Abstract
A large and rapidly increasing body of evidence indicates that microglia-neuron signaling is essential for chronic pain hypersensitivity. Here we show using multiple approaches that microglia are not required for mechanical pain hypersensitivity in female mice; female mice achieve similar levels of pain hypersensitivity using adaptive immune cells, likely T-lymphocytes. This sexual dimorphism suggests that male mice cannot be used as proxies for females in pain research.
It is now well appreciated that immunocompetent cells are importantly involved in pain pathophysiology1. Spinal glial cells are reactive to peripheral inflammation or nerve damage, and can produce symptoms including mechanical hypersensitivity or allodynia. Critical data supporting this hypothesis are that intrathecal injection of drugs disrupting glial functioning can prevent and/or reverse pain behavior in rodents2.
This preclinical literature—as is standard in the pain field3—represents the results of experiments overwhelmingly conducted on male rodents. We previously reported that the involvement of spinal toll-like receptor 4 (TLR4) in the production of mechanical allodynia was male-specific4. Here we investigated the possibility that the underlying reason for this sex difference was that microglia, on which TLR4s are located, may not be required for pain processing in female mice.
Mechanical allodynia was induced in mice of both sexes using spared nerve injury (SNI), a procedure producing persistent neuropathic pain. Seven days after the nerve injury, mice were injected intrathecally with glial inhibitors minocycline, fluorocitrate or propentofylline, and mechanical thresholds were retested over the next 120 min. All three inhibitors produced robust, dose‐dependent reversal of allodynia in male mice; no significant reversal of allodynia was observed in female mice at any dose (Fig. 1a; Supplementary Fig. 1). Similar results were observed for persistent inflammatory pain (Supplementary Fig. 2). Repeated systemic injections of minocycline also reversed mechanical allodynia in male but not female mice (Supplementary Fig. 3). Moreover, in mice tested 28 days post‐SNI, minocycline reversed mechanical allodynia in males but not females (sex × repeated measures: F5,26 = 2.8, p=0.04) (Fig. 1a, right side). As previously demonstrated for TLR4 involvement in chronic pain4, the sex difference appeared to be dependent on testosterone (Supplementary Fig. 4). Reactive microgliosis after SNI was broadly similar between sexes (Supplementary Fig. 5).
Fig. 1. Mechanical allodynia after nerve injury is reversed by microglial inhibition in male but not female mice.
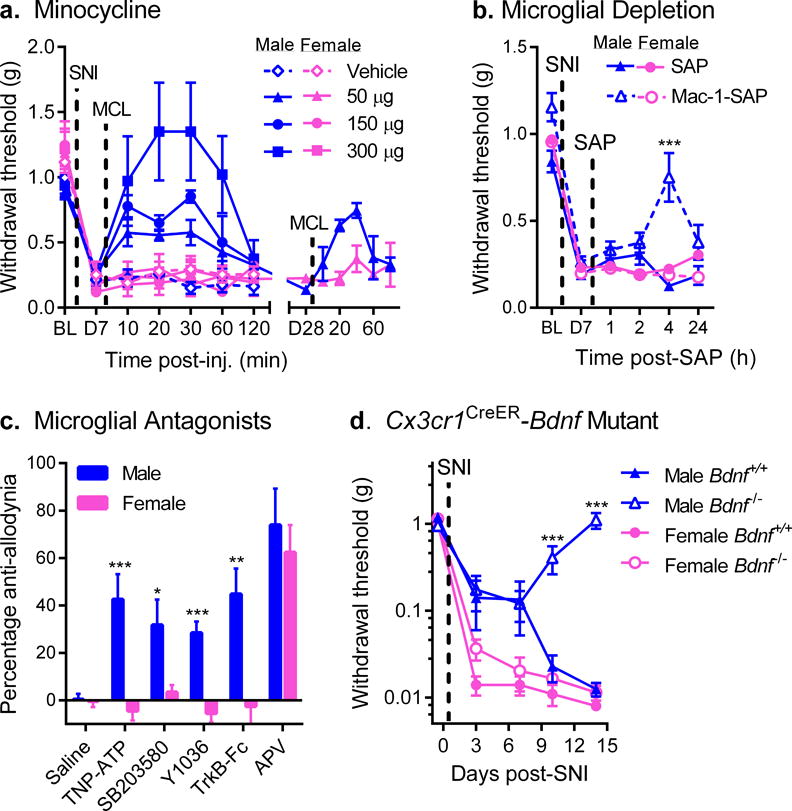
