Abstract
Revealing how molecular mechanisms influence higher brain circuits in primates will be essential for understanding how genetic insults lead to increased risk of cognitive disorders. Traditionally, modulatory influences on higher cortical circuits have been examined using lesion techniques, where a brain region is depleted of a particular transmitter to determine how its loss impacts cognitive function. For example, depletion of catecholamines or acetylcholine from the dorsolateral prefrontal cortex produces striking deficits in working memory abilities. More directed techniques have utilized direct infusions of drug into a specific cortical site to try to circumvent compensatory changes that are common following transmitter depletion. The effects of drug on neuronal firing patterns are often studied using iontophoresis, where a minute amount of drug is moved into the brain using a tiny electrical current, thus minimizing the fluid flow that generally disrupts neuronal recordings. All of these approaches can be compared to systemic drug administration, which remains a key arena for the development of effective therapeutics for human cognitive disorders. Most recently, viral techniques are being developed to be able to manipulate proteins for which there is no developed pharmacology, and to allow optogenetic manipulations in primate cortex. As the association cortices greatly expand in brain evolution, research in nonhuman primates is particularly important for understanding the modulatory regulation of our highest order cognitive operations.
Keywords: Lesion, Microinfusion, Iontophoresis, Viral manipulations, Systemic administration
INTRODUCTION
A great challenge for this century is to discover how molecular mechanisms influence brain circuits, so that we can understand how genetic insults lead to symptoms of disease. This goal is particularly important for the higher cognitive functions of the primate association cortex, which are the target of so many devastating disorders. For example, the layer III pyramidal cells circuits in the dorsolateral prefrontal cortex (dlPFC) generate the mental representations that are the foundation of abstract thought, yet these circuits weaken with normal aging, and degenerate in schizophrenia and Alzheimer’s disease. Thus, it is particularly important to understand the molecular regulation of these newly evolved circuits. The arousal systems (e.g., norepinephrine (NE), dopamine (DA), acetylcholine (Ach), serotonin, orexins, and histamine) project to the cortex from the brainstem and basal forebrain, and release transmitter based on waking/sleep state, and the brain’s own interpretation of environmental events. These neuromodulators alter information processing in the brain, determining the strength of memories and the state of conscious awareness. Recent data indicate that dlPFC circuits in primates are particularly sensitive to changes in these neuromodulatory actions, and that they are regulated at the intracellular level differently than classic synapses in rodents, with mechanisms that are sometimes opposite to those seen in sensory cortical and hippocampal circuits. For example, they are rapidly taken “off-line” by exposure to even quite mild, uncontrollable stress through activation of cAMP signaling, conditions which strengthen many subcortical functions (Arnsten, 2009; Arnsten et al, 2015). As the association cortices greatly expand in brain evolution, many of these questions can only be addressed in nonhuman primates. The following review summarizes some of the approaches to study modulatory and molecular influences on primate cortical function.
Lesions and depletion of neuromodulators
Lesions remain a key research tool, as they can reveal what is necessary for function. The earliest studies of neuromodulatory influences on higher cortical function in monkeys examined the effects of depleting catecholamines from the dlPFC on the performance of a spatial working memory task (Brozoski et al, 1979). This pioneering study compared the effects of the neurotoxin 6-OHDA (with or without treatment to try to protect noradrenergic terminals) to that of dlPFC cortical tissue ablation in rhesus monkeys. Ablation of the dlPFC produced a dramatic and permanent deficit on performance of the working memory task. Remarkably, the 6-OHDA lesions that produced large depletions of both DA and NE produced deficits in working memory performance as severe as those caused by tissue ablation. In contrast, depletions of serotonin from the dlPFC had little effect. This study was the first indication that the correct modulatory state is essential for the functioning of the dlPFC. The study was replicated in marmoset monkeys, and extended to studies of the orbital PFC, where both serotonin and catecholamines were found to be important for function, with qualitative differences in the errors made depending on which monoamines were lesioned (Roberts, 2011; Walker et al, 2009). More recently, research in rhesus monkeys has focused on cholinergic mechanisms, showing that destruction of Ach terminals in the dlPFC also produces significant deficits in working memory performance (Croxson et al, 2011). These lesion studies revealed the importance of catecholamines and Ach to dlPFC function, and inspired further studies of the receptor and intracellular mechanisms underlying their critical actions.
