Abstract
Erectile dysfunction (ED) is a common disorder that can jeopardize quality of life and the partnership of patients and their sexual partners. The advent of oral phosphodiesterase type 5 inhibitors (PDE5Is) has revolutionized a treatment for ED, and they are recognized as the first-line therapy for ED, regardless of its etiology. Mirodenafil, a second-generation PDE5I, has biochemical profiles such as high affinity for PDE5 and high selectivity for PDE5 over other PDE isoforms, compared to other existing PDE5Is such as sildenafil, vardenafil and tadalafil. Available evidence has suggested that doses of 50 and 100 mg mirodenafil effectively improve ED [with improvements in the erectile function domain of the International Index of Erectile Function (IIEF-EF) scores, positive responses to questions 2 of the Sexual Encounter Profiles (SEP2) and questions 3 of the Sexual Encounter Profiles (SEP3): 7.6–11.6 points, 27.72–38.98% and 44.20–67.33%, respectively] in a broad range of patient populations with ED of a variety of underlying etiologies, severities and ages, without any serious treatment-related adverse effects. In the treatment of diabetic ED, a traditionally difficult-to-treat population, 100 mg mirodenafil has been reported to offer favorable efficacy (with improvements in the IIEF-EF scores, and positive responses to the SEP2 and the SEP3: 9.3 points, 36.1% and 61.8%, respectively) and tolerability (mild adverse effects of less than 19.6%), which are comparable with results from clinical studies on other PDE5Is. Mirodenafil appears to be effective, safe and well tolerated in men with both ED and hypertension or lower urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH) who are taking concomitant antihypertensive medications or α1-blockers. Furthermore, recent evidence has indicated that mirodenafil may be a potential option for chronic dosing in the treatment of ED despite its short half-life (T1/2). Most of the available clinical studies have reported that adverse effects (up to 53.7%) caused by 50 and 100 mg mirodenafil are mild or moderate in severity, with headache (1.8–14.8%) and flushing (6.7–24.1%) being the most common. Due to the pharmacodynamic profiles of mirodenafil, its tolerability is expected to be somewhat better than those of the other PDE5Is. However, further well designed studies with larger cohorts of different ethnicities, flexible dosing schedules and long-term follow up are necessary to confirm the favorable efficacy and tolerability profiles of mirodenafil for the treatment of ED.
Keywords: erectile dysfunction, mirodenafil, phosphodiesterase type 5 inhibitor
Introduction
Erectile dysfunction (ED), otherwise known as impotence, is defined as the inability to attain or maintain a penile erection sufficient for successful vaginal intercourse [National Institutes of Health, 1993]. Because the prevalence of ED increases with age, it is now regarded as a major health problem for the increasingly aging population [Shamloul and Ghanem, 2013]. It is estimated that the number of men with ED worldwide in 2025 will be approximately 322 million, with an increase of nearly 170 million men over 25 years [Ayta et al. 1999]. The risk factors of ED include advanced age, hypertension, dyslipidemia, metabolic syndrome, cardiovascular disease, central neuropathologic conditions, psychological factors, diabetes mellitus (DM), radical prostatectomy and the use of medications prescribed for the treatment of depression and hypertension [Kubin et al. 2003; Kupelian et al. 2006; Billups, 2005; McVary et al. 2001; Brown et al. 2005; McVary, 2007].
Among many treatment modalities for ED, phosphodiesterase type 5 (PDE5) inhibitors (PDE5Is) are now recognized as the first-line therapy for most men with ED of a broad spectrum of underlying etiologies and severity. In addition to sildenafil, vardenafil, tadalafil and avanafil that have been approved for the treatment of ED worldwide and udenafil that has been approved in thirteen countries, mirodenafil (Mvix; SK Chemicals Life Science, Seongnam, Korea), a second-generation PDE5I, was launched in Korea in November 2007, and its orally dissolving film (Mvix S) was launched in December 2011. Mirodenafil has been approved only in Korea. Its pharmacokinetic profiles include a time to peak serum concentration (Tmax) of 1.25 hours, a half-life (T1/2) of 2.5 hours and a half-maximal inhibitory concentration (IC50) for PDE5 of 0.34 nmol/l [Palit and Eardley, 2010; Paick et al. 2008a], which would confer a biochemical property of relatively high affinity (potency) for PDE5. While the pharmacokinetic profile is similar to that of sildenafil, mirodenafil appears to be 10 times more selective for PDE5 than sildenafil [Shin et al. 2006]. Several randomized, controlled trials have reported the favorable clinical efficacy and tolerability of mirodenafil in men with ED of a broad range of etiologies or severity [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013]. Thus, the availability of various PDE5Is including mirodenafil can provide multiple treatment regimens from which clinicians and patients may choose to optimize the likelihood of treatment success and improve the patients’ satisfaction with few adverse effects. In this review, we highlight the preclinical and clinical data for the efficacy and tolerability of mirodenafil in men with ED.
Biochemical properties of mirodenafil
Because PDE5Is have similar structures to cyclic guanosine monophosphate (cGMP), they inhibit the degradation of nitric oxide (NO)-derived cGMP through binding competitively to the catalytic site of PDE5 [Eardley et al. 2010]. This allows the maintenance of higher cellular levels of cGMP in the corpus cavernosum and the vessels for sustained activation of NO/cGMP pathway, resulting in increased penile blood flow during sexual stimulation and, thereby, enhanced penile erection [Eardley et al. 2010]. Therapeutic and adverse effects of PDE5Is including mirodenafil depend on their selectivity for PDE5 over other PDE isoforms, on their pharmacokinetic profiles and on the distribution of different PDE isoenzymes in the corpus cavernosum or penile vessels [Cho and Paick, 2014]. Because different PDE isoenzymes are distributed over various tissues and a high concentration of PDE5 is present in the smooth muscle of the corpus cavernosum, the high selectivity for PDE5 over the other PDE isoenzymes is important for the increased therapeutic window of PDE5Is [Corbin and Francis, 2002]. In addition to the selectivity for PDE5 over the other PDE isoenzymes, degree of PDE5 inhibition in tissues other than the corpus cavernosum plays a critical role in determining the tolerability of PDE5Is. Table 1 shows a summary of mirodenafil characteristics.
Table 1.
Summary of mirodenafil characteristics.
| Available dosage, mg | 50 and 100 |
| Pharmacological profiles * | |
| – IC50 for PDE5, nmol | 0.34 |
| – Tmax, hr | 0.67–1.5 |
| – Cmax, ng/ml | 104.9 and 278.2 |
| – AUC, ng/hr/ml | 361.5 and 842.0 |
| – T1/2, hr | − 3.0 |
| – Metabolism | Liver: CYP3A4, CYP2C8 |
| – Active metabolite | SK-3541 |
| – Excretion | Feces (mainly), Urine (< 2%) |
| – Molecular weight, g/mol | 531.666 |
| Inhibitory selectivity (compared to PDE5) $ | |
| – PDE1 | 48,235 |
| – PDE3 | 254,000 |
| – PDE6 | 30 |
| – PDE11 | 10,000 |
Data obtained from a study by Paick et al. (2008a), Noh et al. (2012), Shin et al. (2007), Cho et al. (2013), Kim et al. (2009) and Shin et al. (2009).
Data obtained from a study by Shin et al. (2006).
