Abstract
Objective. To validate the ability of 99mTc-Annexin V to visualize early stage of glucocorticoid-induced femoral head necrosis by comparing with 99mTc-MDP bone scanning. Methods. Femoral head necrosis was induced in adult New Zealand white rabbits by intramuscular injection of methylprednisolone. 99mTc-Annexin scintigraphy and 99mTc-MDP scans were performed before and 5, 6, and 8 weeks after methylprednisolone administration. Rabbits were sacrificed at various time points and conducted for TUNEL and H&E staining. Results. All methylprednisolone treated animals developed femoral head necrosis; at 8 weeks postinjection, destruction of bone structure was evident in H&E staining, and apoptosis was confirmed by the TUNEL assay. This was matched by 99mTc-Annexin V images, which showed a significant increase in signal over baseline. Serial 99mTc-Annexin V scans revealed that increased 99mTc-Annexin V uptake could be observed in 5 weeks. In contrast, there was no effect on 99mTc-MDP signal until 8 weeks. The TUNEL assay revealed that bone cell apoptosis occurred at 5 weeks. Conclusion. 99mTc-Annexin V is superior to 99mTc-MDP for the early detection of glucocorticoid-induced femoral head necrosis in the rabbit and may be a better strategy for the early detection of glucocorticoid-induced femoral head necrosis in patients.
1. Introduction
Glucocorticoid-induced necrosis of the femoral head (also known as glucocorticoid-induced avascular necrosis or aseptic necrosis of the femoral head) was initially reported in the 1950s [1]. In China, it is estimated that there are approximately 5 to 7.5 million patients suffering from femoral head necrosis, with 75,000 to 150,000 new cases each year [2]. Glucocorticoid is the primary cause of nontrauma femoral head necrosis, a condition which seriously reduces the patient's quality of life and ability to work, and eventually requires artificial joint replacement in the majority of cases. If femoral head necrosis can be detected at a very early stage, it may be possible to prevent the collapse of the femoral head, preserving joint function, delaying progression of the disease, leading to an overall reduction in morbidity [3–5].
In previous decades, the pathogenesis of glucocorticoid-induced osteonecrosis has been ascribed to increasing intraosseous pressure and decreased blood perfusion in the bone, fat embolism, or a hypercoagulable state [5]. Recently, it has been demonstrated that glucocorticoid-induced bone of femoral head necrosis is closely associated with bone cell apoptosis, and, using a rabbit model, Hwang et al. demonstrated the early appearance of apoptosis after glucocorticoid treatment [6]. Youm et al. found that high dose glucocorticoid significantly increased apoptosis in osteoblast and osteoclast cells in the femoral head, with subsequent trabecular bone fracture and femoral head collapse; further, apoptosis of osteoblasts reduced bone reconstruction and repair [7, 8], without affecting the vascular supply to the femoral head [8]. In transplant patients, Rojas et al. demonstrated osteoblastic cell apoptosis after high dose glucocorticoid [9], further supporting a role for programmed cell death in glucocorticoid-induced femoral head necrosis in patients and animals.
99mTc-Annexin V is a radionuclide-labeled molecular probe for detecting apoptosis that has been validated in animal experiments and preliminary clinical use [10, 11]. 99mTc-Annexin V imaging successfully detected apoptotic cells in acute myocardial infarction, myocarditis, cardiac transplant rejection, unstable atherosclerotic plaque, and cancer after effective chemotherapy [11]. Recently, 99mTc-Annexin V has been used to monitor the therapeutic effect of antiapoptosis drugs in heart failure patients [12–14]. We hypothesized that 99mTc-Annexin V scintigraphy would successfully image apoptosis in the femoral head and so enable visualization of early stage disease.
In this study, using a rabbit model, glucocorticoid-induced necrosis of the femoral head was imaged with both 99mTc-Annexin V and 99mTc-MDP bone scan. Comparison of the two imaging agents suggests that 99mTc-Annexin V may be superior.
