Abstract
Background:
Psychiatric illnesses like bipolar disorder are increasingly understood to be neurodevelopmental disorders with clinical, psychological, and biological indicators recognizable long before the emergence of the full-blown syndromes.
Methods:
This paper is a selective review of findings from studies of high-risk children of affected parents that inform the knowledge of illness risk and development markers of bipolar disorder. We specifically focus on candidate clinical, biological, and psychological risk indicators that could serve as targets for future early intervention and prevention studies.
Results:
There is convergent evidence from prospective studies that bipolar disorder typically debuts as depressive episodes after puberty. In some high-risk children, sleep and anxiety disorders precede mood disorders by several years and reflect an increased vulnerability. An association between early exposure to adversity (eg, exposure to parental illness, neglect from mother) and increased risk of psychopathology may be mediated through increased stress reactivity evident at both behavioral and biological levels. Inter-related psychological processes including reward sensitivity, unstable self-esteem, rumination, and positive self-appraisal are risk factors for mood disorders. Disturbances in circadian rhythm and immune dysfunction are associated with mood disorders and may be vulnerability markers influenced by these other risk factors.
Conclusions:
There is accruing evidence of a number of measurable and potentially modifiable markers of vulnerability and developing illness in youth at familial risk for bipolar disorder. Longitudinal studies of multiple biological and psychological risk processes in high-risk offspring, both individually and together, will improve our understanding of illness onset and lead to the development of specific early interventions.
Keywords: high-risk, bipolar disorder, biological risk factors, psychological risk factors, early adversity
Introduction
Psychiatric disorders are increasingly being understood as heritable neurodevelopmental disorders with clinical manifestations occurring years prior to any recognizable diagnosable syndromes (Insel, 2012). Adolescence is characterized by accelerated biological, psychological, and social development and marks the beginning of the high-risk period for onset of mood disorders that recur into adulthood (Paus et al., 2008). Psychiatric illness during this important development period has devastating consequences that persist lifelong. Unipolar depression and bipolar disorder (BD) place among the top-ranked causes of morbidity and mortality in youths aged 10 to 24 years (Gore et al., 2011). Moreover, other highly ranked causes of morbidity and mortality in this age group are associated with mood disorders (eg, addictions). Suicide is estimated to be the second leading cause of death in teens and young adults in Western countries, the majority of cases being associated with a psychiatric disorder (Cavanagh et al., 2003) and most often a mood disorder (Windfuhr et al., 2008).
Despite the substantial morbidity and mortality already evident early in the course of mood disorders, it reportedly takes over a decade from the time an individual seeks help until an accurate diagnosis is made and much longer still from the onset of impairing symptoms (Judd and Akiskal, 2003). Clearly there is an urgent need to identify serious mood disorders as early as possible and to develop effective and acceptable early interventions. This paper provides an overview of the early natural history of BD and related mood disorders based on findings from selected prospective high-risk studies and highlights evidence for candidate early intervention targets including: antecedent risk syndromes, early adversity and attachment relationships, psychological processes, and circadian neuroendocrine and immune system disturbance. We close with a discussion of the implications for future clinical and research efforts.
Methods
This paper is intended as a selective review of an emerging field of increasing importance. There are limited published data to directly inform the question of risk indicators amenable to early intervention, and much systematic research remains to be completed. This selective review used perspectives from lead investigators, actively involved in longitudinal prospective research of offspring of bipolar parents, to describe the existing data on promising early intervention targets to inform this topic. Studies were selected based on the authors’ knowledge of the literature and supplemented through a MEDLINE search using the following combination of MeSH terms: high-risk, bipolar disorder, prospective, and longitudinal. We excluded neuroimaging studies in this review, as we felt this was beyond the scope of the manuscript.
Natural History and Clinical Staging
Evidence from a number of prospective studies has demonstrated that, despite the high heritability of mood disorders, offspring of BD parents not uncommonly present with nonspecific psychiatric symptoms and syndromes early in childhood. This is true even in studies that controlled for assortative mating (ie, other parent well) and for socioeconomic effects (eg, isolated populations such as the Amish or Canadian middle class, intact families with accessible public healthcare). These early presentations have included anxiety and sleep disturbances at both the symptom and syndrome level (Shaw, 2005; Duffy et al., 2013). Interestingly, the nature of these early-risk syndromes appears variable. For example, observed anxiety disorders in high-risk children include generalized, separation and social anxiety, panic disturbance, and phobia (Duffy et al., 2013). However, obsessive compulsive and neurodevelopmental disorders are typically seen only in offspring of lithium nonresponsive parents who suffer from a poorly remitting, psychotic subtype of BD (Duffy and Carlson, 2013; Duffy, 2014b).
With longer prospective study, it became evident that these earlier varied presentations in high-risk offspring were signaling vulnerability for mood disorders. Specifically, there is now convergent evidence that high-risk offspring who manifest childhood anxiety and sleep disorders have a significantly elevated risk of developing major mood disorders (ie, depression, BD, schizoaffective-BD) in adolescence and early adulthood (Grof et al., 2009; Duffy et al., 2010; Nurnberger, 2011; Ritter et al., 2012). This raises several questions, including: (1) are these childhood risk syndromes an indicator of specific risk for mood disorder in an immature brain or an indicator of nonspecific vulnerability? (2) are there other indicators of mood disorder vulnerability in high-risk offspring at either or both the psychological and biological level? And (3) are these early presentations responsive to presumably relatively more benign and acceptable interventions that subsequently change the distribution of BD severity and progression in the population (Scott et al., 2013).
