Abstract
The past decade has brought increased awareness of the potential adverse effects of androgen deprivation therapy (ADT) in men with prostate cancer. Arguably the most important and controversial of these is the increased risk of cardiovascular morbidity and mortality. Although multiple observational studies have shown that men treated with ADT are at increased risk of developing atherosclerotic cardiovascular disease, our understanding of the biological mechanisms that might underlie this phenomenon is still evolving. In this review, we discuss some of the mechanisms that have been proposed to date, including ADT-induced metabolic changes that promote the development and progression of atherosclerotic plaques as well as direct local effects of hormonal factors on plaque growth, rupture and thrombosis.
Keywords: androgen deprivation therapy, atherosclerosis, cardiovascular disease, castration, metabolic syndrome, prostate cancer
Introduction
Adenocarcinoma of the prostate is an exquisitely hormone-sensitive malignancy, with its growth being primarily dependent on androgens. Androgen deprivation therapy (ADT), which refers to the disruption of the hypothalamic–pituitary–gonadal axis via surgical or medical means, is therefore the mainstay of treatment of high-risk localized and metastatic prostate cancer. ADT use among men with prostate cancer is widespread, with approximately 40% of men receiving ADT within 6 months of diagnosis [Shahinian et al. 2010].
Although ADT extends the lives of many men with prostate cancer, this benefit comes at a cost of significant side effects. ADT has long been known to cause hot flashes, fatigue, decreased libido, erectile dysfunction and osteopenia [Nguyen et al. 2015a]. More recently, there has been rising awareness that ADT may also cause more silent but potentially lethal adverse effects related to cardiovascular disease (CVD). Although not confirmed by post hoc analyses of randomized trials of ADT, evidence from multiple large observational studies suggests that men treated with ADT are at increased risk of cardiovascular events, including myocardial infarction (MI) and stroke [Zhao et al. 2014; Bosco et al. 2015a]. These studies were the basis for a joint statement issued in 2010 by the American Urological Association, the American Society for Radiation Oncology and the American Heart Association that aimed to raise awareness of the association between ADT and CVD [Levine et al. 2010].
Although further studies are necessary to confirm that the association between ADT and CVD is not attributable to confounding factors, there are several biologically plausible mechanisms by which ADT may promote the development of CVD. ADT is thought to indirectly contribute to the development of CVD by inducing metabolic changes such as hyperglycemia, dyslipidemia and obesity that are well-established causal risk factors for the development of atherosclerosis. Recent research has also revealed that androgens may, via androgen receptor (AR) dependent and AR-independent mechanisms, modulate the local inflammatory response that plays a key role in the development of atherosclerotic plaques as well as eventual plaque destabilization and rupture.
The objective of this review is to briefly summarize the current evidence supporting the link between ADT and CVD, and to provide a thorough review of what is known about the biological mechanisms that may underlie this association.
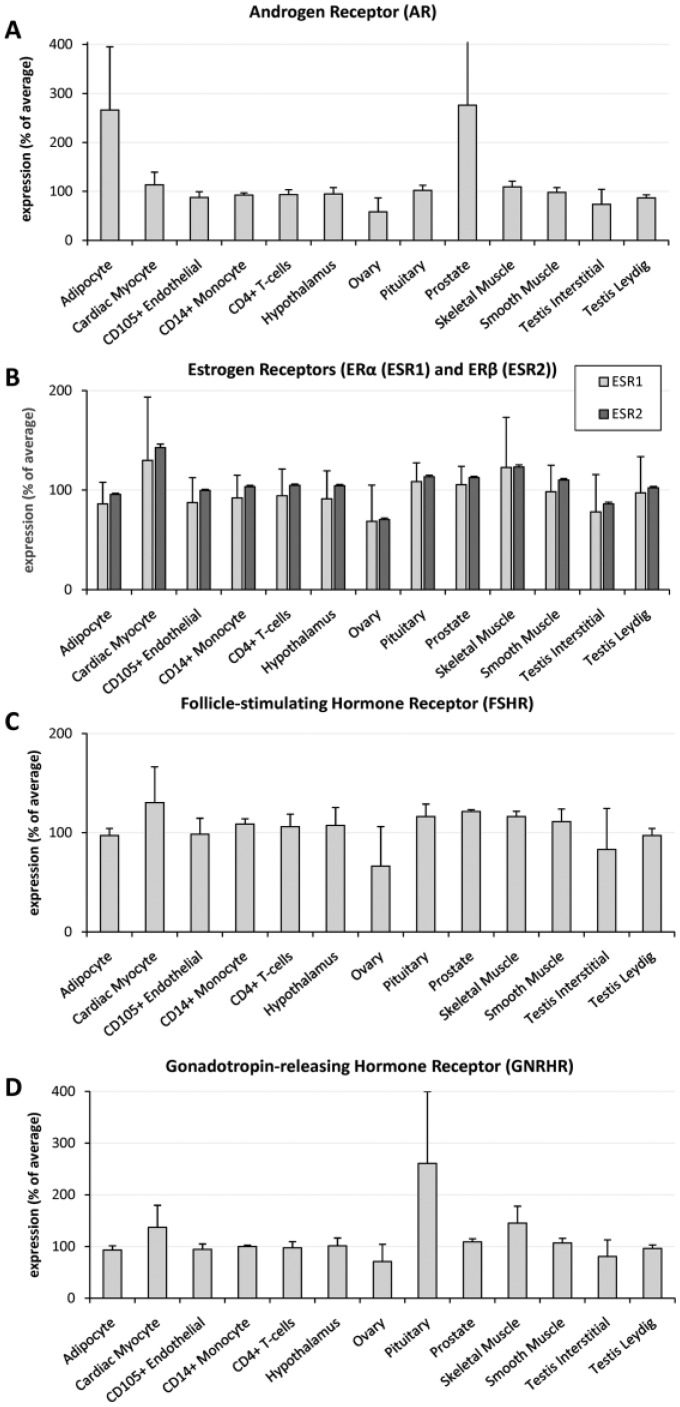
Figure 1 presents the tissue- and cell-specific patterns of messenger RNA (mRNA) expression of the various hormone receptors discussed in this review [Su et al. 2004].
Figure 1.
