Abstract
Antibody-mediated lymphoablation is commonly used in solid organ and hematopoietic cell transplantation. However, these strategies fail to efficiently control pathogenic memory T cells and significantly improve long term transplant outcomes. Understanding the mechanisms of T cell reconstitution is critical for enhancing the efficacy of antibody-mediated depletion in sensitized recipients. Using a murine analog of anti-thymocyte globulin (mATG) in a mouse model of cardiac transplantation, we previously showed that peritransplant lymphocyte depletion induces rapid memory T cell proliferation and only modestly prolongs allograft survival. We now report that T cell repertoire following depletion is dominated by memory CD4 T cells. Additional depletion of these residual CD4 T cells severely impairs the recovery of memory CD8 T cells after mATG treatment. The CD4 T cell help during CD8 T cell recovery depends on the presence of B cells expressing CD40 and intact CD40/CD154 interactions. The requirement for CD4 T cell help is not limited to the use of mATG in heart allograft recipients, and is observed in non-transplanted mice and after CD8 T cell depletion with mAb instead of mATG. Most importantly, limiting helper signals increases the efficacy of mATG in controlling memory T cell expansion and significantly extends heart allograft survival in sensitized recipients. Our findings uncover the novel role for helper memory CD4 T cells during homeostatic CD8 T cell proliferation and open new avenues for optimizing lymphoablative therapies in allosensitized patients.
Introduction
Antibody-mediated lymphoablation is widely used in solid organ transplantation to improve graft function and survival, particularly in highly sensitized patients and patients receiving cadaveric donor and other marginal grafts (1, 2). While donor-reactive T cell memory is a primary reason for the use of induction therapies, memory T cells are more resistant to antibody-mediated depletion than naïve T cells, remain detectable in transplant patients treated with anti-thymocyte globulin (ATG) or anti-CD52 mAb (CAMPATH-1), and are associated with acute rejection episodes (3-6).
We have recently reported that pre-existing memory T cells are a predominant component of anti-donor immune responses in murine heart allograft recipients treated with a rabbit anti-mouse thymocyte globulin (mATG) (7). Peritransplant lymphocyte depletion was followed by rapid memory T cell proliferation and only modestly prolonged allograft survival. Thus, understanding the mechanisms driving the recovery of preexisting memory T cells is vital to improving the efficacy of lymphoablation in sensitized transplant patients.
Helper signals from CD4 T cells promote generation of effector CD8 T cells and are crucial for the generation and maintenance of functional memory CD8 T cells (8, 9). While different models of CD4 T cell/dendritic cell/CD8 T cell interactions have been proposed, they all postulate the central role for CD40/CD154 costimulatory pathway in facilitating CD4 T cell help during antigen-specific responses (8, 10). In addition, the observations made in HIV-infected individuals raise the possibility that the minimal threshold of CD4 T cell numbers is required to support homeostasis of CD8 T cells (11). Nevertheless, the role of CD4 helper T cells during CD8 T cell homeostatic expansion and survival in lymphopenic environment has not been previously addressed.
Previous studies distinguish two types of peripheral T cell homeostatic expansion observed in animal models of lymphopenia (12). Slow lymphopenia-induced proliferation (LIP) is observed when T cells are transferred into irradiated or anti-lymphocyte antibody treated wild type recipients. This type of T cell expansion is critically dependent on IL-7 and self peptide/MHC interactions, but does not require costimulation through CD28/CD80/CD86 or CD40/CD154 pathways (13-16). In contrast, T cell transfer into hosts intrinsically lacking T lymphocytes such as TCRβ−/−, RAG−/− or scid mice results in IL-7-independent fast LIP that is driven by foreign antigens from commensal microorganisms and requires CD28 costimulation (17, 18). Given the distinct mechanisms of LIP depending on the experimental conditions, it is possible that the reconstitution of endogenous T cells following in situ antibody-mediated depletion differs from transferring T cells from intact animals into irradiated, T cell depleted or genetically T cell deficient hosts. This distinction may have important clinical implications as antibody-mediated lymphoablation is commonly used as part of immunosuppression therapies in solid organ and bone marrow transplant recipients and in patients with autoimmune diseases.
Lymphocyte depletion studies using various polyclonal and monoclonal antibodies, including those by our group, revealed that: 1) memory T cells are more resistant to depletion compared to naïve T cells, 2) the depletion is more prominent in circulation than in lymphoid and non-lymphoid tissues; 3) the efficiency of CD4 T cell depletion is generally lower than that of CD8 T cells (4, 7, 19-22). The goal of the current study was to test the role of depletion-resistant memory CD4 T cells in reconstitution of host CD8 T cell repertoire. We report that CD4 T cell help and CD40/CD154 interactions are essential for CD8 T cell recovery following mATG treatment and that B cells are important mediators of this help. The requirement for CD4 T cell help is not limited to the use of mATG in heart allograft recipients, but is a common feature of homeostatic T cell reconstitution following antibody-mediated lymphoablation. Targeting help by residual memory CD4 T cells improves the efficacy of mATG in prolonging heart allograft survival in sensitized recipients.
Materials and Methods
Animals
Male and female C57BlL/6J (H-2b) [B6], BALB/cJ (H-2d) [BALB/c], SJL/J-Pde6brd1 (H2s), C3H/HeJ (H-2k), DBA/1J (H-2q), B6.129S2-Cd4tm1Mak/J (H-2b) [B6.CD4−/−], B6.129P2-Cd40tm1Kik/J (H-2b) [B6.CD40], and B6.129S2-Ighmtm1Cgn/j (H-2b) [B6.μMT] mice, aged 6-8 weeks, were purchased from The Jackson Laboratories (Bar Harbor, ME). Human CD20 transgenic mice (H2b) [B6.hCD20] were provided by Genentech (San Francisco, CA). All animals were maintained and bred in the pathogen-free facility at the Cleveland Clinic.
Heart transplantation
Vascularized heterotopic cardiac transplants were performed as previously described (23). Rejection was defined as a loss of palpable heartbeat and confirmed by laparotomy. Graft tissue sections were stained with H&E or with anti-CD3 and anti-C4d Ab for immunohistochemical analysis as previously described (24).
mATG preparation and recipient treatment
Rabbit anti-mouse thymocyte serum was generated by the Hybridoma Core at the Cleveland Clinic Research Institute by immunizing rabbits with C3H, DBA1 and SJL thymocytes. Total IgG (mATG) were isolated by a sequential ammonium sulfate precipitation. Total protein concentration was measured using BCA assay (ThermoScientific), and the purity was confirmed by SDS-PAGE. Heart allograft recipients were treated with mATG (1mg i.p.) or control rabbit IgG (Equitech-Bio Inc., Kerrville, TX) on days 0 and 4 posttransplant. Non-transplanted mice were injected with 1 mg mATG i.p. on days 0 and 4 of the experiment. For CD4 or CD8 T cell depletion, anti-mouse CD4 mAb (clones GK1.5 and YTS191, BioXCell, West Lebanon, NH) or anti-mouse CD8 mAb (clones TIB105 and YTS169, BioXCell) were injected i.p. on days −3, −2, −1 relative to mATG treatment, 200 μg each per animal per day. When indicated, the recipients were treated i.v. with 1 mg anti-CD154 Ab MR1 1 day prior to the surgery or with 0.1 mg agonistic anti-CD40 mAb FGK4.5 on days 0 and 1 posttransplant (both from BioXCell). For B cell depletion, B6.huCD20 mice were injected i.p. with 0.5 mg anti-human CD20 mAb (Rituximab, Biogen Idec Inc., and Genentech USA, Inc., South San Francisco, California) on d. 0, 1 and then every five days throughout the experiment. To neutralize TNFα, recipients were injected with anti-TNFα Ab (clone XT3.11, 0.5 mg i.v. on days −1 and 1).
