SUMMARY
To survive in the periodontal pocket, Porphyromonas gingivalis, the main causative agent of periodontal disease, must overcome oxidative and nitric oxide (NO) stress. Previously, we reported that, in the presence of NO comparable to stress conditions, the transcriptome of P. gingivalis was differentially expressed, and genes belonging to the PG1178-81 cluster were significantly up regulated. To further evaluate their role(s) in NO stress resistance, these genes were inactivated by allelic exchange mutagenesis. Isogenic mutants P. gingivalis FLL460 (ΔPG1181::ermF) and FLL461 (ΔPG1178-81::ermF) were black-pigmented, with gingipain and hemolytic activities comparable to that of the wild-type strain. Whereas the recovery of these isogenic mutants from NO stress was comparable to the wild-type, there was increased sensitivity to hydrogen peroxide-induced stress. RNA-Seq analysis under conditions of NO stress showed that approximately 5 and 8 percent of the genome was modulated in P. gingivalis FLL460 and FLL461 respectively. The PG1178-81 gene cluster was shown to be part of the same transcriptional unit and is inducible in response to NO stress. In the presence of NO, PG1181, a putative transcriptional regulator, was shown to bind to its own promoter region and that of several other NO responsive genes including PG1181, PG0214 an extracytoplasmic function sigma factor, PG0893 and PG1236. Taken together, the data suggest that PG1181 is an NO responsive transcriptional regulator that may play an important role in the NO stress resistance regulatory network in P. gingivalis.
Keywords: nitric oxide, stress resistance, transcription regulation
INTRODUCTION
Periodontal disease is a chronic infection-induced inflammation condition that can result in the destruction of the tooth supporting tissues (Champagne et al., 2003;Martin et al., 2009). Porphyromonas gingivalis, one of the major Gram-negative etiologic agent of periodontal disease, must overcome the oxidative and nitric oxide (NO) stress environment of the periodontal pocket to maintain colonization, growth and survival. Adaptability and resistance to the diverse stress conditions in the periodontal pocket would require elaborate mechanisms that can quickly be switched on and off, depending on the composition of the environment (Henry et al., 2012). Previous studies showed that P. gingivalis altered its transcriptome when exposed to NO and hydrogen peroxide stress (Boutrin et al., 2012;McKenzie et al., 2012).
The NO detoxification pathway in P. gingivalis appears to comprise the non-enzymatic conversion of NO to hydroxylamine, a toxic nitrogen species, followed by its reduction to ammonia through the action of the hybrid cluster protein (HCP), a 4Fe-4S cluster binding oxidoreductase/ hydroxylamine reductase enzyme (Boutrin et al., 2012;Rodionov et al., 2005). HCP is induced by nitrite and NO, regulated by a FNR-like regulator designated as HcpR, and is important for NO stress resistance in P. gingivalis (Boutrin et al., 2012;Lewis et al., 2012).
In bacteria, the response to NO stress often includes a class of transcriptional regulators that can either act as primary (e.g. NorR, NsrR, NssR) or secondary (e.g. FNR) NO sensors. The primary sensors first detect NO and then regulate the expression of genes that encode for enzymes with NO as a substrate (Bowman et al., 2011). In P. gingivalis, our previous studies revealed that under NO stress, several genes in the PG1181-1178 cluster were among the most up regulated genes (Boutrin et al., 2012). While most of the genes in this cluster are annotated as hypothetical, PG1181 is a putative TetR transcription regulator (www.ncbi.nlm.nih.gov/nuccore/NC_002950). In other bacterial systems, TetR regulators have been reported to play an important role in environmental stress responses including multidrug resistance, biofilm formation, and virulence (Cuthbertson and Nodwell, 2013). In this study, we have further investigated the role of PG1181 in P. gingivalis. The data suggest that in the presence of NO, PG1181 can bind the promoter region of several NO responsive genes that may be important for survival under these conditions.
MATERIAL AND METHODS
Bacteria strains and culture conditions
The strains used in this study are listed in Table 1. P. gingivalis strains were grown in Brain-Heart Infusion (BHI) broth supplemented with 0.5% yeast extract (Difco Laboratories, Detroit, MI), hemin (5μg mL−1), vitamin K (0.5μg mL−1), and cysteine (0.1%; Sigma-Aldrich, St. Louis, MO). P. gingivalis strains were cultured in an anaerobic chamber (Coy Manufacturing, Ann Arbor, MI) in 10% H2, 10% CO2, and 80% N2. E. coli strains were grown in Luria-Bertani broth (LB). Unless otherwise stated, all cultures were incubated at 37°C. Growth rates were determined spectrophotometrically at OD600nm (OD600). Erythromycin, tetracycline and ampicillin concentrations used were 10μg mL−1, 3μg mL−1 and 100μg mL−1 respectively. Hemolysis was determined by growth of the P. gingivalis cells on Brucella Blood agar and incubated anaerobically at 37°C for 7 days.
Table 1.
List of plasmids and strains used in this study
| Plasmids | Strains | Phenotype, genotype, or description | Source of reference |
|---|---|---|---|
| pVA2198 | Spr, ermF-ermAM | (9) | |
| pT-COW | Apr, tetQ | (12) | |
| pFLL460a | Apr, tetQ, PG1181∷Tc | This study | |
| pFLL460b | Apr | This study | |
| pEXP-5-NT/TOPO® | Apr | Invitrogen | |
| Porphyromonas gingivalis W83 | Wild type | (9) | |
| Porphyromonas gingivalis | PG1181-defective mutant | This study | |
| FLL460 | |||
| Complemented Porphyromonas gingivalis FLL460c | Complemented PG1181-defective mutant | This study | |
| Porphyromonas gingivalis FLL461 | PG1181-78-defective mutant | This study | |
| Escherichia coli DH5α | F- φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rk−, mk+) phoA supE44 λ− thi− 1 gyrA96 relA1 | Invitrogen | |
| Escherichia coli TOP10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen | |
| Escherichia coli BL21AI | F- ompT hsdSB (rB-mB-) gal dcm araB∷T7RNAPtetA | Invitrogen |
Gingipain activity
The activity of Arg-X and Lys-X-specific cysteine protease (Rgp and Kgp) activity was determined using BAPNA (Nα-benzoyl-DL-arginine-p-nitroanilide; 4mM) and ALNA (AC-Lys-p-nitroanilide HCl; 4mM) as substrates in an activated protease buffer (0.1M Tris, pH 7.6, 0.2M NaCl, 5mMCaCl2, 10mM NaOH, 9mM L-Cysteine). Substrates were individually added to P. gingivalis culture samples and the end point OD determined at 405nm using a microplate reader (Bio-Rad, CA).
