Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive parenchymal lung disease characterized by a median survival of 3–5 years following diagnosis. The diagnosis is based on clinical, radiological and histopathological evaluation. Therefore, a multidisciplinary team is needed to reach the correct diagnosis. For a long time, supportive care and lung transplantation in selected cases, have been considered the only possible treatments for IPF. In the last decade many studies have investigated IPF pathogenesis, leading to an improved knowledge of the mechanisms underlying the disease and to the approval of two new drugs for IPF treatment (pirfenidone and nintedanib). The therapeutic approach of IPF cannot be limited to the administration of antifibrotic drugs, but it is necessary for improving the quality of life of patients and for facilitating, as far as possible, the performance of normal daily activities and relationships. IPF patients are also afflicted by disease-related complications such as gastroesophageal reflux, pulmonary hypertension, acute exacerbations and an increased risk of developing lung cancer. The clinician who treats IPF patients, should also treat these possible complications to slow disease progression, thus maintaining the possibility of a pulmonary transplantation.
Keywords: pulmonary fibrosis, pirfenidone, nintedanib, exacerbation, management
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive parenchymal lung disease characterized by a median survival of 3–5 years following diagnosis. However, the clinical course of the disease is unpredictable ranging from a slowly progressive decline course for several years to a rapid deterioration and death in some months [King et al. 2001].
IPF annual incidence was estimated at 16.3 per 100,000 using broad criteria and 6.8 per 100,000 using narrow criteria per 100,000 population in the USA by Raghu and colleagues [Raghu et al. 2006 b] and 4.6 per 100,000 population in Europe by Navaratnam and colleagues [Navaratnam et al. 2011]. However, a rising incidence of the disease, perhaps due to the improved capacity to diagnose IPF using computed tomography (CT) imaging, has been observed [Olson et al. 2007].
According to ATS/ERS statement on IPF, diagnosis is based on the presence of a peculiar radiological pattern on high-resolution computed tomography (HRCT), usual interstitial pneumonia (UIP) (Figure 1), specific combination of radiology and histology patterns and exclusion of other known causes of interstitial lung disease (ILD) [Raghu et al. 2015]. Therefore, a multidisciplinary team with experience in dealing with ILDs is required for a correct IPF diagnosis. Usually, patients refer coughing and exertional dyspnea that may result in respiratory failure. It is possible to find digital clubbing and “velcro”-like rales on chest auscultation.
Figure 1.
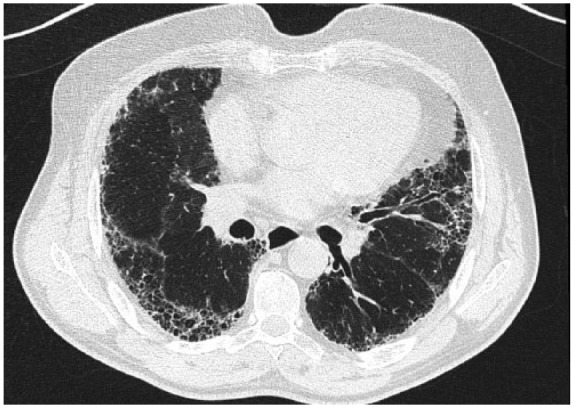
UIP pattern in a 69-year-old woman. The image shows all features of a typical UIP: presence of honey-combing, with reticular pattern and bronchiectasis, predominantly localized in the basal and subpleural areas.
IPF patients are also afflicted by disease-related complications such as gastroesophageal reflux (GER), pulmonary hypertension (PH), acute exacerbations and an increased risk of developing lung cancer (LC). The clinician who treats IPF patients should also treat these possible complications to slow disease progression maintaining the possibility of a pulmonary transplantation.
In this manuscript we will focus on IPF management and on therapeutic issues including lung transplantation.
Clinical trials a lesson from the past
In the last decade many studies have investigated IPF pathogenesis, leading to an improved knowledge of the mechanisms underlying the disease. At the beginning, IPF was supposed to be the final result of an inflammatory process triggered by alveolar injury, evolving into fibrosis through a process involving inflammatory and fibrotic mediators. Corticosteroids and immune-suppressors (azathioprine or prednisone) were recommended as standard treatment by ATS/ERS statement on IPF published in 2000, although a Cochrane review found no studies useful for meta-analysis and no support for their use in IPF [Richeldi et al. 2003]. A new hypothesis maintained IPF as the result of an aberrant reparative response to alveolar epithelial cell injury with migration and differentiation of fibroblasts, excessive deposition of extracellular matrix and wound healing with irreversible architectural distortion of the lung. The 2011 guidelines recommended a ‘weak no’ against corticosteroids and a combination therapy [prednisone, azathioprine and N-acetyl-cysteine (NAC)] so that no proven pharmacological therapy was suggested. Following this statement a multicentre double-blind random placebo-controlled trial, named PANTHER was conducted to demonstrate the efficacy of the association therapy prednisone–azathioprine and NAC in IPF patients. The study was stopped on 21 October 2011, when a press release was published, reporting that the triple therapy arm had been stopped due to an excessive number of deaths, hospitalizations and adverse events [Raghu et al. 2012]. Triple therapy is not only noneffective, but potentially dangerous for IPF patients.
Meanwhile, many other clinical trials have been performed testing the efficacy of different molecules on the basis of pathogenetic observations.
Some studies have suggested that the microenvironment of the fibrotic lung is procoagulant and that some coagulation factors are overexpressed in fibrotic lungs causing fibroblasts proliferation through a mechanism of binding to proteinase activated receptors [Wygrecka et al. 2011]. Based on this evidence new clinical trial were conducted by Kubo and colleagues [Kubo et al. 2005] and Noth and coworkers [Noth et al. 2012] confirming a ‘strong no’ for the use of warfarin in IPF patients.
Many other drugs have been proposed for IPF treatment, such as phosphodiesterase type 5 inhibitors (sildenafil) or an endothelin 1 receptor antagonist (bosentan) because of their supposed antifibrotic action. In particular, two double-blind random placebo-controlled trials testing respectively sildenafil versus placebo (STEP-IPF) [Zisman et al. 2010] and bosentan versus placebo (BUILD 1) [King et al. 2008] failed to show efficacy in clinical trials. Therefore, there is no indication for their use in IPF. Ambrisentan, another endothelin A receptor antagonist, was also tested to reduce IPF progression in a double-blind placebo controlled trial (ARTEMIS-IPF), showing the inefficacy of the drug in the IPF group [Raghu et al. 2013].
The discovery of increased levels of tumor necrosis factor (TNF)-α, a profibrotic cytokine, in lungs of experimental animals led to a clinical trial testing the efficacy of etanercept (TNF-α antagonist) in IPF patients.
