al. 1974, Pérez et al. 1989) involves peripheral vasoconstriction with anaerobic metabolism, particularly in circulations that undergo constriction, such as the femoral vascular bed (Boyle et al. 1990). The latter induces lactate production in peripheral tissues, explaining the fall in the blood pH observed only in this group (Giussani et al. 1993). In contrast, the HA fetal lambs showed similar basal MSAP, but a blunted pressor response during superimposed acute hypoxia relative to LA sheep. This suggests either a lower neutrally triggered chemoreflex drive and sympathetic tone, and/or reduced vasoconstrictor hormones and/or a greater vasodilator influence masking the constrictor responses induced by fetal gestation under chronic hypoxia (Tissot van Patot et al. 2012). In lowland fetal sheep, in response to acute hypoxia the bradycardia and the initial increase in femoral vascular resistance are established carotid chemoreflexes, as both are abolished by selective carotid body denervation (Giussani et al. 1993). Therefore, the lesser bradycardic response in HA fetuses in the present study is likely to be due to a blunted carotid chemoreflex in fetal life triggered by the chronic hypoxia of pregnancy at high altitude. This effect is similar to the blunted chemoreflex hyperventilatory response to hypoxia in highland natives (Monge & León‐Velarde, 1991). Alternatively, additional results in the present manuscript show that femoral arteries from uninstrumented HA fetuses have lower contractile capacity and sensitivity to K+, but an increased response and sensitivity to NA relative to LA fetuses. Thus, the in vivo blunted contractile response could be associated with the decreased contractile capacity of the femoral vascular bed but not to alterations related to vascular adrenergic pathways.
The fetal lambs chronically exposed to HA showed relative higher basal CBF, an effect that should maintain an adequate delivery of oxygen and nutrients to the developing brain in hypoxia (Tissot van Patot et al. 2012). However, carotid blood flow did not have a significant increase in this group during the superimposed acute hypoxia (Pereyra et al. 2007; Tissot van Patot et al. 2012). The increase in CBF during acute hypoxia in LA fetal sheep is known to be mediated by vasodilators, including nitric oxide (NO) (Green et al. 1996; Hunter et al. 2003; Sanhueza et al. 2005). Therefore, the enhanced basal CBF in HA fetuses may indicate a greater NO tone induced by exposure to high altitude. This appears to be the case, since preliminary data from our laboratory show that CBF decreases more in HA than LA fetuses after the infusion of N G‐nitro‐l‐arginine methyl ester, a nitric oxide synthases (NOS) inhibitor (unpublished data). In our study, basal cerebral blood flow in HA fetuses was not different from the LA fetuses, in agreement with the data of Kamitomo et al. (1993), both measurements performed by the microspheres method. Furthermore, there was an increase in cerebral blood flow in HA and LA fetuses, with no difference between them, as also described previously (Kamitomo et al. 1993). In addition, the brain oxygen delivery was not different among HA and LA fetal lambs, suggesting that HA fetuses are able to maintain the brain oxygenation in spite of the low (Kamitomo et al. 1993; Pereyra et al. 2007). Moreover, the ex vivo studies of MCA reactivity in the present paper showed a higher contractile capacity and reduced response to noradrenaline in HA fetal lambs relative to controls. Thus, the increase in the brain blood flow and the fall in the vascular resistance observed in the HA fetus during superimposed hypoxia could be attributed to a blunted sensitivity to catecholamines and/or an augmented vasodilator NO effect, which is known to be an important modulator in the fetal brain (Green et al. 1996). The cerebral and carotid blood flow responses to acute hypoxia in the HA fetus are different from a species adapted to the life in the Andean altiplano, such as the fetal llama. Llama fetuses respond to acute hypoxia decreasing the cerebral metabolism, mediated by reductions in Na+–K+‐ATPase activity and expression of voltage‐gated Na+ channels (NaV) (Ebensperger et al. 2005) and a marked peripheral vasoconstrictor response (Giussani et al. 1996, 1999; Llanos et al. 2003; Sanhueza et al. 2005). Clearly, we are in the presence of dissimilar acquired adaptations to high altitude between sheep and llamas, probably due to differential evolutionary exposure to chronic hypoxia in the Andean highlands (Stanley et al. 1994).