IC50, half-maximal inhibitory concentration; PDE5, phosphodiesterase type 5; Tmax, peak serum concentration; Cmax, maximum concentration; AUC, area under the time–concentration curve; T1/2, half-life.
Chemistry
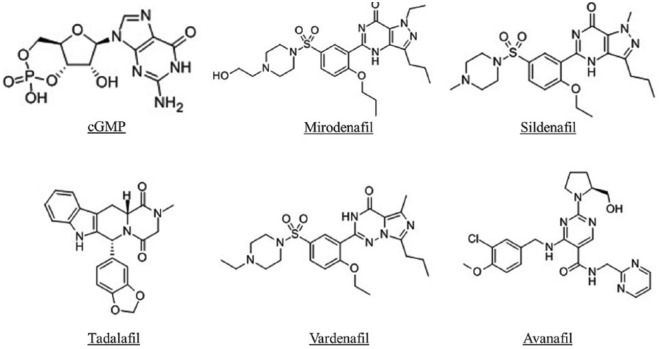
Mirodenafil [5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydro-4H-pyrrolo(3,2-d) pyrimidin-4-one] is a pyrrolopyrimidinone compound and has a molecular structure similar to cGMP, like other PDE5Is (Figure 1) [Shin et al. 2006]. Mirodenafil has a molecular mass of 531.666 g/mol and the product is available in tablet formulation or orally dissolving film of 50 and 100 mg.
Figure 1.
Comparison of chemical structures among different phosphodiesterase type 5 inhibitors (PDE5Is) and cyclic guanosine monophosphate (cGMP).
Pharmacokinetics
Previous clinical studies of healthy male volunteers have shown that mirodenafil of 50–100 mg is rapidly absorbed, reaching maximum concentrations in plasma (Cmax) at 0.67–1.5 hours and is then eliminated with a T1/2 of 1.32–3.0 hours [Paick et al. 2008a; Noh et al. 2012; Shin et al. 2007, 2009; Cho et al. 2013; Kim et al. 2009]. According to a previous preclinical study using rats, the Cmax and the area under the time–concentration curve (AUC) of mirodenafil were dose dependent after oral administration at doses of 10, 20 and 50 mg/kg [Choi et al. 2009]. In another preclinical study using rats, oral bioavailability of SK-3530 based on the total radioactivity was estimated to be 60.4%, 62.2% and 69.9% for 10, 20 and 40 mg/kg doses, respectively, whereas that of parent SK-3530 was calculated to be 24.1%, 30.1% and 43.4% for 10, 20 and 40 mg/kg doses, respectively [Yoo et al. 2007]. This suggested that a considerable amount of mirodenafil was eliminated through an extensive first-pass metabolism, although orally administered mirodenafil was relatively well absorbed in the gastrointestinal tract [Yoo et al. 2007]. Thus, mirodenafil appears to have low oral bioavailability due to a high first-pass effect. A single-dose, randomized, open-label, crossover study of healthy male volunteers demonstrated that the pharmacokinetics (Cmax, AUC, Tmax and T1/2) were not significantly altered by the concurrent administration of mirodenafil with alcohol [Kim et al. 2009]. Also, according to a recent single-dose (50 mg), parallel-group, open-label clinical study of healthy men and those with renal impairment (creatinine clearance of less than 30 ml/min), the Cmax or T1/2 value of the volunteer men with renal impairment was higher or lower than that of the healthy volunteers, but the Tmax and AUC were not different between the two [Noh et al. 2012]. However, there have been no published data on interaction of mirodenafil with food.
Pharmacodynamics
The IC50 for PDE5 of mirodenafil (0.34 nmol) is 10 times less than that of sildenafil (3.50 nmol), indicating that mirodenafil has a 10 times higher affinity or potency than sildenafil [Shin et al. 2006]. However, the higher affinity or potency of mirodenafil for PDE5 does not mean that it has a greater clinical effect, but that less of it is needed for the desired effect [Eardley et al. 2010]. The selectivity of PDE5I for PDE5 over PDE1 is closely related to adverse effects such as flushing, tachycardia and vasodilation. The inhibitory effect of mirodenafil on PDE5 is about 48,000-fold greater than that on PDE1 (selectivity ratio of 48,235), which appears to be greater than the PDE1 selectivity of sildenafil, vardenafil and tadalafil (with selectivity ratios of 80, 690 and >4000, respectively) [Shin et al. 2006; Francis and Corbin, 2003]. Inhibition of PDE3, which is mainly distributed in cardiomyocytes and degraded cyclic adenosine monophosphate (cAMP), is associated with increased heart rates and a positive inotropic effect [Bischoff, 2004]. The PDE3 selectivity ratio of mirodenafil is approximately 254,000, which is greater than those of sildenafil, vardenafil and tadalafil (with selectivity ratios of 4629, 40,000 and >4000) [Shin et al. 2006; Francis and Corbin, 2003]. Because PDE6 is mainly found in the cones/rods of the retina and controls retinal cGMP levels, PDE6 inhibition may cause visual disturbances such as blurred vision and chromatopsia [Bischoff, 2004]. The PDE6 selectivity ratio of mirodenafil is about 30, which appears to be a little greater than that of sildenafil (with selectivity ratio of 11) [Shin et al. 2006]. To date, no visual adverse effects have been reported in men taking mirodenafil. Also, the PDE11 selectivity ratio of mirodenafil is greater than 10,000, which is greater than those of sildenafil, vardenafil and tadalafil (with selectivity ratios of 780, 1620 and 5.5), suggesting a bare possibility of PDE11 inhibition at therapeutic doses of mirodenafil [Shin et al. 2006; Francis and Corbin, 2003]. However, the physiological relevance of PDE11 has not yet been established, although it has been speculated that back pain or myalgia may be associated with PDE11 inhibition [Bischoff, 2004]. Taken together, mirodenafil is a selective PDE5I on the basis of its high selectivity for PDE5 over the other PDE isoforms, although it is difficult to directly compare it with other PDE5Is because of a scarcity of data from head-to-head studies.
Metabolism
Cytochrome P450 3A4 (CYP3A4) plays a predominant role in the N-dealkylation of mirodenafil to SK-3541, a major active metabolite of mirodenafil, with a minor contribution from CYP2C8 [Lee et al. 2007, 2008]. SK-3541 has 10 times lower in vitro potency for inhibition of PDE5 (3 nmol) compared with that of mirodenafil (0.34 nmol) [Choi et al. 2010]. Mirodenafil is mainly excreted into the feces through hepatobiliary metabolism after oral administration [Lee et al. 2007]. Urinary excretion of mirodenafil is very low (less than 2%) and that of SK-3541 is negligible, indicating that mirodenafil or SK-3541 is subject to nonrenal elimination [Lee et al. 2007].
Efficacy of mirodenafil in the general population with erectile dysfunction
According to a preclinical study using an ex vivo tissue model of rabbits, the corpus cavernosum was relaxed in response to mirodenafil in a dose-dependent manner, suggesting therapeutic potential in the treatment of ED [Kim et al. 2011]. A previous phase I study showed that mirodenafil is effective and well tolerated at daily doses up to 200 mg in healthy male volunteers [Paick et al. 2008a].
The clinical efficacy and tolerability of mirodenafil in patients with ED of various underlying etiologies and severities have been assessed in several randomized, placebo-controlled clinical studies [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013]. Tables 2 and 3 list baseline characteristics of the patients, outcome measures and treatment outcomes of the studies.