The drug failed to show any efficacy in slowing disease progression [Raghu et al. 2008].
Interferon gamma 1b was also tested as a possible treatment in a clinical trial (INSPIRE) revealing a lack of minimal benefit compared with placebo, this study was also stopped before its conclusion [King et al. 2009]. In spite of the different therapeutic efforts the last decade was characterized by very disappointing results, until the two CAPACITY trials, concluded in 2011, showed for the first time that pirfenidone was able to slow the progression of IPF. More recently, an antityrosine receptor antagonist, already used for cancer and named nintedanib, has been approved for IPF.
Pirfenidone
Pirfenidone is an oral synthetic molecule with antifibrotic, antioxidant and anti-inflammatory effects. Data from preclinical studies have demonstrated that pirfenidone inhibits the synthesis of cytokines involved in fibrotic and inflammatory processes such as transforming growth factor (TGF)-β and TNF-α [Nakayama et al. 2008]. The first double-blind random clinical trial testing the efficacy of pirfenidone in IPF patients was a phase II study conducted in Japan [Azuma et al. 2005]. The study was stopped because an interim analysis showed an excess of acute exacerbations in the placebo group compared with the pirfenidone arm. These findings have led to a phase III study in Japan, whose results confirmed the efficacy of pirfenidone in slowing the forced vital capacity (FVC) decline in IPF patients [Taniguchi et al. 2010]. The phase II and III studies allowed the approval of pirfenidone in Japan.
At the same time in Europe and North America, pirfenidone was tested in patients affected by mild to moderate IPF in the CAPACITY trials that consisted of two concurrent multinational randomized double-blind placebo-controlled phase III trials (004 and 006) [Noble et al. 2011]. In study 004, pirfenidone significantly reduced FVC decline at week 72, while study 006 failed to meet its primary end point. On the basis of positive results obtained by study 004 and by Japanese trials, the European Medicines Agency (EMA) approved the use of pirfenidone in mild to moderate IPF in Europe. The US Food and Drug Administration (FDA) instead required an additional trial to approve the use of pirfenidone in the USA. Therefore, an additional randomized, double-blind, placebo-controlled trial called ASCEND was conducted to confirm the effect of pirfenidone on disease progression [King et al. 2014]. Compared with the placebo, pirfenidone reduced the decline of FVC at week 52 and the relative risk of death by 43%. In support of these data a Cochrane meta-analysis showed that pirfenidone is the only effective treatment for IPF patients when progression-free survival is the considered endpoint [Spagnolo et al. 2010]. Following ASCEND results, pirfenidone was also approved by the FDA for the treatment of IPF patients regardless of disease severity. The safety of pirfenidone has been demonstrated in CAPACITY and ASCEND studies. The most frequent side effects reported were gastrointestinal and skin-related events, which were always transient and generally mild to moderate in severity, rarely causing discontinuation. The recommended daily dose of pirfenidone is 2.403 mg corresponding to three 267 mg capsules three times a day to be taken during or at the end of the meal. To prevent gastrointestinal adverse events (nausea, diarrhoea, dyspepsia and vomiting), taking pirfenidone with the meal is suggested because co-administration with food decreases the peak concentration of pirfenidone. Pirfenidone can also cause photosensitivity that is directly proportional to sun exposure. This is related to the pirfenidone pharmacokinetic profile as demonstrated by preclinical studies. Experts suggest avoiding exposure to sunlight and protecting the skin with sunscreen (active against UVA and UVB), wearing protective clothes and hats and avoiding the use of other drugs known to cause photosensitivity.
Experts suggest also to treat mild to moderate photosensitivity reaction or rash, by reducing dosage, followed by discontinuation if it persists for 7 days. Allergic reaction with rash are rare and require pirfenidone discontinuation even if some experts suggest adding antihistamine and prednisone [Costabel et al. 2014].
Nintedanib
Nintedanib is an intracellular multiple tyrosine kinase inhibitor directed against platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), which are all supposed to be involved in IPF pathogenesis. Some studies have shown increased serum levels of FGF and PDGF in IPF animal models [Zhuo et al. 2004; Inoue et al. 2002] and the capacity to reduce lung fibrosis in mice through VEGF receptor inhibition [Hamada et al. 2005].
The first tyrosine kinase inhibitor tested in IPF was imatinib mesylate, whose action is directed against c-abl, Bcr-abl, c-kit and PDGF receptor. It has been used in chronic myeloid leukaemia and gastrointestinal stromal tumours. It was proved that imatinib administration inhibits murine lung fibroblasts growth and prevents bleomycin-induced pulmonary fibrosis in mice [Aono et al. 2005]. However, in a placebo-controlled phase II trial of patients affected by mild to moderate IPF, imatinib did not meet the primary endpoint, showing no effect on survival or lung function decline [Daniels et al. 2010].
On the basis of this evidence, a new molecule called BIBF1000, characterized by a kinase inhibition profile against FGF and VEGF was identified [Bisping et al. 2005]. The capacity of BIBF1000 to attenuate fibrosis in bleomycin induced lung fibrosis and inhibit fibroblasts differentiation in vitro was demonstrated [Chaudhary et al. 2007]. These encouraging results led to a randomized double-blind, placebo-controlled phase II trial (TOMORROW) to evaluate the efficacy of BIBF1120, a potent intracellular inhibitor of tyrosine kinases in IPF patients, used in oncology [Hilberg et al. 2008]. Its targets are PDGF, FGF and VEGF receptors involved in IPF pathogenesis. This clinical trial randomized 432 IPF patients, who were assigned capsules of placebo or one of the following doses of BIBF1120: 50 mg once a day, 50 mg twice a day, 100 mg twice a day, 150 mg twice a day.
The primary endpoint was defined as the annual rate of decline of FVC and it was reached by patients group receiving the highest dose of BIBF1120. A reduction of 68.4% in the annual rate of decline in FVC in the group receiving 150 mg of BIBF1120 twice a day was recorded. The incidence of acute exacerbations, defined as secondary endpoint, was lower in the group receiving BIBF1120 150 mg twice a day compared with placebo. BIBF1120 was also tested for its safety showing gastrointestinal adverse events as the principal cause of drug discontinuation, followed by increased liver enzyme levels [Richeldi et al. 2011]. Following the results of this clinical trial, two other randomized double-blind phase III trials (INPULSIS-1, INPULSIS-2) were conducted to evaluate the efficacy and safety of BIBF1120 in IPF patients. BIBF1120 reduced the rate of decline in FVC over the 52 weeks treatment period in both INPULSIS trials reaching the primary endpoint [Richeldi et al. 2014]. The most frequent adverse events were gastrointestinal events, especially diarrhoea that occurred in 61.5% of patients in INPULSIS-1 and 63.2% in INPULSIS-2, and increased liver enzymes levels that occurred in 4.9% of patients in INPULSIS-1 and 5.2% of patients in INPULSIS-2, respectively. Adverse events were reversible without clinically significant consequences. Following INPULSIS-1 and INPULSIS-2 results, nintedanib received EMA and FDA approval for the treatment of IPF patients, regardless of disease severity.
