Abstract
Objective
To assess the effect of 3 insulin analogue regimens on change in carotid intima-media thickness (IMT) in patients with type 2 diabetes.
Design and setting
Investigator-initiated, randomised, placebo-controlled trial with a 2×3 factorial design, conducted at 8 hospitals in Denmark.
Participants and interventions
Participants with type 2 diabetes (glycated haemoglobin (HbA1c)≥7.5% (≥58 mmol/mol), body mass index >25 kg/m2) were, in addition to metformin versus placebo, randomised to 18 months open-label biphasic insulin aspart 1–3 times daily (n=137) versus insulin aspart 3 times daily in combination with insulin detemir once daily (n=138) versus insulin detemir alone once daily (n=137), aiming at HbA1c≤7.0% (≤53 mmol/mol).
Outcomes
Primary outcome was change in mean carotid IMT (a marker of subclinical cardiovascular disease). HbA1c, insulin dose, weight, and hypoglycaemic and serious adverse events were other prespecified outcomes.
Results
Carotid IMT change did not differ between groups (biphasic −0.009 mm (95% CI −0.022 to 0.004), aspart+detemir 0.000 mm (95% CI −0.013 to 0.013), detemir −0.012 mm (95% CI −0.025 to 0.000)). HbA1c was more reduced with biphasic (−1.0% (95% CI −1.2 to −0.8)) compared with the aspart+detemir (−0.4% (95% CI −0.6 to −0.3)) and detemir (−0.3% (95% CI −0.4 to −0.1)) groups (p<0.001). Weight gain was higher in the biphasic (3.3 kg (95% CI 2.7 to 4.0) and aspart+detemir (3.2 kg (95% CI 2.6 to 3.9)) compared with the detemir group (1.9 kg (95% CI 1.3 to 2.6)). Insulin dose was higher with detemir (1.6 IU/kg/day (95% CI 1.4 to 1.8)) compared with biphasic (1.0 IU/kg/day (95% CI 0.9 to 1.1)) and aspart+detemir (1.1 IU/kg/day (95% CI 1.0 to 1.3)) (p<0.001). Number of participants with severe hypoglycaemia and serious adverse events did not differ.
Conclusions
Carotid IMT change did not differ between 3 insulin regimens despite differences in HbA1c, weight gain and insulin doses. The trial only reached 46% of planned sample size and lack of power may therefore have affected our results.
Trial registration number
Keywords: ULTRASONOGRAPHY
Strengths and limitations of this study.
It is not known to what extent different insulin analogue regimens primarily targeting basal or postprandial hyperglycaemia may influence the risk of cardiovascular disease in a differential manner in patients with type 2 diabetes. The Copenhagen Insulin and Metformin Therapy (CIMT) trial was designed to address this question.
Strengths of the CIMT trial include the centrally randomised design and the relatively large population of well-characterised patients.
A limitation is the choice of carotid intima-media thickness as a surrogate risk marker for cardiovascular disease instead of hard clinical outcomes.
The trial only reached 46% of the planned sample size and lack of power may therefore have affected our results.
Introduction
Intensified treatment with insulin eventually becomes necessary to maintain acceptable glycaemic control in most patients with type 2 diabetes (T2D),1 although this intervention has not been proven to reduce the risk of cardiovascular disease.2 3 Insulin regimens targeting postprandial glucose excursions may reduce risk of atherosclerosis compared with regimens primarily targeting fasting glucose levels.4 5 Clinical guidelines have endorsed use of basal insulin preparations as the preferential initial insulin regimen.1 This recommendation is based on reports indicating that use of basal bedtime insulin may be associated with less weight gain and reduced risk of hypoglycaemia.6–8 Nevertheless, treatment with basal insulin may be insufficient, and addition of rapid-acting insulin before meals (basal-bolus therapy) or switching to one or more daily injections of premixed insulin preparations may become necessary to reduce and maintain the glycated haemoglobin (HbA1c) targets.6 9–13 However, large-scale long-term (>12 months) comparisons to guide the clinician's choice between the different insulin regimens are lacking.
Carotid intima-media thickness (IMT) is a risk marker for cardiovascular disease, and has been shown to correlate with established—and to predict future—cardiovascular disease.14–16
The objective of the Copenhagen Insulin and Metformin Therapy (CIMT) trial was to evaluate the effect of 18 months intervention with metformin versus placebo in combination with three insulin analogue regimens on the progression of mean carotid IMT in patients with T2D. The metformin versus placebo comparison was defined as the primary objective and reported in a separate article in this volume of BMJ Open,17 whereas comparisons between insulin regimens were defined as secondary objectives and reported in this article.
Materials and methods
Trial design, participants and randomisation
The CIMT trial is an investigator-initiated, multicentre, randomised superiority trial with a 2×3 factorial design, conducted from May 2008 to December 2012, as previously described.17 18 Participants with T2D, age >30 years, body mass index (BMI) >25 kg/m2, HbA1c≥7.5% (58 mmol/mol), treated with oral antidiabetic drugs for at least 1 year and/or insulin treatment for at least 3 months, where the investigator deemed the patient capable of insulin therapy once daily, were recruited from the eight diabetes outpatient clinics in the Capital Region, Denmark. Exclusion criteria were major cardiovascular disease within the past 3 months, carotid artery stenosis >70%, heart failure, recent cancer, renal or liver disease, alcohol or drug abuse, unstable retinopathy, pregnant or breastfeeding women, fertile women not using birth control measure and allergy towards trial medication. All participants gave written informed consent before participation and, after a screening visit, were centrally randomised according to the 2×3 factorial design. Randomisation was, in the first step, performed according to a computer-generated allocation sequence, using permuted blocks with varying block sizes of four, six and eight, and in the second step, using varying block sizes of three, six and nine. Participants were, in two steps, randomised 1:1:1 to one of three insulin analogue regimens and 1:1 to treatment with metformin versus placebo (see online supplementary figure S1). Randomisation was stratified by age >65 years, insulin treatment at trial entry and treatment at Steno Diabetes Center (recruiting half of the participants). The insulin treatment was open-labelled, whereas participants, investigators and medical staff were blinded to the metformin or placebo intervention.