a) Reversal of established SNI-induced mechanical allodynia by intrathecal minocycline (MCL) in male but not female mice (see also Supplementary Fig. 1a). Symbols represent mean ± SEM 50% withdrawal threshold from von Frey filaments before surgery (BL), 7 days after surgery (pre-injection; D7), and 10–120 min post-injection of minocycline (n=4–5 mice/dose/sex). A different set of mice (n=4 mice/sex) were tested similarly, 28 days post-SNI (D28; right side). b) Male but not female mice treated with Mac-1-SAP display significantly reduced SNI allodynia at 4 h post-treatment. Symbols represent mean ± SEM 50% withdrawal threshold from von Frey filaments before surgery (BL), 7 days after surgery, pre-injection (D7), and 1, 2, 4 and 24 h post-SAP (n=11 mice/sex/condition). c) Intrathecal administration of the P2X inhibitor, TNP-ATP, the p38 MAPK inhibitor, SB203580, the NGF/BDNF inhibitor, Y1036, or the BDNF-sequestering fusion protein, TrkB-Fc, all block SNI-induced allodynia in male but not female mice. The NMDA receptor antagonist, APV, blocks allodynia equally in both sexes. Bars represent mean ± SEM percentage of maximal anti-allodynia (see Methods; n=8 mice/sex/drug). d) Development of SNI-induced mechanical allodynia in male and female mice lacking central nervous system microglial BDNF (i.e., with tamoxifen-induced Cre-loxP-mediated deletion of the Bdnf gene in CX3CR1-positive cells). Mutant (Bdnf−/−) male mice fail to develop full allodynia displayed by female Bdnf−/− and wildtype (Bdnf+/+) mice. Symbols represent mean ± SEM absolute withdrawal threshold from von Frey filaments before and 3, 7, 10 and 14 days post-surgery (n=4–8 mice/sex/genotype). *p<0.05, **p<0.01, ***p<0.001 compared to corresponding female mice by t-test.
Although a common action of minocycline, fluorocitrate and propentofylline is to inhibit glial function, these compounds have many different unrelated actions. To determine whether spinal cord microglia themselves are necessary for allodynia in male but not female mice, saporin toxin conjugated to macrophage antigen complex-1 (Mac-1; integrin CD11b/CD18 receptor) was injected intrathecally in order to transiently deplete microglia. At 4 h post-injection, similar microglial depletion in both sexes (Supplementary Fig. 6) resulted in significant reversal of allodynia in male mice, but was without effect in females (sex × drug × repeated measures: F4,80 = 4.7, p=0.002) (Fig. 1b).
A microglial receptor essential for SNI-induced allodynia is the P2X4 receptor (P2X4R), as previously demonstrated in male rats via the reversal of allodynia by intrathecal TNP‐ATP5. We replicated the effect in male mice, but found no effect of TNP-ATP in female mice (t14 = 4.1, p=0.001) (Fig. 1c). Also reversing allodynia in male but not female mice were inhibitors of key signaling molecules in the spinal cord microglia-to-neuron pain pathway6: p38 MAPK7 (SB203580; t10 = 2.5, p=0.03), and BDNF8 (Y1036 or TrkB-Fc; t10 = 5.5, p<0.001 and t14 = 3.4, p=0.004, respectively) (Fig. 1c). However, SNI-induced allodynia was equally reversed (t10 = 0.6, p=0.56) in male and female mice by intrathecal N-methyl-D-aspartate (NMDA) receptor blockade using APV (Fig. 1c).