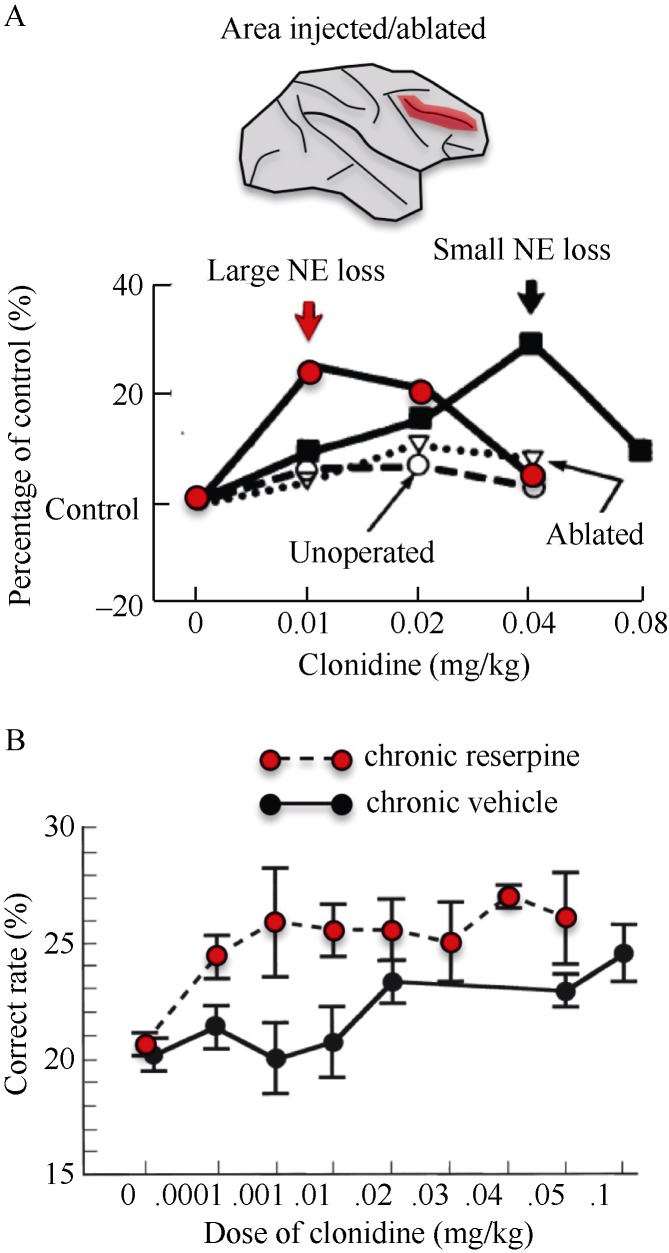
There are several advantages to the lesion approach. As described above, the lesion method is one of the few that can reveal whether a mechanism is necessary for function and the consequences of its removal to cognitive function. Depletion of a transmitter can also help to dissociate drug actions at pre- v.s. post-synaptic receptors, as drugs acting at presynaptic receptors lose their efficacy when the substrate is depleted or destroyed, while post-synaptic actions remain and are often magnified due to post-synaptic super-sensitivity. An example of this is the work showing that clonidine’s beneficial effects on working memory occur at post-synaptic sites in the dlPFC. Clonidine is an alpha-2 adrenoceptor agonist, and initial studies of alpha-2 receptors focused on their presynaptic location (Langer, 1978). As shown in Figure 1, research in rhesus monkeys showed that clonidine’s beneficial effects on cognition were magnified in response to NE depletion in the dlPFC (Arnsten & Goldman-Rakic, 1985), or by more global monoamine depletion with systemic reserpine (Arnsten & Cai, 1993; Cai et al, 1993). Demonstration of a post-synaptic site of action was key for discovering the importance of NE actions on dlPFC neurons.
Figure 1.
Lesion studies demonstrated that stimulation post-synaptic alpha-2 receptors in dlPFC improves working memory function^A: Rhesus monkeys with 6-OHDA lesions or ablations of the dlPFC (red area) were treated with the alpha-2 agonist, clonidine, prior to performing a spatial working memory task. Clonidine’s potency related to the degree of NE depletion from dlPFC, consistent with actions at post-synaptic alpha-2 receptors in this region (Adapted from Arnsten & Goldman-Rakic, 1985); B: Rhesus monkeys were treated with chronic reserpine to deplete monoamines globally, a classic test for pre- v.s. post-synaptic drug actions. Clonidine’s beneficial effects on spatial working memory were enhanced following reserpine treatment, consistent with a post-synaptic site of drug action (Adapted from Cai et al, 1993).
