Abstract
Purpose
We evaluated the efficacy of a mouthwash containing potassium nitrate (KNO3) as its main component, along with sodium fluoride (NaF) and cetylpyridinium chloride (CPC). The primary endpoint was the relief of dentin hypersensitivity (DH) against the cold stimuli. The effects on other DH tests and periodontal inflammation were also evaluated.
Methods
We used a single-center, double-blind, placebo-controlled, randomized design. A total of 82 patients with DH (40 in the test group, 42 placebo controls) were analyzed using visual analog scales (VASs) for a cold test, a tactile test, a compressive air test, and self-reported pain during daily activities, as well as clinical parameters including plaque index, gingival index, modified sulcular bleeding index (mSBI), gingival recession, and probing depth, which were collected at baseline and after four and six weeks of mouthwash use.
Results
VAS scores for cold sensations, tactile sensations, the compressive air test, and self-reported pain significantly decreased from baseline during the six weeks in both groups (P<0.01), and no significant differences between the groups were found. In male patients (10 in the test group and 7 in the control group), both groups showed significant reductions in VAS scores for the cold test over the six weeks, and greater reductions were found in the test group than in the control group between four and six weeks (P=0.01) and between baseline and six weeks (P<0.01). In addition, the mSBI in the test group significantly decreased from baseline during the six weeks (P<0.01), and the changes at four and six weeks from baseline were significantly greater in the test group compared to the control group (P=0.03 and P=0.02, respectively).
Conclusions
A mouthwash containing a mixture of KNO3, NaF, and CPC reduced DH and gingival inflammation, however, the efficacy was comparable to the control group.
Keywords: Dentin hypersensitivity, Mouthwash, Potassium nitrate, Sodium fluoride, Cetylpyridinium chloride, Gingival inflammation
Graphical Abstract
INTRODUCTION
In everyday practice, dentists are frequently faced with patients suffering from dentin hypersensitivity (DH). DH is characterized by short, sharp pain that arises from exposed dentin in response to a stimulus including thermal, osmotic, mechanical, electrical, or chemical stimuli, and cannot be ascribed to any other form of dental defect or pathology [1]. The intensity of the pain may range from minor discomfort to the severe disturbance of daily activities, such as avoiding specific foods and modifying personal hygienic care [2]. Previous studies have reported a wide range of prevalence values (3%–73%), due to heterogeneity in assessment methods and study designs [3]. In a review of available studies, DH was found to be present in 10%–30% of the general population, with a higher incidence among females and a peak in the third and fourth decades of life [3,4,5]. Factors that increase susceptibility to DH appear to include periodontal disease, active periodontal therapy, gingival recession, and erosive toothwear that exposes dentin [6].
Although the mechanism of DH is still unclear, the hydrodynamic theory proposed by Brännström [7] has been widely accepted as an explanation of this pattern of sensitivity. The fluid within the dentinal tubule flows inward or outward when the exposed opening at the dentin surface is affected by stimuli. The change in flow activates the action potential of nerve fibers at the pulp-dentin border and thus generates a painful sensation. DH may be related to the exposure and patency of the dentinal tubules, with factors such as diameter, the number of patent dentinal tubules, and patency status distinguishing sensitive from non-sensitive teeth [8].
The management of DH includes directed therapy to interfere with the mechanism of DH temporarily or permanently. The two main strategies for treating DH are decreasing neural transmission and physically occluding the patent tubule [9]. Attempts have been made to plug the exposed openings of dentinal tubules using chemical agents such as ions, salts, and proteins; physical agents, including restorative materials (dentin sealers); and laser treatments. Some representative agents are stannous fluoride, strontium salts, oxalates, calcium phosphates, and fluorides [10]. Another potential desensitization agent is potassium nitrate (KNO3), which is known to act on the nerves [11,12]. It causes an increased extracellular concentration of potassium ions, which is thought to help depolarize the nerve, thereby reducing nerve excitation and the associated pain sensation.
Toothpastes and mouthwashes containing desensitizing ingredients are the most common products prepared for the home treatment of DH. They are considered to be noninvasive, simple, and cost-effective, and thus are often the first-line approach to treat DH [13]. Recently, multiple mixtures of active ingredients have been used to improve the clinical outcomes of DH treatment, and these ingredients have also been combined with other components with whitening or antiplaque functions for various therapeutic and aesthetic benefits. The mouthwash used in this study was a novel mixture of KNO3 with sodium fluoride (NaF) and cetylpyridinium chloride (CPC) for additive effects in easing DH symptoms and improving oral hygiene, respectively [10,14]. We evaluated its efficacy against DH and its safety, as well as its effects on periodontal inflammation as a secondary outcome.
