Abstract
The function of curcumin on NADPH oxidase-related ROS production and cardiac apoptosis, together with the modulation of protein signalling pathways, was investigated in cardiomyocytes. Primary cultures of neonatal rat cardiomyocytes were exposed to 30 mmol/L high glucose with or without curcumin. Cell viability, apoptosis, superoxide formation, the expression of NADPH oxidase subunits, and potential regulatory molecules, Akt and GSK-3β, were assessed in cardiomyocytes. Cardiomyocytes exposure to high glucose led to an increase in both cell apoptosis and intracellular ROS levels, which were strongly prevented by curcumin treatment (10 μM). In addition, treatment with curcumin remarkably suppressed the increased activity of Rac1, as well as the enhanced expression of gp91phox and p47phox induced by high glucose. Lipid peroxidation and SOD were reversed in the presence of curcumin. Furthermore, curcumin treatment markedly inhibited the reduced Bcl-2/Bax ratio elicited by high glucose exposure. Moreover, curcumin significantly increased Akt and GSK-3β phosphorylation in cardiomyocytes treated with high glucose. In addition, LY294002 blocked the effects of curcumin on cardiomyocytes exposure to high glucose. In conclusion, these results demonstrated that curcumin attenuated high glucose-induced cardiomyocyte apoptosis by inhibiting NADPH-mediated oxidative stress and this protective effect is most likely mediated by PI3K/Akt-related signalling pathway.
1. Introduction
Diabetes mellitus (DM) is becoming a global health problem that is afflicting millions of people. According to the investigation conducted by the International Diabetes Federation (IDF), the incidence of DM is rapidly increasing and the total number of people with DM will reach 592 million in 2035 [1]. Studies have indicated that diabetic people have a 2- to 5-fold increased risk of developing heart failure [2] and that more than 50%–80% of diabetic patients die from diabetic cardiovascular complications [3]. Diabetic cardiomyopathy (DCM), as a major complication of DM, was initially proposed by Rubler in 1972 [4]. DCM is characterized by structural and functional cardiac disorder occurring independently of coronary artery disease and hypertension [5]. Although many research studies have attempted to elucidate its underlying mechanisms, the aetiology of DCM has never been directly determined. Numerous studies utilizing experimental animal models and clinical diabetes patients reported that diabetes enhances cardiomyocyte apoptosis not only simply in animals but also in patients [6, 7]. Thus, cell death by apoptosis likely plays an important role in triggering the pathogenic changes in DCM [8]. Cardiomyocyte apoptosis can cause a loss of cardiac contractile muscle tissue, which eventually leads to left ventricular remodeling [9].
Both type 1 and type 2 DM are associated with long-standing hyperglycemia. Chronic hyperglycemia has been shown to directly participate in the pathogenesis of DM-induced cardiac injury by promoting excessive oxidative stress in the heart [10], which increases cardiomyocyte apoptosis in both human and experimental DCM. Overproduction of reactive oxygen species (ROS) and a diminished antioxidant defence system are linked to enhanced oxidative stress in the heart in DM. Consequently, if the balance between ROS generation and ROS scavenging systems is broken, superoxide accumulates and results in cellular damage or dysfunction. Given the injurious effects of ROS in DCM, increasing attention has been placed on the administration of antioxidant agents as a compensatory therapeutic approach in DCM [11].
Curcumin, a major constituent derived from the root of Curcuma longa, has been used as a spice and food additive in India since ancient times. Today, interest in curcumin has grown rapidly due to its diverse array of biological and pharmacological activities, and it was shown to have the potential to treat inflammatory and cardiovascular diseases and cancer [12]. Curcumin has antioxidant properties that are responsible for its cardioprotective effect by enhancing antioxidant defences and eradicating ROS [13]. Additional studies have indicated that C66, a curcumin analogue, has a protective role against high glucose-induced cardiac damage via inactivation of the JNK pathway [14]. Our early stage study demonstrated that curcumin reduces cardiomyocyte remodeling and improves cardiac dysfunction by inhibiting inappropriate apoptosis in diabetic rats [15], but the mechanism through which curcumin inhibits cardiomyocyte apoptosis and oxidative stress remains unknown.
Therefore, this study was performed to determine the action of curcumin against high glucose-induced cardiac injury and elucidate the molecular mechanism of cardiomyocyte protection by exposing primary neonatal rat cardiomyocytes to a high concentration of glucose.