Lesion studies also have many disadvantages. There are few neurotoxins available for this purpose, and those that do exist are often not very selective and/or effective. For example, the effective 6-OHDA lesion in Brozoski et al (1979) produced a 87% depletion of DA and a 76% depletion of NE, even though treatments were given to try to protect NE terminals. Another major disadvantage of lesions is that it takes time for the depletion to occur, and there are usually compensatory actions that can mask the effects of the lesion. Thus, negative effects are hard to interpret. Nonetheless, they have been foundational to the field, and will remain a bench post for identifying the most important modulatory influences on cortical function.
Microinfusions of drug
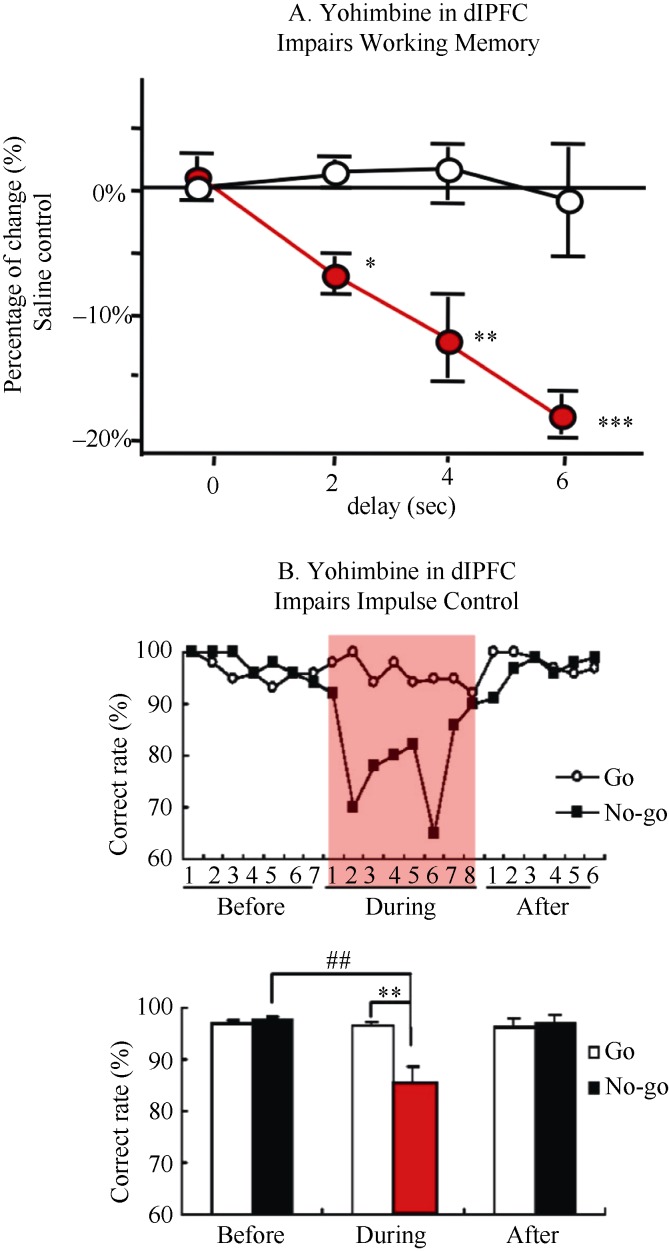
A highly effective tool for examining the contribution of not only a neuromodulator, but its receptors, is the ability to infuse drug into cortex to observe immediate effects on cognitive behavior. For example, blocking alpha-2 adrenoceptors by infusions of yohimbine into the rhesus monkey dlPFC revealed the critical importance of endogenous NE stimulation of these receptors. Yohimbine infusions impaired working memory (Li & Mei, 1994), weakened impulse control (Ma et al, 2003), and induced locomotor hyperactivity (Ma et al, 2005), producing a profile of deficits similar to Attention Deficit Hyperactivity Disorder (Figure 2). Infusions into primate dlPFC have also helped to illuminate how high levels of catecholamine release during stress impair working memory function, as the loss of working memory function can be mimicked by the infusion of NE alpha-1 (Arnsten et al, 1999) or DA D1 (Gamo et al, 2015) receptor agonists into rhesus monkey dlPFC. Conversely, infusions of a D1 receptor antagonist similarly impaired working memory (Sawaguchi & Goldman-Rakic, 1991), consistent with the D1 receptor inverted U dose response (Zahrt et al, 1997). More recently, impairments in associative learning of visual features
Figure 2.