MATERIALS AND METHODS
Patient screening and enrollment
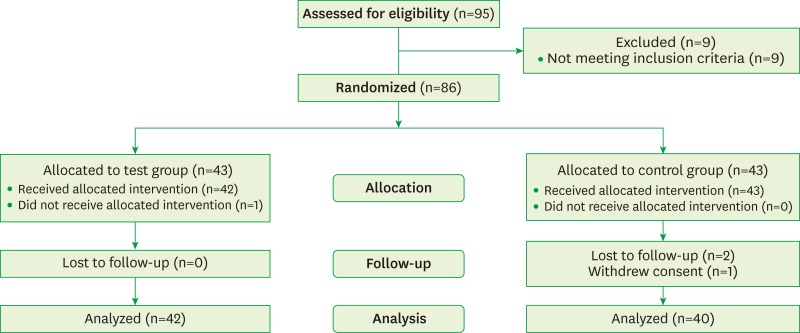
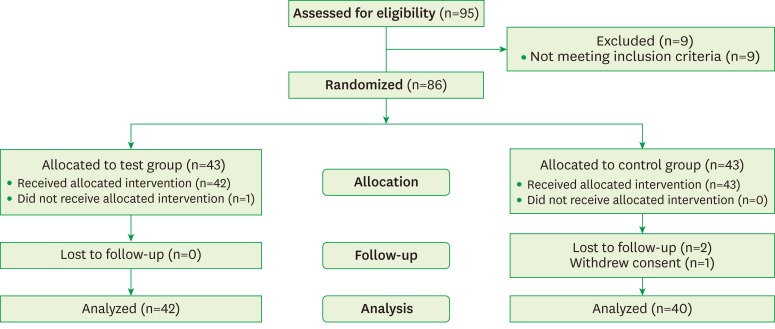
This study was planned with a single-center, double-blind, placebo-controlled, randomized, parallel-group design. The protocol was approved by the Committee on Ethics of Kyung Hee Clinical Research Institute, Kyung Hee University Medical Center, Seoul, Korea (IRB No. KHDIRB-1204-2). The patients were given written explanations of the study, and they provided written informed consent. Subjects were recruited from July 2012 to December 2012, and 95 patients were assessed for eligibility. Subjects of both genders aged 25–65 years who had been diagnosed with generalized chronic incipient periodontitis and had symptoms of DH were screened. They had no specific dental or medical issues other than DH that might have caused spontaneous orofacial or dental pain. DH was diagnosed by self-reported symptoms of sensitivity to cold and hot food or beverages and toothbrushing. It was also confirmed by the compressive air test for an evaporative stimulus. Since nine subjects did not meet the inclusion criteria, 86 subjects were randomly assigned to either the test group, who received a mouthwash containing KNO3, NaF, and CPC as the main components, or a placebo control group who received an identical-appearing mouthwash in which those components (KNO3, NaF and CPC) were not included. At the end of the clinical study, results were analyzed for 82 patients: 42 in the control group (seven males, 35 females) and 40 in the test group (10 males, 30 females) (Figure 1).
Figure 1.
Diagram of patient enrollment.
Clinical evaluation and parameters to assess efficacy toward DH
The visual analog scale (VAS) scores for the cold test throughout the entire dentition was used as the primary outcome. An ice stick wrapped in wet gauze was placed against the buccal surface of all teeth, and VAS scores (from 0 for no pain or discomfort to 10 for intense pain or discomfort; 0–100 mm VAS) were measured. Teeth with restorations that covered the cervical region were excluded. The mean VAS score for each patient was recorded.
