Abstract
Action potential (AP) propagation in myelinated nerves requires clustered voltage gated sodium and potassium channels. These channels must be specifically localized to nodes of Ranvier where the AP is regenerated. Several mechanisms have evolved to facilitate and ensure the correct assembly and stabilization of these essential axonal domains. This review highlights the current understanding of the axon intrinsic and glial extrinsic mechanisms that control the formation and maintenance of the nodes of Ranvier in both the peripheral nervous system (PNS) and central nervous system (CNS).
Schwann cells and oligodendrocytes form sheaths of myelin around the axons of neurons. They also contribute to other important neuronal functions, including the clustering of ion channels at the nodes of Ranvier.
Axons conduct electrical signals, called action potentials (APs), among neurons in a circuit in response to sensory input, and between motor neurons and muscles. In mammals and other vertebrates, many axons are myelinated. Myelin, made by Schwann cells and oligodendrocytes in the peripheral nervous system (PNS) and central nervous system (CNS), respectively, is a multilamellar sheet of glial membrane that wraps around axons to increase transmembrane resistance and decrease membrane capacitance. Although myelin is traditionally viewed as a passive contributor to nervous system function, it is now recognized that myelinating glia also play many active roles including regulation of axon diameter, axonal energy metabolism, and the clustering of ion channels at gaps in the myelin sheath called nodes of Ranvier. Together, the active and passive properties conferred on axons by myelin, result in axons with high AP conduction velocities, low metabolic demands, and reduced space requirements as compared with unmyelinated axons. Thus, myelin and the clustering of ion channels in axons permitted the evolution of the complex nervous systems found in vertebrates. This review highlights the current understanding of the axonal intrinsic and glial extrinsic mechanisms that control the formation and maintenance of the nodes of Ranvier in both the PNS and CNS.
THE ORGANIZATION OF MYELINATED AXONS
Morphology
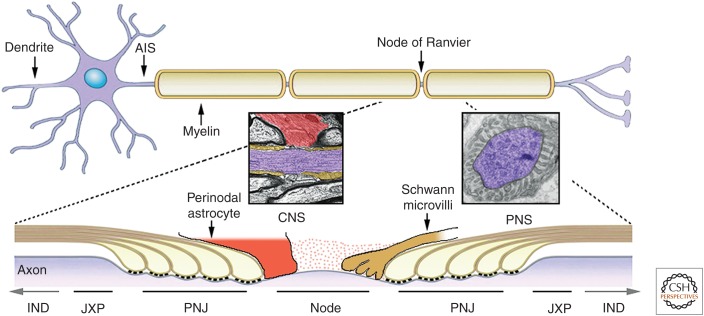
Myelinated axons are divided into distinct domains including nodes of Ranvier, paranodal axoglial junctions (PNJ), juxtaparanodes (JXP), and internodes (Fig. 1). At PNS nodes of Ranvier, microvilli emanate from the outer aspect of the Schwann cells that myelinate the flanking internodes to contact the nodal axolemma (Berthold et al. 1983; Ichimura and Ellisman 1991). In contrast, myelinating oligodendrocytes in the CNS do not form nodal microvilli. Instead, some nodes are contacted by perinodal astrocyte or oligodendrocyte progenitor cell (OPC) processes (Black and Waxman 1988; Butt et al. 1994, 1999). Although microvilli in the PNS contribute to Na+ channel clustering during development, the functions of perinodal astrocytes and OPCs remain obscure. Another important difference between myelinated PNS and CNS axons is that the latter lack a basal lamina. PNS myelinated axons have a basal lamina that completely surrounds individual Schwann cell–axon units and even extends through the nodal gap (Court et al. 2006). In the nodal gap, glial processes are embedded in extracellular matrix (ECM)–rich material (termed the “cement disk” by Ranvier) that participates in node formation and is thought to serve as a cationic pool through the negative charges of sulfated proteoglycan chains (Fig. 2A) (Bekku et al. 2010a; Susuki et al. 2013). Nodes of Ranvier are bordered by the PNJ, a specialized axoglial contact formed between the axolemma and the paranodal loops of the myelinating cells. Here, myelin lamellae split into a series of cytoplasmic loops that are closely apposed to the axon, being separated by a gap of only 2.5–3 nm. These loops spiral around the axon to form septate-like junctions with the axon; these junctions constitute the largest known vertebrate intercellular junction. In electron micrographs of longitudinal sections through the paranodal region, the junctions appear as a series of ladder-like densities (i.e., transverse bands or septa) that arise from the axon and contact the glial membranes (Rosenbluth 2009). PNJ have diverse functions that include attachment of the myelin sheath to the axon, separating the electrical activity of nodal axolemma from internodal axolemma, and functioning as a boundary to limit the lateral diffusion of axonal membrane proteins (Rosenbluth 2009). As detailed below, the PNJ plays essential roles in the formation and maintenance of nodal and JXP membrane domains. The third specialized region in myelinated axons, the so-called JXP, is located beneath the compact myelin at the interface between the PNJ and internode. This region is characterized by clusters of intramembranous particles in freeze–fracture replicas (Stolinski et al. 1981; Tao-Cheng and Rosenbluth 1984) thought to correspond to delayed rectifier K+ channels (Chiu and Ritchie 1980). These channels may stabilize conduction and help to maintain the internodal resting potential, especially during myelination and remyelination (Chiu and Ritchie 1984; Wang et al. 1993; Vabnick and Shrager 1998; Rasband 2010). As described below, the formation of this domain depends on the presence of an intact PNJ. Finally, the internodal axolemma located beneath the compact myelin is also considered a unique domain because there are distinct membrane proteins and structures that comprise this region. In the PNS, the organization of the internodal axolemma is dictated by the overlying myelin sheath. Here the membrane contains two linear rows of juxtaparanodal-type intramembranous particles that flank a paranodal-type aggregate at the inner mesaxon and under the Schmidt–Lanterman incisures (Miller and Pinto da Silva 1977; Stolinski et al. 1985). However, in contrast to the PNJ, no distinct junctional specialization (like transverse bands) is present between the axolemma and the adaxonal Schwann cell membrane at the inner mesaxon. Similar to the JXP, the aggregates found at the juxtamesaxonal (JXM) and juxtaincisural lines correspond to delayed rectifier K+ channels (Arroyo et al. 1999). Hence, the radial organization of paranodal and juxtaparanodal components along the internodes corresponds to the longitudinal (i.e., axial) polarity of these proteins near the nodes of Ranvier. In contrast to the PNS, no juxtamesaxonal organization is detected in the CNS (Arroyo et al. 2001).
Figure 1.