The behavioral sex difference was accompanied by an analogous sex difference in dorsal horn gene expression, whereby SNI upregulated P2rx4 gene expression in male but not female mice (Supplementary Fig. 7). In contrast, SNI increased in dorsal horn expression of other genes associated with microglial reactivity (and upregulation of P2rx4)—Itgam, Emr1, Irf5 and Irf8—equally in both sexes, consistent with the lack of a sex difference in SNI-induced microgliosis (Supplementary Fig. 5). As IRF5 and IRF8 are transcription factors upstream of P2rx4, the point of divergence between males and females is the injury-induced upregulation of P2X4R expression.
To further investigate the requirement for microglial BDNF in mice of both sexes, we created transgenic mice in which BDNF was deleted, in a tamoxifen-dependent manner, in Cx3cr1-positive cells9. We took advantage of the difference in rates of cell turnover between central and peripheral Cx3cr1-expressing populations allowing the periphery to repopulate before testing. Thus, the animals tested lack only BDNF derived only from microglia. These mice displayed normal reactive microgliosis in the dorsal horn in both sexes (Supplementary Fig. 8). Mutant male mice developed modest mechanical allodynia early after SNI surgery, but by 14 days post-surgery allodynia was absent. In contrast, female mutant mice displayed equivalent mechanical allodynia to wildtypes (genotype × sex × repeated measures: F4,92 = 11.9, p<0.001) (Fig. 1d). To determine whether pre-existing SNI-induced mechanical allodynia may be reversed by depleting microglial BDNF in a sex-specific manner, we treated mutant mice with tamoxifen one week after SNI. We found that allodynia was reversed in male but not in female mutants, or wildtypes of either sex (Supplementary Fig. 9).
In these studies, statistically significant sex differences in baseline mechanical sensitivity and allodynia were observed in some cases (Supplementary Fig. 10), but when present did not affect the interpretation of the qualitative (all-or-none) sex differences in the microglial-dependence of allodynia. That all interventions suppressing microglia signaling reversed allodynia in male but not female mice, and that female mice displayed equivalent levels of allodynia compared to males, demand the existence of an alternate pathway playing an analogous role in female mice. The demonstrated involvement of infiltrating T-cells in mechanical allodynia in mice10–12 suggested that female mice might preferentially use adaptive immune cells instead of microglia to produce allodynia after injury, which was tested using T-cell- (and B-cell-) deficient nude (Foxn1nu) and Rag1−/− mice. In contrast to previous observations10, 11, we observed no consistent reduction of mechanical allodynia in male nude or Rag1−/− mice (Supplementary Fig. 10). Wildtype mice (CD-1 and C57BL/6J strains, respectively) displayed the sex difference in glial inhibitor sensitivity described above; in contrast, both male and female nude and Rag1−/− mice were sensitive to glial inhibitor reversal of SNI and complete Freund’s adjuvant (CFA) allodynia (all genotype × sex interactions p<0.05) (Fig. 2a; Supplementary Figs. 11 and 12). These data reveal that in the absence of adaptive immune cells, female mice use the male, glial-dependent pathway, as was the case for testosterone-treated females. We have previously observed this type of “switching” from female to male systems in studies of opioid analgesia and hyperalgesia13–15.
Fig. 2. Mechanical allodynia after nerve injury is mediated by adaptive immune cells in female but not male mice.