Table 2.
Baseline characteristics and efficacy parameters of five randomized placebo-controlled comparative clinical trials.
| Study | Characteristics of participants | Study period | Group | Sample size (n) | Etiology of ED (%) (organic/psychogenic/mixed) | Severity of ED (%) (mild/mild-to-mod/mod/severe) | Prior PDE5I users (%) | Primary efficacy | Secondary efficacy |
|---|---|---|---|---|---|---|---|---|---|
| Paick et al. [2008b] | General | 8 week | Placebo | 30 | 60.0/6.7/33.3 | 36.7/ - /50.0/13.3 | 40.0 | IIEF Q3 | IIEF-EF domain |
| (Phase II) | 50 mg mirodenafil | 30 | 60.0/10.0/30.0 | 20.0/ - /50.0/30.0 | 43.3 | IIEF Q4 | SEP2, 3 | ||
| 100 mg mirodenafil | 30 | 20.0/53.3/26.7 | 26.7/ - /53.3/20.0 | 30.3 | GAQ | ||||
| 150 mg mirodenafil | 29 | 20.7/31.0/48.3 | 48.3/ - /31.0/20.7 | 30.0 | |||||
| Paick et al. [2008a] | General | 12 week | Placebo | 75 | 38.7/8.0/53.3 | 2.7/26.7/45.3/25.3 | 49.3 | IIEF Q3 | All IIEF domains |
| (Phase III) | 50 mg mirodenafil | 73 | 30.1/20.6/49.3 | 4.1/31.5/45.2/19.2 | 52.1 | IIEF Q4 | SEP2, 3 | ||
| 100 mg mirodenafil | 74 | 41.9/10.8/47.3 | 2.7/31.1/39.2/27.0 | 46.0 | GAQ, LSC | ||||
| Paick et al. [2010] | Hypertension | 12 week | Placebo | 53 | 66.0/3.8/30.2 | 3.8/18.9/45.3/32.0 | 43.4 | IIEF-EF domain | All IIEF domains |
| (Phase III) | 100 mg mirodenafil | 54 | 64.8/3.7/31.5 | 1.9/18.5/40.7/38.9 | 38.9 | IIEF Q3, 4 | |||
| SEP2, 3 | |||||||||
| GAQ, LSC | |||||||||
| Park et al. [2010] | DM | 12 week | Placebo | 53 | 50.9/0.0/49.1 | 1.9/18.9/50.9/28.3 | 41.5 | IIEF-EF domain | All IIEF domains |
| (Phase III) | 100 mg mirodenafil | 55 | 40.0/3.6/56.4 | 5.5/20.0/40.0/34.5 | 40.0 | IIEF Q3, 4 | |||
| SEP2, 3 | |||||||||
| GAQ, LSC | |||||||||
| Chung et al. [2013] | General | 12 week | Placebo | 63 | 35.6/20.0/27.8 | NA | 53.3 | IIEF-5 | Qmax, PVR |
| (Phase III) | 50 mg mirodenafil | 71 | 38.9/15.6/30.0 | 57.8 | PEDT | IPSS | |||
| SEP2, 3 | QOL index |
ED, erectile dysfunction; PDE5I, phosphodiesterase type 5 inhibitor; IIEF, International Index of Erectile Function; EF, erectile function; IIEF-EF, EF domain of the IIEF; SEP, Sexual Encounter Profile; GAQ, Global Assessment Question; LSC, Life Satisfaction Checklist; DM, diabetes mellitus; PEDT, Premature Ejaculation Diagnosis Tool; Qmax, maximum flow rate; PVR, post-void residual urine volume; QOL, quality of life; NA, not available; IPSS, International Prostate Symptom Score.
Table 3.
Main efficacy outcomes of mirodenafil in five randomized placebo-controlled trials.
| Study | Group | IIEF-EF or IIEF-5 |
SEP2 (%) |
SEP3 (%) |
GAQ (%) | % Shift to normal EF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End-point | Change | Baseline | End-point | Change | Baseline | End-point | Change | ||||
| Paick et al. [2008b] | Placebo | 14.6 | 17 | 2.4 | 62.8 | 71.1 | 8.3 | 16.8 | 35.4 | 18.6 | 34.5 | 17.2 |
| 50 mg | 12.5 | 20.9 | 8.4 | 45.5 | 83.4 | 37.9 | 12.2 | 57.1 | 44.9 | 75.9 | 24.1 | |
| 100 mg | 13.7 | 24.4 | 10.7 | 57.5 | 94.8 | 37.3 | 8.6 | 71.8 | 63.2 | 86.2 | 51.7 | |
| 150 mg | 15.0 | 23.2 | 8.2 | 62.5 | 90.9 | 28.4 | 12.0 | 68.4 | 6.4 | 82.1 | 48.3 | |
| Paick et al. [2008a] | Placebo | 13.6 | 17.0 | 3.4 | 45.16 | 60.60 | 15.44 | 6.59 | 26.77 | 20.18 | 31.51 | 17.3 |
| 50 mg | 14.5 | 22.1 | 7.6 | 52.92 | 80.64 | 27.72 | 8.63 | 52.83 | 44.20 | 66.67 | 46.6 | |
| 100 mg | 14.0 | 25.6 | 11.6 | 52.97 | 91.95 | 38.98 | 5.87 | 73.20 | 67.33 | 89.04 | 62.2 | |
| Paick et al. [2010] | Placebo | 12.74 | 15.40 | 2.66 | NA | NA | 6.5 | NA | NA | 16.5 | 26.00 | 7.5 |
| 100 mg | 12.85 | 22.20 | 9.35 | 55.8 | 86.0 | 30.2 | 8.7 | 64.0 | 55.3 | 84.31 | 40.7 | |
| Park et al. [2010] | Placebo | 12.6 | 14.0 | 1.4 | 46.3 | 55.2 | 8.9 | 4.5 | 22.3 | 17.8 | 19.1 | 9.4 |
| 100 mg | 12.7 | 22.0 | 9.3 | 45.9 | 82.0 | 36.1 | 7.2 | 69.0 | 61.8 | 76.9 | 32.7 | |
| Chung et al. [2013] | Placebo | 8.92 | 10.33 | 1.41 | 34.4 | 50.7 | 16.3 | 30.0 | 32.0 | 2.0 | NA | NA |
| 50 mg | 9.49 | 14.82 | 5.33 | 32.2 | 5.6 | 43.4 | 26.7 | 55.3 | 28.6 | NA | NA | |
IIEF, International Index of Erectile Function; EF, erectile function; IIEF-EF, EF domain of IIEF; SEP, Sexual Encounter Profile; GAQ, Global Assessment Question; NA, not available.
