Abstract
Purpose
Porphyromonas gingivalis and Tannerella forsythia have been implicated as the major etiologic agents of periodontal disease. These two bacteria are frequently isolated together from the periodontal lesion, and it has been suggested that their interaction may increase each one’s virulence potential. The purpose of this study was to identify proteins on the surface of these organisms that are involved in interbacterial binding.
Methods
Biotin labeling of surface proteins of P. gingivalis and T. forsythia and liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis was performed to identify surface proteins involved in the coaggregating activity between P. gingivalis and T. forsythia.
Results
It was found that three major T. forsythia proteins sized 161, 100, and 62 kDa were involved in binding to P. gingivalis, and P. gingivalis proteins sized 35, 32, and 26 kDa were involved in binding to T. forsythia cells.
Conclusions
LC-MS/MS analysis identified one T. forsythia surface protein (TonB-linked outer membrane protein) involved in interbacterial binding to P. gingivalis. However, the nature of other T. forsythia and P. gingivalis surface proteins identified by biotin labeling could not be determined. Further analysis of these proteins will help elucidate the molecular mechanisms that mediate coaggregation between P. gingivalis and T. forsythia.
Keywords: Bacterial outer membrane proteins, Biotinylation, Periodontitis, Porphyromonas gingivalis, Tannerella forsythia
Graphical Abstract
INTRODUCTION
Bacterial pathogens have evolved sophisticated mechanisms to ensure survival within their hosts by colonizing host tissue, overcoming host resistance, and establishing specific niches [1]. In human periodontal disease, the microorganisms in dental plaque form a unique niche on the tooth surface and in periodontal pockets and mediate destruction of the periodontium [2,3]. Dental plaque is a biofilm that consists of more than 700 different bacterial species [4,5,6,7], and it is believed to interact with the host as a dynamic and complex microbial community. Microbial interaction or cell-cell communication in dental plaque affects the structure and ultimately the behavior of the biofilm. The results of numerous studies strongly suggest that a population shift toward gram-negative anaerobic species in the plaque biofilm is responsible for the initiation and progression of periodontal disease [8,9,10,11]. Therefore, it is important to characterize interactions among dental plaque microorganisms, especially periodontopathic ones, in order to understand the microbial pathogenesis of periodontal disease.
The most prominent example of microbial interaction in dental plaque biofilm is coaggregation, or interbacterial binding. This phenomenon results from cell-to-cell recognition between specific microbial partners in dental plaque. It has been established that most bacteria in the oral cavity can physically interact with one or more specific partners, and this activity may be responsible for the development and formation of the biofilm [12,13]. Coaggregation can occur between early colonizers (i.e., Streptococcus sanguis and Actinomyces viscosus), between early and late colonizers (i.e., Streptococcus gordonii and Fusobacterium nucleatum), and between late colonizers (i.e., Treponema denticola and Porphyromonas gingivalis) [12,13]. In addition, it may facilitate physiological and metabolic interactions between microorganisms, which may lead to synergistic activity between dental plaque microorganisms and modify their potential virulence [14,15,16].
Two gram-negative strict oral anaerobes, Porphyromonas gingivalis and Tannerella forsythia, have been implicated, along with Aggregatibacter actinomycetemcomitans, as the major etiologic agents in periodontal disease [17]. Together with T. forsythia, P. gingivalis is frequently isolated from periodontal lesions [9,18]. In animal models, a mixed infection with these two organisms has significantly enhanced the virulence potential in abscess formation [19,20], suggesting a possible synergistic effect of P. gingivalis and T. forsythia in periodontal infection. Sonicated protein extracts of T. forsythia have been found to stimulate the growth of P. gingivalis in nutrition-depleted medium in a dose-dependent manner [21]. Collectively, these findings suggest a synergistic effect of P. gingivalis and T. forsythia in the pathogenesis of periodontal disease.
The mechanisms involved in coaggregation have been described for interactions between several of the early colonizers of dental plaque biofilm, including S. gordonii, S. sanguis, and A. viscosus [13]; however, similar information is not yet available for most of the periodontopathic pathogens, including P. gingivalis and T. forsythia. Elucidating these mechanisms may help us better understand the formation and development of periodontitis-associated dental plaque and, thereby, the pathogenesis of periodontal infection.
