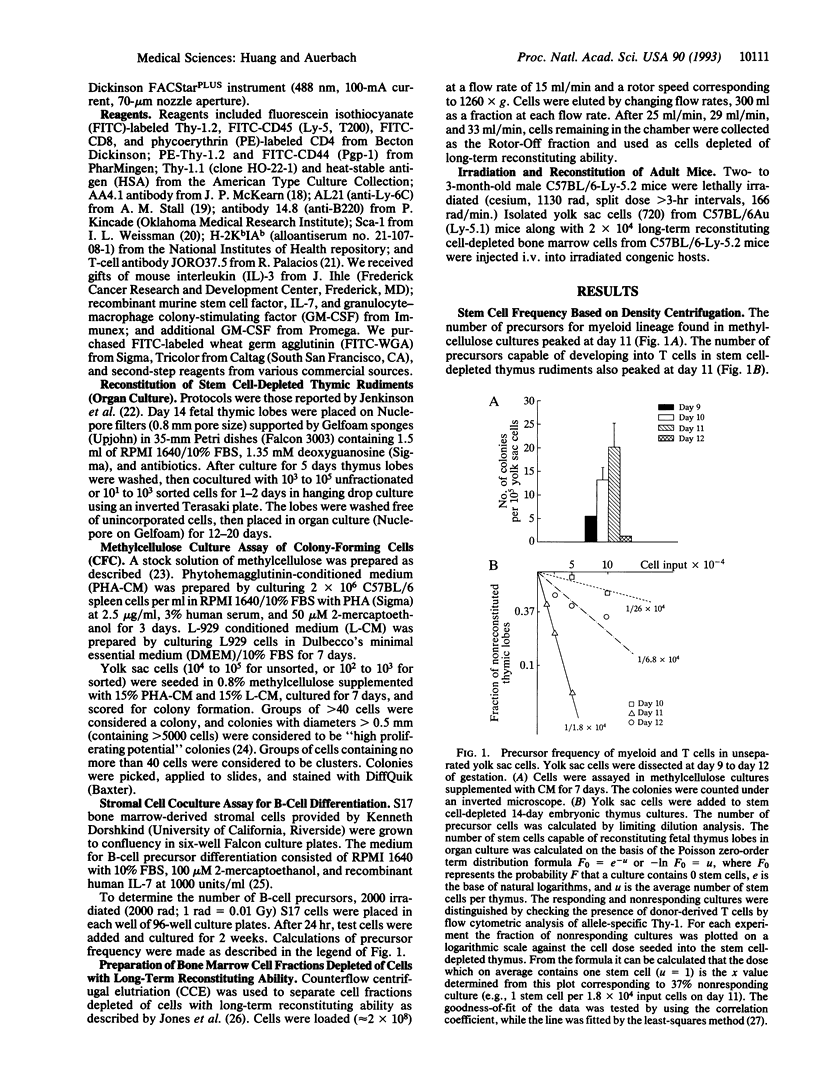
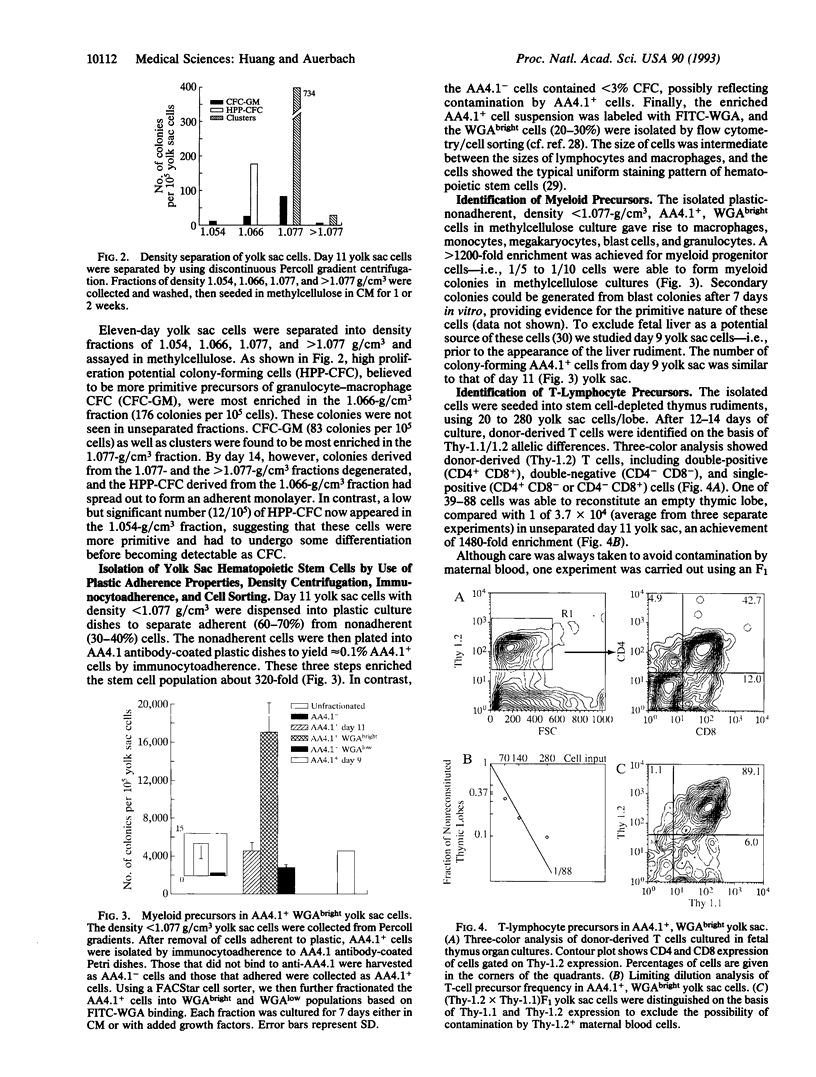
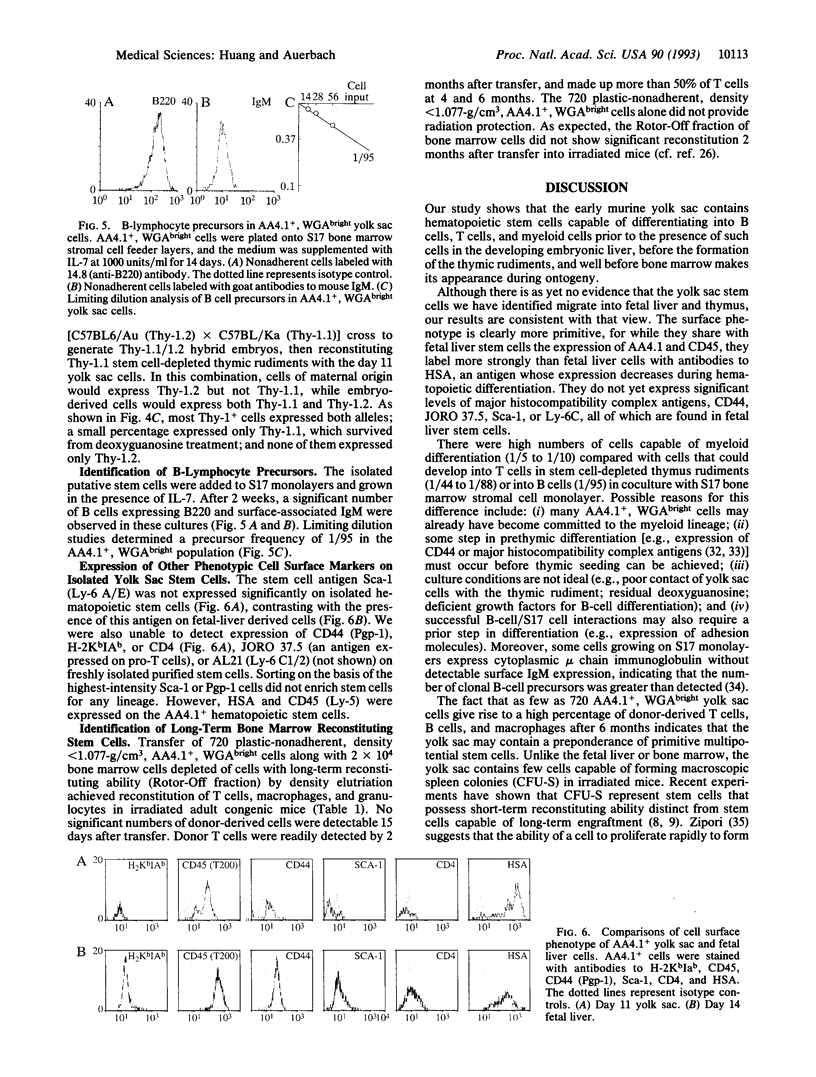
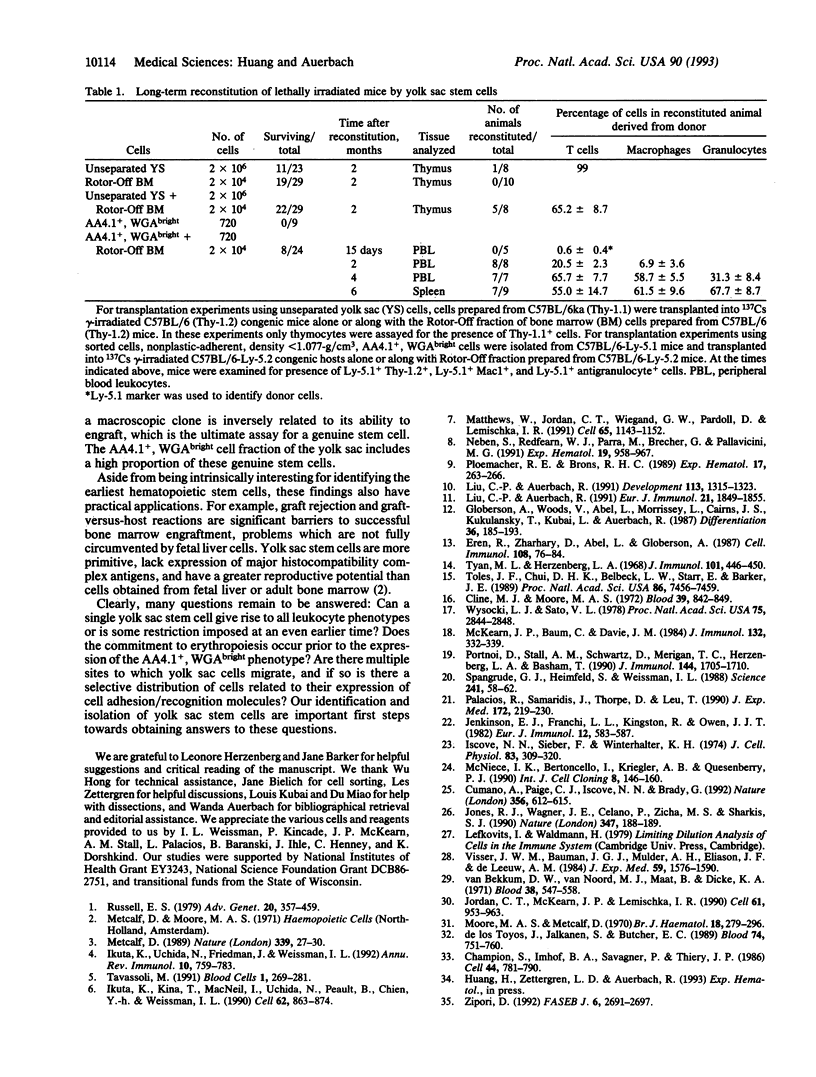
Abstract
The yolk sac is the first site of hematopoiesis in the mammalian embryo. However, little is known about the initial stem cells in the yolk sac. We have isolated hematopoietic stem cells from early mouse embryonic yolk sac by using a sequential protocol of nonadherence to plastic, density gradient centrifugation, immunocytoadherence, and cell sorting. Isolated, nonadherent, density < 1.077-g/cm3, surface antigen AA4.1+, wheat germ agglutinin bright (WGAbright) cells give rise to multiple lineages, including T cells, B cells, and myeloid cells, as detected by using fetal thymus organ culture, S17 stromal feeder layers, or methylcellulose culture colony-forming cells, respectively. AA4.1+, WGAbright cells expressed high levels of heat-stable antigen (HSA) and CD45 (Ly-5) but did not significantly express major histocompatibility complex antigens, CD44, or Sca-1. Peak stem cell concentration is reached by day 11, before stem cells can be found in the liver, omentum, or thymus. In vivo long-term reconstitution of lethally irradiated mice was effected by as few as 720 AA4.1+, WGAbright yolk sac cells, but it required addition of a subset of bone marrow cells capable of providing immediate (short-term) radiation protection. Yolk sac donor-derived T cells, B cells, and