Abstract
Background:
The pharmacokinetics of oral lysergic acid diethylamide are unknown despite its common recreational use and renewed interest in its use in psychiatric research and practice.
Methods:
We characterized the pharmacokinetic profile, pharmacokinetic-pharmacodynamic relationship, and urine recovery of lysergic acid diethylamide and its main metabolite after administration of a single oral dose of lysergic acid diethylamide (200 μg) in 8 male and 8 female healthy subjects.
Results:
Plasma lysergic acid diethylamide concentrations were quantifiable (>0.1ng/mL) in all the subjects up to 12 hours after administration. Maximal concentrations of lysergic acid diethylamide (mean±SD: 4.5±1.4ng/mL) were reached (median, range) 1.5 (0.5–4) hours after administration. Concentrations then decreased following first-order kinetics with a half-life of 3.6±0.9 hours up to 12 hours and slower elimination thereafter with a terminal half-life of 8.9±5.9 hours. One percent of the orally administered lysergic acid diethylamide was eliminated in urine as lysergic acid diethylamide, and 13% was eliminated as 2-oxo-3-hydroxy-lysergic acid diethylamide within 24 hours. No sex differences were observed in the pharmacokinetic profiles of lysergic acid diethylamide. The acute subjective and sympathomimetic responses to lysergic acid diethylamide lasted up to 12 hours and were closely associated with the concentrations in plasma over time and exhibited no acute tolerance.
Conclusions:
These first data on the pharmacokinetics and concentration-effect relationship of oral lysergic acid diethylamide are relevant for further clinical studies and serve as a reference for the assessment of intoxication with lysergic acid diethylamide.
Keywords: LSD, O-H-LSD, pharmacokinetics, pharmacodynamics, plasma, urine
Introduction
Lysergic acid diethylamide (LSD) is a prototypical hallucinogen (Nichols, 2004; Passie et al., 2008). LSD became famous as a psychedelic in the 1960s, and its recreational use continues (Passie et al., 2008). However, no clinical research has been conducted with LSD since the 1970s until recently (Gasser et al., 2014; Kupferschmidt, 2014). Almost no scientific clinical pharmacological data on LSD are available. Specifically, the pharmacokinetics (PK) of oral LSD in humans are unknown. A small PK study administered single intravenous doses of 2 μg/kg in 5 healthy male human subjects (Aghajanian and Bing, 1964). Blood samples were taken up to 8 hours after administration. Plasma concentrations were 6 to 7ng/mL 30 minutes after intravenous administration, 4–6ng/mL at 30–120min, and approximately 1ng/mL at 8 hours. The mean plasma elimination half-life of LSD was estimated at 175 minutes in this previous study. In another study, single oral doses of 160 μg were administered to 13 male human subjects, and blood was sampled nonsystematically at various time points up to a maximum of 2.5 to 5 hours. Plasma levels peaked 40 to 130 minutes after LSD administration, and peaks ranged from 1.8 to 8.8ng/mL (Upshall and Wailling, 1972). The dataset and short sampling time did not allow the calculation of PK parameters.
The aim of the present study was to characterize the single-dose kinetics and PK-pharmacodynamic relationships of LSD in healthy male and female subjects. For clinical and forensic toxicologists, it is important to know the toxicokinetics of LSD and how plasma concentrations of LSD are linked to its dynamic effects and signs of intoxication.
LSD was administered in a single oral dose of 200 μg. The same dose was used in a clinical study (Gasser et al., 2014). The dose used was within the range of doses (50–400 μg) taken for recreational purposes and expected to induce a full “LSD reaction” (Nichols, 2004; Passie et al., 2008). The study also evaluated the acute subjective, autonomic, and endocrine effects of LSD. The pharmacodynamics are reported in detail elsewhere (Schmid et al., 2014), but the PK-pharmacodynamic relationships are presented herein.
Methods
Study Design
The study used a double-blind, placebo-controlled, cross-over design with 2 experimental test sessions in balanced order. The washout periods between sessions were at least 7 days. The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines in Good Clinical Practice and approved by the Ethics Committee of the Canton of Basel, Switzerland and the Swiss Agency for Therapeutic Products (Swissmedic). The administration of LSD to healthy subjects was authorized by the Swiss Federal Office for Public Health, Bern, Switzerland. The study was registered at ClinicalTrials.gov (NCT01878942). All of the subjects provided written informed consent after being given written and oral descriptions of the study, the procedures involved, and the effects and possible risks of LSD administration.
