Abstract
Rationale
3,4-Methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) are synthetic drugs found in so-called “bath salts” products. Both drugs exert their effects by interacting with monoamine transporter proteins. MDPV is a potent uptake blocker at transporters for dopamine and norepinephrine while methylone is a non-selective releaser at transporters for dopamine, norepinephrine and serotonin (5-HT).
Objectives
We hypothesized that prominent 5-HT-releasing actions of methylone would render this drug less reinforcing than MDPV.
Methods
To test this hypothesis, we compared behavioral effects of MDPV and methylone using intravenous (i.v.) self-administration on a fixed-ratio 1 schedule in male rats. Additionally, neurochemical effects of the drugs were examined using in vivo microdialysis in nucleus accumbens, in a separate cohort of rats.
Results
MDPV self-administration (0.03 mg/kg/inj) was acquired rapidly, and reached 40 infusions per session, similar to the effects of cocaine (0.5 mg/kg/inj), by the end of training. By contrast, methylone self-administration (0.3 & 0.5 mg/kg/inj) was acquired slowly, and response rates only reached 20 infusions per session by the end of training. In dose substitution studies, MDPV and cocaine displayed typical inverted U-shaped dose-effect functions, but methylone did not. In vivo microdialysis revealed that i.v. MDPV (0.1 and 0.3 mg/kg) increased extracellular dopamine while i.v. methylone (1 and 3 mg/kg) increased extracellular dopamine and 5-HT.
Conclusions
Our findings support the hypothesis that elevations in extracellular 5-HT in the brain can dampen positive reinforcing effects of cathinone-type drugs. Nevertheless, MDPV and methylone are both self-administered by rats suggesting these drugs possess significant abuse liability in humans.
Keywords: 3, 4-Methylenedioxypyrovalerone (MDPV); methylone; cocaine; self-administration; microdialysis; rats
Introduction
The non-medical use of psychoactive synthetic cathinones, commonly known as “bath salts,” has increased worldwide in recent years (Baumann, 2014; German et al., 2014), often leading to life-threatening medical consequences (Kesha et al., 2013; Wright et al., 2013). Three of the most popular synthetic cathinones, 3,4-methylenedioxypyrovalerone (MDPV), 4-methyl-N-methylcathinone (mephedrone) and 3,4-methylenedioxy-N-methylcathinone (methylone), have been classified by the United States (US) as Schedule I controlled substances (Drug Enforcement Administration, 2011, 2013). The thirty-sixth meeting of the Expert Committee on Drug Dependence, convened by the World Health Organization, was followed by a recommendation to place these three substances in Schedule II of the United Nations Convention on Psychotropic Substances 1971 (World Health Organization, 2015). Despite these introductions of legislative control, MDPV and methylone remain available in the street drug marketplace and are continuing to be abused in the US and elsewhere (Drug Enforcement Administration, 2014; Seely et al., 2013). As a result, it is important to understand the pharmacological actions of these two drugs, particularly with regard to their abuse liability.
MDPV and methylone are both classified as synthetic cathinones, but the drugs are chemically distinct and their molecular mechanisms of action differ. With respect to chemical structure, MDPV possesses a nitrogen-containing pyrrolidine ring and an extended α-carbon alkyl chain, whereas methylone does not. MDPV is an uptake blocker at transporters for dopamine (DAT) and norepinephrine (NET) (Baumann et al., 2013; Cameron et al., 2013; Eshleman et al., 2013; Simmler et al., 2013), similar to cocaine but much more potent. Importantly, MDPV is highly selective for DAT and NET, with little affinity for the serotonin transporter (SERT). By contrast, methylone acts as a non-selective substrate for DAT, NET and SERT, thereby causing the transporter-mediated release of all three monoamine transmitters, similar to the actions of 3,4-methylenedioxymethamphetamine (MDMA) (Baumann et al., 2012; Eshleman et al., 2013; Simmler et al., 2013). MDPV and methylone increase locomotor activity in rodents (Aarde et al., 2013; Gatch et al., 2013; Lopez-Arnau et al., 2012; Marusich et al., 2012) and can increase body temperature, particularly after high-dose administration or at elevated ambient temperatures (Fantegrossi et al., 2012; Lopez-Arnau et al., 2014a, b; Kiyatkin et al., 2015). Both drugs also enhance intracranial self-stimulation (Bonano et al., 2014, Watterson et al., 2012, 2014) and engender conditioned place preference (Karlsson et al., 2014), effects that are indicators of high abuse potential. In studies that have directly compared the in vivo effects of MDPV and methylone, MDPV is typically 3–10 times more potent than methylone (Bonano et al., 2014; Gatch et al., 2013; Karlsson et al., 2014; Marusich et al., 2012; Kiyatkin et al., 2015).
