Abstract
Changes in dental implant materials, structural design, and surface properties can all affect biological response. While bulk properties are important for mechanical stability of the implant, surface design ultimately contributes to osseointegration. This article reviews the surface parameters of dental implant materials that contribute to improved cell response and osseointegration. In particular, we focus on how surface design affects mesenchymal cell response and differentiation into the osteoblast lineage. Surface roughness has been largely studied at the microscale, but recent studies have highlighted the importance of hierarchical micron/submicron/nanosurface roughness, as well as surface roughness in combination with surface wettability. Integrins are transmembrane receptors that recognize changes in the surface and mediate downstream signaling pathways. Specifically, the noncanonical Wnt5a pathway has been implicated in osteoblastic differentiation of cells on titanium implant surfaces. However, much remains to be elucidated. Only recently have studies been conducted on the differences in biological response to implants based on sex, age, and clinical factors; these all point toward differences that advocate for patient-specific implant design. Finally, challenges in implant surface characterization must be addressed to optimize and compare data across studies. An understanding of both the science and the biology of the materials is crucial for developing novel dental implant materials and surface modifications for improved osseointegration.
Keywords: osteoblast, nanotechnology, nanostructures, dental materials, Wnt5a, titanium
Introduction
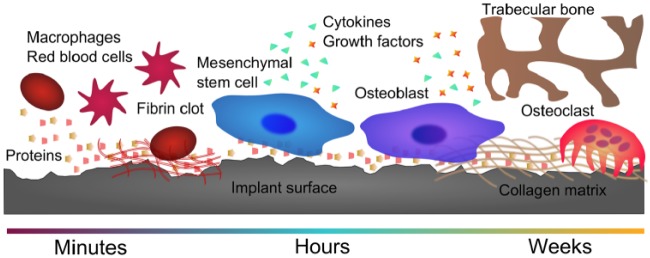
Bone is a dynamic tissue that experiences constant remodeling. When a dental implant is placed, it causes injury to the bone and requires a cascade of events to complete regeneration. Studies on early-phase healing show that implant surface design can contribute to successful osseointegration—or failure—of dental implants (Buser et al. 1991). During early healing, proteins, blood, immune cells, and osteoprogenitor cells interact with the biomaterial (Fig. 1). These interactions ultimately affect implant osseointegration (Claes et al. 2012).
Figure 1.
Biological response timeline on the implant surface. Proteins, blood, immune cells, and osteoprogenitor cells interact with the biomaterial during the early stages of healing. These interactions are surface dependent and can affect osteoblastic differentiation, maturation, and local factor production and, finally, matrix formation and implant osseointegration.
Although many studies have attempted to standardize and characterize mesenchymal stem cells (MSCs), the scientific community is still far from a complete understanding of how these cells contribute to the osseointegration process (Bianco et al. 2013). In this review, we summarize the influence of physical surface parameters on MSC response to dental implant materials. It is our hope that these insights on osteoblastic signaling pathways in response to surface roughness, cell cytoskeletal arrangement, clinical variables contributing to implant osseointegration, and differential biological responses to roughness at different scales can be used for further understanding the cell-material interface in implant dentistry, inspiring the design of a new generation of implants.
Surface Roughness
Surface roughness at the microscale has now become an important parameter in clinical implant design for osseointegration (Coelho et al. 2009). Surface roughness not only increases surface area but also affects cell morphology and increases osteoblastic differentiation, bone formation, and bone remodeling (Schwartz et al. 1997; Wennerberg and Albrektsson 2009). Recent studies show that microtextured titanium surfaces, without additional osteogenic factors, are able to promote osteoblastic differentiation and maturation (Olivares-Navarrete, Hyzy, Hutton, et al. 2010) and implant osseointegration (Cochran et al. 1998).
Although various materials have been studied for use in dental implants, titanium and its alloys are still most commonly used. Our laboratory model is based on 2 titanium surfaces: 1 smooth and 1 rough. Pretreated (PT) surfaces are grade 2 titanium that have undergone a degreasing and acid pretreatment procedure. These surfaces, which are smooth at the microscale, are further processed by sandblasting with large grit and acid etched to produce SLA surfaces possessing approximately a 5-fold increase in surface roughness. The PT and SLA surfaces have allowed us to explore in depth the effect of clinically relevant physical surface properties on cell response and implant osseointegration. We have shown that MSCs and immature osteoblasts consistently exhibit higher osteocalcin, a later marker of osteoblast differentiation, on SLA surfaces versus PT surfaces (Olivares-Navarrete, Hyzy, Park, et al. 2011; Gittens et al. 2013), suggesting enhanced differentiation and maturation of osteoblast lineage cells on rough surfaces as compared with smooth surfaces. In vivo, smooth implants result in fibrous capsule formation over time or osseointegration with low bone-to-implant contact, whereas implants with microroughness are able to achieve osseointegration and higher levels of bone-to-implant contact (Schwartz et al. 2008).