2. Materials and Methods
2.1. Animals
One- to three-day-old Sprague-Dawley rats were obtained from the experimental animal centre at Hubei University of Science and Technology. The Committee of Experimental Animals of Hubei University of Science and Technology approved this study. All animals used in this study were cared for and experimented on in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
2.2. Primary Culture of Neonatal Rat Cardiomyocytes and Treatment
Neonatal rat cardiomyocytes were isolated as previously described with slight modifications [16]. The cardiomyocytes were cultured in DMEM containing 10% FBS (Gibco Life of Cells, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humidified air containing 5% CO2 at 37°C. When the cardiomyocytes reached 70%–80% confluence, the cells were randomized into the experimental groups: 5.5 mmol/L D-glucose as the normal (NG) group, 30 mmol/L D-glucose (Sigma, USA) as the high glucose (HG) group, or identical concentrations of mannitol as an osmotic control group containing 5.5 mmol/L D-glucose plus 24.5 mmol/L mannitol for 24 h in the presence or absence of curcumin (10 μmol/L). A subset of cardiomyocytes were exposed to LY294002 for 1 h before administration of high glucose and curcumin.
2.3. Assessment of Cell Viability
Cell viability was assessed with a Cell Counting Kit-8 assay kit (CCK-8, Dojindo Molecular Technologies, Japan) in 96-well plates following the instructions from the manufacturer.
2.4. Analysis of Biochemical Parameters
Lactate dehydrogenase (LDH) and aspartate amino transferase (AST) released into the culture medium as well as the malondialdehyde (MDA) level and superoxide dismutase (SOD) activity in cells were determined using the associated enzyme activity assay kits (Nanjing Jiancheng Bioengineering Research Institute, China).
2.5. Intracellular ROS Measurement
Intracellular superoxide anions were examined using the fluorescence probe dihydroethidium (DHE). Cardiomyocytes were cultured in a dark chamber at 37°C for 30 min after the application of 10 μmol/L DHE (Life Technology, USA) and were washed twice with PBS. Images of the cardiomyocytes were captured and analysed immediately under an inverted fluorescence microscopy (Olympus IX71, Japan).
Intracellular ROS accumulation was assessed by DCFH-DA staining (Beyotime, China). Cultured cells were incubated in DMEM with 10 μmol/L DCFH-DA at 37°C for 30 min. ROS production was detected by a Bio-Tek fluorometric imaging plate reader (excitation at 485 nm and emission at 528 nm).
Furthermore, intracellular ROS was also measured by high performance liquid chromatography (HPLC) (SHIMADZU, LC-20AD, Japan) using a DHE fluorescent probe as previously described [17]. Briefly, cardiomyocytes were treated with DHE (10 μmol/L) for 30 min and then incubated with 0.1% Triton X-100 dissolved in PBS to permeabilize the cell membrane. Protein determination was performed using a Bicinchoninic Acid (BCA) protein assay kit (Beyotime, China). Then, 100 μL of cell lysate was added to an equal volume of 0.2 mol/L HClO4 in methanol, and the mixture was put on ice for 2 h to precipitate the proteins. Afterwards, the resulting mixture was centrifuged at 20,000 g at 4°C for 30 min. Then the supernatant was collected and neutralized with 1 mol/L potassium phosphate buffer (pH 2.6). The supernatant was spun again for 15 min and subjected to the HPLC analysis (excitation at 490 nm and emission at 596 nm).
2.6. TUNEL Assay
Apoptotic cardiomyocytes were detected using a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay kit obtained from Roche Applied Science. Briefly, 4% paraformaldehyde and 0.1% Triton X-100 were used to fix and permeabilize the cardiomyocytes on glass slides. After rinsing the cardiomyocytes with PBS, they were added and incubated with the TUNEL reagents according to the instructions from the manufacturer. Images were captured under a fluorescence microscope (Olympus BX53, Japan) and the proportion of TUNEL-positive cells was estimated using the following formula: TUNEL-positive cardiomyocytes/total number of cardiomyocytes × 100%.
2.7. Flow Cytometry
Flow cytometry was performed using an Annexin V-FITC Apoptosis Detection Kit (Best Bio, China) following the manufacturer's protocol. Briefly, after being treated with the appropriate drugs, the cardiomyocytes were harvested with trypsin and washed with cold PBS. Afterwards, the collected cardiomyocytes were isolated by centrifugation, resuspended in 500 μL of 1x binding buffer, and treated with 5 μL Annexin V-FITC and 5 μL PI for 15 min at 4°C in the dark. Apoptotic cardiomyocytes were detected by flow cytometer (Becton Dickinson, USA).
2.8. Determination of Rac1 Activity
Rac1 activity was determined using a Rac1 Activation Assay Kit (Millipore, USA) following the instructions from the manufacturer. Cultured cardiomyocytes were homogenized with 1x MLB. Rac-GTP was immunoprecipitated using PAK1-PBD agarose beads coated with an anti-active Rac-GTP mouse monoclonal antibody. Finally, the extracts were analysed by western blot.