Axonal domains along the myelinated axons. A neuron containing the soma, branched dendrites, and a myelinated axon is shown. Axons are myelinated by Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). Action potentials (APs) generated at the axon initial segment (AIS) travel down the axon and are regenerated at the nodes of Ranvier until reaching the nerve terminals. The axonal membrane is divided into distinct domains. The internodes (INDs) (shown partially in the lower cartoon) comprise the majority of the axon and are located beneath the compact myelin sheath. The juxtaparanodes (JXP) are located at the end of the internodes. Near the nodes of Ranvier, the myelin sheath ends with a series of cytoplasmic loops (e.g., paranodal loops) that generate a specialized junction with the axon (paranodal junction [PNJ], often referred to as the axoglial junction). Bordered by the PNJ are the nodes of Ranvier, which are gaps between myelin segments. In the PNS, the nodal axolemma is contacted by microvilli that originate from the outer aspect of the myelinating Schwann cells, whereas, in the CNS, some nodes are contacted by a process from a perinodal astrocyte or oligodendrocyte progenitor cell (OPC). Insets show EM images of a longitudinal section through the node of Ranvier in the CNS (perinodal astrocyte in red, paranodes in brown, and axon in purple) and the PNS (axon in purple). The nodal gap is filled with highly charged extracellular matrix material (dots).
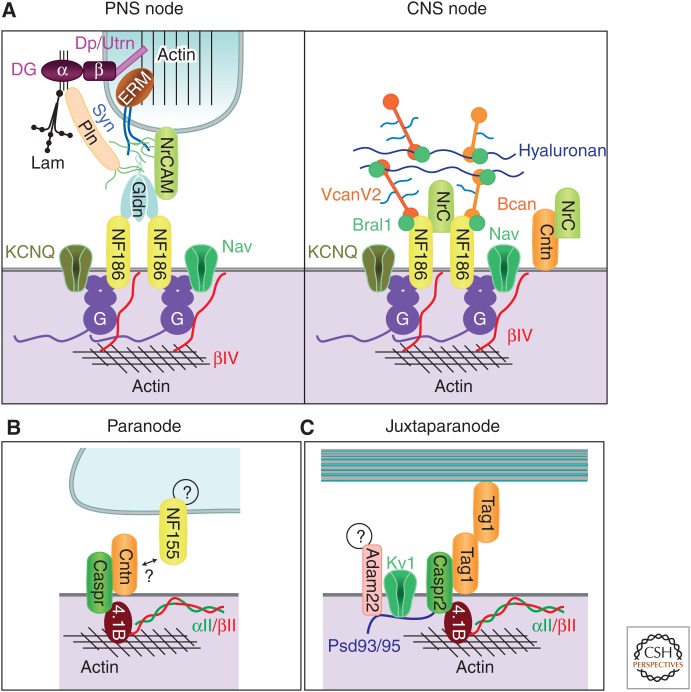
Figure 2.
Molecular organizations of axonal subdomains. Simplified illustration showing some of the molecules and interactions involved in the nodal (A), paranodal (B), and juxtaparanodal (C) domains. PNS nodes are contacted by microvilli of Schwann cells. Dystroglycan (DG) is autocleaved into α and β chains, which remain associated. β-DG interacts with dystrophin (Dp) and utrophin (Utrn). A transmembrane form of NrCAM is present at the microvilli. Interaction of laminins (Lam) and perlecan (Pln) with α-DG requires proper glycosylation of α-DG (thin black lines). Pln and sydecans 3/4 (Syn) are modified by heparan sulfate side chains (green lines). The furin-shed gliomedin (Gldn) trimerizes and is associated with heparan sulfate through its amino-terminal region and collagen-like domain and interacts with NrCAM and NF186 through its olfactomedin domain. G and βIV represent AnkG and βIV spectrin, respectively. In the CNS, the nodal ECM is enriched with shed NrCAM, but its cellular source is unknown. VcanV2 and Bcan interact with Bral1 and hyaluronan through their G1 globular domains and with NF186 through the G3 domains. The intervening regions of VcanV2 and Bcan are modified by chondroitin sulfate side chains (light blue lines). Contactin (Cntn) was found at CNS nodes, but only weakly at a few PNS nodes. The cytoplasmic partners of NF155 at the paranodal junction and ligands of Adam22 at the juxtaparanode are currently unknown. (From Chang et al. 2013; reprinted, with permission, from Elsevier Limited © 2013.)
Composition
The molecular composition of the nodes, paranodes, JXPs, and internodes is a remarkable example of reciprocal neuron–glia interactions mediated by ECM proteins, cell adhesion molecules (CAMs), and cytoskeletal scaffolds, all designed to facilitate ion-channel clustering and the rapid propagation of APs. The molecular compositions of each domain and their protein–protein interactions are described below (Fig. 2).
Nodes of Ranvier
AP propagation depends on the rapid de- and repolarization of the axolemma; these actions are performed by Na+ and K+ channels clustered at nodes (Fig. 2A). However, nodes are not uniform in their ion channel composition. Like synapses with different ionotropic neurotransmitter receptors, nodes of Ranvier can also have one or more different kinds of voltage-gated Na+ and K+ channels, although this diversity is relatively limited. For example, nodes can include Nav1.1, Nav1.2, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 (Fjell et al. 2000; Boiko et al. 2001; Henry et al. 2005; Duflocq et al. 2008; Black et al. 2012). These Na+ channels function as the pore-forming protein subunits that mediate ion flux across the membrane. Na+ channels also interact with the accessory β-subunits Navβ1, Navβ2, and Navβ4 (Chen et al. 2002, 2004; Buffington and Rasband 2013). The β-subunits are covalently linked to Na+ channels through an extracellular disulfide bond (Chen et al. 2012; Buffington and Rasband 2013) to promote the surface expression of Na+ channels and change their biophysical properties. In addition to Na+ channels, nodes of Ranvier are also enriched in K+ channels (Cooper 2011). These include Kv3.1b, KCNQ2, and KCNQ3 (Devaux et al. 2003, 2004), which together regulate neuronal excitability (Battefeld et al. 2014; King et al. 2014). These channels have unique physiologies and together with the diversity of Na+ channels and their accessory subunits emphasize that not all nodes are created equal.
Besides ion channels, nodes of Ranvier are also enriched with the cytoskeletal and scaffolding proteins ankyrin G (ankG) and βIV spectrin that together link the Na+ and K+ channels to the underlying cytoskeleton (Kordeli et al. 1995; Berghs et al. 2000). These scaffolds also link ion channels to extrinsic (glial) interactions through CAMs: the 186-kDa isoform of neurofascin (NF186) and NrCAM (Davis et al. 1996). Nodal CAMs interact with a diverse and rich extracellular matrix. In the PNS, nodal ECM proteins include gliomedin, syndecans, laminins, NG2, and versican. A specialized ECM also exists in the CNS and consists of the chondroitin sulfate proteoglycans brevican, versican, neurocan, and phosphacan, as well as tenascin-R, Bral1, and shed NrCAM (Susuki et al. 2013). Gliomedin, shed NrCAM, versican, brevican, and Bral1 are all direct binding partners of NF186 (Eshed et al. 2005; Susuki et al. 2013). The aforementioned nodal microvilli in the PNS are also specifically enriched with unique proteins including ezrin, radixin, moesin, EBP50, dystrophin, and utrophin (Occhi et al. 2005). In contrast, no specific proteins have been identified at the terminals of perinodal glial processes in the CNS.