The secondary outcomes included VAS scores for the tactile test, compressive air test, and self-reported pain during daily activities, as well as the following clinical examinations of the entire dentition: the plaque index (PI; Silness & Löe) [15], gingival index (GI; Löe & Silness) [16], modified gingival sulcular bleeding index (mSBI; Barnett et al.) [17], gingival recession (GR), and probing depth (PD). In the tactile test (tactile sensitivity), pressure was applied on the labial surface of the cervical area of the tooth with a periodontal probe. In the compressive air test (evaporative air stimulus), an air blast from a dental unit syringe was applied to the tooth surface for one second, and the average score on a 100-mm VAS scale was recorded for each patient (0 for no pain or discomfort to 10 for intense pain or discomfort). The self-reported pain VAS reflected the subjects’ own rating of pain or discomfort during daily routines such as drinking and eating cold/hot beverages or foods, eating sweet or sour foods, breathing cold air, toothbrushing, or performing any oral habits that might induce DH. In the stimuli testing to obtain VAS results, at least five minutes were allowed between the evaluations.
Clinical study design and treatment
At the first visit, patients were screened for DH and assessed for eligibility for the study. For patients who agreed to participate in the study, scaling was performed and toothbrushing instructions using the modified Bass method three times a day were provided. A two-week pre-trial run-in period was included, during which the patients brushed their teeth without using any dentifrice but water. The experiment started with the second visit (baseline visit), when the subjects were randomly allocated to the placebo control group or the test group in a double-blinded design. Both mouthwashes were provided in identical bottles labeled with only the subjects’ study numbers. Baseline data for the VAS scores of each test and for each clinical examination were recorded. Subjects were instructed to brush their teeth three times a day without toothpaste followed by rinsing with 10-15 mL of mouthwash (placebo or test solution) for one minute. Two weeks after the baseline visit, a telephone call was made to confirm that the patient had been using the mouthwash properly and to determine whether the patient had experienced any kind of adverse event. Visits 3 and 4 were made four and six weeks after the baseline visit, respectively, to collect the measurements, evaluate the efficacy of the mouthwash, and check for the safety and oral tolerance of the mouthwash during the period of the study.
Safety
For the safety analysis, all randomized subjects who had been treated with the test or control products at least once were included (43 patients in the test group and 42 patients in the control group). Treatment-emergent adverse events (TEAEs) throughout the study period were screened and reported as the number and proportion of patients who experienced the events, following the guidelines of the Medical Dictionary for Regulatory Activities System Organ Class and Preferred Term. All TEAEs were recorded, regardless of potential causal relationships.
Statistical analysis
Demographic features at baseline were compared between the control and test groups using the Wilcoxon rank-sum test (age analysis) and chi-square test (gender analysis). Complete data sets on VAS scores for the various stimuli and clinical examinations were collected from 82 subjects (40 in the test group and 42 in the control group) at baseline and at four and six weeks, and are presented as means and standard deviations.
The primary efficacy outcome was measured by changes in VAS scores on the cold test over the course of six weeks after the baseline visit. Changes in VAS scores on the tactile test, compressive air test, and self-reported pain, as well as changes in the clinical parameters, were used as secondary outcomes. For the comparison of changes in the primary outcome (VAS from the cold test) between treatments during the study period, repeated measures analysis of variance with the post-hoc Bonferroni test was used for within-group correlations, and the paired t-test was used for pairwise comparisons between the groups according to the normality of the distribution. Otherwise, the Friedman test was used to evaluate the differences in outcomes in the groups over the course of the study period when the data set had a non-normal distribution, which was the case for all parameters except the VAS from the cold test. The Wilcoxon signed-rank test was performed for within-group correlations and the Wilcoxon rank-sum test was used for pairwise comparisons between the groups. The threshold for statistical significance was set at P<0.05.
RESULTS
The demographic features of the patients are presented in Table 1. The mean age of the patients was 49.7±10.8 years in the control group and 46.7±10.1 years in the test group, which was not found to be a significant difference (P=0.15). The gender distribution also did not significantly differ between the groups (P=0.35).
Table 1. Demographic features of the patients enrolled in the study.
| Demographics | Control (n=42) | Test (n=40) | Total (n=82) |
|---|---|---|---|
| Age (yr) | |||
| Mean±SD | 49.7±10.8 | 46.7±10.1 | 48.3±10.5 |
| Median (Min, Max) | 51.5 (27.0, 64.0) | 50.5 (26.0, 62.0) | 51.0 (26.0, 64.0) |
| P-value | 0.15 | ||
| Gender, n (%) | |||
| Male | 7 (16.7) | 10 (25.0) | 17 (20.7) |
| Female | 35 (83.3) | 30 (75.0) | 65 (79.3) |
| P-value | 0.35 |
n, number of patients; SD, standard deviation; Min, minimum; Max, maximum.