Participants
Sixteen healthy subjects (8 men and 8 women; mean age±SD: 28.6±6.2 years; range: 25–51 years) were included. The exclusion criteria are reported in detail elsewhere (Schmid et al., 2014) and included age <25 or >65 years, pregnancy, personal or family (first-degree relative) history of psychotic or major affective disorder, regular use of medications, chronic or acute physical illness, lifetime prevalence of illicit drug use >10 times (except for tetrahydrocannabinol), illicit drug use within the last 2 months, and illicit drug use during the study. Nine subjects were hallucinogen-naive, and the other 7 had limited prior experience with hallucinogenic drugs, including 1 subject who had used LSD once and 2 subjects who had used LSD twice. The subjects were asked to abstain from excessive alcohol consumption between test sessions and particularly limit their use to 1 drink on the day before the test sessions. Additionally, the participants were not allowed to drink xanthine-containing liquids after midnight before the study day. Three subjects were light smokers (<10 cigarettes/d) and were told to maintain their usual smoking habits but not smoke during the sessions. We performed urine drug tests at screening and before each test session using TRIAGE 8 (Biosite, San Diego, CA). No alcohol test was performed.
Study Outline
The test sessions began at 8:15 AM. A urine sample was taken to verify abstinence from drugs of abuse, and a pregnancy test was performed in women. An indwelling intravenous catheter was placed in an antecubital vein for blood sampling, and the subjects completed baseline measurements. LSD (200 µg) or placebo was administered at 9:00 AM. A standardized lunch and dinner was served at 1:30 PM and 5:30 PM, respectively. The subjects were sent home the next day at 9:30 AM after the 24-hour blood sample collection
Drugs
Gelatin capsules that contained 100 µg LSD (D-LSD hydrate with a purity (high-performance liquid chromatography) >99%; Lipomed AG, Arlesheim, Switzerland), and corresponding placebo capsules were prepared with authorization from the Swiss Federal Office for Public Health. LSD was administered in a single absolute dose of 200 µg, corresponding to a dose of 2.84±0.5 µg/kg body weight (mean±SD; range: 2.04–3.85 μg).
Blood and Urine Sampling
Blood was collected into lithium heparin tubes 1 hour before and 0.5, 1, 1.5, 2.5, 3, 4, 6, 8, 10, 12, 16, and 24 hours after LSD administration. Urine (entire volume) was collected during 3 sampling periods: 0 to 8, 8 to 16, and 16 to 24 hours after LSD administration. Blood samples were immediately centrifuged, and plasma and urine were rapidly stored at -20°C until analysis within 2 to 6 months. Long-term stability (6 months) has been shown for LSD and 2-oxo-3-hydroxy-LSD (O-H-LSD) when kept under refrigerated or frozen conditions (Klette et al., 2002; Martin et al., 2013). The recovery (ng) of LSD and O-H-LSD was determined by multiplying the analyte urine concentrations (ng/mL) with the urinary volume (mL) of the respective sampling interval.
Analysis of LSD and O-H-LSD
LSD and O-H-LSD concentrations in plasma and urine were determined using a validated liquid-chromatography-tandem mass-spectrometry method as reported in detail in the supplementary Material online and elsewhere (Dolder et al., 2015). The lower limit of quantification was 0.1ng/mL, and the upper limit of quantification was 10ng/mL for LSD and O-H-LSD in both plasma and urine.
PK
The plasma concentration data were analyzed using noncompartmental methods using Phoenix WinNonlin 6.4 (Certara, Princeton, NJ). Cmax and Tmax values were obtained directly from the observed data. The area under the concentration-time curve (AUC) from 0 to 24 hours after dosing (AUC24) was calculated using the linear-up log-down trapezoidal method. The terminal elimination rate constant (λz) for LSD was estimated by log-linear regression after semilogarithmic transformation of the data using at least the last 3 data points of the terminal linear phase of the concentration-time curve. The terminal half-life was calculated using λz and the equation t 1/2 = ln2/λz. The AUC to infinity was then determined by extrapolation of the AUC24 using λz. We also determined a separate half-life for the Tmax to 12 hour interval, because the rate of elimination changed at 12 hours in many subjects (see supplementary Figure S1 for all plots), and the decrease in plasma concentrations followed first-order kinetics in all subjects from Tmax to 12 hours. For this phase, we estimated the elimination rate constant (λ) for LSD using at least 3 data points of the concentration-time curve. Thus, this half-life does not describe the slower decrease in the concentration of LSD observed in a subset of subjects beyond 12 hours or 16 hours. Individual concentration-time curves show that a slower terminal decrease in LSD concentrations occurred only beyond 12 hours (after eating dinner and during the night) and not concentration-dependent (ie, was not observed below a certain threshold concentration of LSD; see supplementary Figure S1). Renal clearance (mL/h) was calculated as urinary recovery24 urine (ng)/AUC24 (ng∙h/mL).
Statistical Analyses
The analysis of the pharmacokinetic parameters was descriptive, and geometric means and 90% CIs are shown to account for nonnormally distributed data. The study included 8 subjects of each sex; the data are also presented for male and female subjects separately. However, the study was not sufficiently powered (power: 52%) to exclude sex differences in the PK of LSD (PASS Power Analysis, Kaysville, UT).