Paranodal Junctions
PNJ flank nodes of Ranvier and consist of a heterotrimeric CAM complex consisting of the glial, 155-kDa isoform of neurofascin (NF155) and an axonal complex of the glycosylphosphatidyl-inositol (GPI) anchored contactin and Caspr (Fig. 2B) (Charles et al. 2002). These proteins are all essential for the generation of the characteristic septate-like junction at the paranodes (Bhat et al. 2001; Boyle et al. 2001; Gollan et al. 2002; Rosenbluth 2009). Furthermore, the significance of proper paranodal junctions in human health can be seen in the recent identification of human frameshift mutations in Caspr. These mutations caused severe arthrogyposis multiplex congenita, characterized by congenital joint contractures. These patients also had significantly reduced motor nerve conduction velocities (Laquerriere et al. 2014). A specialized cytoskeleton is also found at paranodes that consists of ankyrinB, αII spectrin, βII spectrin, and protein 4.1B (Ogawa et al. 2006). This paranodal cytoskeletal complex plays important roles in regulating the barrier functions of paranodes (see below).
Juxtaparanodes
JXP are characterized mainly by the high-density clustering of Kv1 K+ channels consisting of Kv1.1, Kv1.2, Kv1.4, and KVβ2 subunits (Fig. 2C) (Rasband et al. 2001). Intriguingly, at JXP there is also a CAM interaction homologous to that found at paranodes. GPI-anchored TAG-1 (also known as contactin-2) and Caspr2 form a complex and both are required for proper clustering of JXP Kv1 channels (Poliak et al. 2003). Proteomics of JXP Kv1 channels also revealed ADAM22 at JXP (Ogawa et al. 2010). Caspr2, Kv1 channels, and ADAM22 all have canonical PDZ-binding motifs. Consistent with this fact, the PDZ-domain proteins PSD95 and PSD93 are also enriched at JXPs, although they are dispensable for assembly of the Caspr2/TAG-1/Kv1 channel complex (Horresh et al. 2008), but loss of ADAM22 blocks the clustering of PSD95/93 without affecting Kv1 channel clustering (Ogawa et al. 2010). Thus, the functions of ADAM22 and PSD95/93 remain unknown. Despite the clear dependence of Kv1 channel clustering on Caspr2 and TAG-1, how these ion channels interact with Caspr2 and TAG-1, and how ADAM22 is recruited to JXP domains remains unknown.
Internodes
As described above, the PNS internode has a thin line of alternating JXP–PNJ–JXP proteins that follows the spiral of the inner-mesaxon along the internode (hence, termed the internodal mesaxonal line). These contact sites between the myelin sheath and the axon share molecular features of the JXP and PNJ. Furthermore, the PNS internode is enriched in CAMs of the Cadm (also known as the Necl and SynCAM) family of proteins (Maurel et al. 2007; Spiegel et al. 2007). Specifically, internodal axolemma is enriched in Cadm3 and Cadm2, which forms a ligand pair with internodal Cadm4 found on the adaxonal surface of Schwann cells and oligodendrocytes (Maurel et al. 2007; Spiegel et al. 2007; N Golan, Y Eshed, and E Peles, unpubl.). In the PNS, axon–glial interaction mediated by glial Cadm4 is required for the organization of the axonal membrane, accurate positioning of Caspr at the paranodal area, as well as the clustering of Kv1 channels at the juxtaparanodal region and the internodal mesaxonal line (Golan et al. 2013). The presence of Kv1 channels at the mesaxonal line also depends on the presence of the cytoskeletal adaptor protein 4.1G, which associates with Cadm4 in Schwann cells (Ivanovich et al. 2012).
Taken together, a picture emerges of a highly organized membrane protein distribution found along myelinated axons that uses common kinds of protein complexes important for domain assembly and/or function (see Table 1 in Eshed-Eisenbach and Peles 2013 for a list of nodal components). In the next section, we will discuss how these different types of proteins interact to organize the myelinated axon into discrete domains.
ASSEMBLY OF THE NODES OF RANVIER
Formation of the nodes of Ranvier depends on both axonal–intrinsic and glial–extrinsic mechanisms. The assembly of nodes is orchestrated through the placement of several cytoskeletal and tethering proteins along the axolemma at locations that are determined by the contacting oligodendrocytes and myelinating Schwann cells.
Intrinsic Regulators of Node Formation
In neurons, ankG is required for Na+ channel clustering at the axon initial segment (AIS), which is structurally and functionally similar to nodes, although its assembly is independent of extrinsic mechanisms (Rasband 2010). Because nodal Na+ and K+ channels, NF186, NrCAM, and βIV spectrin all have ankyrin-binding motifs, ankG has been assumed to function at the center of node assembly as a master “protein accumulator.” What evidence supports this conclusion? Mutation of the ankG-binding domain in NF186 blocks its ability to cluster at nodes (Susuki et al. 2013), and the ankG-binding domain in Na+ channels is both necessary and sufficient for channel localization. Chimeras between the Na+ channel’s ankG-binding motif and an unrelated transmembrane protein can cause it to cluster at nodes (Gasser et al. 2012). The affinity of ankG for Na+ channels increases 1000-fold following phosphorylation of the ankG-binding motif by the protein kinase CK2 (also enriched at nodes) (Bréchet et al. 2008). Remarkably, a nearly identical ankyrin-binding motif is found in KCNQ2/Q3 K+ channels and likely evolved by convergent molecular evolution to facilitate efficient node of Ranvier function (Hill et al. 2008). Clustering of βIV spectrin at nodes depends on its 15th spectrin repeat, which functions as its ankG-binding domain (Yang et al. 2007), and mutation of βIV spectrin leads to disrupted nodes, ataxia, and premature death (Yang et al. 2004). Silencing expression of ankG in myelinating DRG-neuron/Schwann cell cocultures blocked the clustering of Na+ channels (Dzhashiashvili et al. 2007). Together, these observations emphasize the importance of intrinsic cytoskeletal and scaffolding protein interactions for proper node assembly and function.