The pharmacological effects of methylone might be expected to mimic those produced by MDMA since the two drugs share similar chemical structures and the ability to release 5-HT from neurons. MDMA is self-administered by animals, but its reinforcing effects are weak when compared to other psychostimulants, most likely due to its stimulation of 5-HT release (De La Garza et al., 2007; Schenk et al., 2007; Bradbury et al., 2013). Accumulating preclinical evidence shows that elevations in extracellular 5-HT in the brain can dampen locomotor and reinforcing effects mediated by dopaminergic stimulants (Wee and Woolverton, 2006; Baumann et al., 2011; Bauer et al., 2013). The fact that MDPV has relatively specific effects on dopamine and norepinephrine, without affecting 5-HT systems, would lead one to predict greater self-administration of this drug when compared to methylone. Somewhat surprisingly, methylone (Watterson et al., 2012; Creehan et al., 2015) and MDPV (Aarde et al., 2013, 2015; Watterson et al., 2014) are both readily self-administered by rats, but methylone appears to be less potent and supports somewhat lower rates of responding than MDPV. Importantly, Creehan et al. (2015) reported much lower rates of responding for methylone in female rats than Watterson et al. (2012) reported in male rats, suggesting that there may be sex differences in responsiveness to the drug.
Methodological differences across the various studies examining self-administration of MDPV and methylone make direct comparison of the results difficult. For example, in studies investigating the reinforcing effects of MDPV in male rats (Aarde et al., 2013; Watterson et al., 2014), subjects were first pre-trained with food prior to drug self-administration, while this was not the case for methylone (Watterson et al., 2012). In addition, Creehan et al. (2015) used female rats to study methylone self-administration. Here we wished to carry out a side-by-side comparison of MDPV and methylone self-administration in male rats, without pre-training for food, and under identical conditions to allow for direct comparison of reinforcing effects. In addition, we used in vivo microdialysis in male rats to compare the effects of the two drugs on extracellular concentrations of dopamine and 5-HT in the nucleus accumbens. Based on our previous neurochemical findings (Baumann et al., 2012; 2013), we predicted that MDPV administration would increase extracellular concentrations of dopamine only, while methylone would increase concentrations of both dopamine and 5-HT. Given the stronger serotonergic effects of methylone, we hypothesized that self-administration of this drug would be less robust when compared to MDPV.
Materials and Methods
Subjects
Male Sprague-Dawley rats, weighing 300–400 g at the beginning of the studies, were used for these experiments. They were housed under standard vivarium conditions of controlled temperature (22 ± 2 °C) and humidity (45 ± 5%) with food and water freely available. Rats were group-housed, two or three per cage, prior to surgery but single-housed thereafter. Rats for self-administration studies were housed under a 12:12 h reverse light-dark cycle (lights on at 2200 h), whereas rats for microdialysis studies were housed under a 12:12 h normal light-dark cycle (lights on at 0700 h). Guidelines of the Institutional Animal Care and Use Committee at the National Institute on Drug Abuse (NIDA)/Intramural Research Program (IRP) and the Guide for the Care and Use of Laboratory Animals were followed at all times.
Drugs
3,4-Methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) were synthesized in racemic form as HCl salts in our laboratories. Chemical and structural analysis included proton nuclear magnetic resonance, gas chromatography/mass spectrometry, thin layer chromatography, and melting point determination. All data confirmed the expected structures. Cocaine hydrochloride (NIDA/IRP, Baltimore, MD), MDPV and methylone were all dissolved in sterile saline prior to administration.
Self-Administration Studies
Rats were anesthetized by a combination of 100 mg/kg ketamine and 10 mg/kg xylazine, (i.p.) and intravenous (i.v.) jugular catheters were implanted according to procedures described previously (Schindler et al., 2011). Briefly, 3 cm of Silastic tubing (Dow Corning, 0.44 mm i.d., 0.9 mm o.d.) was inserted into the right jugular vein and connected to vinyl tubing (Dural Plastics, 0.5 mm i.d., 1.0 mm o.d.) that exited the back at the midscapular region, and was plugged with an obturator. Immediately following catheter implantation, an incision was made posterior to the exit site of the catheter and a 20 mm nylon screw was inserted serving as a back mount. A metal spring, which housed Tygon extension tubing connecting the fluid swivel (Instech, Plymouth Meeting, PA) on the top of the cage to the i.v. catheter, was attached to this mount at the time of self-administration sessions. This tubing was attached to a 10-ml syringe controlled by a motor-driven syringe pump outside the sound attenuation cubicle. The pump (PHM-100, Med Associates) delivered a constant volume (0.2 ml/inj) of drug solution over 2 secs. Concentration of drug was adjusted for each rat’s body weight to give the desired dose. Catheters were flushed before and after each training session with 0.1 ml of a saline solution containing 1.25 units/ml heparin and 0.08 mg/ml gentamicin. If a catheter failed during the study, a second catheter was implanted on the opposite side. After the second catheter failure, the rat was removed from the study.