The primary pharmacodynamic study results were reported elsewhere (Schmid et al., 2014). The a priori hypothesis relating to the PK-pharmacodynamics as defined in the study protocol was that the pharmacodynamic effects of LSD would show no acute pharmacological tolerance (ie, no clockwise hysteresis in the concentration-effect relationship). To assess PK-pharmacodynamic relationships, the LSD-induced effect was determined as a difference from placebo in the same subject at the corresponding time point to control for circadian changes (Schmid et al., 2014). The pharmacodynamic changes after LSD administration for each time point were plotted against the respective plasma concentrations of LSD and graphed as hysteresis curves for each subject. Because pupil size measurements were unavailable at the same time points as plasma levels, pupil size values at 7 and 11 hours were matched with concentrations at 8 and 12 hours. No pupil size measurement was available for the 24-hour time point; therefore, we used the baseline value at t = 0 hours, assuming a return to baseline by 24 hours. The area within the hysteresis (AH) was calculated as AUCC0-Cmax – AUCCmax-C24 using the trapezoidal rule. AH<0 indicates counterclockwise hysteresis (lag time between concentration and effect due to absorption/distribution processes). AH > 0 indicates clockwise hysteresis (tolerance).
To estimate the plasma concentration of LSD at which 50% of the maximal response to LSD is reached (EC50), a sigmoidal concentration-response (variable slope) model was fitted to the plasma concentration-effect data: E = (E max × C p h ) / (C p h + EC 50 h), in which E is the observed effect, C p is the plasma LSD concentration, E max is the maximal effect, and h is the Hill slope using WinNonlin. Because of the hysteresis observed for most plasma-concentration effect curves, an indirect descriptive link model would be needed in which the plasma concentrations are linked to the pharmacodynamic parameter by an effect compartment, providing an estimate of the equilibration half-life between plasma and the effect compartment. However, because insufficient data pairs for the absorption phase (0-Cmax) were available, we directly linked dynamic effects to the plasma concentrations using only data from Cmax up to 24 hours after drug administration for this analysis. Statistical analyses were conducted using NCSS 2004 software (Statistical Software, Kaysville, UT).
Pharmacodynamic Measurements
Pharmacodynamic measures were included in this study to evaluate PK-pharmacodynamic relationships. Subjective effects were assessed repeatedly over time using visual analog scales (VASs) (Hysek et al., 2014), including “any drug effect,” “good drug effect,” and “bad drug effect.” The VASs were presented as 100-mm horizontal lines marked with “not at all” on the left and “extremely” on the right. The VASs were administered 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 16, and 24 hours after drug administration. Vital signs, including blood pressure, heart rate, and body (tympanic) temperature, were assessed repeatedly 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 hours after drug administration using previously reported methods (Hysek et al., 2014). Additionally, pupil size (dark-adapted maximal pupil diameter) was measured 1, 2.5, 4, 7, and 11 hours after drug administration using an infrared pupillometer (PRL-200, NeurOptics, Irvine, CA) under standardized dark-light conditions as previously reported (Hysek and Liechti, 2012).
Results
PK
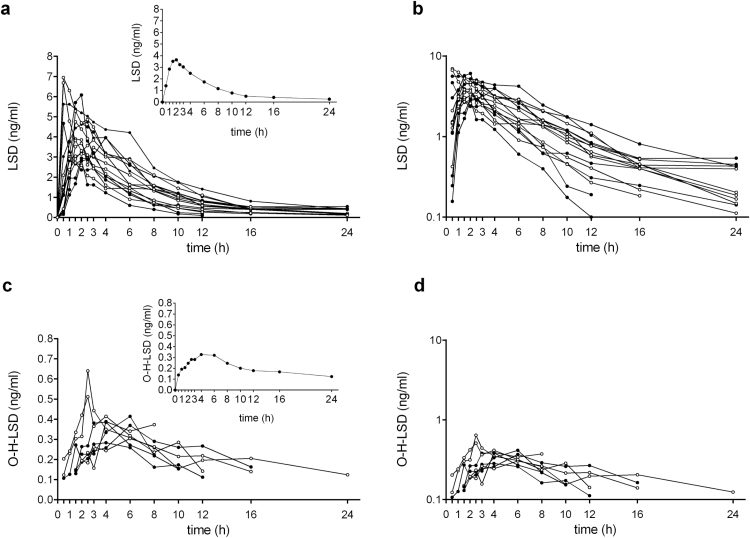
Figure 1 shows the plasma-concentration-time curves for LSD and O-H-LSD. The PK parameters are shown in Table 1. The plasma concentrations of LSD (>0.1ng/mL) could be measured in all of the subjects up to 12 hours, in 14 subjects up to 16 hours, and in 11 subjects up to 24 hours after administration. Concentrations of LSD decreased following first-order kinetics up to 12 hours with a half-life of 3.6±0.9 hours (Figure 1b). In some subjects, a slower decrease in plasma concentrations was observed late in time between 12 and 24 hours. This slower decrease occurred after the subjective effects of LSD had mostly subsided and the individual concentration-time curves showed that the slower decrease was dependent on time >12 hours (after eating dinner and during the night) and not on concentration (ie, below a certain concentration of LSD) (see supplementary Figure S1). The terminal half-life was 8.9±5.9 hours including 4 subjects (S4-S7, see supplementary Figure S1) in whom concentrations of LSD at 24 hours showed no further decrease compared with the 16-hour concentrations.