Recipient sensitization
B6 mice were transplanted with full thickness BALB/c skin allografts and used four weeks later either as heart allograft recipients or for CD4 T cell isolation. CD4 T cells were purified using negative selection murine T cell isolation kit from STEMCELL Technologies (Vancouver, Canada). The equivalent of 5 × 106 CD4+CD44hi cells were injected i.v. into naïve B6 mice followed by BALB/c heart allograft transplantation.
Bone marrow chimeras
Bone marrow was isolated from femurs of wild type B6, B6.CD40−/− and μMT−/− mice. Female μMT−/− mice were irradiated (1100 rads) and injected i.v. with bone marrow cells so that each recipient received 5×106 μMT−/− cells plus 5×106 either wild type or CD40−/− cells. Four weeks later, the expression of CD40 on peripheral blood B220+ and B220− cells was evaluated by flow cytometry.
Adoptive transfer of B cells
B6.CD40−/− mice were transplanted with BALB/c heart allografts and injected with mATG on d.0 and 4 posttransplant. B220+ cells were isolated by flow sorting from spleens of naïve B6 mice (>98% purity) and intravenously injected into mATG treated B6.CD40−/− recipients on d. 5 posttransplant (1 day after last mATG injection). Groups of B6.CD40−/− recipients with or without B cell transfer were additionally injected with agonistic anti-CD40 mAb FGK4.5 (0.1 mg i.v. on d. 6 and 7 posttransplant).
Flow cytometry
Fluorochrome-conjugated Ab were purchased from BD Biosciences (San Diego, CA) or from eBioscience (San Diego, CA). Cells were isolated from peripheral blood, spleen, lymph nodes (mesentheric lymph nodes were processed and evaluated separately from the rest), bone marrow, thymus, lung and liver and stained as previously described (7, 25). At least 200,000 events/sample were acquired on a BD Bioscience LSRII followed by data analysis using FlowJo software (Tree Star Inc., Ashland, OR).
ELISPOT assays
IFNγ ELISPOT assays were performed as previously described using capture and detecting anti-mouse cytokine antibody from BD Biosciences (23, 26, 27). Recipient spleen cells were stimulated with mitomycin C-treated BALB/c or SJL spleen cells for 24 h. The resulting spots were analyzed using an ImmunoSpot Series 4 analyzer (Cellular Technology, Cleveland, OH).
RNA isolation and quantitative real-time PCR analysis
Spleen B cells were purified using negative selection kit from STEMCELL technologies (Vancouver, Canada). Total RNA was isolated from individual samples using TriZol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit, quantitative real-time PCR was done on a 7500 Fast Real-Time PCR System instrument using Taqman Fast Universal PCR Master Mix (2X), No AmpEraseUNG (all from Applied Biosystems, Foster City, CA). Probes and primers were from Taqman gene expression assay reagents (Applied Biosystems): H2-b1 (Mm04205967-gH), TNFα (Mm00443258_m1), CD80 (Mm00711660-m1), and CD86 (Mm00444543-m1). Data were normalized to Mrpl 32 (Mm00777741-sH) RNA amplification and calculated relative to the expression of the target gene in B220+ cells from naïve B6 mice.
Statistical analysis
Heart allograft survival was compared between groups by Kaplan-Meier analysis. Differences between groups during recall immune responses were analyzed using a non-parametric equivalent of one-way ANOVA, the Kruskal-Wallis test. When the overall p value was <0.05, pairwise comparisons were carried out using Mann-Whitney test. A value of p<0.05 was considered statistically significant.
Study approval
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Results
Effector/memory CD4 T cells dominate T cell repertoire following mATG depletion
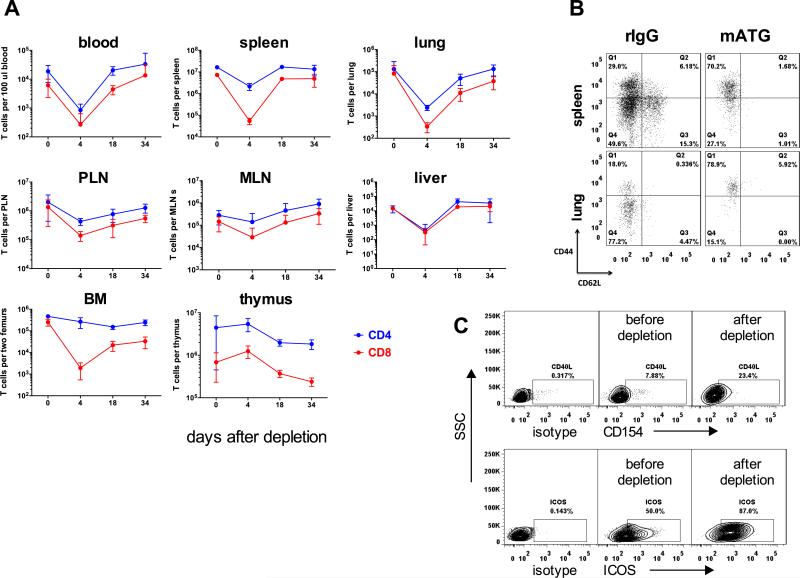
We initially investigated the kinetics of T cell depletion and recovery in naïve non-transplanted B6 mice. mATG treatment markedly decreased the numbers of CD8+ T and CD4+ T cells in circulation and within most analyzed lymphoid and non-lymphoid organs by day 4 after first injection (Figure 1). Consistent with previous reports, CD4+ and CD8+ T cells within the thymus were more resistant to depletion, with the significant reduction in thymocyte numbers achieved only by 10-15 days after first mATG injection (Figure 1A). Regardless of anatomical location, CD4+ cells were predominant subset immediately after depletion (95-99% of all T lymphocytes) and at all time points during gradual T cell recovery, up to one month after mATG treatment (Figure 1A). The depletion-resistant CD4 T cells were enriched for effector/memory CD44hiCD62Llo cells expressing high levels of CD154 and ICOS (Figure 1B and not shown).