bmjopen-2015-008377supp.pdf (463.6KB, pdf)
Intervention, visits and investigations
After local screening and investigations including analysis of carotid IMT at Steno Diabetes Center, prior glucose lowering drugs were discontinued, and participants initiated allocated treatment with one of three insulin regimens: biphasic insulin aspart before main meals one to three times daily (biphasic group) versus insulin aspart before main meals three times daily in combination with bedtime insulin detemir once daily (aspart+detemir group) versus bedtime insulin detemir alone once daily (detemir group), all in combination with metformin 1 g two times daily versus matching placebo (Ref. 17 and see online supplementary figure S1). In all treatment groups, the aim was HbA1c≤7.0% (≤53 mmol/mol). Participants treated with insulin before trial entry were allocated insulin at a daily dose similar to the pretrial dose. Participants not treated with insulin before trial entry were initiated with a daily insulin dose of 0.2 IU/kg. The insulin dose was continuously titrated based on fasting, and preprandial and postprandial plasma glucose levels, with guidance from an insulin titration algorithm (see online supplementary appendix 1). A proportion of participants randomised to detemir once daily experienced unacceptably high blood glucose excursions despite high detemir doses. The degree of this challenge was not expected prior to the trial and it was therefore necessary to make an amendment to the protocol during the trial, which was approved by the Regional Committee on Biomedical Research Ethics by 1 July 2009. The participants were thereafter supplemented with a morning dose of detemir if HbA1c≤7.0% was not reached and plasma glucose levels in the evening were >10 mmol/L despite acceptable fasting plasma glucose levels (4.4–6.1 mmol/L). If acceptable glycaemic response (defined as HbA1c≤7.0% or a reduction in HbA1c of 0.5%) was reached after 3–6 months, twice daily detemir was continued, otherwise treatment was changed to detemir once daily in combination with insulin aspart before one or more meals. Participants were treated with antihypertensives and statins according to guidelines and received aspirin 75 mg/day at the discretion of the investigators.
During the 18-month intervention, participants were seen every third month at their local diabetes clinics. Before termination of trial medication, investigations including carotid IMT were repeated at Steno Diabetes Center.
Ultrasound scans of the carotid arteries were performed by two technicians, and mean and maximum IMT of the common carotid artery were subsequently analysed using specialised software, and averaged from the left and right side. In addition, atherosclerotic plaques in the left and right bifurcation, and the common and internal carotid artery, were quantified. The technicians were blinded with respect to the metformin versus placebo comparison. Regarding insulin, the technicians were not informed about the treatment group. However, we cannot exclude that, occasionally, information about which insulin analogue the participant used was given to the technician. A description (see online supplementary appendix 2) of carotid IMT analysis and reproducibility has been reported.18 19
Outcomes
Primary outcome was change in mean carotid IMT of the common carotid arteries. Secondary outcomes were episodes of hypoglycaemia and serious adverse events. Other prespecified explorative outcomes were changes in maximal carotid IMT, number of atherosclerotic plaques, glycaemic control (HbA1c, fasting plasma glucose, insulin and C peptide), insulin dose and weight. In the preplanned data analyses, carotid intima-media area, relative compliance and incremental elastic modulus were included as explorative outcomes. Other prespecified explorative outcomes will be reported separately.18
Statistical analyses
Sample size estimation was based on the primary hypothesis of metformin being superior to placebo with regard to change in carotid IMT. Owing to the 2×3 factorial designs and the choice of five possible group comparisons, the α value was set to 0.01 for preserving an overall 5% family-wise error. With an estimated 900 participants providing a power of 85% to detect a relevant difference of 0.018 mm in carotid IMT assuming a population SD of 0.075 mm between metformin and placebo during 18 months, comparisons between the three insulin regimens achieves a power of approximately 70% to detect or reject an effect similar to that of statins reported in the meta-analysis by Espeland et al.18 20 Missing data on the primary outcome were imputed using multiple imputation.21 The primary analysis was intention-to-treat of the mean carotid IMT at 18 months adjusted for stratification variables and baseline value of carotid IMT. Secondary analysis was adjusted only for baseline value of carotid IMT. Analysis of explorative outcomes was conducted by a random-effects model for all observations, with a random person effect, including stratification variables and design variables (sex, prior cardiovascular disease, statin treatment and positive glutamic acid decarboxylase antibodies). This model was also used for reporting the changes in mean carotid IMT in each randomisation group, as these changes are not modelled in the specified primary analysis with baseline control.17 The statistical model used for analysis of hypoglycaemic events was a Poisson model for the rate of events for each person and a logistic regression for occurrence of any event. We did not plan to adjust predefined analyses for multiplicity. However, to adjust for multiplicity, we post hoc calculated the significance level adjusted to 0.05/((K+1)/2)) (where K represents the number of prespecified secondary outcomes).22
All analyses were made in the statistical program R (R Core Team. R: A language and environment for statistical computing. 16-1-2014. Vienna, Austria: R Foundation for Statistical Computing, 2013).
Results
Anonymised patient-level data and a detailed account of all statistical analyses can be found at http://bendixcarstensen.com/SDC/CIMT/DOM/CIMT.pdf
Trial participants and interventions
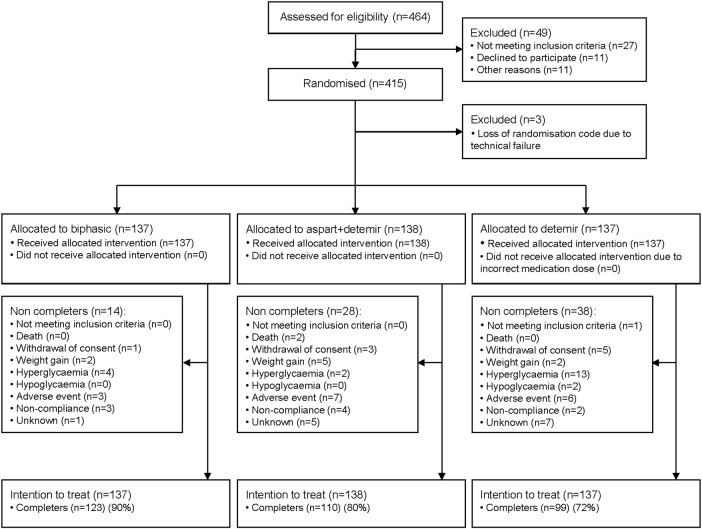
We assessed 464 participants for eligibility to participate and 415 underwent randomisation. The randomisation code for the blinded metformin/placebo treatment was lost for three participants due to server failure during the trial, and those participants were excluded from the analysis. In addition to intervention with metformin/placebo, 137 participants were randomised to the biphasic group (123 (90%) completers), 138 to the aspart+detemir group (110 (80%) completers) and 137 to the detemir group (99 (72%) completers). The number of completers was significantly higher in the biphasic group compared with the aspart+detemir (p=0.024) and detemir (p=0.001) groups. More patients in the detemir group dropped out due to hyperglycaemia as compared with the other treatment groups (figure 1). Whenever possible, participants not completing the trial came to a final visit including measurement of carotid IMT (ie, the number of participants not attending the final visit was 10 (7%) in the biphasic group, 11 (8%) in the aspart+detemir group and 17 (12%) in the detemir group). As shown in table 1, the participants were well matched at trial entry (age of 60–61 years, mean duration of T2D of 13 years and ∼70% receiving insulin treatment at trial entry). In the biphasic group, injections were increased from one to two daily injections in 65 (47%) participants and to three daily injections in 63 (46%) participants. In the aspart+detemir group, insulin detemir was administered once daily according to the trial design. In the detemir group, the number of injections was increased from one time to two times daily in 110 (80%) participants, and additional mealtime insulin aspart was prescribed in 32 (23%) participants.