Local infusions of the alpha-2 receptor antagonist, yohimbine, into the dlPFC of rhesus monkeys impaired cognitive function and top-down control of behavior (The red areas represent yohimbine infusion period)^A: Infusions of yohimbine into the rhesus monkey dlPFC produced profound impairments in working memory performance. In contrast, comparable infusions of the alpha-1 receptor antagonist, prazosin, or the beta receptor antagonist, propranolol, had no effect (Adapted from Li & Mei, 1994); B: Infusions of yohimbine into the rhesus monkey dlPFC impaired impulse control on a Go/No-Go task. Monkeys still performed well on Go trials, but could not inhibit behavior on No-Go trials (Adapted from Ma et al, 2003).
were observed with D1 receptor antagonist infusions into the ventrolateral PFC in rhesus monkey (Puig & Miller, 2012).
There are many advantages to the infusion technique. Infusions can be large enough to influence a large volume of cortex, and thus sufficient to have consequences for behavior (in contrast to iontophoretic application of tiny amounts of drug; see below). The infusion technique can be used with a wide variety of pharmacological compounds, irrespective of whether they cross the blood brain barrier, and thus one can see the functional consequences of blocking v.s. stimulating receptors, enzymes, transporters and/or intracellular signaling pathways. As the drug acts immediately, there is no time for compensatory brain actions, and one is more likely to see the functional ramifications of drug actions.
Iontophoretic application of drug
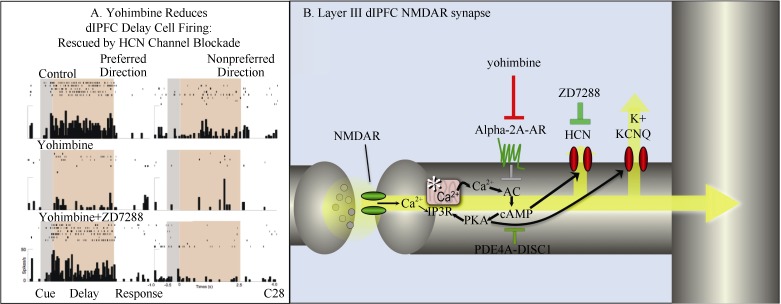
Iontophoresis uses a very small (nA) electrical current to move minute amounts of an electrically charged molecule out of a micropipette and into the brain. As there is no fluid movement, nearby neurons do not move, and one can continue with stable neuronal recordings. This technique has been essential in revealing the cellular basis for catecholamine and cholinergic actions in the primate dlPFC, including intracellular and ionic influences on neuronal physiology during higher cognitive processing. In monkeys performing a spatial working memory task, there are neurons that are able to maintain spatially tuned firing across a delay period when there is no sensory stimulation, i.e., so called Delay cells. Delay cells are thought to reside in deep layer III of the primate dlPFC, where recurrent excitation through NMDA receptor synapses allows them to maintain firing in the absence of sensory stimulation. Immunoelectron microscopy (immunoEM) revealed that alpha-2A adrenoceptors were localized immediately next to HCN channels on dendritic spines in layer III of dlPFC, suggesting that these molecules might interact (Wang et al, 2007). As the open state of HCN channels is increased by cAMP signaling, while alpha-2A adrenoceptors inhibit cAMP production, iontophoretic studies tested for functional interactions. These studies found that blocking alpha-2 receptors by iontophoresis of yohimbine markedly reduced the firing of Delay cells, while co-iontophoresis of ZD7288 to block HCN channels restored firing (Wang et al, 2007) (Figure 3). Thus, the physiological interaction was confirmed.
Figure 3.
Iontophoresis reveals the cellular actions of alpha-2 receptors in the primate dlPFC^A: Iontophoresis of the alpha-2 receptor antagonist, yohimbine, onto a Delay cell in the dlPFC reduced persistent firing in monkeys performing a spatial working memory task. Firing was restored by co-iontohoresis of the HCN channel blocker, ZD7288 (Adapted from Wang et al, 2007); B: Schematic illustration of the key role of alpha-2A adrenoceptors in inhibiting feedforward, cAMP-calcium-K+ channel signaling in layer III of the primate dlPFC. Yohimbine blocks these receptors, opening K+ channels, weakening synaptic efficacy, and reducing task-related neuronal firing needed for working memory.