Future and ongoing clinical trials
Many clinical trials to test the efficacy of new molecules in IPF are currently ongoing. Some studies have shown increased levels of interleukin-13 (IL-13) in bronchoalveolar lavage fluid (BALF) of IPF patients and it has been demonstrated that IL-13 is involved in abnormal epithelial-fibroblast cross talk and that promotes fibroblast collagen production [Murray et al. 2008]. This evidence has led to a phase II clinical trial testing the efficacy of a human anti IL-13 monoclonal antibody in IPF patients and its results are still expected.
Lysil oxidase and lysil oxidase-like (LOLXL) enzymes are involved in the cross-linking of collagen. This represents the principal component of extracellular matrix, whose excessive deposition can lead to IPF. A new molecule called anti-LOXL2 has been proposed. It is a monoclonal antibody directed against LOXL2 that inhibits excessive extracellular matrix deposition [Barry-Hamilton et al. 2010]. Many other new drugs with the aim of inhibiting different known pathways of IPF are being tested at the moment. An emerging opinion is rising among experts, according to which, combination treatments are needed, as no single pathway exists in individual patients. Pirfenidone and nintedanib are pleiotropic drugs, whose action is directed towards different pathways. The development of effective combination therapies amplifying the benefits of available therapies requires a deeper knowledge of IPF pathogenesis leading to a shift to combination therapy like many other lung diseases [Wuyts et al. 2014].
Lung transplantation
Lung transplantation is an accepted form of treatment for patients with chronic, end-stage lung disease who are failing maximal medical therapy, or for whom no effective medical therapy exists.
Despite advances in medical therapy, lung transplantation represents the best opportunity for selected IPF patients [Raghu et al. 2015]. Patients should be referred to the transplantation centre when the criteria required to enter the transplantation list are respected. However there are no clear criteria to guide timing of transplantation but recommendations have been proposed on the basis of pulmonary function tests (PFTs) and on the basis of disease progression. The International Society for Heart and Lung Transplantation (ISHLT) guidelines indicate criteria for transplantation in IPF patients based on respiratory functional data: DLCO < 40% predicted, >10% decline in FVC after 6 months follow up or desaturation under 88% in pulsossimetry during 6-minute walking test (6MWT). Potential candidates for the transplant waiting list should be studied carefully to detect the presence of contraindications [McCartney and Meyer, 2008].
To evaluate the priority on the waiting list, a lung allocation score (LAS score) is used in an effort to measure the urgency (expected lifetime without a transplant) and the benefit (improved survival following the transplant) of the transplant. LAS scores range from 0 to 100 and patients with the higher score i.e. greater predicted survival, have priority. In May 2005, the United Network for Organ Sharing (UNOS) implemented the Lung Allocation Score (LAS), which has also been applied in Europe since 2011 and this has led to an increased number of lung transplantations.
The primary goal of lung transplantation is to provide survival benefit. According to the OPTN Annual Report of 2011, survival at one year post transplantation in US IPF patients from 2005–2006 was 81%, decreasing to 64% at 3 years and to 53% at 5 years post-transplantation. This data shows a poorer prognosis for IPF transplanted patients compared to transplanted patients for other diseases. However, considering the higher waiting list mortality, poor prognosis and poor quality of life of IPF patients, lung transplantation must be considered an efficient treatment.
IPF comorbidities and their treatment
Very often IPF patients have comorbidities that may further worsen their quality of life and the prognosis of the disease. Gastresophageal reflux (GER), pulmonary hypertension (PH) and cardiovascular diseases, acute exacerbations (AEIPF) and an increased risk of developing lung cancer (LC) are common in IPF patients. The therapeutic approach of IPF cannot be limited to the administration of antifibrotic drugs or lung transplantation but a series of measures and actions should also be considered in order to support and improve the quality of life of these patients and to facilitate, as far as possible, the performance of normal daily activities and relationships.
Gastroesophageal reflux
Many studies have shown the higher prevalence of GER in IPF patients compared with patients affected by other ILDs and the general population [Morehead, 2009]. Silent GER was observed in 90% of IPF patients based on 24-hour esophageal pH monitoring [Raghu et al. 2006 a]. GER and IPF also share many risk factors such as aging, male gender and cigarette smoke exposure. The relationship between IPF and GER has been investigated to better understand its role in the pathogenesis, natural history and survival of IPF. According to some studies, repetitive alveolar epithelial cell injury can cause an irregular wound healing process, leading to lung fibrosis. It has been proposed that in some predisposed patients, GER and repetitive micro-aspiration of acid in the airways can cause continuous alveolar epithelial cell injury, leading to a series of mechanism responsible for IPF [Schachter, 2003]. Moreover, it is well known that micro-aspiration is involved in the pathogenesis of other interstitial pneumonia, such as lipoid pneumonia, caused by chronic micro-aspiration of mineral oil. Therefore, micro-aspiration is a possible explanation of IPF pathogenesis. Micro-aspiration is also believed to be involved in IPF acute exacerbations (AEIPF) as proved by Lee and colleagues, who showed high level of pepsin in BALF of AEIPF patients. The discovery of pepsin in BALF was proposed as a biomarker of microaspiration and correlated with AEIPF even if its increased level was not predictive of survival [Lee et al. 2013]. In addition, small case series have shown FVC stabilization in IPF patients affected by GER and treated with medical therapy [Raghu et al. 2006 c], leading to new studies testing the efficacy of GER treatment in IPF progression. Lee and colleagues showed a slower decline of functional capacity in IPF patients affected by GER treated with anti-acid therapy, suggesting that anti-acid therapy may be beneficial to IPF patients [Lee et al. 2013].
Pulmonary hypertension
PH is defined as mean pulmonary artery pressure (mPAP) of >25 mmHg as assessed by right heart catheterization (RHC). It is increasingly recognized as a complication of ILDs and included in group 3 of the 2013 NICE clinical classification of PH [Simonneau et al. 2013]. The real prevalence of PH in IPF remains undefined, with reported prevalence rates varying from 8.1% to 50% in different studies, due to differences in populations and measuring methods (RHC versus echocardiography) [Seeger and Pullamsetti, 2013; Hamada et al. 2007].
PH in IPF is usually mild, with only a small percentage of patients showing mPAP values >40 mmHg [Shorr et al. 2007]. Conventionally, mPAP values >35 mmHg in IPF patients have been defined as ‘severe PH’. This identifies the relatively small group of IPF patients with significant vascular abnormalities and circulatory impairment that substantially worsens exercise capacity.