2.9. Western Blot Analysis
Cardiomyocytes were lysed with 1x RIPA lysis buffer (Cell Signalling Technology, USA). After centrifugation, the lysates were clarified, and the supernatants fractions were isolated. Protein concentrations in cells were defined by the BCA protein assay. Approximately 30–50 μg of protein was loaded and separated by SDS-PAGE gels and then transferred to a PVDF membrane. After blocking the membrane with 5% nonfat milk, the following primary antibodies were used for western blot: Bcl-2, Bax, Akt, GSK-3β, phospho-Akt (Ser473), phospho-GSK-3β (Ser9) (Cell Signalling Technology, USA), gp91phox, p47phox, and β-actin (Santa Cruz Biotechnology, USA). Then the membrane was probed with appropriate secondary antibodies. Finally, the blots were visualized using a chemiluminescence system (Pierce Biosciences, USA). Image analysis software (GeneTools from SynGene) was used to quantify the immunoblots.
2.10. Statistics
The values are expressed as mean ± SD from repeated experiments. Statistical analysis was performed using ANOVA, and a P value of <0.05 was considered to indicate a significant difference for all the values.
3. Results
3.1. Curcumin Inhibited High Glucose-Induced Cardiomyocytes Injury
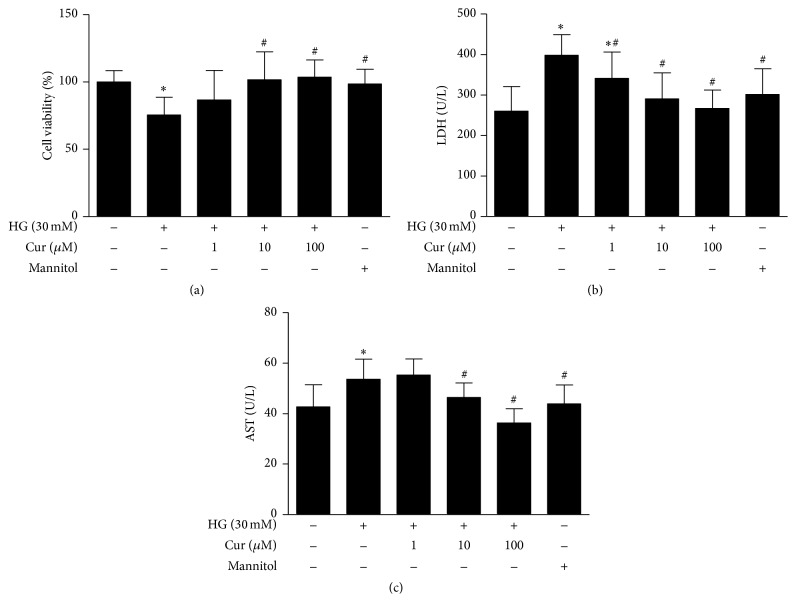
To ascertain the role of curcumin in cell survival, we examined the viability of primary cultured neonatal rat cardiomyocytes incubated with different doses of curcumin for 24 h using a CCK-8 assay. As presented in Figure 1(a), compared with the NG group, cell viability was markedly decreased at the high glucose concentration of 30 mmol/L, and mannitol (30 mmol/L) employed as an osmotic control agent did not mimic the effects of 30 mmol/L D-glucose. In the cardiomyocytes exposed to high glucose, curcumin treatment increased cell viability in a dose-dependent manner.
Figure 1.
Curcumin increased cell viability and inhibited injury in cardiomyocytes exposure to high glucose. (a) Cell viability was examined with a CCK-8 assay. (b) Curcumin decreased the level of LDH in the supernatant. (c) Curcumin decreased the level of AST in the supernatant. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group. n = 10.
LDH and AST are oxidoreductase enzymes that are present in the cytosol of animals and plants. They are highly stable enzymes that can be used to evaluate tissue and cell damage. As shown in Figures 1(b) and 1(c), the amounts of LDH and AST released by the cardiomyocytes were much higher in the HG group than those in the NG group. Interestingly, the cardiomyocytes exposed to high glucose and treated with curcumin released significantly lower amounts of LDH and AST into the medium than the cardiomyocytes undergoing high glucose alone.
3.2. Curcumin Abrogated High Glucose-Induced Cardiomyocytes Apoptosis
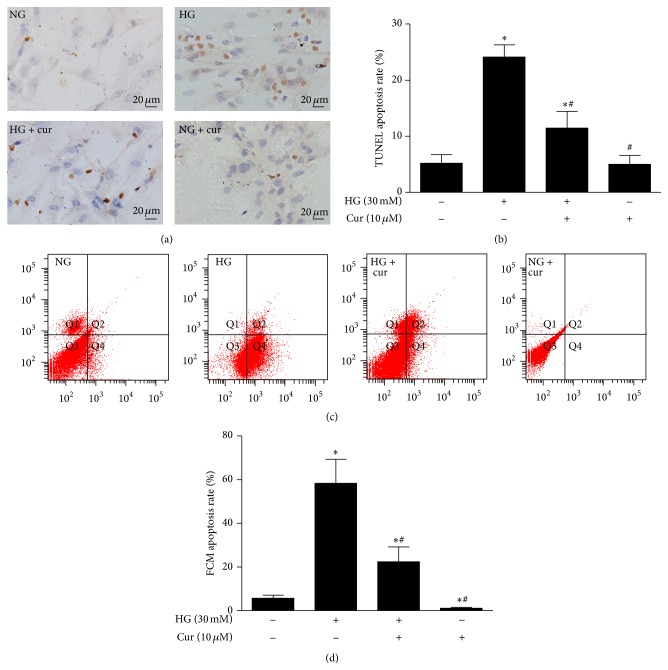
Apoptotic cardiomyocytes were detected by both TUNEL staining and flow cytometry. The TUNEL assay showed few apoptotic cardiomyocytes in the NG group and a greater number of apoptotic cardiomyocytes in the HG group. However, cotreatment with high glucose and curcumin (10 μM) abrogated the increase in TUNEL-positive cells triggered by high glucose (Figures 2(a) and 2(b)).