Extrinsic Regulators of Node Formation
Freeze fracture electron microscopy (EM) studies show developmental and cell contact mediated changes in the axolemma (Tao-Cheng and Rosenbluth 1983). More recent studies revealed dynamic changes in the pattern of both Na+ and K+ channel clustering during development and after demyelination (Dugandzija-Novakovic et al. 1995; Rasband et al. 1998; Vabnick et al. 1999). During PNS nerve development, different nodal domains follow a stereotypical sequence of events. Na+ channels are first clustered at the nodes, followed by the generation of the paranodal junction and only then by the clustering of K+ channels at the juxtaparanodal region (Vabnick et al. 1996, 1999; Schafer et al. 2006). In the CNS, Na+ channel clustering follows formation of paranodes (Rasband et al. 1999). In both the CNS and the PNS, Na+ channels cluster initially at sites adjacent to the edges of processes extended by oligodendrocytes (Rasband et al. 1999; Susuki et al. 2013) and myelinating Schwann cells (Vabnick et al. 1996; Ching et al. 1999). Further longitudinal growth of these processes causes displacement of the clusters until ultimately two neighboring clusters appear to fuse, thus forming a new node of Ranvier. These results indicate that these Na+ channel clusters are positioned by direct glial cell contact. In agreement, Na+ channels are diffuse along retinal ganglion cell axons until they cross the lamina cribrosa and become myelinated, after which they are clustered at nodes (Boiko et al. 2001). Na+ channels are not clustered after ablation of oligodendrocytes (Mathis et al. 2001) or Schwann cells (Vabnick et al. 1997) and are dispersed after demyelination (Dugandzija-Novakovic et al. 1995). Furthermore, nodal Na+ channels are associated with the edges of myelinating Schwann cells in nerves that display shorter internodes as a result of remyelination (Dugandzija-Novakovic et al. 1995), or genetically impaired myelination (Koszowski et al. 1998). These results strongly support the idea that axoglial interactions induce the clustering of Na+ channels at the node of Ranvier. As detailed below, two distinct axoglial contact sites at the nodes and the paranodes control the molecular assembly of the nodes of Ranvier.
Nodal Axoglial Interactions
PNS nodes (Fig. 2A)
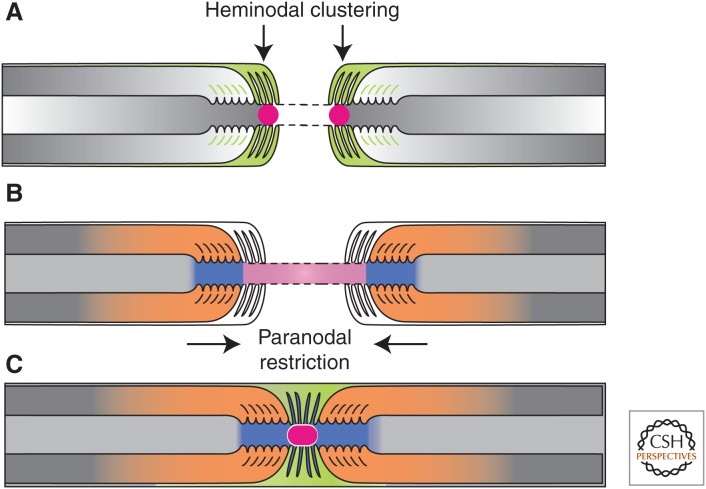
During PNS myelination, heminodal clustering of Nav channels, that is their accumulation at the edges of a growing myelin segment, depends on the interaction of axonal NF186 with glial gliomedin and NrCAM (Fig. 3A) (Eshed et al. 2005; Feinberg et al. 2010). Gliomedin binds to NrCAM present on the microvillar membrane, and also associates with other nodal ECM components to create high avidity CAM-binding multimolecular complexes that drive the accumulation of NF186 in the underlying axolemma (Eshed et al. 2007; Feinberg et al. 2010). As a binding partner for NF186, ankG is recruited to the NF186 complexes, followed by βIV spectrin and Na+ channels (Lambert et al. 1997; Eshed et al. 2005; Dzhashiashvili et al. 2007; Yang et al. 2007). The initial accumulation of NF186 at heminodes as well as its internodal clearance requires its extracellular domain, whereas its stabilization in mature nodes requires its cytoplasmic ankG-binding domain (Zhang et al. 2012). NF186 binds both ankG (Garver et al. 1997) and ECM proteins (Eshed et al. 2005; Susuki et al. 2013), and, thus, plays a key role during node formation in both CNS and PNS (Sherman et al. 2005; Zonta et al. 2008; Thaxton et al. 2011). In the PNS, secretion of gliomedin into the nodal gap and its accumulation on microvilli by binding to NrCAM initiates the molecular assembly of the nodes of Ranvier (Feinberg et al. 2010). Different binding sites for gliomedin and NrCAM on NF186 allow the multimolecular glial complexes to cluster axonal NF186 at heminodes rapidly and efficiently (Labasque et al. 2011). By genetically eliminating the expression of different combinations of nodal and paranodal CAMs, it was shown that Schwann cells govern the assembly of nodes of Ranvier by two distinct contact-dependent mechanisms: (1) active clustering of Na+ channels at heminodes (Fig. 3A), and (2) restricting Na+ channel distribution to the nodal gap (Fig. 3B) (Feinberg et al. 2010). The first mechanism of heminodal clustering requires gliomedin, the glial form of NrCAM, and axonal NF186. The second cooperating mechanism that allows the accumulation of ion channels at mature nodes occurs independently of these axonodal CAMs and depends on the formation of the paranodal junction (see below). These two cooperating processes provide reciprocal backup systems to ensure that Na+ channels are clustered at nodes in the PNS (Feinberg et al. 2010).
Figure 3.
The formation of PNS nodes of Ranvier. (A) Na+ channels (red circle) are trapped at heminodes that are contacted by Schwann cell microvilli ([MV] orange). Axon–glia interaction at this site is mediated by binding of gliomedin and glial NrCAM to axonal NF186. A transmembrane form of glial NrCAM traps gliomedin on Schwann cell microvilli and enhances its binding to axonal NF186. (B) The distribution of Na+ channels is restricted between two forming myelin segments by the PNJ (blue). Three CAMs, NF155 present at the glial paranodal loops, and an axonal complex of Caspr and contactin mediate axon–glia interaction and the formation of the PNJ. (C) These two extrinsic cooperating mechanisms provide reciprocal backup systems and ensure that Na+ channels are found at high density at the nodes. Finally, the entire nodal complex requires stabilization and interaction with cytoskeletal and scaffolding proteins, which constitute a third, intrinsic mechanism for Na+ channel clustering. (From Feinberg et al. 2010; reprinted, with permission, from Elsevier Limited © 2010.)