The phase II, 8-week, randomized, double-blind, placebo-controlled, multicenter, parallel-group clinical trial by Paick and colleagues evaluated the efficacy and tolerability of mirodenafil at three doses (50, 100 and 150 mg) in 119 Korean men aged 19–70 years with at least a 6-month history of ED from organic, psychogenic, or mixed etiology, to determine the optimal dose with respect to effectiveness and patient tolerance [Paick et al. 2008b]. As for the primary efficacy variable of erectile function (EF) domain scores of the International Index of Erectile Dysfunction (IIEF) questionnaire, the 50, 100 and 150 mg mirodenafil groups showed improvements of 8.4, 10.7 and 8.2 points, respectively, which were significantly higher than 2.3 points in the placebo group. The mirodenafil treatment groups demonstrated greater increases from baseline in the rates of successful vaginal penetration [questions 2 of the Sexual Encounter Profile (SEP2)] of 28.4–37.9% compared with 8.3% in the placebo group. Also, the increases from baseline of the rates of successful intercourse completions [questions 3 of the Sexual Encounter Profile (SEP3)] in the mirodenafil treatment groups (44.9–63.2%) were significantly greater than 18.6% in the placebo group. In terms of patients’ responses to the Global Assessment Question (GAQ), the proportion of patients responding positively to the GAQ was significantly greater in the mirodenafil groups (50 mg mirodenafil, 24.1%; 100 mg mirodenafil, 51.7%; and 150 mg mirodenafil, 48.3%), compared with the placebo group (17.2%). Although the 150 mg group experienced significantly more treatment-associated adverse events (TAEs), all adverse events were mild or moderate in severity. Thus, the most efficacious and well tolerated doses were determined to be 100 and 50 mg, respectively.
A phase III, 12-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study by Paick and colleagues investigated the efficacy and tolerability of mirodenafil at two doses (50 and 100 mg) in 223 Korean men aged 19–70 years with a minimum 6-month history of ED of organic, psychogenic, or mixed etiology [Paick et al. 2008a]. Regarding the primary efficacy measures of changes from baseline in scores of the IIEF question 3 (penetration ability) and question 4 (maintenance frequency), the 50 and 100 mg mirodenafil groups showed significantly greater increases in IIEF question 3 scores (placebo, 0.68; 50 mg mirodenafil, 1.16; and 100 mg mirodenafil, 1.63) and IIEF question 4 scores (placebo, 0.80; 50 mg mirodenafil, 1.83; and 100 mg mirodenafil, 2.62), compared with the placebo group. Likewise, mirodenafil improved the secondary efficacy variables such as changes from baseline in SEP2 and SEP3, and patients’ responses to the GAQ. Mirodenafil significantly increased the proportions of ‘yes’ responders to the SEP2 (placebo, 15.44%; 50 mg mirodenafil, 27.72%; and 100 mg mirodenafil, 38.98%) and those to SEP3 (placebo, 20.18%; 50 mg mirodenafil, 44.19%; and 100 mg mirodenafil, 67.33%), compared with the placebo group. Also, the percentages of patients responding positively to the GAQ were greater in the 50 and 100 mg mirodenafil groups (66.67% and 89.04%, respectively), compared with the placebo group (31.51%). When subjects with scores of at least 26 in the IIEF-EF domain were classified as having normal erectile function, 17.3%, 46.6% and 62.2% of the placebo, mirodenafil 50 mg and 100 mg groups, respectively, were considered to recover normal erectile function after treatment.
According to a meta-analysis of three multicenter, randomized, double-blind, placebo-controlled clinical trials by Du and colleagues, the pooled data showed greater increases of 7.32 points in change from baseline of the IIEF-EF domain after the mirodenafil treatment compared with the placebo data [Du et al. 2014]. The pooled data demonstrated that mirodenafil treatment offered greater improvements of 24.73% and 43.78% in positive responses to SEP2 and SEP3 compared with the placebo data. Furthermore, mirodenafil treatment showed a greater increase in positive response to the GAQ compared with the placebo (risk ratio, 3.18). The pooled analysis showed that most adverse events were mild or moderate in severity with flushing and headache being the most common, and that no serious adverse events were reported during the study period. Thus, according to the meta-analysis, mirodenafil was observed to offer significant improvements across all efficacy measures.
Efficacy of mirodenafil in special populations with erectile dysfunction
Diabetic erectile dysfunction population
Three preclinical studies using rat models of diabetic ED have suggested mirodenafil as a potential treatment of diabetic ED [Park et al. 2008, 2011]. A previous study by Park and colleagues using a rat model of streptozotocin-induced diabetic ED demonstrated that daily administration of mirodenafil for 4 weeks activated Akt signaling, which suppressed pro-apoptotic stimuli and maintained erectile function [Park et al. 2008]. Another preclinical study showed that administration of intravenous bolus mirodenafil (1 mg/kg) significantly improved erectile response in a rat model of streptozotocin-induced diabetic ED [Park et al. 2011]. Furthermore, a preclinical study by Lee and colleagues evaluated the pharmacokinetics of mirodenafil at a dose of 20 mg/kg in a streptozotocin-induced diabetic rat model [Lee et al. 2010]. They showed that after oral administration of mirodenafil, the AUC and Cmax were not significantly different between diabetic and controls rats, possibly due to the opposite changes in protein expression of intestinal CYP1A1/2 (increase) and CYP2D (decrease) subfamilies in diabetic rats [Lee et al. 2010].
A phase III, 12-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group, fixed-dose study by Park and colleagues assessed the efficacy and tolerability of 100 mg mirodenafil in 112 Korean men with diabetic ED [Park et al. 2010]. Regarding the primary efficacy variable of the change from baseline in the IIEF-EF domain score, the mirodenafil group demonstrated significantly greater improvement, compared with the placebo group (9.3 ± 6.8 versus 1.4 ± 6.1). Similarly, greater improvements in the mirodenafil groups were observed for the secondary efficacy parameters including the IIEF questions 3 and 4, and all IIEF domains other than IIEF-EF, SEP2, SEP3 and GAQ. Also, the percentage of patients who met the ‘return-to-normal’ definition of IIEF-EF domain scores of at least 26 after 12-week treatment was significantly higher in mirodenafil group (32.7%) than in the placebo group (9.4%).
Erectile dysfunction population with hypertension
A preclinical study by Lee and colleagues assessed the pharmacokinetics of mirodenafil at a dose of 20 mg/kg in a hypertensive rat model [Lee et al. 2011b]. After oral administration of mirodenafil to 6-week-old and 16-week-old spontaneously hypertensive rats (animal models for essential hypertension), the AUC of mirodenafil was significantly greater than that in age-matched controls. This was attributable to the hereditary characteristics of spontaneously hypertensive rats, but not the hypertensive state itself. On the other hand, after oral administration of mirodenafil to 16-week-old deoxycorticosterone acetate–salt-induced hypertensive rats (animal models for secondary hypertension), the AUC of mirodenafil was smaller than that in age-matched controls, which was explained by a significant increase in the intestinal metabolism of mirodenafil compared with the controls. Thus, the pharmacokinetics of mirodenafil does not appear to be affected by essential hypertension itself. According to a recent organ-bath study using corporal tissue strips to evaluate the interaction of mirodenafil with antihypertensive drugs, angiotensin receptor blocker (losartan), calcium channel blocker (nifedipine and amlodipine), α-adrenergic blocker (tamsulosin and doxazosin) except for angiotensin-converting enzyme (ACE) inhibitor (enalapril), enhanced mirodenafil-induced relaxation on phenylnephrine-contracted corpus cavernosum of rabbits, indicating that the combination of PDE5Is with the above-mentioned drugs could be a pharmacologic strategy for simultaneously treating ED and its comorbidities and for increasing response rates to PDE5Is [Lee et al. 2012].