Figure 1.
Pharmacokinetics (PK) of lysergic acid diethylamide (LSD) and 2-oxo-3-hydroxy-LSD (O-H-LSD). (a) Individual LSD plasma concentration-time curves with the geometric mean shown in the inset. Filled circles indicate male subjects, and open circles indicate female subjects. (b) Semilogarithmic plot of the individual concentrations of LSD. Curves are shown separately for each individual in the supplementary Material (supplementary Figure S1). First-order kinetics were observed in all 16 subjects up to 12 hours. LSD levels fell below the lower limit of quantification (0.1ng/mL) in 2 subjects at 16 hours and 5 subjects at 24 hours. Slower elimination was observed between 12 and 24 hours. (c) Individual O-H-LSD plasma concentration-time curves in 8 subjects in whom metabolite concentrations could be determined, with the geometric mean shown in the inset. (d) Semilogarithmic plot of the individual concentrations of O-H-LSD. Curves are shown separately in supplementary Figure S2. LSD was administered at t = 0 hours.
Table 1.
Pharmacokinetic Parameters for LSD and O-H-LSD
| N= | Cmax (ng/ml) Geometric Mean (95%CI) | tmax (h) Median (range) | t1/2 (h) Tmax-12h Mean±SD | t1/2 (h) Terminal Mean±SD | AUC24 (ng·h/ mL) Geometric Mean (90%CI) | AUC∞ (ng·h/ mL) Geometric Mean (90%CI) | CLR (mL/min) Mean±SD | ||
|---|---|---|---|---|---|---|---|---|---|
| LSD | All | 16 | 4.3 (3.8–4.9) | 1.5 (0.5–4) | 3.6±0.9 | 8.9±5.9 | 26 (22–30) | 28 (24–33) | 79±36 |
| LSD | Male | 8 | 4.4 (3.6–5.3) | 1.5 (0.5–4) | 3.5±1.0 | 10.2±6.7 | 25 (18–35) | 28 (20–38) | 88±36 |
| LSD | Female | 8 | 4.2 (3.4–5.3) | 1.5 (0.5–3) | 3.8±0.8 | 7.6±5.1 | 26 (23–30) | 28 (25–32) | 71±36 |
| aO-H-LSD | All | 8 | 0.4 (0.3–0.5) | 4 (2.5–6) | 3.4 (2.6–4.3) | 3.8 (2.8–5.3) |
Abbreviations: AUC, area under the plasma concentration-time curv; AUC∞, AUC from time zero to infinity; AUC24, from time 0–24; CLR, renal clearance; Cmax, maximum observed plasma concentration; T1/2, plasma half-life; Tmax, time to reach Cmax; aO-H-LSD levels were above the limit of detection in only 8 subjects).
The O-H-LSD concentration-time profiles could be determined for only 8 subjects, because metabolite concentrations were not present or fell below the lower limit of quantification in one-half of the subjects (Figure 1c-d). We could not show a difference in the pharmacokinetic profiles of LSD between male and female subjects (Table 1). The concentrations of LSD and O-H-LSD in urine and the urine recovery of LSD and O-H-LSD are shown in Table 2. The mean molar concentrations of O-H-LSD (molecular weight: 355.4) were 23.2, 49.9, and 40.6 pM/mL and 8, 14, and 19 times higher than the mean molar concentrations of LSD (molecular weight: 323.4; 3.0, 3.5, 2.2 pM/mL) in the 0 to 8, 8 to 16, and 16 to 24 hour sampling intervals, respectively. Of the nonmetabolized LSD that was recovered from urine, 56% appeared in urine within the first 8 hours after administration and 45% of the O-H-LSD appeared in urine 8 to 16 hours after LSD administration. Of the orally administered LSD hydrate (200 μg or 618nM), 13% was eliminated in urine as O-H-LSD (28.3 μg or 79.5nM) within 24 hours. Only 1% (2.1 μg or 6.4nM) of the dose of LSD was eliminated in urine as LSD within 24 hours. The renal clearance of LSD was 1.32±0.6mL/min or approximately 1.6% of the apparent total clearance after oral administration (CL/F), assuming an oral bioavailability of 71% (see Discussion). No significant differences in LSD or O-H-LSD urine concentrations were observed between male and female subjects (Table 2). The urine recovery of O-H-LSD was greater in male subjects than in female subjects during the 8 to 16 hour sampling period, but no significant differences were observed in the overall 0 to 24 hour sampling (Table 2).