2. Materials and Methods
2.1. Animals
Adult New Zealand white rabbits were purchased from Di Dunlop Biological Resources Development Co. (Xi'an, China). Rabbits were maintained and used according to the guidelines of Inner Mongolian Medical University Animal Care and Use Committee. The animal study protocols were approved by the committee. Rabbits were housed singly and supplied with standard diet.
2.2. Generation of a Rabbit Model of Glucocorticoid-Induced Femoral Head Necrosis
Experiments were performed on 20 rabbits (body weight 2.5 ± 0.3 kg, 10 male, 10 female). Animals were acclimated in the vivarium for one week and injected intramuscularly with methylprednisolone (7.5 mg/kg), twice weekly for 8 weeks to generate femoral head necrosis. Six control rabbits received saline by the same route and schedule.
2.3. 99mTc-Annexin V Scintigraphy and 99mTc-MDP Bone Scans
Annexin V was labeled in-house as previously described [10–14], to a radiochemical purity of 90%. 0.5 mCi (18.5 MBq) was injected via the ear vein and imaging was performed 1 hour later. Bone scintigraphy was performed 48 hours prior to 99mTc-Annexin V imaging. 99mTc-MDP (0.5 mCi [18.5 MBq], radiochemical purity 95%) was injected via the ear vein and imaged 2 hours subsequently.
Imaging was performed with a clinical dual-head detector SPECT/CT (Millennium VG, Hawkeye; GE Healthcare) equipped with low-energy and high-resolution collimators (peak energy 140 keV, window width 20%). Animals were anesthetized by inhalation of isoflurane (1.5–2%)-air mixture for a 6 min planar scan. SPECT data was acquired into a 128 × 128 matrix.
The images were processed using a postprocessing system workstation. Tracer uptake was expressed as the ratio of signal in limb joints to mediastinum (T/N). And images were read by two nuclear medicine physicians who were blinded to the pathologic findings. In case of disagreement, a third nuclear medicine physician made a diagnostic conclusion.
2.4. Preparation of Femoral Head Paraffin Sections for Histological Assay
Rabbits were sacrificed either by intravenous injection of sodium pentobarbital or air embolism. The femoral heads were resected and stored in 10% formalin solution. Femoral heads were decalcified by immersing in Surgipath II decalcification solution (10% formaldehyde, 8% formic acid, and 1% methanol; Surgipath, Richmond, USA) for approximately 1 week and subsequently embedded in paraffin. Blocks were sectioned (4 μm slices) every 1 mm throughout the bone.
2.5. TUNEL Assay
Terminal deoxynucleotidyl transferase- (TDT-) mediated dUTP nick end labeling (TUNEL) was performed according to the kit manufacturer's instructions (Thermo Scientific, USA) for tissue sections. Slices were dewaxed with freshly prepared 3% H2O2 solution at room temperature for 10 min and treated with proteinase K at 37°C for 10 min. Tissue was covered with 20 μL labeling buffer containing TDT (1 μL) and DIG-dUTP (1 μL) and incubated for 2 h at 37°C in 100% humidity. Biotinylated antidigoxigenin antibody was applied (50 μL per specimen, 37°C for 30 min) followed by SABC (10 μL, 37°C, 30 min) and finally stained with hematoxylin for 2 s. TUNEL-positive cells were identified through the nucleus, which was either stained tan or brown. Five fields were randomly selected and the osteoblast apoptosis index was calculated as the ratio of apoptotic to total cells.
2.6. Statistical Analysis
SPSS19.0 software (IBM, USA) was used for statistical analysis. Data was expressed as mean ± standard deviation. Student's t-test was used to compare differences between groups, and a P value less than 0.05 was considered statistically significant.
3. Results
Five weeks following methylprednisolone injection, rabbits displayed decreased activity, malaise, a decrease in lower limb body support, and reduced food intake. All animals developed femur head necrosis as confirmed by H&E staining and TUNEL at 8 weeks (Figure 1).
Figure 1.