Figure 1. Effector/memory CD4 T cells dominate T cell repertoire following mATG depletion.
Naïve C57BL/6 mice were injected with mATG or control rIgG (1.0 mg i.p. on days 0, 4) and sacrificed at indicated time points after depletion. A. The absolute numbers of CD4+ and CD8+ T cells in various anatomical compartments are presented as mean ±SD for 3-4 mice per group. The experiment was performed twice with similar results. B-C. The phenotype of residual CD4+ T cells on d. 4 after first mATG injection. Representative flow cytometry plots from two independent experiments (N = 3-4 mice per group) are shown after gating on CD4+ cells. The numbers indicate percentages of cells within gates.
CD8 T cell recovery following mATG depletion is dependent on CD4 T cell help
We have previously reported that despite causing massive T cell depletion, mATG failed to induce long-term allograft survival due to the rapid recovery of pre-existing memory T cells and the development of robust anti-donor T cell immune responses. Given the prominence of effector/memory CD4 T cells following mATG depletion, we investigated the role of these cells in the reconstitution of the T cell repertoire in B6 (H-2b) recipients of BALB/c (H-2d) heart allografts.
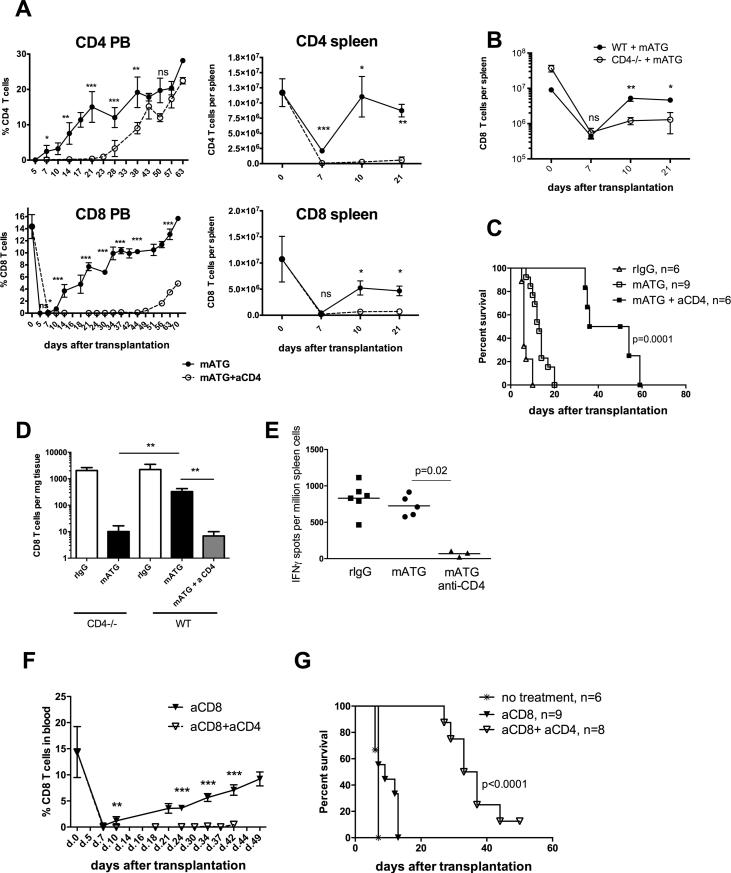
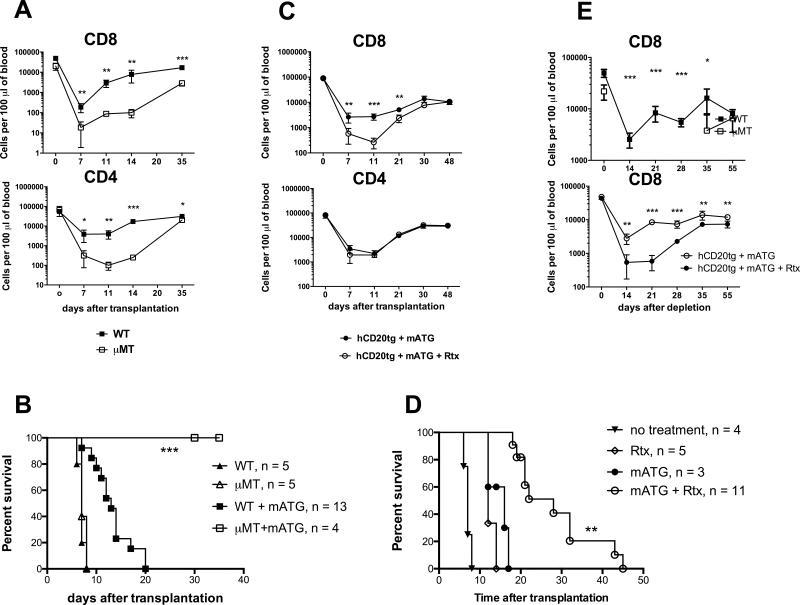
Additional depletion of CD4+ T cells with anti-CD4 mAb or the use of B6.CD4−/− recipients severely impaired the recovery of memory CD8+ T cells following mATG lymphoablation (Figure 2A-B). The delayed reconstitution of CD8 T cells was associated with prolongation of heart allograft survival (Figure 2C). At the time of rejection, the numbers of graft-infiltrating CD8 T cells and the priming of donor-reactive T cell in the spleen were significantly decreased by CD4 cell depletion (Figure 2D-E). The CD4 T cell-depleted recipients with prolonged allograft survival had high serum titers of donor-reactive IgG alloantibody and diffuse C4d deposition in the graft capillaries (not shown) suggesting that antibody and not T cells are the main effector mechanism of graft tissue injury.
Figure 2. CD8 T cell recovery following antibody-mediated depletion is dependent on CD4 T cell help.
A. B6 recipients of BALB/c heart allografts were treated with mATG (d. 0, 4) with or without additional CD4 T cell depletion (d. −3, −2, −1). T cell recovery was monitored in peripheral blood and in the spleen. B. Spleen CD8 T cell reconstitution in B6.CD4−/− mice transplanted with BALB/c heart allografts and treated with mATG. C. Kaplan-Meier plot represents heart allograft survival in recipients depicted in (A). D. Numbers of graft infiltrating CD8 T cells on day 10 posttransplant. Graphs represent mean ±SD in two independent experiments with total of 6-9 animals per group. E. Recipient spleen cells were tested in a recall IFNγ ELISPOT assay against donor BALB/c or third party SJL stimulator cells. The results are presented as frequencies of donor-reactive IFNγ secreting cells in individual recipients and are representative of two independent experiments with total N = 6-9 mice per group. F-G. B6 recipients of BALB/c heart allografts were treated with depleting anti-mouse CD8 mAb (d. −3, −2, −1) with or without CD4 T cell depletion and evaluated for CD8 T cell recovery (F) and heart allograft survival (G). Data are presented as mean ±SD, N = 6-9 animals per group. *P < 0.05; **P < 0.01; ***P < 0.001.