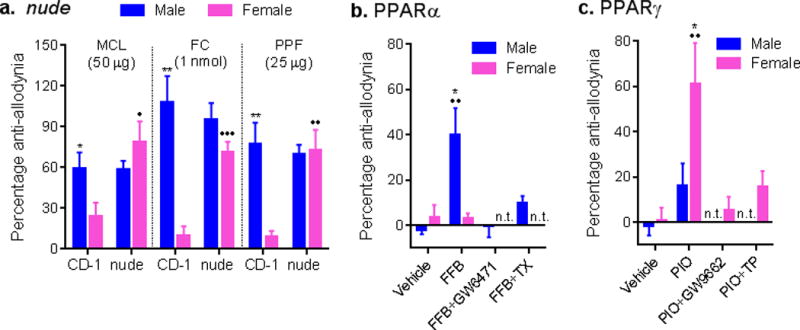
a) Reversal of mechanical allodynia after SNI by intrathecally administered glial inhibitors minocycline (MCL), fluorocitrate (FC) and propentofylline (PPF) in male but not female CD-1 mice, but in immunocompromised nude mice of both sexes. Bars represent mean ± SEM percentage of maximal anti-allodynia (n=4–7 mice/sex/drug/genotype). b) Male-specific reversal of allodynia from the PPARα ligand, fenofibrate (FFB) is blocked by the PPARα antagonist, GW6471, and by castration (TX). Bars as in graph a (n=4–6 mice/sex/condition). c) Female-specific reversal of allodynia from the PPARγ ligand, pioglitazone (PIO) is reversed by the PPARγ antagonist, GW9662, and by testosterone proprionate (TP). Bars as in graph a (n=7–10 mice/sex/condition). *p<0.05, **p<0.01 compared to corresponding female mice by t-test. •p<0.05, ••p<0.01, •••p<0.001 compared to same-sex wildtype (CD-1) or vehicle group by t-test. n.t. = not tested.
If the absence of adaptive immune cells causes female mice to use a glial-dependent pathway, then restoring these cells may enable the mice to switch to the glial-independent pathway. We performed adoptive splenocyte transfer in female Rag1−/− mice (Supplementary Fig. 13a), followed by evaluation of the effects of minocycline (50 μg) on CFA-induced allodynia. The adoptive splenocyte transfer had no impact on allodynia levels (Supplementary Fig. 10). However, female Rag1−/−mice receiving the adoptive transfer were rendered insensitive to minocycline, in contrast to vehicle-treated Rag1−/− mice, which were sensitive to minocycline (Supplementary Fig. 13b).
We considered the possibility that the observed sex difference may derive from the almost two-fold higher resident T-lymphocyte population in the periphery of female mice compared to males16. We confirmed that female CD-1 mice indeed have higher levels of lymphocytes and CD4+ and CD8+ cells in the blood than do males (Supplementary Fig. 14). Furthermore, we found that in the lumbar spinal cord 7 days after SNI, mRNA levels of the T-cell markers CD3e, CD4, and CD8a were higher in female CD-1 mice than in males (Supplementary Fig. 15).
A known immune system-related sex difference that may explain our observations is the reported sexually dimorphic expression of peroxisome proliferator activated receptors (PPARs) α and γ in mouse and human T-cells17. Testosterone increases PPARα but decreases PPARγ expression in T-cells, leading to decreased production of interferon-γ but higher production of interleukin-17A17. Both cytokines have been previously associated with pain10, 18, but a recent study observed that after nerve injury T-cells infiltrating the spinal cord are predominantly Th1 (i.e., interferon-γ-releasing) cells19. Perhaps, therefore, infiltrating T-cells in male mice are less able to mediate hypersensitivity, resulting in the adoption of an alternative mechanism (i.e., microglia). We observed that the PPARα agonist, fenofibrate, reversed SNI-induced allodynia in male but not in female mice (sex × drug: F1,16 = 12.6, p=0.003), an effect blocked by the PPARα antagonist, GW6471 (Fig. 2b). Conversely, the PPARγ agonist, pioglitazone, reversed SNI-induced allodynia in female but not male mice (sex × drug: F1,35 = 3.9, p=0.05), an effect blocked by the PPARγ antagonist, GW9662 (Fig. 2c). Importantly, castration of male mice also prevented the anti-allodynic effect of fenofibrate (male only groups: F3,15 = 10.8, p<0.001; Fig. 2b), and administrating testosterone proprionate to female mice prevented the anti-allodynic effect of pioglitazone (female only groups: F3,33 = 6.7, p=0.001; Fig. 2c).