Ten training chambers were used (ENV-008CT, Med Associates, St. Albans VT). Each chamber was enclosed in a sound-attenuation cubicle equipped with a fan to provide ventilation and stable background noise (ENV-018M, Med Associates). Each chamber had a grid floor and two nose-poke response holes (ENV-114BM, Med Associates), one on each side of a food trough (not used in this study). The nose-poke holes could be illuminated from inside the hole by a dim yellow light. A houselight (ENV-215M, Med Associates) was situated above the nose-poke holes. Experimental events were controlled by a MED-PC computer system (Med Associates).
Following surgery and at least 7 days of recovery, the catheters were connected to the extension tubing, and the rats were placed in the training chambers every weekday. Sessions began with the illumination of the houselight and the two nose-poke holes. A nose-poke in the active hole was reinforced on a fixed-ratio 1 schedule with an infusion of drug, the houselight and nose-poke hole lights were turned off and a 20 sec timeout began. Nose-pokes in the inactive hole were recorded, but had no scheduled consequence. During the timeout, responses were recorded but not reinforced. Following the timeout, the houselight and nose-poke hole lights were turned on and active nose-pokes were again reinforced. Whether the right or left nose-poke was active was counterbalanced across subjects. Sessions lasted for 2 h. Initial acquisition doses were 0.03 mg/kg/inj for MDPV, 0.3 or 0.5 mg/kg/inj for methylone, and 0.5 mg/kg/inj for cocaine. The training doses of MDPV and methylone were chosen based on the findings of others (e.g., Watterson et al., 2012; 2014) while the training dose of cocaine was based on our previous experience. Most rats received at least 10 days of acquisition training, except those rats trained on 0.5 mg/kg/inj methylone where most rats received 17 days of training.
Following acquisition training, dose-effect testing began for all the groups except those originally trained on 0.5 mg/kg/inj methylone. The dose of each drug was varied to 3 additional doses. Each dose was available for self-administration for 3 days, with results for days 2 and 3 averaged for analysis. Following dose-effect testing, the rats were returned to the original training dose. After responding stabilized, saline was substituted for each drug and training continued until responding decreased.
Microdialysis Studies
Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and each rat received a surgically-implanted jugular catheter and an intracranial guide cannula as previously described (Baumann et al., 2012). Briefly, approximately 3 cm of Silastic tubing was inserted into the right jugular vein and connected to vinyl tubing that exited the back at the midscapular region, and was plugged with an obturator. Immediately following catheter implantation, the rat was placed into a stereotaxic apparatus and an intracerebral guide cannula (CMA/12, Harvard Apparatus, Holliston, MA) was surgically implanted. The guide cannula was aimed at the nucleus accumbens, according the coordinates: 1.7 mm lateral and 1.6 mm anterior to bregma (reference zero), and 6.0 mm ventral to the dura surface. Rats were allowed at least one week for recovery from surgery. On the evening before an experiment, a 2 × 0.5 mm dialysis probe (CMA/12, Harvard Apparatus) was inserted into the guide cannula, and an extension tube was attached to the jugular catheter. Each rat was placed into its own enclosure and connected to a tethering system. The enclosure was equipped with photobeams to detect locomotor activity (Truscan, Harvard Apparatus). Probes were perfused overnight with Ringer’s salt solution pumped at a flow rate of 0.6 μl/min. On the next morning, dialysate samples were collected at 20-min intervals. Samples were immediately assayed for dopamine and 5-HT by HPLC-ECD as described elsewhere (Baumann et al., 2011).
Rats were randomly assigned to groups receiving either drug or saline injections. Once three stable baseline samples were obtained, rats received two sequential i.v. injections of drug: one dose at time zero, followed by a 3-fold higher dose 60 min later. Drugs were administered i.v. to mimic the self-administration route, and the low drug doses were ~3-fold greater than the training dose self-administered. For MDPV, rats received 0.1 mg/kg followed by 0.3 mg/kg. For methylone, rats received 1.0 mg/kg followed by 3 mg/kg. Control rats received sequential i.v. injections of saline (1 ml/kg) according to the same schedule. Microdialysis samples were collected every 20 min throughout the post-injection period for 120 min. At the end of the experiments, rats were euthanized with CO2 and decapitated. Brains were cut on a cryostat (CM1950, Leica Biosystems, Buffalo Grove, IL) and examined to verify placement of microdialysis probe tips within the nucleus accumbens. Only those rats with correct placements were included in data analyses.