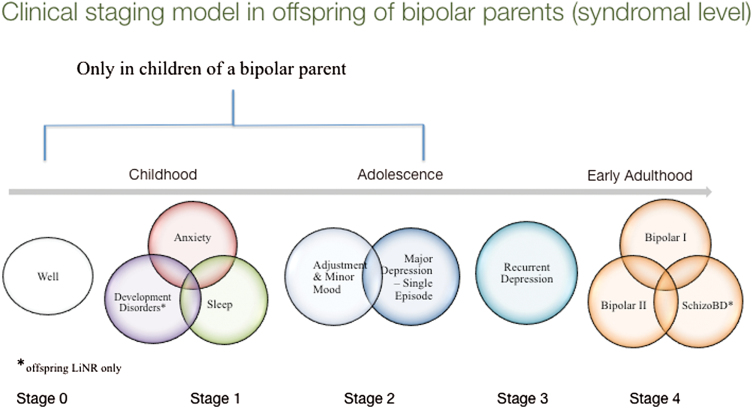
Based on almost 20 years of longitudinal observation of well-characterized BD parents divided into lithium-responsive subtypes and their family members, together with convergent findings from other longitudinal high-risk studies (Birmaher et al., 2009; Egeland et al., 2012; Mesman et al., 2013), Duffy and colleagues (2014) recently proposed a clinical staging model (Figure 1) describing the natural history of BD. This clinical staging model illustrates that in some high-risk offspring, early childhood risk syndromes transition into vulnerability to stress (adjustment disorders) and minor mood disorders during puberty. In mid- to late adolescence major depressive episodes onset, and typically several years later diagnosable activated episodes emerge (Duffy, 2014b, 2015; Duffy et al., 2014). This model is not intended to capture all variability across individual cases of BD development but serves as an aggregate description based on several hundred prospectively followed offspring of parents systematically assessed and treated and largely suffering from Bipolar I Disorder (Duffy, 2014a). The model incorporates an important but neglected aspect of heterogeneity of BD by including 2 prototypical trajectories: lithium-responsive classical episodic illness and lithium nonresponsive psychotic spectrum BD (Duffy and Grof, 2001; Grof et al., 2009).
Figure 1.
The clinical trajectory into bipolar disorder (BD) in high-risk offspring studied prospectively. LiNR, offspring of lithium nonresponder. Adapted from Duffy (2015).
A developmental approach to understanding the emergence of psychiatric disorders is important to the aim of achieving earlier accurate identification of youth on a specific trajectory to develop a serious psychiatric illness. Furthermore, by taking into account the natural history of specific disorders, one can identify targeted and novel prevention and early intervention opportunities (McGorry et al., 2010; Duffy and Carlson, 2013). To minimize false positives and over-diagnosis of normative or transient disturbance in youth, the proposed model limits the early clinical stages to youth at confirmed familial risk of BD, reflecting the high heritability of the disorder.
Promising Early Intervention Targets
Early Child Development
Mood disorders are complex and likely result from interplay between genetic, epigenetic, and environmental risk factors, which in turn predict psychological and biological vulnerability (Etain et al., 2008; Heim, 2012). Twin studies have established an independent effect of early environmental risk factors on mood disorder risk beyond genetic susceptibility (Kendler et al., 2000; Nelson et al., 2002; Silberg et al., 2010). From birth to approximately 7 years of age, children undergo major brain maturation in critical regions related to emotional regulation and cognition (Paus et al., 1999; Keverne, 2004). Stress during this critical time can have lasting negative effects through alterations in brain structure and function (Murray et al., 2004). The hypothalamic-pituitary-adrenal (HPA) axis, centrally important in the development of mood disorders, continues to develop for the first few months after birth and is especially vulnerable during this time, while the presence of attachment relationships in the following months act to regulate HPA axis activity (Doom and Gunnar, 2013).
Reports of childhood abuse and neglect are commonly reported in some studies of adult patients with BD (Garno et al., 2005). Evidence suggests that early childhood stressors may contribute to the substantial morbidity associated with BD. In particular, individuals with BD and a history of physical and sexual abuse reportedly have an earlier age of onset (Daruy-Filho et al., 2011), worsened clinical presentation and psychosocial functioning (Daglas et al., 2014), higher rate of comorbid substance abuse (Garno et al., 2005), and a higher number of suicide attempts (Daruy-Filho et al., 2011). Experiencing emotional and sexual abuse may in part explain why certain individuals with BD express suicidal behavior while some do not (Etain et al., 2008). Although less studied, it has been reported that rates of BD are higher among high-risk children with a history of childhood abuse compared with those without (Goldstein et al., 2010).
Until recently, little attention has been paid to how having a parent with BD impacts risk of developing psychopathology beyond the genetic susceptibility. This is despite compelling evidence of a significant association between exposure to parental unipolar depression in childhood and risk of mood disorder later in life (Fergusson et al., 1995; Hammen and Brennan, 2003; Murray et al., 2011; Naicker et al., 2012). Depending on the duration, severity, and timing of the illness episodes in the parents, children of an affected parent may be exposed to an irritable, agitated parent during a manic episode and a sad, distracted, and sometimes suicidal parent during a depressive episode. Furthermore, there may be emotional loss associated with parental separation either through divorce, hospitalization, or both.
In line with the observation of a critical period for HPA axis development and stress reactivity (Doom and Gunnar, 2013), we recently reported evidence that a higher exposure to parental BD during the first 2 years of life was significantly associated with the development of mood disorders in high-risk offspring (S. Goodday, A. Levy, G. Flowerdew, J. Horrocks, M. Ellenbogen, P. Grof, and A. Duffy, unpublished observations). A higher duration of exposure to parental illness during the first decade of life was also significantly associated with the development of substance use disorders (Goodday, 2015). We have also reported that perceived neglect from mother was a significant predictor of mood disorder in high-risk offspring after taking into account the amount of exposure to parental BD during the first decade of life, life stress, and offspring temperament (Doucette et al., 2014). Interestingly, other longitudinal studies of offspring of depressed mothers have reported similar findings, where poor support from the mother early in childhood was a significant predictor of unipolar depression later in life (Murray et al., 2011).
In addition to temperament, mechanisms underlying the association between early childhood adversity and development of BD have focused on disruptions in attachment and epigenetic mechanisms (Heim, 2012). Attachment relationships begin to formulate in the fourth to fifth months after birth, and evidence suggests that this relationship may have protective effects over HPA axis regulation (Doom and Gunnar, 2013). For example, even minor variations in maternal sensitivity during the first few months of life have been shown to significantly impact infant cortisol reactivity (Albers et al., 2008). Lack of expected nurturing from caregivers during this sensitive time may have lasting effects on the regulation of emotional systems (Doom and Gunnar, 2013). Findings on attachment style among offspring of BD parents have been mixed, although this may reflect differences in study populations and methods. Specifically, high risk offspring of BD parents with minimal psychiatric comorbidity and higher socio-economic status show more similar attachment patterns to controls and to the general population (Reichart et al., 2007; Doucette et al., 2013), while children from families of lower socio-economic status and higher rates of parental comorbid substance use and personality disorders have more negative attachment to parents (Chang et al., 2001; Romero et al., 2005).