Figure 1.
Flow of participants through trial.
Table 1.
Baseline characteristics of participants by allocation group
| Biphasic (n=137) | Aspart+detemir (n=138) | Detemir (n=137) | |
|---|---|---|---|
| Age (years) | 61.1 (5.6) | 60.2 (9.3) | 60.5 (8.9) |
| N (%) male | 90 (65.7) | 96 (69.6) | 95 (69.3) |
| Weight (kg) | 95.6 (14.8) | 97.5 (15.0) | 98.5 (15.1) |
| Body mass index* | 31.8 (4.4) | 32.2 (3.9) | 32.4 (4.3) |
| Waist–hip ratio | 1.00 (0.08) | 1.00 (0.08) | 1.01 (0.09) |
| N (%) smokers | 27 (19.7) | 19 (13.8) | 17 (12.6) |
| Median (IQR) alcohol consumption (units/week) | 2 (0–5) | 2 (0–5) | 2 (0–6) |
| N (%) Caucasians | 133 (97.1) | 133 (96.4) | 136 (99.3) |
| Diabetes and complications | |||
| Duration of type 2 diabetes (years) | 12.9 (6.5) | 12.9 (6.5) | 12.9 (6.2) |
| N (%) GAD65 antibodies ≥25 U/mL | 10 (7.3) | 9 (6.5) | 11 (8.0) |
| HbA1c (%) | 8.6 (1.0) | 8.5 (1.0) | 8.5 (1.1) |
| HbA1c (mmol/mol) | 70 (11) | 69 (11) | 69 (12) |
| Fasting p-glucose (mmol/L) | 10.7 (3.5) | 9.9 (3.1) | 10.4 (3.1) |
| Median (IQR) fasting p-insulin (pmol/L) | 68 (38–103) | 75 (45–132) | 65 (37–114) |
| Median (IQR) fasting C peptide (pmol/L) | 782 (418–1150) | 828 (507–1217) | 877 (466–1280) |
| N (%) prior CVD† | 35 (25.6) | 29 (21.0) | 36 (26.3) |
| N (%) microalbuminuria | 35 (26.5) | 23 (17.0) | 30 (22.2) |
| N (%) macroalbuminuria | 5 (3.8) | 8 (5.9) | 7 (5.2) |
| eCCr‡ (mL/min) | 129 (45) | 130 (45) | 124 (43) |
| N (%) simple retinopathy | 38 (28.4) | 42 (31.3) | 42 (31.3) |
| N (%) proliferative retinopathy | 10 (7.5) | 6 (4.5) | 9 (6.7) |
| N (%) prior laser treatment | 16 (11.9) | 7 (5.1) | 14 (10.5) |
| N (%) autonomous neuropathy | 23 (16.9) | 22 (16.1) | 24 (17.7) |
| N (%) peripheral neuropathy | 48 (35.0) | 46 (33.6) | 60 (44.1) |
| Blood pressure and lipids | |||
| Systolic blood pressure (mm Hg) | 138.5 (15.1) | 139.8 (14.8) | 139.8 (16.1) |
| Diastolic blood pressure (mm Hg) | 82.2 (9.2) | 82.4 (9.3) | 81.8 (9.4) |
| Heart rate (bpm) | 77 (13) | 76 (11) | 77 (11) |
| Total cholesterol (mmol/L) | 4.1 (0.9) | 4.2 (1.0) | 4.1 (1.0) |
| LDL cholesterol (mmol/L) | 2.1 (0.8) | 2.3 (0.8) | 2.2 (0.8) |
| Median (IQR) VLDL cholesterol (mmol/L) | 0.7 (0.5–1.0) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) |
| HDL cholesterol (mmol/L) | 1.2 (0.4) | 1.1 (0.3) | 1.1 (0.3) |
| Median (IQR) triglycerides (mmol/L) | 1.5 (1.0–2.3) | 1.6 (1.2–2.1) | 1.6 (1.2–2.4) |
| Medication | |||
| N (%) metformin§ | 111 (81.0) | 114 (82.6) | 118 (86.8) |
| N (%) insulin§ | 94 (68.6) | 96 (69.6) | 95 (69.3) |
| N (%) sulfonylurea§ | 39 (28.5) | 40 (29.0) | 37 (27.2) |
| N (%) other antihyperglycaemic drug§ | 17 (12.4) | 17 (12.3) | 25 (18.3) |
| N (%) RAS blockade | 106 (77.4) | 102 (73.9) | 100 (73.0) |
| N (%) other antihypertensive drug | 79 (57.7) | 73 (52.9) | 81 (59.1) |
| N (%) statin | 116 (84.7) | 116 (84.1) | 119 (86.9) |
| N (%) aspirin | 80 (58.4) | 79 (57.3) | 72 (52.6) |
| Carotid ultrasound measures | |||
| Mean carotid IMT (mm) | 0.786 (0.121) | 0.796 (0.148) | 0.798 (0.139) |
| Maximal carotid IMT (mm) | 0.949 (0.140) | 0.954 (0.165) | 0.965 (0.155) |
| Relative compliance×103 (mm/Hg) | 2.5 (1.0) | 2.6 (1.1) | 2.6 (1.1) |
| Incremental elastic modulus (mm Hg) | 2377 (1073) | 2314 (1012) | 2348 (1009) |
| Carotid intima-media area (mm2) | 18.64 (4.10) | 19.28 (5.14) | 19.49 (4.89) |
| Median (IQR) number of plaques¶ | 3 (2–4) | 2 (1–4) | 3 (2–5) |
Values are means (SDs) unless stated otherwise.
*Body mass index is calculated as weight (kg) divided by height (m)2.
†Prior CVD was defined as one or more of the following: myocardial infarction, heart surgery, ischaemic heart disease, heart insufficiency, vascular surgery, stroke, transitory cerebral ischaemia and amputation.
‡Calculated by the Cockcroft-Gault equation: eCCr=((140−age)×weight (kg)×constant)/serum creatinine (μmol/L), constant female: 1.04, male: 1.23.
§All antihyperglycaemic drugs were terminated at randomisation.
¶Sum of plaques in left and right bifurcation, common and internal carotid artery.
CVD, cardiovascular disease; eCCr, estimated creatinine clearance; GAD, glutamic acid decarboxylase; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; IMT, intima-media thickness; LDL, low-density lipoprotein; RAS, renin angiotensin system; VLDL, very low-density lipoprotein.