The major advantage of iontophoresis is that it is the only technique that allows the reliable observation of drug effects on neuronal firing in cognitively-engaged monkeys. As the drug has immediate actions, there is no time for the brain to mount compensatory counteractions, and a mechanism can be probed effectively. Washout periods can be used to see if neuronal firing returns to normal (although some second messenger actions have long-lasting effects whereby neuronal firing does not normalize within the confines of a testing session). The ability to iontophorese multiple compounds also allows for testing of physiological interactions not possible with other techniques. The amount of drug released in brain with iontophoresis is sufficient to influence a group of cells, e.g., a minicolumn or local microcircuit, but usually not adequate to influence behavior. These features can be both a strength and/or a weakness. The absence of changes in behavior allows for easier interpretation of the data (changes in neuronal firing are not simply due to behavioral changes), and one can observe the changes in neuronal firing in the context of its normal circuitry. However, there are times when one would like to manipulate a single neuron independent of its microenvironment, and this is not possible with this technique.
The major disadvantage of iontophoresis is that it requires the use of electrically charged compounds. As drugs are purposefully synthesized to be lipophilic to promote brain penetration, this can limit the pharmacological arsenal available for this technique. The absence of behavioral changes can also be problematic if one wants to know how the manipulation would alter cognitive performance.
Viral manipulations
Viral manipulations allow the genetic manipulation of protein expression, including proteins for which there is no developed pharmacology. These powerful genetic methods have been tremendously successful in rodents, but are just beginning to be applied in primates. The current focus of the field is on the development of genetic manipulations to allow optogenetic manipulations in monkeys, e.g., to selectively activate excitatory neurons in the primary visual cortex (Nassi et al, 2015). However, there is a great need to extend this technology to be able to knockdown or overexpress signaling proteins for which there are currently no pharmacological tools, e.g. as has been done in rodent PFC. For example, viral knockdown of DISC1 (Disrupted In SChizophrenia) in rat medial PFC lowers the threshold for stress-induced PFC dysfunction, possibly by unanchoring PDE4A and dysregulating cAMP signaling (Gamo et al, 2013).
There will be enormous advantages to the application of these techniques in primate brain, including the opportunity to manipulate a wide universe of molecules with immediate relevance to genetic insults in human cognitive disorders. As higher cortical circuits are often regulated differently than circuits in rodents, this will be especially important for understanding how genetic insults lead to symptoms of higher cognitive dysfunction in established brain circuits.
However, the current disadvantages of this technology are daunting. Applying these methods to methods is still in its infancy, and there is a critical need to verify efficacy and selectivity, as methods that work in rodent neurons can fail to have the same effect in primate tissue. This process is very expensive, and has been particularly challenging in the current NIH funding environment. Even when successful, the method will have its challenges, as the genetic changes take days/weeks to express, and thus the brain has a chance to undergo compensatory/reactive changes. For example, one cannot perform the rapid before vs. after methods that are so powerful for single unit recordings with iontophoresis. Nonetheless, this arena represents a critical direction for future research.
Comparison to systemically administered compounds
Finally, it can be important to examine changes in neuronal firing following systemic administration of drug, as this has direct relevance to human patients taking medications. Some types of agents have the same effect whether they are administered systemically, or by local iontophoresis. For example, systemic administration of the alpha-2 agonist, clonidine, enhances the firing of dlPFC Delay cells (Li et al, 1999), similar to the enhancing effects of iontophoretically applied guanfacine, a more selective alpha-2A-adrenoceptor agonist (Wang et al, 2007). However, this correspondence between systemic and local application is not always seen, e.g. systemic administration of the NMDAR blocker, ketamine, increases the firing of dlPFC Response cells, while local NMDAR blockade reduces Response cell firing (Wang et al, 2013).
The great advantage of systemic administration of drug is that it allows direct comparison to human medications. For example, clonidine and guanfacine are both now approved for the treatment of ADHD, and their beneficial effects on dlPFC neuronal firing likely contribute to their therapeutic actions (Arnsten & Jin, 2012). Systemic administration also becomes necessary when drugs cannot be iontophoresed due to lack of electric charge. However, global actions throughout brain and body provide very little mechanistic information unless coupled with some of the techniques described above.
Summary
In summary, we have made great progress in understanding the molecular needs of the primate dlPFC, using a variety of complementary approaches to reveal intracellular mechanisms and create new treatments for human cognitive disorders. However, we will need to adapt viral technology to bridge our understanding of primate cortex with the genetic revolution.
Contributor Information
Min WANG, Department of Neurobiology, School of Medicine, Yale University, New Haven, CT, 06510, USA.
