Abstract
Background:
Environmental enrichment alters susceptibility in developing drug addiction. We have demonstrated that rats raised in an enriched condition are more sensitive than rats raised in an impoverished condition to nicotine-induced locomotor activity, and this is associated with alterations of phosphorylated extracellular signal-regulated kinase 1/2 within the prefrontal cortex. This study determined the impact of microRNA-221 in the prefrontal cortex on phosphorylated extracellular signal-regulated kinase 1/2 and the enriched environment-dependent behavioral changes in response to nicotine.
Methods:
A microRNA array was conducted to profile microRNA expression in the prefrontal cortex of enriched condition and impoverished condition rats in response to repeated nicotine (0.35mg/kg, s.c.) administration. microRNA-221 in the prefrontal cortex, nucleus accumbens, and striatum was further verified by quantitative real-time PCR. Lentiviral-mediated overexpression of microRNA-221 in PC12 cells and the medial prefrontal cortex was performed to determine the effects of microRNA-221 on nicotine-mediated phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated cAMP-response element-binding protein, and locomotor activity.
Results:
microRNA-221 was profoundly upregulated in the prefrontal cortex but not in nucleus accumbens and striatum of enriched condition rats relative to impoverished condition rats following repeated administration of nicotine. Overexpression of lentiviral-microRNA-221 attenuated nicotine-induced increase in phosphorylated extracellular signal-regulated kinase 1/2 in PC12 cells. Lentiviral-microRNA-221 overexpression in the medial prefrontal cortex further increased locomotor activity in impoverished condition but not in enriched condition rats in response to repeated nicotine administration. Accordingly, lentiviral-microRNA-221 attenuated nicotine-induced increases in phosphorylated extracellular signal-regulated kinase 1/2 and phosphorylated cAMP-response element-binding protein in the medial prefrontal cortex of impoverished condition but not enriched condition rats.
Conclusion:
These findings suggest that environmental enrichment, via upregulation of prefrontal microRNA-221 expression, suppresses the nicotine-induced activation of extracellular signal-regulated kinase and cAMP-response element-binding protein, which provides a potential mechanism underlying enhanced locomotor sensitivity to nicotine.
Keywords: Environmental enrichment, nicotine, ERK, medial prefrontal cortex, microRNA
Introduction
Exposure to environmental enrichment during childhood and adolescence reduces the adverse effects of abused drugs and the susceptibility to drug addiction (Leshner, 2000; Rhee et al., 2003; Stairs and Bardo, 2009). Due to the ethical considerations of studying environmental factors in humans, animal models have been used widely to identify variables aiding in the characterization and treatment of individuals vulnerable to drug abuse. In an enriched environment animal model, rats are raised in 1 of the 3 different conditions: an enriched condition (EC), an impoverished condition (IC), and a standard condition (SC), which differ in novelty, handling, social cohorts, and physical activity. EC animals have reduced sensitivity to the reinforcing effects of nicotine (Gomez et al., 2015) but display enhanced sensitivity to the locomotor effects induced by both acute and repeated nicotine administration (Gomez et al., 2012, 2015). However, the underlying molecular mechanisms for these behavioral changes are largely unknown.
Nicotine, through activation of nicotinic acetylcholine receptors, stimulates neurotransmitter release and induces synaptic activity-dependent neuroadaptations (Laviolette and van der Kooy, 2004). The prefrontal cortex (PFC) is a critical brain region in mediating psychostimulant-induced behavioral alterations, as it receives dense ascending dopaminergic projections from the nucleus accumbens (NAc) and the ventral tegmental area while also sending reciprocal descending glutamatergic projections (Dowd et al., 1998). Disruptions to the PFC alter nicotine-mediated behaviors (Rezvani et al., 2008), indicating that the PFC represents a fundamental role in the mesocorticolimbic circuitry to regulate nicotine-mediated behaviors. Activation of extracellular signal-regulated kinase1/2 (ERK1/2), a key integrator of the dopamine (DA)/D1 receptor and glutamate/NMDA receptor signaling, induces long-term cellular alterations and adaptive behaviors in response to most drugs of abuse (Valjent et al., 2001, 2004). Nicotine, through DA/D1 receptors, induces the phosphorylation of DA- and cAMP-regulated phosphoprotein-32 (pDARPP-32) and ERK1/2 (pERK1/2), subsequently triggering the activation of the cAMP-response element-binding protein (CREB) (Nakayama et al., 2001; Brunzell et al., 2003; Hamada et al., 2004; Valjent et al., 2005a, 2005b), potentially underlying nicotine-mediated behaviors. Nicotine-activated ERK1/2 is implicated to facilitate synaptic plasticity and mediate the behavioral effects of nicotine (Raybuck and Gould, 2007). We have recently demonstrated that, in the PFC, EC rats exhibit altered sensitivity in the levels of DA- and cAMP-regulated phosphoprotein-32, pERK1/2, and phosphorylated CREB (pCREB) in response to repeated nicotine administration and nicotine self-administration (Gomez et al., 2012, 2015).Thus, the enrichment-mediated compensatory alterations of D1 receptor/DARPP-32 and ERK1/2 signaling pathways in the PFC may contribute to environmental enrichment-dependent reduction of susceptibility to nicotine dependence.
Recent evidence indicates that microRNAs (miRs) play a crucial role in determining the vulnerability to drug addiction. For example, extended cocaine self-administration increased miR-212 in the striatum, and the overexpression of miR-212 reversed cocaine-taking behavior in a brain-derived neurotrophic factor and CREB-dependent manner (Hollander et al., 2010; Im et al., 2010). Additionally, in the medial PFC (mPFC), an upregulation of miR-206 was observed in alcohol-dependent rats, and miR-206 overexpression in drug-naïve animals resulted in escalating alcohol consumption (Tapocik et al., 2014). Herein, we hypothesize that a distinct miR expression profile in the PFC may be responsible for the enrichment-dependent signaling pathways and behavioral sensitivity in response to repeated nicotine administration. In this study, we profiled miR expression in the PFC of rats raised in different rearing conditions and identified the functional relevance of miR-221 in nicotine-mediated ERK1/2 activity and behavioral sensitivity to nicotine. The current study provides new mechanistic insights into understanding how, at both the neurobiological and behavioral levels, environmental enrichment reduces nicotine addiction.
Methods
Animals
Male Sprague-Dawley rats at the age of 21 days, obtained from Harlan Laboratories, Inc. (Indianapolis, IN), were randomly assigned to EC, IC, or SC housing conditions from postnatal day 21 to day 53 based on our previous reports (Wooters et al., 2011; Gomez et al., 2012, 2015). All rats were housed with food and water ad libitum in a colony room in the Division of Laboratory Animal Resources at the University of South Carolina. The colony room was maintained at 21±2ºC, 50±10% relative humidity on a 12-hour-light/-dark cycle (lights on at 7:00 am). All of the experimental procedures using animals were performed according to the National Institutes of Health (NIH) guidelines for AAALAC-accredited facilities and approved by the Institutional Animal Care and Use Committee at the University of South Carolina in compliance with animal welfare assurance.