Table 2.
Urinary Elimination of LSD and O-H-LSD
| N= | LSD | O-H-LSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–8 hours | 8–16 hours | 16–24 hours | 0–24 hours | 0–8 hours | 8–16 hours | 16–24 hours | 0–24 hours | |||
| Urinary concentrations (ng/mL) | ||||||||||
| all | 16 | 0.96±0.8 | 1.1±1.8 | 0.70±0.6 | 8.3±4.7 | 17.7±11 | 14.4±10 | |||
| male | 8 | 0.78±0.4 | 0.82±0.2 | 0.66±0.6 | 6.6±3.4 | 22.7±14 | 11.2±7 | |||
| female | 8 | 1.1±1.0 | 1.5±2.6 | 0.74±0.7 | 9.9±5.4 | 12.7±5.3 | 17.6±12 | |||
| Urinary volume (L) | ||||||||||
| all | 16 | 1.4±0.7 | 0.79±0.4 | 0.47±0.3 | ||||||
| male | 8 | 1.8±0.8 | 0.86±0.5 | 0.63±0.2 | ||||||
| female | 8 | 1.1±0.5 | 0.71±0.4 | 0.30±0.2 | ||||||
| Urinary recovery (nM) Ae0-24 | ||||||||||
| all | 16 | 3.6±2.6 | 2.0±1.7 | 0.82±0.5 | 6.4±2.9 | 28.3±15 | 35.9±27 | 15.3±7.8 | 79.5±41 | |
| male | 8 | 3.8±2.4 | 2.0±0.8 | 1.1±0.6 | 6.8±2.6 | 29.3±19 | 49.6±33 | 17.1±8.6 | 96.1±51 | |
| female | 8 | 3.5±2.8 | 2.0±2.3 | 0.58±0.3 | 6.0±3.3 | 27.3±13 | 22.3±9* | 13.4±6.8 | 62.9±18 | |
Abbreviations: Ae, amount eliminated in nM; LSD, lysergic acid diethylamide; O-H-LSD, 2-oxo-3-hydroxy-LSD.
*Significant difference from men (P<.05). Values are mean±SD.
PK-Pharmacodynamic Relationship
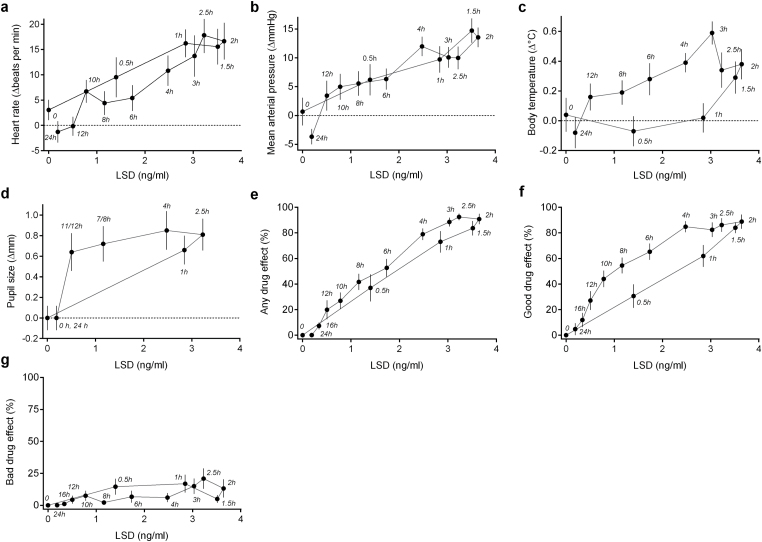
Figure 2 shows the effects of LSD as a function of plasma concentration. There was a close relationship between the LSD concentration and its dynamic effects overt time. No hysteresis was found for heart rate (Figure 2a), blood pressure (Figure 2b), or bad drug effect (Figure 2g). The 95% CIs of the mean of the area within the hysteresis loops (AH) overlapped with 0 for heart rate (4.4 beats×ng/min×mL [-13 to +22]), blood pressure (-5 mgHg×ng/min×mL [-24 to +13]), and bad drug effect (5%×ng/min×mL [-29 to +38]), indicating no hysteresis. Counterclockwise hysteresis (negative AH value) was observed, attributable to relatively higher plasma levels compared with the dynamic effects during the assumed drug absorption phase (0–2 hours) for body temperature (Figure 2c), pupil size (Figure 2d), any drug effect (Figure 2e), and good drug effect (Figure 2f). Mean AH values (95% CI) were the following: body temperature (-1°C×ng/min×mL [-1.5 to -0.5]), pupil size (-1.4 mm×ng/min×mL [-2.2 to -0.7]), any drug effect (-78%×ng/min×mL [-113 to -43]), and good drug effect (-106%×ng/min×mL [-151 to -61]). The decline of the response to LSD and plasma concentration over time followed a sigmoidal Emax dose-response curve for any drug effect and good drug effect. The EC50 mean±SD values were 1.3±0.7ng/mL for any drug effect and 1.0±0.5ng/mL for good drug effect. Heart rate, blood pressure, body temperature, and bad drug effect linearly increased with plasma concentrations of LSD and did not show an Emax (Figure 2a-c, g). Not enough values were available to fit changes in pupil size. No clockwise hysteresis was observed for any of the concentration-effect curves, meaning that the dynamic values were higher later in time at a given plasma concentration and consistent with no acute tolerance to the effects of LSD. LSD produced acute adverse effects, including difficulty concentrating, headache, exhaustion, and dizziness lasting up to 24 hours and as reported elsewhere (Schmid et al., 2014). There were no severe adverse effects.