Taken together, the findings suggest that early-life adversity, including exposure to parental psychopathology very early in life, mother-child interactions, and trauma such as abuse and neglect, plays a significant role in the risk of BD in those already at familial risk and may contribute to a worsened prognosis. These factors could be potential targets for earlier identification of offspring at ultra-high risk for developing BD and related mood disorders and serve as preventive intervention targets.
Psychological Processes
There is an increasing awareness of the importance of psychological processes in relation to the development and course of BD. Understanding of such processes is relevant to both development of comprehensive biopsychosocial models of BD and to the development and refinement of effective psychological therapies. We have shown that temperamental elevations in emotionality are associated with a greater risk of mood disorders in children of a BD parent and that such emotionality is linked with duration of exposure to parental illness (Doucette et al., 2013, 2014). Specific psychological processes implicated in BD, which might provide promising early intervention targets, include impulsivity, reward sensitivity, rumination, and cognitive style. Each of these processes is discussed briefly in turn below.
Impulsivity has been defined as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences” (Moeller et al., 2001). There is evidence for elevated impulsivity in BD, on both self-report and computerized assessments, across euthymic and manic phases in particular (Moeller et al., 2001; Swann et al., 2003, 2009). Higher rates of impulsivity in BD are also associated with comorbid problems including substance use and suicidality (Swann et al., 2004, 2005). There have also been suggestions that impulsivity may be an early characteristic of developing psychopathology in young child offspring of BD parents (Fergus et al., 2003; Birmaher et al., 2010).
Research into reward sensitivity has developed from 3 complimentary fields of investigation. First, there is evidence that dopamine, which is implicated in reward seeking and reward sensitivity (Wise and Rompre, 1989; Panksepp et al., 2002), can induce mania symptoms in both healthy controls and individuals with BD (Van Kammen and Murphy, 1975; Johanson and Uhlenhuth, 1980; Halbreich et al., 1981; Nurnberger et al., 1982; Sernyak and Woods, 1993). Second, research with BD patients suggests that goal attainment life events can trigger a manic episode (Johnson et al., 2000). Third, the behavioral activation system, which governs individual approach behavior in response to signals of possible reward/goal attainment, has been theorized to play a key role in BD with respect to both mania and depression (Depue and Iacono, 1989; Depue et al., 1989; Urosevic et al., 2008). It is worth noting that impulsivity and reward sensitivity are clearly inter-related phenomena. Individuals at behavioral risk of BD show preference for immediate rewards over delayed rewards, and on electroencephalography this bias is associated with greater differentiation between delayed and immediate outcomes in early attention-sensitive and later reward-sensitive (feedback-related negativity) components (Mason et al., 2012).
Prospective studies have shown that high-behavioral activation system sensitivity predicts greater likelihood of a BD spectrum diagnosis in the following year in college students (Alloy et al., 2012) as well as interacting with impulsiveness to predict academic attainment (Nusslock et al., 2008) and both BD and substance use disorder onsets over a similar period (Alloy et al., 2009). Additionally, in the presence of goal-striving events, individuals with BD, but not controls, exhibit increase manic but not depressive symptoms (Nusslock et al., 2007). In individuals with BD and those at risk, goals are regarded as more important, set more ambitiously, and with greater perceived likelihood of success than in controls (Meyer and Krumm-Merabet, 2003; Meyer et al., 2004; Johnson et al., 2005; Johnson and Carver, 2006). Furthermore, there is recent fMRI evidence of disturbed reward processes in healthy offspring of a BD parent on a monetary incentive delay task compared with controls (Singh et al., 2014).
Rumination is indicated by repetitive patterns of thinking typically about negative affect or stressful events and inferences about their causes (Smith and Alloy, 2008). Rumination has been widely reported in unipolar depression both as a prospective risk factor for onset as well as predictive of worse outcomes in individuals with depression (Nolen-Hoeksema, 2000; Spasojevic and Alloy, 2001). There is also evidence that rumination is elevated in individuals with BD even during remission (Jones et al., 2006b; Thomas et al., 2007) and in individuals at behavioral risk (Knowles et al., 2005). This pattern of increased rumination has also been observed in teenage offspring of a BD parent (ie, who have not yet transitioned to BD) (Jones et al., 2006b; Pavlickova et al., 2014). More recently, researchers have begun to investigate positive rumination, described as repetitive thinking with respect to one’s own achievements and positive mood (Feldman et al., 2008). Positive rumination is positively associated with behavioral risk for mania (Feldman et al., 2008) and is elevated in BD compared with unipolar depressed or control participants (Johnson et al., 2008).
Research into cognitive styles suggests evidence for unstable self-esteem and elevated positive self-appraisal in BD (Bentall et al., 2005; Jones et al., 2006a; Knowles et al., 2007). Prospectively, low explicit self-esteem appears to predict increased risk of both mania and depression in individuals diagnosed with BD (Pavlickova et al., 2013). We have also observed low and unstable explicit self-esteem in teenage offspring of bipolar parents (Jones et al., 2006b; Pavlickova et al., 2014). Although these phenomena seem to be specifically associated with the presence of depressive symptoms (Pavlickova et al., 2015), there is some evidence that manic symptoms in high-risk teenagers are associated with low implicit self-esteem (Pavlickova et al., 2014b).
The exploration of positive self-appraisal emerged from a model of BD that attempted to understand how circadian vulnerabilities (described below in 3) might translate in the patterns of symptoms and behavior observed in bipolar mood episodes (Jones, 2001). This proposed that mania symptoms in particular are more likely to occur when the individual applies a positive self-dispositional appraisal to experiences of significant circadian disturbance. This appraisal style has been observed in adults with BD (Jones et al., 2006a, 2006b) as well as in adolescent and adult individuals at behavioral risk (Cooke and Jones, 2009; Johnson and Jones, 2009) and has been associated with the extent to which positive experiences such as inspiration are linked to risk of mania (Jones et al., 2014). Regression analysis of the relative contributions of positive self-dispositional appraisal and familial risk in relation to mania risk in teenagers indicated that positive self-appraisal was the most significant predictor (Espie et al., 2012).