Nanostructures and resulting nanoroughness on surfaces are defined by ASTM International as having structures that are 1 to 100 nm in at least 1 dimension (Mansoori and Soelaiman 2005). Although it has been shown by our laboratory and others that micron- and submicron-scale roughness is important for osteoblast differentiation and maturation in vitro and osseointegration in vivo, only recently has nanoroughness been recognized as a possible contributing factor to these phenomena (Mendonça et al. 2008; Gittens, Olivares-Navarrete, Schwartz, et al. 2014). From a biological perspective, surface nanostructures are intriguing because they have the potential to affect protein adsorption and the resulting integrin attachment, focal adhesion formation, and cellular response to a biomaterial (Gittens, Olivares-Navarrete, Schwartz, et al. 2014).
In addition to smooth PT and rough SLA surfaces, our laboratory has used a hydrophilic SLA surface, which has a comparable microstructure as SLA, to assess the effects of wettability on cell response. The modified SLA (modSLA) surface is processed in a nitrogen atmosphere and stored in isotonic sodium chloride to prevent exposure to atmospheric hydrocarbons. Hydrophilic modSLA surfaces have spontaneously formed nanostructures in addition to their already existing microroughness, which were formed during aging of the surfaces in saline (Wennerberg et al. 2013). Prior to this finding, “nano” was considered in surface analysis but not as a convoluting factor. Most research had focused on nanoroughness or surface energy separately, without considering the possibility of a synergistic effect. These discoveries led us to further attempt to delineate effects of surface nanotopography and wettability (Park, Olivares-Navarrete, et al. 2012; Park, Wasilewski, et al. 2012; Olivares-Navarrete, Rodil, et al. 2015).
Multiscale Surface Roughness
Recent studies have highlighted the need for hierarchical surface roughness, occurring at both the micron- and submicron scale, to be present for osteoblasts to respond synergistically to surface energy and topography (Rupp et al. 2006; Zhao et al. 2007). To understand the effects of nanostructures and hierarchical surface roughness, we developed a novel method of generating nanostructures on clinically relevant microrough surfaces, using a thermal oxidation method (Gittens et al. 2011). Smooth PT surfaces were thermally oxidized at 740 °C for 45, 90, or 180 min. Nanostructures were homogeneously distributed on the surface, ranging from 60 to 360 nm in diameter depending on oxidation time. SLA surfaces showed a similar distribution of submicron and nanostructures across the surface. Osteocalcin, osteoprotegerin, and vascular endothelial growth factor (VEGF) protein levels were all upregulated in osteoblast cultures on combined micro/nanorough surfaces when compared with smooth, nanorough-only, and microrough-only surfaces. The ability to mimic bone, which also has hierarchical roughness, is thought to contribute to the positive biological response to these surfaces with multiscale roughness (Gittens, Olivares-Navarrete, Schwartz, et al. 2014).
Determining the specific role of nanoscale roughness on cell response is confounded by the complexity of the system. Responses of cells in the osteoblast lineage to surface topography vary among cell lines and osteoblast maturation state (Wang et al. 2012; Gittens et al. 2013; Olivares-Navarrete et al. 2014). MG63 osteoblast-like cells are commonly used for in vitro studies (Gittens et al. 2011; Pae et al. 2011; Vandrovcova et al. 2012). MG63 cells, which were initially isolated from a human osteosarcoma, exhibited increased maturation and local factor production on combined nano/microrough titanium surfaces, but human MSCs exhibited a less robust response (Gittens et al. 2013). Because all surfaces were relatively hydrophobic in this study, the impact of surface energy in comparison to that of nanotopography is unknown. These studies not only highlight the importance of experimental design in understanding biological response to materials but also show the need to assess multiple variables to fully comprehend this complex system.
Surface topography is also important for 3-dimensional (3D) constructs. Studies using electrospun titanium 3D scaffolds showed that cell proliferation is dependent on surface microroughness, while osteoblastic differentiation and local factor production depend on both surface microroughness and electrospun nanofiber diameter (Wang et al. 2012). As is the case on 2-dimensional substrates, integrin α2β1 signaling mediates the cellular response to roughness of the 3D surfaces (Wang et al. 2015). These 3D materials served as early prototypes for production of trabecular porosity-inspired Ti-6Al-4V constructs produced by additive manufacturing. Osteoblasts showed porosity-dependent responses in proliferation, differentiation, and local factor production when grown on constructs with interconnected porosity ranging from 15% to 70% (Cheng et al. 2014). These studies suggest 3D porous implants as a possible option for increasing implant osseointegration in compromised patients.