macrophages were readily identified 6 months after transfer of yolk sac-derived stem cells. We suggest that, because of their cell surface phenotype as well as their capacity to differentiate in vitro and in vivo, the cells isolated from the mouse embryonic yolk sac may include the most primitive hematopoietic pluripotential stem cells yet identified.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Champion S., Imhof B. A., Savagner P., Thiery J. P. The embryonic thymus produces chemotactic peptides involved in the homing of hemopoietic precursors. Cell. 1986 Mar 14;44(5):781–790. doi: 10.1016/0092-8674(86)90844-5. [DOI] [PubMed] [Google Scholar]
- Cumano A., Paige C. J., Iscove N. N., Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992 Apr 16;356(6370):612–615. doi: 10.1038/356612a0. [DOI] [PubMed] [Google Scholar]
- Eren R., Zharhary D., Abel L., Globerson A. Ontogeny of T cells: development of pre-T cells from fetal liver and yolk sac in the thymus microenvironment. Cell Immunol. 1987 Aug;108(1):76–84. doi: 10.1016/0008-8749(87)90194-8. [DOI] [PubMed] [Google Scholar]
- Globerson A., Woods V., Abel L., Morrissey L., Cairns J. S., Kukulansky T., Kubai L., Auerbach R. In vitro differentiation of mouse embryonic yolk sac cells. Differentiation. 1987;36(3):185–193. doi: 10.1111/j.1432-0436.1987.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y. H., Weissman I. L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62(5):863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Uchida N., Friedman J., Weissman I. L. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Jenkinson E. J., Franchi L. L., Kingston R., Owen J. J. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982 Jul;12(7):583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Wagner J. E., Celano P., Zicha M. S., Sharkis S. J. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990 Sep 13;347(6289):188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Auerbach R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development. 1991 Dec;113(4):1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991 Jun 28;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Baum C., Davie J. M. Cell surface antigens expressed by subsets of pre-B cells and B cells. J Immunol. 1984 Jan;132(1):332–339. [PubMed] [Google Scholar]
- McNiece I. K., Bertoncello I., Kriegler A. B., Quesenberry P. J. Colony-forming cells with high proliferative potential (HPP-CFC). Int J Cell Cloning. 1990 May;8(3):146–160. doi: 10.1002/stem.5530080302. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970 Mar;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Neben S., Redfearn W. J., Parra M., Brecher G., Pallavicini M. G. Short- and long-term repopulation of lethally irradiated mice by bone marrow stem cells enriched on the basis of light scatter and Hoechst 33342 fluorescence. Exp Hematol. 1991 Oct;19(9):958–967. [PubMed] [Google Scholar]
- Palacios R., Samaridis J., Thorpe D., Leu T. Identification and characterization of pro-T lymphocytes and lineage-uncommitted lymphocyte precursors from mice with three novel surface markers. J Exp Med. 1990 Jul 1;172(1):219–230. doi: 10.1084/jem.172.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons R. H. Separation of CFU-S from primitive cells responsible for reconstitution of the bone marrow hemopoietic stem cell compartment following irradiation: evidence for a pre-CFU-S cell. Exp Hematol. 1989 Mar;17(3):263–266. [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. Embryonic and fetal hemopoiesis: an overview. Blood Cells. 1991;17(2):269–286. [PubMed] [Google Scholar]
- Toles J. F., Chui D. H., Belbeck L. W., Starr E., Barker J. E. Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7456–7459. doi: 10.1073/pnas.86.19.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D. The renewal and differentiation of hemopoietic stem cells. FASEB J. 1992 Jun;6(9):2691–2697. doi: 10.1096/fasebj.6.9.1612293. [DOI] [PubMed] [Google Scholar]
- de los Toyos J., Jalkanen S., Butcher E. C. Flow cytometric analysis of the Hermes homing-associated antigen on human lymphocyte subsets. Blood. 1989 Aug 1;74(2):751–760. [PubMed] [Google Scholar]
- van Bekkum D. W., van Noord M. J., Maat B., Dicke K. A. Attempts at identification of hemopoietic stem cell in mouse. Blood. 1971 Nov;38(5):547–558. [PubMed] [Google Scholar]