So far, the exact mechanisms by which P. gingivalis and T. forsythia interact have not been adequately studied, although it has been suggested that these mechanisms are mediated through protein–protein interactions [11,22]. In our previous study [23], it was observed that cell-cell contact between P. gingivalis and T. forsythia was confirmed by electron and epifluoresence microscopic studies. In addition, it was suggested that the coaggregation is a protein-protein interaction, as protease treatment of P. gingivalis and T. forsythia completely blocked the coaggregating activity. The purpose of this study was to identify the surface proteins involved in interbacterial binding between P. gingivalis and T. forsythia.
MATERIALS AND METHODS
Bacterial strains
T. forsythia ATCC 43037 cells were grown on agar plates containing 3.8% heart infusion broth (Becton Dickinson, Sparks, MD, USA), 0.005% N-acetylmuramic acid (Fisher Scientific, Pittsburgh, PA, USA), 5% fetal bovine serum (FBS) (Fisher Scientific), 5 µg/mL of hemin (Fisher Scientific), 0.5 µg/mL of menadione (Fisher Scientific), and 5% yeast extract (Becton Dickinson). Cells of P. gingivalis strain 381 were grown on blood agar plates containing 3% tryptic soy agar (Becton Dickinson,), 0.5% yeast extract, 5 µg/mL of hemin, 1 µg/mL of menadione, and 5% sheep’s blood. These bacteria were cultivated under an anaerobic atmosphere (10% H2, 5% CO2, and 85% N2) at 37°C in a Forma anaerobic chamber (Thermo Scientific, Waltham, MA, USA), as described elsewhere.
Immunofluorescence microscopy
The bonds between the bacterial cells and the biotin-labeled surface proteins of the counterpart cells were visualized as follows. Surface proteins from T. forsythia or P. gingivalis cells were labeled with biotin, as described below, and were mixed with the counterpart P. gingivalis or T. forsythia cells, respectively, for 30 minutes. Bound cells were placed on glass slides, heat-fixed, and washed with methanol. After being blocked with 1% BSA/TBS for 30 minutes, the bacterial cells were incubated with a 1:100 dilution of streptavidin–fluorescein isothiocyanate (FITC) (Thermo Scientific). All the procedures were performed at room temperature. The bacterial cells were observed using a Zeiss LSM 510 confocal microscope (Zeiss, Oberkochen, Germany).
Biotinylation and identification of surface proteins bound to counterpart bacterial cells
The surface proteins of T. forsythia and P. gingivalis were labeled with biotin as follows. Bacterial cells were cultivated, harvested, and washed three times with PBS. Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific) was added to the individual bacterial cells at a final concentration of 1 mg/mL, and the cells were incubated at RT for 30 minutes by rotation. Unbound biotin was removed by washing the cells three times with 0.1 M of glycine. Biotinylated surface proteins from T. forsythia were extracted with 2% deoxycholic acid (Thermo Fisher Scientific), dialyzed in 10 mM of PBS, and then mixed with whole P. gingivalis cells for 2 hours at RT. The resulting P. gingivalis cells labeled with biotinylated T. forsythia surface proteins were then separated by SDS-PAGE and were transferred to a nitrocellulose membrane, as described elsewhere. Subsequently, biotinylated T. forsythia surface proteins were detected with the use of streptavidin–horseradish peroxidase (HRP) (Thermo Fisher Scientific) and 4-chloro-1-naphthol (4-CN) (Thermo Fisher Scientific), according to the manufacturer’s instructions. The same procedures were performed for identifying the biotinylated surface proteins of P. gingivalis that are involved in the interaction with T. forsythia cells.