Figure 2.
Curcumin inhibited high glucose-induced cardiomyocytes apoptosis. (a) Representative images of apoptotic cardiomyocytes stained by TUNEL (magnification = 400x, bar is 20 μm). (b) Quantitative analysis of TUNEL staining. (c) Representative images of apoptotic cardiomyocytes observed using FCM. (d) Quantitative analysis of the FCM results. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group.
The flow cytometric analysis also suggested that the administration of curcumin resulted in an evident decrease in the number of apoptotic bodies compared to cardiomyocytes exposed to high glucose that were not treated with curcumin (Figures 2(c) and 2(d)).
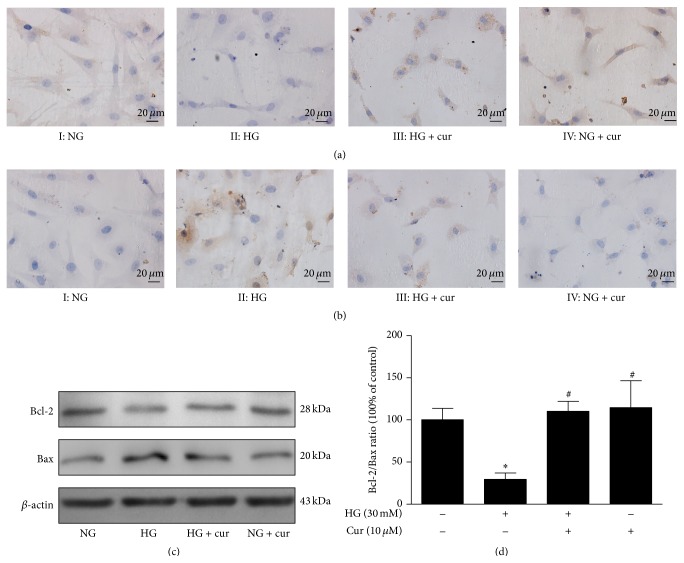
It is well known that apoptotic-related proteins regulate the progression of apoptosis. Thus, we performed further experiments to investigate if these regulatory proteins contributed to the effect of the inhibition of high glucose-induced apoptosis by curcumin. As indicated by immunohistochemical staining (Figures 3(a) and 3(b)), in contrast to the NG group, the HG group had reduced Bcl-2 expression and enhanced Bax expression. As we predicted, curcumin significantly enhanced Bcl-2 expression and reduced Bax expression in cardiomyocytes exposed to high glucose. Western blot showed that the Bcl-2/Bax ratio was remarkably reduced in the HG group compared to the NG group (Figures 3(c) and 3(d)). After treatment with curcumin, Bcl-2 expression was elevated, Bax expression was greatly reduced, and the Bcl-2/Bax ratio was significantly upregulated.
Figure 3.
Curcumin regulated the expression of Bax and Bcl-2 in cardiomyocytes exposed to high glucose. (a) Representative images of Bcl-2 by immunohistochemical staining (magnification = 400x, bar is 20 μm). (b) Representative images of Bax by immunohistochemical staining (magnification = 400x, bar is 20 μm). (c) Representative images of Bax and Bcl-2 expression by western blot. (d) Quantitative analysis of the Bcl-2/Bax ratio. n = 3. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group.