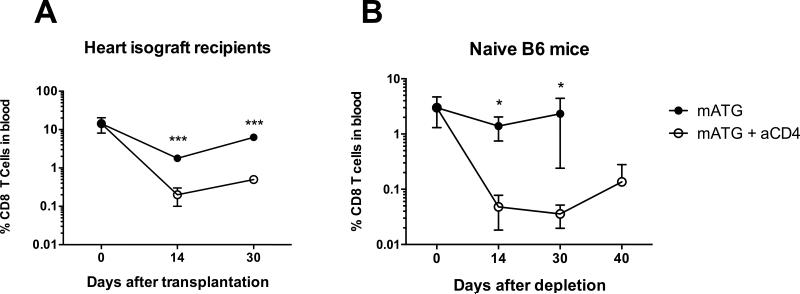
Similar to findings in mATG treated recipients, CD4 T cell depletion inhibited the CD8 T cell recovery and heart allograft rejection following treatment with anti-CD8 mAb (Figure 2F-G). Thus, the requirement for CD4 cell help is not limited to mATG depletion but is a common feature of antibody-mediated lymphoablation. In addition, memory CD8 T cell reconstitution was dependent on CD4 T cell help in B6 isograft recipients and in non-transplanted B6 mice treated with mATG (Figure 3). These results indicate that CD4 T cell help is required for homeostatic proliferation of memory CD8 T cells following antibody-mediated lymphoablation.
Figure 3. CD4 T cell help is required for homeostatic proliferation of CD8 T cells.
B6 heart isograft recipients (A) or naïve non-transplanted B6 mice (B) were treated with mATG with or without CD4 T cell depletion and monitored for CD8 T cell recovery. Percentages of CD8+ T cells in peripheral blood are presented as mean ±SD with N = 3-4 animals per group.
CD40/CD154 signaling is necessary and sufficient for CD8 T cell recovery following antibody-mediated depletion
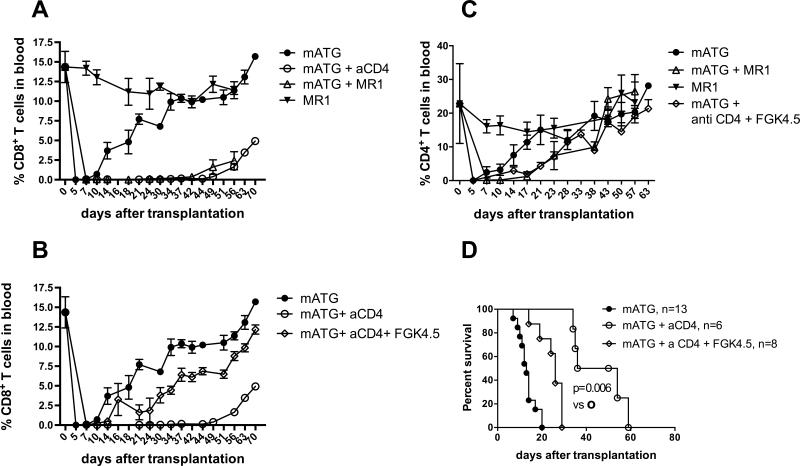
As CD40/CD154 interactions are central for CD4+ T cell help and 20-25% of depletion-resistant memory CD4 T cells express CD154 (Figure 1C), we tested the role of this pathway during homeostatic recovery of memory CD8+ T cell in mATG treated allograft recipients. Treatment with blocking anti-CD154 mAb MR1 in addition to mATG inhibited memory CD8 T cell reconstitution in B6 heart allograft recipients to the levels observed in CD4 T cell-depleted mice (Figure 4A). Conversely, the injection of agonistic anti-CD40 mAb FGK4.5 (100 μg i.v. on d. 0 and 1) restored expansion of memory CD8 T cells and accelerated heart allograft rejection following mATG treatment of CD4 T cell depleted heart allograft recipients (Figure 4B and 4D). Thus, memory CD4 T cell help necessary for CD8 T cell recovery following lymphoablation requires intact CD40/CD154 signaling pathway. The kinetics of CD4 T cell reconstitution was not significantly altered by CD154 blockade or by the injection of agonistic anti-CD40 mAb (Figure 4C).
Figure 4. CD40/CD154 signaling is necessary and sufficient for CD8 T cell recovery following antibody-mediated depletion.
A. B6 recipients of BALB/c heart allografts were treated with mATG with or without anti-CD154 mAb (clone MR1, 1 mg i.v. on day −1). The percentages of CD8+ T cell were evaluated in peripheral blood at indicated time points. Data are presented as mean ±SD, N = 5 mice/group. P values are shown for comparisons between mATG alone (closed circles) versus mATG + anti-CD154 mAb (open circles) treated groups. *P < 0.05; **P < 0.01; ***P < 0.001. B. B6 recipients of BALB/c heart allografts were depleted of CD4 T cells and treated with mATG with or without the injection of agonistic anti-CD40 mAb (clone FKG4.5; 100 μg i.v. on days 0, 1). The percentages of CD8+ T cells in peripheral blood are presented as mean ±SD, N =5-8 mice per group. P values are shown for comparisons between mATG + anti-CD mAb (open circles) versus mATG + anti-CD4 mAb + agonistic anti-CD40 mAb (open diamonds) treated groups. *P < 0.05; **P < 0.01; ***P < 0.001. C. The percentages of CD4+ T cells in peripheral blood of recipients shown in panels A and B are presented as mean ±SD. N = 5-8 mice per group. D. Kaplan-Meier survival curves represent heart allograft survival in recipients depleted of CD4 T cells and treated with mATG with or without the injection of agonistic anti-CD40 mAb. N = 8-11 mice per group.
B cells expressing CD40 are required for rapid homeostatic recovery of CD8 T cells
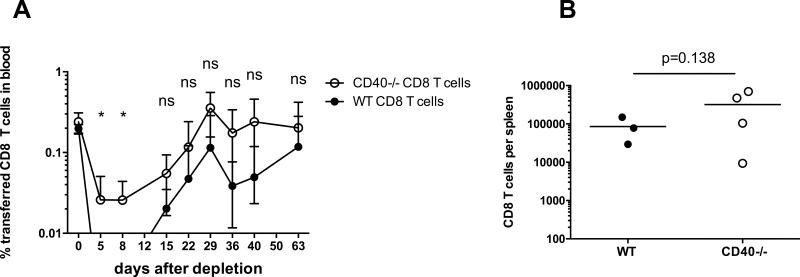
Next we investigated the identity of the CD40 expressing cells receiving cognate help from residual memory CD4 T cells. Activated CD8 T cells were previously shown to express CD40 and to receive helper signals directly from CD4 T cells (28). To test the possibility of direct CD40/CD154 interactions between CD4 and CD8 T lymphocytes in our model, we co-transferred tracer populations of congenic CD40−/− and WT CD8 T cells into B6 mice followed by BALB/c heart transplantation and mATG treatment. CD40−/− and WT CD8 T cells injected into the same recipients recovered equally well (Figure 5). These results suggest that CD4 T cell help for memory CD8 T cell recovery is mediated by a distinct population of CD40+ cells, with B cells, dendritic cells (DCs) and macrophages being the most likely candidates.