In-gel enzymatic digestion of proteins
Detected protein bands were excised from the gel, transferred to 100 µL of 0.01 M of dithiothreitol/0.1 M of Tris (pH 8.5), and incubated at 55°C for 1 to 2 hours. After cooling, the gel bands were incubated with 100 µL of 0.03 M of iodoacetamide/0.1 M of Tris (pH 8.5) for 30 minutes in the dark, were washed twice with 200 µL of 0.05 M of Tris (pH 8.5)/30% acetonitrile for 20 minutes with shaking, and were then washed once with 100 µL of acetonitrile for 2 to 3 minutes until the gel turned an opaque white. After removing the acetonitrile, the gel pieces were dried for 20 to 30 minutes in a Speed-Vac Concentrator (Thermo Scientific). Gels were digested by adding 0.08 to 0.10 µg of modified trypsin (sequencing grade, Roche Molecular Biochemicals, Indianapolis, IN, USA) in 15 to 20 µL of 0.025 M of Tris (pH 8.5) or enough volume to completely hydrate the gel. The tubes were placed in a heating block at 32°C and left overnight. Peptides were extracted with 50 µL of 50% acetonitrile/2% trifluoroacetic acid, and the combined extracts were either dried or reduced in volume in a Speed-Vac Concentrator to about 10 µL pending further analysis.
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis
LC-MS/MS analysis was performed on a Micromass hybrid quadrupole/time-of-flight mass spectrometer with a nanoelectrospray source (Waters, Milford, MA, USA). Capillary voltage was set at 1.8 kV and cone voltage at 32 V; the collision energy was set according to the mass and charge of the ion, from 14 eV to 50 eV. Chromatography was performed on an LC Packings HPLC with a C18 PepMap column using a linear acetonitrile gradient with a flow rate of 200 nL/min. Raw data files were processed using MassLynx ProteinLynx software (Boston, MA, USA), and .pkl files were submitted for a database search at http://www.matrixscience.com using the Mascot scoring algorithm. The peptide sequences of the identified proteins were determined from a database containing whole genome sequences of T. forsythia (available at http://www.ncbi.nlm.nih.gov/genome).
RESULTS
Confirmation of biotin-labeled surface proteins bound to P. gingivalis and T. forsythia cells
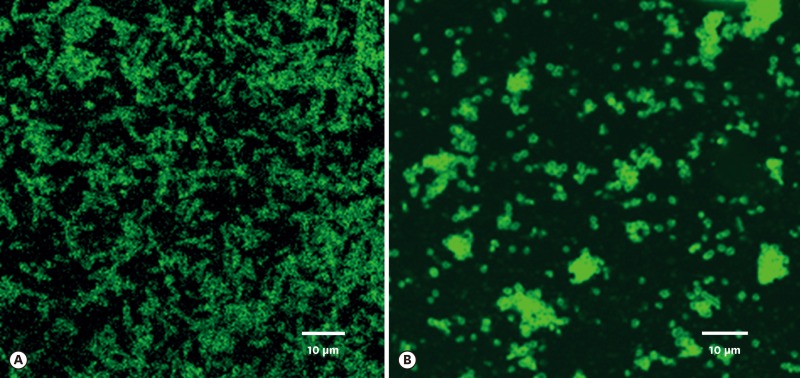
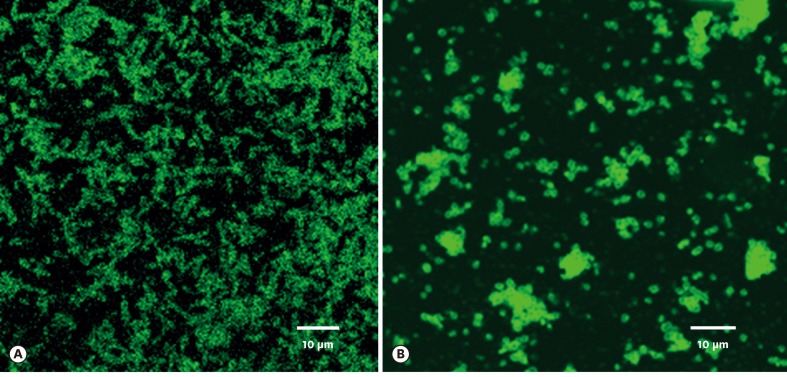
The surface proteins of T. forsythia and P. gingivalis cells were labeled with Sulfo-NHS-LC-Biotin. Unbound biotins were removed by washing three times with 100 mM of glycine. To check whether the surface proteins could bind to the counterpart cells, biotinylated T. forsythia and P. gingivalis cells were dissolved in 2% deoxycholic acid and underwent dialysis in 10 mM of phosphate buffers to remove the detergents in the solution. T. forsythia lysates and P. gingivalis lysates were incubated with P. gingivalis and T. forsythia cells, respectively, at 4°C overnight. The unbound lysates were removed by centrifugation and washed twice with PBS. T. forsythia cells and P. gingivalis cells that were bound with P. gingivalis and T. forsythia surface proteins, respectively, were visualized on confocal microscopy with streptavidin-FITC (Figure 1).