The VAS scores for all stimuli were significantly lower (P<0.01) at four and six weeks compared to baseline in both groups; however, no significant differences were found between the groups (Table 2). In the VASs of the cold test and self-reported pain, a statistically significant reduction between four and six weeks (P<0.01) was also found in each group, whereas in the tactile and compressive air tests, a significant effect was seen only in the control group (P<0.01). The primary outcome of changes in the VAS score on the cold test was also analyzed by gender (Table 3). In male patients, a significant difference was observed in the change in the VAS score between groups during the study period (P<0.01). Statistically significant reductions in VAS scores compared to baseline were seen in both groups at four weeks (P<0.01 in both groups) and six weeks (P<0.01 in the test group, P=0.01 in the control group). However, a significant reduction between four and six weeks was found only in the test group (-1.80±1.42, P<0.01), whereas the control group had a nonsignificant change (0.21±1.41, P=0.70). The changes in the VAS score between baseline and six weeks (-4.00±1.65 in the test group and -1.67±1.21 in the control group), and between four and six weeks (-1.80±1.42 in the test group and 0.21±1.41 in the control group), were significant between the test and control groups (P<0.01 and P=0.01, respectively). In female patients, a significant reduction in the VAS for the cold test was observed at four and six weeks compared to baseline in both groups (P<0.01); however, no significant differences were found between the groups.
Table 2. Visual analog scale (VAS) scores for the cold test, tactile test, compressive air test, and self-reported pain at baseline and at four and six weeks in the control (n=42) and test (n=40) groups.
| Test stimuli | Observation periods | P-valuea) | ||
|---|---|---|---|---|
| Baseline | 4 wk | 6 wk | ||
| Cold test | ||||
| Test group | 6.31±1.73 | 4.64±2.36b) | 3.82±2.38b,c) | |
| Change from baseline | -1.68±1.71 | -2.39±1.87 | 0.43 | |
| Control group | 5.96±1.98 | 4.28±2.12b) | 3.58±1.84b,c) | |
| Change from baseline | -1.68±1.69 | -2.49±1.97 | ||
| Tactile test | ||||
| Test group | 2.45±2.00 | 1.07±1.59b) | 0.83±1.48b) | |
| Change from baseline | -1.38±1.90 | -1.62±2.04 | 0.40 | |
| Control group | 2.23±2.03 | 1.15±1.50b) | 0.26±0.74b,c) | |
| Change from baseline | -1.08±2.17 | -1.97±1.93 | ||
| Compressive air test | ||||
| Test group | 4.91±1.64 | 3.29±2.18b) | 2.81±1.98b) | |
| Change from baseline | -1.61±1.58 | -2.10±1.97 | 0.38 | |
| Control group | 4.53±1.78 | 3.08±1.74b) | 2.53±1.66b,c) | |
| Change from baseline | -1.44±1.93 | -2.00±2.24 | ||
| Self-reported pain | ||||
| Test group | 5.87±1.29 | 4.73±2.14b) | 3.60±2.54b,c) | |
| Change from baseline | -1.13±1.91 | -2.27±2.42 | 0.36 | |
| Control group | 5.92±1.21 | 4.26±1.83b) | 3.79±1.98b,c) | |
| Change from baseline | -1.66±1.89 | -2.13±2.09 | ||
Values are presented as mean±standard deviation.
n, number of patients.
a) P-value for repeated measures analysis of variance (the cold test) and the Friedman test (tactile test, compressive air test, and self-reported pain).
b)Statistically significant difference from the baseline in each group (P<0.05; paired t-test for the cold test, the Wilcoxon signed-rank test for the tactile test, compressive air test, and self-reported pain).
c)Statistically significant difference from the four-week values in each group (P<0.05; paired t-test for the cold test, the Wilcoxon signed-rank test for the tactile test, compressive air test, and self-reported pain).