Usually, patients with IPF and severe PH have significantly lower values for DLCO and arterial oxygenation at rest, lower exercise capacity, and decline of arterial oxygenation upon exercise, independent of lung function tests [Boutou et al. 2011; Minai et al. 2012]. Severe PH has a significant impact on outcomes, with an almost threefold increased risk of death. Once diagnosed, the median survival time in PH-IPF patients is estimated to be 2.5–3.5 years [Nadrous et al. 2005].
Routine measurements from PFTs may not accurately correlate with survival in patients with IPF [King et al. 2001; Martinez et al. 2005], because there is only poor or even no correlation between PH severity and lung function impairment or HRCT fibrosis score. Surrogate markers, such as reduced DLCO, rapid desaturation upon exercise, need for supplemental oxygen, poor performance on the 6MWT, large right heart dilation on chest radiography, enlargement of pulmonary vessels on HRCT (Figure 2) or high brain natriuretic peptide (BNP) levels, should raise suspicion of the presence of PH and the need for confirmatory and RHC [Shorr et al. 2007; Lettieri et al. 2006].
Figure 2.
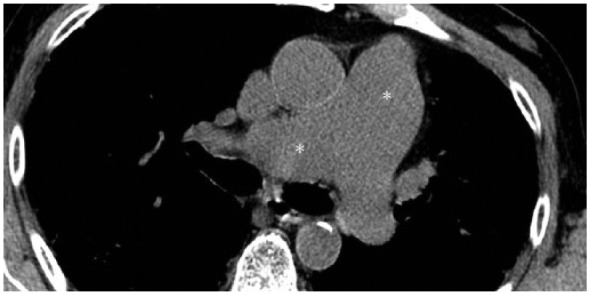
Pulmonary hypertension in a UIP patient. Enlargement of pulmonary vessels (white asterisks) are well depicted in the mediastinum.
RHC is the gold standard for diagnosis of PH and it should be performed in order to evaluate patients for lung transplantation. Echocardiography is frequently inaccurate in patients with ILD. It should be performed as the initial screening test to confirm the suspicion of PH and to exclude concomitant cardiac diseases, or during follow up, to monitor right ventricular function [Arcasoy et al. 2003].
Currently, there are no treatments approved for PH in IPF. Even if PH-IPF seems to share some pathobiological features with PAH, there are no data to support the use of vasoactive therapy approved for PAH in PH-IPF patients. In addition, several concerns have been raised about the potential unfavourable profile of these drugs in IPF patients, supposing a worsening in gas exchange due to the interference with hypoxic vasoconstriction causing ventilation/perfusion mismatch [Agusti et al. 1993]. Long-term randomized controlled trials focusing on patients with severe PH and restrictive lung diseases are needed and are ongoing. Only this approach will provide reliable data for the use of PAH-approved drugs in these patients. Currently, for severe PH-IPF patients, referral to expert PAH centres is strongly recommended [Seeger et al. 2013].
Cancer in IPF
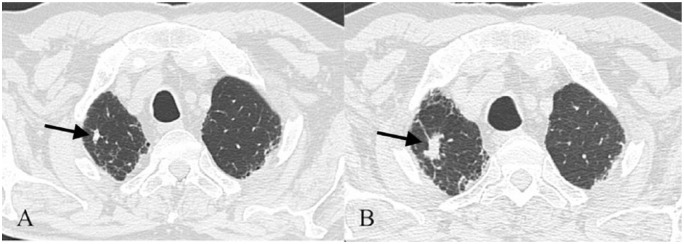
LC occurs with higher prevalence in patients affected by IPF, ranging from 4.8% to 48% [Le Jeune et al. 2007]. The cumulative incidence of LC in IPF was estimated in a retrospective study showing 3.3%, 15.4% and 54.7% of incidence of LC, respectively, after 1, 5 and 10 years of follow up [Ozawa et al. 2009] (Figure 3).
Figure 3.
UIP pattern in 74-year-old man. A small nodular lesion is well depicted on Figure A (black arrow); 18 months later (Figure B) nodule (black arrow) - located in the right upper lobe - enlarges in size, and shows an irregular shape, with speculated margins. Lesion has been reported as a lung cancer in a scar tissue.
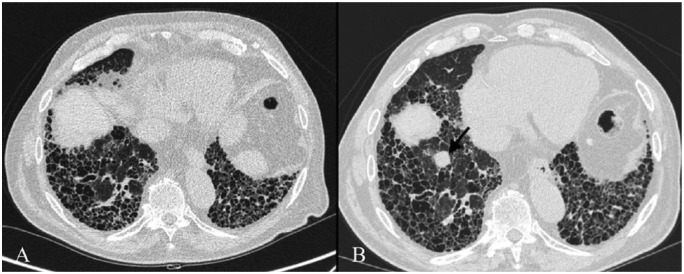
The cause of cancer and IPF association is unknown but common risk factors were identified as cigarette smoke, male gender and age [Aubry et al. 2002]. HRCT is used in IPF follow up and brought considerable advantage in the early discovery of LC in these patients. Usually, cancer lesions are identified in peripheral areas of the lung and in the lower lobes in correspondence with honeycombing lesions or fibrotic areas [Kishi et al. 2006]. Moreover, the most frequent histological type of cancer lesion is represented by squamous cell carcinoma [Park et al. 2001] (Figure 4).
Figure 4.
Right basal lung cancer in a 76-year-old man with UIP. Figure A clearly shows a typical UIP pattern; on figure B, HRCT performed 15 months later demonstrates a nodular lesion in the right basal lung (black arrow), which was not evident in the previous control.
The survival in patients affected by LC and IPF is unclear. A recent study has proved that mortality in IPF complicated by LC is due not only to IPF progression but also to complications of LC treatment. It has been estimated that 80% of IPF patients with LC experienced exacerbation following treatment or diagnostic procedures for LC.
Therefore, clinical decisions are very difficult to make. Contrasting opinions are present in literature concerning whether or not to treat these lesions. The high risk of IPF exacerbation following radiotherapy or surgical treatment of cancer lesions has been shown in some studies, according to which surgical treatment regardless of age, sex and disease stage caused a reduction in survival. Therefore, complications can be considered as lethal as cancer itself [Lee et al. 2014 b]. On the other hand, some authors have demonstrated that lung resection can improve survival in a selected group of patients despite the high risk of IPF exacerbations [Kumar et al. 2013]. In particular, Watanabe and colleagues suggest surgical treatment of cancer lesions in IPF patients affected by stage I LC although long-term results of these patients are worse than patients without IPF [Watanabe et al. 2008].