Environmental Conditions
EC rats were group-housed (10–15 per cage) in a metal cage (120 cm×60cm × 45cm) with daily handling. For the EC rats, 12 hard, nonchewable, plastic objects were randomly placed in the cage. One-half of the objects were replaced with new objects daily, and the remaining one-half was rearranged to increase the novelty. IC rats were individually housed in wire mesh hanging cages (25 cm×18cm × 17cm) with solid metal sides and a wire mesh floor. SC rats were pair-housed in a clear polycarbonate cage (43cm × 20cm × 20cm) in accordance to standard housing conditions set in the NIH Guide for the 1996 version of the NIH Guide for the Care and Use of Laboratory Animals. IC and SC rats were neither handled nor exposed to any object. Rats were maintained in their respective environmental conditions throughout all experiments.
RNA Isolation and miR Expression Profiling
For profiling miR expression, beginning at 54 days of age, EC, IC, and SC rats (8 rats/each housing condition) received daily saline or nicotine (0.35mg/kg, freebase, s.c.) injection for 15 days. Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline (0.9% sodium chloride). Twenty-four hours after the last injection, brain tissues (approximately 30mg) from PFC, NAc, and dorsal striatum were placed in 1mL of RNAlater and total RNA isolation was performed using a miRNeasy Mini Kit (Qiagen, Valencia, CA). RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA), and RNA Integrity Numbers ranged from 8.1 to 9.4. RNA Integrity Number is a measure of RNA quality that ranges from 0 (totally degraded RNA) to 10 (intact RNA).
For the miR microarray, RNA samples from the PFCs of all rats were 3’-end labeled, and miRs were purified using the Micro Bio-spin 6 columns from Bio-Rad (Hercules, CA). Labeled miR samples were vacuum dried, resuspended in nuclease-free water, and hybridized to rat miR microarrays Release 16.0 (8x15K, #G4473B, Agilent) at 55°C for 20 hours. Both labeling and hybridizations were performed using the miR Complete Labeling and Hyb Kit (5190- 0456, Agilent). Arrays were scanned using an Agilent Microarray Scanner (G2565CA), and the data were extracted from images with the Feature Extractor Software (v.10.7.3.1; Agilent). Final array data were uploaded into GeneSpring GX 11.5.1, log base 2 transformed, and normalized using the shift to the 75th percentile algorithm. Additionally, the data were median baseline transformed. Quality control of the data using principal components and correlation analysis, as well as other metrics available in the software, resulted in the elimination of one sample from the EC-nicotine group and one sample from IC-saline group. Subsequently, data were filtered based on expression. Only spots with signals greater than the 20th percentile in at least 66% of the samples in any given experimental condition were considered for further analysis. A cutoff value of 2-fold was used to determine differences in miR levels.
Quantification of miRs Using Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) analyses of miR expression were performed to confirm the identified miR-221 and miR-483 in the PFC and verify whether these miRs are also upregulated in NAc and dorsal striatum. Complementary DNAs (cDNAs) were first synthesized from the isolated RNA samples in the PFC, NAc, and dorsal striatum of rats with previously repeated saline or nicotine injection through miRCURY LNA Universal RT microRNA PCR Universal cDNA synthesis Kit (EXIQON, Woburn, MA). Following cDNA synthesis, miRs-221 and 483 in the PFC, NAc, and dorsal striatum were quantified using qPCR with EXIQON LNATM PCR primer sets for rno-miR-221 (AGCU ACAUUGUCUGCUGGGUUUC) and rno-miR-483 (CACUCCUCCC CUCCCGUCUUGU). All reactions were normalized to miR-423-3p (primer set: AGCUCGGUCUGAGGCCCCUCAGU), a stably expressed candidate miR used as an endogenous reference gene (control). Comparisons among groups were performed using the method of 2-ΔΔCt where the threshold cycle is at a significant detectable increase in fluorescence. ΔCt values were calculated by subtracting the Ct value for the endogenous control (miR-423-3p) from the Ct value of the miR of interest (miR-221 or miR-483). The 2-ΔΔCt value was calculated by subtracting the ΔCt value of the control sample from the ΔCt value of the experimental sample.
Bioinformatic Analysis of miR Target Genes
A list of target transcripts for miR-221 (310 transcripts) and miR-483 (51 transcripts) were obtained from the TargetScan website (Friedman et al., 2009). These targets were analyzed by a comparison analysis using the Ingenuity Pathways Analysis software to determine which miR was more likely to produce a behavioral phenotype. The canonical pathways and the biological functions results from the 2 target transcript sets were compared.
Generation of Lentiviral (LV) Overexpressing miR-221
Viral constructs were purchased from Systems Biosciences (SBI, Mountain View, CA), and consisted of pCDH-CMV-rno-miR-1-EF1-copGFP and pCDH-CMV-rno-miR-221-EF1-copGFP. Expression plasmids containing full-length miR-1 and miR-221 with green fluorescent protein (copGFP) reporter genes were under the control of cytomegalovirus (CMV) and elongation factor-1 (EF1) promoters. MiR-1 was selected as control for miR-221 based on previous reports showing no effects of miR-1 on the signaling pathways tested in the current study (Lagos-Quintana et al., 2002; Mishima et al., 2007; Hollander et al., 2010). Plasmid DNA from the viral constructs was propagated using One Shot Top 10 chemically competent E. coli (C4040, Life Technologies, Grand Island, NY). A plasmid isolation kit (Qiagen) was used to purify plasmid DNA, and sequencing was confirmed by restriction enzyme mapping and DNA sequencing at the University of South Carolina Viral Vector Core facility. Lentiviruses were then produced in HEK 293T cells using a third-generation packaging system (pMDLg/pRRE+pRSV-Rev+pVSV-G) as described previously (Matsubara et al., 1998). HEK-293FT cells (Life Technologies) were maintained using high-glucose Dulbecco's modified Eagle medium (DMEM), penicillin/streptomycin (100U/mL), 1% sodium pyruvate, 1% nonessential amino acids, and 2mM glutamine. Cells were transfected with 5 µg of specific miR plasmid DNA, 1mL 150mM NaCl, 20 µl Δ8.91 + VSV-G mix, and 30 µL PEI-transfection reagent. Media containing virus particles (10mL) were harvested 48 to 72 hours posttransfection. Viral-containing medium was then filtered through 0.45-µm polyvinylidene fluoride filters and combined with PC12 cell medium. PC12 cells were subsequently incubated for 24 hours and fresh media was replaced. GFP visualization was verified using an Olympus IX81 fluorescent microscope and confirmation of miR-221 overexpression was confirmed by qPCR.
Overexpression of miR-221 and Nicotine-Mediated ERK Activity in PC12 Cells
PC12 cells (#CRL-1721.1, ATCC, Manassas, VA) were maintained in high-glucose DMEM (Life Technologies) supplemented with penicillin/streptomycin (100U/mL), 2.5% bovine calf serum (Hyclone Thermo Scientific, Logan, UT), 15% horse serum (Life Technologies), and 2mM glutamine (Life Technologies). Cells transfected with LV-miR-1 and LV-miR-221 were plated in 6-well poly-d lysine-coated plates at a density of ~2 to 5×106 cells/well. Cells with no transfection were used as a negative control. Culture media were replaced with serum-free media for approximately 3 hours prior to nicotine exposure. Cells were exposed to the optimized concentrations of nicotine (1 or 100 µM) for 10 minutes, washed twice with ice-cold 1× phosphate buffered saline, scraped, and then collected. Cells were then sonicated, centrifuged for 15 minutes at 12000rpm at 4°C, and subjected to Western-blot analysis.