The combination of nanoroughness and wettability of surfaces plays a pivotal role in the early stages of implant healing. Distinct nanostructures on a hydrophobic surface can trap air bubbles, thus influencing the adsorption profile of proteins onto the surface and the resulting cellular adhesion and healing cascade (Gittens, Scheideler, et al. 2014). To investigate the early mechanisms of wound healing on biomaterial surfaces, researchers recently compared protein adsorption and blood coagulation on hydrophobic and hydrophilic microrough commercially pure Ti, hydrophobic and hydrophilic micro/nanorough commercially pure Ti, hydrophobic microrough titanium zirconium alloy, and hydrophilic micro/nanorough titanium zirconium alloy surfaces (Kopf et al. 2015). Fibrinogen and fibronectin adsorption increased on hydrophilic micro/nanorough surfaces as compared with any of the other surfaces, regardless of the material. The presence of micro/nanoroughness alone was able to increase protein adsorption in comparison with hydrophilic surfaces without nanostructures but not as much as the combination of hydrophilicity and nanostructures. In contrast, hydrophilicity alone was the main contributing factor to blood coagulation, and the combination of hydrophilicity and micro/nanoroughness increased coagulation the most. These results point toward the dynamic interplay between nanoroughness and hydrophilicity on the early implant response, corroborating the importance of implant surface design on biological response.
Signaling Pathways
Several biological pathways have emerged as critical for MSC and osteoblast cell response to surface roughness (Fig. 2). Osteoinductive factors were first reported by Marshall Urist in 1965 (Urist 1965), leading to the cloning of the gene for BMP2 (Wozney et al. 1988). BMP2 is now used clinically for bone regeneration in a variety of applications, including sinus lifts (Esposito et al. 2008). We have shown that osteoblasts produce BMP2 when cultured on microtextured Ti and Ti-6Al-4V surfaces, suggesting that they can influence osteoblast differentiation in other cells not on the surface via paracrine regulation (Olivares-Navarrete, Hyzy, Hutton, et al. 2011; Olivares-Navarrete et al. 2014). MSCs treated with conditioned medium from osteoblasts cultured on microrough surfaces were driven toward an osteogenic lineage, supporting this hypothesis (Olivares-Navarrete, Hyzy, Hutton, et al. 2010). Subsequent studies showed that signaling via α2β1 integrins also induced secretion of Dkk2, which had a paracrine effect on MSCs (Olivares-Navarrete, Hyzy, Wieland, et al. 2010; Olivares-Navarrete, Hyzy, Park, et al. 2011).
Figure 2.
Signaling pathways involved in cellular response to implant materials. Integrins are transmembrane receptors that aid in attachment and contribute to differentiation of mesenchymal stem cells on implant surfaces. BMPs and Wnts are important proteins involved in the osteoblastic differentiation pathway. As cells differentiate and mature and bone is formed, local factors are secreted, such as OCN, OPG, BMPs, VEGF, and FGF2.
Mechanisms regulating MSC differentiation and maturation down an osteoblastic pathway on microrough and hydrophilic surfaces involve a variety of signaling pathways. The Wnt signaling pathway is important in embryonic development and for cell proliferation and differentiation. Although the canonical Wnt pathway signals through Wnt3a and β-catenin, our laboratory has found that it is the noncanonical pathway, which signals through Wnt5a and calcium, that results in the response of MSCs to surface roughness (Olivares-Navarrete, Hyzy, Park, et al. 2011). While treatment with Wnt3a maintained the mesenchymal phenotype, treatment with Wnt5a upregulated integrin subunits α2 and β1, BMPs 2 and 4, and osteoblast differentiation markers on rough titanium surfaces as compared with control rough surfaces. Silencing Wnt5a upregulated Wnt3a expression in MSCs. This and other studies suggest that the noncanonical Wnt5a can inhibit the Wnt3a pathway on rough implant surfaces (Baksh et al. 2007; Olivares-Navarrete, Hyzy, Hutton, et al. 2011). Dkk2, an inhibitor of the Wnt canonical pathway, is secreted by osteoblasts grown on microrough titanium surfaces, and secretion of this protein is thought to exert its paracrine effects on MSC differentiation distal to the implant site (Olivares-Navarrete, Hyzy, Hutton, et al. 2010). MG63 osteoblasts grown on microrough SLA surfaces also had increased expression of canonical Wnt inhibitor AXIN2 and BMPs 2 and 4 when compared with tissue culture polystyrene and smooth PT surfaces (Olivares-Navarrete, Hyzy, Hutton, et al. 2011). Further work suggests that while canonical Wnt signaling is involved in early osteoblast differentiation, Ca2+-dependent Wnt5a signaling, as well as Dkk2, BMPs, and integrins, regulates osteoblast differentiation on hydrophilic surfaces with hierarchical roughness (Olivares-Navarrete, Hyzy, Wieland, et al. 2010; Olivares-Navarrete, Hyzy, Hutton, et al. 2011; Olivares-Navarrete, Hyzy, Park, et al. 2011).