Relative mRNA expression of hormone receptors in different human cells and tissues.
mRNA expression is presented as a percentage of average expression in all human tissues examined using high-density oligonucleotide arrays in a panel of 79 human tissues [Su et al. 2004]. For AR expression, data from two probesets were used; for GnRHR three, for ERα nine, and ERβ five. For FSHR expression only one probeset was available. Source: BioGPS.
Risk of CVD in men treated with ADT
CVD is a major cause of noncancer-related mortality in men with prostate cancer. An analysis of Surveillance, Epidemiology, and End Results (SEER) Medicare linked data revealed that CVD accounted for approximately a quarter of deaths among men with prostate cancer occurring in the mid-1990s [Lu-Yao et al. 2004]. This spurred a great deal of interest within the research community to identify potential causes of CVD in this population, with particular attention being paid to the role of ADT as a contributing factor.
The majority of the available evidence indicating a positive association between ADT and CVD is observational in nature. Post hoc analyses of randomized trials of ADT have failed to show an association between ADT and CVD. Although individual trials were likely underpowered to detect a difference in the incidence of cardiovascular events, a recent meta-analysis pooling eight randomized trials in which men were assigned to ADT or control showed no increased risk of cardiovascular events among those receiving ADT [relative risk (RR) 0.93, 95% confidence interval (CI) 0.79–1.10] [Nguyen et al. 2011]. In contrast, multiple observational studies have linked ADT use to an increased risk of cardiovascular events. A meta-analysis of six such studies, which in aggregate included a total of 119,625 patients who received ADT and 150,974 patients who did not receive ADT, showed that the risk of cardiovascular mortality was 17% higher among those receiving ADT (HR 1.17, 95% CI 1.04–1.32) [Zhao et al. 2014]. Similarly, a meta-analysis of 8 observational studies published by Bosco and colleagues showed a similar increase in risk among patients who underwent orchiectomy (RR 1.44, 95% CI 1.28–1.62) and those who received either a gonadotropin-releasing hormone (GnRH) agonist (RR 1.38, 95% CI 1.29–1.48) or anti-androgen (RR 1.21, 95% CI 1.07–1.37) compared with patients who did not receive ADT [Bosco et al. 2015a]. A subsequent large observational study by O’Farrell and colleagues not included in these meta-analyses likewise showed an increased risk of CVD with ADT [O’Farrell et al. 2015]. In this study comparing 41,362 Swedish men with prostate cancer starting on ADT with 187,785 men without prostate cancer, the risk of incident CVD was higher among men who underwent orchiectomy (HR 1.16, 95% CI 1.08–1.25) or were treated with a GnRH agonist (HR 1.21, 95% CI 1.18–1.25) than the control group of men without prostate cancer [O’Farrell et al. 2015]. Other studies have confirmed that this increase in risk applies not only to MI but also to peripheral artery disease and stroke [Azoulay et al. 2011; Hu et al. 2012].
The risk of cardiovascular events in men using ADT may also be related to the presence or absence of pre-existing CVD. In the Swedish cohort described by O’Farrell and colleagues, men with 2 or more prior cardiovascular events, with the most recent event occurring within 1 year of ADT initiation, experienced the greatest increase in risk of additional cardiovascular events after starting ADT (HR 1.91, 95% CI 1.66–2.20 versus HR 1.21, 95% CI 1.18–1.25 for all men treated with GnRH agonists) [O’Farrell et al. 2015]. Such effect modification was also reported in a study that showed an increased risk of all-cause mortality with neoadjuvant ADT prior to radiation therapy among men with a history of MI or ischemic congestive heart failure, but not among men without such a history [Nanda et al. 2009]. These findings are clinically relevant as CVD is highly prevalent in this relatively elderly population. In a recent study using SEER-Medicare data, 32% of men with metastatic prostate cancer had a history of CVD at the time of ADT initiation [Gandaglia et al. 2015]; in a separate study of 5149 men with localized prostate cancer, 22% reported a history of heart disease at the time of diagnosis [Marr et al. 2006].
Methodological limitations may account for the discrepant findings of observational and randomized studies with respect to the association between ADT and CVD. Randomized trials recruit a very specific patient population, so their findings may have limited generalizability. Patients in the control arm in some of the randomized trials did receive ADT, only in a deferred, rather than immediate, manner, thus attenuating any potential effect of ADT on CVD risk. Cardiovascular events were only analyzed post hoc in these randomized trials, so the extent to which events were identified and verified is uncertain. In contrast, nonrandomized studies, while observing very large numbers of patients for long periods of time, are prone to confounding by factors that are related both to ADT use and CVD. While some of these factors, such as age and tumor characteristics, are known and can be accounted for using appropriate statistical methods, it is plausible that other unknown factors linking ADT and CVD exist and await recognition.
ADT and atherosclerosis
Atherosclerosis is characterized by vascular lesions that progress from fatty streaks to plaques demonstrating arterial intimal thickening, inflammatory cell accumulation, vascular smooth muscle cell proliferation, and extracellular lipid and fibrous tissue deposition [Falk et al. 2013]. While most plaques remain asymptomatic and represent subclinical disease, some eventually rupture, leading to thrombosis and resultant MI or stroke.
Many animal studies have established a link between androgen deprivation and the development and progression of atherosclerosis. Testosterone has been demonstrated to have beneficial effects in mouse models of atherosclerotic disease, mostly using low-density lipoprotein receptor-deficient (LDLR-/-), apolipoprotein E-deficient (ApoE-/-) and/or AR-knockout (ARKO) mice. The findings from these studies suggest that androgens exert their effects on the vasculature via AR-dependent as well as AR-independent mechanisms, with the latter potentially being mediated by aromatization to estrogens.
In a study by Bourghardt and colleagues, male ApoE-/- ARKO mice underwent prepubertal orchiectomy or sham operation and were subsequently placed on a high-fat diet [Bourghardt et al. 2010]. Testosterone levels were further manipulated by exogenous testosterone supplementation. The atherosclerotic lesion area in the aortic root of ARKO mice was significantly larger than in wildtype controls. While testosterone supplementation reduced atherosclerosis in both wildtype and ARKO mice, the effect on atherosclerotic lesion and necrotic core areas was more pronounced in wildtype than in ARKO mice, suggesting that the atheroprotective properties of testosterone are mediated both through AR-dependent and AR-independent pathways [Bourghardt et al. 2010]. In another study using ARKO mice, selective AR deficiency in the vascular endothelium or smooth muscle did not result in an increase in neointimal lesion size following injury to the femoral artery [Wu et al. 2014]. In contrast, neointimal lesion size was increased in orchiectomized mice compared with those that underwent sham surgery, suggesting that androgens exert a protective effect on the vasculature in an AR-independent manner [Wu et al. 2014].