How do different components reach PNS nodes of Ranvier? In general, targeting proteins to specific membrane domains is achieved either by selective transport, or random transport followed by specific retention in the correct domain or removal of mislocalized proteins. Retention can be accomplished by anchoring to large molecular scaffolds or by restricting lateral diffusion (Jensen et al. 2011; Lasiecka and Winckler 2011). At PNS nodes during development CAMs are clustered from a cell surface pool, whereas the accumulation of Na+ channels and ankG requires vesicular transport from the cell soma (Zhang et al. 2012). These results are consistent with the model that Na+ channel targeting to the axolemma is ankG-dependent, but gliomedin-induced CAM clustering requires a mobile surface pool of CAMs.
CNS nodes (Fig. 2A)
As in the PNS, specific glial-extrinsic mechanisms contribute to CNS node of Ranvier formation (Fig. 2A). One of the first studies to investigate how Na+ channels are clustered in CNS axons concluded that a soluble factor secreted by oligodendrocytes promotes Na+ channel clustering (Kaplan et al. 1997). However, other evidence suggested that contact by oligodendrocytes intiates node formation (Rasband et al. 1999). In contrast to the PNS, in which contact-dependent mechanisms are necessary to assemble nodes (see above), a combination of both contact-dependent interactions and secreted factors participate in CNS node formation (Susuki et al. 2013). Furthermore, the sequence of contact-dependent events in the CNS is distinct from that in the PNS. First, myelination begins in the CNS together with the formation of the PNJ (Rasband et al. 1999; Susuki et al. 2013). As described above, the PNJ functions as a barrier to restrict membrane proteins between the adjacent myelin segments. Thus, the initiating event in CNS node formation is the axoglial interactions that occur at the PNJ. After PNJ assembly, Na+ channels accumulate at the edges of the nascent myelin sheath together with NF186, ankG, and βIV spectrin (Susuki et al. 2013). The nodal ECM proteins, although sufficient to induce clustering of nodal proteins alone, then bind to NF186 and likely stabilize the entire nodal protein complex rather than initiate its assembly. Together, although some of the ECM proteins are unique to the CNS, these observations reveal two extrinsic, glial mechanisms for ion channel clustering similar to the PNS: the primary PNJ-barrier mechanism, and a secondary NF186-ECM mechanism. These two mechanisms work in concert with the intrinsic ankG-βIV spectrin clustering mechanism described above. The idea that multiple independent, yet overlapping, processes contribute to CNS node of Ranvier formation was tested by generating mice, in which two of these overlapping mechanisms were disrupted simultaneously (Susuki et al. 2013). This was necessary because loss of only a single mechanism (e.g., PNJ, ECM, or cytoskeleton) results in mild impairments of channel clustering rather than overt loss. If multiple mechanisms contribute to CNS node formation, we reasoned that loss of a single one might not disrupt node formation, and that the two remaining mechanisms could compensate to cluster Na+ channels. We further reasoned that loss of two mechanisms might cause profound disruption of nodes if the remaining mechanism cannot completely rescue nodes. It was found that mice with two mechanisms disrupted at once had profound disruptions in CNS node of Ranvier formation, impaired nerve conduction, and early lethality compared with wild-type mice or mice lacking just a single clustering mechanism (Susuki et al. 2013). Taken together, these observations support the conclusion that three mechanisms work together to assemble CNS nodes of Ranvier.
CAM-Mediated Formation of a Cytoskeletal Barrier at the Paranodal Junction
The ECM and cytoskeletal mechanisms described above work through ankG. This is consistent with the observation that the ankG-binding domain in Na+ channels is both necessary and sufficient for nodal Na+ channel clustering (Gasser et al. 2012). But how do PNJ function to restrict axonal membrane proteins to distinct domains (Fig. 3B)? Loss of Caspr, contactin, or NF155 all result in disrupted paranodes with absence of transverse bands. Although these paranodal mutants still have clustered Na+ channels (caused by the redundant ECM/NF186 and cytoskeletal mechanisms), they fail to exclude JXP proteins from PNJ domains. Thus, paranodal CAMs are essential for the barrier function of the PNJ. How do these CAMs restrict JXP proteins from paranodes? Two mechanisms have been proposed. First, as the PNJ develops, the CAMs that define this domain acquire biochemical properties consistent with lipid raft-associated proteins (Schafer et al. 2004). Thus, the lipid membrane environments flanking each node of Ranvier are unique from those found at nodes and JXP domains, and the unique biochemical properties of these PNJ lipid domains can influence how membrane proteins partition among nodal, PNJ, and JXP membrane domains. Indeed, mutants lacking myelin lipids have paranodal abnormalities and impaired paranodal barriers with Kv1 K+ channels found in paranodal domains (Dupree et al. 1999; Ishibashi et al. 2002). Second, the paranodal CAMs assemble a specialized paranodal cytoskeleton consisting of protein 4.1B, αII spectrin, and βII spectrin (Ogawa et al. 2006; Horresh et al. 2010). Previous studies of how AIS form revealed the existence of an intraxonal boundary, or barrier, consisting of αII spectrin, βII spectrin, and ankyrinB that functioned to restrict ankG and βIV spectrin to the proximal axon (Galiano et al. 2012). Loss of the boundary permitted ankG to extend into the distal axon. Because paranodes share many of these same proteins, it was proposed that the paranodal cytoskeleton may also function as a repeating boundary to restrict membrane proteins (i.e., ion channels) to JXP and nodes. This possibility was directly tested using mice that lacked paranodal βII spectrin or paranodal 4.1B. In βII spectrin mutant mice, although the paranodal CAMs remained at paranodes, and PNJ still had transverse bands, Kv1 K+ channels were no longer restricted to JXP domains (Zhang et al. 2013). Thus, paranodal CAMs, their specialized lipid environments, and transverse bands are not sufficient to form paranodal barriers. Instead, the paranodal submembranous cytoskeleton constitutes the paranodal cytoskeletal barrier. In further support of this, the interaction between Caspr and protein 4.1B at paranodes has been shown to be important for the generation of a robust paranodal membrane barrier (Horresh et al. 2010).