These studies demonstrate that surface properties are able to regulate MSC fate through a positive-feedback loop among the calcium-dependent Wnt5a pathway, integrin α2β1, and BMPs. Recent work suggests that 1α,25-dihydroxyvitamin D3, or 1α,25(OH)2D3, which also synergistically affects osteoblast response in combination with surface roughness, may compete with Wnt5a to regulate proliferation and differentiation in osteoblasts. This may have implications in patients receiving vitamin D treatment (Boyan et al. 1998; Doroudi et al. 2014).
It is clear that soluble factors produced by cells in response to surface topographic cues can influence differentiation of cells not on the surface. When grown in coculture with osteoblasts plated on titanium surfaces, human MSCs were differentiated toward osteoblastic phenotype and showed higher levels of osteocalcin, VEGF, and TGF-β1. These effects were higher when the osteoblasts were cultured on modSLA surfaces than on SLA surfaces (Olivares-Navarrete, Hyzy, Hutton, et al. 2010). These results point toward the indirect effects of titanium surface micro/nanoroughness and hydrophilicity on cells distal from the implant site. MG63 cells show higher alkaline phosphatase–specific activity and osteocalcin production as well as higher BMP2 and noggin levels when grown on modSLA surfaces, which are hydrophilic and have nanoroughness, than on microrough-only SLA surfaces. Addition of exogenous BMP2 or knockdown of noggin in cultures enhanced osteoblast maturation, suggesting paracrine regulation of osteoblast maturation (Olivares-Navarrete, Hyzy, Pan, et al. 2015). Angiogenic factors VEGF-A and FGF-2 are both increased significantly on modSLA surfaces in comparison with smooth or microrough-only surfaces, and conditioned media from cultures grown on modSLA stimulate tube formation in cultures of human umbilical vein endothelial cells to a greater extend than media from SLA cultures, suggesting that the combination of roughness and hydrophilicity can enhance blood vessel formation (Raines et al. 2010).
The influence of surface roughness extends indirectly beyond the cellular level to the microenvironment by regulating inflammation and bone remodeling. Rough SLA and modSLA titanium surfaces decreased production of proinflammatory interleukins 6, 8, and 17 and increased anti-inflammatory interleukin 10 by MG63 cells (Hyzy et al. 2013). MSCs also produce reduced levels of proinflammatory cytokines and increased levels of anti-inflammatory cytokines when grown on microtextured surfaces than on smooth surfaces (Olivares-Navarrete, Hyzy, Slosar, et al. 2015). Factors produced by these cells also regulate osteoblast recruitment and activity, thereby delaying bone resorption during the early phase of bone formation. Osteoprotegerin, a decoy receptor for the osteoclast-activating RANKL, is elevated on microrough surfaces (Schwartz et al. 2009). In addition, TGF-β1 is increased, which stimulates bone matrix synthesis and inhibits osteoclasts (Bonewald and Mundy 1990; Kieswetter et al. 1996).
Production of these factors is mediated by signaling through α2β1 integrins. Single knockdown of α2 and double knockdown of α2β1 integrin subunits result in decreased osteoprotegerin, TGF-β1, and PKC levels on rough surfaces. Silencing integrin α2 increases VEGF-A levels and alkaline phosphatase–specific activity on rough surfaces when compared with the response of wild-type cells.
Cell Morphology and Integrin Signaling
Along with biological signals, surface roughness may trigger changes in the cytoskeleton and resulting morphology, causing a change in planar cell polarity and downstream activation of gene transcription and osteoblast differentiation and maturation. Morphologic analysis revealed that osteoblasts grown on rough SLA surfaces exhibited lower cell length, width, area, and circularity but higher aspect ratios than cells grown on smooth PT surfaces (Lai et al. 2014). These changes in cell morphology on rough surfaces correlated with increased osteoblast differentiation marker osteocalcin, as well as α2 and β1 integrin subunits. When α2-silenced cells were cultured on these surfaces the change in morphology was lost, indicating the importance of signaling by α2β1 in mediating cell shape and, ultimately, cell phenotype.