Similarly, using LDLR-/- mice fed a high-fat diet, Nathan and colleagues showed orchiectomy to result in larger aortic root atherosclerotic lesions than sham operation [Nathan et al. 2001]. Orchiectomized rats supplemented with testosterone but not testosterone and anastrazole, an aromatase inhibitor, developed smaller lesions than orchiectomized rats not supplemented with either agent, suggesting that testosterone does not inhibit atherogenesis directly but rather through conversion to 17β-estradiol [Nathan et al. 2001]. In the same study, atherosclerotic lesion size correlated inversely with circulating 17β-estradiol levels and supplementation with 17β-estradiol also attenuated early atherogenesis in male mice to the same extent as administration of testosterone [Nathan et al. 2001].
Further insight into the role of estrogens was provided by a study using the testicular feminized mouse (Tfm) model, which demonstrates a nonfunctional AR and low serum testosterone levels [Nettleship et al. 2007]. Tfm mice develop a pro-atherogenic lipid profile and aortic fatty streaks on a cholesterol-enriched diet, an effect not observed in male littermate controls. Because aortic fatty streaks were observed not only in Tfm mice but also in surgically castrated male mice, the authors suggest that fatty streak formation is a consequence of low endogenous testosterone levels and not a result of the absence of functional AR [Nettleship et al. 2007]. Moreover, long-term physiological testosterone replacement therapy induced a significant reduction in fatty streak formation in Tfm mouse compared with controls. The degree of aortic lipid deposition observed in Tfm mice receiving physiological testosterone was significantly increased by cotreatment with either anastrazole or fulvestrant, an estrogen receptor-α (ERα) antagonist, suggesting that the non-AR mediated atheroprotective effects of testosterone are largely related to the aromatase-induced conversion of testosterone to 17β-estradiol and subsequent activation of ERα [Nettleship et al. 2007].
Although these animal studies provide robust evidence for a causal effect of androgen deprivation on the development of atherosclerosis, similar studies in humans are lacking. Several studies have assessed surrogate markers of atherosclerosis in men on ADT. Smith and colleagues demonstrated an increase in augmentation index, a measure of large artery stiffness, in 22 men with prostate cancer after initiation of GnRH agonist therapy [Smith et al. 2001]. In a study of 43 men with localized prostate cancer randomized to receive either goserelin, a GnRH agonist or bicalutamide (a nonsteroidal AR antagonist), carotid-brachial and carotid-femoral artery pulse wave velocity, another validated measure of arterial stiffness that correlates with cardiovascular events and mortality, increased in men treated with both ADT modalities [Dockery et al. 2009]. In men without prostate cancer, low serum free testosterone concentrations have been shown to be inversely associated with progression of atherosclerosis as measured by carotid artery intima-media thickness, even after adjustment for traditional CVD risk factors [Muller et al. 2004].
Atherogenic metabolic changes induced by ADT
The putative detrimental effects of ADT on the development and progression of atherosclerotic CVD have classically been ascribed to ADT-induced atherogenic metabolic disturbances. Obesity, diabetes mellitus (DM) and dyslipidemia are well-established risk factors for atherosclerotic CVD [Gaziano and Gaziano, 2012]. There is also evidence that visceral adiposity, hyperglycemia, high low density lipoprotein (LDL) and low high density lipoprotein (HDL) levels have a causal effect on atherosclerotic plaque development [Libby, 2012; Bentzon et al. 2014]. Often, these metabolic perturbations occur together and form the metabolic syndrome, the root cause of which is likely insulin resistance [Reaven, 1988; Ferrannini et al. 1991].
Metabolic syndrome, like CVD, is common in men with prostate cancer starting ADT. In a study of 5149 men with localized prostate cancer in the CaPSURE database, pre-existing hypertension and DM were reported by 44% and 13% of men, respectively [Marr et al. 2006]. Among a subset of 2952 men, 71% were found to be either overweight or obese [Kane et al. 2005]. In a separate study, 123 of 539 patients (22.9%) starting on ADT had metabolic syndrome as per the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria prior to initiating treatment [Morote et al. 2015].
It is also apparent that ADT exacerbates these often pre-existing metabolic derangements. A recent meta-analysis of nine observational studies showed that prostate cancer patients treated with ADT had a 75% higher risk of developing incident metabolic syndrome than patients not treated with ADT (RR 1.75, 95% CI 1.27–2.41) [Bosco et al. 2015b]. Five of these nine studies also reported an increased risk of DM in patients treated with ADT. Similarly, a cohort study of 12,191 US men with localized prostate cancer showed ADT use to be associated with a 60% higher risk of developing incident DM (HR 1.61, 95% CI 1.38–1.88) [Tsai et al. 2015], a finding that has been replicated in an Asian population [Teoh et al. 2015]. ADT also increases hemoglobin A1c levels among men with pre-existing DM [Keating et al. 2014], and experimental studies have demonstrated that acute androgen deprivation in both healthy male volunteers and men with prostate cancer produces a marked increase in insulin resistance as measured by various insulin sensitivity indices [Basaria et al. 2006; Smith et al. 2006; Rubinow et al. 2012]. However, to our knowledge, no randomized trials have studied the effect of ADT on blood glucose and lipid levels. Thus, the evidence supporting the popular notion that ADT causes adverse metabolic changes is subject to the same limitations as the evidence for a link between ADT and CVD.
A likely contributing factor to insulin resistance in men with metabolic syndrome is an increase in fat mass leading to central obesity. ADT decreases lean body mass, while increasing both visceral and subcutaneous fat [Hamilton et al. 2011; Hara et al. 2013]. These changes are thought to be caused by loss of androgen-mediated inhibition of stem cell differentiation into adipocytes [Chazenbalk et al. 2013]. Androgens also appear to stimulate lipolysis and inhibit triglyceride synthesis from free fatty acids within adipocytes [Gupta et al. 2008].