The increased potassium contractile response in the MCAs of HA fetal sheep may reflect increases in both the wall thickness and L‐type calcium channel function (Gilbert et al. 2003), raising the calcium flux into the fetal smooth muscle cells, triggering contraction. In contrast, in the femoral circulation, decreases in femoral wall thickness and/or L‐type calcium channel function may have taken place. Moreover, NA and Phe contractile responses in the MCAs from HA fetuses were completely blunted compared to LA fetal sheep. Arteries from chronically hypoxic fetuses are exposed to enhanced plasma concentrations of catecholamine (Kitanaka et al. 1989). Further, chronic hypoxia reduced the capacity of NA to cause contraction, mainly due to hypoxic downregulation of adrenergic and lesser IP3 receptor densities (Gilbert et al. 2003), resulting in lower intracellular Ca2+ concentration and smooth muscle contraction in HA MCAs. In marked contrast, in HA fetal sheep the catecholamine response is clearly enhanced in the femoral circulation ex vivo. This finding may be partially explained by upregulation of the α1‐adrenergic receptor in these arteries ex vivo, as described for smooth muscle cells isolated from fresh or cultured segment of mesenteric arteries in vitro (Cao et al. 2006).
The reduced levels of umbilical blood flow measured in the HA fetus in the present study could suggest an impaired placental vasodilator function at altitude, possibly due to an umbilical or chorionic endothelial dysfunction (Krause et al. 2012; Herrera et al. 2014). In fact intrauterine growth restricted fetuses show impaired eNOS function in umbilical vessels (Krause et al. 2012). Further, in the presence of oxidative stress, reactive oxygen species will decrease the NO bioavailability with increased peroxynitrites resulting in endothelial cell damage or placental apoptosis and reduced umbilical perfusion (Miller et al. 1996; Giussani et al. 2012). Accordingly, Thakor et al. (2010) reported that treatment of sheep with antioxidants such as melatonin and vitamin C could increase umbilical conductance via increasing NO bioavailability. The decrease in umbilical blood flow promotes a lower O2 and nutrients delivery to the fetus, and impaired removal of waste products towards the mother in the chronic hypoxic pregnancy, an important cause of IUGR, as determined in this study.
We believe that our findings highlight the need to diagnose, in human fetuses, the increase in carotid and decrease in umbilical blood flow observed in our studies. These indices are already in use in some health centres in the Alto Andino and widely used in hospitals and clinics at lowlands. Additionally, the lack of further increase in carotid blood flow during the fall of fetal , as may occur during the uterine contractions of labour at the end of highland pregnancy, may signal exhausted mechanisms to augment the cerebral blood flow. The latter could eventually be added to the clinical repertoire used to diagnose the fragile chronically hypoxic fetus.
In conclusion, this study determined for the first time the fetal cardiovascular responses to chronic and acute hypoxia in sheep at high altitude. We reported markedly blunted cardiovascular responses to acute superimposed hypoxia in highland relative to lowland fetal sheep. Whether these blunted cardiovascular responses are beneficial or detrimental during development under chronic hypoxia is still unclear. More studies are required to determine if these effects are short or long lasting after delivery and if the changes are genetically fixed by selection pressure, epigenetically determined or simply a long term physiological acclimatization to a chronic hypoxic milieu.
We speculate that blunting the cardiovascular responses in chronically hypoxic fetuses reflects a change in the focus of the compensatory mechanisms towards cells, tissues and molecules, instead of mounting major cardiovascular modifications, which require a high oxygen consumption, oxygen not easily available in the Andean altiplano.
Additional information
Competing interests
No conflicts of interest are declared by the authors.