LV-miR Overexpression into the mPFC
Due to its profound role in goal-directed actions, impulsivity, and reward processing (Perry et al., 2011), the mPFC was selected for overexpression of LV-miR-221. On postnatal day 54, EC and IC rats were anesthetized by intraperitoneal injections of ketamine (66mg/kg), xylazine (1.33mg/kg), and equithesin (0.5mL/kg). LV-miR-1 or LV-miR-221 (4–5×107 infection units/mL) was injected bilaterally into the mPFC (1.5 µL/infusion; 2 infusions/side) at the intended injection sites relative to bregma (AP +3.0mm, ML ± 0.6mm, DV – 2.6mm from dura). LV injection placement was confirmed by slicing the brain on a cryostat (15 µm/slice), mounting in slides, and examined by GFP expression within the PFC using an Olympus I×81 fluorescent microscope.
Nicotine-Mediated Locomotor Activity
Habituation and nicotine-mediated locomotor activity were determined as described in our previous studies (Gomez et al., 2012, 2015). One week after intra-mPFC LV injections, all rats were habituated to the locomotor activity chambers for 2 consecutive days. Twenty-four hours later, baseline activity was measured for 60 minutes following a saline injection. Thereafter for a total of 15 days, rats received daily saline or nicotine (0.35mg/kg, s.c.) injections. The dose of nicotine and the timeline for treatment were chosen based on previous studies from our laboratory and others showing that it produces the greatest nicotine-mediated activity and sensitization (Addy et al., 2007; Midde et al., 2011; Gomez et al., 2012). In addition, a 15-day nicotine treatment differentially altered D1R/cAMP/DARPP-32 and pERK/pCREB in the PFC from EC, IC, and SC animals (Gomez et al., 2012, 2015). Locomotor activity was measured every other day for 30 minutes prior to the injection and 60 minutes postinjection. To test the functional role of miR-221 in nicotine-mediated activity in EC and IC rats, 3 groups (LV-miR-1 saline, LV-miR-1 nicotine, and LV-miR-221 nicotine) were designed for the EC or IC group. A LV-miR-221 saline group was not included in the current study because (1) a pilot experiment showed no differences in baseline locomotor activity between LV-miR-1 and LV-miR-221 groups (data not shown), and (2) our in vitro data showing no difference in basal levels of pERK1/2 between LV-miR-1 and LV-miR-221. On the nonlocomotor testing days, rats were still transported to the same room that rats were injected for locomotor testing and then returned to home cages after injection. Twenty-four hours after the last injection, rats were decapitated, brains were removed, and the PFC, NAc, and dorsal striatum were dissected in a chilled matrix for qPCR and Western-blot analyses.
Western-Blot Analysis
Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (approximately 40 µg per PFC tissue samples; 5 µg per PC12 cell samples), blots were probed with primary antibodies: ERK 1/2 (1:7500, V114A; Promega Madison, WI), pERK 1/2 (1:1000, SC-16982R; Santa Cruz Biotechnology), CREB (1:10000, 9104), and pCREB (1:5000, 9196, Cell Signaling, Beverly, MA) and then blotted with secondary affinity-purified, horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgGs. After detection and quantification, each blot was stripped and reprobed with β-tubulin (H-235, Santa Cruz Biotechnology) to monitor protein loading among samples. Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Scion image software (Scion Corp., Frederick, MD).
Data Analysis
Data are presented as mean ± SEM, and n represents the number of independent experiments or subjects for each group. Data from microarray and qPCR were analyzed by a factorial ANOVA with housing condition (EC, IC, or SC) and treatment (saline or nicotine) as between-subjects factors. One-way ANOVAs were subsequently performed after the factorial ANOVAs to determine a significant effect for treatment across groups. For in vitro overexpression of LV-miR study in PC12 cells, 1-way ANOVAs were used to evaluate data from qPCR confirmation analysis of miR-221 along with concentration- and time-dependent effects of nicotine. Mixed-factors ANOVAs were conducted on EC and IC groups in the nicotine-mediated locomotor experiments, where groups (LV-miR-1 Saline, LV-miR-1 Nicotine, and LV-miR-221 Nicotine) were the between-subject factors and day was the within-subjects factor. To determine the effects of miR-221 on nicotine-mediated phospho-proteins levels, 1-way ANOVAs on pERK1, pERK2, and pCREB levels were performed within each housing condition. Simple effect comparisons (Bonferroni and Tukey) were conducted between groups where appropriate. All statistical analyses were performed using IBM SPSS Statistics version 20, and α level was set at P<.05 for all analyses.
Results
Repeated Nicotine Administration Upregulates miR-221 and miR-483 in the PFC of EC Rats
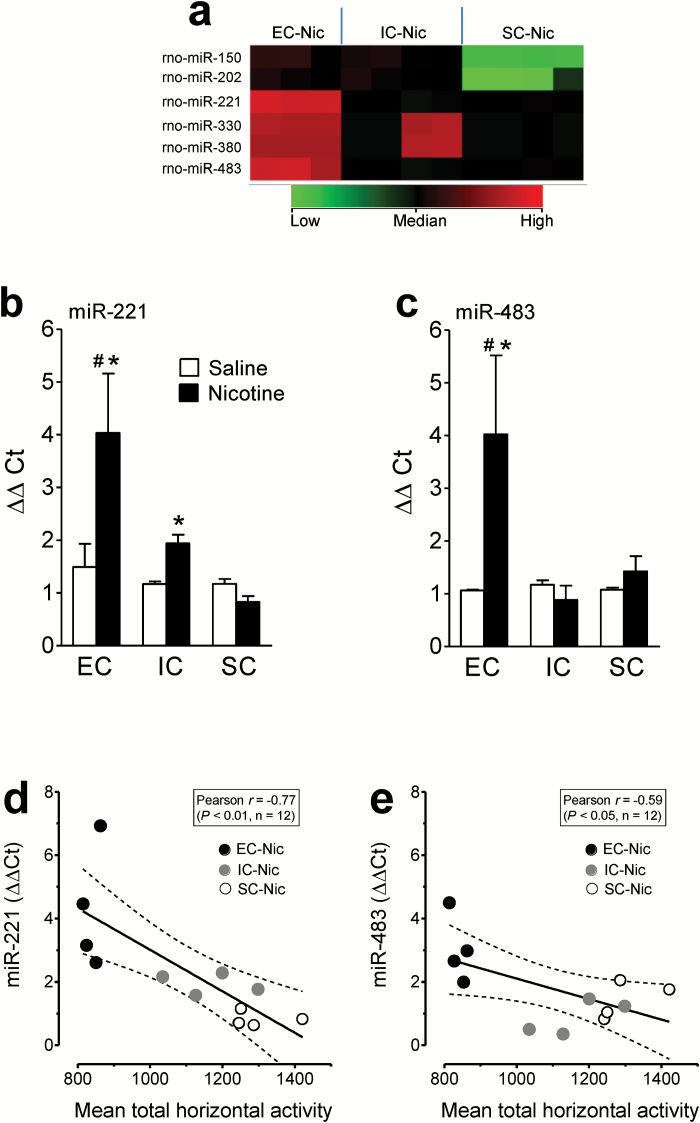
As shown in Figure 1a, analysis of miR microarray data revealed significant increases in the expression levels of 6 miRs (miRs-150, 202, 330, 380, 221, and 483) in the PFC of EC rats relative to IC and SC rats after repeated nicotine injection. No significant differences in the expression levels of these 6 miRs in the PFC, NAc, and dorsal striatum were observed among EC, IC, and SC groups after saline injection (data not shown). Among the 6 upregulated miRs, miR-221 and miR-483 exhibited the greatest magnitude of change from their respective saline controls. Therefore, the expression levels of miR-221 or miR-483 in the PFC, NAc, and dorsal striatum were further validated in EC, IC, and SC rats by qPCR. The expression levels of miR-221 in the PFC were increased in EC rats (170±20%, P<.001) and IC rats (66±7%, P<.05) but not SC rats relative to their respective saline controls (Figure 1b). Similarly, repeated nicotine injection increased the miR-483 level (278±89%, F(1, 8) = 3.9, P<.05) in EC rats but not in IC and SC rats relative to their respective saline controls (Figure 1c). No difference in the expression levels of miR-221 and miR-483 was observed in the NAc or dorsal striatum among EC, IC, and SC groups after repeated administration of saline or nicotine (Table 1). In addition, the expression levels of miR-221 and miR-483 in the PFC of EC, IC, and SC rats were negatively correlated with their respective horizontal activity on day 15 following repeated nicotine injection (miR-221: Figure 1d, P<.01, Pearson r = -0.77 and miR-483: Figure 1e, P<.05, Pearson r = -0.59).