Tomassetti and colleagues suggest sublobar resection as a safer surgical treatment of stage I LC in mild IPF patients compared with lobectomy even if the risk of LC recurrence is high [Tomassetti et al. 2015]. Therefore, to treat LC in a minimally invasive way, early diagnosis of LC by HRCT scan would be necessary. Chemotherapy is not considered safe and the median survival of IPF patients affected by LC stage III/IV treated with chemotherapy is estimated to be 8.5 months from diagnosis [Tomasetti et al. 2015]. Some studies propose the benefits of chemotherapy in advanced non-small cell lung cancer (NSCLC) patients with IPF even if well-controlled studies are needed to clarify the efficacy of chemotherapy and surgical treatments. Surgical and chemotherapy treatments of LC in IPF patients are characterized by an increased incidence of complications, as lethal as the cancer itself. Consequently, their indiscriminate use is still discussed.
Acute exacerbation
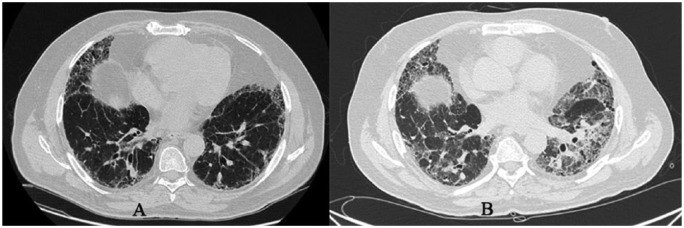
AEIPF is defined by the IPF Clinical Research Network as a condition characterized by the following features: subjective worsening of dyspnea within 30 days, presence of new bilateral opacities on HRCT of the chest, no evidence of pulmonary infection in bronchoalveolar lavage and the exclusion of other alternative causes (Figure 5). The 1- and 3-year incidences have been evaluated at 14% and 21% respectively in a retrospective study conducted by Song et al. [Song et al. 2011]. Many hypotheses about the aetiology of AEIPF exist, such as aspiration of gastric context, viral infection, drug toxicity or seasonal variation. However, the real aetiology is still unknown. Some reports suggest that surgical lung biopsy [Kondoh et al. 2006] and any kind of thoracic surgery may be precipitating factors for AEIPF [Sakamoto et al. 2011]. Unfortunately the prognosis is poor and the median survival is estimated at 2.2 months from the onset. The 5-year survival rate was estimated at 18.4%. Nearly 50% of patients were admitted to an intensive unit care of which nearly 80% died [Song et al. 2011]. An early identification of AEIPF is fundamental because of poor prognosis. An accurate medical history is needed to exclude reversible factors responsible for the acceleration of diseases, such as heart failure, drug toxicity or pulmonary infection. Echocardiography is necessary to exclude the presence of congestive heart failure, which can mimic AEIPF [Bradley et al. 2008] and to evaluate PH, which is associated with a significant risk of AEIPF [Judge et al. 2012].
Figure 5.
Pulmonary exacerbation in a 75-year-old woman with UIP. Diffuse ground-glass areas and pulmonary consolidations are shown in the figure.
There are no clear guidelines regarding the treatment of AEIPF and this is due to the lack of clinical trials and to the lack of a consistent definition of AEIPF. Therefore, management is based on case series or on expert recommendation. In recent guidelines for the management of IPF there is a weak recommendation for the use of pulses of methylprednisolone. In a recent report a small group of AEIPF patients was treated with initial steroid pulses of methylprednisolone 1000 mg for 3 days, and on day 4 placed on an escalating dose of cyclophosphamide with an initial dose of 500 mg intravenously, increased by 200 mg every 2 weeks to reach the maximum dose of 1500 mg. The 1-year survival was estimated to be 33% [Morawiec et al. 2011].
A recent retrospective study was conducted on 26 patients with rapidly progressive ILD including AEIPF. The mean survival time of patients treated with polymyxin added to steroid pulse therapy, was longer compared with patients treated without polymyxin. These results suggest that the administration of polymyxin on the first day of steroid pulse therapy can improve the prognosis of rapidly progressive ILDs including AEIPF. However, clinical trials are needed to confirm the result [Takada et al. 2014].
Polymyxin B fibre column is an extracorporeal technique used to reduce blood toxins in sepsis. It is administered in AE-IPF because of increased blood levels of inflammatory cytokines in these patients. Even if the mechanism through which polymyxin improves oxygenation in AEIPF is unclear, the level of cytokines was decreased by the treatment [Nakamura et al. 2004].
OSAS in IPF
Obstructive sleep apnoea (OSA) is the most common sleep-related breathing disorder and affects approximately 15–26% of the population [Young et al. 2009]. It is characterized by the presence of apnoeas and hypopnoeas responsible for oxygen desaturations and repeated arousals that cause significant respiratory, cardiovascular, metabolic and cognitive complications, impaired sleep quality, chronic disabling symptoms and, in severe cases, increased mortality. Many symptoms caused by sleep fragmentation, such as daytime fatigue and insomnia, are very common in patients with IPF and from this observation many studies have been conducted to evaluate the correlation of IPF with OSA [Krishnan et al. 2008; Rasche and Orth, 2009]. Recently, some authors have reported a high incidence of OSA in IPF patients, estimated between 59% and 88% [Lancaster et al. 2009; Kolilekas et al. 2013], leading to more attention being given to sleep disorders and their clinical impact on these patients. These studies focus on the primary role that OSA plays in worsening sleep quality and daytime symptoms, which is already compromised in IPF patients by the presence of cough, arousals, desaturations, increased respiratory drive and altered relationship between REM and NREM sleep [Rasche et al. 2009]. Although the reasons for this association between OSA and IPF remain unclear, several hypotheses have to be considered. First of all, it is believed that restrictive lung diseases reduce upper airway tone, increasing collapsibility due to reduced caudal traction and that increased BMI can also contribute to the genesis of OSA. Also, alterations of ventilatory drive, frequent in IPF patients, are likely to play a role [Troy and Corte, 2014]. Last to be considered is the hypothesis that OSA, through mechanisms of oxidative stress, micro-aspiration from GER and insults due to mechanical traction, would create a chronic subclinical lung injury that, in susceptible individuals could open the door to IPF [Lederer et al. 2012; Lee et al. 2014 a].
Latest evidence in literature shows the importance of investigating the presence of OSA, thereby treating it to improve sleep and life quality and reducing complications and mortality in subjects suffering from such a devastating disease as IPF [Mermigkis et al. 2015]. Whether OSA treatment can stop or slow the progression of IPF remains an interesting topic of study.
Pulmonary rehabilitation
In 2011, ATS, ERS, JRS and ALAT jointly declared that, despite the low level of recommendation, pulmonary rehabilitation (PR) should be recommended to most IPF patients and its prescription should not be considered a reasonable choice only in a minority of patients [Raghu et al. 2011].