These observations—made independently by multiple experimenters in three different laboratories—suggest that there are two separable mechanisms of immune-neural interactions in the spinal cord of CD-1 and C57BL/6 mice. One important end result of microglial pain processing is P2X4R-induced release of BDNF by microglia8, which we show to be essential only in male mice. We note that T-cells can also release a host of other proalgesic mediators that might similarly enhance neuronal activity1. The topic of immune system involvement in chronic pain pathophysiology is one of the most active in the pain field; that this sex difference has not been observed until now is very surprising indeed. An important implication of the present findings is that distinct strategies targeting neuroimmune signaling might be required for the treatment of chronic pain in men versus women.
ONLINE METHODS
Mice
Subjects were naïve, young adult (7–12 week-old) mice of both sexes, except for one experiment using 4 week-old mice of both sexes. CD-1® (Crl:ICR) and nude CD-1 (Crl:CD1-Foxn1nu) mice were purchased from Charles River Laboratories (Boucherville, QC or Durham, NC), C57BL/6J were purchased from The Jackson Laboratory (Bar Harbor, Maine), and Rag1−/− mice (Rag1tm1Mom) were obtained from Dr. Maya Saleh (McGill University). Castrated and ovariectomized CD-1 mice were purchased (surgeries performed by supplier) from Charles River Laboratories; at least two weeks elapsed from surgery to the start of testing. All mice were housed in standard polycarbonate cages in groups of 2–5 same-sex littermates in a temperature-controlled (20 ± 1 °C) environment (14:10 h light/dark cycle; lights on at 07:00 h); tap water and food (Harlan Teklad 8604) were available ad libitum. All procedures were approved by local animal care and use committees and were consistent with national guidelines. In pharmacological studies, mice were assigned to experimental groups using within-cage randomization. Experimenters were blinded to drug and dose, and sex where possible; blinding to sex was not possible in behavioral experiments.
von Frey Testing
Behavioral testing was performed by experimenters of both genders20; no differences were noted. The up-down method of Dixon21 was used to estimate 50% withdrawal thresholds using nylon monofilaments (Stoelting Touch Test), calibrated monthly. All experiments took place during the light cycle, no earlier than 09:00 h and no later than 16:00 h. Mice were placed in custom-made Plexiglas cubicles (5 × 8.5 × 6 cm) on a perforated metal floor, and were permitted to habituate for at least 1 h prior to testing. Filaments were applied to the plantar surface of the hind paw for 1 s and responses were recorded. Two consecutive measures were taken on each hind paw at each time point, and averaged. Although data were collected on both hind paws, only data from the hind paw ipsilateral to the injury (neuropathic or inflammatory; see below) are presented, as no significant effects of sex, drug or genotype on the contralateral paw were observed in any experiment.
In one study, von Frey filaments were used to estimate absolute withdrawal thresholds. Mice were tested using an ascending series, starting with the lowest von Frey filament (0.008 g) until threshold was reached, determined as reflex withdrawal occurring twice in ten stimulations22.
Neuropathic Surgeries
Some mice received unilateral SNI23 on the left side, one day following baseline von Frey testing. Surgery was performed under isoflurane/oxygen anesthesia. We spared the sural nerve, and thus von Frey testing before and after SNI occurred on the lateral aspect of the hind paw. In one experiment conducted in a different laboratory (SB203580), mice received unilateral chronic constriction injury24 (CCI) on the left side instead, and were tested with von Frey filaments aimed at the mid-plantar hind paw. In most experiments mice were retested for mechanical allodynia on day 7 post-surgery; in one experiment (shown in Fig. 1a, right side) mice were retested on day 28 post-surgery. Microglial-specific Bdnf mutants (see below) were retested 3, 7, 10 and 14 days post-surgery in the prevention experiment, and 1, 4, 5, 6, 7 and 8 weeks post-surgery in the reversal experiment.
Complete Freund’s Adjuvant (CFA)
Some mice received unilateral injections of CFA (50% in 20 μl) into the plantar surface of the left hind paw. von Frey fibers before and after CFA were aimed at the mid-plantar hind paw. In all CFA experiments mice were retested for mechanical allodynia on day 3 post-injection.