Figure 1.
Surface proteins of P. gingivalis and T. forsythia bound to T. forsythia (A) and P. gingivalis cells (B), respectively, as observed under epifluorescence microscopy using FITC-conjugated streptavidin antibody. Fluorescence-labeled rod-shaped T. forsythia cells are clearly evident (A). Fluorescence-labeled coccobacilli-shaped P. gingivalis cells can be seen (B). For details, refer to the main text.
Identification of surface proteins bound to T. forsythia and P. gingivalis
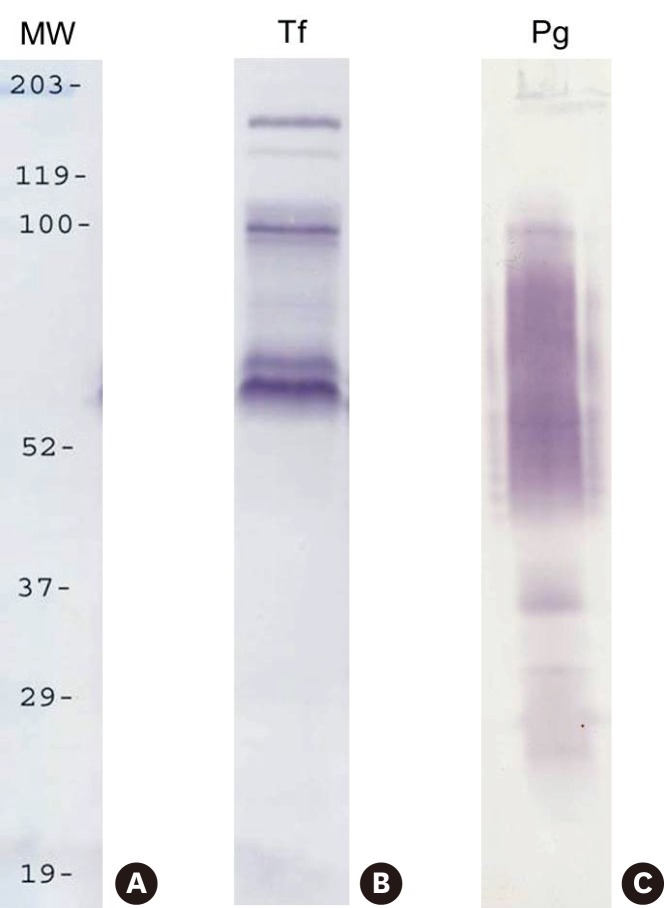
T. forsythia and P. gingivalis cells to which the surface proteins of P. gingivalis and T. forsythia cells, respectively, were attached were dissolved in 3% SDS buffer. The resultant total proteins were separated in 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. Biotinylated proteins were then detected with streptavidin-HRP and 4-CN. It was found that three major T. forsythia proteins having molecular masses of 161, 100, and 62 kDa were involved in binding to P. gingivalis cells, and that P. gingivalis proteins having molecular masses of 35, 32, and 26 kDa were involved in binding to T. forsythia cells (Figure 2).
Figure 2.