Figure 1.
Environmental enrichment increases expression of specific miRs in the PFC of rats in response to repeated administration of nicotine. (a) Expression analysis using Agilent GeneSpring showed that miRs levels were significantly enhanced in the prefrontal cortex (PFC) of enriched condition (EC) rats treated repeatedly with nicotine (Nic; 0.35mg/kg, s.c., 15 days). Data are expressed as mean ± SEM of log 2 normalized intensity values. Columns (left to right): 1–3, EC rats with nicotine treatment (EC-Nic, n=3); 4–7, impoverished condition (IC) rats with nicotine treatment (IC-Nic, n=4); 8–11, standard condition (SC) rats with nicotine treatment (SC-Nic, n=4). Rows (left top to bottom) display a list of 6 microRNAs (miRs) that were found to be significant among groups with over 2-fold expression. Quantitative real-time PCR (qPCR) validation of miR-221 (b) and miR-483 (c) from the PFC of EC, IC, and SC rats with repeated saline or nicotine injections. 2-ΔΔCt values were calculated by subtracting the ΔCt value of the control sample (miR-423-3p) from the ΔCt of miR-221 or miR-483. Two-way ANOVA on ΔΔCt value of miR-221 revealed significant main effects of housing condition (F(2, 18) = 6.4, P<.01) and treatment (F(1, 18) = 5.8, P<.05), and a housing × treatment interaction (F(2, 18) = 4.2, P<.05). Two-way ANOVA on ΔΔCt value of miR-483 revealed a significant housing condition × treatment interaction (F(2, 18) = 3.7, P<.05). There was a trend for main effects of housing condition (F(2, 18) = 3.3, P=.059) and treatment (F(1, 18) = 3.8, P= .068). Data are expressed as mean ± SEM. * P<.05, denotes difference between the nicotine- and saline-treated groups. #P<.05, denotes difference within nicotine-treated groups among EC, IC, and SC rats. n=4 rats/group. Total horizontal activity counts were presented as mean values of each rat within 60-minute session on day 15. Correlation of the expression levels (ΔΔCt) of miR-221 (d) and miR-483 (e) in the PFC with total horizontal activity in EC, IC, and SC rats following a 15-day nicotine administration. Dashed lines represent the 95% confidence interval of the liner regression fit (solid line).
Table 1.
ΔΔCt Values for miR-221 and miR-483 in Different Brain Regions of EC, IC, and SC Rats after Repeated Saline or Nicotine Administration (0.35mg/kg for 15 days)
| miR-221 | ||||||
|---|---|---|---|---|---|---|
| EC-Sal | EC-Nic | IC-Sal | IC-Nic | SC-Sal | SC-Nic | |
| PFC | 1.49±0.44 | 4.03±1.13*# | 1.17±0.05 | 1.94±0.16* | 1.17±0.09 | 0.83±0.11 |
| NAc | 1.13±0.08 | 1.64±0.09 | 1.24±0.09 | 1.04±0.37 | 1.14±0.07 | 0.98±0.06 |
| STR | 1.04±0.01 | 0.61±0.14 | 1.20±0.16 | 1.15±0.40 | 1.09±0.06 | 1.14±0.17 |
| miR-483 |
||||||
| EC-Sal | EC-Nic | IC-Sal | IC-Nic | SC-Sal | SC-Nic | |
| PFC | 1.07±0.02 | 4.02±1.50*# | 1.17±0.09 | 0.89±0.27 | 1.08±0.04 | 1.43±0.29 |
| NAc | 1.06±0.05 | 1.01±0.28 | 1.17±0.09 | 1.12±0.45 | 1.18±0.08 | 1.02±0.14 |
| STR | 1.03±0.01 | 0.76±0.21 | 1.17±0.06 | 1.78±0.45 | 1.19±0.11 | 2.48±1.00 |
NAc, nucleus accumbens; PFC, prefrontal cortex; STR, striatum.
*P<.05 denotes difference within rearing groups between the nicotine- and saline-treated groups. #P<.05 denotes difference between housing groups. n=4 rats/group.
Bioinformatic Analysis of miR Target Genes
Figure 2 compares miR-221 and miR-483 target gene sets. The canonical pathways analysis produced a significant P value for the mitogen-activated protein kinase (MAPK)/ERK signaling pathway from the miR-221 target list (-log[P value] = 3.30), but not for the miR-483 target list (-log[P value] = 0.43). Similar results were obtained from the Neurotrophin-3/Trk signaling pathway (miR-221 -log[P value] = 2.50; miR-483 -log[P value] = 0.82). The target set for miR-221 produced a significant result for behavior in the biological functions analysis. These results were largely driven by genes involved in learning (P=7.24E-6; 22 targets) and by Locomotion (P=1.58E-4; 17 targets). The target set for miR-483 contained no genes involved in either behavior. These results suggest that miR-221 may contribute to the enriched environment-mediated behavior in response to nicotine.
Figure 2.
Ingenuity pathway analysis of miR-221 and miR-483 target genes. (a) -log(P values) for significantly overrepresented canonical pathways (mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase [ERK] and neurotrophin-3 (NT3)/Trk signaling) and biological functions (learning and locomotion) among miR-221 target genes. (b) Combined MAPK/ERK signaling and neurotrophin/Trk signaling pathways. Yellow symbols are targets of miR-221. (c) miR-221 target genes implicated in learning. (d) miR-221 target genes implicated in locomotion.