A phase III, 12-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group, fixed-dose trial by Paick and colleagues was performed to determine the efficacy and tolerability of 100 mg mirodenafil in 107 Korean men with a minimum 6-month history of ED who were receiving at least one antihypertensive medication in a stable dose for a duration of at least 4 weeks [Paick et al. 2010]. In regard to the primary efficacy variable of changes from baseline in the IIEF-EF domain scores, the mirodenafil group demonstrated significantly greater increase in the scores at 12 weeks compared with the placebo group. Similarly, superior improvements in the mirodenafil group were observed for the secondary efficacy variables such as scores of the IIEF question 3 and question 4, the other four domains of IIEF, and percentages of patients responding positively to the SEP2, SEP3 and GAQ, compared with the placebo group. After the 12-week treatment, 40.7% of the mirodenafil group was observed to have a IIEF-EF score of at least 26, representative of normal erectile function, compared with only 7.5% in the placebo group. Mirodenafil was generally well tolerated in the subjects taking antihypertensive medications, without any serious TAEs during the study or thereafter.
Daily dosing for erectile dysfunction
A phase III, 12-week, randomized, double-blind, parallel-group comparative study assessed the efficacy and tolerability of once-daily administration of 50 mg mirodenafil in the treatment of ED [Chung et al. 2013]. In terms of the primary efficacy variables of the IIEF-5 scores, Premature Ejaculation Diagnosis Tool (PEDT) scores, SEP2 and SEP3, the mirodenafil group showed significant improvements in all of the variables, compared with the control group. Similar results were observed in the comparison of the secondary efficacy variables such as the total International Prostate Symptom Score (IPSS), quality of life (QOL) index, subtotal voiding symptoms score of the IPSS and maximum flow rate (Qmax) between the mirodenafil and placebo groups. However, the mirodenafil treatment did not improve subtotal storage symptoms score of the IPSS and post-void residual urine volume (PVR) compared with placebo. No significant changes in systolic blood pressure (SBP), diastolic BP (DBP) and pulse rate were observed during the study period. All of the adverse effects were mild to moderate.
Erectile dysfunction population with lower urinary tract symptoms/benign prostatic hyperplasia
A multicenter, open-label, prospective, noncomparative study by Lee and colleagues evaluated the efficacy and tolerability of the combination of daily administered α1-blockers (tamsulosin 0.2 mg or alfuzosin) with 100 mg mirodenafil (twice a week) in 121 patients with both lower urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH) and ED, who already received stable α1-blocker therapy for more than 3 months [Lee et al. 2011a]. After the start of the study, mean IPSS significantly decreased from 15.60 ± 7.79 at baseline to 11.29 ± 5.77 at 4 weeks and 9.01 ± 4.86 at 8 weeks, and mean quality of life index decreased from 3.22 ± 0.89 at baseline to 2.86 ± 0.90 at 4 weeks and 2.42 ± 0.96 at 8 weeks. Also, mean IIEF-5 score significantly increased from 7.78 ± 4.30 at baseline to 11.22 ± 4.19 at 4 weeks and 15.22 ± 4.00 at 8 weeks. In terms of the SEP2, SEP3 and GAQ, 62.24%, 56.12% and 72.45% of the patients reported ‘yes’ at 4 weeks, and 80.82%, 84.93% and 90.41% reported ‘yes’ at 8 weeks, respectively. However, no significant improvement in Qmax or PVR was observed. No significant change in BP or heart rate (HR) was observed during the study. The TAEs were minimal and self-limited.
Another prospective, multicenter, open-label trial assessed the efficacy and tolerability of daily co-administered α1-blocker and 50 mg mirodenafil in patients with both LUTS/BPH and ED, who were already receiving stable α1-blocker therapy for at least 4 weeks [Bang et al. 2013]. Although the improvement of total IPSS after the initial α1-blocker monotherapy of 4 weeks was significant (from 23.70 to 18.70), the addition of 50 mg mirodenafil to the α1-blocker resulted in further reduction in total IPSS at 4 and 8 weeks after the co-administration (14.30 and 13.72, respectively). In terms of efficacy in the treatment of ED, the mean IIEF-5 scores significantly improved at 4 and 8 weeks after the additional mirodenafil medication (16.23 ± 5.80 and 16.16 ± 5.07, respectively), compared with before the co-administration (10.94 ± 5.70). Although the daily co-administered α1-blocker and 50 mg mirodenafil did not reduce the PVR, the Qmax improved at the end of the study period. There were no significant changes in SBP/DBP and HR during the study period. Also, no severe or serious adverse events were reported during the study period.
Erectile dysfunction population with other comorbidities
Penile rehabilitation strategies, such as chronic dosing with PDE5Is, have been suggested to prevent the functional and structural alterations in the penis induced by cavernous nerve (CN) injury during radical prostatectomy [Barazani et al. 2015]. A preclinical study using a rat model of CN injury showed that chronic dosing of 10 mg/kg or 20 mg/kg mirodenafil for 8 weeks improved the protein expression of neuronal NO synthase (NOS) or endothelial NOS and cGMP, and that it improved the erectile function by preserving the smooth muscle content and inhibiting the fibrosis of the corpus cavernosum [Kim et al. 2010]. To date, however, no clinical data have been published on the efficacy of mirodenafil in patients with ED after radical prostatectomy.
A previous preclinical study using a rabbit model of acute spinal cord injury (SCI) evaluated the effect of PDE5Is at three doses (0.3 mg/kg, 1.0 mg/kg, or 3.0 mg/kg), on erectile response and compared between mirodenafil and sildenafil [Jung et al. 2008]. Interestingly, the onset of erection with mirodenafil was faster than with sildenafil, and the erectogenic potential of mirodenafil was significantly better than that of sildenafil at the same dose.
Safety/tolerability profiles
Based on available clinical data on mirodenafil for the treatment of ED, it is generally well tolerated with a few mild or moderate TAEs. All the reported TAEs of mirodenafil are listed in Table 4. Although most of the clinical studies reported more frequent TAEs in the mirodenafil group than placebo group, they were generally mild to moderate in severity, with flushing (3.3–24.1%) and headache (1.8–14.8%) being most common. Severe TAE was reported only in one patient (facial flushing) [Paick et al. 2008a]. These TAEs are related to the vasodilation, which is attributable to the selectivity of PDE5I for PDE5 over other PDEs such as PDE1. Thus, the adverse effects of PDE5Is such as mirodenafil are closely associated with inhibition of PDE5 expressed in nonpenile tissue or cross-reactivity with the other PDEs. Mirodenafil does not appear to be associated with adverse effects such as back pain or myalgia. In most of the studies, there were few cases that dropped out due to the TAEs.
Table 4.