3.3. Curcumin Decreased High Glucose-Induced ROS Generation, Reduced MDA Content, and Increased SOD Activity in Cardiomyocytes
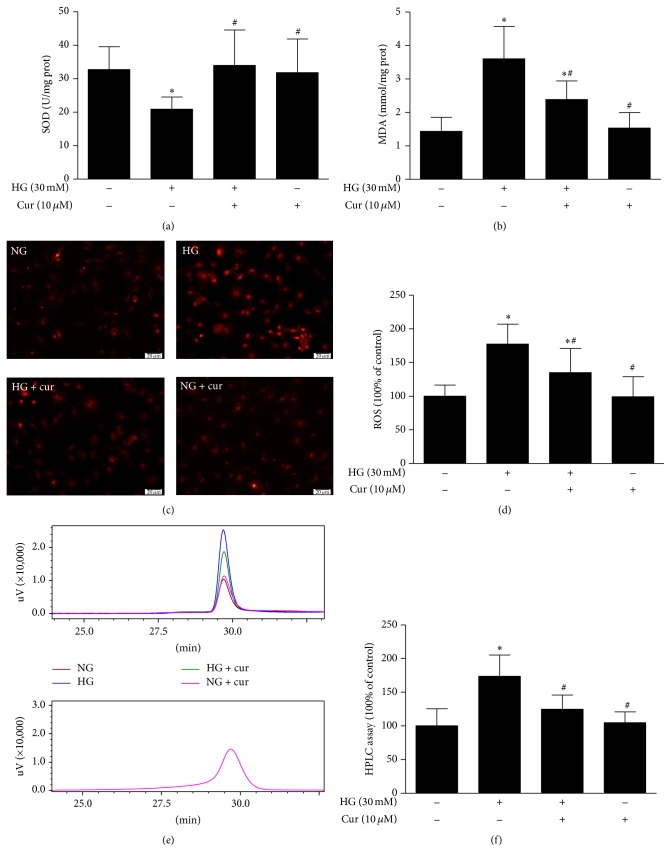
MDA formation and SOD activity, indexes of lipid superoxide, and oxygen free radical levels were measured in the cardiomyocytes. The MDA level in cardiomyocytes was significantly increased in the HG group. In contrast, SOD activity was found to be decreased when comparing the HG group with the NG group. The cardiomyocytes exposed to high glucose with curcumin markedly decreased the MDA level and enhanced SOD activity compared to those not treated with curcumin (Figures 4(a) and 4(b)).
Figure 4.
Curcumin suppressed high glucose-induced cardiomyocytes oxidative stress. (a) Curcumin enhanced SOD activity in cardiomyocytes (n = 12). (b) Curcumin reduced MDA level in cardiomyocytes (n = 10). (c) Representative images of DHE staining (n = 3). (d) Quantification of DCFH-DA staining (n = 11-12). (e) Representative images of HPLC assay (n = 4–6). (f) Quantification of HPLC assay. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group.
ROS (a key executor of oxidative stress) was measured by DHE staining, DCFH-DA staining, and HPLC assay, which indicated that the ROS level in the HG group was much higher than that in the NG group. However, the HG-induced increase in ROS was strongly blocked by treatment with curcumin (Figures 4(c)–4(f)).
3.4. Curcumin Attenuated High Glucose-Induced Expression of NADPH Oxidase Isoforms in Cardiomyocytes
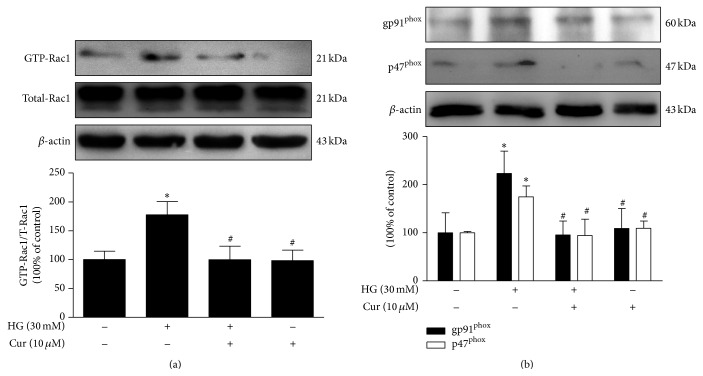
Because NADPH oxidase activation is directly related to increased oxidant production induced by hyperglycemia [18], we evaluated Rac1 activity and gp91phox and p47phox (NADPH subunits) expression. As presented in Figure 5, Rac1 activity in cardiomyocytes was much higher in the HG group than in the NG group. Moreover, increased expression of gp91phox and p47phox was detected in the HG group, while Rac1 activity and the expression of gp91phox and p47phox were markedly inhibited by curcumin in the cardiomyocytes exposed to high glucose. These data suggest that the protective role of curcumin against HG-induced cardiac injury is largely through inhibition of NADPH oxidase-mediated ROS production.
Figure 5.
Curcumin decreased high glucose-induced Rac1 activity and the expression of gp91phox and p47phox. (a) Rac1 activity assay. (b) Western blots analysis of gp91phox and p47phox expression. n = 3. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group.