Figure 2.
Lysergic acid diethylamide (LSD) effects plotted against LSD plasma concentrations (geometric means). The pharmacodynamic values are the mean±SEM differences from placebo at each time point in 16 subjects. The time of sampling is noted next to each point (in hours after LSD administration). Heart rate (a), mean arterial pressure (b), and bad drug effect (g) showed no hysteresis. Counterclockwise hysteresis was observed for body temperature (c), pupil size (d), any drug effect (e), and good drug effect (f), consistent with a delay between plasma concentration and effect. For most dynamic variables, maximal plasma concentrations (at approximately 2 hours) coincided with maximal dynamic effects. The dynamic changes then gradually decreased over time with decreasing plasma levels. No evidence of acute tolerance (clockwise hysteresis) was observed for any of the dynamic effects of LSD.
Discussion
The present study determined the single-dose PK of oral LSD in humans. The concentrations of LSD were maximal after 1.5 hours (median) and gradually declined to very low levels by 12 hours. We observed first-order kinetics of LSD up to 12 hours in all subjects and an inconsistent slower decrease in concentrations thereafter in some subjects. This could be attributable to redistribution from tissue or due to less precise quantification of the very low plasma levels of LSD at 12 to 24 hours (ie, close to the lower limit of quantification). The half-life of 3.6 hours during the first 12 hours after drug administration is close to the 3 hours previously observed in a small study that used intravenous LSD administration (Aghajanian and Bing, 1964). Only 1% of the orally administered LSD was eliminated renally. LSD is almost completely metabolized in rats, guinea pigs, and monkeys (Axelrod et al., 1957; Siddik et al., 1979). In humans, the major metabolite of LSD detectable in urine is O-H-LSD (Klette et al., 2000; Poch et al., 2000; Canezin et al., 2001). In the present study, O-H-LSD was detected in blood plasma at very low concentrations and in only one-half of the subjects. The urine concentrations of O-H-LSD in the present study were approximately 10, 15, and 20 times higher than those of LSD at 0 to 8, 8 to 16, and 16 to 24 hours after LSD administration. Similarly, in LSD-positive forensic urine samples, O-H-LSD concentrations are higher than those of LSD, and O-H-LSD can be detected for a longer time than LSD after LSD administration (Reuschel et al., 1999; Klette et al., 2000; Poch et al., 2000). In the present study, 13% of the orally administered LSD was recovered from urine as O-H-LSD within 24 hours. LSD is metabolized to O-H-LSD by cytochrome P450 enzymes, but the specific enzymes and mechanisms are unknown (Klette et al., 2000). To our knowledge, it is unknown whether O-H-LSD is pharmacologically active.
The oral bioavailability of LSD can be crudely estimated using the previous data on intravenous LSD administration (Aghajanian and Bing, 1964) and our data on oral LSD. After intravenous LSD administration (2 μg/kg of the free base in 5 male subjects), a mean total plasma exposure (AUC∞) of 31.4 ng∙mL/h was obtained (15.7 ng∙mL/h per μg/kg free base), calculated based on the published plasma concentration profile (Aghajanian and Bing, 1964). After oral LSD administration in the present study (2.5 μg/kg free base in 8 male subjects), the mean AUC∞ was 28 ng∙mL/h (11.2 ng∙mL/h per μg/kg free base). Based on these data, the oral bioavailability of LSD is approximately 71%. In the present study, LSD was administered after a light meal. When ingested with a “full breakfast,” oral LSD was reported to result in lower plasma concentrations compared with administration on an empty stomach (Upshall and Wailling, 1972). However, these observations were made in only 2 to 3 subjects (Upshall and Wailling, 1972) and would need confirmation. Remaining to be tested is whether food reduces or delays the absorption of oral LSD. Additionally, the PK profiles were similar in male and female subjects. However, the study was too underpowered to statistically exclude sex differences in the PK of LSD.