Table 3. Visual analog scale (VAS) for the cold test according to gender at baseline and at four weeks and six weeks in the control (n=42) and test (n=40) groups.
| Gender VAS for the cold test |
Observation periods | P-valuea) | ||
|---|---|---|---|---|
| Baseline | 4 wk | 6 wk | ||
| Male | ||||
| Test group (n=10) | 6.30±1.44 | 4.10±2.48b) | 2.30±2.07b,c) | |
| Change from baseline | -2.20±1.92 | -4.00±1.65d) | <0.01 | |
| Control group (n=7) | 4.60±2.02 | 2.71±1.63b) | 2.93±2.05b) | |
| Change from baseline | -1.88±0.68 | -1.67±1.21 | ||
| Female | ||||
| Test group (n=30) | 6.32±1.84 | 4.82±2.33b) | 4.33±2.28b) | |
| Change from baseline | -1.50±1.61 | -1.99±1.83 | 0.26 | |
| Control group (n=35) | 6.24±1.88 | 4.60±2.08b) | 3.71±1.79b,c) | |
| Change from baseline | -1.64±1.85 | -2.53±1.96 | ||
Values are presented as mean±standard deviation.
n, number of patients.
a) P-value for repeated measures analysis of variance.
b)Statistically significant difference from baseline in each group (paired t-test, P<0.05).
c)Statistically significant difference from the four-week values in each group (paired t-test, P<0.05).
d)Statistically significant difference in the change from baseline compared to the control group (paired t-test, P<0.05).
Regarding the clinical parameters, the mSBI showed a significant difference between the treatment groups during the six-week study period (Friedman test, P=0.02) (Table 4). The test group showed a statistically significant reduction at four and six weeks compared to baseline (P<0.01 for both periods), whereas the control group did not (P=0.83 and P=0.38, respectively). In addition, the change in the mSBI between baseline and four weeks (-0.25±0.51 in the test group and -0.01±0.37 in the control group), and between baseline and six weeks (-0.25±0.48 in the test group and -0.05±0.39 in the control group), significantly differed (P=0.03 and P=0.02, respectively). Compared to baseline, the GI also showed significant reductions at four weeks (P<0.01 in the test group, P=0.04 in the control group) and six weeks (P<0.01 in both groups) in both groups. However, no significant differences between the groups were found in pairwise comparisons. The PI of the control group was significantly lower at four and six weeks compared to baseline (P<0.01 and P=0.02, respectively); however, no significant differences were found between the test and control groups. The GR and PD did not change significantly during the time periods or between groups, although a significant reduction of PD was found at 4 weeks from baseline in the control group (P<0.01).
Table 4. Results of clinical periodontal examinations at baseline and at four weeks and six weeks in the control (n=42) and test (n=40) groups.
| Periodontal examinations | Observation periods | P-valuea) | ||
|---|---|---|---|---|
| Baseline | 4 wk | 6 wk | ||
| PI | ||||
| Test group | 0.50±0.55 | 0.37±0.48 | 0.35±0.57 | |
| Change from baseline | -0.13±0.49 | -0.15±0.54 | 0.69 | |
| Control group | 0.54±0.62 | 0.25±0.38b) | 0.32±0.46b) | |
| Change from baseline | -0.29±0.54 | -0.22±0.61 | ||
| GI | ||||
| Test group | 0.63±0.75 | 0.26±0.43b) | 0.22±0.40b) | |
| Change from baseline | -0.36±0.71 | -0.40±0.74 | 0.19 | |
| Control group | 0.59±0.63 | 0.45±0.61b) | 0.34±0.53b) | |
| Change from baseline | -0.14±0.43 | -0.25±0.62 | ||
| mSBI | ||||
| Test group | 0.29±0.50 | 0.04±0.15b) | 0.04±0.17b) | |
| Change from baseline | -0.25±0.51c) | -0.25±0.48c) | 0.02 | |
| Control group | 0.24±0.46 | 0.23±0.50 | 0.19±0.46 | |
| Change from baseline | -0.01±0.37 | -0.05±0.39 | ||
| GR | ||||
| Test group | 1.21±0.87 | 1.24±0.84 | 1.25±0.85 | |
| Change from baseline | 0.02±0.32 | 0.04±0.39 | 0.05 | |
| Control group | 0.92±0.78 | 0.88±0.71 | 0.88±0.69 | |
| Change from baseline | -0.04±0.33 | -0.04±0.18 | ||
| PD | ||||
| Test group | 1.46±0.48 | 1.40±0.46 | 1.30±0.54 | |
| Change from baseline | -0.05±0.32 | -0.15±0.50 | 0.15 | |
| Control group | 1.61±0.53 | 1.47±0.53b) | 1.50±0.46 | |
| Change from baseline | -0.14±0.32 | -0.11±0.46 | ||
Values are presented as mean±standard deviation.
n, number of patients; PI, plaque index; GI, gingival index; mSBI, modified gingival sulcular bleeding index; GR, gingival recession; PD, probing depth.
a) P-value using the Friedman test.
b)Statistically significant difference from baseline in each group (Wilcoxon signed-rank test, P<0.05).
c)Statistically significant difference in change from baseline compared to the control group (Wilcoxon rank-sum test, P<0.05).