MAINTAINING EXCITABLE DOMAINS IN MYELINATED AXONS
The interactions of the nodal complex with cytoskeletal and ECM components, as well as the formation of the paranodal barrier, all play a role in both assembly and maintenance of the nodes. NF186 is dispensable for the assembly of AIS and nodes (Hedstrom et al. 2007; Zonta et al. 2008; Feinberg et al. 2010), but is critical for the maintenance of the AIS (Zonta et al. 2011), as well as of nodes in the PNS (Zhang et al. 2012) and the CNS (Desmazieres et al. 2014). Interestingly, CNS nodes are more susceptible to disruption after genetic deletion of NF186 in adult mice than PNS nodes, an observation that likely reflects the major contribution of the PNJ in the CNS. In the PNS, removal of gliomedin and NrCAM, and hence the glial clustering signal operating through NF186, resulted in gradual loss of Na+ channels and other axonal components that form the nodes (Amor et al. 2014). These results revealed that continuous axon–glia interaction mediated by these molecules is required for long-term maintenance of Na+ channels at nodes of Ranvier (Amor et al. 2014). In the CNS, perinodal ECM components accumulate after Na+ channels are clustered and, thus, likely play a stabilizing role (Susuki et al. 2013). This highly variable and redundant ECM always contains BralI, which is a brain-specific link protein that stabilizes the binding of lecticans and hyaluronic acid (Cicanic et al. 2012). In BralI knockout mice, changes in the nodal ECM (i.e., brevican, versicanV2, and hyaluronan are missing) do not result in changes in Na+ channel clustering at the node. However, BralI mice show slower AP conduction (Bekku et al. 2010b), probably because the BralI-associated ECM serves as an extracellular ion pool that facilitates nodal APs. Thus, the nodal ECM, at least in the CNS, is crucial for maintaining functional nodes. Given the analogy between CNS and PNS nodes, it is likely that, in addition to gliomedin and NrCM, other ECM components that bind NF186 are involved in stabilization and maintenance of PNS nodes. The involvement of other glial-derived ligands in preserving the nodal complex in the PNS is an attractive idea in light of the observation that Na+ channels were still present in about two-thirds of the nodes in adult mice lacking both gliomedin and NrCAM (Amor et al. 2014) or NF186 (Desmazieres et al. 2014). The nodal gap in the PNS contains several proteins that could cooperate with gliomedin and NrCAM in maintaining the composition of the nodal axolemma (Eshed-Eisenbach and Peles 2013). Some candidates include the adhesion receptor dystroglycan (Saito et al. 2003; Occhi et al. 2005), the heparan sulfate proteoglycans (HSPGs) syndecan 3, syndecan 4 (Goutebroze et al. 2003; Melendez-Vasquez et al. 2005), and perlecan (Bangratz et al. 2012), the ECM components collagen XXVIII (Grimal et al. 2010) and collagen V (Melendez-Vasquez et al. 2005), and the gliomedin-related protein myocilin (Kwon et al. 2013). Gliomedin binds HSPGs (Eshed et al. 2007), and this interaction could further contribute to nodal stabilization, similar to the role HSPGs play in CNS nodes (Dours-Zimmerman et al. 2009; Bekku et al. 2010b; Susuki et al. 2013).
Given their significant role in node assembly, it is not surprising that the PNJs are also required for node maintenance. Thus, in mutant mice with paranodal abnormalities, such as mice lacking Caspr (Rios et al. 2003), the galactolipids galactocerebroside (GalC) and sulfatide (Dupree et al. 1998; Rosenbluth et al. 2003), or NF155 (Pillai et al. 2009) nodes, gradually become larger and more irregular in shape. Although the paranodal junction-dependent barrier forms in PNS nodes, this mechanism is not always sufficient to maintain Na+ channel clusters at the axolemma in mature nodes. Interestingly, although the initial accumulation of NF186 at heminodes in the PNS requires its extracellular domain, its cytoplasmic ankyrin G-binding domain mediates its transport and stabilization in mature nodes, suggesting that additional mechanisms, likely involving ankG, regulate nodal maintenance (Zhang et al. 2012). In addition, axon–glial interactions at nodes also control nodal stability and the organization by preventing the intrusion of the adjacent paranodal loops onto the nodal axolemma (Thaxton et al. 2011; Amor et al. 2014; Desmazieres et al. 2014). Maintaining the exquisite molecular organization of myelinated axons, and particularly the nodal environ is of interest given the presence of autoantibodies to nodal CAMs in Guillain–Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and multiple sclerosis (Mathey et al. 2007; Pruss et al. 2011; Devaux 2012; Devaux et al. 2012; Ng et al. 2012). The presence of such antibodies in animal models of these neuropathies leads to the disorganization of the nodes of Ranvier, formation of some binary nodes, lengthening of the nodal gap, and reduced nerve conduction (Lonigro and Devaux 2009). In addition to the direct disruption of nodes by autoimmune attack described above, loss of the myelin sheath (e.g., stroke and spinal cord injury), dysmyelination (e.g., Charcot–Marie–Tooth disease), or hypomyelination (e.g., Pelizaeus–Merzbacher disease) all include, as a common sequela, the aberrant clustering of Na+ and K+ channels. Furthermore, genetic studies have identified mutations in ion channels (e.g., KCNQ2, Nav1.1, and Nav1.6), scaffolding proteins (e.g., ankG and αII spectrin), and CAMs (e.g., Caspr and Caspr2), which are found at and near nodes of Ranvier (for review, see Faivre-Sarrailh and Devaux 2013 and Susuki 2013). Together, these observations emphasize the necessity of nodes for human health, and suggest the proper organization of nodes of Ranvier and their associated domains is an essential consideration for any therapeutic effort aimed at nervous system repair or preservation after disease or injury.
CONCLUDING REMARKS
In conclusion, the cellular and molecular mechanisms that have evolved to facilitate the assembly and maintenance of membrane domains along myelinated axons provide exquisite examples of how myelinating glia shape and influence the function and efficiency of neurons. Although a great deal is known about both the extrinsic and intrinsic mechanisms regulating assembly and maintenance of nodes, paranodes, and JXPs, much work remains to be performed to have a full understanding of how these domains are assembled and maintained, and how to both preserve and repair these domains after injury.
ACKNOWLEDGMENTS
We thank Dr. Yael Eshed-Eisenbach for discussion and comments. Work performed in the authors’ laboratories is supported by the National Institutes of Health, NINDS Grants (NS50220 to E.P., NS044916 to M.N.R., and NS069688 to M.N.R.), the Israel Science Foundation, the United States–Israel Binational Science Foundation, the European Research Projects on Rare Diseases (E-Rare-2), the National Multiple Sclerosis Society, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. E.P. is the Incumbent of the Hanna Hertz Professorial Chair for Multiple Sclerosis and Neuroscience.