The summary of adverse effects caused by mirodenafil in five randomized placebo-controlled trials.
| Study |
Paick et al. [2008b] |
Paick et al. [2008a] |
Paick et al. [2010] |
Park et al. [2010] |
Chung et al. [2013] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Placebo | 50 mg | 100 mg | 150 mg | Placebo | 50 mg | 100 mg | Placebo | 100 mg | Placebo | 100 mg | Placebo | 50 mg |
| Flushing (%) | 0.0 | 16.7 | 6.7 | 20.7 | 4.0 | 9.5 | 16.2 | 3.6 | 24.1 | 1.8 | 7.1 | 0.0 | 3.3 |
| Headache (%) | 0.0 | 3.3 | 6.7 | 20.7 | 3.3 | 8.1 | 10.8 | 1.8 | 14.8 | 0.0 | 1.8 | 2.2 | 4.4 |
| Nasal congestion (%) | 0.0 | 0.0 | 3.3 | 3.4 | 1.1 | 2.2 | |||||||
| Nasopharyngitis (%) | 1.1 | 1.1 | |||||||||||
| Eye redness (%) | 0.0 | 3.3 | 3.3 | 3.4 | 0.0 | 2.7 | 4.1 | 1.8 | 1.8 | ||||
| Dyspepsia (%) | 0.0 | 0.0 | 3.3 | 3.4 | 0.0 | 0.0 | 2.7 | 1.8 | 3.7 | 1.8 | 1.8 | 2.2 | 4.4 |
| Nausea (%) | 0.0 | 4.1 | 2.7 | 0.0 | 3.6 | ||||||||
| Dizziness (%) | 3.3 | 0.0 | 3.3 | 3.4 | 1.3 | 0.0 | 2.7 | 1.8 | 0.0 | 1.8 | 1.8 | 0.0 | 1.1 |
| Arthralgia (%) | 0.0 | 1.8 | |||||||||||
| Palpitation (%) | 0.0 | 2.7 | 0.0 | ||||||||||
| Chest discomfort (%) | 0.0 | 2.7 | 0.0 | ||||||||||
| Back pain (%) | 0.0 | 2.2 | |||||||||||
| Others (%) | 0.0 | 6.7 | 10.0 | 13.8 | 0.0 | 5.4 | 0.0 | 0.0 | 1.8 | 1.1 | 1.1 | ||
Considering the vasodilatory effect of PDE5Is such as mirodenafil, clinicians might pay attention to its tolerability in men with ED who receive concomitant medications. To date, most of the clinical studies demonstrated that mirodenafil did not have an adverse effect on laboratory tests, electrocardiogram (ECG), or vital signs in men with ED, even in those who were taking concomitant medications such as antihypertensive medications or α1-blockers [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013; Lee et al. 2011a; Bang et al. 2013]. In men with ED who were receiving at least one antihypertensive medication (ACE inhibitor, angiotensin II-receptor inhibitor, α-blocker, β-blocker, calcium-channel blocker or diuretics) at a stable dose for a duration of at least 4 weeks, SBP and DBP along with HR were not significantly altered from baseline values in the 100 mg mirodenafil group, as well as in the placebo group. According to the two clinical investigations that examined the efficacy and tolerability of co-administered α1-blockers (tamsulosin or silodosin or alfuzosin or doxazosin) and 50–100 mg mirodenafil in patients with both LUTS/BPH and ED, mirodenafil treatment (100 mg twice a week or 50 mg once daily) did not cause significant hemodynamic changes such as SBP/DBP and HR over the course of the trial [Lee et al. 2011a; Bang et al. 2013].
Comment
According to the current guidelines for ED, oral PDE5Is are now accepted as the first-line treatment option for ED, due to advantages such as favorable efficacy, tolerability, and ease of use [Montague et al. 2005; Hatzimouratidis et al. 2010]. Because all available PDE5Is have an appropriate onset or duration of action and favorable success rates, clinicians may consider trying all available PDE5Is until it is known which one has the best effects on the patient’s erections with the least overall adverse effects [Shamloul and Ghanem, 2013]. In this respect, the introduction of various PDE5Is with varying biochemical properties can provide additional treatment options to help in choosing the right PDE5I for treatment of different patients in daily clinical practice. Thus, mirodenafil may be a helpful option for treatment of a variety of patients with ED, as supported by its favorable efficacy and tolerability profiles in the treatment of ED of broad-spectrum etiology and severity [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013; Lee et al. 2011a; Bang et al. 2013].
Most of the available evidence suggests that mirodenafil is effective, safe and well tolerated in the treatment of ED [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013; Lee et al. 2011a; Bang et al. 2013]. The recent meta-analysis by Du and colleagues demonstrated that mirodenafil improved the IIEF-EF domain score by 7.32 points, which is comparable with data from pivotal studies of sildenafil (6.00 points), tadalafil (7.49 points), vardenafil (7.11 points) and udenafil (5.66 points) [Du et al. 2014; Yuan et al. 2013]. As measured by the SEP2 and SEP3, mirodenafil could improve the rates of successful vaginal penetration and successful intercourse completion by 22.14% and 43.78%, respectively [Du et al. 2014]. These results are comparable with data of the pooled analysis of sildenafil (8.73% and 17.25%), tadalafil (27.73% and 36.17%), vardenafil (27.40% and 36.25%) and udenafil (26.22% and 28.29%) [Yuan et al. 2013]. In regard to the GAQ, the mirodenafil group (risk ratio, 3.18) had approximately 3.18 times higher positive responses to GAQ than the placebo group, which is consistent with data from prior studies of sildenafil (risk ratio, 3.20), tadalafil (risk ratio, 3.31), vardenafil (risk ratio, 3.32) and udenafil (risk ratio, 3.02) [Yuan et al. 2013]. However, further head-to-head comparative studies between the PDE5Is are needed because it is difficult to directly compare the efficacy between different PDE5Is due to differences in baseline characteristics of patients, inclusion criteria, outcome measures, dosing schedules and treatment periods. Furthermore, four of the five randomized clinical trials of mirodenafil excluded the nonresponders to PDE5Is [Paick et al. 2008a, 2008b, 2010; Park et al. 2010], while only a few sildenafil trials, half of vardenafil trials and only a third of tadalafil trials excluded men with ED that did not respond to previous PDE5Is [Tsertsvadze et al. 2009]. Thus, the difference could limit the comparison of treatment outcomes of mirodenafil trials with those of historical trials of vardenafil, tadalafil or sildenafil.
The pathophysiology of diabetic ED is multifactorial, including neuropathy, endothelial dysfunction, cavernosal smooth muscle structural/functional changes and hormonal changes. Thus, the patients with ED are considered to be one of the difficult-to-treat populations [Hatzimouratidis and Hatzichristou, 2009, 2014]. Mirodenafil (100 mg) treatment showed statistically significant improvements in changes from baseline in the IIEF-EF domain scores, compared with placebo (9.3 versus 1.4 points) [Park et al. 2010]. Similarly, greater improvements in the mirodenafil group were observed for the SEP2 (36.1% versus 8.9%) and SEP3 (61.8% versus 17.8%), compared with the placebo group [Park et al. 2010]. In accordance with the results for mirodenafil, a recent Cochrane review demonstrated that sildenafil treatment provided a greater increase in the IIEF-EF domain score of 7.19 points compared with placebo [Vardi and Nini, 2007]. Also, according to a multicenter, randomized, placebo-controlled trial by Sáenz de Tejada and colleagues, treatment with 10 and 20 mg tadalafil resulted in greater improvements of 6.3 and 7.2 points, respectively, in the IIEF-EF domain score compared with placebo [Sáenz de Tejada et al. 2002]. Also, the increases from baseline of the positive responses to SEP2 and SEP3 in 10 mg (22.2% and 28.4%) and 20 mg tadalafil (22.6% and 29.1%) groups were significantly greater compared with the placebo group (−4.1% and 1.9%). According to a phase III, multicenter, randomized, placebo-controlled trial by Goldstein and colleagues, treatment with 10 and 20 mg vardenafil resulted in improvements of 4.5 and 6.4 points, respectively, in the IIEF-EF domain score compared with placebo [Goldstein et al. 2003]. Also, treatment with 10 and 20 mg vardenafil showed greater increases from baseline in positive responses to SEP2 (10 mg, 26.2%; 20 mg, 19.2%) and SEP3 (10 mg, 31.0%; 20 mg, 29.0%) compared to placebo. A phase III, multicenter, placebo-controlled, randomized trial by Moon and colleagues showed that 100 and 200 mg udenafil improved the IIEF-EF domain score by 5.8 and 7.01 points, respectively, compared with placebo [Moon et al. 2011]. The treatment with 100 and 200 mg udenafil provided significantly greater increases from baseline in the positive response to SEP2 (23.84% and 31.07%) and SEP3 (45.57% and 55.56%) compared with placebo. Taken together, the efficacy of mirodenafil in the treatment of diabetic ED was comparable with that of other PDE5Is. However, the exclusion of nonresponders to PDE5Is and a scarcity of head-to-head comparative studies make the comparison between different PDE5Is difficult.