The metabolic changes induced by ADT differ in some ways from those of metabolic syndrome, with the most prominent difference relating to serum lipid profile [Smith et al. 2008]. While both metabolic syndrome and androgen deprivation are associated with increased total cholesterol and LDL levels [Braga-Basaria et al. 2006], the latter is associated with an increase, rather than a decrease, in serum HDL levels [Goldberg et al. 1985; Moorjani et al. 1987; Behre et al. 1994]. HDL is thought to protect against the development of atherosclerosis by modulating cholesterol efflux from macrophages within atheromas [Rader et al. 2009]. This efflux capacity has been shown to predict severity of atherosclerosis as determined by carotid intima-media thickness and coronary angiography independent of HDL levels [Khera et al. 2011]. Importantly, Langer and colleagues demonstrated that incubation of human monocyte-derived macrophages in the presence of testosterone at increasing concentrations increases cholesterol efflux, thus facilitating removal of excess cholesterol from atherosclerotic lesions [Langer et al. 2002]. Therefore, although ADT may induce an increase in serum HDL levels, it could ostensibly compromise reverse cholesterol transport from the arterial wall to the liver.
Direct hormonal effects on atherosclerotic plaque stability and rupture
Sex hormones may also play a more direct role in the pathophysiology of atherosclerosis by modulating the local inflammatory process that is a key player in plaque progression and rupture. Several processes are associated with plaque vulnerability, but arguably the most important of these is local inflammation mediated by the activity of cells from the monocyte-macrophage lineage, which have the capacity to degrade the protective fibrous cap [Shah et al. 1995; Libby and Theroux, 2005].
While macrophages are the principal mediators of atherosclerotic plaque progression, their specific effect depends on their phenotype [De Paoli et al. 2014]. Depending on the stimulus, macrophages can differentiate into pro-inflammatory M1 macrophages, which are involved in cholesterol loading and produce factors that contribute to plaque rupture and thrombosis, or anti-inflammatory M2 macrophages, which synthesize matrix repair proteins that stabilize plaques [Shah et al. 1995; De Paoli et al. 2014]. The macrophage pool in atherosclerotic plaques is comprised of a complex mix of macrophage subsets and there is evidence, mainly from in vitro studies, to suggest that macrophage subsets exhibit phenotypic plasticity in response to various factors [Butcher and Galkina, 2012]. In mice, switching from the M2 (atheroprotective) to M1 (atherogenic) subtype within atherosclerotic lesions has been observed [Khallou-Laschet et al. 2010].
Studies of the effects of androgens on macrophage function and the local inflammatory process within the atherosclerotic plaque have yielded conflicting results. In orchiectomized rabbits fed a high-cholesterol diet, administration of dihydrotestosterone (DHT) reduced the formation of lipid-laden macrophages, or foam cells, in the aortic intima, while culturing of wildtype rabbit macrophages with DHT resulted in decreased expression of pro-inflammatory cytokines [Qiu et al. 2010]. Similarly, in a study by Corcoran and colleagues, production of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin (IL) 1 by human monocyte-derived macrophage cells was reduced by culturing the cells in testosterone at both physiologic and supraphysiologic concentrations [Corcoran et al. 2010]. In a rat model, monocyte adhesion to aortic endothelial cells exposed to the inflammatory mediator lipopolysaccharide was impaired by the presence of testosterone [Campelo et al. 2012a]. Similarly, an in vitro study using human aortic endothelial cells showed TNF-α-induced expression of vascular cell adhesion molecule 1 (VCAM-1), which is important for monocyte adhesion to the vascular endothelium, to be decreased in a dose-dependent manner by testosterone [Hatakeyama et al. 2002]. Taken together, these studies suggest that androgen deprivation could promote monocyte-endothelial cell binding and the resultant local inflammatory response.
Other studies, however, have produced contradictory results. Several studies have shown androgens to promote binding of leukocytes to the endothelium [McCrohon et al. 1999; Filgueira et al. 2012], an effect that may be facilitated by an increase in VCAM-1 expression in human endothelial cells [Mukherjee et al. 2002; Zhang et al. 2002; Death et al. 2004]. One study employing LDLR-/- mice found reduced atherosclerosis in mice lacking AR in monocytes/macrophages compared with wildtype LDLR-/- controls [Huang et al. 2014]. Importantly, there was no difference in the extent of atherosclerosis between wildtype LDLR-/- controls and mice lacking AR in endothelial or vascular smooth muscle cells, suggesting that the putative detrimental effect of androgens on atherosclerosis development in this mouse model was mediated by cells of the monocyte-macrophage lineage in an AR-dependent manner. Androgens have also been shown to increase expression of atherosclerosis-related genes and lipid-loading of human male macrophages [Ng et al. 2003]. These discrepant results could be partly explained by differential effects of androgens on the local inflammatory response at physiologic and supraphysiologic levels. Further research is necessary to clarify the effect of androgens and androgen deprivation on the local inflammatory processes that contribute to atherosclerotic plaque development and rupture.
Rupture of atherosclerotic plaques exposes platelets to highly thrombogenic factors, resulting in the formation of a clot that blocks coronary blood flow. There is evidence that androgens may inhibit this process by stimulating endothelial production of nitric oxide, which inhibits platelet aggregation, and inhibiting platelet secretion of thromboxane A2, which has the opposite effect [Li et al. 2007a, 2007b; Campelo et al. 2012b]. Testosterone may also stimulate fibrinolysis and resultant clot degradation by increasing expression of tissue plasminogen activator [Jin et al. 2007].
Other androgen-mediated effects on the cardiovascular system
Beyond modulating the inflammatory process that leads to atherosclerosis and plaque rupture-induced thrombosis, androgens have also been shown to affect vascular and cardiac function in other ways. For instance, androgens directly induce arterial dilatation. Acute testosterone administration in men undergoing exercise stress testing increases time to development of ST depression, suggesting an increase in coronary blood blow [Webb et al. 1999a]. This finding was confirmed by a subsequent study in which men with coronary artery disease were administered a testosterone infusion directly into the coronary arteries during angiography, which resulted in an increase in coronary artery diameter and blood flow [Webb et al. 1999b]. It has been proposed that this effect is mediated by the opening of calcium-dependent potassium channels and the closure of calcium channels [Perusquia and Villalon, 1999; Deenadayalu et al. 2001, 2012; Ding and Stallone, 2001; Oloyo et al. 2011]. Paradoxically, another recent study found ADT administration to promote vasodilation [Nguyen et al. 2015b].