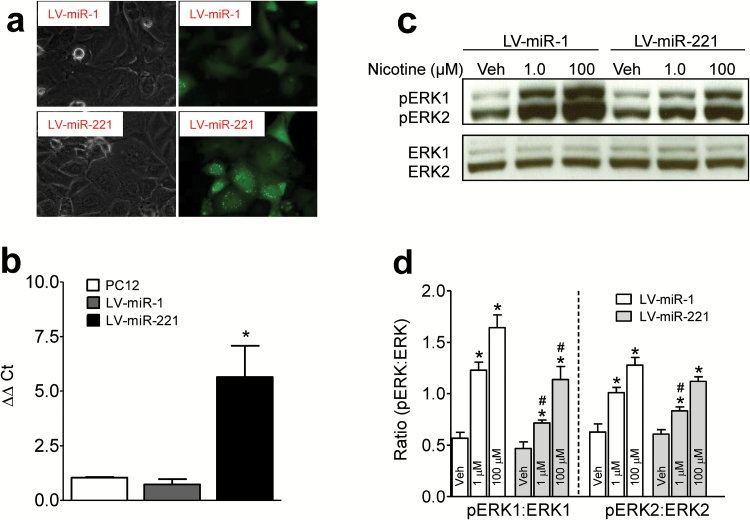
Overexpression of miR-221 in Vitro Diminishes Nicotine-Induced Increase in pERK1/2
LV transfection in PC12 cells was validated by GFP expression (Figure 3a). Overexpression of LV-miR-221 in PC12 cells relative to cells with no transfection and transfected with LV-miR-1 was confirmed by qPCR (Figure 3b, F(2,6) = 10.8, P<.05). The effects of overexpression of miR-221 or miR-1 on the regulation of pERK1/2 levels in PC12 cells in response to nicotine were examined (Figure 3c-d). Two-way ANOVA revealed significant main effects of LV condition (pERK1: F(1, 18) = 27.2, P<.001 and pERK2: F(1, 18) = 6.5, P<.05), nicotine concentration (pERK1: F(2,18) = 50.0, P<.001 and pERK2: F(2,18) = 51.6, P<.001), and a LV condition × nicotine concentration interaction for pERK1 (F(2,18) = 3.7, P<.05). One-way ANOVA revealed that nicotine (1 or 100 µM) increased pERK1 within LV-miR-1 group (F(2. 9) = 35.2, P<.001) and LV-miR-221 group (F(2,9) = 16.7, P < .01), respectively. Similarly, nicotine (1 or 100 µM) increased pERK2 within the LV-miR-1 group (F(2. 9) = 22.6, P<.001) and LV-miR-221 group (F(2,9) = 35.9, P < .01), respectively. Overexpression of miR-221 significantly diminished pERK1 at nicotine concentrations of 1 µM (F(1, 6) = 38.5, P<.01) and 100 µM (F(1, 6) = 8.0, P<.05), and pERK2 at 1 µM nicotine (F(1, 6) = 7.7, P<.05) relative to miR-1, suggesting that nicotine-induced ERK1/2 activity is regulated by miR-221. No changes in basal pERK1, pERK2, and total ERK1/2 were found after LV treatments.
Figure 3.
Overexpression of miR-221 diminishes nicotine-induced increases in phosphorylated extracellular signal-regulated kinase1/2 (pERK1/2) levels. (a) Confirmation of lentiviral (LV)-miR-221 expression in PC12 cells: Image (40×) of phase contrast (left panels) and GFP expression (right panels) in PC12 cells transfected with LV-miR-1 or LV-miR-221, respectively. (b) Quantitative real-time PCR (qPCR) verification of increased miR-221 levels in PC12 cells transfected with LV-miR-1 or LV-miR-221. PC12 cells with no transfection were used as a negative control. Histobars represent means ± SEM of 3 independent experiments. 2-ΔΔCt values were calculated by subtracting the ΔCt values of the control sample (miR-423-3p) from the ΔCt of the experimental sample (miR-221). *P<.001, compared with PC12 cells with no transfection or LV-miR-1. Transfected cells were first exposed to nicotine (1 µM or 100 µM) or vehicle (Veh) for 10 minutes and then pERK1/2 levels were determined. (c) Representative immunoblots of pERK1/2 and ERK1/2 in LV-miR-1 or LV-miR-221 transfected cells following exposure to nicotine or Vehicle (Veh). Total ERK1/2 and pERK1/2 were run at the same time with the same amount of proteins. (d) The ratio of pERK1/2 to total ERK1/2 in corresponding PC12 cell groups. Data are presented as the percentage of pERK1/2 to total ERK1/2 densitometry values of immunoreactivity. Histobars represent means ± SEM of 4 independent experiments. * P<.05, compared to the respective vehicle controls. #P<.05, compared to the respective LV-miR-1 group within same concentration of nicotine.
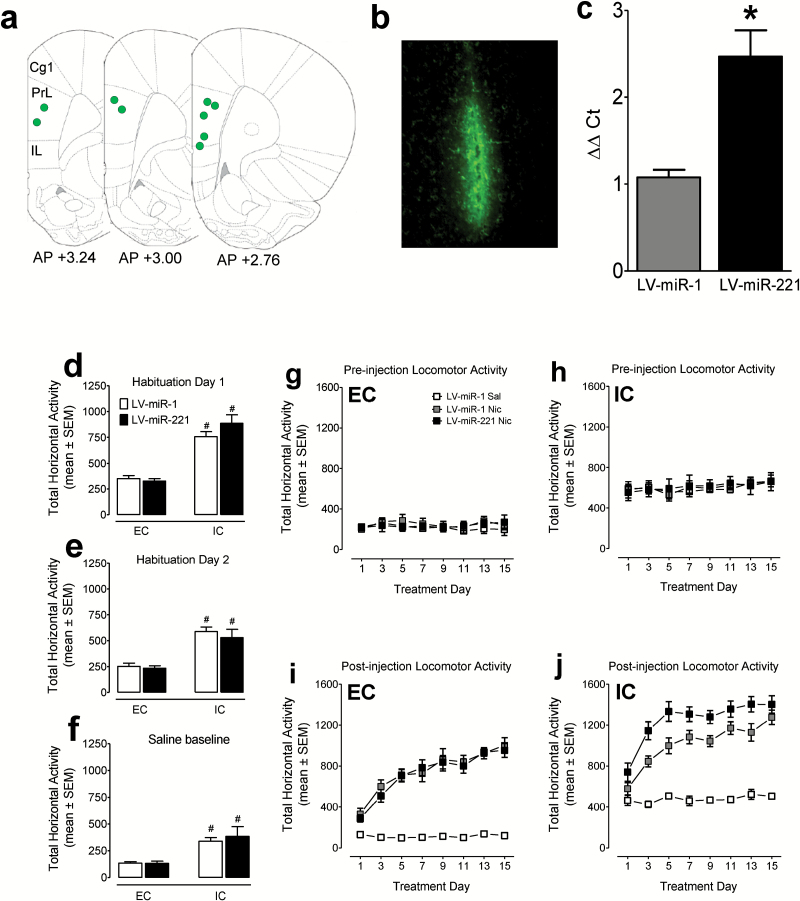
Overexpression of miR-221 in the mPFC Enhances Nicotine-Mediated Locomotor Activity
Overexpression of miR-221 in the mPFC of EC and IC rats was confirmed by representative injection sites with GFP expression (Figure 4a-b) and qPCR verification of increased miR-221 (Figure 4c).