Hypertension is a strong risk factor for ED [Shamloul and Ghanem, 2013]. Also, several antihypertensive medications such as beta-blockers and thiazides are involved in the development of ED [Shamloul and Ghanem, 2013]. Although a frequent clinical issue is the possible interaction between PDE5Is and antihypertensive drugs, the PDE5Is are usually well tolerated in men with ED who are taking antihypertensive medications [Corona et al. 2011]. The clinical trial by Paick and colleagues showed that mirodenafil (100 mg) treatment provided significantly greater increases from baseline in the IIEF-EF scores compared with placebo (9.35 versus 2.66) [Paick et al. 2010]. Also, the mirodenafil treatment showed significantly greater increases in the success rates (proportions of ‘yes’ responders) of the SEP2 (30.18% versus 6.50%) and SEP3 (55.30% versus 16.48%), compared with placebo [Paick et al. 2010]. In accordance with these results, similar results for the IIEF-EF domain scores, SEP2 and SEP3 were obtained with 25–100 mg sildenafil (6.2 points), 20 mg tadalafil (8.1 points, 34.3% and 45.1%), 5–20 mg vardenafil (8.9 points, 32.4% and 38.0%), 200 mg udenafil (8.06 points, 26.7% and 50.5%) [Blonde, 2006; Kloner et al. 2005; Shabsigh et al. 2007; Paick et al. 2009]. In regard to the tolerability of mirodenafil concomitantly administered with antihypertensive medications, no significant alterations of SBP/DBP or HR in the mirodenafil group were observed [Paick et al. 2010]. Furthermore, co-administration of mirodenafil and antihypertensive medications did not significantly increase the incidences of TAEs such as vasodilatory symptoms (flushing or headache) or hypotensive symptoms (dizziness). The results of mirodenafil are comparable with those from published studies on already available PDE5Is such as sildenafil, tadalafil and vardenafil [Blonde, 2006; Kloner et al. 2005; Shabsigh et al. 2007; Paick et al. 2009; Kloner et al. 2001; van Ahlen et al. 2005]. Taken together, the PDE5Is, including mirodenafil, appear to be effective, safe and well tolerated, even in hypertensive men with ED who are receiving concomitant antihypertensive medications. However, the exclusion of nonresponders to PDE5Is and a scarcity of head-to-head comparative studies make the comparison between different PDE5Is difficult.
There are several preclinical and clinical evidences supporting a potential benefit of daily or chronic administration of PDE5Is through improvement in endothelial dysfunction, although which patients with ED are more likely to benefit from chronic dosing rather than on-demand treatment remains to be determined [Behr-Roussel et al. 2005; Porst et al. 2006, 2008]. Chronic PDE5I dosing may have the following potential benefits: (i) recovery of nonresponders to on-demand therapy; (ii) endothelial and penile rehabilitation, especially in complicated patients; (iii) more spontaneity and naturalness; and (iv) treatment of LUTS/BPH [Bruzziches et al. 2013]. Thus, a recent randomized, double-blind, clinical trial by Chung and colleagues evaluated the efficacy and tolerability of once-daily administration of 50 mg mirodenafil for 12 weeks in the treatment of ED [Chung et al. 2013]. The mirodenafil treatment provided significantly greater improvements in the IIEF-5 scores (14.82 points versus 10.33 points), SEP2 (43.0% and 17.0%) and SEP3 (29.0% and 4.5%), compared with placebo, which was comparable with the data obtained with chronic dosing of 2.5–10 mg tadalafil, the only drug currently approved for daily dosing in the treatment of ED (IIEF-5, 4.9–8.8 points; SEP2, 24.3–39.4%; SEP3, 31.2–50.1%). Despite the short T1/2 of mirodenafil, the clinical trial by Chung and colleagues reported the favorable efficacy and tolerability of chronic mirodenafil dosing in the treatment of ED and LUTS. They suggested several plausible explanations for the findings as follows: (i) because significant improvements of endothelial molecular markers were observed in patients with ED after once-daily dosing of sildenafil with the pharmacokinetic properties similar to mirodenafil, which had the higher AUC and Cmax in both plasma and corpus cavernosum than sildenafil in rats, could be expected to improve endothelial function in a fashion similar to that of once-daily treatment with sildenafil [Chung et al. 2013; Konstantinopoulos et al. 2009; Lee et al. 2009]; (ii) as the concentrations of mirodenafil in the corpus cavernosum were higher than of those in the plasma, effects of once-daily treatment cannot be determined by just measuring the blood pharmacokinetics of maximum concentration and T1/2 of the agent [Chung et al. 2013; Lee et al. 2009]; and (iii) it is possible that PDE5Is may also affect the body at levels lower than the minimal effective concentration. In addition, once-daily treatment with agents that have short half-lives can be effective due to pharmacodynamic factors such as drug distribution and reabsorption [Chung et al. 2013]. However, there has been a scarcity of data on the advantage or protective effect of daily administered mirodenafil on endothelium or the related mechanisms in preclinical or clinical studies, besides a previous preclinical study [Kim et al. 2010]. Therefore, further studies are needed to draw a conclusion about it.
ED is a common complication in patients with SCI. Thus, owing to the overwhelming interest patients with SCI have in sexual function, it is important for clinicians working with this population to be aware of the treatment options (such as PDE5Is) available to improve ED [Rizio et al. 2012]. According to the previous studies, sildenafil, vardenafil and tadalafil all have demonstrated efficacy in treating ED in men with SCI, without any serious adverse effects [Del Popolo et al. 2004; Giuliano et al. 2007; Soler et al. 2007; Ergin et al. 2008]. A randomized controlled trial by Ergin and colleagues showed that sildenafil produced higher levels of successful sexual stimulation, successful intercourse rates (60–70% versus < 25%) and the positive response rates to GAQ (87.5% versus 50.0%), compared with placebo [Ergin et al. 2008]. Another randomized controlled trial by Giuliano and colleagues reported that the tadalafil treatment provided significantly greater improvements in the IIEF-EF domain scores (9.2 points versus 0.2 points), the positive responses to SEP2 (75.4% versus 41.1%), SEP3 (47.6% versus 16.8%), GAQ (84.6% versus 19.5%) and ejaculatory frequency (assessed by the IIEF question 9), compared with placebo [Giuliano et al. 2007]. A previous randomized, blinded, crossover clinical trial comparing sildenafil with tadalafil for ED in SCI patients by Del Popolo and colleagues showed that tadalafil significantly increased the percentage of successful intercourse attempts (67.9%) at post-dose 24 hours, compared with 17.9% with sildenafil, while no significant differences were observed in up to 12 hours [Del Popolo et al. 2004]. Interestingly, a previous clinical study comparing the treatment efficacy for ED among sildenafil, vardenafil and tadalafil in SCI patients by Soler and colleagues showed that all of the three PDE5Is significantly improved the IIEF-EF domain scores compared with baseline, and a good rigidity (rigidity enough for penetration) was reported in 85% of the patients on sildenafil, 74% of the patients on vardenafil and 72% of the patients on tadalafil [Soler et al. 2007]. Also, the mean duration of erection in the patients on sildenafil, vardenafil and tadalafil was 34, 28 and 26 minutes, respectively [Soler et al. 2007]. In accordance with the above-mentioned results, a preclinical study using a rabbit model of acute SCI for comparing the effect of mirodenafil on erectile response with that of sildenafil showed that both mirodenafil and sildenafil produced erectile responses [Jung et al. 2008]. Interestingly, the onset of erection with mirodenafil was faster than with sildenafil, and the erectogenic potential of mirodenafil was significantly better than that of sildenafil at the same dose. Thus, mirodenafil might be a useful option for the treatment of ED secondary to SCI, although further well designed, comparative, clinical trials are needed.