Histological images demonstrating methylprednisolone-induced femoral head necrosis. (a) Representative H&E stained section of a femoral head, 8 weeks after methylprednisolone administration, showing destruction of the bone structure (2 rabbits were examined). (b) Representative H&E stained section of a femoral head section from a control rabbit treated with saline (2 rabbits were examined). (c) TUNEL assay of a femoral head, 8 weeks after methylprednisolone administration. Apoptotic cells (indicated by arrows) are stained brown. (d) TUNEL assay of a femoral head from a control rabbit treated with saline.
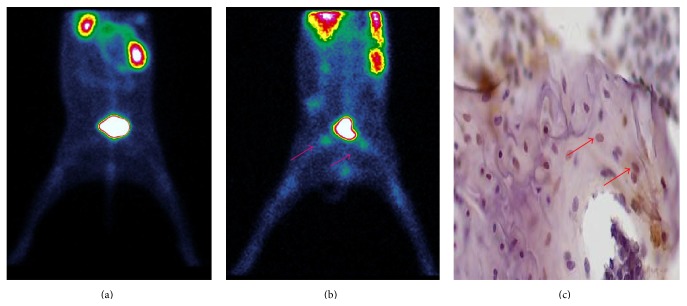
Planar scintigraphy performed 1 day before and 8 weeks after methylprednisolone administration in 6 rabbits showed that 99mTc-Annexin V accumulation in the femoral heads had significantly increased. (Representative 99mTc-Annexin V images are presented in Figures 2(a) and 2(b).) Subsequent histology indicated the presence of elevated levels of apoptosis (Figure 2(c)).
Figure 2.
Representative 99mTc-Annexin V images of methylprednisolone-induced femoral head necrosis. (a) 99mTc-Annexin V image of a saline-treated rabbit as control. (b) 99mTc-Annexin V image of a rabbit after 8 weeks of prednisolone treatment. Radioactivity accumulates in the femoral head regions as indicated by the arrows. (c) TUNEL assay of a femoral head section, 8 weeks after prednisolone administration, with apoptotic cells stained brown, as indicated by the arrow.
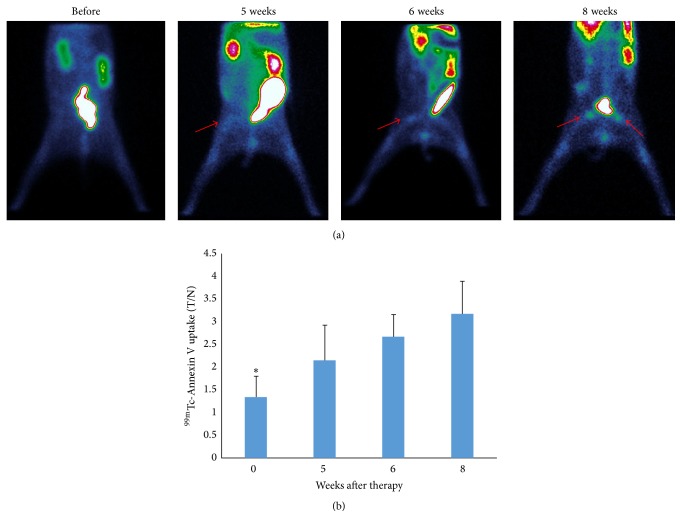
To observe the disease development process, we performed serial 99mTc-Annexin V scans in 6 rabbits (3 male, 3 female). Scans were collected before treatment and 5–8 weeks after the initiation of methylprednisolone treatment. As shown in Figure 3(a), increased 99mTc-Annexin V uptake was observed as early as approximately 5 weeks after commencing methylprednisolone. 99mTc-Annexin V uptake, expressed as T/N, was significantly increased over baseline at all observed time points (Figure 3(b)).
Figure 3.