We found a close relationship between the plasma concentrations of LSD and physiologic response or psychotropic effects of LSD over time. Estimated EC50 values for the psychotropic effects were in the range of 1.0 to 1.3ng/mL (approximately 3–4nM). The unbound fraction of LSD in human plasma is unknown. In cats, the unbound fraction was 0.2, and LSD concentrations in cerebrospinal fluid were similar to free LSD plasma concentrations (Axelrod et al., 1957). Thus, LSD concentrations of 0.6 to 0.8nM could be expected in cerebrospinal fluid. These values are in the range of the binding affinity of LSD at the 5-hydroxytryptamine-2A (5-HT2A) receptor (Ki = 0.4–1.3nM, respectively) (Titeler et al., 1988; Egan et al., 1998) and also close to the EC50 for the functional stimulant activity of LSD at the receptor in vitro (EC50 = 7.2nM) (Egan et al., 1998). Pupil size was also strongly increased at low concentrations of LSD. We previously showed that pupil diameters were significantly larger compared with placebo until the last pupil measurement at 11 hours after LSD administration. In contrast, elevations in blood pressure, heart rate, and body temperature were only significant up to 5 hours after LSD administration compared with placebo, as reported elsewhere (Schmid et al., 2014). Additionally, the increases in heart rate, blood pressure, body temperature, and bad drug effects showed no ceiling effect in the concentration-effect curves, in contrast to the other dynamic effects of LSD. Heart rate, body temperature, blood pressure, and bad drug effects would likely increase further with higher doses of LSD, whereas the pupillary or good subjective effects can be expected to be similar to those seen in the present study. The hypertensive effects of LSD may result from 5-HT2A and/or α1-adrenergic receptor-mediated vasoconstrictive effects at higher doses (Dyer and Gant, 1973; Blessing and Seaman, 2003).
No evidence of acute tolerance was observed, which would become apparent as clockwise hysteresis in the concentration-response curve and has been shown for 3,4-methylenedioxymethamphetamine (MDMA) (Hysek et al., 2011). In contrast and as typically expected for most drugs, counterclockwise hysteresis was observed early in time until the end of the assumed drug absorption phase. No similar studies on the PK-pharmacodynamic relationship of LSD have been performed. Only one other small study measured plasma LSD concentrations and concomitant pharmacodynamic effects (Aghajanian and Bing, 1964). LSD was administered intravenously in 5 male subjects. To obtain a crude index of performance, subjects were given one of a series of equivalent tests, consisting of simple addition problems, after each blood sample was drawn (Aghajanian and Bing, 1964). After the distribution phase (30 minutes after intravenous LSD administration), the impairments in performance declined in parallel with the plasma levels of LSD, also suggesting a close temporal relationship between the PK and pharmacodynamics of LSD (Aghajanian and Bing, 1964). In contrast to the single-dose administration in the present study, tolerance to the subjective effects of LSD with repeated daily LSD administration has been reported (Abramson et al., 1956; Belleville et al., 1956). However, a gradual increase in head twitches and catatonic postures and no tolerance was observed up to 3 to 4 days after continuous LSD administration in rats (Ellison et al., 1980). Also in contrast to our findings with LSD, we observed pronounced acute tolerance to the psychotropic and cardiostimulant effects of MDMA using the same methodology (Hysek et al., 2011). As a result, the pharmacodynamic effects of MDMA last significantly shorter than would be expected based on plasma levels. The subjective and cardiostimulant effects of MDMA last only 5 hours despite its long half-life of 10 hours (Hysek et al., 2011). In contrast, the subjective drug effects of LSD lasted for 12 hours in most subjects and up to 16 hours in some subjects in the present study despite LSD’s shorter half-life. Thus, subjects with MDMA in blood may no longer be clinically intoxicated, whereas subjects with quantifiable LSD concentrations in plasma are clinically intoxicated. A mechanistic explanation for this acute tolerance in the case of MDMA is that it mainly produces its acute effects through the release of endogenous serotonin and norepinephrine (ie, as an indirect serotonergic and noradrenergic agonist). In contrast, LSD is thought to produce its psychotropic hallucinogenic effects through a direct interaction with the 5-HT2A receptor (ie, as a direct serotonergic agonist), resulting in pharmacodynamic effects to which no acute tolerance was observed in our study.
In summary, we show first data on the PK and PK-pharmacodynamic relationship of oral LSD in human subjects. The PK profiles exhibit first-order kinetics of LSD up to 12 hours. LSD produces physiological and psychotropic effects lasting up to 12 hours, closely related to the plasma concentrations of LSD and inhibiting no acute tolerance. The findings are important for further clinical studies and serve as a reference for the assessment of intoxication with LSD.
Statement of Interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
The authors thank Stefan Borgwardt, Felix Müller, and Florian Enzler for their assistance with conducting the clinical study; Stephan Krähenbühl for comments on the manuscript; and Michael Arends for editorial assistance. Supported by the University Hospital Basel, Switzerland, and Swiss National Science Foundation (grant no. 320030_1449493).