Androgens may also have anti-arrhythmic properties. Men are known to have shorter QT intervals than women and recent evidence suggests that hormonal factors underlie this difference. A study of 727 men participating in the National Health and Nutrition Examination Survey (NHANES) showed a significantly shorter QT interval among men in the highest quartile of serum testosterone concentration compared to men in the lowest quartile [Zhang et al. 2011]. This effect may be produced by shortening of the action potential duration by activation of delayed rectifier potassium currents and inhibition of L-type calcium currents [Bai et al. 2005]. The clinical significance of these findings in terms of their relationship to the risk of clinically significant arrhythmias, especially in the setting of ischemia, is to date unknown.
GnRH antagonists and risk of CVD
It should not be assumed that all modes of androgen deprivation, namely orchiectomy, GnRH agonists and GnRH antagonists, have the same effect on the cardiovascular system. A recent meta-analysis of six randomized controlled trials in which men with metastatic prostate cancer were randomized to receive either a GnRH agonist or GnRH antagonist showed that, among men with a past history of CVD, GnRH antagonists were associated with a significantly lower risk of cardiovascular events within the first year of starting therapy (HR 0.44, 95% CI 0.26–0.74) [Albertsen et al. 2014]. In a recent study by our group in which we compared LDLR-/- mice treated with orchiectomy, sham surgery, sham surgery plus GnRH antagonist (degarelix), or sham surgery plus GnRH agonist (leuprolide), mice treated with the GnRH antagonist demonstrated less visceral fat accumulation, increased glucose tolerance and smaller atherosclerotic plaques than either orchiectomized mice or those treated with the GnRH agonist [Hopmans et al. 2014]. Furthermore, GnRH antagonist therapy was associated with a smaller volume of necrosis within the plaque core, a marker of plaque instability [Hopmans et al. 2014].
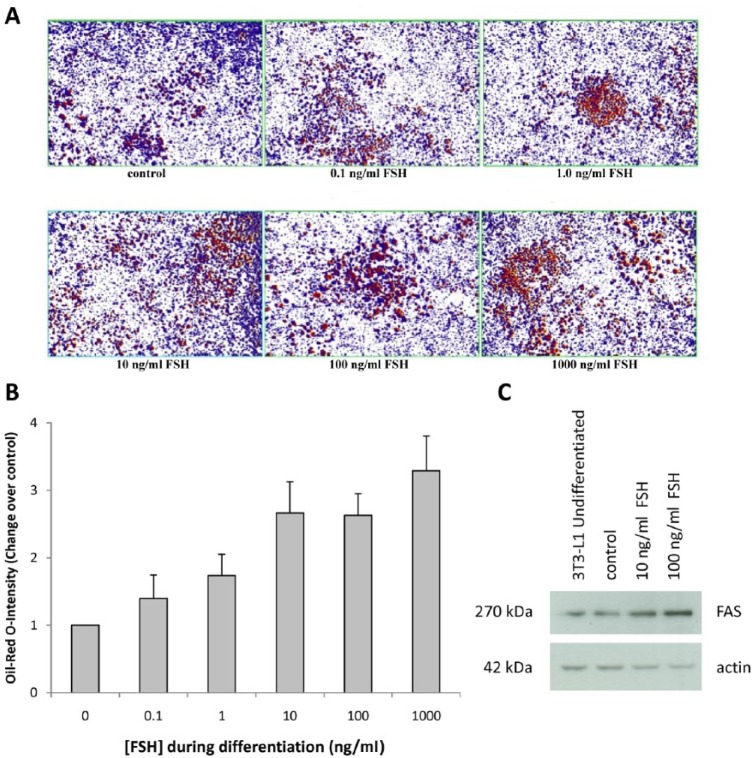
The differing effects of GnRH agonists and antagonists on the development of atherosclerosis may be explained by their differing effects on follicle stimulating hormone (FSH) levels. FSH is a gonadotropin that is released by the anterior pituitary in response to GnRH. The three main androgen deprivation modalities have markedly different effects on serum FSH: patients receiving the GnRH-antagonist degarelix exhibit a rapid decrease in FSH to <90% of normal levels, while GnRH agonists induce an initial increase in FSH levels followed by a gradual decrease to approximately 50% of normal levels [Klotz et al. 2008]. Orchiectomy leads to very high FSH levels due to the loss of inhibin secretion by Sertoli cells [Tomic, 1987]. In an elegant series of experiments, Liu and colleagues demonstrated that FSH was capable of promoting fat storage and lipogenesis, both in vitro and in vivo [Liu et al. 2015]. Treatment of 3T3-L1 preadipocytes with FSH accelerated the formation of lipid droplets, a trend that was reversed using small interfering RNA (siRNA) specific against the FSH receptor [Liu et al. 2015]. Moreover, treatment with recombinant FSH upregulated genes encoding for proteins involved in lipogenesis, such as fatty acid synthase [Liu et al. 2015]. These findings have been confirmed in our laboratory; treatment with increasing concentrations (0.1–1000 ng/ml) of human recombinant FSH induced in vitro differentiation of preadipocyte 3T3-L1 into fat cells as assessed by Oil Red O staining (Figures 2A and 2B) and increased protein expression of fatty acid synthase (Figure 2C). Furthermore, Lui and colleagues showed in vivo that administration of recombinant FSH to mice treated with GnRH agonist resulted in a significant increase in body weight and accumulation of dysfunctional fat as evidenced by increased leptin levels [Liu et al. 2015]. Dysfunctional fat plays a key pathogenic role in the development of metabolic syndrome and CVD [Hajer et al. 2008; Franssen et al. 2011], further supporting the notion that FSH may play an important role in the pathogenesis of ADT-induced CVD.
Figure 2.
In vitro FSH treatment induces differentiation of murine preadipocyte 3T3-L1 into fat cells as assessed by Oil Red O-staining (A, B) and increases the expression of fatty acid synthase (C).
The 3T3-L1 mouse fibroblast cell line was plated in six-well plates in DMEM media supplemented with 10% fetal bovine serum. The cells were differentiated as described by Basseri and colleagues [Basseri et al. 2013]. Different concentrations of recombinant human FSH (0.1–1000 ng/ml) were added during differentiation. Following 8 days of differentiation, cells were stained with Oil Red O and protein lysates were prepared for Western blot analysis. Eight representative microscopic images per well were taken (at 4× magnification) to semi-quantitatively determine the staining intensity of adipose cells using ImageScope software and the positive pixel count algorithm according to Duivenvoorden et al. [2013].