Sensitivity to nitric oxide (NO) and hydrogen peroxide (H2O2)
Fresh cultures of P. gingivalis strains were grown from overnight cell cultures. P. gingivalis strains were grown to early exponential phase (OD600:~0.3) in BHI broth. Stress agent (Diethylamine (DEA) NONOate: 15μL, 24mM stock concentration; H2O2: 10μL, 0.25mM final concentration) was added to the cultures and further incubated at 37°C anaerobically. The OD600 was measured at various intervals (1–6h) for 24h. Untreated wild type and mutant strains cell cultures grown under the same conditions were used as controls.
RNA-Sequencing (RNA-Seq) library construction and sequencing
The library was generated using RiboMinus Transcriptome Isolation Kit (Life Technologies, CA) and NEXTflex RNA-Seq Kit (Bioo Scientific, TX) following the vendors' protocols. Briefly, 2 μg total RNA (20 μl) was hybridized with 4 μl of RiboMinus probe in 100 μl hybridization buffer at 37 °C for 5 min, which was then cooled on ice for at least 30 seconds. The sample was transferred into an Eppendorf tube containing RiboMinus Beads resuspended in 100 μl hybridization buffer, which was incubated at 37 °C for 15 min to allow beads to bind with probes. The tube was placed on a magnetic separator for 1 min to pellet the rRNA-probe complex. The supernatant containing RiboMinus RNA was transferred into a new tube. The RiboMinus RNA was concentrated by ethanol precipitation and was resuspended in 14 μl nuclease free water. Five μl of NEXTflex RNA Fragmentation buffer (Bioo Scientific, TX) was mixed with the 14 μl RiboMinus RNA. The mixture was incubated at 95 °C for 10 min and then chilled on ice immediately. The first and second strand cDNAs were synthesized following the Bioo Scientific protocol and the NEXTflex RNA-Seq barcode was ligated to double strand cDNA after the end repair and adenylation for multiplexing. The library was amplified by PCR. The quality of each RNA-Seq library was checked using Agilent Bioanaylzer and DNA 1000 Nano chip and the library was quantified using Qubit (Life Technology, CA). The libraries (20 samples) were loaded into a single lane of Illumina V3 flow cell for cluster generation and the sequencing was done on Illumina HiSeq 2000 with 100 × 100 BP of pair ends from 5' and 3'.
RNA-Seq data analysis
The quality of reads was checked using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were filtered by Trimmomatic-0.22 (http://www.usadellab.org/cms/index.php?page=trimmomatic), including removing: 1) reads with sequencing adapters; 2) the 5'-end 5 bases of each read; 3) reads with more than 20 bases of `N' nucleotides; 4) reads with low quality; and 5) reads with length less than 36 bases after trimmed. Trimmed reads were mapped to P. gingivalis W83 genome (NCBI accession ID: NC_002950) using Bowtie2 (http://bowtiebio.sourceforge.net/bowtie2/index.shtml). The mapped reads were then processed using Cufflinks (http://cufflinks.cbcb.umd.edu/) for transcript assembly. Read counts for each annotated gene in the P. gingivalis W83 genome assembly version ASM758v1 were calculated, normalized as reads per million mapped reads (RPM), and then transformed into log2 scale. To avoid infinite values, 1 count was added to each gene before log2 transformation. Differentially expressed genes (DEGs) were determined with a non-stringent p-value cutoff of ≤ 0.05 and a fold change (FC) ≥ 2 (both up regulated and down regulated) compared with wide type W83. The Gene Ontology (GO) functional clustering and pathway analyses were done using NCBI DAVID Bioinformatics Tool (http://david.abcc.ncifcrf.gov/home.jsp).
Real-Time PCR analysis
Total RNA samples were obtained from 15 min untreated and DEA NONOate-treated (as in NO sensitivity experiments) cultures using a total RNA isolation kit (Promega, WI). Additional DNase treatment was carried out using DNase (Ambion, Austin, TX). cDNA was synthesized using a Transcriptor High Fidelity cDNA Synthesis kit (Roche, IN) and processed as previously reported (Boutrin et al., 2012). Real-time PCR analysis was also used to study the expression of a selection of genes in the complemented FLL460 strain under similar stress conditions. The Real-Time primers used are listed in the Supplementary material. The absolute quantification method was used for data analysis and referenced to the 16s gene.
Determination of co-transcription for the PG1181-78 locus
RNA extraction and cDNA synthesis were carried out as described in the Real-Time PCR analysis section above. Purified cDNA samples were subsequently subjected to PCR using primers for PG1181, PG1180, PG1179, and PG1178 (Supplementary material), and gel electrophoresis used for analysis of transcripts.
Determination of co-transcription for the PG0214-18 locus
RNA extraction and cDNA synthesis were carried out as described in the Real-Time PCR analysis section above. Purified cDNA samples were subsequently subjected to PCR using primers for PG0214, PG0215, PG0216, PG0217, and PG0218 (Supplementary material), and gel electrophoresis used for analysis of transcripts.
Gene Ontology analysis
The Gene Ontology (GO) analysis was carried out based on DEGs using the DAVID functional annotation tool (Huang et al., 2007). DAVID Bioinformatic tool allowed the classification of a large DEG list into functional related gene groups and ranking of the importance of significant genes under our experimental conditions. Significantly differentially expressed gene list obtained for the experimental conditions were uploaded to DAVID using Gene Ontology biological process annotation category. Fisher Exact p <0.05 was set to be considered significantly enriched in the annotation categories.
SDS-PAGE and immunoblot analysis
SDS-PAGE was used to separate and analyze protein samples. Proteins from the SDS-PAGE were transferred to a nitrocellulose membrane (Schleicher & Schuell, NH) using a Semi-Dry Trans-blot apparatus (Bio-Rad, CA) at 15 V for 30 min. Poly-histidine containing blots were probed using anti-His mouse antibodies, and their presence subsequently revealed using anti-mouse antibodies, and the Western Lightning® Plus-ECL kit (PerkinElmer, MA) as outlined by the manufacturers.