Some studies have been recently conducted to evaluate the effectiveness of PR in IPF patients but unfortunately there was no standardization of the type (cycle ergometer, treadmill, and/or arts training), the duration (6–12 weeks) and the intensity (50–80% of the “peak work rate”) of exercise. Therefore, despite the lack of an ideal protocol, data have confirmed that PR is useful in IPF patients. An improvement in exercise tolerance, particularly a transient increase in the distance travelled in the walk test or a decrease in heart rate during the test and a clear improvement of the resistance to the cycle ergometer can be observed. Positive effects were also seen on both the quality of life as demonstrated by the results of the SF-36 and SGRQ questionnaires, and on dyspnea as evaluated by CRQ and TDI questionnaires. This showed a significant improvement in dyspnea or at least a trend towards reducing dyspnea [Nishiyama et al. 2008; Swigris et al. 2010]. A study of a case series of 34 patients showed that the effects of PR do not last for more than six months, except for a small subset of patients in more advanced stage in which some positive effects were still present after 6 months [Salhi et al. 2010].
However, there is scientific evidence that a longer duration of rehabilitation cycle can increase the duration of the effects: rehabilitation courses of 12 or even 24 weeks provide a more lasting effect [Jastrzebski et al. 2006].
PR plays a different role in patients who are candidates for lung transplantation. In this case PR is useful for improving the preparation of the patient for surgical and post-operative stress. In this sense, there is some evidence of the remarkable utility of PR in candidates for lung transplantation. Recently, a group of 345 patients in a PR program before lung transplantation was analysed. The patients with more benefits from PR showed a general continuation of exercise capacity after transplantation and, above all, a favourable postoperative course [Li et al. 2013].
Conclusion
IPF is a chronic progressive disease leading to lung failure and death. For a long time supportive care and lung transplantation in selected cases, have been considered the only possible treatments for IPF. Recent studies have increased our understanding of IPF and allowed for the discovery of two new drugs (pirfenidone and nintedanib) that may slow the rate of decline in lung function. These encouraging results have stimulated other studies on IPF pathogenesis that will facilitate the selection of therapeutic targets, thus leading to new clinical trials.
It is well known that comorbidities, such as LC and cardiovascular disease, can affect the prognosis of patients with IPF even if little is known about their impact on disease progression. However, it is important to recognize and to treat comorbidities as soon as possible because this may play a role in optimizing the survival of IPF patients.
Pharmacological treatment together with comorbidities treatment will help to turn IPF from a fatal disease into a chronic and treatable one.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Silvia Puglisi, Regional Centre for Interstitial and Rare Lung Diseases, Department of Clinical and Experimental Medicine, University of Catania, Via S. Sofia 78 Catania, 95123, Italy silviapuglisi@fastwebnet.it.
Sebastiano Emanuele Torrisi, Regional Centre for Interstitial and Rare Lung Diseases, Department of Clinical and Experimental Medicine, University of Catania, Via S. Sofia 78 Catania, 95123, Italy torrisiseby@hotmail.it.
Virginia Vindigni, Regional Centre for Interstitial and Rare Lung Diseases, Department of Clinical and Experimental Medicine, University of Catania, Via S. Sofia 78 Catania, 95123, Italy v-vindigni@hotmail.it.
Riccardo Giuliano, Regional Centre for Interstitial and Rare Lung Diseases, Department of Clinical and Experimental Medicine, University of Catania, Via S. Sofia 78 Catania, 95123, Italy dottor.giuliano@gmail.com.
Stefano Palmucci, Radiodiagnostic and Radiotherapy Unit-University Hospital “Policlinico-Vittorio Emanuele” Via Santa Sofia 78, Catania 95123-Italy spalmucci@sirm.org.
Massimiliano Mulè, University of Catania - Division of Cardiology Ferrarotto Hospital, Catania, Italy medimaxim@gmail.com.
Carlo Vancheri, Regional Centre for Interstitial and Rare Lung Diseases, Department of Clinical and Experimental Medicine, University of Catania, Via S. Sofia 78 Catania, 95123, Italy.
References
- Agusti A., Rodriguez-Roisin R. (1993) Effect of pulmonary hypertension on gas exchange. Eur Respir J 6: 1371–1377. [PubMed] [Google Scholar]
- Aono Y., Nishioka Y., Inayama M., Ugai M., Kishi J., Uehara H., et al. (2005) Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 171: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Arcasoy S., Christie J., Ferrari V., Sutton M., Zisman D., Blumenthal N., et al. (2003) Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 167: 735–740. [DOI] [PubMed] [Google Scholar]
- Aubry M., Myers J., Douglas W., Tazelaar H., Washington Stephens T., Hartman T., et al. (2002) Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis. Mayo Clin Proc 77: 763–770. [DOI] [PubMed] [Google Scholar]
- Azuma A., Nukiwa T., Tsuboi E., Suga M., Abe S., Nakata K., et al. (2005) Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 171: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Barry-Hamilton V., Sangler R., Marshall D., McCauley S., Rodriguez H., Oyasu M., et al. (2010) Allosteric inhibition of lixil oxidase like 2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Bisping G., Kropff M., Wenning D., Dreyer B., Bessonov S., Hilberg F., et al. (2005) Targeting receptor kinases by a novel indolinone derivative in multiple myeloma: abrogation of stroma-derived interleukin-6secretion and induction of apoptosis in cytogenetically defined subgroups. Blood 107: 2079–2089. [DOI] [PubMed] [Google Scholar]
- Boutou A., Pitsiou G., Trigonis I., Papakosta D., Kontou P., Chavouzis N., et al. (2011) Exercise capacity in idiopathic pulmonary fibrosis: the effect of pulmonary hypertension. Respirology 16: 451–458. [DOI] [PubMed] [Google Scholar]
- Bradley B., Branley H., Egan J., Greaves M., Hansell D., Harrison N., et al. (2008) Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 63(Suppl. 5): v1–v58. [DOI] [PubMed] [Google Scholar]
- Chaudhary N., Roth G., Hilberg F., Muller-Quernheim J., Prasse A., Zissel G., et al. (2007) Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J 29: 976–985. [DOI] [PubMed] [Google Scholar]
- Costabel U., Bendstrup E., Cottin V., Dewint P., Egan J., Ferguson J., et al. (2014) Pirfenidone in idiopathic pulmonary fibrosis: expert panel discussion on the management of drug-related adverse events. Adv Ther 31: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C., Lasky J., Limper A., Mieras K., Gabor E., Schroeder D., et al. (2010) Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebocontrolled trial results. Am J Respir Crit Care Med 181: 604–610. [DOI] [PubMed] [Google Scholar]