DMEM, Dulbecco’s Modified Eagle Medium; FAS, fatty acid synthase; FSH, follicle-stimulating hormone.
Another potential explanation for the differences in cardiovascular toxicity between GnRH agonists and antagonists may relate to the GnRH receptor, which is also expressed on T lymphocytes (Figure 1) [Chen et al. 1999]. Activation of these receptors by GnRH agonists stimulates T-cell proliferation and differentiation into the type 1 T cell (Th1) or pro-inflammatory phenotype [Tanriverdi et al. 2005], which may contribute to destabilization of atherosclerotic plaques.
There are no prospective, randomized clinical trials designed to compare cardiovascular events in prostate cancer patients receiving GnRH agonists or antagonists to provide more robust evidence for the hypothesized safer cardiovascular profile of GnRH antagonists.
Conclusion
In observational studies, ADT is associated with an increased risk of developing CVD, although this has not been confirmed by post hoc analyses of previously published randomized trials. ADT has consistently been shown to induce metabolic changes that mimic, although are not identical to, metabolic syndrome, and this represents the most well-established mechanism that may link ADT to atherosclerotic CVD. Evidence that androgens may have a positive effect on cardiovascular health by inhibiting the local inflammatory process that contributes to atherosclerotic plaque development and rupture is inconsistent, with studies showing both beneficial and harmful effects of androgens on macrophage function and the local inflammatory process. Further research is needed to define the effect of both physiologic and supraphysiologic doses of androgens on processes such as local macrophage function and platelet aggregation. Limited post hoc evidence indicates that GnRH antagonists may have less of a detrimental effect on cardiovascular health than GnRH agonists, although this requires confirmation in well-designed and adequately powered prospective clinical trials. The mechanisms by which different forms of ADT might influence atherosclerotic CVD risk should be a focus of future research.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: J.H.P. has received honoraria and speaker fees from Ferring Pharmaceuticals for work related to concepts discussed in this article. J.H.P. and W.D. have also received investigator-initiated research funding from Ferring Pharmaceuticals. D.P.L. has received honoraria and consulting fees from Ferring Pharmaceuticals for work related to concepts discussed in this article. P.Z. declares no conflicts of interest in preparing this article.
Contributor Information
Piotr Zareba, Division of Urology, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Wilhelmina Duivenvoorden, Division of Urology, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Darryl P. Leong, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada
Jehonathan H. Pinthus, Department of Surgery, Juravinski Hospital and Cancer Centre, 711 Concession St, Hamilton, Ontario L8V 1C3, Canada; Division of Urology, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
References
- Albertsen P., Klotz L., Tombal B., Grady J., Olesen T., Nilsson J. (2014) Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 65: 565–573. [DOI] [PubMed] [Google Scholar]
- Azoulay L., Yin H., Benayoun S., Renoux C., Boivin J., Suissa S. (2011) Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer. Eur Urol 60: 1244–1250. [DOI] [PubMed] [Google Scholar]
- Bai C., Kurokawa J., Tamagawa M., Nakaya H., Furukawa T. (2005) Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation 112: 1701–1710. [DOI] [PubMed] [Google Scholar]
- Basaria S., Muller D., Carducci M., Egan J., Dobs A. (2006) Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 106: 581–588. [DOI] [PubMed] [Google Scholar]
- Basseri S., Lhotak S., Fullerton M., Palanivel R., Jiang H., Lynn E., et al. (2013) Loss of TDAG51 results in mature-onset obesity, hepatic steatosis, and insulin resistance by regulating lipogenesis. Diabetes 62: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behre H., Bockers A., Schlingheider A., Nieschlag E. (1994) Sustained suppression of serum LH, FSH and testosterone and increase of high-density lipoprotein cholesterol by daily injections of the GnRH antagonist cetrorelix over 8 days in normal men. Clin Endocrinol 40: 241–248. [DOI] [PubMed] [Google Scholar]
- Bentzon J., Otsuka F., Virmani R., Falk E. (2014) Mechanisms of plaque formation and rupture. Circ Res 114: 1852–1866. [DOI] [PubMed] [Google Scholar]
- Bosco C., Bosnyak Z., Malmberg A., Adolfsson J., Keating N., Van Hemelrijck M. (2015a) Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol 68: 386–396. [DOI] [PubMed] [Google Scholar]
- Bosco C., Crawley D., Adolfsson J., Rudman S., Van Hemelrijck M. (2015b) Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PLoS One 10: e0117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourghardt J., Wilhelmson A., Alexanderson C., De Gendt K., Verhoeven G., Krettek A., et al. (2010) Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology 151: 5428–5437. [DOI] [PubMed] [Google Scholar]
- Braga-Basaria M., Muller D., Carducci M., Dobs A., Basaria S. (2006) Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. Int J Impot Res 18: 494–498. [DOI] [PubMed] [Google Scholar]
- Butcher M., Galkina E. (2012) Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol 3: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo A., Cutini P., Massheimer V. (2012a) Cellular actions of testosterone in vascular cells: mechanism independent of aromatization to estradiol. Steroids 77: 1033–1040. [DOI] [PubMed] [Google Scholar]
- Campelo A., Cutini P., Massheimer V. (2012b) Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J Endocrinol 213: 77–87. [DOI] [PubMed] [Google Scholar]
- Chazenbalk G., Singh P., Irge D., Shah A., Abbott D., Dumesic D. (2013) Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 78: 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Jeung E., Stephenson M., Leung P. (1999) Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab 84: 743–750. [DOI] [PubMed] [Google Scholar]
- Corcoran M., Meydani M., Lichtenstein A., Schaefer E., Dillard A., Lamon-Fava S. (2010) Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol 206: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Death A., McGrath K., Sader M., Nakhla S., Jessup W., Handelsman D., et al. (2004) Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology 145: 1889–1897. [DOI] [PubMed] [Google Scholar]
- Deenadayalu V., Puttabyatappa Y., Liu A., Stallone J., White R. (2012) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302: H115–H123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenadayalu V., White R., Stallone J., Gao X., Garcia A. (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281: H1720–H1727. [DOI] [PubMed] [Google Scholar]