Creation of P. gingivalis FLL460 (ΔPG1181∷ermF) and FLL461 (ΔPG1178-81∷ermF) mutants
The PG1181 and PG1178-81 genes were inactivated following the method of Dou et al. and as previously reported (Boutrin et al, 2012). The primers used are listed in the Supplementary material. The PCR program included one 5min cycle at 94°C, followed by 30 cycles of 30s at 94°C, 40s at 53°C and 49°C for PG1181 and PG1178-81 respectively, and 3min 10s at 68°C, with a 6min final extension at 68°C. The obtained fragment was electroporated in P. gingivalis as mentioned in Abaibou et al., (Abaibou et al., 2001) and the transformed cells plated on BHI agar with 10μg mL−1 of erythromycin. The plates were incubated for 7 days at 37°C. Colony PCR and DNA sequencing were used to confirm appropriate gene replacement in erythromycin resistant mutant colonies. RT-PCR was also performed on mutant RNA samples to confirm the absence of gene expression for PG1181 and PG1181-78.
Complementation of the P. gingivalis FLL460 (ΔPG1181∷ermF) mutant
A DNA fragment containing the PG1181 open reading frame and its upstream promoter region were amplified from P. gingivalis W83 chromosomal DNA using oligonucleotide primers engineered with BamH1 restriction sites (Supplementary material). The BamHI restriction site was created at the end of both primers to facilitate the subcloning of the PCR fragment. The BamHI-digested pT-COW (Gardner et al., 1996) and BamHI-digested PCR fragment were ligated together and used to transform E. coli DH5α cells. The purified recombined plasmids designated pFLL460a was used to transform the P. gingivalis FLL460 (ΔPG1181∷ermF) mutant by electroporation. The transformed cells were grown on BHI agar plates in the presence of erythromycin and tetracycline for 7–10 days at 37°C.
Cloning and over-expression of recombinant PG1181
The pEXP5-NT/TOPO expression vector (Invitrogen, Carlsbad, CA) encoding a N-terminal 6X histidine tag was used to generate the recombinant protein. Briefly, the 0.6kb PG1181 open reading frame was amplified from P. gingivalis chromosomal DNA using PG1181F and PG1181R primers (Supplementary material). The obtained fragment was cloned into TOP10 cells (Invitrogen, Carlsbad, CA) as per manufacturer's protocol, and successful transformants identified through antibiotic resistance on LB plates with 100μg/ml ampicillin. Recombinant plasmids isolated from the latter were screened for correct orientation using PCR and DNA sequence using DNA sequencing. A plasmid designated pFLL460b, with correct orientation and sequence, was chosen for expression of recombinant PG1181 in BL21 AI cells (Table 1; Invitrogen, Carlsbad, CA). After transformation of competent BL21 AI cells with pFLL460b, recombinant PG1181 was over-expressed as per manufacturer's protocol for toxic genes. Cell lysate and supernatant were analyzed for the presence and solubility of over-expressed protein using SDS-PAGE. After confirmation of protein solubility, cell lysate was obtained through harvesting of cells by centrifugation at 10,000 rpm, pellet re-suspension in Tris-HCl (pH7.4), 1mg/ml DNAse treatment and multiple French press passes in the presence of protease inhibitor (Roche, IN). Cellular debris was removed by centrifugation at 15,000 rpm and the supernatant kept for further experiments. Detection of poly-histidine recombinant protein was done using SDS-PAGE and immunoblot. Additional confirmation of the recombinant protein was done using mass spectrometry.
Purification of recombinant PG1181
Debris-free cell lysate from recombinant protein overexpression was passed through a HisPur™ Cobalt Spin column (Pierce, IL) for purification under native conditions as per manufacturer's protocol. Fractions of purified proteins were examined using SDS-PAGE, pooled and concentrated by buffer exchange dialysis with 10mM Tris-HCl (pH 7.4).
In-silico analysis of PG1181
The phylogenetic analysis of twenty nine annotated transcriptional regulators from the genome of P. gingivalis W83 was done using MEGA version 4.0 (Tamura et al., 2007). The phylogenetic distance was calculated using the Kimura 2 Parameter model (Kimura, 1980). For clustering, the Neighbor - joining method used bootstrap values based on 1000 replicates (Saitou and Nei, 1987). The modeling of the protein was performed using the Modeller software package (Eswar et al., 2007). The models were validated using the WHATIF program (Vriend, 1990).
Spectrum of optical absorption of PG1181
To investigate the absorption properties of PG1181, a wavelength scan of the purified protein preparation was done using the NanoDrop 2000 Spectrophotometer (Thermo Scientific, MA) according to the manufacturer's protocol. Iron sulfate (FeSO4) and Bovine Serum Albumin (BSA) preparations were used as controls.
Promoter prediction studies
Inter-regions (IR) preceding the genes of interest were studied for promoter region prediction using the BDGP Neural Network Promoter Prediction software (http://www.fruitfly.org/seq_tools/promoter.html). The score cutoff was set at 0.80.
Electrophoretic mobility shift assay (EMSA) studies
A selection of the modulated genes revealed by RNASeq was analyzed for promoter region prediction using the BDGP: Neural Network Promoter Prediction tool (http://www.fruitfly.org/seq_tools/promoter.html; minimum promoter score 0.8). The predicted promoter regions were amplified by PCR using their respective primers (Supplementary material). The resulting PCR fragments were purified and labeled with biotin using the Biotin 3' end DNA labeling kit (Thermo Scientific, MA). The EMSA assay was performed according to the manufacturer's guidelines (Thermo Scientific, MA). Briefly, 1 μg of purified recombinant PG1181 was mixed with 10 fmol of biotin-DNA, binding buffer (10 mM Tris, 50 mM KCl, 1 mM DTT; pH7.5), and incubated at room temperature for 30 min. The samples were resolved in 6% of polyacrylamide non-denaturing gel and analyzed using the Chemiluminescence kit (Thermo Scientific, MA). Vim A, a non DNA-binding protein from P. gingivalis, was used as a negative control.