Taken overall, there is accumulating evidence for the importance of temperament, impulsivity, reward sensitivity, rumination, and self-appraisal processes in the development and recurrence of BD. It is therefore important to further evaluate the ability of markers of these processes to serve as predictors, separately and in combination, of risk for development of BD. In addition, structured psychological therapies have already been shown to have benefits in more recent onset BD (Scott et al., 2006; Jones et al., 2015). Further refinement of such interventions to better target these processes may improve the efficacy in BD and also provide options for preventative interventions in high-risk groups (Berk et al., 2007).
Circadian Neuroendocrine Immune Disturbances
Disruption in circadian rhythm is emerging as an important indicator of susceptibility to BD (Baethge et al., 2003; Murray and Harvey, 2010; Edgar and McClung, 2013; Geoffrey, 2014). The endogenous circadian system modulates mood and the sleep-wake cycle in mammals (Baethge et al., 2003; Murray and Harvey, 2010). The circadian system is a network of open feedback loops governed by the circadian pacemaker located in the hypothalamic suprachiasmatic nucleus and influenced by a number of inputs including exposure to light, arousal levels, and social and psychological cues (Harvey et al., 2011). Cells of the suprachiasmatic nucleus are themselves genetically regulated by transcriptional and posttranscriptional clock proteins (Azzi et al., 2014), and epigenetic mechanisms underlie the substantial plasticity in circadian behavior (Stevenson and Prendergast, 2013; Azzi et al., 2014). Prospective data have shown that changes in sleep and circadian rhythm precede and predict the onset of mood episodes (depressive and manic) in BD patients (Bauer et al., 2008). Emotional regulation and circadian disturbances are associated with neurotransmitter systems (Lamont et al., 2007; McClung, 2007) and genes that are implicated in the etiology of BD (Mitterauer, 2000; Benedetti et al., 2007; McClung, 2007; Harvey, 2008). Lithium and other maintenance treatments are known to stabilize circadian function (Klemfuss, 1992; Li et al., 2002; Yin et al., 2006), and circadian clock genes have been associated with response to lithium prophylaxis in BD patients (Rybakowski et al., 2014). Of specific relevance here is evidence that circadian disturbance persists during euthymic periods in remitted BD patients (Millar et al., 2004; Harvey et al., 2005; Geoffroy et al., 2014) and may be a trait marker and candidate endophenotype in high-risk children (Nurnberger et al., 1988; Jones et al., 2006b; Ritter et al., 2012; Ng et al., 2015). Specifically, remitted BD patients have shown longer sleep latency, higher fragmentation index, lower sleep quality, and lower sleep efficiency compared with controls (Geoffroy et al., 2014). While most studies have been completed in BD adults, there is some evidence of differences in sleep patterns in high-risk compared with control offspring (Jones et al., 2006b) and in healthy individuals at behavioral risk (Ankers and Jones, 2009). Also, we have reported that sleep disorders are childhood antecedents predicting an increased risk of mood disorders in high-risk offspring (Grof et al., 2009; Ritter et al., 2012; Duffy et al., 2014), consistent with the hypothesis that circadian disturbance is a vulnerability marker in youth at familial risk.
Chronic inflammation and dysregulation of the immune response is a known disease mechanism in a variety of disorders, including cancer, dementia, and more recently mood disorders. There is accruing evidence from clinical and animal studies of an association between mood episodes of both depressive and manic polarity and low-grade chronic inflammation (Raison and Miller, 2011; Leonard and Maes, 2012). Proinflammatory cytokines such as IL-1β, IL-6, and TNFα have been associated with depressive and anxious behavioral states when administered both systemically and centrally in animals (Anisman et al., 2005; Song et al., 2006; Song and Wang, 2011). Increased serum levels of these inflammatory mediators and acute phase proteins have been associated with acute episodes of depression and mania in humans (Goldstein et al., 2009; Kauer-Sant’Anna et al., 2009; Song et al., 2009; Berk et al., 2011; Maes et al., 2012). Furthermore, markers of cell-mediated immune activation have been associated with depression (Maes, 2011; Maes et al., 2012) and BD (Munkholm et al., 2013).
Cytokines and other immune mediators directly and indirectly access the brain and widely influence brain function. Cytokines interact with every pathophysiological domain implicated in mood disorders. As reviewed by McClung (2013), mechanisms mediating the association between a proinflammatory state and mood disorders include circadian disturbance (Muller-Oerlinghausen et al., 2005), activation of the HPA axis (Raison and Miller, 2011), and modulation of monoamine neurotransmitter systems (Felger and Lotrich, 2013). With progressive burden of illness, there is evidence of amplified changes in inflammatory markers, increased oxidative stress, mitochondrial dysfunction, and decreased neurotrophic factors (Berk et al., 2011; Maes et al., 2011; Fries et al., 2012; Frey et al., 2013). Lithium and other mood stabilizers regulate inflammatory mediators and circadian rhythm (Murray and Harvey, 2010), while several classes of antidepressants normalize the proinflammatory bias in treatment-responsive patients (Maes, 2001; Song et al., 2009; Berk et al., 2011; Raison et al., 2013).
Most studies of inflammatory markers have been cross-sectional in nature and involved adult BD patients and are therefore confounded by substantial burden of illness effects. It is conceivable that recognizable differences in cytokine overactivity are present in high-risk individuals during the early stages of illness development. Padmos and colleagues (2008) reported increased expression of proinflammatory genes in remitted adults with BD and their high-risk offspring, especially in those who went on to develop depressive disorders. In a more recent cross-sectional analysis of prospectively assessed high-risk offspring, these authors reported evidence of increased inflammatory gene expression in monocytes during adolescence and early adulthood in high-risk offspring compared with controls (Mesman et al., 2015). Similarly, in a cross-sectional pilot study of prospectively assessed high-risk offspring, we reported evidence of increased IL-6 and BDNF plasma protein levels in high-risk offspring compared with controls and in high-risk offspring in the earlier compared with the later clinical stages of illness development (Duffy, 2014b). These associations were moderated by specific genetic variants.
Collectively, findings suggest that, for at least a subset of high-risk individuals, perturbations in the circadian and neuro-immune system influencing emotional functioning are promising candidate markers of vulnerability in high-risk youth associated with the subsequent onset of mood disorders (Maes, 2011; Insel, 2012; Raison and Miller, 2013; Ng et al., 2015). Furthermore, preliminary evidence suggests that changes in these markers parallel development and progression of mood disorders, and the strength of these associations may be influenced by genetic variants (Duffy, 2014b).