Change in carotid IMT and other ultrasound-derived variables
Mean carotid IMT changes did not differ between the three groups (biphasic vs aspart+detemir p=0.27, biphasic vs detemir p=0.82, aspart+detemir vs detemir p=0.19, figure 2 and table 2). The change in mean carotid IMT during the 18 months of intervention in the biphasic group was −0.009 mm (95% CI −0.022 to 0.004), p=0.17, in the detemir group −0.012 mm (95% CI −0.025 to 0.000), p=0.06, and in the aspart+detemir group 0.000 mm (95% CI −0.013 to 0.013), p=0.99. Change in maximum carotid IMT did not differ between the insulin groups. No between-group differences were observed in other ultrasound derived variables (data not shown). The interaction between metformin/placebo and insulin regimens did not reach statistical significance (p=0.085) as also reported in the detailed account of all statistical analyses at http://bendixcarstensen.com/SDC/CIMT/DOM/CIMT.pdf
Figure 2.
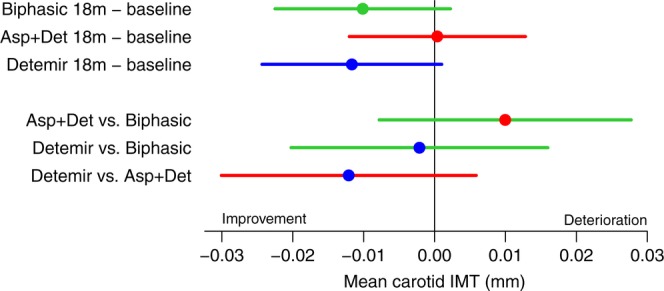
Changes from baseline and differences between treatments in mean carotid intima-media thickness (IMT) (mean (95% CI)) during 18 months treatment with three insulin regimens: biphasic (green), aspart (Asp)+detemir (Det) (red) and detemir (blue), from the random-effects model with baseline as covariate using multiply imputed data, adjusted for stratification variables.
Table 2.
Changes in outcome from trial entry to 18 months*
| Biphasic (n=137) | Aspart+detemir (n=138) | Detemir (n=137) | p Value biphasic vs aspart+detemir | p Value biphasic vs detemir | p Value aspart+detemir vs detemir | |
|---|---|---|---|---|---|---|
| Carotid ultrasound measures | ||||||
| Mean carotid IMT (mm)† | −0.009 (−0.022 to 0.004) | 0.000 (−0.013 to 0.013) | −0.012 (−0.025 to 0.000) | 0.2720 | 0.8173 | 0.1871 |
| Maximal carotid IMT (mm) | −0.010 (−0.025 to 0.005) | 0.001 (−0.014 to 0.016) | −0.016 (−0.031 to −0.001) | 0.2935 | 0.5734 | 0.1095 |
| Body composition | ||||||
| Weight (kg) | 3.3 (2.7 to 4.0) | 3.2 (2.6 to 3.9) | 1.9 (1.3 to 2.6) | 0.8102 | 0.0026 | 0.0055 |
| Body mass index (kg/m2) | 1.1 (0.9 to 1.3) | 1.0 (0.8 to 1.2) | 0.6 (0.4 to 0.8) | 0.7037 | 0.0021 | 0.0071 |
| Waist−hip ratio | 0.00 (−0.01 to 0.01) | 0.01 (0.00 to 0.02) | 0.01 (0.00 to 0.02) | 0.0926 | 0.0484 | 0.7516 |
| Glycaemic control | ||||||
| HbA1c (%) | −1.00 (−1.16 to −0.83) | −0.45 (−0.61 to −0.28) | −0.26 (−0.43 to −0.09) | <0.001 | <0.001 | 0.1304 |
| HbA1c (mmol/mol) | −10.9 (−12.7 to −9.1) | −4.9 (−6.7 to −3.1) | −2.8 (−4.7 to −1.0) | <0.001 | <0.001 | 0.1304 |
| HbA1c at 18 months (%) | 7.78 (7.56 to 8.00) | 8.19 (7.98 to 8.42) | 8.42 (8.20 to 8.64) | 0.0019 | <0.001 | 0.1074 |
| HbA1c at 18 months (mmol/mol) | 62 (59 to 64) | 66 (64 to 69) | 69 (66 to 71) | 0.0019 | <0.001 | 0.1074 |
| N (%) participants with HbA1c≤7.0% at end-of-trial | 38 (28) | 31 (22) | 12 (9) | 0.306 | 0.000 | 0.005 |
| Fasting p-glucose, mmol/L | −2.0 (−2.6 to −1.4) | −1.3 (−1.9 to −0.7) | −2.4 (−3.1 to −1.8) | 0.1108 | 0.3347 | 0.0109 |
| Fasting p-insulin (relative change from baseline) | 0.92 (0.78 to 1.09) | 0.57 (0.48 to 0.67) | 0.52 (0.44 to 0.62) | <0.001 | <0.001 | 0.5103 |
| Fasting C peptide (relative change from baseline) | 0.85 (0.76 to 0.94) | 0.70 (0.63 to 0.78) | 0.60 (0.54 to 0.67) | 0.0110 | <0.001 | 0.0480 |
| Insulin dose, end-of-trial (IU/kg/day) | 0.96 (0.86 to 1.08) | 1.15 (1.02 to 1.29) | 1.58 (1.40 to 1.78) | 0.0064 | <0.001 | <0.001 |
| Insulin dose, end-of-trial (IU/day) | 95 (84 to 108) | 116 (102 to 132) | 158 (139 to 180) | 0.080 | <0.001 | <0.001 |
| Insulin dose (relative change from baseline) | 1.96 (1.82 to 2.11) | 2.27 (2.10 to 2.45) | 3.37 (3.11 to 3.65) | 0.006 | <0.001 | <0.001 |
| Blood pressure and lipids | ||||||
| Systolic blood pressure (mm Hg) | −6.7 (−9.4 to −3.9) | −6.0 (−8.8 to −3.1) | −3.5 (−6.4 to −0.6) | 0.7282 | 0.1249 | 0.2391 |
| Diastolic blood pressure (mm Hg) | −3.3 (−4.8 to −1.8) | −2.9 (−4.4 to −1.4) | −3.5 (−5.1 to −1.9) | 0.6996 | 0.8795 | 0.5998 |
| Heart rate (bpm) | −1.3 (−3.1 to 0.4) | −0.5 (−2.3 to 1.2) | −3.0 (−4.8 to −1.2) | 0.5168 | 0.1853 | 0.0532 |
| Total cholesterol (mmol/L) | 0.17 (0.03 to 0.31) | 0.05 (−0.09 to 0.19) | 0.19 (0.05 to 0.33) | 0.2323 | 0.8254 | 0.1603 |
| LDL cholesterol (mmol/L) | 0.15 (0.04 to 0.27) | 0.06 (−0.06 to 0.18) | 0.17 (0.05 to 0.29) | 0.2634 | 0.9012 | 0.2187 |
| VLDL cholesterol (relative change from baseline) | 0.96 (0.90 to 1.03) | 1.02 (0.95 to 1.09) | 1.01 (0.94 to 1.09) | 0.2821 | 0.3111 | 0.9578 |
| HDL cholesterol (mmol/L) | 0.03 (−0.00 to 0.07) | −0.01 (−0.05 to 0.03) | −0.01 (−0.05 to 0.02) | 0.1112 | 0.0840 | 0.8722 |
| Triglycerides (relative change from baseline) | 0.97 (0.91 to 1.05) | 1.00 (0.93 to 1.08) | 1.02 (0.95 to 1.10) | 0.5826 | 0.3541 | 0.6989 |
The variables p-insulin, C peptide, insulin dose, VLDL cholesterol and triglycerides did not meet the criteria of a normal distribution and were accordingly log transformed. Therefore, these variables are presented in table 2 with relative change from baseline instead of absolute change.