Data Analysis
For self-administration studies, only responses during the reinforcement period were used for analysis. Timeout responses were not included, but were generally very low following the first couple of days of training. Data from the self-administration experiments were analyzed using a linear mixed-effects model. For the acquisition and extinction analysis, day and response (active/inactive) were treated as within-subject factors. For the dose-effect study, dose was treated as a within-subject factor. Dose/hour was calculated by multiplying the total number of infusions by the dose/infusion and dividing by the 2 hour session time. For microdialysis studies, all neurotransmitter concentrations (pg/μl) and motor activity measures (cm/20 min) were normalized to a percentage of control values based on three pre-injection baseline samples and the corresponding 20-min activity bins. Microdialysis data were analyzed using two-way analysis-of-variance (treatment x time). Post-hoc analyses were performed using t-tests with Bonferroni corrections.
Results
Self-administration
Figure 1 shows acquisition data for MDPV (0.03 mg/kg/inj), methylone (0.3 mg/kg/inj) and cocaine (0.5 mg/kg/inj). Both active/reinforced and inactive responses are shown. Timeout responses are not included. On the first day of training, rats took over 40 injections of MDPV (43.2 ± 10.2) and maintained that level of responding throughout training. On the first day of training, inactive responses were high as well (29.4 ± 9.5), but decreased rapidly after day 1. Statistical analysis showed a significant effect of the interaction of response (active/inactive) and day (F9,312 = 3.17, p < .01). Post-hoc analysis showed that active and inactive responses were significantly different (solid symbols in Figure 1) for every day beyond day 1 (p < .001).
Figure 1.
Acquisition and extinction of self-administration of 0.03 mg/kg/inj MDPV (top panel, acquisition n = 17–20, extinction n = 4–12), 0.3 mg/kg/inj methylone (middle panel, acquisition n = 15, extinction n = 6–9) and 0.5 mg/kg/inj cocaine (bottom panel, acquisition n = 14–16, extinction n = 7–9). The first 10 days of acquisition are shown, followed by the first 6 days of extinction. Active responses and inactive responses do not include timeout responses. Therefore, active responses are equivalent to infusions. Filled symbols for active responses are significantly different from inactive on the same day. A = acquisition, E = Extinction
In contrast to MDPV, reinforced responses for methylone were below 20 on day 1 (14.4 ± 3.3) and slowly increased over time to a maximum of 25.5 ± 4.9 by day 10. Inactive responses were similar to active responses over the first few days of training (8.5 ± 1.8 on day 1), but a separation between active and inactive responses did eventually develop. Statistical analysis again showed a significant interaction of response (active/inactive) and day (F9,246 = 3.14, p < .001). Post-hoc analysis showed that active and inactive response differed on day 7–10 (solid symbols, p < 0.01).
For cocaine, active/reinforced responses were initially similar to those of MDPV, averaging 32.1 ± 11.6 active responses on day 1. Inactive responses were also high initially (38.0 ± 5.7 on day 2), but dropped over the ensuing days of training. Because of the variability in the inactive response data, the statistical analysis failed to show any significant effects. When only active responses were compared across the 3 drugs, statistical analysis showed a significant effect of drug (F2,47 = 7.8, p < 0.003), but not for either the day factor or the interaction. Post-hoc analysis showed all three drugs differed (cocaine vs MDPV, p < 0.01, cocaine vs methylone and MDPV vs methylone, p < 0.001). Active responses for MDPV were slightly but consistently higher for MDPV when compared to cocaine. Active responses for methylone were consistently well below those for both MDPV and cocaine.
Watterson et al. (2012) reported much higher levels of self-administration for methylone than were observed here. At a dose of 0.5 mg/kg/inj, they reported rates of up to 80 infusions per 2 h session by day 10 of training, and this level of responding continued throughout the 20 days of training. To more closely replicate the Watterson et al. (2012) procedure, we trained a separate group of rats on 0.5 mg/kg/inj methylone and continued training beyond 10 days. Due to catheter limitations, most rats were only able to continue training for 14–17 days. Figure 2 shows the results for the 0.5 mg/kg/inj group. Over the first 10 days of training the results for 0.5 mg/kg/inj are comparable to those for the 0.3 mg/kg/inj methylone dose (see Figure 1). After 10 days, there was only a small increase in the number of injections for the 0.5 mg/kg/inj group. Statistical analysis showed a significant interaction of response (active/inactive) and day (F16,244 = 6.3, p < 0.0001). Post-hoc analysis showed active and inactive responses differed on days 6, 7 and 9–17 (solid symbols in Figure 2).