Figure 5. CD40 expression on CD8 T cells is not required for efficient CD8 T cell recovery after mATG depletion.
CD8+ T cells from B6.CD40+/+.Thy1.1 and B6.CD40−/− CD45.2+ mice were mixed at 1:1 ratio and intravenously injected into congenic B6.CD45.1.Thy1.2 mice (8 × 106 of each cell type/mouse) followed by BALB/c heart allograft placement and mATG treatment. A. The percentages of tracer CD8 T cell populations (Thy1.1+CD45.2+ WT and Thy1.1−CD45.2+ CD40−/−) within total live peripheral blood cells were determined by flow cytometry and presented as mean ± SEM, N = 4 mice per group. B. The numbers of transferred CD8 T cells in the spleens of individual recipients and group means were determined on day 63 after mATG treatment.
We initially performed mATG treatment of B cell-deficient μMT recipients of cardiac allografts. The lack of B cells lead to significant delay in the reconstitution of both CD8 and CD4 T cells and significantly prolonged heart allograft survival compared to wild type mice (Figure 6A-B). To avoid possible artifacts associated with the use of μMT mice, we used B6 mice expressing huCD20tg under B220 promoter (B6.huCD20tg) as heart allograft recipients. In these mice, the treatment with anti-human CD20 mAb (Rituximab) depleted >80% of circulating B cells and >70% of spleen B cells ((29-31) and data not shown) and markedly inhibited the expansion of recipient memory CD8, but not CD4 T cells at days 7, 11 and 21 post-transplant compared to non-depleted recipients (Figure 6B). The delayed CD8 T cell recovery was associated with prolonged heart allograft survival in B6.huCD20tg recipients treated with mATG + Rituximab compared to mATG or Rituximab monotherapies (MST of 28 d. vs 16 and 12 d. respectively, p < 0.001, Figure 6D). Despite ongoing administration of Rituximab, the numbers of peripheral CD8 T cells were comparable in B cell depleted and non-depleted recipients at 30 days posttransplant and thereafter (Figure 6C), and all recipients rejected their heart allografts by d. 45 (Figure 6D). Importantly, B cell deficiency or B cell depletion resulted in significant inhibition of CD8 T cell expansion in naïve, non-transplanted mice, demonstrating the critical role for B cells during homeostatic recovery of CD8 lymphocytes (Figure 6C).
Figure 6. B cell deficiency or depletion compromises homeostatic recovery of CD8 T cells.
A-B. B6.WT or B6.μMT mice were transplanted with BALB/c heart allografts and treated with mATG. Numbers of peripheral blood T cells (A) and heart allograft survival (B) were evaluated in 4-13 mice per group. *** p < 0.0001 for WT and μMT recipients treated with mATG. C-D. B6.huCD20tg mice were transplanted with BALB/c heart allografts, injected with mATG (days 0, 4) and either treated with Rituximab (Rtx, 0.5 mg i.p. every 5 days starting on day 0) or left untreated. Numbers of peripheral blood T cells (C) and heart allograft survival (D) were evaluated in 3-11 mice per group. ** p < 0.001 for recipients treated with mATG alone vs mATG + Rtx. E. mATG depletion was performed in naïve non-transplanted B6.WT and B6.mMT mice (top) or in naïve non-transplanted B6.huCD20tg mice with or without Rtx treatment (bottom).
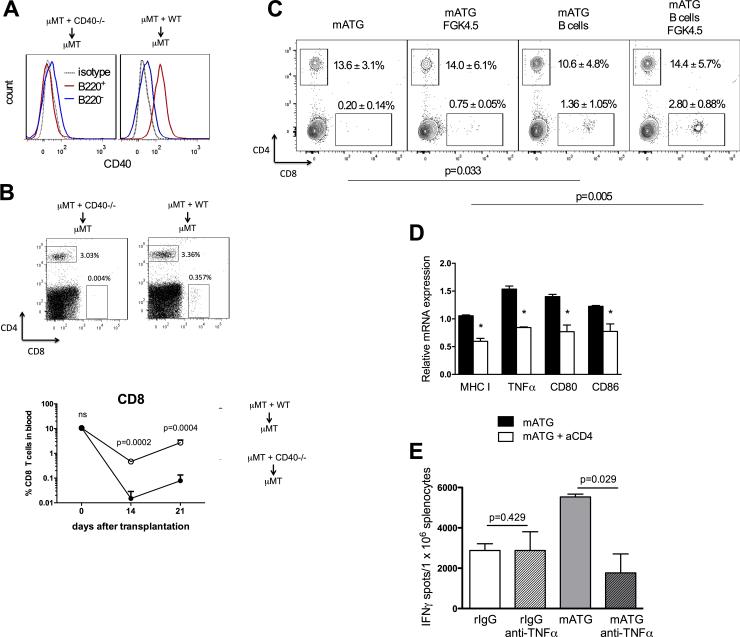
To test whether B cells require CD40 expression to mediate CD4 T cell help, we used two complementary approaches. First, we generated mixed bone marrow chimeras in which only B cells lacked CD40 (Figure 7A). Despite intact CD40 expression on B220− cells, these animals had markedly reduced recovery of CD8 T ell compartment following mATG treatment compared to control chimeras indicating that CD40 expression on B cells and not other cell subsets is critical for CD8 T cell reconstitution (Figure 7B). Second, we adoptively transferred B cells isolated from naïve B6.WT mice into B6.CD40−/− recipients of BALB./s heart allografts treated with mATG. Consistent with CD154 blocking experiments, CD40-deficient recipients had minimal numbers of CD8+ T cells at 6 weeks posttransplant. CD8 T cell reconstitution was significantly improved by B cell transfer immediately after mATG depletion and was further accelerated by treatment with agonistic anti-CD40 mAb (Figure 7C).
Figure 7. CD40 expression on B cells and TNFα are required for CD8 T cell reconstitution.