Closing Remarks and Future Directions
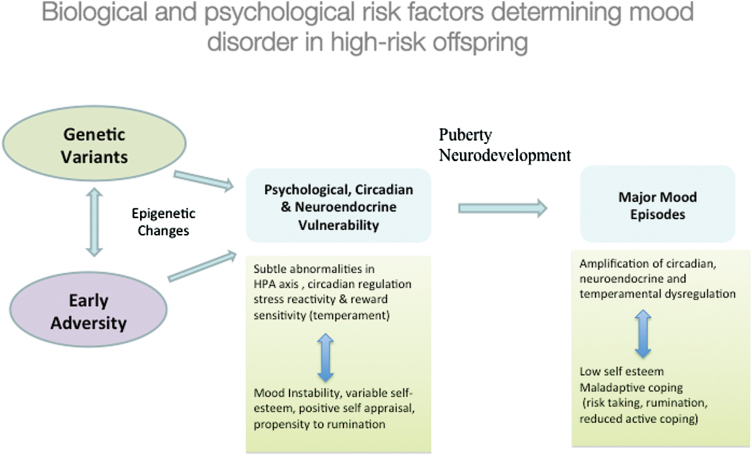
BD is a multi-gene disorder, and it is becoming increasingly clear that in the majority of cases, identifying genetic variants associated with increased susceptibility to BD is going to be insufficient to fully understand how those at familial risk develop illness. We now realize that genetic vulnerability may manifest in a variety of interactive pathways, including genetically determined sensitivity to early adversity, higher emotional reactivity to stress programmed during critical periods of development, unstable or vulnerable psychological processes and maladaptive coping styles that act both as risk and perpetuating factors, and through genetically mediated circadian and neuroendocrine pathways moderated by other risk exposures (Figure 2). Longitudinal studies of children at confirmed familial risk for developing BD provide an ideal approach to investigate these interactive processes at different stages of biological, psychological, and illness development, assessing both the individual and combined influences on risk and illness progression. The high-risk approach also provides the opportunity for identifying and understanding factors that contribute to resiliency in the face of risk and which may not be simply the inverse of the identified risk factors (eg, low compared with high emotionality). Longitudinal high-risk studies have already provided substantial evidence that BD is developing many years prior to the first diagnosable activated (ie, manic/hypomanic) episode and jeopardizes normative emotional, psychological, social, and likely neurobiological development.
Figure 2.
The onset of major psychiatric disorders like bipolar disorder (BD) are likely the result of a dynamic interplay between predisposition and other risk factors, compounded by the illness burden and compensatory processes. HPA, hypothalamic pituitary adrenal.
The proposed aggregate clinical staging model requires refinement based on systematic longitudinal studies of multiple candidate vulnerability and illness progression markers in parallel in well-characterized high-risk offspring. This information could inform a comprehensive risk model that takes into account genetically sensitive intermediate pathways, allowing for the placement of an individual on an illness continuum and refined monitoring of illness progression as routinely occurs in other areas of medicine (McGorry, 2013). Moreover, a fuller understanding of the important early exposures that interact with genetic risk and the psychological processes that mediate between genetic risk and mood disorder onset would provide the necessary basis for developing and refining targeted prevention and early intervention strategies.
This review was limited in a number of important ways that should be mentioned. First, this was not intended to be a systematic review of the literature but rather a selective review of promising early intervention targets focusing on the findings from longitudinal prospective offspring studies. Findings from neuroimaging studies were not included in this review, as the practical application to early intervention is at this time not clear and beyond the scope of the paper. Second, the published studies discussed vary in terms of recruitment strategies, inclusion and exclusion criteria, and methods of assessment, which can affect outcomes of interest and therefore should be kept in mind (Duffy et al., 2011).
Practical implications of the already available evidence suggest that parents and parents-to-be suffering from BD should be provided with intensive medical, psychological, educational, and family support to ensure the best quality of remission, prevent postpartum recurrences, maintain stability of circadian rhythm, and facilitate healthy attachments with their newborn. Furthermore, children of BD parents should be monitored for early risk syndromes, including circadian disturbance, stress/emotional reactivity, anxiety disorders, and psychological vulnerability (rumination, unstable self-esteem, impulsivity, and reward sensitivity/risk taking). Finally, there is sufficient evidence to justify the development of specialized high-risk and early intervention programs that engage children and teenagers who are at identifiable (familial, clinical) increased risk of serious mood disorders and provide education about risk factors (ie, substance abuse), sleep regulation, optimal diet, healthy coping, exercise, and suitability for evidence-based step-wise intervention as needed.
Statement of Interest
None.
Acknowledgments
Some of the high-risk research cited was supported by an operating grant (MOP 102761) from the Canadian Institutes for Health Research.
We thank our research participants and their family members for their commitment to the high-risk research.