Figure 2.
Acquisition of self-administration of a higher dose of methylone 0.5 mg/kg/inj (n = 5–9). Training for the higher dose of methylone was continued beyond 10 days and the first 17 days of acquisition is shown. Filled symbols for active responses are significantly different from inactive responses on the same day. For comparison, the results for 0.3 mg/kg/inj methylone from Figure 1 are shown as solid (active responses) and dashed (inactive responses) lines.
When saline was substituted for drug, responding decreased for MDPV, methylone, and cocaine (Figure 1). Responding dropped rapidly for MDPV, but relatively slowly for cocaine. For methylone there was an immediate decrease in responding. Statistical analysis showed that for MDPV the interaction of response (active/inactive) and day was significant (F5,80 = 3.8, p < .01) with post-hoc tests showing that only the difference in active/inactive responses on day 1 was significant (solid symbol). For methylone, neither the main effects of response and day, nor the interaction were significant. For cocaine, the main effects were both significant (day F5,76 = 4.47, p < .01; response F1,8 = 18.14, p < .01), but the interaction was not.
Figure 3 shows the results for the dose-effect manipulation. The top panel shows the average number of reinforced responses. For comparison, the average number of saline infusions for the last day of extinction is shown for each drug. For both MDPV and cocaine, results typical for stimulant drug self-administration were observed, with peak infusions occurring at lower doses and rates of responding decreasing with increases in dose. However, for methylone responding was relatively flat across doses. For all three drugs there was a significant effect of dose (MDPV F4,20 = 21.95, p < .0001; methylone F4,27 = 3.79, p < 0.05; cocaine F4,32 = 22.9, p < 0.0001). The number of infusions taken were significantly above saline for multiple doses for both MDPV (0.003, 0.01 and 0.03 mg/kg/inj, solid symbols) and cocaine (0.1, 0.3 mg/kg/inj, solid symbols), but for methylone, only the 0.1 mg/kg/inj dose maintained infusion rates above saline (p < 0.05, solid symbol).
Figure 3.
Dose-effect functions for infusions (top panel) and intake in Dose/Hr (bottom panel) is shown for MDPV (n = 4–11), methylone (n = 4–13) and cocaine (n = 7–12). Dose was manipulated following initial acquisition of the self-administration response. Solid symbols in the top panel indicate differences from the appropriate saline (S) point. Solid symbols in bottom panel are significantly different from open symbols for same drug, but do not differ from each other for the same drug.
The bottom panel of Figure 3 shows the average hourly intake for each drug. For cocaine, the pattern of responding led to fairly steady rates of intake across doses, while for methylone rates of intake increased with increasing dose. Rate of intake for MDPV increased most dramatically from 0.003 mg/kg/inj, a dose that did not reliably maintain responding, to 0.01 mg/kg/inj. Above 0.01 mg/kg/inj intake increased gradually. Again, for all three drugs there was a significant effect of dose (MDPV F3,14 = 15.37, p < .0001; methylone F3,19 = 35.72, p < 0.0001; cocaine F3,24 = 14.4, p < 0.0001). For both MDPV and cocaine, the intake at the lowest dose was significantly different from the other doses (p < 0.01, open symbol), but the higher doses did not differ from each other. For methylone, the 0.01 and 0.03 doses did not differ from each other and the 0.1 and 0.3 doses did not differ. All other comparisons were significant (p < 0.01).