Figure 4.
Overexpression of miR-221 in the medial prefrontal cortex (mPFC) increases nicotine-mediated locomotor activity in impoverished condition (IC) rats. At the age of 54 days, enriched condition (EC) and IC rats received bilateral injections of either LV-miR-1 or LV-miR-221 into the mPFC. (a) Representative placement mapping for coronal sections showing LV injection sites within the mPFC, where green circles represent injection sites. Cg 1, cingulate; PrL, prelimbic, IL, infralimbic cortices. (b) Coronal section (10× magnification) of lentiviral (LV) injections tagged with GFP expression into the mPFC. (c) Quantitative real-time PCR (qPCR) validation of miR-221 overexpression from the mPFC of rats injected bilaterally with either LV-miR-1 or LV-miR-221. n =7 rats/groups. *P<.05, compared with LV-miR-1. One week after lentiviral injection surgery, all rats were habituated to the locomotor activity chambers for two 60-minute sessions, once per day with no injection (d-e). Twenty-four hours after the second habituation session, all rats were injected subcutaneously with saline and placed into the activity chambers for 60 minutes to measure baseline activity (f). #P<.001, compared with EC group (n = 5–10 rats/group). Twenty-four hours after saline baseline measurement, all rats were administered nicotine (Nic, 0.35mg/kg, s.c.) or saline (Sal) on days 1 to 15. Total horizontal activity during the 30-minute preinjection habituation period was recorded in EC (g) and IC (h) rats across sessions. Total horizontal activity in EC (i) and IC (j) rats during the 60-minute postinjection period following repeated saline (Sal) or nicotine (Nic) injections.
One week after LV injection surgery, all rats received two 60-minute habituations in locomotor chambers (Figure 4d-e). A housing condition × miR × day ANOVA (2×2 × 2) revealed main effects of housing condition (F(1, 25) = 51.9, P<.001) and day (F(1, 25) = 41.4, P<.001) with no significant main effect of miR and housing × miR interaction, suggesting no difference in basal activity between LV-miR-1 and LV-miR-221 groups. Twenty-four hours after the second habituation session, all rats received a saline injection and were then placed into the locomotor chambers for 60 minutes to measure baseline activity (Figure 4f). The housing condition × miR ANOVA (2×2) revealed a main effect of housing condition (F(1, 25) = 24.1, P<.001); however, the main effects of miR and a housing condition × miR interaction were not significant. EC rats exhibited less basal horizontal activity than IC rats during all habituation sessions (Figure 4d-f, P<.01, Bonferroni t test).
On each locomotion testing day, all rats were habituated in the locomotor chambers for 30 minutes prior to saline or nicotine injection (Figure 4g-h). A housing condition × group × day ANOVA (2×3 × 8) revealed significant main effects of housing condition (F(1, 23) = 133, P<.001), day (F (7, 161) = 7.9, P<.001), and housing × day interaction (F(1, 23) = 18.8, P<.001). EC rats exhibited less basal horizontal activity than IC rats across all sessions (P<.01, Bonferroni t-test). No significant difference in basal activity was found between LV-miR-1 and LV-miR-221 groups in EC or IC group, further demonstrating miR-221 overexpression does not alter basal activity.
Following repeated saline or nicotine injection, total horizontal activity in the EC and IC rats during the 60-minute postinjection period is shown in Figure 4i-j). In the EC group, a group × day ANOVA (3×8) within the EC group revealed significant main effects of group (F(2, 10) = 71.6, P<.001), day (F(7, 70) = 34.3, P<.001), and group × day interaction (F(14, 70) = 9.3, P<.001). Nicotine increased locomotor activity in LV-miR-1 and LV-miR-221 groups relative to the LV-miR-1 saline group (P<.01, Bonferroni t test); however, no differences in nicotine-mediated activity between LV-miR-1 and LV-miR-221 groups were found (Figure 4i), suggesting that miR-221 does not alter nicotine-mediated activity in the EC group. In the IC group, a group × day ANOVA (3×8) revealed significant main effects of group (F(2, 13) = 26.0, P<.001), day (F(7, 91) = 39.8, P<.001), and group × day interaction (F(14, 91) = 2.3, P<.05). Bonferroni t test showed that repeated nicotine injection increased locomotor activity in the LV-miR-1 and LV-miR-221 groups relative to the LV-miR-1 saline group (P<.01). Specifically, when the nicotine-mediated activity data were expressed as a percentage change relative to the respective saline controls, on day 1 acute nicotine increased activity in LV-miR-1 (252%) and LV-miR-221 (221%) of EC rats (Figure 4i), and in LV-miR-1 (124%) and LV-miR-221 (159%) of IC rats (Figure 4j). On day 15, nicotine administration increased activity in LV-miR-1 (834%) and LV-miR-221 (793%) of EC group (Figure 4i), and in LV-miR-1 (252%) and LV-miR-221 (278%) of IC rats (Figure 4j). Posthoc tests showed that the nicotine-mediated activity was greater in LV-miR-221 IC rats than that in LV-miR-1 IC rats (Figure 4j, F (1, 10) = 8.8, P<.05), demonstrating that miR-221 overexpression in the mPFC of IC rats produce a greater locomotor sensitivity to nicotine.
Overexpression of miR-221 in the mPFC Attenuates Nicotine-Induced pERK1 and pCREB Increases
Neither pERK1/2 nor ERK1/2 was altered in EC rats among groups (Figure 5a, c). In contrast, a significant difference in pERK1 was found in IC rats among these groups (F (2, 15) = 5.4, P<.05) (Figure 5b, d). Posthoc analysis revealed that repeated nicotine injection increased pERK1 in LV-miR-1 IC rats (F(1, 10) = 5.6, P<.05), which was attenuated in LV-miR-221 IC rats (F(1, 10) = 12.9, P<.05). No differences in pERK2 levels were observed in IC rats among groups. Similar to pERK1/2, repeated nicotine and/or miR-221 overexpression in the mPFC did not alter pCREB levels in EC rats among miR-1 saline-, miR-1 nicotine-, and miR-221 nicotine-treated animals (Figure 6a, c). However, a significant difference in pCREB was obtained in IC rats (F (2, 15) = 4.9, P<.05) (Figure 6b, d). Repeated nicotine injections increased pCREB in IC rats with LV-miR-1 injection (F(1, 10) = 8.0, P<.05), which was inhibited by LV-miR-221 (F(1, 10) = 8.9, P<.05). Thus, these data suggest that overexpression of miR-221 in the mPFC of IC rats alters nicotine-mediated pERK1 and pCREB, which may be, at least in part, responsible for the increased nicotine-mediated locomotor sensitivity observed in LV-miR-221 IC rats.
Figure 5.