Serial 99mTc-Annexin V scans in methylprednisolone treated rabbits. (a) Scans were performed before therapy and 5–8 weeks after initiation by methylprednisolone. Increasing 99mTc-Annexin V uptake is observed as early as approximately 5 weeks after the start of prednisolone treatment. (b) 99mTc-Annexin V uptake, expressed as T/N, significantly increased with time.
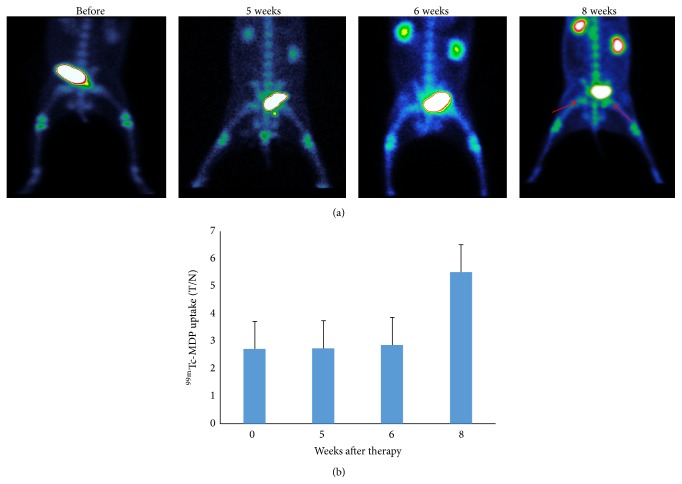
99mTc-MDP bone scans were performed in the same group of animals two days prior to 99mTc-Annexin V imaging. Abnormal femoral head uptake of 99mTc-MDP was not observed until 8 weeks, when it was significantly higher than pretreatment (P < 0.01) (Figure 4).
Figure 4.
Serial 99mTc-MDP bone scans in methylprednisolone treated rabbits. Animals from Figure 3 were scanned with 99mTc-MDP, two days prior to 99mTc-Annexin V imaging. (a) Abnormal 99mTc-MDP is found by 8 weeks, as indicated by the arrow. (b) A significant increase in 99mTc-MDP is observed only at 8 weeks after methylprednisolone exposure, ∗ P < 0.01 at 8 weeks versus the rest of the time points. There were no significant differences in 99mTc-MDP uptake between pretherapy (week 0) and 5 or 6 weeks.
In a separate group, 6 rabbits were treated with methylprednisolone and sacrificed at 5, 6, and 8 weeks after treatment (two animals per time point and four control animals). The TUNEL assay revealed a significantly higher apoptotic index in the treated femoral heads at each endpoint (P < 0.05) relative to the controls (Figure 5).
Figure 5.
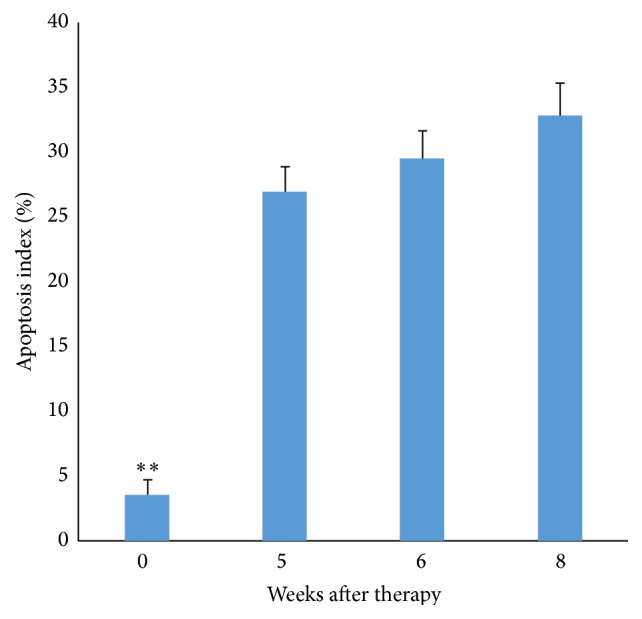
Apoptotic frequency as a function of time after treatment by TUNEL assay. The frequency of apoptosis as assessed by TUNEL in rabbits sacrificed at 5, 6, and 8 weeks after methylprednisolone treatment. Control animals were treated with saline. Each treated point represents 4 femoral heads and 8 in the control group, ∗∗ P < 0.001.