A-B. μMT + CD40−/− ➔ μMT (lack CD40 only on B cells) and μMT + WT ➔ μMT (express CD40) mixed bone marrow chimeras were generated as described in the Methods section. CD40 expression on B220+ vs B220− cells was evaluated by flow cytometry at four weeks after bone marrow reconstitution (A). The chimeric mice were transplanted with BALB/c heart allografts and treated with mATG on days 0 and 4 after transplantation. The percentages of CD4+ and CD8+ T cells in peripheral blood were determined by flow cytometry on days 14 and 21 after transplantation (B). Representative histograms (A), dot plots (B, top) and mean ±SD (B, bottom) are shown for 3-4 animals/group. C. B6.CD40−/− mice were transplanted with BALB/c heart allografts and treated with mATG on days 0 and 4 posttransplant. Splenic B cells from naïve B6 mice were adoptively transferred into recipients on day 5 posttransplant. Groups of recipients with or without B cell transfer were injected with agonistic anti-CD40 mAb FGK4.5 on days 6 and 7. The percentages of CD4 and CD8 T cells in blood were analyzed on day 42 by flow cytometry. P values are shown to compare percentages of CD8+ T cells. D. Non-transplanted B6 mice were treated with mATG (d. 0 and 4) with or without anti-CD4 mAb. Spleen B cells were isolated on day 14 after first mATG injection and mRNA expression was analyzed by qRT-PCR. E. B6 recipients of BALB/c heart allografts were treated with mATG or rIgG alone or in combination with anti-TNFα Ab (XT3.11, 0.5 mg i.v. on d. −1 and d. 1). Anti-donor IFNγ production by recipient spleen cells was measured at d. 10 posttransplant. All panels represent mean ±SD for 4-9 mice/group analyzed at each time point. *P < 0.05; **P < 0.01; ***P < 0.001.
We compared gene expression in B cells isolated from mATG treated recipients with or without additional CD4 depletion. Eliminating CD4 T cell help led to decreased B cell expression of MHC class I, CD80, CD86, and TNFα (Figure 7D). The expression levels of IL-12 and IL-15 were not significantly different between “helped” and “helpless” B cells (data not shown). To begin examining the influence of TNFα on CD8 T cell reconstitution, we administered neutralizing anti-TNFα Ab into B6 recipients of BALB/c heart allografts treated with mATG or control rIgG. TNFα neutralization decreased the numbers of donor-reactive IFNγ producing cells in the spleens of mATG treated recipient, but had little effect on the anti-donor IFNγ response in control treated mice (Figure 7E). These results suggest that upon receiving CD4 T cell help, B cells promote CD8 T cell expansion through both cognate interactions and secreted cytokines, and that TNFα facilitates recovery of the T cell repertoire in recipients treated with mATG.
Limiting CD4 T cell help inhibits CD8 T cell reconstitution and improves the efficacy of mATG induction therapy in sensitized allograft recipients
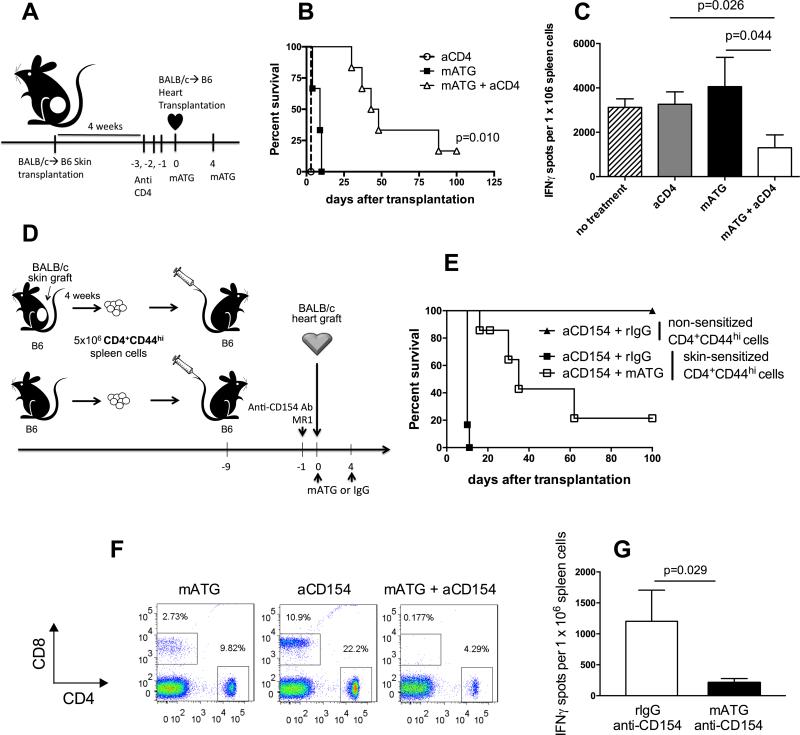
We reasoned that therapeutic interference with CD4 T cell helper signals should inhibit pathogenic memory T cell recovery and improve allograft survival following mATG induction therapy. First, we tested this hypothesis in donor skin-sensitized heart allograft recipients. B6 mice were transplanted with BALB/c skin allografts followed by BALB/c heart transplantation four weeks later (Figure 8A). Neither mATG nor anti-CD4 mAb alone prevented rapid rejection of heart allografts in skin-sensitized recipients. In contrast, CD4 T cell depletion improved the efficacy of mATG and the combination therapy significantly prolonged allograft survival in this stringent model of allosensitization (median survival time, MST, of 46 days, N = 6, Figure 8B). At the time of rejection (or sacrifice at 100 d.), recipients treated with mATG plus anti-CD4 mAb had significantly decreased frequencies of donor-reactive IFNγ-producing T cells compared to recipients receiving either monotherapy (Figure 8C).
Figure 8. Inhibition of CD4 T cell help improves the efficacy of mATG induction therapy in sensitized allograft recipients.
A. B6 mice were sensitized with BALB/c skin allografts four weeks prior to BALB/c heart allograft transplantation. Sensitized heart allograft recipients were treated either with mATG alone, anti-CD4 mAb alone or mATG + anti-CD4 mAb. B. Heart allograft survival, N = 3-6 mice/group. C. Recipients anti-donor T cell responses analyzed by IFNγ ELISPOT assay at the time of rejection. Responder spleen cells were stimulated with donor BALB/c or third party SJL stimulator cells. The results are presented as mean ±SD for 3-5 mice per group. D. B6 mice were injected with 5 × 106 CD4+CD44hi cells isolated either from BALB/c skin-sensitized or naïve B6 mice followed by BALB/c heart allograft transplantation. Treatment groups included mATG alone (1 mg i.p. on days 0 and 4) or mATG + anti-CD154 mAb (clone MR1, 1 mg i.v. on day −1). E. Heart allograft survival, N = 4-6 mice per group. F. The percentages of CD8+ and CD4+ T cells among total live splenocytes at day 10 after transplantation. Flow cytometry plots are representative of 4-6 mice analyzed per group. G. Anti-donor spleen T cells responses measured by IFNγ ELISPOT assay at the time of rejection. Data are presented as mean ±SD for 4 mice per group.