Overexpression of miR-221 in the medial prefrontal cortex (mPFC) attenuates nicotine-mediated phosphorylated extracellular signal-regulated kinase1/2 (pERK1) levels in impoverished condition (IC) rats. After locomotor sensitization paradigm, levels of pERK1/2 and ERK1/2 in the PFC of enriched condition (EC) and IC rats that received either lentiviral (LV)-miR-1 or LV-miR-221 were determined. Top panels show representative immunoblots of pERK1/2 and ERK1/2 immunoreactivity in the PFC of EC (a) and IC (b) rats after the last injection of nicotine (Nic; 0.35mg/kg, s.c.) or saline (Sal). Bottom panels show the ratio of pERK1/2 levels to total ERK1/2 levels in the PFC of corresponding EC (c) and IC (d) rats. Data are presented as the ratio of pERK1/2 to total ERK1/2 densitometry values of immunoreactivity. n =4–6 rats/group. *P<.05, compared with LV-miR-1 Sal group. #P<.05, compared with LV-miR-1 Nic group.
Figure 6.
LV-miR-221 overexpression reduces nicotine-mediated phosphorylated cAMP-response element-binding protein (pCREB) levels in impoverished condition (IC) rats. Protein expression levels of pCREB were determined after nicotine sensitization paradigm in the prefrontal cortex (PFC) of enriched condition (EC) and IC rats that received either lentiviral (LV)-miR-1 or LV-miR-221. Top panels show representative immunoblots of pCREB and CREB immunoreactivity in the PFC of EC (a) and IC (b) rats after the last injection of nicotine (Nic; 0.35mg/kg, s.c.) or saline (Sal). Bottom figures show the ratio of pCREB levels to total CREB levels in the PFC of EC (c) and IC (d) rats. Data are presented as the ratio of pCREB to total CREB densitometry values of immunoreactivity. n =4–6 rats/group. *P<.05, compared with LV-miR-1 Sal group. #P<.05, compared with LV-miR-1 Nic group.
Discussion
The novel finding in the present study is that prefrontal miR expression plays a critical role in environmental enrichment-dependent enhancement of locomotor sensitivity to nicotine. Repeated nicotine administration resulted in a regionally selective upregulation of 6 miRs within the PFC of EC but not IC and SC rats. miR-221 was upregulated approximately 2.7-fold in the PFC of EC rats but only slightly increased in IC rats after repeated nicotine injections. Our recent study has demonstrated that EC rats had attenuated nicotine-induced phosphorylation of ERK1/2, with enhanced locomotor sensitivity to repeated nicotine administration (Gomez et al., 2015). Lentiviral-mediated overexpression of miR-221 in PC12 cells diminished nicotine-mediated increases in ERK1/2 phosphorylation. Furthermore, overexpression of miR-221 in the mPFC of IC rats resulted in an EC-like behavioral response to nicotine, demonstrating that miR-221 contributes to enrichment-induced behavioral effects of nicotine. miR-221–mediated enhancement of nicotine-induced locomotor activity in IC rats was accompanied by attenuation of nicotine-augmented pERK1 and pCREB in the PFC by LV-miR-221. Taken together, these findings support the notion that the enrichment-dependent increase in nicotine-mediated locomotor sensitivity and the enrichment-induced attenuation of nicotine-induced pERK1/2 increases may be attributed to upregulated miR-221 expression in the PFC.
Repeated administration of drugs produces behavioral sensitization (Benwell and Balfour, 1992; Cadoni and Di Chiara, 2000), which represents neuropharmacological changes primarily in the mesocorticolimbic system (Brondello et al., 1999; Valjent et al., 2000). Under the behavioral sensitization paradigm, EC rats display increased sensitivity to nicotine compared with IC and SC rats when the baseline activity is lower in EC rats than in IC and SC rats (Gomez et al., 2012, 2015). We and others have demonstrated that exposure to environmental enrichment leads to compensatory neurobiological adaptations (Bowling et al., 1993; Del Arco et al., 2007; Green et al., 2010), particularly in the PFC (Gomez et al., 2012, 2015), which may be implicated in protection against maladaptive behaviors associated with drug abuse. miRs are important in the regulation of neuronal plasticity underlying drug-mediated behaviors (Hollander et al., 2010; Im et al., 2010; Chandrasekar and Dreyer, 2011; Bahi and Dreyer, 2013; Tapocik et al., 2014). In this study, we identified that the manipulation of environmental factors distinctly affects nicotine-induced miRs in the PFC. Among the 6 miRs elevated in the PFC of EC rats with repeated nicotine administration, the upregulation of miR-221 and miR-483 is the most pronounced. Particularly, the enhancement of miR-221 and miR-483 by nicotine is specific to the environmental enrichment condition, since both miRs were not altered in IC or SC rats after repeated nicotine administration (Figure 1). Additionally, these identified miRs were found only in the PFC but not in the NAc and dorsal striatum (Table 1). Further, the expression levels of miR-221 or miR-483 were negatively correlated with nicotine-mediated locomotor activity in EC, IC, and SC rats, suggesting that these miRs are associated with nicotine-mediated activity in rats under different environment conditions. This conclusion was further supported by the results from bioinformatic analysis. Noteworthy, intravenous nicotine self-administration is the most reliable and direct measure of nicotine reinforcement in animals (Corrigall, 1999). We have recently demonstrated that EC rats self-administered less nicotine relative to IC rats (Gomez et al., 2015), indicating a nicotine-resistant phenotype of environmental enrichment. More importantly, profound elevation of miR-221 expression in the PFC was also validated in EC rats at the end of nicotine self-administration (A. M. Gomez, D. Altomare, W. L. Sun, and J. Zhu, unpublished observations). Thus, the increase of miR-221 expression in the PFC may contribute to the enrichment-induced behavioral alterations implicated in both nicotine-mediated locomotor activity and nicotine self-administration.
Environmental enrichment differentially alters nicotine-augmented ERK-CREB signaling in the PFC, which is associated with nicotine-mediated behavioral locomotion (Gomez et al., 2012, 2015). In this study, we mainly focused on miR-221, because, through bioinformatic analysis of miR target genes, we identified that miR-221 is highly related to the MAPK/ERK signaling pathway. miR-221 expression has been shown to regulate ERK1/2 activity (Kim et al., 2002; Terasawa et al., 2009; Hamada et al., 2012; Zhang et al., 2013). ERK activation is implicated in cocaine- and amphetamine-induced locomotor activity, as well as cocaine-mediated conditioned place preference (Muda et al., 1996; Nishi et al., 2002; Valjent et al., 2006a, 2006b), indicating a critical role for ERK in the behavioral adaptations produced by psychostimulants. Our data show that overexpression of miR-221 in PC12 cells attenuates the nicotine-mediated increase in ERK1/2 phosphorylation. Furthermore, overexpression of miR-221 in the mPFC has no effect on basal locomotor activity in EC and IC rats but enhances nicotine-mediated locomotor activity in IC rats only. This behavioral adaptation in IC rats corresponded to the attenuating effect of prefrontal LV-miR-221 on nicotine-mediated pERK1 and pCREB in the mPFC. The failure to further change nicotine-mediated locomotor activity and ERK activity in EC rats with LV-miR-221 could be due to a maximal effect of the prefrontal endogenous miR-221 expression on nicotine-mediated ERK activity. Further investigation in the effect of inhibition of miR-221 expression in the mPFC on nicotine-mediated locomotion and ERK activity in EC rats is warranted. Thus, repeated nicotine administration may engage a novel feed forward circuit in the PFC of EC rats, relative to IC rats, in which upregulation of miR-221 expression inhibits the MAPK/ERK signaling pathway, thereby increasing locomotor sensitivity to nicotine.