Author contributions
The experiments in this study were performed in Putre Research Station of the International Center for Andean Studies (INCAS) and in the Program of Pathophysiology, ICBM, Faculty of Medicine, University of Chile. EAH, RTR, BJK, GE, RVR, DAG, JTP and AJLL conceived and designed the experiments. EAH, RTR, BJK, GE, RVR, DAG, JTP and AJLL collected, analysed and interpreted the experimental data. EAH, RTR, DAG, JTP and AJLL drafted the article. All authors revised and approved the final version of the article.
Funding
This work was supported by National Fund for Scientific and Technological Development (FONDECYT‐Chile), Grant nos 1120605, 1130424, 1140647, 1151119; and The Wellcome Trust Collaborative Research Initiative, Grant no. 072256.
Acknowledgements
We are grateful for the technical assistance of Carlos Brito and Gabino Llusco, for their excellent technical assistance and to Felipe Beñaldo in preparing the manuscript.
E. A. Herrera and R. T. Rojas contributed equally to this work.
References
- Alonso JG, Okai T, Longo LD & Gilbert RD (1989). Cardiac function during long‐term hypoxemia in fetal sheep. Am J Physiol Heart Circ Physiol 257, H581–H589. [DOI] [PubMed] [Google Scholar]
- Beall CM (2007). Two routes to functional adaptation: Tibetan and Andean high‐altitude natives. Proc Natl Acad Sci USA 104, 8655–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet L & Gunn AJ (2009). The fetal heart rate response to hypoxia: insights from animal models. Clin Perinatol 36, 655–672. [DOI] [PubMed] [Google Scholar]
- Boyle DW, Hirst K, Zerbe GO, Meschia G & Wilkening RB (1990). Fetal hind limb oxygen consumption and blood flow during acute graded hypoxia. Pediatr Res 28, 94–100. [DOI] [PubMed] [Google Scholar]
- Browne VA, Julian CG, Toledo‐Jaldin L, Cioffi‐Ragan D, Vargas E & Moore LG (2015). Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 370, 20140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y‐X, Xu C‐B, Luo G‐G & Edvinsson L (2006). Up‐regulation of α1A‐adrenoceptors in rat mesenteric artery involves intracellular signal pathways. Basic Clin Pharmacol Toxicol 98, 61–67. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA & Rudolph AM (1974). Cardiovascular response to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120, 817–824. [DOI] [PubMed] [Google Scholar]
- Ebensperger G, Ebensperger R, Herrera EA, Riquelme RA, Sanhueza EM, Lesage F, Marengo JJ, Tejo RI, Llanos AJ & Reyes RV (2005). Fetal brain hypometabolism during prolonged hypoaxemia in the llama. J Physiol 563, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AJ, Gardner DS, Edwards CM, Fowden AL & Giussani DA (2006). Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol 291, H3023–H3034. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sferruzzi‐Perri AN, Coan PM, Constancia M & Burton GJ (2009). Placental efficiency and adaptation: endocrine regulation. J Physiol 587, 3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RD, Pearce WJ & Longo LD (2003). Fetal cardiac and cerebrovascular acclimatization responses to high altitude, long‐term hypoxia. High Alt Med Biol 4, 203–213. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ & Bennet L (1993). Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461, 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HH, Spencer JA, Moore PJ, Bennet L & Hanson MA (1994). Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late‐gestation sheep fetus. J Physiol 477, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, McGarrigle HHG, Gaete CR, Sanhueza EM, Hanson MA & Llanos AJ (1996). Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. Am J Physiol Regul Integr Comp Physiol 271, R73–R83. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Sanhueza EM, Hanson MA, Blanco CE & Llanos AJ (1999). Adrenergic and vasopressinergic contributions to the cardiovascular response to acute hypoxaemia in the llama fetus. J Physiol 515, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S & Barker DJ (2001). Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49, 490–494. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Zachary Blake E, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7, e31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Glantz SA & Slinker BK (2001). Primer of Applied Regression and Analysis of Variance, 2nd edn, pp. 418–507. McGraw‐Hill, New York. [Google Scholar]