In another model of recipient sensitization, we injected B6 mice with polyclonal memory CD4 T cells isolated either from the spleens of BALB/c skin allograft recipients or from naïve B6 mice (Figure 8D) (23, 27). Consistent with our previous studies, anti-CD154 Ab induced long-term heart allograft survival in recipients containing memory CD4 T cells from naïve but not from donor skin-sensitized mice (MST of 100 vs 10 days, N = 4-6 per group, Figure 8E) (23). The addition of anti-CD154 Ab to mATG treatment markedly prolonged graft survival in recipients injected with donor-reactive memory CD4 T cells compared to either treatment alone (MST of 35 days, N = 5, Fig. 8E). The graft prolongation was associated with a delay in CD8 T cell recovery (Figure 8F). Although at the time of rejection all recipients had comparable levels of peripheral CD8 T cells (not shown), the combination of anti-CD154 and mATG significantly reduced the frequencies of donor-reactive T cells in the spleen of rejecting recipients (Figure 8G). Allograft rejection was associated with high serum titers of anti-donor alloantibody and C4d deposition in the graft (data not shown). Thus, targeting CD4 T cell help improves the efficacy of mATG induction therapy in sensitized recipients.
Discussion
The discovery of lymphopenia-induced T cell proliferation raised a concern that despite initial immunosuppressive effects, lymphoablative therapies may eventually lead to unpredictable shifts in repertoire and accumulation of pathogenic T cells (32). Later studies confirmed that memory-like T cells generated via homeostatic expansion prevent the induction of transplantation tolerance (33). Furthermore, overt lymphoablation was reported to break stable tolerance induced by costimulatory blockade (34). Our group and others reported that donor-reactive memory T cells rapidly expand following antibody-mediated lymphoablation, dominate reconstituted T cell repertoire and contribute to the transplant loss (3-7). Nevertheless, the mechanisms underlying homeostatic recovery of pathogenic memory T cells remained poorly understood.
Here we demonstrate for the first time that help by memory CD4 T cells is a critical requirement for memory CD8 T cell LIP. Importantly, CD4 T cells are essential for CD8 T cell recovery even in naïve, non-transplanted animals. Furthermore, the requirement for CD4 T cell help is not limited to treatment with polyclonal mATG, and is likely to be the common feature of antibody-mediated T cell depletion. While previous studies identified several factors driving LIP T cell proliferation, the CD8 T cell recovery appeared to be independent of CD4 T cells (12-16, 35, 36). However, many of these studies were performed in irradiated or genetically T cell deficient mice and relied on TCR transgenic T cells. Under these conditions, investigating the contribution of CD4 T cell help to homeostatic T cell recovery is problematic. For instance, compared to wild type mice, RAG-deficient hosts have severely impaired secondary lymphoid organ structures and altered levels of cytokines driving T cell expansion such as IL-7, IL-2 and IL-15. Notably, Neujahr et al. demonstrated efficient proliferation of CD8 T cells transferred into wild type mice treated with depleting anti-CD4 and anti-CD8 monoclonal antibodies (4). However, the depletion of CD4 T cell compartment achieved in this study did not exceed 85% (similar to that observed after mATG monotherapy) and allowed for ample help by residual CD4 T cells.
We identified CD40-expressing B cells as central mediators of CD4 T cell help during CD8 T cell LIP. This aspect of B cell function is distinct from previously described capacity of B cells to influence T cell responses through antigen presentation and cytokine production or to facilitate de novo generation of memory T cells during antigen-specific priming (37). The initial experiments suggest the potential role for TNFα during CD8 T cell reconstitution (Figure 7E). Ongoing studies in our laboratory are focused on the nature of B cell-mediated helper signals such as the requirement for TCR/MHC cognate interactions and costimulation and production of cytokines, including but not limited to TNFα. Notably, our data indicate that CD4 T cell reconstitution following lymphoablation does not require B cells or intact CD40/CD154 interactions suggesting distinct mechanisms of LIP for CD4 and CD8 memory T cells.
The role of B cells during lymphopenia may not be limited to enabling CD4 T cell help. Through production of TNFα and lymphotoxin (LTα), B cells regulate organization of secondary lymphoid organs (SLO) including development of B cell follicles and T cell zones in spleen (38-41). Thus, B cell depletion may affect memory T cell recovery by altering SLO structure. Similarly, CD4 T cell depletion in HIV patients leads to disruptions in the follicular reticular cell (FRC) network and may decrease peripheral CD8 T cell numbers due to lack of their maintenance in the SLO (42, 43). The importance of SLO structure and the specific roles of individual cell types supporting this structure in lymphopenic host remain to be determined.
Our previous studies using tracer populations of naïve and memory T cells demonstrated that following mATG treatment, rapid homeostatic expansion of preexisting memory T cells is the dominant mechanism of T cell repertoire reconstitution. However, it is possible that thymic output contributes to T cell recovery in mATG treated heart allograft recipients, especially at later time points. While the possible influence of residual CD4 T cells on thymopoiesis and the generation of single positive (SP) CD8 T cells under lymphopenic conditions is an interesting question, it is beyond the scope of our current study.
The most important implication of our study is the potential to increase the efficacy of ATG induction in targeting memory T cells and improving allograft survival in sensitized recipients. As a proof of principle, CD4 T cell depletion at the time of mATG treatment markedly prolonged heart allograft survival in robust models of recipient sensitization (Figure 8). While CD4 T cell depletion with monoclonal antibody at the time of ATG induction may not be feasible in clinical transplantation, our heart allograft survival data suggest that targeting CD40 signaling or B cell depletion are viable alternatives. Costimulatory blockade and B cell depletion are currently used as maintenance immunosuppression and for treatment of antibody-mediated rejection, but have not been considered to enhance the efficacy of ATG induction therapies. Several reagents targeting CD40 have been successfully tested in non-human primates and are in various stages of clinical trials, and studies developing non-thromboembolytic anti-CD40L reagents are underway (44-46). The main rationale for using Rituximab as part of preventive immunosuppressive protocols is inhibiting alloantibody responses rather than targeting memory T cells. While Rituximab showed promise in high risk ABO-incompatible transplants or in recipients containing high serum DSA titers (47, 48), the data on Rituximab induction in non-sensitized renal transplant patients are more controversial and require further validation (49, 50).
Our data indicate that inhibiting CD4 T cell help during mATG treatment delays the reconstitution and/or functions of donor-specific memory CD8 T cells compared to the entire T cell repertoire (Figure 8) implying a possible role for immune regulation. Previous studies in clinical and experimental transplantation suggest that T regs may facilitate the graft prolonging effects of ATG (51, 52). In our preliminary experiments, the proportion of Tregs within the recovering T cell repertoire was higher in mATG depleted than in non-depleted recipients and was further increased by treatment with anti-CD154 Ab (data not shown). However, it remains unknown whether the role of Tregs is limited to inhibition of donor antigen-specific responses or if Tregs diminish homeostatic T cell recovery as previously shown (53, 54). Regardless of the mechanisms, limiting CD4 T cell help provides a unique opportunity to specifically target pathogenic anti-donor memory T cells while sparing protective immunity in ATG treated recipients.