References
- Abramson HA, Jarvik ME, Gorin MH, Hirsch MW. (1956) Lysergic acid diethylamide (LSD-25): XVII tolerance development and its relationship to a theory of psychosis. J Psychol 41:81–105. [Google Scholar]
- Aghajanian GK, Bing OH. (1964) Persistence of lysergic acid diethylamide in the plasma of human subjects. Clin Pharmacol Ther 5:611–614. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Brady RO, Witkop B, Evarts EV. (1957) The distribution and metabolism of lysergic acid diethylamide. Ann N Y Acad Sci 66:435–444. [DOI] [PubMed] [Google Scholar]
- Belleville RE, Fraser HF, Isbell H, Wikler A, Logan CR. (1956) Studies on lysergic acid diethylamide (LSD-25): I. Effects in former morphine addicts and development of tolerance during chronic intoxication. AMA Arch Neurol Psychiatry 76:468–478. [PubMed] [Google Scholar]
- Blessing WW, Seaman B. (2003) 5-hydroxytryptamine2A receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 117:939–948. [DOI] [PubMed] [Google Scholar]
- Canezin J, Cailleux A, Turcant A, Le Bouil A, Harry P, Allain P. (2001) Determination of LSD and its metabolites in human biological fluids by high-performance liquid chromatography with electrospray tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 765:15–27. [DOI] [PubMed] [Google Scholar]
- Dolder PC, Liechti ME, Rentsch KM. (2015) Development and validation of a rapid turboflow LC-MS/MS method for the quantification of LSD and 2-oxo-3-hydroxy LSD in serum and urine samples of emergency toxicological cases. Anal Bioanal Chem 407:1577–84. [DOI] [PubMed] [Google Scholar]
- Dyer DC, Gant DW. (1973) Vasoconstriction produced by hallucinogens on isolated human and sheep umbilical vasculature. J Pharmacol Exp Ther 184:366–375. [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. (1998) Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology 136:409–414. [DOI] [PubMed] [Google Scholar]
- Ellison G, Ring M, Ross D, Axelrood B. (1980) Cumulative alterations in rat behavior during continuous administration of LSD or mescaline: absence of tolerance? Biol Psychiatry 15:95–102. [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, Brenneisen R. (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 202:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. (2012) Effects of MDMA alone and after pretreatement with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology 224:363–376. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, Huwyler J, Liechti ME. (2011) The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clin Pharmacol Ther 90:246–255. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Grouzmann E, Liechti ME. (2014) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone and in combination. Int J Neuropsychopharmacol 17:371–381. [DOI] [PubMed] [Google Scholar]
- Klette KL, Anderson CJ, Poch GK, Nimrod AC, ElSohly MA. (2000) Metabolism of lysergic acid diethylamide (LSD) to 2-oxo-3-hydroxy LSD (O-H-LSD) in human liver microsomes and cryopreserved human hepatocytes. J Anal Toxicol 24:550–556. [DOI] [PubMed] [Google Scholar]
- Klette KL, Horn CK, Stout PR, Anderson CJ. (2002) LC-MS analysis of human urine specimens for 2-oxo-3-hydroxy LSD: method validation for potential interferants and stability study of 2-oxo-3-hydroxy LSD under various storage conditions. J Anal Toxicol 26:193–200. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K. (2014) High hopes. Science 345:18–23. [DOI] [PubMed] [Google Scholar]
- Martin R, Schurenkamp J, Gasse A, Pfeiffer H, Kohler H. (2013) Determination of psilocin, bufotenine, LSD and its metabolites in serum, plasma and urine by SPE-LC-MS/MS. Int J Legal Med 127:593–601. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181. [DOI] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. (2008) The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther 14:295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch GK, Klette KL, Anderson C. (2000) The quantitation of 2-oxo-3-hydroxy lysergic acid diethylamide (O-H-LSD) in human urine specimens, a metabolite of LSD: comparative analysis using liquid chromatography-selected ion monitoring mass spectrometry and liquid chromatography-ion trap mass spectrometry. J Anal Toxicol 24:170–179. [DOI] [PubMed] [Google Scholar]
- Reuschel SA, Eades D, Foltz RL. (1999) Recent advances in chromatographic and mass spectrometric methods for determination of LSD and its metabolites in physiological specimens. J Chromatogr B Biomed Sci Appl 733:145–159. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Müller F, Borgwardt S, Liechti ME. (2014) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Siddik ZH, Barnes RD, Dring LG, Smith RL, Williams RT. (1979) The fate of lysergic acid DI[14C]ethylamide ([14C]LSD) in the rat, guinea pig and rhesus monkey and of [14C]iso-LSD in rat. Biochem Pharmacol 28:3093–3101. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. (1988) Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology 94:213–216. [DOI] [PubMed] [Google Scholar]
- Upshall DG, Wailling DG. (1972) The determination of LSD in human plasma following oral administration. Clin Chim Acta 36:67–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.