- Gonzales GF, Tapia V & Carrillo CE (2008). Stillbirth rates in Peruvian populations at high altitude. Int J Gynaecol Obstet 100, 221–227. [DOI] [PubMed] [Google Scholar]
- Green LR, Bennet L & Hanson MA (1996). The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol 497, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes VR, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA & Llanos AJ (2007). High altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292, R2234–R2240. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA & Llanos AJ (2010). Long‐term exposure to high‐altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299, R1676–R1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Ebensperger G, Riquelme R, Díaz M, Reyes V, Torres‐Farfan C & Llanos A (2011). Respuesta cardiopulmonar a cambios de oxigenación en recién nacidos de ovejas de diferentes altitudes. Avances Ciencias Veterinarias 26, 53–63. [Google Scholar]
- Herrera EA, Kane AD, Hansell JA, Thakor AS, Allison BJ, Niu Y & Giussani DA (2012). A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J Physiol 590, 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Krause BJ, Ebensperger G, Reyes RV, Casanello P, Parra‐Cordero M & Llanos AJ (2014). The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front Pharmacol 5, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann MA, Payne BD, Hoffman JIE & Rudolph AM (1977). Blood flow measurements with radionuclide‐labeled particles. Prog Cardiov Dis 20, 55–79. [DOI] [PubMed] [Google Scholar]
- Huang ST, Vo KC, Lyell DJ, Faessen GH, Tulac S, Tibshirani R, Giaccia AJ & Giudice LC (2004). Developmental response to hypoxia. FASEB J 18, 1348–1365. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Blood AB, White CR, Pearce WJ & Power GG (2003). Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus. J Physiol 549, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Gilbert LD & Longo LD (1993). Effects of long‐term, high altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169, 701–707. [DOI] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Hansell JA & Giussani DA (2012). Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol 590, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA & Moore LG (2003). Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediat Res 54, 20–25. [DOI] [PubMed] [Google Scholar]
- Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK & Longo LD (1989). Fetal responses to long‐term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256, R1348–R1354. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Prieto CP, Munoz‐Urrutia E, San Martin S, Sobrevia L & Casanello P (2012). Role of arginase‐2 and eNOS in the differential vascular reactivity and hypoxia‐induced endothelial response in umbilical arteries and veins. Placenta 33, 360–366. [DOI] [PubMed] [Google Scholar]
- León‐Velarde F, Villafuerte FC & Richalet JP (2010). Chronic mountain sickness and the heart. Prog Cardiovasc Dis 52, 540–549. [DOI] [PubMed] [Google Scholar]
- Lichty JA, Ting TY, Bruns PD & Dyar E (1957). Studies of babies born at high altitudes. I. Relation of altitude to birth weight. J Dis Child 93, 666–669. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Green JR, Creasy RK & Rudolph AM (1980). Increased heart rate response to parasympathetic and beta adrenergic blockade in growth‐retarded fetal lambs. Am J Obstet Gynecol 136, 808–813. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Riquelme RA, Sanhueza EM, Hanson MA, Blanco CE, Parer JT, Herrera EA, Pulgar VM, Reyes RV, Cabello G & Giussani DA (2003). The fetal llama versus the fetal sheep: different strategies to withstand hypoxia. High Alt Med Biol 4, 193–202. [DOI] [PubMed] [Google Scholar]
- Londero AP, Bertozzi S, Visentin S, Fruscalzo A, Driul L & Marchesoni D (2013). High placental index and poor pregnancy outcomes: a retrospective study of 18,386 pregnancies. Gynecol Endocrinol 29, 666–669. [DOI] [PubMed] [Google Scholar]
- Longo LD, Hull AD, Long D & Pearce WJ (1993). Cerebrovascular adaptations to high altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264, R65–R72. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Voelker CA, Olister S, Thompson JH, Zang XJ, Rivera D, Eloby‐Childress S, Liu X, Clark DA & Pierce MR (1996). Fetal growth retardation in rats may results from apoptosis: role of peroxynitrite. Free Radic Biol Med 21, 619–629. [DOI] [PubMed] [Google Scholar]