4. Discussion
Animal models of glucocorticoid-induced femoral head osteonecrosis mimic human disease and are thus critically important for in-depth studies of both pathogenesis and therapy. Importantly, we have successfully established that, in methylprednisolone-induced femoral head osteonecrosis in rabbits, the pathogenesis was closely related to bone cell apoptosis (Figure 1). We have also used this model to validate that 99mTc-Annexin V apoptosis imaging is superior to 99mTc-MDP for the early detection of the disease (Figures 2–4).
Glucocorticoid treatment is the leading cause of nontraumatic femoral head necrosis [15]. However, in the asymptomatic early stage, the disease is difficult to diagnose with current imaging modalities. In 1971, Harrington et al. [16] were the first to describe bone avascular necrosis in renal transplant patients treated with glucocorticoids. Subsequently, the key role of apoptosis in the pathogenesis of glucocorticoid-induced necrosis was established [7]. Accordingly, in this study we hypothesized that apoptosis imaging may visualize early pathological changes in the femoral head. 99mTc-Annexin V successfully detected the onset of femoral head necrosis after 5 weeks of continuous glucocorticoid administration (Figure 3) and was much more sensitive than a 99mTc-MDP bone scan (Figure 4), which is currently considered the gold standard for the clinical diagnosis of femoral head necrosis. The imaging results were consistent with the TUNEL assay which demonstrated the presence of apoptosis 5 weeks after methylprednisolone administration.
Annexin V specifically binds to phosphatidylserine on the surface of early apoptotic cells and is widely used to detect apoptosis [17]. Radionuclide-labeled Annexin V has been used for the in vivo detection of apoptosis [18–20] and has been the subject of clinical trials in the US and Europe designed to assess chemotherapy of small-cell lung cancer [21]. To the best of our knowledge, 99mTc-Annexin V has not yet been used to detect very early stage glucocorticoid-induced femoral head osteonecrosis.
99mTc-Annexin V apoptosis imaging was found to be superior to 99mTc-MDP bone scintigraphy for the early detection of glucocorticoid-induced femoral head osteonecrosis (Figures 3 and 4) and may be used to observe the therapeutic effect of novel treatment strategies. We are in the process of using 99mTc-Annexin V SPECT to study and evaluate patients who have received high dose glucocorticoid for various reasons.
5. Conclusions
99mTc-Annexin V apoptosis imaging is superior to 99mTc-MDP bone imaging for the early detection of glucocorticoid-induced femoral head necrosis in the rabbit and may be a better strategy for detecting early stage femoral head necrosis induced by glucocorticoid in patients.
Acknowledgments
This study was supported by Natural Science Foundation of China (81360224), the Inner Mongolia Major Basic Science Research Program award for a project entitled Hypoxic Microenvironment of Lung Cancer Metastases and Hypoxia Targeted Therapy and Inner Mongolia Science and Technology Innovation project (2015cztcxyd03).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Xiaolong Wang and Yu Liu contributed equally to this paper.