Our results established a functional link between miR-221 and ERK1/2 activity. First, consistent with the previous in vitro study (Nakayama et al., 2002), acute nicotine dose dependently increased pERK1/2 in PC12 cells. Overexpression of miR-221 in PC12 cells diminished nicotine-augmented pERK1/2 levels without altering the basal ERK1/2 phosphorylation, indicating that the suppressive effect of miR-221 on pERK1/2 is dependent on nicotine stimulation. Further, overexpression of miR-221 in the mPFC attenuated nicotine-induced ERK activity, which was profoundly observed in pERK1 relative to pERK2. Two major isoforms of ERK, ERK1 and ERK 2, are very similar in sequence (Yoon and Seger, 2006) but have distinct functions. ERK1-knockout mice exhibit an enhanced response to sensitization and conditioned place preference to cocaine and morphine (Mazzucchelli et al., 2002; Ferguson et al., 2006). These findings suggest a critical regulatory role for ERK1 in molecular and behavioral changes in response to drugs of abuse. CREB has been identified as a potential molecular target for ERK activation (Greengard et al., 1999). Similar to pERK1, overexpression of miR-221 in the mPFC also reduced the nicotine-induced pCREB in the PFC of IC rats, suggesting a functional correlation between miR-221 and ERK-CREB signaling in response to nicotine.
In conclusion, the current results suggest a novel role of miR-221 within the mPFC as a mediator in enrichment-induced increased sensitivity to nicotine. Future studies will be essential in characterizing: (1) the mechanisms underlying brain region specific miR-221 upregulation in response to nicotine within EC rats, (2) the specific target(s) of miR-221 that mediate attenuation in nicotine-mediated pERK1/2 activity, such as neurotrophin-3/Trk signaling, and (3) the functional relevance of miR-221 in nicotine self-administration. Ultimately, the present study showed that the rearing environment has remarkable effects in altering the downstream signaling mechanisms that mediate nicotine-mediated behavior and further provide a role for the implication of prefrontal miRs in mediating nicotine addiction.
Statement of Interest
None.
Acknowledgments
This research was supported by grants from the National Institute of Health (DA035714, DA024275, and DA026721) and a Bioinformatics Pilot Project grant provided by the South Carolina INBRE (P20GM103499) and ASPIRE I Award from the Office of the Vice President for Research, University of South Carolina. We would like to acknowledge Dr. Charles F. Mactutus for providing the locomotor chambers that were used in this study.
Michael Shtutman: NIGMS P20GM109091; Jun Zhu: R01DA035714, R03DA024275, and R03DA026721.
References
- Addy NA, Fornasiero EF, Stevens TR, Taylor JR, Picciotto MR. (2007) Role of calcineurin in nicotine-mediated locomotor sensitization. J Neurosci 27:8571–8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. (2013) Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci 38:2328–2337. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Pouyssegur J, McKenzie FR. (1999) Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286:2514–2517. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. (2003) In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84:1431–1441. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. (2000) Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. European Journal of Pharmacology 387:23–25. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. (2011) Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology 36:1149–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. (1999) Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1:11–20. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. (2007) Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm 114:43–48. [DOI] [PubMed] [Google Scholar]
- Dowd S, Sneddon AA, Keyse SM. (1998) Isolation of the human genes encoding the pyst1 and Pyst2 phosphatases: characterisation of Pyst2 as a cytosolic dual-specificity MAP kinase phosphatase and its catalytic activation by both MAP and SAP kinases. J Cell Sci 111:3389–3399. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. (2006) Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology 31:2660–2668. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Midde NM, Mactutus CF, Booze RM, Zhu J. (2012) Environmental enrichment alters nicotine-mediated locomotor sensitization and phosphorylation of DARPP-32 and CREB in rat prefrontal cortex. PLoS One 7:e44149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Sun WL, Midde NM, Harrod SB, Zhu J. (2015) Effects of environmental enrichment on ERK1/2 phosphorylation in the rat prefrontal cortex following nicotine-induced sensitization or nicotine self-administration. Eur J Neurosci 41:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. (2010) Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23:435–447. [DOI] [PubMed] [Google Scholar]
- Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. (2004) Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem 90:1094–1103. [DOI] [PubMed] [Google Scholar]
- Hamada N, Fujita Y, Kojima T, Kitamoto A, Akao Y, Nozawa Y, Ito M. (2012) MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int 60:743–750. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. (2010) Striatal microRNA controls cocaine intake through CREB signalling. Nature 466:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kwon HB, Kim YS, Ryu JH, Kim KS, Ahn Y, Lee WJ, Choi KY. (2002) Isolation and characterization of a Drosophila homologue of mitogen-activated protein kinase phosphatase-3 which has a high substrate specificity towards extracellular-signal-regulated kinase. Biochem J 361:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12:735–739. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65. [DOI] [PubMed] [Google Scholar]
- Leshner AI. (2000) Vulnerability to addiction: new research opportunities. Am J Med Genet 96:590–591. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Kawabata Y, Takumi Y, Usami S, Shinkawa H, Haruta A, Matsuda K, Tono T. (1998) Quantitative immunogold cytochemistry reveals sources of glutamate release in inner ear ischemia. Acta oto-laryngologica Supplementum 539:48–51. [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. (2002) Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34:807–820. [DOI] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. (2011) Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav 98:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T. (2007) RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res 1131:37–43. [DOI] [PubMed] [Google Scholar]
- Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. (1996) The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem 271:27205–27208. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. (2001) Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem 79:489–498. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T. (2002) Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem 83:1372–1379. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. (2002) Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J Neurochem 81:832–841. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. (2011) Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev 65:124–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. (2007) Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci 121:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. (2008) Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience 154:885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. (2003) Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry 60:1256–1264. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. (2009) Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav 92:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M. (2014) microRNA-206 in Rat Medial Prefrontal Cortex Regulates BDNF Expression and Alcohol Drinking. J Neurosci 34:4581–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa K, Ichimura A, Sato F, Shimizu K, Tsujimoto G. (2009) Sustained activation of ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. FEBS J 276:3269–3276. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. (2000) Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20:8701–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. (2001) Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci 14:342–352. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. (2004) Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19:1826–1836. [DOI] [PubMed] [Google Scholar]
- Valjent E, Herve D, Girault JA. (2005a) [Drugs of abuse, protein phosphatases, and ERK pathway]. Med Sci (Paris) 21:453–454. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. (2005b) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A 102:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. (2006a) Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. (2006b) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A 103:2932–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. (2011) Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res 219:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Seger R. (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun K, Lei S, Zhong Y, Deng H, Ou W, Wu C. (2013) [Anti-microRNA-221 enhances radiosensitivity of colorectal carcinoma cells by up-regulating PTEN]. Nan fang yi ke da xue xue bao. Journal of Southern Medical University 33:728–732. [PubMed] [Google Scholar]