Cell-surface labeling with biotin. The surface proteins of P. gingivalis and T. forsythia were labeled with Sulfo-NHS-LC-Biotin and mixed with T. forsythia and P. gingivalis cells, respectively. Subsequently, the mixtures were subjected to SDS-PAGE, and then transferred onto nitrocellulose membranes. Biotinylated P. gingivalis and T. forsythia surface proteins were detected with streptavidin–HRP and 4-CN. Lanes: MW, molecular weight marker; Tf, biotinylated T. forsythia surface proteins bound to P. gingivalis; Pg, biotinylated P. gingivalis surface proteins bound to T. forsythia
LC-MS/MS analysis of the 100-kD T. forsythia protein that was bound to P. gingivalis revealed that this protein was actually a TonB-linked outer membrane protein (118 kD). However, the nature of the other T. forsythia and P. gingivalis surface proteins could not be determined by LC-MS/MS analysis.
DISCUSSION
The purpose of this study was to identify surface proteins that are involved in interbacterial binding between P. gingivalis and T. forsythia. So far, the molecular mechanisms responsible for the interaction between P. gingivalis and T. forsythia have not been elucidated. Yoneda et al. [19] found that mixed infections with P. gingivalis and T. forsythia had a synergistic effect in causing abscess formation in mice and suggested a role for gingipains of P. gingivalis in this effect. Gingipains are cysteine proteinases known to produce usable peptides and amino acids through the digestion of host proteins. Their biological functions include disturbance of host immune systems, impairment of the bactericidal functions of polymorphonuclear leukocytes, acquisition of heme, and maturation of fimbriae [24]. Furthermore, Kamaguchi et al. [25] revealed the role of adhesion domains within gingipains in the coaggregating activity between P. gingivalis and Prevotella intermedia. Therefore, the gingipains of P. gingivalis may be involved in interactions with other microorganisms in dental plaque biofilms. On the other hand, the molecular components of T. forsythia involved in interbacterial binding have not yet been identified, except for a surface (S-) layer. Shimotahira et al. [26] showed that the S-layer is in fact involved in coaggregation with Streptococcus sanguis, Streptococcus salivarius, and P. gingivalis, because the S-layer–deficient mutants exhibited reduced coaggregation with these microorganisms.
In our previous study, we showed that T. forsythia possesses a unique surface (S-) layer that is involved in hemagglutination, in the attachment to and invasion of epithelial cells, and in abscess formation in mice [27]. We subsequently determined that the S-layer is composed of two large glycoprotein subunits [28]. Being an outermost surface structure and a hemagglutinin, the S-layer could presumably be involved in the coaggregation of T. forsythia with P. gingivalis. In addition, the S-layer–deficient mutant appeared to exhibit reduced coaggregating activity with P. gingivalis, as compared with that of the wild-type strain [26]. These findings suggest that the S-layer is, at least in part, involved in coaggregation with P. gingivalis. Considering that heat treatment of T. forsythia significantly increased its coaggregating activity [23], it is likely that the carbohydrate components of the S-layer are involved in the surface interaction with P. gingivalis. However, the results of the present study did not provide direct evidence that the S-layer of T. forsythia is involved in a surface–surface interaction with P. gingivalis, since biotin-labeling of the S-layer was not confirmed in SDS-PAGE. It can be postulated that the S-layer was not adequately labeled with biotin and was therefore not identified in the surface interaction with P. gingivalis.
One of the T. forsythia surface proteins bound to P. gingivalis cells was identified; however, other biotin-labeled T. forsythia and P. gingivalis surface proteins could not be determined by means of LC-MS/MS, since there were probably too many minor protein bands or non-specific backgrounds associated with the major proteins to allow a precise sequence determination. The single identified T. forsythia surface protein was TonB-linked outer membrane protein (Gene ID: TF1439). Although the function of this surface protein has not yet been determined, it has been shown to be produced only in vivo in patients with chronic periodontitis [29]. Therefore, along with its involvement in the coaggregation with P. gingivalis and in vivo expression inside the host, the TF1439 protein may be involved in chronic periodontitis. Based on our results, it can be postulated that T. forsythia binds to P. gingivalis in the actual disease process, enhancing the virulence potential of these two bacteria, but this possibility will require further testing.