- De Paoli F., Staels B., Chinetti-Gbaguidi G. (2014) Macrophage phenotypes and their modulation in atherosclerosis. Circ J 78: 1775–1781. [DOI] [PubMed] [Google Scholar]
- Ding A., Stallone J. (2001) Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol 91: 2742–2750. [DOI] [PubMed] [Google Scholar]
- Dockery F., Bulpitt C., Agarwal S., Vernon C., Rajkumar C. (2009) Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. J Androl 30: 410–415. [DOI] [PubMed] [Google Scholar]
- Duivenvoorden W., Beatty L., Lhotak S., Hill B., Mak I., Paulin G., et al. (2013) Underexpression of tumour suppressor LKB1 in clear cell renal cell carcinoma is common and confers growth advantage in vitro and in vivo. Br J Cancer 108: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E., Nakano M., Bentzon J., Finn A., Virmani R. (2013) Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 34: 719–728. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Haffner S., Mitchell B., Stern M. (1991) Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia 34: 416–422. [DOI] [PubMed] [Google Scholar]
- Filgueira F., Lobato N., DosSantos R., Oliveira M., Akamine E., Tostes R., et al. (2012) Endogenous testosterone increases leukocyte–endothelial cell interaction in spontaneously hypertensive rats. Life Sci 90: 689–694. [DOI] [PubMed] [Google Scholar]
- Franssen R., Monajemi H., Stroes E., Kastelein J. (2011) Obesity and dyslipidemia. Med Clin North Am 95: 893–902. [DOI] [PubMed] [Google Scholar]
- Gandaglia G., Sun M., Popa I., Schiffmann J., Trudeau V., Shariat S., et al. (2015) Cardiovascular mortality in patients with metastatic prostate cancer exposed to androgen deprivation therapy: a population-based study. Clin Genitourin Cancer 13: e123–e130. [DOI] [PubMed] [Google Scholar]
- Gaziano T., Gaziano J. (2012) Global burden of cardiovascular disease. In: Bonow R., Mann D., Zipes D., Libby P. (eds), Braunwald’s Heart Disease: Textbook of Cardiovascular Medicine, 9th edition. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- Goldberg R., Rabin D., Alexander A., Doelle G., Getz G. (1985) Suppression of plasma testosterone leads to an increase in serum total and high density lipoprotein cholesterol and apoproteins A-I and B. J Clin Endocrinol Metab 60: 203–207. [DOI] [PubMed] [Google Scholar]
- Gupta V., Bhasin S., Guo W., Singh R., Miki R., Chauhan P., et al. (2008) Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol 296: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajer G., van Haeften T., Visseren F. (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959–2971. [DOI] [PubMed] [Google Scholar]
- Hamilton E., Gianatti E., Strauss B., Wentworth J., Lim-Joon D., Bolton D., et al. (2011) Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol 74: 377–383. [DOI] [PubMed] [Google Scholar]
- Hara N., Ishizaki F., Saito T., Nishiyama T., Kawasaki T., Takahashi K. (2013) Decrease in lean body mass in men with prostate cancer receiving androgen deprivation therapy: mechanism and biomarkers. Urology 81: 376–380. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H., Nishizawa M., Nakagawa A., Nakano S., Kigoshi T., Uchida K. (2002) Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett 530: 129–132. [DOI] [PubMed] [Google Scholar]
- Hopmans S., Duivenvoorden W., Werstuck G., Klotz L., Pinthus J. (2014) GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol Oncol 32: 1126–1134. [DOI] [PubMed] [Google Scholar]
- Hu J., Williams S., O’Malley A., Smith M., Nguyen P., Keating N. (2012) Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol 61: 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Pang H., Wang L., Niu Y., Luo J., Chang E., et al. (2014) New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis. Hypertension 63: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Lin J., Fu L., Mei Y., Peng G., Tan X., et al. (2007) Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol 85: 246–251. [DOI] [PubMed] [Google Scholar]
- Kane C., Bassett W., Sadetsky N., Silva S., Wallace K., Pasta D., et al. (2005) Obesity and prostate cancer clinical risk factors at presentation: data from CaPSURE. J Urol 173: 732–736. [DOI] [PubMed] [Google Scholar]
- Keating N., Liu P., O’Malley A., Freedland S., Smith M. (2014) Androgen-deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol 65: 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A., Clement M., et al. (2010) Macrophage plasticity in experimental atherosclerosis. PLoS One 5: e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera A., Cuchel M., de la, Llera-Moya M., Rodrigues A., Burke M., Jafri K., et al. (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L., Boccon-Gibod L., Shore N., Andreou C., Persson B., Cantor P., et al. (2008) The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 102: 1531–1538. [DOI] [PubMed] [Google Scholar]
- Langer C., Gansz B., Goepfert C., Engel T., Uehara Y., von Dehn G., et al. (2002) Testosterone up-regulates scavenger receptor B1 and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun 296: 1051–1057. [DOI] [PubMed] [Google Scholar]
- Levine G., D’Amico A., Berger P., Clark P., Eckel R., Keating N., et al. (2010) Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 121: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Li X., Li J., Deng X., Li Y. (2007a) Inhibition of oxidative-stress-induced platelet aggregation by androgen at physiological levels via its receptor is associated with the reduction of thromboxane A2 release from platelets. Steroids 72: 875–880. [DOI] [PubMed] [Google Scholar]
- Li S., Li X., Li J., Deng X., Li Y., Cong Y. (2007b) Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thromb Res 121: 127–134. [DOI] [PubMed] [Google Scholar]
- Libby P. (2012) The vascular biology of atherosclerosis. In: Bonow R., Mann D., Zipes D., Libby P. (eds), Braunwald’s Heart Disease: Textbook of Cardiovascular Medicine, 9th edition Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- Libby P., Theroux P. (2005) Pathophysiology of coronary artery disease. Circulation 111: 3481–3488. [DOI] [PubMed] [Google Scholar]
- Liu X., Chan H., Ding G., Cai J., Song Y., Wang T., et al. (2015) FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell 14: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Yao G., Stukel T., Yao S. (2004) Changing patterns in competing causes of death in men with prostate cancer: a population based study. J Urol 171: 2285–2290. [DOI] [PubMed] [Google Scholar]
- Marr P., Elkin E., Arredondo S., Broering J., DuChane J., Carroll P. (2006) Comorbidity and primary treatment for localized prostate cancer: data from CaPSURE. J Urol 175: 1326–1331. [DOI] [PubMed] [Google Scholar]