Amy F.T. ARNSTEN, Department of Neurobiology, School of Medicine, Yale University, New Haven, CT, 06510, USA
REFERENCES
- [1].Arnsten AFT. 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6): 318-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arnsten AFT, Cai JX. 1993. Post-synaptic alpha-2 receptor stimulation improves working memory in aged monkeys: Indirect effects of yohimbine vs. direct effects of clonidine. Neurobiology of Aging, 14(6): 597-603. [DOI] [PubMed] [Google Scholar]
- [3].Arnsten AFT, Goldman-Rakic PS. 1985. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science, 230(4731): 1273-1276. [DOI] [PubMed] [Google Scholar]
- [4].Arnsten AFT, Jin LE. 2012. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. The Yale Journal of Biology and Medicine, 85(1): 45-58. [PMC free article] [PubMed] [Google Scholar]
- [5].Arnsten AFT, Mathew R, Ubriani R, Taylor JR, Li B-M. 1999. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biological Psychiatry, 45(1): 26-31. [DOI] [PubMed] [Google Scholar]
- [6].Arnsten AFT, Raskind M, Taylor FB, Connor DF. 2015. The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for Post-Traumatic Stress Disorder. Neurobiology of Stress, 1(1): 89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brozoski T, Brown RM, Rosvold HE, Goldman PS. 1979. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science, 205(4409): 929-931. [DOI] [PubMed] [Google Scholar]
- [8].Cai JX, Ma Y, Xu L, Hu X. 1993. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha-2 adrenergic agonist clonidine. Brain Research, 614(1-2): 191-196. [DOI] [PubMed] [Google Scholar]
- [9].Croxson PL, Kyriazis DA, Baxter MG. 2011. Cholinergic modulation of a specific memory function of prefrontal cortex. Nature Neuroscience, 14(12): 1510-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gamo NJ, Duque A, Paspalas CD, Kata A, Fine R, Boven L, Bryan C, Lo T, Anighoro K, Bermudez L, Peng K, Annor A, Mansson E, Taylor SR, Patel K, Simen AA, Arnsten AFT. 2013. Role of disrupted in schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Translational Psychiatry, 3: e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gamo NJ, Lur G, Higley MJ, Wang M, Paspalas CD, Vijayraghavan S, Yang Y, Ramos BP, Peng K, Kata A, Boven L, Lin F, Roman L, Lee D, Arnsten AFT. 2015. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with HCN channels. Biological Psychiatry, Feb 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Langer SZ. 1978. Presynaptic receptors. Nature, 275: 479-480. [DOI] [PubMed] [Google Scholar]
- [13].Li BM, Mao ZM, Wang M, Mei ZT. 1999. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology, 21(5): 601-610. [DOI] [PubMed] [Google Scholar]
- [14].Li BM, Mei ZT. 1994. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behavioral and Neural Biology, 62(2): 134-139. [DOI] [PubMed] [Google Scholar]
- [15].Ma CL, Arnsten AFT, Li BM. 2005. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha-2-adrenoceptors in monkeys. Biological Psychiatry, 57(2): 192-195. [DOI] [PubMed] [Google Scholar]
- [16].Ma CL, Qi XL, Peng JY, Li BM. 2003. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Neuroreport, 14: 1013-1016. [DOI] [PubMed] [Google Scholar]
- [17].Nassi JJ, Avery MC, Cetin AH, Roe AW, Reynolds JH. 2015. Optogenetic activation of normalization in alert macaque visual cortex. Neuron, 86(6): 1504-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Puig MV, Miller EK, 2012. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron, 74(5): 874-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roberts AC. 2011. The importance of serotonin for orbitofrontal function. Biological Psychiatry, 69(12): 1185-1191. [DOI] [PubMed] [Google Scholar]
- [20].Sawaguchi T, Goldman-Rakic PS. 1991. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science, 251(4996): 947-950. [DOI] [PubMed] [Google Scholar]
- [21].Walker SC, Robbins TW, Roberts AC. 2009. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cerebral Cortex, 19(4): 889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley AG, Nou E, Mazer JA, McCormick DA, Arnsten AFT. 2007. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell, 129(2): 397-410. [DOI] [PubMed] [Google Scholar]
- [23].Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang X-J, Arnsten AF. 2013. NMDA receptors subserve working memory persistent neuronal firing In dorsolateral prefrontal cortex. Neuron, 77(4): 736-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. 1997. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. The Journal of Neuroscience, 17(21): 8528-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]