- Hamada N., Kuwano K., Yamada M., Hagimoto N., Hiasa K., Egashira K., et al. (2005) Antivascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol 175: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Hamada K., Nagai S., Tanaka S., Handa T., Shigematsu M., Nagao T., et al. (2007) Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 131: 650–656. [DOI] [PubMed] [Google Scholar]
- Hilberg F., Roth G., Krssak M., Kautschitsch S., Sommergruber W., Tontsch-Grunt U., et al. (2008) BIBF1120: triple angiokinase inhibitor with sustained receptor blockade and good anti tumor efficacy. Cancer Res 68: 4774–4782. [DOI] [PubMed] [Google Scholar]
- Inoue Y., King T., Jr, Barker E., Daniloff E., Newman L. (2002) Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med 166: 765–773. [DOI] [PubMed] [Google Scholar]
- Jastrzebski D., Gumola A., Gawlik R., Kozielski J. (2006) Dyspnea and quality of life in patients with pulmonary fibrosis after six weeks of respiratory rehabilitation. J Physiol Pharmacol 57(Suppl. 4): 139–148. [PubMed] [Google Scholar]
- Judge E., Fabre A., Adamali H., Egan J. (2012) Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J 40: 93–100. [DOI] [PubMed] [Google Scholar]
- King T., Jr, Albera C., Bradford W., Costabel U., Hormel P., Lancaster L., et al. (2009) Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet 374: 222–228. [DOI] [PubMed] [Google Scholar]
- King T., Jr, Behr J., Brown K., du Bois R., Lancaster L., de Andrade J., et al. (2008) BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 177: 75–81. [DOI] [PubMed] [Google Scholar]
- King T., Jr, Bradford W., Castro-Bernardini S., Fagan E., Glaspole I., Glassberg M., et al. (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- King T., Tooze J., Schwarz M., Brown K., Cherniack R. (2001) Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 164: 1171–1181. [DOI] [PubMed] [Google Scholar]
- Kishi K., Homma S., Kurosaki A., Motoi N., Yoshimura K. (2006) High-resolution computed tomography findings of lung cancer associated with idiopathic pulmonary fibrosis. J Comput Assist Tomogr 30: 95–99. [DOI] [PubMed] [Google Scholar]
- Kolilekas L., Manali E., Vlami K., Lyberopoulos P., Triantafillidou C., Kagouridis K., et al. (2013) Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med 9: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh Y., Taniguchi H., Kitaichi M., Yokoi T., Johkoh T., Oishi T., et al. (2006) Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 100: 1753–1759. [DOI] [PubMed] [Google Scholar]
- Krishnan V., McCormack M., Mathai S., Agarwal S., Richardson B., Horton M., et al. (2008) Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest 134: 693–698. [DOI] [PubMed] [Google Scholar]
- Kubo H., Nakayama K., Yanai M., Suzuki T., Yamaya M., Watanabe M., et al. (2005) Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest 128: 1475–1482. [DOI] [PubMed] [Google Scholar]
- Kumar P., Goldstraw P., Yamada K., Nicholson A., Wells A. (2013) Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 125: 1321–1327. [DOI] [PubMed] [Google Scholar]
- Lancaster L., Mason W., Parnell J., Rice T., Loyd J., Milstone A., et al. (2009) Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest 136: 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer D., Jelic S., Basner R., Bhattacharya J. (2012) Is obstructive sleep apnea a cause ofidiopathic pulmonary fibrosis? Arch Pathol Lab Med 136: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Collard H., Anstrom K., Martinez F., Noth I., Roberts R., et al. (2013) Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomized controlled trials. Lancet Respir Med 1: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Mira-Avendano I., Ryu J., Daniels C. (2014a) The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med 108: 955–967. [DOI] [PubMed] [Google Scholar]
- Lee T., Park J., Lee H, Cho Y., Yoon H., Lee J., et al. (2014b) Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 108: 1549–1555. [DOI] [PubMed] [Google Scholar]
- Le Jeune I., Gribbin J., West J., Smith C., Cullinan P., Hubbard R. (2007) The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 101: 2534–2540. [DOI] [PubMed] [Google Scholar]
- Lettieri C., Nathan S., Barnett S., Ahmad S., Shorr A. (2006) Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 129: 746–752. [DOI] [PubMed] [Google Scholar]
- Li M., Mathur S., Chowdhury N., Helm D., Singer L. (2013) Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant 32: 626–632. [DOI] [PubMed] [Google Scholar]
- Martinez F., Safrin S., Weycker D., Starko K., Bradford W., King T., Jr, et al. (2005) The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 142: 963–967. [DOI] [PubMed] [Google Scholar]
- McCartney J., Meyer K. (2008) Optimizing post-transplant outcomes in lung transplantation. Expert Rev Respir Med 2:183–199. [DOI] [PubMed] [Google Scholar]
- Mermigkis C., Bouloukaki I., Antoniou K., Papadogiannis G., Giannarakis I., Varouchakis G., et al. (2015) Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep Breath 19: 385–391. [DOI] [PubMed] [Google Scholar]
- Minai O.A., Santacruz J.F., Alster J.M., Budev M.M., McCarthy K. (2012) Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir Med 106: 1613–1621. [DOI] [PubMed] [Google Scholar]
- Morawiec E., Tillie-Leblond I., Pansini V., Salleron J., Remy-Jardin M., Wallaert B. (2011) Exacerbations of idiopathic pulmonary fibrosis treated with corticosteroids and cyclophosphamide pulses. Eur Respir J 38: 1487–1489. [DOI] [PubMed] [Google Scholar]
- Morehead R. (2009) Gastro-oesophageal reflux disease and non-asthma lung disease. Eur Respir Rev 18: 233–243. [DOI] [PubMed] [Google Scholar]
- Murray L., Argentieri R., Farrel F., Bracht M., Sheng H., Whitaker B., et al. (2008) Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGF beta1, IL13 and CCl2. Int J Biochem Cell Biol 40: 2174–2182. [DOI] [PubMed] [Google Scholar]
- Nadrous H., Pellikka P., Krowka M., Swanson K., Chaowalit N., Decker P., et al. (2005) Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 128: 2393–2399. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Kawagoe Y., Matsuda T., Shoji H., Ueda Y., Tamura N., et al. (2004) Effect of polymyxin B-immobilized fiber on blood metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels in acute respiratory distress syndrome patients. Blood Purif 22: 256–260. [DOI] [PubMed] [Google Scholar]
- Nakayama S., Mukae H., Sakamoto N., Kakugawa T., Yoshioka S., Soda H., et al. (2008) Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci 82: 210–217. [DOI] [PubMed] [Google Scholar]
- Navaratnam V., Fleming K.M., West J., Smith C.J., Jenkins R.G., Fogarty A., Hubbard R.B. (2011) The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 66(6): 462–467. [DOI] [PubMed] [Google Scholar]