References
- Albers EM, Riksen-Walraven JM, Sweep FC, de Weerth C. (2008) Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry 49:97–103. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, Sylvia LG, Harmon-Jones E. (2009) Bipolar spectrum–substance use co-occurrence: behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J Pers Soc Psychol 97:549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, Abramson LY. (2012) High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. J Abnorm Psychol, 121:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, Hayley S. (2005) Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des 11:963–972. [DOI] [PubMed] [Google Scholar]
- Ankers D, Jones SH. (2009) Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. J Clin Psychol 65:1071–1086. [DOI] [PubMed] [Google Scholar]
- Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, Kramer A, Brown SA. (2014) Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Neurosci 17:377–382. [DOI] [PubMed] [Google Scholar]
- Baethge C, Tondo L, Bratti IM, Bschor T, Bauer M, Viguera AC, Baldessarini RJ. (2003) Prophylaxis latency and outcome in bipolar disorders. Can J Psychiatry 48:449–457. [DOI] [PubMed] [Google Scholar]
- Bauer M, Glenn T, Whybrow PC, Grof P, Rasgon N, Alda M, Marsh W, Sagduyu K, Schmid R, Adli M. (2008) Changes in self-reported sleep duration predict mood changes in bipolar disorder. Psychol Med 38:1069–1071. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, Colombo C, Smeraldi E. (2007) Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet 144B:631–635. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Kinderman P, Manson K. (2005) Self-discrepancies in bipolar disorder: comparison of manic, depressed, remitted and normal participants. Br J Clin Psychol 44: 457–473. [DOI] [PubMed] [Google Scholar]
- Berk M, Hallam KT, McGorry PD. (2007) The potential utility of a staging model as a course specifier: a bipolar disorder perspective. J Affect Disord 100:279–281. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS. (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. (2009) Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry 66:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Monk K, Kalas C, Obreja M, Hickey MB, Iyengar S, Brent D, Shamseddeen W, Diler R, Kupfer D. (2010) Psychiatric disorders in preschool offspring of parents with bipolar disorder: The Pittsburgh Bipolar Offspring Study (BIOS). Am J Psychiatry 167:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. (2003) Psychological autopsy studies of suicide: a systematic review. Psychol Med 33:395–405. [DOI] [PubMed] [Google Scholar]
- Chang KD, Blasey C, Ketter TA, Steiner H. (2001) Family environment of children and adolescents with bipolar parents. Bipolar Disord 3:73–78. [DOI] [PubMed] [Google Scholar]
- Cooke LS, Jones SH. (2009) An evaluation of cognitions, mood and behaviours in late adolescents: a study of associations with risk for bipolar disorder. Pers Indiv Differ 46:314–318. [Google Scholar]
- Daglas R, Conus P, Cotton SM, Macneil CA, Hasty MK, Kader L, Berk M, Hallam KT. (2014) The impact of past direct-personal traumatic events on 12-month outcome in first episode psychotic mania: trauma and early psychotic mania. Aust Nz J Psychiatry 48:1017–1024. [DOI] [PubMed] [Google Scholar]
- Daruy-Filho L, Brietzke E, Lafer B, Grassi-Oliveira R. (2011) Childhood maltreatment and clinical outcomes of bipolar disorder. Acta Psychiat Scand 124:427–434. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. (1989) Neurobehavioral aspects of affective disorders. Annu Rev Psychol 40:457–492. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, Arbisi P. (1989) General behavior inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. J Abnorm Psychol 98:117–126. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR. (2013) Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol 25:1359–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette S, Horrocks J, Grof P, Keown-Stoneman C, Duffy A. (2013) Attachment and temperament profiles among the offspring of a parent with bipolar disorder. J Affect Disord 150:522–526. [DOI] [PubMed] [Google Scholar]
- Doucette S, Levy A, Flowerdew G, Horrocks J, Grof P, Ellenbogen M, Duffy A. (2014) Early parent-child relationships and risk of mood disorder in a Canadian sample of offspring of a parent with bipolar disorder: findings from a 16-year prospective cohort study. Early Interv Psychiatry DOI: 10.1111/eip.12195. [DOI] [PubMed] [Google Scholar]
- Duffy A.(2014a) Author’s reply. Br J Psychiatry 204:494. [DOI] [PubMed] [Google Scholar]
- Duffy A.(2014b) Towards a comprehensive clinical staging model for bipolar disorder: integrating the evidence. Can J Psychiatry 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A. (2015) Early identification of recurrent mood disorders in youth: the importance of a developmental approach. Evid Based Ment Health 18:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A, Alda M, Hajek T, Sherry SB, Grof P. (2010) Early stages in the development of bipolar disorder. J Affect Disord 121:127–135. [DOI] [PubMed] [Google Scholar]
- Duffy A, Carlson GA. (2013) How does a developmental perspective inform us about the early natural history of bipolar disorder? J Can Acad Child Adolesc Psychiatry 22:6–12. [PMC free article] [PubMed] [Google Scholar]
- Duffy A, Doucette S, Lewitzka U, Alda M, Hajek T, Grof P. (2011) Findings from bipolar offspring studies: methodology matters. Early Interv Psychiatry 5:181–191. [DOI] [PubMed] [Google Scholar]
- Duffy A, Grof P. (2001) Psychiatric diagnoses in the context of genetic studies of bipolar disorder. Bipolar Disord 3:270–275. [DOI] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. (2013) Childhood anxiety: an early predictor of mood disorders in offspring of bipolar parents. J Affect Disord 150:363–369. [DOI] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. (2014) The developmental trajectory of bipolar disorder. Br J Psychiatry 204:122–128. [DOI] [PubMed] [Google Scholar]
- Edgar N, McClung CA. (2013) Major depressive disorder: a loss of circadian synchrony? Bioessays 35:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland JA, Endicott J, Hostetter AM, Allen CR, Pauls DL, Shaw JA. (2012) A 16-year prospective study of prodromal features prior to BPI onset in well Amish children. J Affect Disord 142:186–192. [DOI] [PubMed] [Google Scholar]
- Espie J, Jones SH, Vance YH, Tai SJ. (2012) Brief report: a family risk study exploring bipolar spectrum problems and cognitive biases in adolescent children of bipolar parents. J Adolescence 35:769–772. [DOI] [PubMed] [Google Scholar]
- Etain B, Henry C, Bellivier F, Mathieu F, Leboyer M. (2008) Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord 10:867–876. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, Johnson SL. (2008) Responses to positive affect: a self-report measure of rumination and dampening. Cognit Ther Res 32:507–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus EL, Miller RB, Luckenbaugh DA, Leverich GS, Findling RL, Speer AM, Post RM. (2003) Is there progression from irritability/dyscontrol to major depressive and manic symptoms? A retrospective community survey of parents of bipolar children. J Affect Disord 77:71–78. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. (1995) Maternal depressive symptoms and depressive symptoms in adolescents. J Child Psychol Psychiatry 36:1161–1178. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant’Anna M, Yatham LN, Kapczinski F, Young LT. (2013) Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust Nz J Psychiatry 47:321–332. [DOI] [PubMed] [Google Scholar]
- Fries GR, Pfaffenseller B, Stertz L, Paz AV, Dargel AA, Kunz M, Kapczinski F. (2012) Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep 14:667–675. [DOI] [PubMed] [Google Scholar]
- Garno JL, Goldberg JF, Ramirez PM, Ritzler BA. (2005) Impact of childhood abuse on the clinical course of bipolar disorder. Br J Psychiatry 186:121–125. [DOI] [PubMed] [Google Scholar]