References
- 1.Pietrogande V., Mastromarino R. Osteopat da prolungata trattamento cortisonico. Orthop Traum Appar Mat. 1957;25:791–810. [Google Scholar]
- 2.Chen Y. Progress in the etiology of femoral head necrosis. Huaxi Medicine Journal. 2009;24(7):1899–1901. [Google Scholar]
- 3.Weinstein R. S. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41(2):183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K. L., Mok C. C. Glucocorticoid-induced avascular bone necrosis: diagnosis and management. The Open Orthopaedics Journal. 2012;6(1):449–457. doi: 10.2174/1874325001206010449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drescher W., Schlieper G., Floege J., Eitner F. Steroid-related osteonecrosis-an update. Nephrology Dialysis Transplantation. 2011;26(9):2728–2731. doi: 10.1093/ndt/gfr212. [DOI] [PubMed] [Google Scholar]
- 6.Hwang Y., Park J., Choi S. H., Kim G. Traumatic and non-traumatic osteonecrosis in the femoral head of a rabbit model. Laboratory Animal Research. 2011;27(2):127–131. doi: 10.5625/lar.2011.27.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youm Y.-S., Lee S.-Y., Lee S.-H. Apoptosis in the osteonecrosis of the femoral head. Clinics in Orthopedic Surgery. 2010;2(4):250–255. doi: 10.4055/cios.2010.2.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhardt A. W., Yeager-Jones A., Blair H. C. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid-treated rabbits. Endocrinology. 2001;142(3):1333–1340. doi: 10.1210/en.142.3.1333. [DOI] [PubMed] [Google Scholar]
- 9.Rojas E., Carlini R. G., Clesca P., et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney International. 2003;63(5):1915–1923. doi: 10.1046/j.1523-1755.2003.00938.x. [DOI] [PubMed] [Google Scholar]
- 10.Blankenberg F. G. In vivo detection of apoptosis. Journal of Nuclear Medicine. 2008;49(6) supplement 2:81S–95S. doi: 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- 11.Boersma H. H., Kietselaer B. L. J. H., Stolk L. M. L., et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. Journal of Nuclear Medicine. 2005;46(12):2035–2050. [PubMed] [Google Scholar]
- 12.Taki J., Higuchi T., Kawashima A., et al. Effect of postconditioning on myocardial 99mTc-Annexin-V uptake: comparison with ischemic preconditioning and caspase inhibitor treatment. Journal of Nuclear Medicine. 2007;48(8):1301–1307. doi: 10.2967/jnumed.106.037408. [DOI] [PubMed] [Google Scholar]
- 13.Doue T., Ohtsuki K., Ogawa K., et al. Cardioprotective effects of erythropoietin in rats subjected to ischemia-reperfusion injury: assessment of infarct size with 99mTc-Annexin V. Journal of Nuclear Medicine. 2008;49(10):1694–1700. doi: 10.2967/jnumed.107.050260. [DOI] [PubMed] [Google Scholar]
- 14.Keitselaer B. L. J. H., Reutelingsperger C. P. M., Boersma H. H., et al. Noninvasive detection of programmed cell loss with 99mTc-labeled Annexin A5 in heart failure. Journal of Nuclear Medicine. 2007;48(4):562–567. doi: 10.2967/jnumed.106.039453. [DOI] [PubMed] [Google Scholar]
- 15.Canalis E., Delany A. M. Mechanisms of glucocorticoid action in bone. Annals of the New York Academy of Sciences. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrington K. D., Murray W. R., Kountz S. L., et al. Avascular necrosis of bone after renal transplantation. Journal of Bone and Joint Surgery. 1971;53(2):203–215. [PubMed] [Google Scholar]
- 17.Vermes I., Haanen C., Steffens-Nakken H., Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. Journal of Immunological Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 18.Blankenberg F. G., Katsikis P. D., Tait J. F., et al. Imaging of apoptosis (programmed cell death) with 99mTc-Annexin V. Journal of Nuclear Medicine. 1999;40(1):184–191. [PubMed] [Google Scholar]
- 19.Lev N., Melamed E., Offen D. Apoptosis and Parkinson's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(2):245–250. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 20.D'Arceuil H., Rhine W., De C. A., et al. 99mTc annexin V imaging of neonatal hypoxic brain injury. Stroke. 2000;31(11):2692–2700. doi: 10.1161/01.str.31.11.2692. [DOI] [PubMed] [Google Scholar]
- 21.Belhocine T. Z., Blankenberg F. G. 99mTc-Annexin A5 uptake and imaging to monitor chemosensitivity. Methods in Molecular Medicine. 2005;111:363–380. doi: 10.1385/1-59259-889-7:363. [DOI] [PubMed] [Google Scholar]