Footnotes
Editors: Ben A. Barres, Marc R. Freeman, and Beth Stevens
Additional Perspectives on Glia available at www.cshperspectives.org
REFERENCES
- Amor V, Feinberg K, Eshed-Eisenbach Y, Vainshtein A, Frechter S, Grumet M, Rosenbluth J, Peles E. 2014. Long-term maintenance of Na+ channels at nodes of Ranvier depends on glial contact mediated by gliomedin and NrCAM. J Neurosci 34: 5089–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS. 1999. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvβ2, and Caspr. J Neurocytol 28: 333–347. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS. 2001. Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res 305: 53–66. [DOI] [PubMed] [Google Scholar]
- Bangratz M, Sarrazin N, Devaux J, Zambroni D, Echaniz-Laguna A, René F, Boërio D, Davoine CS, Fontaine B, Feltri ML, et al. 2012. A mouse model of Schwartz–Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking. Am J Pathol 180: 2040–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A, Tran BT, Gavrilis J, Cooper EC, Kole MH. 2014. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J Neurosci 34: 3719–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekku Y, Vargova L, Goto Y, Vorisek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fassler R, Ninomiya Y, Sykova E, et al. 2010a. Bral1: Its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci 30: 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekku Y, Vargová L, Goto Y, Vorísek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fässler R, Ninomiya Y, Syková E, et al. 2010b. Bral1: Its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci 30: 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, et al. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol 151: 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold CH, Nordborg C, Hildebrand C, Conradi S, Sourander P, Lugnegard H. 1983. Sural nerve biopsies from workers with a history of chronic exposure to organic solvents and from normal control cases. Morphometric and ultrastructural studies. Acta Neuropathol 62: 73–86. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30: 369–383. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. 1988. The perinodal astrocyte. Glia 1: 169–183. [DOI] [PubMed] [Google Scholar]
- Black JA, Frezel N, Dib-Hajj SD, Waxman SG. 2012. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. 2001. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30: 91–104. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. 2001. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30: 385–397. [DOI] [PubMed] [Google Scholar]
- Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. 2008. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol 183: 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Rasband MN. 2013. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J Neurosci 33: 6191–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Berry M. 1994. Astrocyte associations with nodes of Ranvier: Ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J Neurocytol 23: 486–499. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. 1999. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26: 84–91. [PubMed] [Google Scholar]
- Chang KJ, Rasband MN. 2013. Excitable domains of myelinated nerves: Axon initial segments and nodes of Ranvier. Curr Top Membr 72: 159–192. [DOI] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. 2002. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol 12: 217–220. [DOI] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ III, Kazen-Gillespie KA, et al. 2002. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc Natl Acad Sci 99: 17072–17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O’Malley HA, Bharucha V, Meadows LS, et al. 2004. Mice lacking sodium channel β1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci 24: 4030–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, Davis TH, Salzer JL, Isom LL. 2012. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel α and β2 subunits. J Biol Chem 287: 39061–39069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Zanazzi G, Levinson SR, Salzer JL. 1999. Clustering of neuronal sodium channels requires contact with myelinating Schwann cells. J Neurocytol 28: 295–301. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. 1980. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature 284: 170–171. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. 1984. On the physiological role of internodal potassium channels and the security of conduction in myelinated nerve fibres. Proc R Soc Lond B Biol Sci 220: 415–422. [DOI] [PubMed] [Google Scholar]
- Cicanic M, Sykova E, Vargova L. 2012. Bral1: “Superglue” for the extracellular matrix in the brain white matter. Int J Biochem Cell Biol 44: 596–599. [DOI] [PubMed] [Google Scholar]
- Cooper EC. 2011. Made for “anchorin”: Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. Semin Cell Dev Biol 22: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Wrabetz L, Feltri ML. 2006. Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol 16: 501–507. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. 1996. Molecular composition of the node of Ranvier: Identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain–) and NrCAM at nodal axon segments. J Cell Biol 135: 1355–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazieres A, Zonta B, Zhang A, Wu LM, Sherman DL, Brophy PJ. 2014. Differential stability of PNS and CNS nodal complexes when neuronal neurofascin is lost. J Neurosci 34: 5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ. 2012. Antibodies to gliomedin cause peripheral demyelinating neuropathy and the dismantling of the nodes of Ranvier. Am J Pathol 181: 1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. 2003. Kv3.1b is a novel component of CNS nodes. J Neurosci 23: 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. 2004. KCNQ2 is a nodal K+ channel. J Neurosci 24: 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Odaka M, Yuki N. 2012. Nodal proteins are target antigens in Guillain–Barre syndrome. J Peripher Nerv Syst 17: 62–71. [DOI] [PubMed] [Google Scholar]
- Dours-Zimmerman MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. 2009. Versican V2 assembles the extracellular matrix surrounding the nodes of Ranvier in the central nervous system. J Neurosci 29: 7731–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. 2008. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci 39: 180–192. [DOI] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P. 1995. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci 15: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Blight A, Suzuki K, Popko B. 1998. Myelin galactolipids are essential for proper node of Ranvier formation in the CNS. J Neurosci 18: 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. 1999. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol 147: 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. 2007. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol 177: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed-Eisenbach Y, Peles E. 2013. The making of a node: A co-production of neurons and glia. Curr Opin Neurobiol 23: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR Jr, Peles E. 2005. Gliomedin mediates schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron 47: 215–229. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Carey DJ, Peles E. 2007. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol 177: 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Devaux JJ. 2013. Neuro-glial interactions at the nodes of Ranvier: Implication in health and diseases. Front Cell Neurosci 7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Sabanay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, et al. 2010. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron 65: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Hjelmstrom P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA. 2000. Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. Neuroreport 11: 199–202. [DOI] [PubMed] [Google Scholar]
- Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. 2012. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 149: 1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. 1997. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol 137: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A, Ho TS-Y, Cheng X, Chang KJ, Waxman SG, Rasband MN, Dib-Hajj S. 2012. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J Neurosci 32: 7232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan N, Kartvelishvily E, Spiegel I, Salomon D, Sabanay H, Rechav K, Vainshtein A, Frechter S, Maik-Rachline G, Eshed-Eisenbach Y, et al. 2013. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot–Marie–Tooth neuropathy. J Neurosci 33: 10950–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. 2002. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol 157: 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutebroze L, Carnaud M, Denisenko N, Boutterin MC, Girault JA. 2003. Syndecan-3 and syndecan-4 are enriched in Schwann cell perinodal processes. BMC Neurosci 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimal S, Puech S, Wagener R, Venteo S, Carroll P, Fichard-Carroll A. 2010. Collagen XXVIII is a distinctive component of the peripheral nervous system nodes of Ranvier and surrounds nonmyelinating glial cells. Glia 58: 1977–1987. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. 2007. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol 178: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MA, Sorensen HJ, Johnson LR, Levinson SR. 2005. Localization of the Nav1.8 sodium channel isoform at nodes of Ranvier in normal human radicular tooth pulp. Neurosci Lett 380: 32–36. [DOI] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. 2008. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet 4: e1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. 2008. Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci 28: 14213–14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I, Bar V, Kissil JL, Peles E. 2010. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci 30: 2480–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Ellisman MH. 1991. Three-dimensional fine structure of cytoskeletal-membrane interactions at nodes of Ranvier. J Neurocytol 20: 667–681. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H. 2002. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci 22: 6507–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic A, Horresh I, Golan N, Spiegel I, Sabanay H, Frechter S, Ohno S, Terada N, Möbius W, Rosenbluth J, et al. 2012. The cytoskeletal adapter protein 4.1G organizes the internodes in peripheral myelinated nerves. J Cell Biol 196: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Rasmussen HB, Misonou H. 2011. Neuronal trafficking of voltage-gated potassium channels. Mol Cell Neurosci 48: 288–297. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA., 1997. Induction of sodium channel clustering by oligodendrocytes. Nature 386: 724–728. [DOI] [PubMed] [Google Scholar]