RESULTS
Sensitivity of P. gingivalis FLL460 and FLL461 to NO stress
The PG1178-81 gene cluster in P. gingivalis was highly up regulated under NO stress (Boutrin et al., 2012). To further evaluate their functional significance, isogenic mutants defective in these genes were created. The isogenic mutants P. gingivalis FLL460 (ΔPG1181∷ermF) and FLL461 (ΔPG1178-81∷ermF) were black-pigmented and had growth rates, β hemolytic and gingipain activities comparable to that of the wild type. For both mutants, recovery from NO stress was comparable to the wild-type (Figure 1). Complementation of the mutant strain FLL460 further decreased its sensitivity to NO stress (Figure 1). There were several unsuccessful attempts to complement FLL461 which could be likely due to the large size of the DNA sequence between PG1178 and PG1181.
Figure 1. Sensitivity of FLL460 and FLL461 to NO stress.

P. gingivalis strains were grown to early exponential phase (OD600:~0.3) in BHI broth. Stress agent NO (0.36μmol) was added to the cultures and further incubated at 37°C anaerobically. The OD600 was measured at various intervals for 24h. Untreated wild type and mutant strains cell cultures grown under the same conditions were used as controls.
Sensitivity of P. gingivalis FLL460 and FLL461 isogenic mutants to hydrogen peroxide (H2O2)
The involvement of the proteins PG1178-81 in P. gingivalis oxidative stress resistance is unknown. Therefore, we estimated the significance of these proteins to oxidative stress by studying the sensitivity of their mutants to hydrogen peroxide stress. The mutants' sensitivity to hydrogen peroxide stress was similar for both strains and lower than that of the wild type (Figure 2).
Figure 2. Sensitivity of FLL460 and FLL461 to H2O2 stress.

P. gingivalis strains were grown to early exponential phase (OD600:~0.3) in BHI broth. Stress agent H2O2 (0.25mM) was added to the cultures and further incubated at 37°C anaerobically. The OD600 was measured at various intervals for 24h. Untreated wild type and mutant strains cell cultures grown under the same conditions were used as controls.
Transcriptome analysis of FLL460 and FLL461 P. gingivalis strains under NO stress
In order to further evaluate the role of PG1181 and the PG1178-81 cluster in NO stress resistance, we carried out a whole-transcriptome analysis by RNA-Seq. Under normal conditions, the deletion of the PG1181 gene caused a 58.6- and 3.5-fold up regulation respectively in the expression of the genes PG0893 and PG1236, and a down regulation in the gene expression of PG0214-18 and PG1269-70 in FLL460, when compared to the wild type. Under similar conditions, PG1858, PG1505, PG1556, PG0495, PG1019-20 and PG1857 were the most up regulated, while PG1178-80 and PG0214-8 were significantly down regulated in FLL461. Under NO stress, a similar group of genes was the most up regulated (e.g. PG0045, PG1208, PG1118, PG0010, PG0520-01, PG1775-79, PG1190, PG0195, PG0421 and PG1546) and down regulated (e.g. PG0214-18, PG0419, PG0914, PG1718 and PG1320) for both mutants in comparison to the wild-type. Transcriptome analysis of FLL460 under NO stress indicated that 5 percent of the genes were ≧ 2-fold up regulated and 8 percent of the genes were ≧ 2-fold down regulated. In FLL461 and under NO stress, 7 percent of the genes were ≧2-fold up regulated and more than fifteen percent of the genes were ≧ 2-fold down regulated. The patterns of gene expression were confirmed by Real-Time PCR (see Table 2).
Table 2.
Real-Time PCR analysis of selected genes modulated in NO transcriptome studies
| Gene ID | Average fold change^ |
|---|---|
|
| |
| PG0195 | 1.5 ± 0.4* (FLL460) |
| 16 ± 7 (FLL461) | |
|
| |
| PG0045 | 1.9 ± 0.1 (FLL460) |
| 7 ± 3.6 (FLL461) | |
|
| |
| PG1208 | 3 ± 1 (FLL460) |
| 3 ± 2(FLL461) | |
|
| |
| PG1236 | −20 ± 16 (FLL460) |
| −9 ± 2 (FLL461) | |
|
| |
| PG0214 | −154 ± 17 (FLL460) |
| −13 ± 3 (FLL461) | |
|
| |
| PG0215 | −40 ± 11 (FLL460) |
| −7 ± 0.5(FLL461) | |
|
| |
| PG0419 | −50 ± 0 (FLL460) |
| −13 ± 2 (FLL461) | |
|
| |
| PG0914 | −27 ± 13 (FLL460) |
| −15 ± 4 (FLL461) | |
NO stress vs. Control with P.gingivalis W83 vs. Listed mutant;
Standard deviation.
Mutants showed modulation in expression of genes mostly involved in DNA binding, metabolic process, recombination and repair, regulation of transcription, and transposition functions
Functional analysis of gene expression profiles in FLL460 showed that, under normal conditions, the mutation caused modulations mainly in the expression of genes involved in DNA binding, transcription, replication, recombination and repair, and transposase functions. Under NO stress, the mutation caused changes mostly in the expression of genes dealing with DNA binding, DNA metabolic process, DNA recombination and repair, and transposition functions. For FLL461, under normal conditions, the mutation resulted in the modulation in expression of genes mostly involved in export functions and thioredoxin-like proteins. Under NO stress, most of the genes with modulated expression had DNA binding, DNA recombination, regulation of transcription and transposase functions.
Transcriptome analysis of selected genes in FLL460, FLL461 and the complemented FLL460 P. gingivalis strains under NO stress
Since the RNASeq results suggested that PG1181 is involved in the regulation of PG0214 and PG1237, the transcriptome of FLL460, FLL461 and complemented FLL460 strains was further explored for the expression of other σ factor genes, such as PG1827, PG0162, PG0985, PG1318, PG1660, and a selection of genes previously reported to be involved in NO stress resistance. Alongside PG0214, all the other σ factors tested showed a decrease in gene expression in the PG1181 mutants compared to the complemented strain under NO stress conditions (Figure 4). Similarly, the expression of other genes shown to be involved in NO stress resistance, such as PG0893 and its regulator PG1053, and PG1237 were also lower in the PG1181 mutants than in the complemented strain, when compared to the wild type, under NO stress conditions (Figure 4). Additionally, PG1240, another TetR regulator found in P. gingivalis showed a comparable pattern of expression (Figure 4).