To more clearly determine the specific contributions of topography and chemistry, we compared responses of human MSCs and MG63 cells to smooth and microtextured titanium and to the same surfaces coated with a nanofilm of graphitic carbon (Olivares-Navarrete, Rodil, et al. 2015). Osteogenic differentiation and maturation were enhanced on rougher surfaces, regardless of the chemistry. Gene expression of integrin α1, α2, and β1 subunits were upregulated on rough SLA surfaces, and α1 and α2 were further upregulated on the hydrophilic rough modSLA surface compared with smooth PT. Silencing of the α2 integrin subunit in osteoblasts abolished surface roughness–dependent expression of mRNAs for integrin β1 and osteocalcin regardless of surface chemistry. Production of prostaglandin E2, osteoprotegerin, and TGF-β1, as well as the response to 1α,25(OH)2D3, was also decreased for integrin α2–silenced cells. In contrast, silencing integrin α1 in osteoblasts led to a surface chemistry–dependent response, where the response to roughness was significantly lower in comparison with wild-type cells on titanium but not on graphitic carbon–coated surfaces. Our study suggests that the β1 subunit is involved in roughness recognition, whereas the alpha subunits are responsible for surface chemistry recognition on microrough surfaces (Olivares-Navarrete et al. 2008; Olivares-Navarrete, Rodil, et al. 2015).
Our studies also suggest that different mechanisms may be involved when osteoblasts are grown on microtextured Ti with homogenous nanofeatures imposed on the microtopography. Human osteoblasts had higher expression of mRNAs for osteocalcin, bone sialoprotein, BMPs 2 and 4, noggin, and gremlin 1 on microrough and combined nano/microrough surfaces in comparison with smooth or nanorough-only titanium alloy surfaces (Gittens, Olivares-Navarrete, Hyzy, et al. 2014). However, integrins α1 and α2, traditionally associated with osteoblast response to surface roughness on titanium, were downregulated on combined nano/microrough surfaces, while αV and β3 expression was increased.
Whereas α2 binds mostly to collagen and laminin, αv interacts more with vitronectin, osteopontin, and bone sialoprotein (Clover et al. 1992). These studies point toward a surface topography–specific integrin response that is critical for activating downstream signaling for osteoblast development. Potential pathways and temporal regulation have yet to be investigated for MSCs on surfaces with hierarchical roughness.
Clinical Variables
MSCs are a heterogeneous population isolated from a variety of tissues, most commonly from bone marrow, and are defined by the presence of a set of cell surface markers and by demonstration of their ability to differentiate along a number of mesenchymal cell lineages depending on the culture medium that is used (Bianco et al. 2008). They are frequently used for biological testing of implant materials, but donor variability and culture conditions can contribute to differences in apparent osteogenic potential (Siddappa et al. 2007). Most studies on implant surfaces have not differentiated between male and female cells in vitro and commonly use only male animals in vivo. However, in clinical situations, sex is an important factor that affects musculoskeletal health (Tosi et al. 2006). We have shown that female osteoblasts are sensitive to surface microroughness and that 17β-estradiol (E2) plays a role in modulating their response (Lohmann et al. 2002). Although male and female cells both show increasing production of osteocalcin, TGF-β1, osteoprotegerin, and prostaglandin E2 on rough SLA versus smooth tissue culture polystyrene and PT surfaces, only female osteoblasts show a roughness-dependent increase in differentiation and local factor production in response to treatment with E2 and E2 that is conjugated to bovine serum albumin (Olivares-Navarrete, Hyzy, Chaudhri, et al. 2010). In contrast, the effect of 1α,25(OH)2D3 on increasing osteoblast differentiation and local factor production was more evident in male cells (Olivares-Navarrete, Hyzy, Chaudhri, et al. 2010; Olivares-Navarrete, Hyzy, Boyan, et al. 2015). These studies highlight the importance of sex-specific hormones in regulating response to implant surfaces.
In addition, age can affect healing and implant osseointegration. In vitro observations showing age-dependent differences in cell response to surface roughness support in vivo observations. Titanium implants placed in the femoral intramedullary canal resulted in less bone-to-implant contact and vascularization in 9-mo-old mice in comparison with 2-mo-old mice (Olivares-Navarrete et al. 2012). These results suggest that MSCs may also be less active in contributing toward bone healing in aged mice. Therefore, implant surface parameters that may increase osseointegration for one population may not achieve the same clinical effects in a different population. Patient factors can play an important role in implant healing and osseointegration, and elucidating the differences among patient populations can help design more effective, personalized treatment plans.
Challenges in Standards for Characterization of Implant Surfaces
It is still unclear how nanotopography contributes to the biological response to surface energy. The lack of standard terminology and characterization of nanostructures may contribute to the conflicting reports on the beneficial effects of nanotopography. Many studies that have shown an effect of specific nanostructures on osteoblast differentiation have used models in which these structures are formed either by employing lithographic methods to define patterns on plastic substrates or by anodizing titanium to create regular-shaped features (Martínez et al. 2009; Zhao et al. 2010). In contrast, etching and saline storage of titanium and Ti-6Al-4V generates random surface nanofeatures (Cheng et al. 2014; Wennerberg et al. 2013). When these are superimposed on microtextured surfaces, a complex topography results. Common roughness algorithms cannot take all these factors into consideration (Table). Thus, surfaces with different nanostructure geometries can still have the same roughness algorithm value. A recent study conducted by our laboratory showed that skewness (symmetry as evaluated by elevations or depressions on a surface) and kurtosis (sharpness of peaks) of microrough titanium surfaces are also factors that may predict osteoblast lineage cell response to varying surfaces (Olivares-Navarrete et al. 2014). Well-defined standards for characterization of nanostructures are important and necessary for comparing surfaces and eliciting biological response to physical parameters.