- Geoffrey PA, Etain B, Sportiche S, Bellivier F. (2014) Circadian biomarkers in patients with bipolar disorder: promsing putative predictors of lithium response. Int J Bipolar Disord 2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Boudebesse C, Bellivier F, Lajnef M, Henry C, Leboyer M, Scott J, Etain B. (2014) Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. J Affect Disord 158:1–7. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. (2009) Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry 70:1078–1090. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Axelson DA, Kalas C, Monk K, Brent DA, Kupfer DJ, Birmaher B. (2010) Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adol Psychiatry 49:388–396. [PMC free article] [PubMed] [Google Scholar]
- Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, Sawyer SM, Mathers CD. (2011) Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet 377:2093–2102. [DOI] [PubMed] [Google Scholar]
- Grof P, Duffy A, Alda M, Hajek T. (2009) Lithium response across generations. Acta Psychiatr Scand 120:378–385. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Asnis G, Ross D, Endicott J. (1981) Amphetamine-induced dysphoria in postmenopausal women. Br J Psychiatry 138:470–473. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. (2003) Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry 60: 253–258. [DOI] [PubMed] [Google Scholar]
- Harvey AG. (2008) Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry 165:820–829. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. (2005) Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry 162:50–57. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, Soehner A. (2011) Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev 31:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C BE. (2012) Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 233:102–111. [DOI] [PubMed] [Google Scholar]
- Insel TR. (2012) Next-generation treatments for mental disorders. Sci Transl Med 4:155ps119. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. (1980) Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 71:275–279. [DOI] [PubMed] [Google Scholar]
- Johnson S, McKenzie G, McMurrich S. (2008) Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cog Ther Res 32:702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS. (2006) Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognit Ther Res 30:377–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Jones S. (2009) Cognitive correlates of mania risk: are responses to success, positive moods, and manic symptoms distinct or overlapping? J Clin Psychol 65:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero CJ, Carver CS. (2005) Cognitive, behavioral, and affective responses to reward: links with hypomanic symptoms. J Soc Clin Psychol 24:894–906. [Google Scholar]
- Jones S, Mansell W, Waller L.(2006a) Appraisal of hypomania-relevant experiences: development of a questionnaire to assess positive self-dispositional appraisals in bipolar and behavioural high risk samples. J Affect Disord 93:19–28. [DOI] [PubMed] [Google Scholar]
- Jones S, Dodd A, Gruber J. (2014) Development and validation of a new multidimensional measure of inspiration: associations with risk for bipolar disorder. PLoS One 9:e91669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH. (2001) Circadian rhythms, multilevel models of emotion and bipolar disorder: an initial step towards integration? Clin Psychol Rev 21:1193–1209. [DOI] [PubMed] [Google Scholar]
- Jones SH, Tai S, Evershed K, Knowles R, Bentall R.(2006b) Early detection of bipolar disorder: a pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord 8:362–372. [DOI] [PubMed] [Google Scholar]
- Jones SH, Smith G, Mulligan LD, Lobban F, Law H, Dunn G, Welford M, Kelly J, Mulligan J, Morrison AP. (2015) Recovery-focused cognitive-behavioural therapy for recent-onset bipolar disorder: randomised controlled pilot trial. Br J Psychiatry 206:58–66. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS. (2003) The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disorders 73:123–131. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, Yatham LN. (2009) Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol 12:447–458. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. (2000) Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 57:953–959. [DOI] [PubMed] [Google Scholar]
- Keverne EB. (2004) Understanding well-being in the evolutionary context of brain development. Phil Trans R Soc B 359:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemfuss H. (1992) Rhythms and the pharmacology of lithium. Pharmacol Ther 56:53–78. [DOI] [PubMed] [Google Scholar]
- Knowles R, Tai S, Christensen I, Bentall R. (2005) Coping with depression and vulnerability to mania: a factor analytic study of the Nolen-Hoeksema (1991) Response Styles Questionnaire. Br J Psychiatry 44:99–112. [DOI] [PubMed] [Google Scholar]
- Knowles R, Tai S, Jones SH, Highfield J, Morriss R, Bentall RP. (2007) Stability of self-esteem in bipolar disorder: comparisons among remitted bipolar patients, remitted unipolar patients and healthy controls. Bipolar Disord 9:490–495. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. (2007) The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci 9:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M. (2012) Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36:764–785. [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS. (2002) Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord 4:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. (2001) The immunoregulatory effects of antidepressants. Hum Psychopharmacol 16:95–103. [DOI] [PubMed] [Google Scholar]
- Maes M. (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Neuropsychopharm Bio Psychiatry 35:664–675. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M. (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Neuropsychopharm Bio Psychiatry 35:676–692. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Ringel K. (2012) Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Progress in neuro-psychopharmacology & biological psychiatry 36:169–175. [DOI] [PubMed] [Google Scholar]
- McClung CA. (2007) Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther 114:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. (2013) How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry 74:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD. (2013) The next stage for diagnosis: validity through utility. World Psychiatry 12:213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Goldstone S, Yung AR. (2010) Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry 55:486–497. [DOI] [PubMed] [Google Scholar]
- Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MH. (2013) The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry 170:542–549. [DOI] [PubMed] [Google Scholar]
- Mesman E, Hillegers MH, Ambree O, Arolt V, Nolen WA, Drexhage HA. (2015) Monocyte activation, brain-derived neurotrophic factor (BDNF), and S100B in bipolar offspring: a follow-up study from adolescence into adulthood. Bipolar Disord 17:39–49. [DOI] [PubMed] [Google Scholar]
- Meyer B, Beevers CG, Johnson SL. (2004) Goal appraisals and vulnerability to bipolar disorder: a personal projects analysis. Cog Ther Res 28:173–182. [Google Scholar]
- Meyer TD, Krumm-Merabet C. (2003) Academic performance and expectations for the future in relation to a vulnerability marker for bipolar disorders: the hypomanic temperament. Pers Indiv Differ 35:785–796. [Google Scholar]
- Millar A, Espie CA, Scott J. (2004) The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord 80:145–153. [DOI] [PubMed] [Google Scholar]
- Mitterauer B. (2000) Clock genes, feedback loops and their possible role in the etiology of bipolar disorders: an integrative model. Med Hypotheses 55:155–159. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- Muller-Oerlinghausen B, Felber W, Berghofer A, Lauterbach E, Ahrens B. (2005) The impact of lithium long-term medication on suicidal behavior and mortality of bipolar patients. Arch Suicide Res 9:307–319. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Vedel Kessing L. (2013) Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord 144:16–27. [DOI] [PubMed] [Google Scholar]