Figure 4. Transcriptome study for a selection of key genes in FLL460, FLL461 and the complemented FLL460 strains under NO stress.
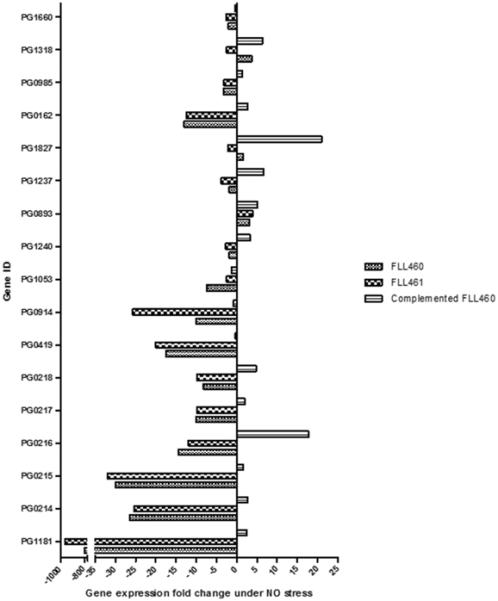
Fold changes in the expression of all P. gingivalis σ factors and a series of genes implicated in NO stress resistance were investigated in NO-stressed samples from the FLL460, FLL461 and complemented FLL460 strains. Genes involved in NO-stress response are PG1181, PG0419, PG0914, PG1053, PG1240, PG0893 and PG1237. Genes encoding sigma factors and sigma factors-related proteins are PG0214-8, PG1827, PG0162, PG0985, PG1318 and PG1660.
Transcription study for the gene cluster PG1178-81
Our previous study suggested that the expression of the genes belonging to the PG1181-78 cluster were similarly modulated under NO stress (Boutrin et al., 2012). The transcription pattern of these genes under normal and NO stress conditions was therefore investigated. PG1181, PG1180, PG1179, PG1178 and PG1178-81 were not expressed under normal conditions, but only under NO stress. Also, a full transcript was obtained for PG1178-81, but only under NO stress. Thus, these results indicate that the PG1178-81 genes may be co-expressed as part of an operon that is not expressed under normal conditions but is induced under NO stress.
Transcription study for the gene cluster PG0214-18
Since our results showed a common pattern in the modulation of the expression of the gene cluster PG0214-18, the cluster was investigated for possible co-transcription. All the genes from the cluster were expressed under normal conditions, and a full transcript was obtained for PG0214-18. These results suggest that the PG0214-18 genes are constitutively co-expressed as part of an operon.
In-silico analysis of PG1181
The phylogenetic analysis of 29 annotated transcriptional regulators from the genome of P. gingivalis W83 showed 9 related clusters (Figure 3a). Cluster 1 was made of putative general transcriptional regulators. Cluster 2 included PG1181, PG0857 a putative transcriptional regulator, PG0465 a ferric uptake transcriptional regulator, PG0148 a sigma 54- dependent transcriptional regulator, and PG1040 a putative transcriptional regulator. Cluster 3 enclosed AraC and LuxR families of transcriptional regulators. Cluster 4 included a MarR family transcription regulator and PG1053, the hcpR regulator involved in nitrosative stress resistance (Lewis et al., 2012). PG1240, a TetR family transcriptional regulator and PG0396, a Crp Fnr family regulator were found in Cluster 5. Clusters 6–9 involved the AraC, Crp, GntR and AsnC families of transcriptional regulators. However, these clusters did not show very close molecular relatedness to the Cluster 3 AraC family of transcriptional regulators (PG1447, PG0826). Also, Clusters 5 and 8 showed other annotated TetR family transcriptional regulators, PG1240 and PG1501 respectively. PG1181 showed close molecular relatedness to PG0857, a putative transcriptional regulator, followed by PG0465 (ferric uptake transcriptional regulator), PG0148 (Sigma 54 dependent transcriptional regulator), and PG1040 (putative transcriptional regulator). PG1181 and PG0465 possessed structural similarity of a helix-turn-helix transcriptional regulator motif and had DNA binding roles.
Figure 3a. Phylogenetic analysis of P. gingivalis W83.
The phylogenetic analysis of transcriptional regulators in P. gingivalis W83 showed 9 related clusters. The Bootstrap values are shown in nodes. PG1181 showed close molecular relatedness to PG0857, a putative transcriptional regulator, followed by PG0465 (ferric uptake transcriptional regulator), PG0148 (Sigma 54 dependent transcriptional regulator), and PG1040 (putative transcriptional regulator). The other two TetR transcriptional regulators showed divergence from PG1181 and clustered in 5 (PG1240) and 8 (PG1501).
Domain prediction showed a characteristic N terminal helix-turn-helix (HTH) motif panning between the amino acid positions 7 to 53. Protein modeling showed alpha helices spanning the majority of the protein's secondary structure. The HTH domain consisted of the characteristic tetra-helical model with four alpha helices (Figure 3b), which showed DNA-binding affinity. The orientation of the binding motif of the functional model protein showed an inverted orientation. Since TetR family regulators are often exclusively alpha helical, these results suggest PG1181 may be a TetR regulator (Cuthbertson and Nodwell, 2013).
Figure 3b. PG1181 structure.

HTH transcriptional regulator motif (yellow) spanning regions are found between amino acid positions 7 to 53. The domain contains a characteristic tetra-helical model (yellow).
Spectrum of optical absorption of PG1181
A wavelength scan of PG1181 was done to investigate the presence of an iron cluster, as iron clusters have been reported in other transcription regulators (Green et al., 2014). Similarly to BSA, PG1181 showed two peaks: one close to 200nm and another at 250–260nm.
Promoter prediction studies
The IR preceding the genes of interest were analyzed for detection of possible promoter region sequences using the BDGP Neural Network Promoter Prediction software. Two promoter regions were predicted for PG0195 IR, two for PG1181 IR, one for PG0914 IR, four for PG1118 IR, six for PG0045, two for PG0010, four for PG0214, three for PG1236, IR and one for PG0893 IR.