In conclusion, our study uncovers previously overlooked but clinically relevant mechanisms underlying reconstitution of the T cell repertoire following antibody-mediated depletion. These findings may guide future development of improved lymphoablative strategies to target preexisting memory T cells and to avoid the rapid recurrence of undesirable T cell responses including alloreactivity and autoimmunity.
Acknowledgments
Grant support: NIH 1P01 AI087586 (A.V.) and NIH 1R56AI113142 (A.V.)
References
- 1.Golshayan D, Pascual M. Tolerance-inducing immunosuppressive strategies in clinical transplantation: an overview. Drugs. 2008;68:2113–2130. doi: 10.2165/00003495-200868150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Haudebourg T, Poirier N, Vanhove B. Depleting T-cell subpopulations in organ transplantation. Transpl Int. 2009;22:509–518. doi: 10.1111/j.1432-2277.2008.00788.x. [DOI] [PubMed] [Google Scholar]
- 3.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, Sogawa H, Murakami T, Strom TB, Colvin RB, Sachs DH, Benichou G, Cosimi AB, Kawai T. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 5.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 6.Zeevi A, Husain S, Spichty KJ, Raza K, Woodcock JB, Zaldonis D, Carruth LM, Kowalski RJ, Britz JA, McCurry KR. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7:471–475. doi: 10.1111/j.1600-6143.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annual review of immunology. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 9.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 11.Margolick JB, Munoz A, Donnenberg AD, Park LP, Galai N, Giorgi JV, O'Gorman MR, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 12.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. Journal of immunology. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 18.Andrade RM, Wessendarp M, Portillo JA, Yang JQ, Gomez FJ, Durbin JE, Bishop GA, Subauste CS. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-alpha. Journal of immunology. 2005;175:6014–6021. doi: 10.4049/jimmunol.175.9.6014. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, Siders WM, Kaplan JM. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128:260–270. doi: 10.1111/j.1365-2567.2009.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marco MR, Dons EM, van der Windt DJ, Bhama JK, Lu LT, Zahorchak AF, Lakkis FG, Cooper DK, Ezzelarab MB, Thomson AW. Post-transplant repopulation of naive and memory T cells in blood and lymphoid tissue after alemtuzumab-mediated depletion in heart-transplanted cynomolgus monkeys. Transpl Immunol. 2013;29:88–98. doi: 10.1016/j.trim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzek MC, Neff KS, Luong M, Smith KA, Culm-Merdek K, Richards SM, Williams JM, Perricone M, Garman RD. In vivo characterization of rabbit anti-mouse thymocyte globulin: a surrogate for rabbit anti-human thymocyte globulin. Transplantation. 2009;88:170–179. doi: 10.1097/TP.0b013e3181abc061. [DOI] [PubMed] [Google Scholar]
- 22.Yokota N, Daniels F, Crosson J, Rabb H. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation. 2002;74:759–763. doi: 10.1097/00007890-200209270-00005. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. Journal of immunology. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 24.Gorbacheva V, Ayasoufi K, Fan R, Baldwin WM, 3rd, Valujskikh A. B cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) mediate CD40-independent help by memory CD4 T cells. Am J Transplant. 2015;15:346–357. doi: 10.1111/ajt.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabant M, Gorbacheva V, Fan RY, H., Valujskikh A. CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 doi: 10.1111/ajt.12432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. Journal of immunology. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 27.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 28.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 29.Abe T, Ishii D, Gorbacheva V, Kohei N, Tsuda H, Tanaka T, Dvorina N, Nonomura N, Takahara S, Valujskikh A, Baldwin WM, 3rd, Fairchild RL. Anti-huCD20 antibody therapy for antibody-mediated rejection of renal allografts in a mouse model. Am J Transplant. 2015;15:1192–1204. doi: 10.1111/ajt.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 31.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 32.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iida S, Suzuki T, Tanabe K, Valujskikh A, Fairchild RL, Abe R. Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection. Am J Transplant. 2013;13:2268–2279. doi: 10.1111/ajt.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 36.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 37.Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1970–1980. doi: 10.1111/j.1600-6143.2010.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annual review of immunology. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The sequential role of lymphotoxin and B cells in the development of splenic follicles. The Journal of experimental medicine. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. The Journal of experimental medicine. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumanov AV, Grivennikov SI, Kruglov AA, Shebzukhov YV, Koroleva EP, Piao Y, Cui CY, Kuprash DV, Nedospasov SA. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood. 2010;116:3456–3464. doi: 10.1182/blood-2009-10-249177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng M, Paiardini M, Engram JC, Beilman GJ, Chipman JG, Schacker TW, Silvestri G, Haase AT. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badell IR, Thompson PW, Turner AP, Russell MC, Avila JG, Cano JA, Robertson JM, Leopardi FV, Strobert EA, Iwakoshi NN, Reimann KA, Ford ML, Kirk AD, Larsen CP. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant. 2012;12:126–135. doi: 10.1111/j.1600-6143.2011.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XL, Menoret S, Le Mauff B, Angin M, Anegon I. Promises and obstacles for the blockade of CD40-CD40L interactions in allotransplantation. Transplantation. 2008;86:10–15. doi: 10.1097/TP.0b013e31817c4b97. [DOI] [PubMed] [Google Scholar]
- 46.Lowe M, Badell IR, Thompson P, Martin B, Leopardi F, Strobert E, Price AA, Abdulkerim HS, Wang R, Iwakoshi NN, Adams AB, Kirk AD, Larsen CP, Reimann KA. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takagi T, Ishida H, Shirakawa H, Shimizu T, Tanabe K. Evaluation of low-dose rituximab induction therapy in living related kidney transplantation. Transplantation. 2010;89:1466–1470. doi: 10.1097/TP.0b013e3181dc0999. [DOI] [PubMed] [Google Scholar]
- 48.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 49.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, Smith KG. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyden G, Genberg H, Tollemar J, Ekberg H, Persson NH, Tufveson G, Wadstrom J, Gabel M, Mjornstedt L. A randomized, doubleblind, placebo-controlled, study of single-dose rituximab as induction in renal transplantation. Transplantation. 2009;87:1325–1329. doi: 10.1097/TP.0b013e3181a235fd. [DOI] [PubMed] [Google Scholar]
- 51.D'Addio F, Yuan X, Habicht A, Williams J, Ruzek M, Iacomini J, Turka LA, Sayegh MH, Najafian N, Ansari MJ. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90:260–269. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, Ames S, Lerner S, Ebcioglu Z, Nair V, Dinavahi R, Sehgal V, Heeger P, Schroppel B, Murphy B. Immune reconstitution following rabbit antithymocyte globulin. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen S, Ding Y, Tadokoro CE, Olivares-Villagomez D, Camps-Ramirez M, Curotto de Lafaille MA, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. The Journal of clinical investigation. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. Journal of immunology. 2008;180:7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]