Among the 86 randomized patients, one patient in the control group did not receive any treatment, and therefore 85 patients were included in the safety analysis. One patient (2.38%) in the control group experienced one adverse event, namely, nasal congestion from a respiratory system disorder. This symptom was mild and transient, did not result in the patient’s withdrawal from the study, and was not considered to be related to the product. The rest of the patients had no adverse events. The product was judged to be safe.
DISCUSSION
Potassium salts, including KNO3, are the most popular substance used for the treatment of DH and act by disrupting the nerve transmission leading to sensations of pain [12]. Although the effect of potassium salts on DH has been controversial, a recent meta-analysis indicated that symptoms of DH may be eased by the use of potassium-containing toothpaste [18,19]. However, these results were mostly limited to the toothpaste form of delivery and focused on the role of a single component without any other additives that could enhance the therapeutic effects. In the present study, the efficacy of a mouthwash containing KNO3 as a main active ingredient, along with NaF and CPC, was evaluated in patients with DH. Fluoride salts have been reported to be effective to treat DH [20], and CPC has been shown to inhibit plaque formation [21]. Our formulation was primarily designed to reduce hypersensitivity and secondarily to improve periodontal inflammation.
Most home care products for the treatment of DH have been formulated as toothpaste, and several clinical trials and systematic reviews have evaluated the effects of desensitizing toothpaste versus placebo [18,19]. However, a number of conventional components are also present in toothpaste and could have influenced sensitivity positively or negatively [20]. Few results have been reported from studies utilizing a mouthwash solution as an adjunct to toothbrushing with toothpaste [21], and evaluating the outcomes of such studies is challenging due to the use of both hygiene strategies [22]. In order to minimize this problem, the patients in this study removed plaque only by mechanical brushing without toothpaste and then rinsed with the solution.
We found that the VAS scores measuring the sensitivity for various stimuli decreased significantly over the study period in both the test and control groups. Across the entire study population, no differences in VAS scores were found between the two groups. However, compared to females, males in the test group showed a significantly greater reduction in VAS score change on the cold test (primary outcome) at six weeks from the baseline than was observed in the control group (-4.00±1.65 in the test group, -1.67±1.21 in the control group). This finding must be interpreted with caution because the baseline scores differed between the groups. The VAS scores were higher in the test group at the starting point (6.30±1.44 in the test group, 4.60±2.02 in the control group), and these values dramatically decreased to become similar to those of the control group at six weeks.
Along with addressing the limited extent of standardization in the baseline data, it would be necessary to increase the amount of male subjects included in future studies to clarify gender discrepancies in the effects of this treatment on DH, since the sample of male subjects in this study was small (10 in the test group and eight in the control group).
A considerable placebo effect was found in the present study, corresponding to previous clinical trials of DH treatments that reported placebo effects of approximately 30%–40% depending on the parameter [22,23,24]. This phenomenon is a response to the intervention itself and consists of complex physiologic and psychological manifestations of the patient’s desire for symptom relief [25]. Moreover, positive emotions and motivation can activate central pain inhibition and release endorphins, which in turn modulate painful stimuli from the peripheral system [26]. A wash-in period was used before the experimental phase in order to minimize the variability in DH regression caused by previous medication and to evaluate the placebo effect, if only observationally [22]. It is possible that another phenomenon, the Hawthorne effect, could have occurred, contributing to the regression of DH in both groups [22]. The Hawthorne effect refers to responses to non-interventional procedures that involve frequent recalls during the study, improved oral hygiene, and patient compliance. As the patients were required not to use toothpaste during daily brushing, instructions for the proper method of toothbrushing were prepared beforehand. Better oral hygiene may have allowed saliva to reach the patent dentinal tubule and occluded the tubule with mineral depositions from the saliva [27,28]. Therefore, DH may have regressed due to improvements in self-performed mechanical plaque control, which could partly explain the limited differences between the groups. The placebo response could have masked the actual therapeutic effects of the mouthwash.