EMSA studies
The binding activity of rPG1181 with different predicted promoter regions was studied to determine the targets of the putative transcription regulator. The identity of rPG1181 was confirmed by protein size on SDS gel, mass spectrometry analysis and the presence of the His-tag. Specific retardation in DNA migration was found only for reactions containing NO-treated rPG1181 and the IRs predicted to contain promoter regions for the PG0214, PG0893, PG1181 and PG1236 genes. PG1236 IR studies considering the binding of rPG1181 to the predicted promoter region for PG1236-7 showed two sets of retardation bands, one of them only found in the reaction without rPG1181. As a control, similar experiments with the PG1236 IR and VimA, a non-DNA binding protein from P. gingivalis, showed one type of retardation bands similar to one of those obtained with rPG1181. Therefore, one of the sets of the retardation bands observed for the rPG1181-PG1236 IR interaction was due to non-specific binding to the DNA sequence, suggesting that the other set of retardation bands may be due to specific binding of rPG1181 to the PG1236 IR. Thus, our results suggest that, only under NO stress, rPG1181 can specifically bind to its own gene IR and that of PG0893, PG0214, and PG1236 to regulate the transcription of these genes.
DISCUSSION
Previous studies of the transcriptome of P. gingivalis W83 under NO stress revealed that the PG1178-81 genes were significantly up regulated under those conditions (Boutrin et al., 2012). In this study, we further explored the induction pattern of these genes under NO stress and found that the expression of these genes occurred only under these conditions. This further confirms their role in the NO stress response, which appears to be auto-regulated. While the P. gingivalis PG1181 gene was the most highly up regulated under conditions of NO stress, an isogenic mutant defective in this gene showed a similar sensitivity compared to the wild-type under those conditions. Because PG1181 is predicted to be a transcriptional regulator, this could indicate that the response of P. gingivalis to NO stress may involve a complex regulatory network with multiple pathways to facilitate the survival of the organism under those conditions. Transcriptome studies of the PG1181-defective mutant showed the up regulation of several genes (PG1236, PG1178-80 and PG1269-70), including the PG0893 gene encoding the hydroxylamine reductase protein (HCP), which is already shown to be implicated in NO stress resistance. In addition to a complex regulatory network facilitating survival, the up regulation of the above genes may have contributed to the recovery from NO stress (Boutrin et al., 2012).
The inactivation of the PG1181 gene and others in the PG1178-81 operon appears to be involved in the dysregulation of multiple environmental stress response genes. Its impact on the regulatory network of the organism is also demonstrated with its effect on the extracytoplasmic function sigma factor and other transcriptional regulators (PG1240 and PG1237), some of which are known to play a role in the response of P. gingivalis to environmental stress (Lewis et al., 2012;Wu et al., 2009;van Kessel et al., 2013;Tsai and Winans, 2010). Thus, it was not surprising that the isogenic mutants defective in the PG1178-81 transcriptional unit showed more resistance to oxidative stress compared to the parent strain. The genes most up regulated in both P. gingivalis FLL460 and FLL461 mutants included PG0045 (htpG), PG1208 (dnaK), PG1118 (clpB), PG0195 (rubrerythrin), PG0010 (clpC), PG1775 (grpE), PG1776 (dnaJ) and PG1777-9 (hypothetical). All these genes encode for proteins involved in protein repair and stability, often crucial for stress resistance. dnaK-J chaperones, clpB-C, htpG and grpE heat shock proteins have been shown to work as a molecular machinery actively involved in the disaggregation and reactivation of protein aggregates caused by stress (Mogk et al., 2003;Doyle and Wickner, 2009;Winkler et al., 2012). Rubrerythrins have been reported to offer an efficient antioxidant defense strategy by being involved in the reduction of superoxide, its related species and reactive nitrogen species (Henry et al., 2012;Mydel et al., 2006). PG1777 and PG1779 are currently classified as hypothetical proteins and their role not well established. However, a recent study from our laboratory has indicated that PG1777 may bind iron to further protect DNA from Fenton chemistry-mediated DNA damage, and could be part of the repair process of iron-sulfur clusters (McKenzie et. al, personal communication). The grpE-dnaJ-PG1777- PG1778-PG1779 genes were also thought to form an operon supporting the stress resistance functions of the DnaK-DnaJ-GrpE protein chaperone system (McKenzie et. al, personal communication). These stress-response proteins have been found to be involved in several forms of oxidative stress (Okano et al., 2006;Meuric et al., 2008;Henry et al., 2012;Boutrin et al., 2012).
The conserved sequences of PG1181 suggest it may be a member of the TetR/AcrR families of transcription regulators (http://www.ncbi.nlm.nih.gov). The TetR family of regulators usually binds their operator sequence composed of 10–30 bp palindromic sequences to repress the target genes and the de-repression occurs when the regulators bind their cognate ligands (Ramos et al., 2005;Willems et al., 2008;Yu et al., 2010). Similar to the typical of TetR regulator, PG1181also carries a HTH DNA-binding motif at its N-terminal site (Ramos et al., 2005). In these regulators, the N-terminal is involved with DNA-binding through the HTH domain, and the C-terminal is responsible for ligand-binding, as part of a signal recognition mechanism (Deng et al., 2013;Ramos et al., 2005). The TetR family proteins are believed to form dimers with a similar domain structure composed of 10 amino acid alpha helices per monomer (Yu et al., 2010). The function of the protein is mediated by conformational changes that increase or decrease the distance between the DNA recognition helices (Orth P et al., 2007). It is still unclear if PG1181 may function as a typical TetR-like regulator. Our results suggest that PG1181 binds to its own promoter region and those of other genes that were modulated during the exposure of P. gingivalis to NO stress. It is noteworthy that PG1181 displayed specific binding to the promoter region of these genes only in the presence of NO. This is in contrast to other TetR regulators that acquire derepressive function in the presence of the environmental signal (Deng et al., 2013;Tahlan et al., 2008;Yu et al., 2010;Ramos et al., 2005;Cuthbertson and Nodwell, 2013). Thus, it is likely that in PG1181, NO signaling at the C-terminal is needed for the protein to specifically bind to the target DNA sequence to facilitate gene expression. There are examples of reverse TetR regulators which are known to bind DNA more strongly in the presence of their ligand than in its absence (Yu et al., 2010). Due to the DNA-binding properties of PG1181, it is unclear if this putative transcriptional regulator may be recognized as part of this family. This is the subject of further investigation in the laboratory.