- Nishiyama O., Kondoh Y., Kimura T., Kato K., Kataoka K., Ogawa T., et al. (2008) Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 13: 394–399. [DOI] [PubMed] [Google Scholar]
- Noble P., Albera C., Bradford W., Costabel U., Glassberg M., Kardatzke D., et al. (2011) Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- Noth I., Anstrom K., Calvert B., de Andrade J., Flaherty K., Glazer C., et al. (2012) A placebocontrolled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A., Swigris J., Lezotte D., Norris J., Wilson C., Brown K. (2007) Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 176: 277–284. [DOI] [PubMed] [Google Scholar]
- Ozawa Y., Suda T., Naito T., Enomoto N., Hashimoto D., Fujisawa T., et al. (2009) Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 14: 723–728. [DOI] [PubMed] [Google Scholar]
- Park J., Kim D.S., Shim T.S., Lim C.M., Koh Y., Lee S.D., et al. (2001) Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J 17(6): 1216–1219. [DOI] [PubMed] [Google Scholar]
- Raghu G., Anstrom K., King T. (2012) Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 366: 1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G., Behr J., Brown K., Egan J., Kawut S., Flaherty K., et al. (2013) Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 158: 641–649. [DOI] [PubMed] [Google Scholar]
- Raghu G., Brown K., Costabel U., Cottin V., du Bois R., Lasky J., et al. (2008) Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med 178: 948–955. [DOI] [PubMed] [Google Scholar]
- Raghu G., Collard H., Egan J., Martinez F., Behr J., Brown K., et al. (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G., Freudenberger T., Yang S., Curtis J., Spada C., Hayes J., et al. (2006a) High prevalence of abnormal acid reflux in idiopathic pulmonary fibrosis. Eur Respir J 27: 136–142. [DOI] [PubMed] [Google Scholar]
- Raghu G., Rochwerg B., Zhang Y., Cuello Garcia C., Azuma A., Behr J., et al. (2015) An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 192: e3–e19. [DOI] [PubMed] [Google Scholar]
- Raghu G., Weycker D., Edelsberg J., Bradford W., Oster G. (2006b) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816. [DOI] [PubMed] [Google Scholar]
- Raghu G., Yang S., Spada C., Hayes J., Pellegrini C. (2006c) Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest 129: 794–800. [DOI] [PubMed] [Google Scholar]
- Rasche K., Orth M. (2009) Sleep and breathing in idiopathic pulmonary fibrosis. J Physiol Pharmacol 60(Suppl. 5): 13–14. [PubMed] [Google Scholar]
- Richeldi L., Costabel U., Selman M., Kim D., Hansell D., Nicholson A., et al. (2011) Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 365: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Richeldi L., Davies H., Ferrara G., Franco F. (2003) Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 3: CD002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L., du Bois R., Raghu G., Azuma A., Brown K., Costabel U., et al. (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Homma S., Mun M., Fujii T., Kurosaki A., Yoshimura K. (2011) Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med 50: 77–85. [DOI] [PubMed] [Google Scholar]
- Salhi B., Troosters T., Behaegel M., Joos G., De-rom E. (2010) Effects of pulmonary rehabilitation in patients with restrictive lung diseases. Chest 137: 273–279. [DOI] [PubMed] [Google Scholar]
- Schachter L., Dixon J., Pierce R., O’Brien P. (2003) Severe gastroesophageal reflux is associated with reduced carbon monoxide diffusing capacity. Chest 123: 1932–1938. [DOI] [PubMed] [Google Scholar]
- Seeger W., Pullamsetti S. (2013) Mechanics and mechanisms of pulmonary hypertension-Conference summary and translational perspectives. Pulm Circ 3: 128–136. [PMC free article] [PubMed] [Google Scholar]
- Seeger W., Adir Y., Barberà J.A., Champion H., Coghlan J.G., Cottin V., et al. (2013) Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 62(25 Suppl): D109-D116. [DOI] [PubMed] [Google Scholar]
- Shorr A., Wainright J., Cors C., Lettieri C., Nathan S. (2007) Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 30: 715–721. [DOI] [PubMed] [Google Scholar]
- Simonneau G., Gatzoulis M., Adatia I., Celermajer D., Denton C., Ghofrani A., et al. (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62(Suppl. 25): D34–D41. [DOI] [PubMed] [Google Scholar]
- Song J., Hong S., Lim C., Koh Y., Kim D. (2011) Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 37: 356–363. [DOI] [PubMed] [Google Scholar]
- Spagnolo P., Del Giovane C., Luppi F., Cerri S., Balduzzi S., Walters E., et al. (2010) Non steroid agents for idiopathic pulmonary fibrosis. Eur Respir J 35: 821–829. [DOI] [PubMed] [Google Scholar]
- Swigris J., Brown K., Behr J., duBois R., King T., Raghu G., et al. (2010) The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med 104: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T., Asakawa K., Sakagami T., Moriyama H., Kazama J., Suzuki E., et al. (2014) Effects of direct hemoperfusion with polymyxin B-immobilized fiber on rapidly progressive interstitial lung diseases. Intern Med 53: 1921–1926. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Ebina M., Kondoh Y., Ogura T., Azuma A., Suga M., et al. (2010) Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 35: 821–829. [DOI] [PubMed] [Google Scholar]
- Tomassetti S., Gurioli C., Ryu J., Decker P., Ravaglia C., Tantalocco P., et al. (2015) The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 147: 157–164. [DOI] [PubMed] [Google Scholar]
- Troy L., Corte T. (2014) Sleep disordered breathing in interstitial lung disease: a review. World J Clin Cases 2: 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Higami T., Ohori S., Koyanagi T., Nakashima S., Mawatari T. (2008) Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg 136: 1357–1363. [DOI] [PubMed] [Google Scholar]
- Wuyts W., Antoniou K., Borensztajn K., Costabel U., Cottin V., Crestani B., et al. (2014) Combination therapy: the future of management for idiopathic pulmonary fibrosis? Lancet Respir Med 2: 933–942. [DOI] [PubMed] [Google Scholar]
- Wygrecka M., Kwapiszewska G., Jablonska E., von Gerlach S., Henneke I., Zakrzewicz D., et al. (2011) Role of protease-activated receptor 2 in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183: 1703–1714. [DOI] [PubMed] [Google Scholar]
- Young T., Palta M., Dempsey J., Peppard P., Nieto F., Hla K. (2009) Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108: 246–249. [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y., Zhang J., Laboy M., Lasky J. (2004) Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 286: L182–L188. [DOI] [PubMed] [Google Scholar]
- Zisman D., Schwarz M., Anstrom K., Collard H., Flaherty K., Hunninghake G., et al. (2010) A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]