To adjust for multiplicity, the significance level can be adjusted to 0.05/(K+1)/2 (where K represents the number of prespecified secondary outcomes) equalling, in this case, an α=0.0045.
*Intention-to-treat, mixed model analyses with random-effect person adjusted for stratification variables, results are presented as mean (95% CI).
†Between-group differences are analysed using multiple imputation of missing values.
HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; IMT, intima-media thickness; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Glycaemic control, weight and insulin doses
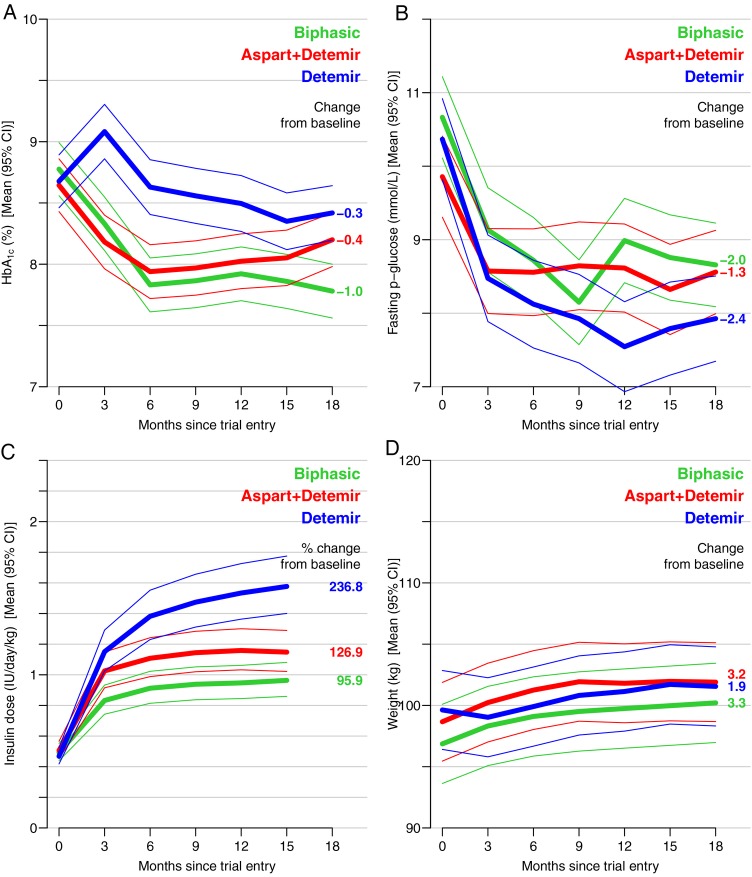
HbA1c was reduced in all three groups (p<0.01), but significantly more in the biphasic compared with the aspart+detemir and detemir groups, table 2 and figure 3. HbA1c levels at the end of the trial were not optimal, but lower in the biphasic compared with the aspart+detemir and detemir groups, p (between groups) <0.001 (table 2). Fasting plasma glucose levels were reduced in all three groups (table 2 and figure 3), however, significantly more so in the detemir (−2.4 mmol/L (95% CI −3.1 to −1.8) compared with the aspart+detemir group (−1.3 mmol/L (−1.9 to −0.7), p (between groups) = 0.01). Fasting plasma C peptide and insulin levels decreased in all three groups, but was less reduced in the biphasic compared with the aspart+detemir and detemir groups (table 2). The variables p-insulin, C peptide, insulin dose, very low-density lipoprotein (VLDL) cholesterol and triglycerides did not meet the criteria of a normal distribution, and were accordingly log transformed. Therefore, these variables are presented in table 2 with relative change from baseline instead of absolute change. Weight increased in all three groups; however, the weight gain was significantly more pronounced in the biphasic (3.3 kg (95% CI 2.7 to 4.0)) and aspart+detemir groups (3.2 kg (2.6 to 3.9)) compared with the detemir group (1.9 kg (1.3 to 2.6), table 2 and figure 3). The prescribed insulin dose increased progressively during the 18 months in all three groups. End-of-trial insulin dose was lowest in the biphasic (0.96 IU/kg/day (95% CI 0.86 to 1.08)) and highest in the detemir (1.58 IU/kg/day (1.40 to 1.78)), whereas the aspart+detemir group was in between (1.15 IU/kg/day (1.02 to 1.29), table 2 and figure 3). Insulin dose represents the dose prescribed by the investigator at each visit for the following 3 months, hence the last insulin dose registered is at 15 months (figure 3), being the last dose prescribed for the last 3 months in the trial.
Figure 3.
Changes (mean (95% CI)) during 18 months treatment with three insulin regimens: biphasic (green), aspart+detemir (red) and detemir (blue) in glycated haemoglobin (HbA1c) (A), fasting plasma glucose (B), insulin dose (C) and weight (D). Numbers on the right hand side of the graphs indicate the absolute/relative changes from trial entry to end-of-trial.
Hypoglycaemia and serious adverse events
No differences were found in the number of participants with at least one episode of severe hypoglycaemia (table 3). The total number of severe hypoglycaemic episodes was 4 in the biphasic compared with 16 episodes in the aspart+detemir group (p=0.01) and 7 episodes in the detemir group (p=0.38). The total number of non-severe hypoglycaemic episodes during 18 months differed between all three groups (p<0.001), with most episodes in the biphasic (2823) and fewest episodes in the detemir (2115) group (table 3). No differences were found with regard to serious adverse events (table 3 and see online supplementary tables S1 and S2). Numbers of adverse events are shown in online supplementary tables S3 and S4.