Microdialysis
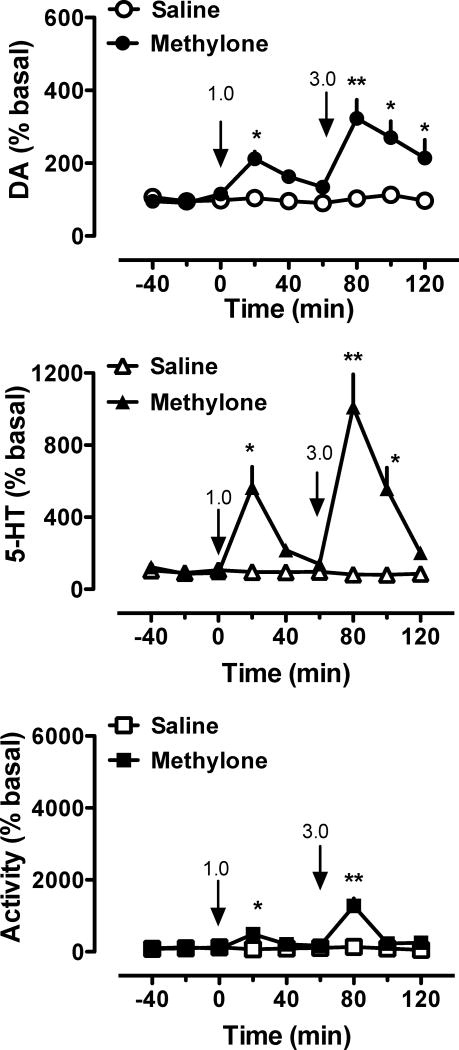
Figure 4 depicts the effects of MDPV or saline injections on extracellular concentrations of dopamine and 5-HT in nucleus accumbens of conscious rats, along with corresponding locomotor activity. MDPV significantly increased dialysate dopamine at both doses tested (F8,99 = 11.68, p < 0.0001), but had no effect on dialysate 5-HT (F8,99 = 0.2). The elevations in dopamine produced by MDPV were 2-fold and 4-fold above basal preinjection values after the 0.1 and 0.3 mg/kg doses, respectively. As shown in the bottom panel of Figure 4, MDPV markedly increased locomotion in a dose-dependent manner (F8,99 = 21.17, p < 0.0001), and motor activation was temporally correlated with elevations in extracellular dopamine. Figure 5 shows the effects of methylone injections on extracellular concentrations of dopamine and 5-HT. In contrast to MDPV, methylone significantly increased concentrations of both dopamine (F8,99 = 5.32, p < 0.0001) and 5-HT (F8,99 = 11.92, p < 0.0001), with the relative increases in 5-HT being much greater. For example, the rise in dialysate 5-HT after 3.0 mg/kg was 8-fold above basal preinjection values, while the corresponding elevation in dialysate dopamine was only about 3-fold. Methylone produced a dose-related increase in locomotor activity (F8,99 = 12.05, p < 0.01), though this effect was smaller in magnitude when compared to MDPV.
Figure 4.
Effects of MDPV (n = 7) or saline vehicle (N=7) on extracellular concentrations of dopamine (DA, top panel) and serotonin (5-HT, middle panel) in the nucleus accumbens, and on horizontal locomotor activity (activity, bottom panel), in conscious rats undergoing microdialysis. Drugs or saline were administered i.v. at the indicated times (arrows) and doses (mg/kg). *p < 0.05, **p < 0.01 = significant difference from saline (n = 6) at the corresponding time point.
Figure 5.
Effects of methylone (n = 7) or saline vehicle (N=7) on extracellular concentrations of dopamine (DA, top panel) and serotonin (5-HT, middle panel) in the nucleus accumbens, and horizontal locomotor activity (activity, bottom panel), in conscious rats undergoing microdialysis. Drugs or saline were administered i.v. at the indicated times (arrows) and doses (mg/kg). *p < 0.05, **p < 0.01 = significant difference from saline (n = 6) at the corresponding time point.
Discussion
A major aim of the present study was to directly compare the reinforcing and neurochemical effects of the bath salts constituents MDPV and methylone. Although these drugs are both classified as synthetic cathinones, they are quite different in terms of their selectivity and mechanism at monoamine transporters. MDPV is a potent uptake blocker at DAT and NET with minimal activity at SERT (Baumann et al., 2013; Cameron et al., 2013; Eshleman et al., 2013; Simmler et al., 2013), whereas methylone is a non-selective transporter substrate that evokes the release of dopamine, norepinephrine and 5-HT (Baumann et al., 2012; Eshelman et al., 2013; Simmler et al., 2013). We hypothesized that the serotonergic effects of methylone would render this drug less reinforcing when compared to MDPV. It was found that rats acquired MDPV self-administration rapidly, within the first few days of training, and the drug maintained high rates of responding (40 infusions per 2 h session). The acquisition of methylone, on the other hand, occurred slowly over the course of many days, and the number of responses (20 infusions per 2 h session) was much less than for MDPV. In dose-substitution studies, MDPV and cocaine gave typical inverted U-shaped dose-effect functions, but methylone did not. Microdialysis findings revealed that MDPV produces selective increases in dialysate dopamine while methylone increases dialysate dopamine and 5-HT, with larger effects on 5-HT. Collectively, the present findings support the hypothesis that elevations in central 5-HT can dampen reinforcing effects mediated by mesolimbic dopamine.