Intrathecal Injections
Immediately following post-SNI or post-CFA testing on day 7 or day 3, respectively, mice were removed from their cubicles, lightly anesthetized using isoflurane/oxygen, and given intrathecal injections of drugs25, in a volume of 5 or 10 μl over 30 s, using a 30-gauge needle.
Drugs
Minocycline (50–300 μg, i.t.), fluorocitrate (0.5–1.5 nmol, i.t.), propentofylline (25–75 μg, i.t.), TNP-ATP sodium salt (5.0 μg, i.t.), (2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate (APV; 0.5 μg, i.t.), and fenofibrate (200 μg, i.t.) were purchased from Sigma. The p38 inhibitor, 4-(4-fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580; 30 μg, i.t.) and Y1036 (5 μg, i.t.) were obtained from Calbiochem. Recombinant human TrkB-Fc (three i.t. injections of 0.5 μg, 24 h apart) was purchased from R&D Systems. Pioglitazone potassium salt (300 μg, i.t.) was purchased from Cedarlane. GW 9662 (2 mg/kg, i.p.) was obtained from Tocris, and administered 90 min prior to pioglitazone. GW 6471 (10 mg/kg, i.p.) was obtained from Sigma, and administered immediately prior to fenofibrate. All drugs were dissolved in physiological saline or 10–20% dimethyl sulfoxide. Doses were determined in pilot experiments. In one experiment, minocycline was injected systemically at a dose of 25 mg/kg, i.p.
Allodynia and Anti-Allodynia Data Transformation
SNI- (day 7 post-surgery) and CFA- (day 3 post-injection) induced allodynia (reported in Supplementary Fig. 10) was quantified as percentage of maximum possible allodynia using the formula: percentage allodynia = [(baseline threshold − post-injury threshold)/baseline threshold] × 100. Reversal of allodynia by drugs (i.e., anti-allodynia) was quantified with respect to the area under the threshold-time curve (using the trapezoidal method) over the post-injection testing period (120 min in all cases except the SB203580 experiment, which was 6 h). Data are reported as percentage of the maximum possible anti-allodynia, calculated for each mouse as a ratio of its actual anti-allodynia compared to a hypothetical situation in which the drug brought withdrawal thresholds to their original baseline at all post-injection time points.
Testosterone Treatment
Testosterone proprionate was purchased from Sigma and dissolved in polyethylene glycol. This solution was placed into an Alzet osmotic minipump (Model 2002, 0.5 μl/h for 14 days) and implanted subcutaneously between the scapulae under isoflurane/oxygen anesthesia. The pump delivered an effective dose of 250 μg/day, and testing began after the full 14 days of treatment. In another experiment, testosterone (2 mg/kg, s.c.) was injected 48 h prior to pioglitazone.
Iba1 Immunohistochemistry
Immediately following behavioral testing, mice were perfused transcardially with 4% paraformaldehyde for subsequent immunohistochemical processing. Lumbar spinal cord was cryosectioned and stained with anti-ionized calcium-binding adaptor molecule 1 (Iba1) polyclonal antibody (1:1000, Wako Chemicals, Cat. #019-19741), anti-NeuN (1:2000, Millipore, Cat. #MAB377), and/or anti-GFAP (1:2000, Sigma, Cat. #G3893). Spleen tissue was stained with Iba1 and anti-CD3 (1:500, Serotec, Cat. #MCA1477). YFP expression in Cx3cr1-Cre mice was revealed with anti-GFP (1:500 Santa Cruz, Cat. #sc390394). All tissue sections were subsequently incubated with appropriate fluorescently labelled secondary antibodies (1:1000, Jackson), and confocal images were acquired using a Zeiss Axiovert microscope and Perkin Elmer Volocity software. The number of Iba-1-positive microglial cells was quantified with Image Pro Plus software within a defined area of interest on the spinal cord dorsal horn (lamina I–III). The scorer was blinded to drug treatment.