3.5. Curcumin Activated the PI3K/Akt/GSK-3β Signalling Pathway in Cardiomyocytes
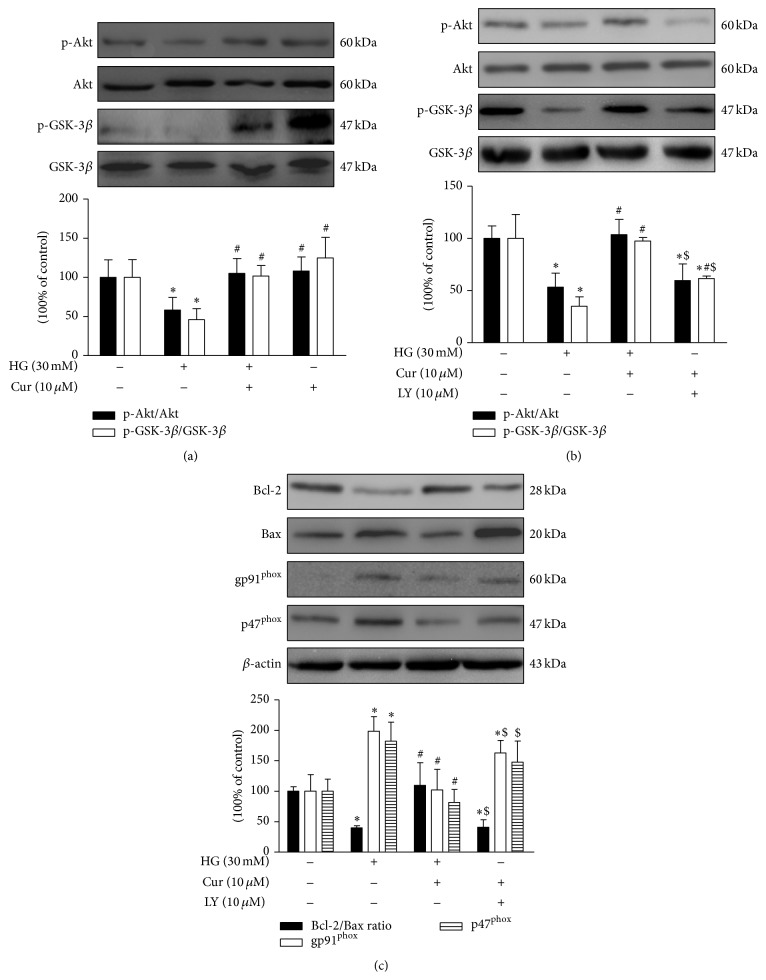
The activation of the PI3K/Akt signalling pathways is well known to inhibit HG-induced apoptosis [19]. Therefore, we targeted the PI3K/Akt signalling pathway to determine the mechanism through which curcumin inhibits HG-induced apoptosis. Akt and GSK-3β phosphorylation were markedly decreased in the HG group as compared with the NG group. Cardiomyocytes treated with curcumin showed a remarkable increase in expression of Akt and GSK-3β phosphorylation. Pretreatment with the PI3K inhibitor LY294002 reversed the increased effect of curcumin on Akt and GSK-3β phosphorylation. In parallel with that, the expression of Bcl-2 and Bax regulated by curcumin was abolished by treatment with LY294002 and curcumin failed to reduce gp91phox and p47phox expression levels when LY294002 was applied. These results indicate that PI3K/Akt signalling may be involved in the inhibition of apoptosis by curcumin in cardiomyocytes exposed to high glucose (Figure 6).
Figure 6.
Curcumin inhibited high glucose-induced apoptosis and oxidative stress via activation of Akt in cardiomyocytes. (a) Curcumin upregulated Akt and GSK-3β phosphorylation levels. (b) Increased Akt and GSK-3β phosphorylation by curcumin were abolished by treatment with LY294002. (c) The effects on the Bcl-2/Bax ratio and the expression of gp91phox and p47phox by curcumin were blocked by treatment with LY294002. n = 3. Values are presented as mean ± SD. ∗ P < 0.05 versus NG group. # P < 0.05 versus HG group. $ P < 0.05 versus curcumin treatment group.
4. Discussion
DCM is frequently seen in asymptomatic diabetic patients. It is now recognized as left ventricular dysfunction associated with increasing the danger of heart failure without hypertension and coronary artery disease or valvular heart diseases [6]. DM-induced left ventricular dysfunction includes impaired systolic and diastolic function, but diastolic dysfunction can occur prior to systolic dysfunction and can be characterized as the early phase of DCM [20]. Several studies have shown a close correlation between left ventricular diastolic dysfunction and myocardial apoptosis and have shown that cardiac function can be improved by sufficient control of myocardial apoptosis [21]. Massive loss of cardiomyocytes due to various apoptotic stimuli occurs, resulting in fibrosis and, eventually, heart failure due to the lack of cardiomyocyte proliferation greatly limiting the generation of new cardiomyocyte. In this context, a clear reduction in cardiomyocyte apoptosis is regarded as a latent therapeutic strategy for the treatment of DCM. In the current work, we found that curcumin lessened cardiomyocyte apoptosis induced by high glucose and observed a curcumin-induced reduction in Bax, which plays a crucial role in mitochondrion-mediated apoptosis by being inserted into the mitochondrial outer membrane and resulting in the release of proapoptotic factors. In contrast, Bcl-2 is an antiapoptotic protein that prevents Bax oligomerization and was increased by curcumin treatment. Thus, curcumin's cardioprotective effects are possibly mediated by normalization of the Bcl-2/Bax ratio.