- Monge C & León‐Velarde F (1991). Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 71, 1135–1172. [DOI] [PubMed] [Google Scholar]
- Moore LG (2001). Human genetic adaptation to high altitude. High Alt Med Biol 2, 257–279. [DOI] [PubMed] [Google Scholar]
- Moore LG (2003). Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4, 141–156. [DOI] [PubMed] [Google Scholar]
- Parer JT (1980). The effect of acute maternal hypoxia on fetal oxygenation and the umbilical circulation in the sheep. Eur J Obstet Gynecol Reprod Biol 10, 125–136. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Atlagitch MA, Diaz R, Bruzzone MA, Behn C & Raggi LA (2005). Effect of hypobaric hypoxia on lamb intrauterine growth: comparison between high‐ and low‐altitude native ewes. Reprod Fertil Dev 17, 497–505. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Atlagich M, Díaz R, Cepeda R, González C, De los Reyes M, Bruzzone ME, Behn C & Raggi LA (2006). Ovine placenta at high altitudes: comparison of animals with different times of adaptation to hypoxic environment. Anim Reprod Sci 95, 151–157. [DOI] [PubMed] [Google Scholar]
- Pereyra J, Tomimatsu T, Hatran DP, Mcgill LL & Longo LD (2007). Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol 578, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez R, Espinoza M, Riquelme R, Parer JT & Llanos AJ (1989). Arginine vasopressin mediates cardiovascular responses to hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 156, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Prinzen FW & Bassingthwaighte JB (2000). Blood flow distributions by microsphere deposition methods. Cardiovasc Res 45, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BS & Bocking AD (1998). Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol 119A, 717–723. [DOI] [PubMed] [Google Scholar]
- Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova‐Davies T, Spasic‐Boskovic O, Dunster C, Mudway IS, Kelly FJ, Burton J, Poston L & Giussani DA (2012). Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza EM, Riquelme RA, Herrera EA, Giussani DA, Blanco CE, Hanson MA & Llanos AJ (2005). Vasodilator tone in llama fetus: the role of nitric oxide during normoxemia and hypoxemia. Am J Physiol Regul Integr Comp Physiol 289, 776–783. [DOI] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT & Ge R (2010). Genetic evidence for high‐altitude adaptation in Tibet. Science 329, 72–75. [DOI] [PubMed] [Google Scholar]
- Soria R, Julian CG, Vargas E, Moore LG & Giussani DA (2013). Graduated effects of high‐altitude hypoxia and highland ancestry on birth size. Pediatr Res 74, 633–638. [DOI] [PubMed] [Google Scholar]
- Stanley HF, Kadwell M & Wheeler JC (1994). Molecular evolution of the family Camelidae: a mitochondrial DNA study. Proc Biol Sci 256, 1–6. [DOI] [PubMed] [Google Scholar]
- Tan W, Riggs KW, Thies RL & Rurak DW (1997). Use of an automated fluorescent microsphere method to measure regional blood flow in the fetal lamb. Can J Physiol Pharmacol 75, 959–968. [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Seron‐Ferre M & Giussani DA (2010). Melatonin and vitamin C increase umbilical blood flow via nitric oxide‐dependent mechanisms. J Pineal Res 49, 399–406. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Allison BJ, Niu Y, Botting KJ, Serón‐Ferré M, Herrera EA & Giussani DA (2015). Melatonin modulates the fetal cardiovascular defense response to acute hypoxia. J Pineal Res 59, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot van Patot MC, Ebensperger G, Gassmann M & Llanos AJ (2012). The hypoxic placenta. High Alt Med Biol 13, 176–184. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang YB, Zhang F, Lin H, Wang X, Wan N, Ye Z, Weng H, Zhang L, Li X, Yan J, Wang P, Wu T, Cheng L, Wang J, Wang DM, Ma X & Yu J (2011). On the origin of Tibetans and their genetic basis in adapting high‐altitude environments. PLoS One 6, e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW & Chandel NS (2011). Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300, C385–C393. [DOI] [PMC free article] [PubMed] [Google Scholar]