- McCrohon J., Jessup W., Handelsman D., Celermajer D. (1999) Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation 99: 2317–2322. [DOI] [PubMed] [Google Scholar]
- Moorjani S., Dupont A., Labrie F., Lupien P., Brun D., Gagne C., et al. (1987) Increase in plasma high-density lipoprotein concentration following complete androgen blockage in men with prostatic carcinoma. Metabolism 36: 244–250. [DOI] [PubMed] [Google Scholar]
- Morote J., Gomez-Caamano A., Alvarez-Ossorio J., Pesqueira D., Tabernero A., Gomez Veiga F., et al. (2015) The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol 193: 1963–1969. [DOI] [PubMed] [Google Scholar]
- Mukherjee T., Dinh H., Chaudhuri G., Nathan L. (2002) Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci U S A 99: 4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., van den Beld A., Bots M., Grobbee D., Lamberts S., van der Schouw Y. (2004) Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation 109: 2074–2079. [DOI] [PubMed] [Google Scholar]
- Nanda A., Chen M., Braccioforte M., Moran B., D’Amico A. (2009) Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA 302: 866–873. [DOI] [PubMed] [Google Scholar]
- Nathan L., Shi W., Dinh H., Mukherjee T., Wang X., Lusis A., et al. (2001) Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A 98: 3589–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleship J., Jones T., Channer K., Jones R. (2007) Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation 116: 2427–2434. [DOI] [PubMed] [Google Scholar]
- Ng M., Quinn C., McCrohon J., Nakhla S., Jessup W., Handelsman D., et al. (2003) Androgens up-regulate atherosclerosis-related genes in macrophages from males but not females: molecular insights into gender differences in atherosclerosis. J Am Coll Cardiol 42: 1306–1313. [DOI] [PubMed] [Google Scholar]
- Nguyen P., Alibhai S., Basaria S., D’Amico A., Kantoff P., Keating N., et al. (2015a) Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 67: 825–836. [DOI] [PubMed] [Google Scholar]
- Nguyen P., Jarolim P., Basaria S., Zuflacht J., Milian J., Kadivar S., et al. (2015b) Androgen deprivation therapy reversibly increases endothelium-dependent vasodilation in men with prostate cancer. J Am Heart Assoc 4: e001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P., Je Y., Schutz F., Hoffman K., Hu J., Parekh A., et al. (2011) Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 306: 2359–2366. [DOI] [PubMed] [Google Scholar]
- O’Farrell S., Garmo H., Holmberg L., Adolfsson J., Stattin P., Van Hemelrijck M. (2015) Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol 33: 1243–1251. [DOI] [PubMed] [Google Scholar]
- Oloyo A., Sofola O., Nair R., Harikrishnan V., Fernandez A. (2011) Testosterone relaxes abdominal aorta in male Sprague-Dawley rats by opening potassium (K(+)) channel and blockade of calcium (Ca(2+)) channel. Pathophysiology 18: 247–253. [DOI] [PubMed] [Google Scholar]
- Perusquia M., Villalon C. (1999) Possible role of Ca2+ channels in the vasodilating effect of 5beta-dihydrotestosterone in rat aorta. Eur J Pharmacol 371: 169–178. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Yanase T., Hu H., Tanaka T., Nishi Y., Liu M., et al. (2010) Dihydrotestosterone suppresses foam cell formation and attenuates atherosclerosis development. Endocrinology 151: 3307–3316. [DOI] [PubMed] [Google Scholar]
- Rader D., Alexander E., Weibel G., Billheimer J., Rothblat G. (2009) The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 50: S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607. [DOI] [PubMed] [Google Scholar]
- Rubinow K., Snyder C., Amory J., Hoofnagle A., Page S. (2012) Acute testosterone deprivation reduces insulin sensitivity in men. Clin Endocrinol 76: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Falk E., Badimon J., Fernandez-Ortiz A., Mailhac A., Villareal-Levy G., et al. (1995) Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92: 1565–1569. [PubMed] [Google Scholar]
- Shahinian V., Kuo Y., Gilbert S. (2010) Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med 363: 1822–1832. [DOI] [PubMed] [Google Scholar]
- Smith J., Bennett S., Evans L., Kynaston H., Parmar M., Mason M., et al. (2001) The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86: 4261–4267. [DOI] [PubMed] [Google Scholar]
- Smith M., Lee H., McGovern F., Fallon M., Goode M., Zietman A., et al. (2008) Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer 112: 2188–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Lee H., Nathan D. (2006) Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 91: 1305–1308. [DOI] [PubMed] [Google Scholar]
- Su A., Wiltshire T., Batalov S., Lapp H., Ching K., Block D., et al. (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 101: 6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi F., Gonzalez-Martinez D., Hu Y., Kelestimur F., Bouloux P. (2005) GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol 142: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh J., Chiu P., Chan S., Poon D., Cheung H., Hou S., et al. (2015) Risk of new-onset diabetes after androgen deprivation therapy for prostate cancer in the Asian population. J Diabetes 7: 672–680. [DOI] [PubMed] [Google Scholar]
- Tomic R. (1987) Pituitary function after orchiectomy in patients with or without earlier estrogen treatment for prostatic carcinoma. J Endocrinol Invest 10: 479–482. [DOI] [PubMed] [Google Scholar]
- Tsai H., Keating N., Van Den Eeden S., Haque R., Cassidy-Bushrow A., Ulcickas Yood M., et al. (2015) Risk of diabetes among patients receiving primary androgen deprivation therapy for clinically localized prostate cancer. J Urol 193: 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C., Adamson D., de Zeigler D., Collins P. (1999a) Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol 83: 437–439. [DOI] [PubMed] [Google Scholar]
- Webb C., McNeill J., Hayward C., de Zeigler D., Collins P. (1999b) Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 100: 1690–1696. [DOI] [PubMed] [Google Scholar]
- Wu J., Hadoke P., Mair I., Lim W., Miller E., Denvir M., et al. (2014) Modulation of neointimal lesion formation by endogenous androgens is independent of vascular androgen receptor. Cardiovasc Res 103: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ouyang P., Post W., Dalal D., Vaidya D., Blasco-Colmenares E., et al. (2011) Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 174: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang L., Dou Y., Zhao J., Jiang T., Qiao Z., et al. (2002) Testosterone and estradiol modulate TNF-alpha-induced expression of adhesion molecules in endothelial cells. Methods Find Exp Clin Pharmacol 24: 125–130. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhu S., Sun L., Meng F., Zhao L., Zhao Y., et al. (2014) Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One 9: e107516. [DOI] [PMC free article] [PubMed] [Google Scholar]