Mac1-Saporin Lesioning
Mac-1-saporin mouse/human toxin (15 μg in 8.8 μl) and saporin control (8.8 μg in 8.8 μl) were purchased in solution from Advanced Targeting Systems. Doses were determined from pilot experiments. Drugs were administered via i.t. injection as described above.
Construction of Microglial-specific Bdnf Mutants (Cx3cr1CreER × loxP-Bdnf)
Mice expressing tamoxifen-inducible Cre recombinase (CreER) under the endogenous CX3CR1 promoter (a generous gift of Dr Wenbiao Gan, New York University School of Medicine) were crossed with mice possessing loxP sites flanking the BDNF coding region (Bdnftm3Jae/J; The Jackson Laboratory, Cat. #00439). Progeny heterozygous for CreER expression were crossed with loxP-Bdnf mice such that all experimental mice were homozygous for floxed Bdnf.
Cre expression was induced by oral administration of tamoxifen (two doses of 10 mg of tamoxifen in 0.5 ml corn oil 48 h apart, and 5% glucose daily subcutaneously9). Non-tamoxifen-treated animals received corn oil as a vehicle control. Specificity was established using qPCR and FACS. In order to allow for repopulation of peripheral Cx3cr1-expressing populations, tamoxifen was administered one month prior to SNI in the prevention experiment shown in Fig. 1d, and 1 week post-SNI, at the peak of behavioural allodynia, in the reversal experiment shown in Supplementary Fig. 9.
Adoptive Splenocyte Transfer
Spleens harvested from female wildtype (C57BL/6) mice using aseptic technique were placed in sterile, ice-cold RPMI-1640 medium (Sigma) and transferred to a cell culture hood for sterile disruption into a single-cell suspension. After centrifugation (800 × g for 5 min at 4 °C), red blood cells were lysed by re-suspending cells in RBC lysis buffer (Sigma) and incubating on ice for 5 min. Buffer was diluted with cold RPMI and cells were centrifuged as above and resuspended in ice-cold RPMI, then counted on a hemocytometer using trypan blue (Sigma) exclusion as a measure of viability. Cells were centrifuged as above and resuspended in sterile 0.9% saline. Unfractionated splenocytes (1 × 107 cells in 0.15 ml) were injected into the tail vein of awake, loosely restrained recipient female Rag1−/− mice. Control female Rag1−/− mice received vehicle injection into the tail vein. CFA was injected 5 days later to both groups, and to wildtype mice of both sexes.
To confirm reconstitution of Rag1−/− mice with wildtype splenocytes, 12 days post-adoptive transfer, spleen tissue was immunolabeled with antibodies against Iba1 and CD3 (see above).
FACS
Blood samples were collected from naïve male and female CD-1 mice. Erythrocytes were removed from whole blood with ACK lysing buffer (Gibco). Leukocyte suspensions and blocking were prepared as described previously26. Samples were then stained with mouse CD4 (1:50, BD Pharmingen, Cat. #553048) and CD8 (1:50, eBioscience, Cat. #46-0081-82) antibodies conjugated by FITC and PE, respectively. Cellular events were acquired using a LSR Fortessa flow cytometer (BD). The number of CD4+ or CD8+ T cells was counted among 1×104 events isolated from each blood sample. Data were analyzed using Flow Jo software.
qPCR (microglial markers)
Male and female CD-1 mice were euthanized 7 days after SNI or sham surgery (n=8 mice/sex/surgical condition) and transcardially perfused with 10 ml RNAlater solution (Life Technologies). The ipsilateral dorsal horn from sciatic territory of the lumbar spinal cord were dissected out and collected in RNAlater solution. RNA was isolated by digesting tissues in TRIZOL® (Life Technologies) and cDNA synthesized using the SuperScript VILO® cDNA kit (Life Technologies). Ten ng per reaction were used for RT-qPCR using pre-designed Taqman probes for P2rx4 (#Mm00501787), Emr1 (#Mm00802529), Irf5 (#Mm00496477), Irf8 (#Mm00492567), and Itgam (#Mm00434455). qPCR were performed for 40 cycles (95 °C for 1 s, 60 °C for 20 s). Levels of the target genes were normalized against the average of four housekeeping genes (Abt1, Eef2, Gapdh, and Hprt1), and interpreted using the comparative ΔΔCt method.