In the present study, we noted robust self-administration of MDPV from the very beginning of training. High rates of drug intake were observed with clear discrimination between active and inactive responses by day 2 of training. The MDPV dose-effect function was typical of psychomotor stimulants, with response rates peaking at 0.01 mg/kg/inj. At higher doses, response rates decreased while intake increased only slightly. The results shown here confirm previous reports of avid self-administration of MDPV in male rats (Aarde et al., 2013, 2015; Watterson et al., 2014). In terms of absolute response rates, we found that MDPV maintained rates comparable to cocaine (40 infusions per 2 h session), although MDPV was about 10-times more potent than cocaine. The high rates of infusions for MDPV observed here are similar to those reported by Aarde et al. (2013), but less than those reported by Watterson et al. (2014) who found rates around 60 infusions per 2 h session for an MDPV training dose of 0.05 mg/kg/inj. We demonstrate here that rats tend to regulate their intake of MDPV and cocaine, with intake for both drugs not differing significantly at the 3 highest doses tested. The present inverted U-shaped dose-effect functions with MDPV and cocaine are consistent with our previous results using cocaine as a training drug in rats (Panlilio et al., 2003). When saline was substituted for drug, rates of responding for both MDPV and cocaine decreased, and the discrimination between the active and inactive responses was lost. One potential caveat in our data set is that cocaine-trained rats exhibited many more inactive responses when compared to MDPV-trained rats. A close inspection of the results showed that variability for inactive responses in cocaine-trained rats was due primarily to certain rats that showed high levels of inactive responses on individual days of training, particularly day 2. We observed that inactive responses were rare among the rats responding for MDPV after the first day of training.
In contrast to MDPV and cocaine, we found that responding for methylone was low initially, with little discrimination between active and inactive responses. Responding did slowly increase over the course of several days, and discrimination between active and inactive responses eventually emerged. However, even when the dose of methylone was increased from 0.3 to 0.5 mg/kg/inj, and additional days of training were allowed, responding remained low relative to MDPV and cocaine. When saline was substituted for methylone, responding immediately decreased and the discrimination between the responses was lost. In general, less responding was seen for methylone in the present experiments (i.e., 20 infusions per 2 h session) than in a previous study using methylone at comparable training doses (i.e., up to 80 infusions per 2 h session) (Watterson et al., 2012). The reason for this discrepancy is not clear since both studies used the same male rat strain, trained rats during the dark phase of the light cycle (i.e., active phase), omitted food pre-training, and had similar schedule parameters. Watterson et al. (2012) used lever press responses in their study, rather than the nose-poke responses used in our study, but nose-poke responding typically facilitates, rather than suppresses, training. Watterson et al. did find that methylone was not as effective in maintaining responding as MDPV when compared across studies (Watterson et al., 2012, 2014). Furthermore, it should be noted that a recent study by Creehan et al. (2015) demonstrated that female rats acquire methylone self-administration in a slow and steady manner, over the course of two weeks of training, with response rates in the range of 20 infusions per 2 h session. Thus, the recent methylone data from female rats reported by Creehan et al. agree closely with what we found here in male rats. In our study, when the methylone dose was manipulated, little change in responding was observed, leading to large changes in total intake. Watterson et al. (2012) trained separate groups of rats at different doses of methylone rather than manipulating dose after initial training. They found clearer differences in responding between doses than observed here, even over a similar dose range. Nevertheless, while responding for methylone was low in our study, self-administration behavior was observed, just not to the same extent as that of MDPV and cocaine. With regard to potency, the present data agree with published self-administration studies in rats, which consistently find that methylone is about 10-times less potent than MDPV (Aarde et al., 2013, 2015; Watterson et al., 2012, 2014).
The microdialysis data reported here may provide clues to the neurochemical mechanisms underlying differences in the behavioral effects of MDPV versus methylone. However, it should be noted that microdialysis experiments were carried out during the light phase of the circadian cycle whereas self-administration experiments were carried out during the dark phase. The different timing for the neurochemical and behavioral studies represents a limitation to direct comparison of the results. We found that MDPV and methylone both produce rapid elevations in extracellular dopamine upon i.v. administration. In fact, the rise in dialysate dopamine produced by 0.1 mg/kg MDPV was nearly identical to the rise produced by 1.0 mg/kg methylone (i.e., 2-fold increase). On the other hand, MDPV had no discernable effect on extracellular 5-HT while methylone produced large increases in 5-HT that exceeded its effects on dopamine. The microdialysis data for MDPV reported here are the first to show this drug fails to affect extracellular concentrations of 5-HT in vivo at pharmacologically-relevant doses. The microdialysis data with methylone agree with our previous findings that used lower doses of i.v. administered drug (i.e., 0.1 and 0.3 mg/kg i.v.) to demonstrate that methylone and mephedrone exhibit MDMA-like neurochemical effects in male rats (Baumann et al., 2012). In the present study, MDPV and methylone both increased locomotor activity. However, MDPV induced much larger increases in hyperactivity than methylone, even though the drugs had similar effects on extracellular dopamine. Our previous microdialysis experiments have shown that elevations in extracellular dopamine in the nucleus accumbens are tightly correlated with the extent of locomotor activity produced by stimulant drugs (Baumann et al., 2008, 2011; Zolkowska et al. 2009). Thus, one interpretation of the current microdialysis and locomotor data is that elevations in extracellular 5-HT can reduce locomotor stimulant effects mediated by extracellular dopamine. Future studies could address this hypothesis by examining microdialysis and locomotor activity responses for other types of stimulant drugs in rodent models.