In conclusion, the interbacterial binding between P. gingivalis and T. forsythia appears to be mediated by multiple protein–protein interactions. One T. forsythia outer membrane protein (TonB-linked outer membrane protein) was identified as a surface protein involved in this coaggregating activity. Other unidentified surface proteins should be determined from T. forsythia and P. gingivalis in order to elucidate the molecular mechanisms that mediate coaggregation between these two periodontal pathogens.
ACKNOWLEDGEMENTS
This study was partially supported by Chonnam National University (2014) and the Chonnam National University Hospital Research Institute of Clinical Medicine (Grant CRI 14020-1).
Footnotes
CONFLICT OF INTEREST: No potential conflicts of interest relevant to this article were reported.
References
- 1.Finlay BB, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 3.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 4.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 6.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96:14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 9.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 10.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 12.Kolenbrander PE. Coaggregations among oral bacteria. Methods Enzymol. 1995;253:385–397. doi: 10.1016/s0076-6879(95)53033-9. [DOI] [PubMed] [Google Scholar]
- 13.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschbaum M, Schultze-Mosgau S, Pfister W, Eick S. Mixture of periodontopathogenic bacteria influences interaction with KB cells. Anaerobe. 2010;16:461–468. doi: 10.1016/j.anaerobe.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 16.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 18.Kamma JJ, Nakou M, Manti FA. Predominant microflora of severe, moderate and minimal periodontal lesions in young adults with rapidly progressive periodontitis. J Periodontal Res. 1995;30:66–72. doi: 10.1111/j.1600-0765.1995.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoneda M, Hirofuji T, Anan H, Matsumoto A, Hamachi T, Nakayama K, et al. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: involvement of gingipains in a synergistic effect. J Periodontal Res. 2001;36:237–243. doi: 10.1034/j.1600-0765.2001.036004237.x. [DOI] [PubMed] [Google Scholar]
- 20.Takemoto T, Kurihara H, Dahlen G. Characterization of Bacteroides forsythus isolates. J Clin Microbiol. 1997;35:1378–1381. doi: 10.1128/jcm.35.6.1378-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoneda M, Yoshikane T, Motooka N, Yamada K, Hisama K, Naito T, et al. Stimulation of growth of Porphyromonas gingivalis by cell extracts from Tannerella forsythia . J Periodontal Res. 2005;40:105–109. doi: 10.1111/j.1600-0765.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 22.Yao ES, Lamont RJ, Leu SP, Weinberg A. Interbacterial binding among strains of pathogenic and commensal oral bacterial species. Oral Microbiol Immunol. 1996;11:35–41. doi: 10.1111/j.1399-302x.1996.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Um HS, Lee SW, Park JH, Nauman RK. Coaggregation between Porphyromonas gingivalis and Tannerella forsythia . J Korean Acad Periodontol. 2006;36:265–272. [Google Scholar]
- 24.Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- 25.Kamaguchi A, Ohyama T, Sakai E, Nakamura R, Watanabe T, Baba H, et al. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia . Microbiology. 2003;149:1257–1264. doi: 10.1099/mic.0.25997-0. [DOI] [PubMed] [Google Scholar]
- 26.Shimotahira N, Oogai Y, Kawada-Matsuo M, Yamada S, Fukutsuji K, Nagano K, et al. The surface layer of Tannerella forsythia contributes to serum resistance and oral bacterial coaggregation. Infect Immun. 2013;81:1198–1206. doi: 10.1128/IAI.00983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabet M, Lee SW, Nauman RK, Sims T, Um HS. The surface (S-) layer is a virulence factor of Bacteroides forsythus . Microbiology. 2003;149:3617–3627. doi: 10.1099/mic.0.26535-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee SW, Sabet M, Um HS, Yang J, Kim HC, Zhu W. Identification and characterization of the genes encoding a unique surface (S-) layer of Tannerella forsythia . Gene. 2006;371:102–111. doi: 10.1016/j.gene.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Yoo JY, Kim HC, Zhu W, Kim SM, Sabet M, Handfield M, et al. Identification of Tannerella forsythia antigens specifically expressed in patients with periodontal disease. FEMS Microbiol Lett. 2007;275:344–352. doi: 10.1111/j.1574-6968.2007.00906.x. [DOI] [PubMed] [Google Scholar]