- Murray G, Harvey A. (2010) Circadian rhythms and sleep in bipolar disorder. Bipolar Disord 12:459–472. [DOI] [PubMed] [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P. (2011) Maternal postnatal depression and the development of depression in offspring up to 16 years of age. J Am Acad Child Adol Psychiatry 50:460–470. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. (2004) A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizo Res 71:405–416. [DOI] [PubMed] [Google Scholar]
- Naicker K, Wickham M, Colman I. (2012) Timing of first exposure to maternal depression and adolescent emotional disorder in a national Canadian cohort. PloS one 7:e33422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. (2002) Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry 59:139–145. [DOI] [PubMed] [Google Scholar]
- Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH. (2015) Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev 20:46–58. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (2000) The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 109:504–511. [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Gershon ES, Simmons S, Ebert M, Kessler LR, Dibble ED, Jimerson SS, Brown GM, Gold P, Jimerson DC, Guroff JJ, Storch FI. (1982) Behavioral, biochemical and neuroendocrine responses to amphetamine in normal twins and ‘well-state’ bipolar patients. Psychoneuroendocrinology 7:163–176. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. (1988) Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharm 1:217–223. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, McInnis M, Reich W, Kastelic E, Wilcox HC, Glowinski A, et al. (2011) A high-risk study of bipolar disorder. Childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry 68:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. (2007) A goal-striving life event and the onset of hypomanic and depressive episodes and symptoms: perspective from the behavioral approach system (BAS) dysregulation theory. J Abnorm Psychol 116:105–115. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB, Abramson LY, Harmon-Jones E, Hogan ME. (2008) Impairment in the achievement domain in bipolar spectrum disorders: role of behavioral approach system hypersensitivity and impulsivity. Minerva Pediatr 60:41–50. [PubMed] [Google Scholar]
- Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, de Ridder D, Kupka RW, Nolen WA, Drexhage HA. (2008) A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry 65:395–407. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. (2002) The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction 97:459–469. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. (1999) Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283:1908–1911. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlickova H, Varese F, Turnbull O, Scott J, Morriss R, Kinderman P, Paykel E, Bentall RP. (2013) Symptom-specific self-referential cognitive processes in bipolar disorder: a longitudinal analysis. Psychol Med 43:1895–1907. [DOI] [PubMed] [Google Scholar]
- Pavlickova H, Turnbull O, Bentall RP. (2014) Discrepancies between explicit and implicit self-esteem and their relationship to symptoms of depression and mania. Psychol Psychother Theor Res Pract 87: 311–323. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. (2011) Is depression an inflammatory disorder? Curr Psychiatry Rep 13:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. (2013) Malaise, melancholia and madness: the evolutionary legacy of an inflammatory bias. Brain Behav Immun 31:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichart CG, van der Ende J, Hillegers MH, Wals M, Bongers IL, Nolen WA, Ormel J, Verhulst FC. (2007) Perceived parental rearing of bipolar offspring. Acta Psychiatr Scand 115:21–28. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Lewtschenko N, Pfeiffer S, Leopold K, Bauer M, Pfennig A. (2012) The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. J Neural Transm 119:1173–1184. [DOI] [PubMed] [Google Scholar]
- Romero S, Delbello MP, Soutullo CA, Stanford K, Strakowski SM. (2005) Family environment in families with versus families without parental bipolar disorder: a preliminary comparison study. Bipolar Disord 7:617–622. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, Hauser J. (2014) Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord 16:151–158. [DOI] [PubMed] [Google Scholar]
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H. (2006) Cognitive-behavioural therapy for bipolar disorder. Br J Psychiatry 188:488–489. [DOI] [PubMed] [Google Scholar]
- Scott J, Leboyer M, Hickie I, Berk M, Kapczinski F, Frank E, Kupfer D, McGorry P. (2013) Clinical staging in psychiatry: a cross-cutting model of diagnosis with heuristic and practical value. Br J Psychiatry 202:243–245. [DOI] [PubMed] [Google Scholar]
- Sernyak MJ, Woods SW. (1993) Chronic neuroleptic use in manic-depressive illness. Psychopharmacol Bull 29:375–381. [PubMed] [Google Scholar]
- Shaw JA EJ, Endicott J, et al. (2005) A 10-year prospective study of prodromal patterns for bipolar disorder among Amish youth. J Am Acad Child Adolesc Psychiatry 44:1104–1111. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Maes H, Eaves LJ. (2010) Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. J Child Psychol Psychiatry 51:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. (2014) Reward processing in healthy offspring of parents with bipolar disorder. JAMA psychiatry 71:1148–1156. [DOI] [PubMed] [Google Scholar]
- Smith JM, Alloy LB. (2009) A roadmap to rumination: a review of the definition, assessment, and conceptualization of this multifaceted construct. Clin Psychol Rev 29:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE. (2006) The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry 39:88–99. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard BE, Luo H. (2009) Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry 42:182–188. [DOI] [PubMed] [Google Scholar]
- Song C, Wang H. (2011) Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Neuropsychopharm Bio Psychiatry 35:760–768. [DOI] [PubMed] [Google Scholar]
- Spasojevic J, Alloy LB. (2001) Rumination as a common mechanism relating depressive risk factors to depression. Emotion 1:25–37. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. (2013) Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad Sci U S A 110:16651–16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. (2003) Impulsivity and phase of illness in bipolar disorder. J Affect Disord 73:105–111. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG. (2004) Impulsivity: a link between bipolar disorder and substance abuse. Bipolar Disord 6:204–212. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. (2005) Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry 162:1680–1687. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. (2009) Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord 11:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Knowles R, Tai S, Bentall RP. (2007) Response styles to depressed mood in bipolar affective disorder. J Affect Disord 100:249–252. [DOI] [PubMed] [Google Scholar]
- Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB. (2008) Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clin Psychol Rev 28:1188–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kammen DP, Murphy DL. (1975) Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 44:215–224. [DOI] [PubMed] [Google Scholar]
- Windfuhr K, While D, Hunt I, Turnbull P, Lowe R, Burns J, Swinson N, Shaw J, Appleby L, Kapur N. (2008) Suicide in juveniles and adolescents in the United Kingdom. J Child Psychol Psychiatry 49:1155–1165. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. (2006) Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 311:1002–1005. [DOI] [PubMed] [Google Scholar]