Among the clinical periodontal parameters, PI levels exhibited similar decreases in the two groups, which is common in home care clinical trials, and is likely to have been due to the Hawthorne effect caused by improved tooth-cleaning habits [29]. These findings differ somewhat from those of previous home use studies of CPC, which has been reported to lead to plaque inhibition but not a significant reduction in gingivitis [30]. In the present study, the effects on GI were partially reflected by the significant reduction in the mSBI in the test group. However, the changes in clinical parameters were not as consistent as those of other parameters, showed limited or nonsignificant differences between the groups, and overall, the evidence was too weak to confirm that the mouthwash had an anti-inflammatory effect independent of the Hawthorne effect. The role of CPC applied in a mixture with KNO3 and NaF to reduce DH and gingivitis should further be evaluated.
We used a randomized, double-blind, placebo-controlled design, which was recommended in the guidelines for the design and conduct of clinical trials of DH by Holland et al. [1]. It was also reported that the median trial duration was eight weeks from 45 clinical studies of DH conducted between 1956 and 1992, which was regarded to be suitable to allow maximum efficacy of the active ingredients and minimize placebo effects. However, testing agents vary in their contents and the effects may vary between products; the optimum duration should be determined by a prior pilot study. In studies that have estimated the lasting effects of desensitization agents, a four-week exposure time has widely been used with a range of two to 12 weeks [3]. Some previous studies using mouthwash containing single or multiple acting gradients including KNO3 and CPC had the exposure period of eight weeks and the change of outcome appeared at four weeks, whether the difference was significant from the control or not [3,21]. In our study, exposure time was set as six weeks to fully elucidate the effects of testing mouthwash and also increase patients’ compliance to the study which might be harmed with the protracted trial.
Mouthwash has become accepted as a preferable delivery vehicle compared to pastes and gels due to their ease of use, and access to all areas of the mouth, and ability to circumvent the discomfort that could occur when brushing the sensitive dentin surface because the osmolality of toothpaste may increase pain sensations [31]. Furthermore, the addition of agents that have functions other than desensitization (e.g., they improve plaque status, gingivitis, the caries index, teeth whitening) could increase the clinical benefits of using the mouthwash. Within the limitations of this study, it can be concluded that a mouthwash containing a mixture of KNO3, NaF, and CPC was a safe and easy-to-use vehicle with potential therapeutic effects in reducing the symptoms of DH and simultaneously improving gingival inflammation.
ACKNOWLEDGMENTS
This study was supported by a grant from Dong-A Pharmaceutical.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article is reported.
References
- 1.Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 2.Matthews DC, Rocchi A, Gafni A. Factors affecting patients’ and potential patients’ choices among anaesthetics for periodontal recall visits. J Dent. 2001;29:173–179. doi: 10.1016/s0300-5712(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 3.Shiau HJ. Dentin hypersensitivity. J Evid Based Dent Pract. 2012;12:220–228. doi: 10.1016/S1532-3382(12)70043-X. [DOI] [PubMed] [Google Scholar]
- 4.Bartold PM. Dentinal hypersensitivity: a review. Aust Dent J. 2006;51:212–218. [PubMed] [Google Scholar]
- 5.Fischer C, Fischer RG, Wennberg A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio de Janeiro, Brazil. J Dent. 1992;20:272–276. doi: 10.1016/0300-5712(92)90043-c. [DOI] [PubMed] [Google Scholar]
- 6.Gillam DG, Seo HS, Bulman JS, Newman HN. Perceptions of dentine hypersensitivity in a general practice population. J Oral Rehabil. 1999;26:710–714. doi: 10.1046/j.1365-2842.1999.00436.x. [DOI] [PubMed] [Google Scholar]
- 7.Brännström M, Aström A. The hydrodynamics of the dentine; its possible relationship to dentinal pain. Int Dent J. 1972;22:219–227. [PubMed] [Google Scholar]
- 8.Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284. doi: 10.1111/j.1600-051x.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 9.Scherman A, Jacobsen PL. Managing dentin hypersensitivity: what treatment to recommend to patients. J Am Dent Assoc. 1992;123:57–61. doi: 10.14219/jada.archive.1992.0107. [DOI] [PubMed] [Google Scholar]