In summary, a direct comparison between MDPV and methylone demonstrated that MDPV supports robust self-administration in rats. Although methylone also supported self-administration, this drug is not as potent or efficacious as MDPV, maintaining lower rates of intake and not showing a clear dose-effect function. Based on the findings presented here, it is tempting to speculate that elevations in extracellular 5-HT produced by methylone can reduce its reinforcing effects in rats, but this view may be an oversimplification. The recent article by Creehan et al. (2015) is noteworthy because it compared the self-administration of MDMA, methylone and mephedrone in female rats. These three “empathogen” drugs are non-selective transporter substrates that increase the release of dopamine, norepinephrine and 5-HT in vitro (Baumann et al. 2012; Eshleman et al., 2013; Simmler et al, 2013). Importantly, the 5-HT-releasing ability of these drugs is more prevalent than their effects on dopamine in vivo (Baumann et al., 2008, 2012; Kehr et al., 2011; Wright et al., 2012). Despite the shared mechanism of action among these drugs, Creehan et al. (2015) showed that mephedrone displays significantly greater reinforcing effects than either methylone or MDMA. Such findings indicate that other factors in addition to 5-HT release, including drug pharmacokinetics, effects on noradrenergic systems, or non-transporter sites of action, must influence the self-administration behavior of MDMA-like drugs. Finally, our self-administration data from rats are consistent with the known misuse of MDPV and methylone in humans (e.g., Johnson and Johnson, 2014), and suggest that MDPV is more apt to be abused in a compulsive manner due to its potent and selective dopaminergic effects.
Acknowledgments
This research was supported by the Intramural Research Program of NIH, NIDA.
Footnotes
The authors have no conflicts of interest to report.
We dedicate this paper to our colleague and friend Steven R. Goldberg who passed away suddenly on November 25, 2014.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–40. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2015;232:1867–77. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–62. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH. Awash in a sea of ‘bath salts’: implications for biomedical research and public health. Addiction. 2014;109:1577–9. doi: 10.1111/add.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–17. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–25. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte G, Schenk S. Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addict Biol. 2014;19:874–84. doi: 10.1111/adb.12069. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168:1750–7. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–7. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2007;189:425–34. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76:65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration. Establishment of drug codes for 26 substances. Final rule. Fed Regist. 2013;78:664–666. [PubMed] [Google Scholar]
- Drug Enforcement Administration. National Forensic Laboratory Information System Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2010–2013. U.S. Drug Enforcement Administration; Springfield, VA: 2014. https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS_SR_CathCan_508.pdf. [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–15. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–73. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs. 2014;46:369–78. doi: 10.1080/02791072.2014.962717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–58. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci. 2013;58:1654–9. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacology. 2015;40:436–45. doi: 10.1038/npp.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Abad S, Pubill D, Camarasa J, Escubedo E. Repeated doses of methylone, a new drug of abuse, induce changes in serotonin and dopamine systems in the mouse. Psychopharmacology (Berl) 2014;231:3119–29. doi: 10.1007/s00213-014-3493-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–20. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Serotonergic impairment and memory deficits in adolescent rats after binge exposure of methylone. J Psychopharmacol. 2014;28:1053–63. doi: 10.1177/0269881114548439. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–13. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology (Berl) 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC. MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci. 2007;26:3229–36. doi: 10.1111/j.1460-9568.2007.05932.x. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Cogan ES, Thorndike EB, Panlilio LV. Rapid delivery of cocaine facilitates acquisition of self-administration in rats: an effect masked by paired stimuli. Pharmacol Biochem Behav. 2011;99:301–6. doi: 10.1016/j.pbb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233:416–22. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J Addict Res Ther. 2012;S9:002. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–43. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Technical Report Series no. 991. Geneva, Switzerland: 2015. WHO Expert Committee on Drug Dependence: thirty-sixth report. http://apps.who.int/iris/bitstream/10665/153834/1/WHO_TRS_991_eng.pdf?ua=1. [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TH, Cline-Parhamovich K, Lajoie D, Parsons L, Dunn M, Ferslew KE. Deaths involving methylenedioxypyrovalerone (MDPV) in Upper East Tennessee. J Forensic Sci. 2013;58:1558–62. doi: 10.1111/1556-4029.12260. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–46. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]