qPCR (T cell markers)
Lumbar spinal cords were harvested from male and female CD-1 mice (n=5–6 mice/sex), 7 days post-SNI surgery. Tissues were snap frozen in dry ice. Total RNA was extracted with the phenol-chloroform method. Reverse transcription was performed using with SuperScript III Reverse Transcriptase (Invitrogen) with 500 ng of total RNA. Real time quantitative PCR reactions (in triplicate) were processed with a Rotor-Gene Q real-time PCR cycler (Qiagen) using SYBR Green mix from Qiagen (RT² SYBR Green FAST Mastermix). The levels of Cd3, Cd4, and Cd8 were normalized against the housekeeping gene, Gapdh, and interpreted using the comparative Ct method. qPCR primers were designed based on gene sequences from GeneBank database on NCBI (Cd3: NM_007648.4; Cd4: NM_013488.2; Cd8: NM_001081110.2), and synthesized by IDT (Integrated DNA Technologies).
Statistics
A criterion α level of 0.05 was adopted in all experiments. Data were analyzed by t-test (two-sided in all cases) or repeated measures ANOVA, as appropriate, after determining the normality (Shapiro-Wilk test) and homoscedasticity (Levene’s test) of the experimental data. Posthoc testing was performed using Tukey’s test. In five cases, data points were excluded as statistical outliers (Standardized residuals >3); in no case does their inclusion alter experiment conclusions. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications in the field27.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MWS, JSM), the Louise and Alan Edwards Foundation (JSM), and the U.S. National Institutes of Health (R-RJ). MWS holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain, and is the Anne and Max Tanenbaum Chair in Molecular Medicine at the Hospital for Sick Children. JSM holds a Canada Research Chair (Tier I) in the Genetics of Pain, and is the E.P. Taylor Professor of Pain Studies at McGill University.
Footnotes
AUTHOR CONTRIBUTIONS
RES, SB, R-RJ, MWS, and JSM conceived of the study. RES designed most experiments; JCSM and JZ designed certain experiments. RES, JCSM, SR, ST, SB, JKA, LFM, J-SA, SGS, DC, MY, XQS, HH, NJP, PJB, YT, and AK collected and analyzed data. RES, JCSM, SB, MWS, and JSM wrote the paper.
COMPETING FINANCIAL INTERESTS STATEMENT
The authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this article.
A supplementary methods checklist is available.
References
- 1.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 3.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Sorge RE, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 6.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin S, Zhuang Z, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coull JAM, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 9.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costigan M, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 14.Juni A, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology. 2010;112:181–188. doi: 10.1097/ALN.0b013e3181c53849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogil JS, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang MA, et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain. 2011;12:370–383. doi: 10.1016/j.jpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Draleau KS, et al. Phenotypic identification of spinal cord-infiltrating CD4+ T lymphocytes in a murine model of neuropathic pain. J Pain Relief. 2014;(Suppl 3):003. doi: 10.4172/2167-0846.S3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorge RE, et al. Olfactory exposure to males, including human males, stresses rodents. Nat Meth. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia evoked by unilateral ligation of the fifth and sixth lumbar nerves in the rat. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 22.Bourquin AF, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.e11–14.e14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Shields SD, Eckert WA, III, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 24.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 25.Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Rainone A, Shi XQ, Fournier S, Zhang J. A new animal model of spontaneous autoimmune peripheral polyneuropathy: implications for Guillain-Barré syndrome. Acta Neuropathol Commun. 2014;2:5. doi: 10.1186/2051-5960-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogil JS, et al. Screening for pain phenotypes: analysis of three congenic mouse strains on a battery of nine nociceptive assays. Pain. 2006;126:24–34. doi: 10.1016/j.pain.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