Cumulative evidence suggests that both cardiomyocyte apoptosis and oxidative stress contribute to the pathogenesis and development of diabetic cardiovascular complications [22]. Previous researches have provided direct evidence that the sustained generation of ROS during oxidative stress leads to cardiomyocyte apoptosis, which contributes to the development of DCM [23]. Emerging evidence has confirmed that activation of NADPH oxidase-generated ROS signalling is related to apoptosis in cardiomyocytes exposed to a hyperglycemic environment [24]. NADPH oxidase contains two membrane-bound subunits (gp91phox and p22phox) and four cytosolic regulatory subunits, including p40phox, p47phox, p67phox, and Rac1. Rac1 plays a crucial role in the assembly of NADPH oxidase, which generates superoxide [25] and is a central factor in NADPH-mediated cardiomyocytes apoptosis in response to high glucose levels [26]. Therefore, the deleterious consequences of overactivation of NADPH oxidase in the form of diabetic cardiovascular complications have been well established. NADPH oxidase can impair the redox balance, thus inducing or exacerbating intracellular oxidative stress and resulting in abnormal ROS production. Therefore, inhibition of excessive ROS produced by NADPH oxidase appears to be another possible target for preventing the development of DCM [27]. Consistent with previous studies, we also found that lipid peroxidation levels were enhanced when the cardiomyocytes were exposed to high glucose, which was accompanied by an elevation in ROS generation as a result of the activation of NADPH oxidase. High glucose triggers NADPH oxidase activation by improving Rac1 activation and enhancing gp91phox and gp47phox expression. In agreement with earlier studies that showed that curcumin has a multitude of cardioprotective effects attributed to its efficient antioxidant capacity, we found that treatment with curcumin suppressed a hyperglycemia-induced rise in ROS generation through inactivation of NADPH oxidase.
Impaired Akt/GSK-3β signalling pathway has been shown to be involved in the development of metabolic disorders. Akt is responsible for the modulation of cardiovascular functions linked with cardiac growth and survival, contractile function, and coronary angiogenesis [28]. In particular, Akt1 has been demonstrated to play an antagonist role against pathological cardiac hypertrophy, which is an inevitable precursor of heart failure. Consistent with these observations, Akt1−/− mice displayed multiple heart defects, including enhanced cardiac growth and insufficient cardiomyocyte contractility [29]. Furthermore, Akt2 exerts a particularly important impact on the regulation of glucose metabolism and supports cell survival by restraining apoptosis via activation or inactivation of a number of target proteins involved in the process of apoptotic cascades [30, 31]. GSK-3β, a critical downstream element of the Akt pathway, participated in physiological and pathological processes such as regulation of glycogen synthesis and disposal, as well as cell death [32]. It is well accepted that glucose utilization is decreased and FFA oxidation is increased in the diabetic heart [33]. This substrate utilization shift has been known to contribute to the pathogenesis of DCM. Thus, activating Akt phosphorylation and inhibiting GSK-3β activity may be considered as cardioprotective actions, as they maintain the physiological growth and functions of the heart and promote cell survival. In the present study, our results indicated that cardiomyocytes exposed to high glucose have strikingly decreased expression levels of Akt and GSK-3β phosphorylation, which is in accordance with the previous report [34]. Interestingly, the beneficial roles exerted by curcumin in high glucose-induced cardiac injury such as enhancement of Akt and GSK-3β phosphorylation, reduction of gp91phox and p47phox expression, and regulatory apoptosis-related proteins were negated by the application of LY294002, which indicate that the PI3K/Akt/GSK-3β signalling pathway may be responsible for the inhibition of high glucose-induced cardiac injury by curcumin.
In summary, curcumin exerts cardioprotection against high glucose-induced cardiomyocyte apoptosis, and these effects were shown to possibly be due to efficient prevention of NADPH oxidase-derived oxidative stress and preservation of Akt and GSK-3β phosphorylation in vitro. Therefore, curcumin may be a feasible novel drug for the treatment of DCM.
Acknowledgments
This work was supported by the following grants: Hubei Province Natural Science Fund Project of outstanding youth project awarded to Dr. Chao Liu (no. 2012FFA005), New Century Excellent Talents Project of Ministry of Education awarded to Dr. Chao Liu (no. NCET-13-0781), the National Natural Science Foundation of China awarded to Dr. Wei Yu (no. 81500296), the Hubei Province Natural Science Fund Project awarded to Dr. Wei Yu (no. 2014CFB382), and the Hubei Province Education Office Fund Project awarded to Dr. Wei Yu (no. D20152802).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Wei Yu and Wenliang Zha contributed equally to this work.