Table.
Commonly Used Terms and Definitions for Surface Roughness.
| Term | Definition |
|---|---|
| Px | Primary values (no filter) |
| Wx | Waviness (low-pass filter) |
| Rx | 2-dimensional roughness (high-pass filter, line) |
| Sx | 3-dimensional roughness (high-pass filter, area) |
| RSa | Average roughness, an arithmetic average value |
| RSc | Mean z height |
| RSsk | Skewness, a measure of asymmetry. Skewness of zero indicates a symmetrical distribution of peaks, whereas nonzero values indicate a weighted distribution toward the right (positive values) or left (negative values) |
| RSku | Kurtosis, a measure of tailedness. Values >3 indicate tails that asymptomatically approach zero, whereas values <3 indicate a uniform distribution without tails. |
| RSq | Root-mean-squared roughness |
| RSt | Total roughness, absolute peak-valley |
| RSz | Maximum peak-valley |
| RSp | Maximum peak height |
| RSv | Maximum valley depth |
A challenge in nanostructure characterization is the limited number of high-resolution techniques available for quantitative nanostructure characterization. Contact profilometry analysis can provide information in only a 2-dimensional line scan but not for a 3D area. Although atomic force microscopy is able to capture the nanoroughness of an otherwise smooth area, it does not have the ability to provide information for clinically relevant surfaces with preexisting microroughness. Though qualitative, scanning electron microscopy is still the gold standard in capturing and assessing nanotopography. Most nanofeatures are analyzed manually via ImageJ or another image-processing software, although development is underway for automated image analysis (Frase et al. 2007; Gittens et al. 2011; Wang et al. 2012). Development of these techniques can allow for better comparisons among studies with varying nanostructure shape and dimension.
On surfaces with roughness at any scale, quantitative evaluation of surface energy can also present a challenge. Typical sessile drop contact angle measurements evaluate surface energy assuming a smooth surface (Rupp et al. 2014). However, the scale of roughness can contribute to droplet-enveloping features or spreading and therefore result in inaccurate contact angle measurements. Smaller droplets that may sit on a “smooth” portion of the rough surface can be affected by line tension and evaporation, while large droplets that compensate for the larger waviness of a surface can be affected by gravity-induced deformations. More sophisticated techniques—such as the Wilhelmy balance method, which immerses the sample into a wetting liquid and takes into consideration the sample weight and buoyancy to calculate the surface tension—may be a more suitable method for assessing wettability of complex surfaces. An alternative method for hydrophobic materials, the captive bubble technique, submerges the surface in a liquid and evaluates the interaction of an air bubble on the surface. It is important to note the nuances and shortcomings associated with each surface technique, especially when comparing across studies.
Conclusion
The field of implant dentistry has progressed tremendously since the discovery of osseointegration. However, for compromised patients, such as smokers or those with a history of chronic periodontitis, implant success is significantly reduced in comparison with success in healthy patients (Karoussis et al. 2003). As new characterization and manufacturing techniques are developed, we will be able to understand cellular response to implant surfaces with better clarity and produce a generation of implants that address patient needs.
While various factors can affect biological response to titanium implant surfaces, roughness at the micro-, submicro-, and nanoscales and hydrophilicity seem to contribute the most to favorable osteoblast response and resulting implant osseointegration. As we begin to understand contributions of each property to protein, cellular, immune, and overall host response, we can begin to design early-loading, longer-lasting dental implants for a wide demographic of patients.
Author Contributions
B.D. Boyan, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript. A. Cheng, R. Olivares-Navarrete, Z. Schwartz, contributed to conception, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Sharon Hyzy for contributions to the work described in this review.