- King CH, Lancaster E, Salomon D, Peles E, Scherer SS. 2014. Kv7.2 regulates the function of peripheral sensory neurons. J Comp Neurol 522: 3262–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. 1995. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem 270: 2352–2359. [DOI] [PubMed] [Google Scholar]
- Koszowski AG, Owens GC, Levinson SR. 1998. The effect of the mouse mutation claw paw on myelination and nodal frequency in sciatic nerves. J Neurosci 18: 5859–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Johnson TV, Joe MK, Abu-Asab M, Zhang J, Chan CC, Tomarev SI. 2013. Myocilin mediates myelination in the peripheral nervous system through ErbB2/3 signaling. J Biol Chem 288: 26357–26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasque M, Devaux JJ, Leveque C, Faivre-Sarrailh C. 2011. Fibronectin type III-like domains of neurofascin-186 protein mediate gliomedin binding and its clustering at the developing nodes of Ranvier. J Biol Chem 286: 42426–42434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Davis JQ, Bennett V. 1997. Morphogenesis of the node of Ranvier: Co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci 17: 7025–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laquérriere A, Maluenda J, Camus A, Fontenas L, Dieterich K, Nolent F, Zhou J, Monnier N, Latour P, Gentil D, et al. 2014. Mutations in CNTNAP1 and ADCY6 are responsible for severe arthrogryposis multiplex congenita with axoglial defects. Hum Mol Genet 23: 2279–2289. [DOI] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B. 2011. Mechanisms of polarized membrane trafficking in neurons—Focusing in on endosomes. Mol Cell Neurosci 48: 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonigro A, Devaux JJ. 2009. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain 132: 260–273. [DOI] [PubMed] [Google Scholar]
- Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T, et al. 2007. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med 204: 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Denisenko-Nehrbass N, Girault JA, Borrelli E. 2001. Essential role of oligodendrocytes in the formation and maintenance of central nervous system nodal regions. Development 128: 4881–4890. [DOI] [PubMed] [Google Scholar]
- Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. 2007. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol 178: 861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. 2005. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia 52: 301–308. [DOI] [PubMed] [Google Scholar]
- Miller RG, Pinto da Silva P. 1977. Particle rosettes in the periaxonal Schwann cell membrane and particle clusters in the axolemma of rat sciatic nerve. Brain Res 130: 135–141. [DOI] [PubMed] [Google Scholar]
- Ng JK, Malotka J, Kawakami N, Derfuss T, Khademi M, Olsson T, Linington C, Odaka M, Tackenberg B, Pruss H, et al. 2012. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 79: 2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, et al. 2005. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci 25: 9418–9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. 2006. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci 26: 5230–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. 2010. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci 30: 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. 2009. Spatiotemporal ablation of myelinating glia-specific neurofascin (NfascNF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res 87: 1773–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, et al. 2003. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 162: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Schwab JM, Derst C, Gortzen A, Veh RW. 2011. Neurofascin as target of autoantibodies in Guillain–Barre syndrome. Brain 134: e173. [DOI] [PubMed] [Google Scholar]
- Rasband MN. 2010. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11: 552–562. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. 1998. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci 18: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. 1999. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci 19: 7516–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. 2001. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci 98: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. 2003. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci 23: 7001–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. 2009. Multiple functions of the paranodal junction of myelinated nerve fibers. J Neurosci Res 87: 3250–3258. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J, Dupree JL, Popko B. 2003. Nodal sodium channel domain integrity depends on the conformation of the paranodal junction, not on the presence of transverse bands. Glia 41: 318–325. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. 2003. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38: 747–758. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. 2004. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci 24: 3176–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Custer AW, Shrager P, Rasband MN. 2006. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol 2: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, et al. 2005. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48: 737–742. [DOI] [PubMed] [Google Scholar]
- Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E. 2007. A central role for Necl4 (SynCAM4) in Schwann cell–axon interaction and myelination. Nat Neurosci 10: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolinski C, Breathnach AS, Martin B, Thomas PK, King RH, Gabriel G. 1981. Associated particle aggregates in juxtaparanodal axolemma and adaxonal Schwann cell membrane of rat peripheral nerve. J Neurocytol 10: 679–691. [DOI] [PubMed] [Google Scholar]
- Stolinski C, Breathnach AS, Thomas PK, Gabriel G, King RH. 1985. Distribution of particle aggregates in the internodal axolemma and adaxonal Schwann cell membrane of rodent peripheral nerve. J Neurol Sci 67: 213–222. [DOI] [PubMed] [Google Scholar]
- Susuki K. 2013. Node of Ranvier disruption as a cause of neurological diseases. ASN Neuro 5: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Chang KJ, Zollinger DR, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann MT, Oses-Prieto JA, Burlingame AL, Seidenbecher CI, et al. 2013. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 78: 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Rosenbluth J. 1983. Axolemmal differentiation in myelinated fibers of rat peripheral nerves. Brain Res 285: 251–263. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Rosenbluth J. 1984. Extranodal particle accumulations in the axolemma of myelinated frog optic axons. Brain Res 308: 289–300. [DOI] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA. 2011. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron 69: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Shrager P. 1998. Ion channel redistribution and function during development of the myelinated axon. J Neurobiol 37: 80–96. [PubMed] [Google Scholar]
- Vabnick I, Novakovic SD, Levinson SR, Schachner M, Shrager P. 1996. The clustering of axonal sodium channels during development of the peripheral nervous system. J Neurosci 16: 4914–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Messing A, Chiu SY, Levinson SR, Schachner M, Roder J, Li C, Novakovic S, Shrager P. 1997. Sodium channel distribution in axons of hypomyelinated and MAG null mutant mice. J Neurosci Res 50: 321–336. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. 1999. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci 19: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. 1993. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365: 75–79. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. 2004. βIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci 24: 7230–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. 2007. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol 176: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bekku Y, Dzhashiashvili Y, Armenti S, Meng X, Sasaki Y, Milbrandt J, Salzer JL. 2012. Assembly and maintenance of nodes of Ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron 73: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Susuki K, Zollinger DR, Dupree JL, Rasband MN. 2013. Membrane domain organization of myelinated axons requires βII spectrin. J Cell Biol 203: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. 2008. Glial and neuronal isoforms of neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol 181: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Desmazieres A, Rinaldi A, Tait S, Sherman DL, Nolan MF, Brophy PJ. 2011. A critical role for neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron 69: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]