- 10.Walters PA. Dentinal hypersensitivity: a review. J Contemp Dent Pract. 2005;6:107–117. [PubMed] [Google Scholar]
- 11.Kim S. Hypersensitive teeth: desensitization of pulpal sensory nerves. J Endod. 1986;12:482–485. doi: 10.1016/S0099-2399(86)80203-5. [DOI] [PubMed] [Google Scholar]
- 12.Nagata T, Ishida H, Shinohara H, Nishikawa S, Kasahara S, Wakano Y, et al. Clinical evaluation of a potassium nitrate dentifrice for the treatment of dentinal hypersensitivity. J Clin Periodontol. 1994;21:217–221. doi: 10.1111/j.1600-051x.1994.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen PL, Bruce G. Clinical dentin hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2:1–12. [PubMed] [Google Scholar]
- 14.Ashley FP, Skinner A, Jackson P, Woods A, Wilson RF. The effect of a 0.1% cetylpyridinium chloride mouthrinse on plaque and gingivitis in adult subjects. Br Dent J. 1984;157:191–196. doi: 10.1038/sj.bdj.4805458. [DOI] [PubMed] [Google Scholar]
- 15.Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 16.Löe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Barnett M, Ciancio S, Mather M. The modified papillary bleeding index: comparison with gingival index during the resolution of gingivitis. J Prev Dent. 1980;6:135–138. [Google Scholar]
- 18.Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;19:CD001476. doi: 10.1002/14651858.CD001476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae JH, Kim YK, Myung SK. Desensitizing toothpaste versus placebo for dentin hypersensitivity: a systematic review and meta-analysis. J Clin Periodontol. 2015;42:131–141. doi: 10.1111/jcpe.12347. [DOI] [PubMed] [Google Scholar]
- 20.Addy M, Dowell P. Dentine hypersensitivity--a review. Clinical and in vitro evaluation of treatment agents. J Clin Periodontol. 1983;10:351–363. doi: 10.1111/j.1600-051x.1983.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 21.Yates R, West N, Addy M, Marlow I. The effects of a potassium citrate, cetylpyridinium chloride, sodium fluoride mouthrinse on dentine hypersensitivity, plaque and gingivitis. A placebo-controlled study. J Clin Periodontol. 1998;25:813–820. doi: 10.1111/j.1600-051x.1998.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 22.West NX, Addy M, Jackson RJ, Ridge BD. Dentine hypersensitivity: controls and placebo response. A comparisons of the effect of strontium acetate and potassium nitrate toothpastes on dentine hypersensitivity. J Clin Periodontol. 1997;24:209–215. doi: 10.1111/j.1600-051x.1997.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 23.Uchida A, Wakano Y, Fukuyama O, Miki T, Iwayama Y, Okada H. Controlled clinical evaluation of a 10% strontium chloride dentifrice in treatment of dentin hypersensitivity following periodontal surgery. J Periodontol. 1980;51:578–581. doi: 10.1902/jop.1980.51.10.578. [DOI] [PubMed] [Google Scholar]
- 24.Chesters R, Kaufman HW, Wolff MS, Huntington E, Kleinberg I. Use of multiple sensitivity measurements and logit statistical analysis to assess the effectiveness of a potassium-citrate-containing dentifrice in reducing dentinal hypersensitivity. J Clin Periodontol. 1992;19:256–261. doi: 10.1111/j.1600-051x.1992.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 25.Paquette DW, Fiorellini JP. Clinical trials and the evaluation of new periodontitis therapies. Curr Opin Periodontol. 1994;2:87–98. [PubMed] [Google Scholar]
- 26.Trowbridge HO, Silver DR. A review of current approaches to in-office management of tooth hypersensitivity. Dent Clin North Am. 1990;34:561–581. [PubMed] [Google Scholar]
- 27.Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod. 1986;12:465–474. doi: 10.1016/S0099-2399(86)80201-1. [DOI] [PubMed] [Google Scholar]
- 28.Suge T, Kawasaki A, Ishikawa K, Matsuo T, Ebisu S. Effects of plaque control on the patency of dentinal tubules: an in vivo study in beagle dogs. J Periodontol. 2006;77:454–459. doi: 10.1902/jop.2006.050159. [DOI] [PubMed] [Google Scholar]
- 29.Addy M. Evaluation of clinical trials of agents and procedures to prevent caries and periodontal disease: choosing products and recommending procedures. Int Dent J. 1995;45:185–196. [PubMed] [Google Scholar]
- 30.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–498. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 31.Mantzourani M, Sharma D. Dentine sensitivity: past, present and future. J Dent. 2013;41(Suppl 4):S3–17. doi: 10.1016/S0300-5712(13)70002-2. [DOI] [PubMed] [Google Scholar]