Footnotes
Sponsors of the electronic and paper versions: Institut Straumann AG, Basel, Switzerland. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award AR052102, AB Dental (Asdod, Israel), Institut Straumann AG (Basel, Switzerland), and Titan Spine LLC (Mequon, Wisconsin). The content is solely the responsibility of the authors and does not necessarily represent the official views of these organizations. A.C. is funded by the National Science Foundation Graduate Research Fellowship. Z.S. is a consultant for AB Dental. B.D.B. is a consultant for Titan Spine.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Baksh D, Boland GM, Tuan RS. 2007. Cross-talk between wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem. 101(5):1109–1124. [DOI] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. 2013. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 19(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. 2008. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2(4):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L, Mundy GR. 1990. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res. 1990(250):261–276. [PubMed] [Google Scholar]
- Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Schwartz Z. 1998. Titanium surface roughness alters responsiveness of mg63 osteoblast-like cells to 1 alpha,25-(oh)2d3. J Biomed Mater Res. 39(1):77–85. [DOI] [PubMed] [Google Scholar]
- Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. 1991. Influence of surface characteristics on bone integration of titanium implants: a histomorphometric study in miniature pigs. J Biomed Mat Res. 25(7):889–902. [DOI] [PubMed] [Google Scholar]
- Cheng A, Humayun A, Cohen DJ, Boyan BD, Schwartz Z. 2014. Additively manufactured 3d porous ti-6al-4v constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 6(4):045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Recknagel S, Ignatius A. 2012. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 8(3):133–143. [DOI] [PubMed] [Google Scholar]
- Clover J, Dodds RA, Gowen M. 1992. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 103 (Pt 1):267–271. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. 1998. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 40(1):1–11. [DOI] [PubMed] [Google Scholar]
- Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G, Thompson VP, Lemons JE. 2009. Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 88(2):579–596. [DOI] [PubMed] [Google Scholar]
- Doroudi M, Olivares-Navarrete R, Hyzy SL, Boyan BD, Schwartz Z. 2014. Signaling components of the 1α,25(oh)2d3-dependent pdia3 receptor complex are required for wnt5a calcium-dependent signaling. Biochim Biophys Acta. 1843(11):2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Kwan S, Worthington HV, Coulthard P. 2008. Interventions for replacing missing teeth: Bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 3:CD003607. [DOI] [PubMed] [Google Scholar]
- Frase CG, Buhr E, Dirscherl K. 2007. CD characterization of nanostructures in SEM metrology. Meas Sci Technol. 18(2):510. [Google Scholar]
- Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, Schwartz Z, Sandhage KH, Boyan BD. 2011. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 32(13):3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittens RA, Olivares-Navarrete R, Cheng A, Anderson DM, McLachlan T, Stephan I, Geis-Gerstorfer J, Sandhage KH, Fedorov AG, Rupp F, et al. 2013. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 9(4):6268–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittens RA, Olivares-Navarrete R, Hyzy SL, Sandhage KH, Schwartz Z, Boyan BD. 2014. Superposition of nanostructures on microrough titanium–aluminum–vanadium alloy surfaces results in an altered integrin expression profile in osteoblasts. Connect Tissue Res. 55 Suppl 1:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD. 2014. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 10(8):3363–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, Boyan BD. 2014. A review on the wettability of dental implant surfaces ii: Biological and clinical aspects. Acta Biomater. 10(7):2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyzy SL, Olivares-Navarrete R, Hutton DL, Tan C, Boyan BD, Schwartz Z. 2013. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous bmp-2. Acta Biomater. 9(3):5821–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Bragger U, Hammerle CH, Lang NP. 2003. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the iti dental implant system. Clin Oral Implants Res. 14(3):329–339. [DOI] [PubMed] [Google Scholar]
- Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, Boyan BD. 1996. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like mg-63 cells. J Biomed Mater Res. 32(1):55–63. [DOI] [PubMed] [Google Scholar]
- Kopf BS, Ruch S, Berner S, Spencer ND, Maniura-Weber K. 2015. The role of nanostructures and hydrophilicity in osseointegration: in-vitro protein-adsorption and blood-interaction studies. J Biomed Mater Res A. 103(8):2661–2672. [DOI] [PubMed] [Google Scholar]
- Lai M, Hermann CD, Cheng A, Olivares-Navarrete R, Gittens RA, Bird MM, Walker M, Cai Y, Cai K, Sandhage KH, et al. 2014. Role of alpha2beta1 integrins in mediating cell shape on microtextured titanium surfaces. J Biomed Mater Res A. 103(2):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann CH, Tandy EM, Sylvia VL, Hell-Vocke AK, Cochran DL, Dean DD, Boyan BD, Schwartz Z. 2002. Response of normal female human osteoblasts (nhost) to 17β-estradiol is modulated by implant surface morphology. J Biomed Mater Res. 62(2):204–213. [DOI] [PubMed] [Google Scholar]
- Mansoori GA, Soelaiman TA. 2005. Nanotechnology—an introduction for the standards community. J ASTM Int. 2(6):1–21. [Google Scholar]
- Martínez E, Engel E, Planell JA, Samitier J. 2009. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat. 191(1):126–135. [DOI] [PubMed] [Google Scholar]