Table 3.
Hypoglycaemia and serious adverse events during 18 months according to insulin regimens
| p Value |
||||||
|---|---|---|---|---|---|---|
| Biphasic (n=137) | Aspart+detemir (n=138) | Detemir (n=137) | Biphasic vs aspart+detemir | Biphasic vs detemir | Aspart+detemir vs detemir | |
| Severe hypoglycaemia (number of patients with at least 1 event, N (%)) | 3 (2.2) | 5 (3.6) | 6 (4.4) | 0.4859 | 0.3205 | 0.7500 |
| Severe hypoglycaemia (number of events (rate among patients with at least 1 event)) | 4 (1.3) | 16 (3.2) | 7 (1.2) | 0.0141 | 0.3770 | 0.0707 |
| Non-severe hypoglycaemia (number of patients with at least one event, N (%)) | 110 (80.3) | 108 (78.2) | 95 (69.3) | 0.6778 | 0.0375 | 0.0927 |
| Non-severe hypoglycaemia (number of events (rate among patients with at least 1 event)) | 2823 (25.7) | 2570 (23.8) | 2115 (22.3) | <0.001 | <0.001 | <0.001 |
| Serious adverse events excluding severe hypoglycaemia (number of patients with at least 1 event, N (%)) | 37 (27.0) | 31 (22.5) | 31 (22.6) | 0.3826 | 0.4072 | 0.9658 |
| Serious adverse events excluding severe hypoglycaemia (number of events (rate among patients with at least 1 event)) | 64 (1.7) | 45 (1.5) | 44 (1.4) | 0.0656 | 0.0588 | 0.9555 |
Severe hypoglycaemia is defined as a hypoglycaemic episode where help from a third person was needed.
Non-severe hypoglycaemia defined as an episode with either symptoms of hypoglycaemia and/or measurement of plasma glucose ≤3.9 mmol/L.
Blood pressure and lipids
Blood pressure was significantly reduced in all three groups during the trial, without any between-group differences (table 2). Low-density lipoprotein cholesterol level increased by 0.15 mmol/L (95% CI 0.04 to 0.27), p=0.010 in the biphasic and 0.17 (95% CI 0.05 to 0.29), p=0.007 in the detemir group, without significant between-group differences (table 2). Triglyceride as well as high-density lipoprotein and VLDL cholesterol levels did not change significantly in any of the three groups (table 2).
The prespecified sensitivity analyses described in the statistical analyses section did not noticeably change the results (analyses available at http://bendixcarstensen.com/SDC/CIMT/DOM/CIMT.pdf).
Discussion
In this randomised clinical trial, we observed no significant differences in the change of mean carotid IMT in participants with T2D during 18 months of treatment between the three insulin analogue regimens with or without adjunct metformin treatment. All three insulin regimens reduced HbA1c level, however, the reduction of HbA1c was significantly more pronounced with biphasic insulin aspart despite lower doses of insulin. Weight gain was greater in the biphasic and in the aspart+detemir group compared with the insulin detemir only group. The highest number of non-severe hypoglycaemic episodes was reported from the biphasic group, whereas severe hypoglycaemic episodes occurred most often in the aspart+detemir group.
Randomised trials evaluating the effect of different insulin regimens on carotid IMT are sparse. Bibra et al23 reported that 24 months treatment with human regular insulin before main meals was associated with reduced postmeal plasma glucose excursion and lower carotid IMT compared with conventional therapy using premixed insulin in patients with T2D. The ORIGIN-Grace study evaluated insulin glargine titrated to a fasting plasma glucose target of 5.3 mmol/L versus standard glycaemic care in 1184 patients with impaired fasting glucose or early T2D, and reported a significant improvement in maximum carotid IMT of the common carotid artery in the glargine group compared with standard care after a median follow-up of 4.9 years.24 However, the risk of clinical cardiovascular disease did not differ between the groups in the ORIGIN trial after a median follow-up of 6.2 years.25 Postprandial hyperglycaemia in patients with T2D has been speculated to be harmful with respect to development and progression of atherosclerosis,5 26 27 and treatments targeting postprandial glucose excursion have been suggested to be associated with significantly improved carotid IMT.28 29 In order to test this, we compared patients treated with the basal-bolus insulin regimen with patients treated with basal insulin only. Furthermore, we included the more pragmatic treatment option to reduce postprandial hyperglycaemia with a premixed combination of 30% rapid-acting and 70% intermediate-acting insulin aspart administered between one and three times daily, as required. Our results, however, do not support a beneficial cardiovascular effect of targeting postprandial hyperglycaemia.
Despite higher glycaemic levels and insulin doses, participants randomised to basal insulin detemir were the only group showing a non-significant improvement in carotid IMT during the intervention period (p=0.06). The extent to which intensified glycaemic control reflected by a reduction in HbA1c level is associated with a reduced cardiovascular risk in T2D remains controversial.30 In the HEART2D trial, survivors of acute myocardial infarction were randomised to treatment with prandial versus basal insulin, and no difference in risk for future cardiovascular events was reported.31 We found inferior glycaemic control in patients randomised to insulin detemir with or without combination with insulin aspart compared with premixed insulin aspart, but no significant differences between treatments in carotid IMT after 18 months. The fact that 23% of the patients in the detemir group needed an additional insulin aspart injection reflects the increasing recognition during the trial that insulin detemir was an ineffective way to control blood glucose in T2D patients with long mean duration of about 13 years. Theoretically, this could to some extent have masked a beneficial effect of meal rapid-acting insulin administration on outcomes including carotid IMT. However, given the much more widespread use of meal rapid acting insulin in patients on multiple injections, we find it highly unlikely to have influenced our results. Accordingly, the results from randomised clinical trials do not, in contrast to some epidemiological studies, support a simple relation between glycaemic levels and risk of clinical cardiovascular disease. Hyperinsulinaemia has been proposed as an isolated risk factor for atherosclerosis in people with and without diabetes.32 33 Experimental studies have provided some support for this hypothesis.34–36 However, despite quantitatively major differences in total insulin doses with the highest doses in the detemir-based regimens, no significant differences in progression rates of carotid IMT were detected. In accordance, the UK Prospective Diabetes Study (UKPDS) did not support hyperinsulinaemia as being a risk factor of cardiovascular disease in patients with T2D.37 Lastly, increased BMI is a risk factor of cardiovascular disease.38 Whether the less weight gain in the detemir group to some extent may be reflected in the trend towards a more beneficial effect on carotid IMT changes in this group cannot be revealed from the present data. The Treating To Target in Type 2 diabetes (4-T) trial compared the metabolic effects of three insulin treatment modalities in patients with T2D during 3 years.6 39 The initial treatment arms included basal insulin detemir, premixed insulin aspart as well as insulin aspart administered before meals. Our finding of improved HbA1c among patients treated with premixed insulin aspart compared with insulin detemir only is consistent with the 1-year outcome data of the 4-T trial, although the absolute differences in HbA1c in our trial were markedly higher.6 39 This may be explained by differences in participant characteristics, with those in the 4-T trial being insulin naïve prior to the trial and treated with insulin in combination with metformin and sulfonylurea.