References
- 1.Guariguata L., Whiting D. R., Hambleton I., Beagley J., Linnenkamp U., Shaw J. E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kannel W. B., McGee D. L. Diabetes and cardiovascular disease. The Framingham study. The Journal of the American Medical Association. 1979;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z. V., Hill J. A. Diabetic cardiomyopathy: catabolism driving metabolism. Circulation. 2015;131(9):771–773. doi: 10.1161/circulationaha.115.015357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubler S., Dlugash J., Yuceoglu Y. Z., Kumral T., Branwood A. W., Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. The American Journal of Cardiology. 1972;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 5.Poornima I. G., Parikh P., Shannon R. P. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circulation Research. 2006;98(5):596–605. doi: 10.1161/01.res.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 6.Frustaci A., Kajstura J., Chimenti C., et al. Myocardial cell death in human diabetes. Circulation Research. 2000;87(12):1123–1132. doi: 10.1161/01.RES.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 7.He C., Zhu H., Li H., Zou M.-H., Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62(4):1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarquini R., Lazzeri C., Pala L., Rotella C. M., Gensini G. F. The diabetic cardiomyopathy. Acta Diabetologica. 2011;48(3):173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 9.Kajstura J., Fiordaliso F., Andreoli A. M., et al. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50(6):1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 10.Modesti A., Bertolozzi I., Gamberi T., et al. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes. 2005;54(2):394–401. doi: 10.2337/diabetes.54.2.394. [DOI] [PubMed] [Google Scholar]
- 11.Fiordaliso F., Bianchi R., Staszewsky L., et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. Journal of Molecular and Cellular Cardiology. 2004;37(5):959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Zingg J.-M., Hasan S. T., Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. BioFactors. 2013;39(1):101–121. doi: 10.1002/biof.1072. [DOI] [PubMed] [Google Scholar]
- 13.Izem-Meziane M., Djerdjouri B., Rimbaud S., et al. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: protective effect of curcumin. The American Journal of Physiology—Heart and Circulatory Physiology. 2012;302(23):665–674. doi: 10.1152/ajpheart.00467.2011. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y., Wang Y., Zhao Y., et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63(10):3497–3511. doi: 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- 15.Yu W., Wu J., Cai F., et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0052013.e52013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younce C. W., Burmeister M. A., Ayala J. E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. American Journal of Physiology—Cell Physiology. 2013;304(6):C508–C518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- 17.Zielonka J., Hardy M., Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radical Biology and Medicine. 2009;46(3):329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Zhu H., Shen E., Wan L., Arnold J. M. O., Peng T. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59(8):2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Babcock S. A., Hu N., Maris J. R., Wang H., Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function. BMC Medicine. 2012;10, article 40 doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.DeBosch B., Treskov I., Lupu T. S., et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113(17):2097–2104. doi: 10.1161/circulationaha.105.595231. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Huang Z., Wang L., et al. Sitagliptin alleviated myocardial remodeling of the left ventricle and improved cardiac diastolic dysfunction in diabetic rats. Journal of Pharmacological Sciences. 2015;127(3):260–274. doi: 10.1016/j.jphs.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo W.-W., Wang W.-J., Tsai C.-Y., Way C.-L., Hsu H.-H., Chen L.-M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. International Journal of Cardiology. 2013;168(1):270–280. doi: 10.1016/j.ijcard.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 24.Tsai K.-H., Wang W.-J., Lin C.-W., et al. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. Journal of Cellular Physiology. 2012;227(4):1347–1357. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 25.Hordijk P. L. Regulation of NADPH oxidases: the role of Rac proteins. Circulation Research. 2006;98(4):453–462. doi: 10.1161/01.res.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 26.Shen E., Li Y., Li Y., et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58(10):2386–2395. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X., Chen R.-C., Yang Z.-H., et al. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food and Chemical Toxicology. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Katare R. G., Caporali A., Oikawa A., Meloni M., Emanuel C., Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circulation: Heart Failure. 2010;3(2):294–305. doi: 10.1161/CIRCHEARTFAILURE.109.903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z.-Z., Tschopp O., Di-Poï N., et al. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Molecular and Cellular Biology. 2005;25(23):10407–10418. doi: 10.1128/mcb.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H., Mu J., Kim J. K., et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 31.DeBosch B., Sambandam N., Weinheimer C., Courtois M., Muslin A. J. Akt2 regulates cardiac metabolism and cardiomyocyte survival. Journal of Biological Chemistry. 2006;281(43):32841–32851. doi: 10.1074/jbc.m513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Feng W., Xue W., et al. Inactivation of GSK-3β by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009;58(6):1391–1402. doi: 10.2337/db08-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razeghi P., Young M. E., Cockrill T. C., Frazier O. H., Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106(4):407–411. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 34.Guo S., Yao Q., Ke Z., Chen H., Wu J., Liu C. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Molecular and Cellular Endocrinology. 2015;412:85–94. doi: 10.1016/j.mce.2015.05.034. [DOI] [PubMed] [Google Scholar]