- Mendonça G, Mendonça DB, Aragão FJ, Cooper LF. 2008. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials. 29(28):3822–3835. [DOI] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Berg ME, Schneider JM, Hotchkiss K, Schwartz Z, Boyan BD. 2014. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann Biomed Eng. 42(12):2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Boyan BD, Schwartz Z. 2015. Regulation of osteoblast differentiation by acid-etched and/or grit-blasted titanium substrate topography is enhanced by 1,25(oh)2d3 in a sex-dependent manner. Biomed Res Int. 2015:365014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Chaudhri RA, Zhao G, Boyan BD, Schwartz Z. 2010. Sex dependent regulation of osteoblast response to implant surface properties by systemic hormones. Biol Sex Differ. 1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z. 2011. Role of non-canonical wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 7(6):2740–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z. 2010. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 31(10):2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Pan Q, Dunn G, Williams JK, Schwartz Z, Boyan BD. 2015. Osteoblast maturation on microtextured titanium involves paracrine regulation of bone morphogenetic protein signaling. J Biomed Mater Res A. 103(5):1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, Boyan BD, Schwartz Z. 2011. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a wnt-integrin feedback loop. Biomaterials. 32(27):6399–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. 2015. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine. 40(6):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Wieland M, Boyan BD, Schwartz Z. 2010. The roles of wnt signaling modulators dickkopf-1 (dkk1) and dickkopf-2 (dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials. 31(8):2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Raines AL, Hyzy SL, Park JH, Hutton DL, Cochran DL, Boyan BD, Schwartz Z. 2012. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 27(8):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. 2008. Integrin α2β1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 105(41):15767–15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Rodil SE, Hyzy SL, Dunn GR, Almaguer-Flores A, Schwartz Z, Boyan BD. 2015. Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces. Biomaterials. 51:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae A, Kim SS, Kim HS, Woo YH. 2011. Osteoblast-like cell attachment and proliferation on turned, blasted, and anodized titanium surfaces. Int J Oral Maxillofac Implants. 26(3):475–481. [PubMed] [Google Scholar]
- Park JH, Olivares-Navarrete R, Wasilewski CE, Boyan BD, Tannenbaum R, Schwartz Z. 2012. Use of polyelectrolyte thin films to modulate osteoblast response to microstructured titanium surfaces. Biomaterials. 33(21):5267–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Wasilewski CE, Almodovar N, Olivares-Navarrete R, Boyan BD, Tannenbaum R, Schwartz Z. 2012. The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors. Biomaterials. 33(30):7386–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. 2010. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 31(18):4909–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp F, Gittens RA, Scheideler L, Marmur A, Boyan BD, Schwartz Z, Geis-Gerstorfer J. 2014. A review on the wettability of dental implant surfaces I: theoretical and experimental aspects. Acta Biomater. 10(7):2894–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. 2006. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 76(2):323–334. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Kieswetter K, Dean DD, Boyan BD. 1997. Underlying mechanisms at the bone-surface interface during regeneration. J Periodontal Res. 32(1, Pt 2):166–171. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD. 2009. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials. 30(20):3390–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, Boyan BD. 2008. Effect of micrometer-scale roughness of the surface of ti6al4v pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 90(11):2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa R, Licht R, van Blitterswijk C, de Boer J. 2007. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 25(8):1029–1041. [DOI] [PubMed] [Google Scholar]
- Tosi LL, Boyan BD, Boskey AL. 2006. Does sex matter in musculoskeletal health? A workshop report. Orthop Clin North Am. 37(4):523–529. [DOI] [PubMed] [Google Scholar]
- Urist MR. 1965. Bone: formation by autoinduction. Science. 150(3698):893–899. [DOI] [PubMed] [Google Scholar]
- Vandrovcova M, Hanus J, Drabik M, Kylian O, Biederman H, Lisa V, Bacakova L. 2012. Effect of different surface nanoroughness of titanium dioxide films on the growth of human osteoblast-like mg63 cells. J Biomed Mater Res A. 100(4):1016–1032. [DOI] [PubMed] [Google Scholar]
- Wang X, Gittens RA, Song R, Tannenbaum R, Olivares-Navarrete R, Schwartz Z, Chen H, Boyan BD. 2012. Effects of structural properties of electrospun tio2 nanofiber meshes on their osteogenic potential. Acta Biomater. 8(2):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwartz Z, Gittens RA, Cheng A, Olivares-Navarrete R, Chen H, Boyan BD. 2015. Role of integrin α2β1 in mediating osteoblastic differentiation on three-dimensional titanium scaffolds with submicron-scale texture. J Biomed Mater Res A. 103(6):1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T. 2009. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 20 Suppl 4:172–184. [DOI] [PubMed] [Google Scholar]
- Wennerberg A, Svanborg LM, Berner S, Andersson M. 2013. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implants Res. 24(2):203–209. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. 1988. Novel regulators of bone formation: Molecular clones and activities. Science. 242(4885):1528–1534. [DOI] [PubMed] [Google Scholar]
- Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. 2007. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 28(18):2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mei S, Chu PK, Zhang Y, Wu Z. 2010. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials. 31(19):5072–5082. [DOI] [PubMed] [Google Scholar]