Strengths and limitations
The strength of the CIMT trial is the randomised design and the fact that all ultrasound scans were centrally performed by only two technicians, with good reproducibility.19 40 41 A limitation is the use of carotid IMT instead of clinical events as primary outcome, as previously discussed.17 In terms of sample size, a rather conservative approach was applied due to the plan of multiple comparisons between intervention groups testing the effect of adjunct metformin treatment versus placebo and the three insulin regimens simultaneously. We therefore used an α of 0.01 and obtained a power of 70% of the insulin comparisons, while a conventional approach using an α of 0.05 might have increased the overall family-wise type 1 error rate for the five planned comparisons to 0.25.18 Owing to slow inclusion rates, which to some extent may be explained by a sustained strike among nurses, the introduction of liraglutide as a competing treatment option and/or concerns about reduced glucose-lowering efficacy of detemir in some patients with T2D, we were only able to include approximately half of the participants originally planned for in this trial.17 However, the total number of participants in the current trial was twofold higher than the total number of participants included in a trial of repaglinide versus glyburide reporting an effect of 12 months treatment with repaglinide on carotid IMT.28 Our sample size estimation was also conservative compared with the Carotid Atherosclerosis: MEtformin for insulin ResistAnce (CAMERA) trial evaluating the effect of 18 months treatment with metformin versus placebo in 173 patients without diabetes with established cardiovascular disease, also using carotid IMT as the primary outcome.42 In the CAMERA trial, a sample size of 180 participants was estimated, compared with our 900, illustrating the difficulties in performing sample size estimation when reliable data are not available. Another limitation was failure to reach good glycaemic control. Despite provision of a detailed guideline with a treat-to-target approach, obtaining adequate glycaemic control proved difficult in many patients. Especially, insulin detemir administered once daily was not sufficient to lower glucose levels in the majority of participants, although insulin doses were titrated aggressively.
Conclusion
In conclusion, despite differences in HbA1c levels, weight gain and insulin doses, we did not find any significant differences in the progression rates of carotid IMT when treating patients with long-term T2D with three different frequently used insulin analogues regimens targeting postprandial and/or fasting plasma glucose. Further research is warranted addressing the question to what extent different insulin analogue regimens targeting basal and/or postprandial hyperglycaemia may influence the risk of cardiovascular disease in a differential manner.
Acknowledgments
The authors thank all the patients for their participation, the staff at the Clinical Research Unit, Steno Diabetes Center, for taking care of all major investigations at trial entry and termination, the Good Clinical Practise Unit in Copenhagen, for trial monitoring, and all trial staff at the participating departments. The authors thank Per Winkel, the Copenhagen Trial Unit, for collaboration in the development of the statistical analysis plan. They also thank Novo Nordisk for their unrestricted grant supporting this trial.
Footnotes
Contributors: All the authors (except BC) contributed to the design, conduct and acquisition of data. LL-C and BC conducted the data analysis, and provided the tables and figures. LL-C, BC, LT, TPA, SM, AV, CG and JW drafted the manuscript and conducted interpretation of data. All the authors had full access to all the data and the statistical report in the trial, and can take responsibility for the integrity of the data and the accuracy of the data analysis. All the authors revised and approved the final version of the manuscript. LL-C and BC are guarantors.
Funding: The investigators received an unrestricted grant from Novo Nordisk A/S to enable conduct of the trial. Novo Nordisk did not decide on the trial design, conduct, data analyses, interpretation, or writing of the manuscript. Novo Nordisk was allowed to comment on the protocol, on protocol changes during the trial, and on the manuscript prior to submission.
Competing interests: The trial received an unrestricted grant from Novo Nordisk A/S for the submitted work; LL-C, LT, TPA, AV, OP, TWB, BC, SSL and TJ have reported shares in Novo Nordisk A/S. LL-C, LT, TPA, AV, TWB and SSL have reported former employment, and BC is employed at Steno Diabetes Center, which is a diabetes hospital and academic institution owned by Novo Nordisk. SSL owns shares in dynamically traded investment funds, which may own stocks from pharmaceutical companies. SSL is employed at Boehringer Ingelheim Pharma GmbH & Co, KG, Ingelheim, Germany. SSL's contribution was his alone and does not necessarily reflect the official position of Boehringer Ingelheim. AV has received fees from Novo Nordisk. TWB is employed at Novo Nordisk A/S. BT is member of advisory board for Eli Lilly. OS has received fees from AstraZeneca, Sanofi, MSD, Boehringer-Ingelheim, Eli Lilly and NovoNordisk. CH has received fees from Bristol-Myers Squibb. LB has received fees from and attended advisory for Novo Nordisk A/S. SM has served as a consultant or adviser to: Novartis Pharma, Novo Nordisk, Merck Sharp & Dome, Sanofi-Aventis, AstraZeneca, Johnson & Johnson, Rosche, Mankind, Boehringer-Ingelheim, Zeeland, E Lilly, Intarcia Therapeutics and Bristol-Meyer Squibb, has received fees for speaking from Novo Nordisk, Merck, Sharp & Dome, AstraZeneca, Johnson and Johnson, Rosche, Shering-Ploug, Sanofi-Aventis, Novartis Pharma, Eli Lilly, Bristol-Meyer Squibb and Boehringer Ingelheim, and has received two research grants from Novo Nordisk.