The expression profile for the PG1181 regulatory network including its ability to bind the promoter region of PG0214, PG0893 and PG1236 may imply that the regulation of the hydroxylamine reductase or HCP gene PG0893, which our previous report showed to be crucial for the process of NO detoxification in P. gingivalis, is not solely limited to the previously identified HcpR regulator (Boutrin et al., 2012;Lewis et al., 2012). Similarly, under NO stress conditions, the transcription of the LuxR regulator gene PG1237 seemingly involves PG1181, and the regulation of PG0214 may not be limited to its auto-regulation with the putative anti-sigma factor PG0215 (Lewis et al., 2012;Dou et al., 2010). These findings suggest that PG1181 is involved in NO detoxification, sensing and transcription regulation pathways, emphasizing the diversity of roles of TetR regulators, and the complexity of NO stress resistance strategies used by P. gingivalis. Also, since PG0214 was significantly down regulated in the absence of PG1181 we cannot rule out the possibility that it may also have transcription activator functions. Conversely, since PG0893 was up regulated in the absence of the protein, PG1181 may behave as a transcription repressor for the gene. To our knowledge, there has been no report of TetR regulators with sensing properties, and both positive and negative regulatory functions. The other reported primary NO sensors involved in NO resistance are NsrR, NorR, and NssR, which exert different mechanisms of sensing and activation. NsrR was found to belong to the Rrf2 family of transcription regulators, control the expression of genes important for growth during NO and nitrosative stress, and possess an iron-sulfur cluster and three conserved C-terminal Cys residues that act as cluster ligands. However, its mechanism of action remains unclear (Green et al., 2014;Tucker et al., 2008). NorR was shown to be a σ54-dependent transcriptional regulator, with a N-terminal GAF domain that contains a non-heme iron center that reversibly binds to NO, an AAA+ ATPase domain, and a C-terminal helix-turn-helix DNA-binding domain. Upon NO binding, the AAA+ domain becomes free to interact with the σ54 subunit of the RNA polymerase to activate the targeted gene transcription, usually norVW or hmp (Green et al., 2014;Bush et al., 2011;Bush et al., 2015). NssR was reported to be a regulator from the cyclic-AMP receptor protein (CRP) family, control the expression of a small operon including cgb and ctb globin-encoding genes, and contain one cysteine and tyrosine residues which may be involved in NO sensing (Green et al., 2014). According to our results, PG1181 does not seem to share any of the properties found for NssR, NorR and NssR, or possess any iron cluster, making its mechanisms of sensing and activation unclear. This is a novel finding that requires further studies to gain adequate understanding. A variety of ligand-induced mechanisms of function for TetR regulators have been reported, and based on our new findings, further studies are warranted to establish those pertinent to PG1181 (Ramos et al., 2005;Gu et al., 2008;Grkovic et al., 2003;Frenois et al., 2006;Su and Yu, 2007;Tahlan et al., 2008;Le et al., 2011;Muhl et al., 2009;Aleksandrov et al., 2008).
This study has confirmed the role of PG1181 as a TetR transcription regulator involved in NO stress resistance. Under NO stress, PG1181 appears to be directly involved in the transcription regulation of its own gene and that of genes associated with PG0214, PG0893, and PG1236 IRs. It may also be indirectly implicated in the regulation of several genes crucial to NO stress resistance, such as σ factors PG1827, PG0162, PG0985, PG1318, and PG1660, which are also associated with virulence factors. Further investigations on the entire molecular organization and DNA-binding mechanisms of this protein are, thus, warranted to fully understand its biochemical properties, and provide knowledge for the creation of pharmaceutical strategies against the development of periodontal disease.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Xiaojin Li for his help with the library for RNA-Seq, and Dr. Lawrence Sandberg for his help with the Mass Spectrometry analysis.
This work was supported by Public Health Services Grants R56DE13664, DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR (to H.M.F).
Reference List
- Abaibou H, Chen Z, Olango GJ, Liu Y, Edwards J, Fletcher HM. vimA gene downstream of recA is involved in virulence modulation in Porphyromonas gingivalis W83. Infect Immun. 2001;69:325–335. doi: 10.1128/IAI.69.1.325-335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov A, Schuldt L, Hinrichs W, Simonson T. Tet repressor induction by tetracycline: a molecular dynamics, continuum electrostatics, and crystallographic study. J Mol Biol. 2008;378:898–912. doi: 10.1016/j.jmb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Boutrin MC, Wang C, Aruni W, Li X, Fletcher HM. Nitric oxide stress resistance in Porphyromonas gingivalis is mediated by a putative hydroxylamine reductase. J Bacteriol. 2012;194:1582–1592. doi: 10.1128/JB.06457-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman LA, McLean S, Poole RK, Fukuto JM. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv Microb Physiol. 2011;59:135–219. doi: 10.1016/B978-0-12-387661-4.00006-9. [DOI] [PubMed] [Google Scholar]
- Bush M, Ghosh T, Sawicka M, Moal IH, Bates PA, Dixon R, Zhang X. The structural basis for enhancer-dependent assembly and activation of the AAA transcriptional activator NorR. Mol Microbiol. 2015;95:17–30. doi: 10.1111/mmi.12844. [DOI] [PubMed] [Google Scholar]
- Bush M, Ghosh T, Tucker N, Zhang X, Dixon R. Transcriptional regulation by the dedicated nitric oxide sensor, NorR: a route towards NO detoxification. Biochem Soc Trans. 2011;39:289–293. doi: 10.1042/BST0390289. [DOI] [PubMed] [Google Scholar]
- Champagne C, Buchanan W, Reddy M, Preisser J, Beck J, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontology 2000. 2003;31:167–180. doi: 10.1034/j.1600-0757.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- Cuthbertson L, Nodwell JR. The TetR family of regulators. Microbiol Mol Biol Rev. 2013;77:440–475. doi: 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Li C, Xie J. The underling mechanism of bacterial TetR/AcrR family transcriptional repressors. Cell Signal. 2013;25:1608–1613. doi: 10.1016/j.cellsig.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Dou Y, Osbourne D, McKenzie R, Fletcher HM. Involvement of extracytoplasmic function sigma factors in virulence regulation in Porphyromonas gingivalis W83. FEMS Microbiol Lett. 2010;312:24–32. doi: 10.1111/j.1574-6968.2010.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SM, Wickner S. Hsp104 and ClpB: protein disaggregating machines. Trends Biochem Sci. 2009;34:40–48. doi: 10.1016/j.tibs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, et al. Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci. 2007;Chapter 2(Unit) doi: 10.1002/0471140864.ps0209s50. [DOI] [PubMed] [Google Scholar]