Ethics approval: The protocol was approved by the Regional Committee on Biomedical Research Ethics (H-D-2007-112) and the Danish Medicines Agency, registered within ClinicalTrials.gov (NCT00657943), and conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB et al. . Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. 10.1007/s00125-012-2534-0 [DOI] [PubMed] [Google Scholar]
- 2.Abraira C, Colwell JA, Nuttall FQ et al. . Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care 1995;18:1113–23. 10.2337/diacare.18.8.1113 [DOI] [PubMed] [Google Scholar]
- 3.Hemmingsen B, Lund SS, Gluud C et al. . Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 2011;343:d6898 10.1136/bmj.d6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabet Med 2008;25:1151–6. 10.1111/j.1464-5491.2008.02565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito K, Ciotola M, Carleo D et al. . Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008;93:1345–50. 10.1210/jc.2007-2000 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Farmer AJ, Davies MJ et al. . Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–47. 10.1056/NEJMoa0905479 [DOI] [PubMed] [Google Scholar]
- 7.Yki-Järvinen H, Kauppila M, Kujansuu E et al. . Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:1426–33. 10.1056/NEJM199211123272005 [DOI] [PubMed] [Google Scholar]
- 8.Yki-Järvinen H, Ryysy L, Nikkila K et al. . Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1999;130:389–96. 10.7326/0003-4819-130-5-199903020-00002 [DOI] [PubMed] [Google Scholar]
- 9.Giugliano D, Maiorino MI, Bellastella G et al. . Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care 2011;34:510–17. 10.2337/dc10-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebl A, Prager R, Binz K et al. . Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab 2009;11:45–52. 10.1111/j.1463-1326.2008.00915.x [DOI] [PubMed] [Google Scholar]
- 11.Liebl A, Prusty V, Valensi P et al. . Ten years of experience with biphasic insulin aspart 30: from drug development to the latest clinical findings. Drugs 2012;72:1495–520. 10.2165/11635490-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock J, Ahmann AJ, Colon G et al. . Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008;31:20–5. 10.2337/dc07-1122 [DOI] [PubMed] [Google Scholar]
- 13.Vaag A, Lund SS. Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. Eur J Endocrinol 2012;166:159–70. 10.1530/EJE-11-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenz MW, Markus HS, Bots ML et al. . Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–67. 10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 15.Sibal L, Agarwal SC, Home PD. Carotid intima-media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab Syndr Obes 2011;4:23–34. 10.2147/DMSO.S8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz MW, Polak JF, Kavousi M et al. . Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053–62. 10.1016/S0140-6736(12)60441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundby-Christensen L, Tarnow L, Boesgaard TW et al. . Metformin versus placebo in combination with insulin analogues in patients with type 2 diabetes mellitus—the randomised, blinded Copenhagen Insulin and Metformin Therapy (CIMT) trial. BMJ Open 2016;6:e008376 10.1136/bmjopen-2015-008376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundby Christensen L, Almdal T, Boesgaard T et al. . Study rationale and design of the CIMT trial: the Copenhagen Insulin and Metformin Therapy trial. Diabetes Obes Metab 2009;11:315–22. 10.1111/j.1463-1326.2008.00959.x [DOI] [PubMed] [Google Scholar]
- 19.Lundby-Christensen L, Almdal TP, Carstensen B et al. . Carotid intima-media thickness in individuals with and without type 2 diabetes: a reproducibility study. Cardiovasc Diabetol 2010;9:40 10.1186/1475-2840-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espeland MA, O'leary DH, Terry JG et al. . Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med 2005;6:3 10.1186/1468-6708-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molenberghs G, Kenward MG. Missing data in clinical studies. West Sussex: John Wiley & Sons Ltd, 2007. [Google Scholar]
- 22.Jakobsen JC, Wetterslev J, Winkel P et al. . Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 2014;14:120 10.1186/1471-2288-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibra Hv, Siegmund T, Ceriello A et al. . Optimized postprandial glucose control is associated with improved cardiac/vascular function—comparison of three insulin regimens in well-controlled type 2 diabetes. Horm Metab Res 2009;41:109–15. 10.1055/s-0028-1112136 [DOI] [PubMed] [Google Scholar]
- 24.Lonn EM, Bosch J, Diaz R et al. . Effect of insulin glargine and n-3FA on carotid intima-media thickness in people with dysglycemia at high risk for cardiovascular events: the glucose reduction and atherosclerosis continuing evaluation study (ORIGIN-GRACE). Diabetes Care 2013;36:2466–74. 10.2337/dc12-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstein HC, Bosch J, Dagenais GR et al. . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28. 10.1056/NEJMoa1203858 [DOI] [PubMed] [Google Scholar]
- 26.Cavalot F, Pagliarino A, Valle M et al. . Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011;34:2237–43. 10.2337/dc10-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceriello A, Hanefeld M, Leiter L et al. . Postprandial glucose regulation and diabetic complications. Arch Intern Med 2004;164:2090–5. 10.1001/archinte.164.19.2090 [DOI] [PubMed] [Google Scholar]
- 28.Esposito K, Giugliano D, Nappo F et al. . Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004;110:214–19. 10.1161/01.CIR.0000134501.57864.66 [DOI] [PubMed] [Google Scholar]
- 29.Barbieri M, Rizzo MR, Marfella R et al. . Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013;227:349–54. 10.1016/j.atherosclerosis.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 30.Hemmingsen B, Lund SS, Gluud C et al. . Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013;11:CD008143 10.1002/14651858.CD008143.pub3 [DOI] [PubMed] [Google Scholar]
- 31.Raz I, Wilson PW, Strojek K et al. . Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–6. 10.2337/dc08-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Després JP, Lamarche B, Mauriège P et al. . Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334:952–7. 10.1056/NEJM199604113341504 [DOI] [PubMed] [Google Scholar]
- 33.Hemmingsen B, Lund SS, Wetterslev J et al. . Oral hypoglycaemic agents, insulin resistance and cardiovascular disease in patients with type 2 diabetes. Eur J Endocrinol 2009;161:1–9. 10.1530/EJE-09-0167 [DOI] [PubMed] [Google Scholar]
- 34.Falholt K, Cutfield R, Alejandro R et al. . The effects of hyperinsulinemia on arterial wall and peripheral muscle metabolism in dogs. Metabolism 1985;34:1146–9. 10.1016/0026-0495(85)90161-1 [DOI] [PubMed] [Google Scholar]
- 35.Nigro J, Osman N, Dart AM et al. . Insulin resistance and atherosclerosis. Endocr Rev 2006;27:242–59. 10.1210/er.2005-0007 [DOI] [PubMed] [Google Scholar]
- 36.Montagnani M, Golovchenko I, Kim I et al. . Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 2002;277:1794–9. 10.1074/jbc.M103728200 [DOI] [PubMed] [Google Scholar]
- 37.Adler AI, Neil HA, Manley SE et al. . Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom Prospective Diabetes Study (UKPDS 47). Am Heart J 1999;138Pt 1):S353–9. 10.1016/S0167-0115(99)90005-4 [DOI] [PubMed] [Google Scholar]
- 38.Whitlock G, Lewington S, Sherliker P et al. . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holman RR, Thorne KI, Farmer AJ et al. . Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–30. 10.1056/NEJMoa075392 [DOI] [PubMed] [Google Scholar]
- 40.Savović J, Jones HE, Altman DG et al. . Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157: 429–38. 10.7326/0003-4819-157-6-201209180-00537 [DOI] [PubMed] [Google Scholar]
- 41.Wood L, Egger M, Gluud LL et al. . Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601–5. 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preiss D, Lloyd SM, Ford I et al. . Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:116–24. 10.1016/S2213-8587(13)70152-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-008377supp.pdf (463.6KB, pdf)