- Frenois F, Baulard AR, Villeret V. Insights into mechanisms of induction and ligands recognition in the transcriptional repressor EthR from Mycobacterium tuberculosis. Tuberculosis (Edinb) 2006;86:110–114. doi: 10.1016/j.tube.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Russell JB, Wilson DB, Wang GR, Shoemaker NB. Use of a modified Bacteroides-Prevotella shuttle vector to transfer a reconstructed beta-1,4-D-endoglucanase gene into Bacteroides uniformis and Prevotella ruminicola B(1)4. Appl Environ Microbiol. 1996;62:196–202. doi: 10.1128/aem.62.1.196-202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Rolfe MD, Smith LJ. Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence. 2014;5:794–809. doi: 10.4161/viru.27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovic S, Hardie KM, Brown MH, Skurray RA. Interactions of the QacR multidrug-binding protein with structurally diverse ligands: implications for the evolution of the binding pocket. Biochemistry. 2003;42:15226–15236. doi: 10.1021/bi035447+. [DOI] [PubMed] [Google Scholar]
- Gu R, Li M, Su CC, Long F, Routh MD, Yang F, et al. Conformational change of the AcrR regulator reveals a possible mechanism of induction. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:584–588. doi: 10.1107/S1744309108016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LG, McKenzie RM, Robles A, Fletcher HM. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012;7:497–512. doi: 10.2217/fmb.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, et al. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Le TB, Stevenson CE, Fiedler HP, Maxwell A, Lawson DM, Buttner MJ. Structures of the TetR-like simocyclinone efflux pump repressor, SimR, and the mechanism of ligand-mediated derepression. J Mol Biol. 2011;408:40–56. doi: 10.1016/j.jmb.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Lewis JP, Yanamandra SS, naya-Bergman C. HcpR of Porphyromonas gingivalis is required for growth under nitrosative stress and survival within host cells. Infect Immun. 2012;80:3319–3331. doi: 10.1128/IAI.00561-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Page RC, Kaye EK, Hamed MT, Loeb CF. Periodontitis severity plus risk as a tooth loss predictor. J Periodontol. 2009;80:202–209. doi: 10.1902/jop.2009.080363. [DOI] [PubMed] [Google Scholar]
- McKenzie RM, Johnson NA, Aruni W, Dou Y, Masinde G, Fletcher HM. Differential response of Porphyromonas gingivalis to varying levels and duration of hydrogen peroxide-induced oxidative stress. Microbiology. 2012;158:2465–2479. doi: 10.1099/mic.0.056416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuric V, Gracieux P, Tamanai-Shacoori Z, Perez-Chaparro J, Bonnaure-Mallet M. Expression patterns of genes induced by oxidative stress in Porphyromonas gingivalis. Oral Microbiol Immunol. 2008;23:308–314. doi: 10.1111/j.1399-302X.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwülbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol. 2003;50:585–595. doi: 10.1046/j.1365-2958.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- Muhl D, Jessberger N, Hasselt K, Jardin C, Sticht H, Burkovski A. DNA binding by Corynebacterium glutamicum TetR-type transcription regulator AmtR. BMC Mol Biol. 2009;10:73. doi: 10.1186/1471-2199-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, III, et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano S, Shibata Y, Shiroza T, Abiko Y. Proteomics-based analysis of a counter-oxidative stress system in Porphyromonas gingivalis. Proteomics. 2006;6:251–258. doi: 10.1002/pmic.200401338. [DOI] [PubMed] [Google Scholar]
- Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat Struct Biol. 2007;7:215–219. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang X, et al. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Dubchak IL, Arkin AP, Alm EJ, Gelfand MS. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol. 2005;1:e55. doi: 10.1371/journal.pcbi.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Su CC, Yu EW. Ligand-transporter interaction in the AcrB multidrug efflux pump determined by fluorescence polarization assay. FEBS Lett. 2007;581:4972–4976. doi: 10.1016/j.febslet.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahlan K, Yu Z, Xu Y, Davidson AR, Nodwell JR. Ligand recognition by ActR, a TetR-like regulator of actinorhodin export. J Mol Biol. 2008;383:753–761. doi: 10.1016/j.jmb.2008.08.081. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tsai CS, Winans SC. LuxR-type quorum-sensing regulators that are detached from common scents. Mol Microbiol. 2010;77:1072–1082. doi: 10.1111/j.1365-2958.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Hicks MG, Clarke TA, Crack JC, Chandra G, Le Brun NE, et al. The transcriptional repressor protein NsrR senses nitric oxide directly via a [2Fe-2S] cluster. PLoS One. 2008;3:e3623. doi: 10.1371/journal.pone.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel JC, Ulrich LE, Zhulin IB, Bassler BL. Analysis of activator and repressor functions reveals the requirements for transcriptional control by LuxR, the master regulator of quorum sensing in Vibrio harveyi. MBio. 2013;4 doi: 10.1128/mBio.00378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend G. WHAT IF: a molecular modeling and drug design program. J Mol Graph. 1990;8:52–6. 29. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- Willems AR, Tahlan K, Taguchi T, Zhang K, Lee ZZ, Ichinose K, et al. Crystal structures of the Streptomyces coelicolor TetR-like protein ActR alone and in complex with actinorhodin or the actinorhodin biosynthetic precursor (S)-DNPA. J Mol Biol. 2008;376:1377–1387. doi: 10.1016/j.jmb.2007.12.061. [DOI] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A. Chaperone networks in protein disaggregation and prion propagation. J Struct Biol. 2012;179:152–160. doi: 10.1016/j.jsb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin X, Xie H. Regulation of hemin binding proteins by a novel transcriptional activator in Porphyromonas gingivalis. J Bacteriol. 2009;191:115–122. doi: 10.1128/JB.00841-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Reichheld SE, Savchenko A, Parkinson J, Davidson AR. A comprehensive analysis of structural and sequence conservation in the TetR family transcriptional regulators. J Mol Biol. 2010;400:847–864. doi: 10.1016/j.jmb.2010.05.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