The association between ED and LUTS has biologic plausibility given the interrelationships of the known pathophysiologic mechanisms of the two conditions. Although the pathophysiologic mechanisms for the relationship between LUTS and ED in men remains to be fully elucidated, the NO/NOS pathway, endothelin-1, autonomic overactivity, Rho-kinase activity and autonomic adrenergic hyperactivity have been suggested as common links [Gacci et al. 2012]. A recent meta-analysis of available prospective and cross-sectional studies on the use of PDE5Is alone or in combination with α1-adrenergic blockers in patients with LUTS/BPH showed that they significantly improved LUTS and erectile function [Gacci et al. 2012]. Also, co-administered α1-blockers and PDE5I significantly improved the IIEF score, total IPSS and Qmax compared with α1-blockers alone, without a significant increase in TAEs [Gacci et al. 2012]. In accordance with the data, the two multicenter, open-label, prospective, noncomparative studies showed that the addition of mirodenafil to α1-blockers significantly improved the IIEF-5 and total IPSS at the end of the study compared with baseline (α1-blockers alone), without any serious TAEs [Lee et al. 2011a; Bang et al. 2013]. The clinical trial by Bang and colleagues showed an improvement in Qmax at the end of the study after the co-administration [Bang et al. 2013], whereas the study by Lee and colleagues did not [Lee et al. 2011a]. Thus, the addition of PDE5I such as mirodenafil to an α1-blocker appears to further improve erectile function and LUTS without a significant increase in adverse effects, although the exact mechanism remains to be elucidated. In terms of the use of PDE5Is alone for the treatment of LUTS, Chung and colleagues showed that once-daily administration of 50 mg mirodenafil for 12 weeks alleviates the total IPSS, QOL index, subtotal voiding symptoms score of the IPSS and Qmax compared with placebo [Chung et al. 2013]. In accordance with this, McVary and colleagues revealed that once-daily administration of sildenafil 50–100 mg for 12 weeks significantly improved the total IPSS, QOL index and Benign Prostatic Hyperplasia Impact Index compared with placebo [McVary et al. 2007]. Despite the short half-lives of mirodenafil or sildenafil, the studies showed the positive results in the management of LUTS. They suggested some possible mechanisms for the findings as follows. (i) Zhao and colleagues reported a significantly higher concentration of PDE5Is in prostatic tissue compared to plasma at 1 hour after the administration in patients who underwent transurethral prostatectomy for BPH [Zhao et al. 2011]. (ii) As the concentrations of mirodenafil in the corpus cavernosum were higher than of those in the plasma, effects of once-daily treatment cannot be determined by just measuring the blood pharmacokinetics of maximum concentration and T1/2 of the agent; it is possible that PDE5Is may also affect the body at levels lower than the minimal effective concentration. (iii) Once-daily treatment with agents that have short half-lives can be effective due to pharmacodynamic factors such as drug distribution and reabsorption. However, further well-designed studies are necessary to draw a definite conclusion about it.
According to the recent meta-analysis for oral PDE5Is, the adverse events caused by PDE5Is were generally mild with flushing, headache and dyspepsia being the most common [Yuan et al. 2013]. Consistent with this, the TAEs caused by mirodenafil were generally mild to moderate in severity, with flushing and headache being most common [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013]. Furthermore, concomitant use of mirodenafil with antihypertensive medications or α1-blockers did not cause significant hemodynamic changes such as SBP/DBP and HR during the period of the studies [Paick et al. 2010; Lee et al. 2011a; Bang et al. 2013]. Thus, most studies on mirodenafil suggest that it is well tolerated and safe in a broad spectrum of patient populations, including those with hypertension and those who are taking an α1-blocker [Paick et al. 2008a, 2008b, 2010; Park et al. 2010; Chung et al. 2013]. Given the 10-fold higher potency of mirodenafil over sildenafil and the higher selectivity ratios of mirodenafil for PDE5 over the other PDE isoforms compared with those of other existing PDE5Is (sildenafil, vardenafil and tadalafil) [Shin et al. 2006; Francis and Corbin, 2003], the tolerability of mirodenafil is expected to be better than those of the other existing PDE5Is. In accordance with this, the overall incidence of TAEs caused by mirodenafil (24.3–37.0%) appears to be somewhat lower than those by sildenafil (45.0%), vardenafil (41.5%) and tadalafil (44.0%), according to a meta-analysis by Tsertsvadze and colleagues [Paick et al. 2008a, 2010; Tsertsvadze et al. 2009]. Also, there has been no report of serious cardiovascular events associated with mirodenafil, compared with sildenafil (0.5%), vardenafil (0.2%) and tadalafil (0.3%) [Tsertsvadze et al. 2009]. Unlike sildenafil (3.6%), there has been no report of visual disturbance caused by mirodenafil. Furthermore, the incidence of myalgia or back pain associated with mirodenafil was negligible, compared with tadalafil (4.0–5.0%) [Tsertsvadze et al. 2009]. Thus, the tolerability profiles of mirodenafil in the treatment of ED appear to be somewhat better than those of the other existing PDE5Is due to the differences in pharmacodynamic profiles. However, because it is largely unproven due to the absence of head-to-head studies, more well designed studies of mirodenafil as well as comparative studies among different classes of PDE5Is are needed to validate it.
It has been reported that a significant portion of men quit taking currently available PDE5Is due to various reasons, such as high cost, adverse effects and lack of efficacy [Hanson-Divers et al. 1998; Souverein et al. 2002; Gonzalgo et al. 2003]. Therefore, it may be suggested that room for improvement still exists in the treatment of ED. Also, besides efficacy, tolerability and preference for a medication could be determined by other factors such as mode of action, food interaction and cost [Paick et al. 2008b]. Therefore, the addition of new PDE5Is such as mirodenafil that have various pharmacologic profiles would benefit some men who were dissatisfied with the pre-existing PDE5Is. However, there is a lack of data on the outcomes of mirodenafil for the treatment of ED in difficult-to-treat populations such as patients with post-prostatectomy ED. Also, there has been a scarcity of clinical data on efficacy and tolerability of mirodenafil for the treatment of ED in ethnic populations other than Korean populations. Furthermore, there have been no long-term studies of mirodenafil in the treatment of ED. Lastly, the fixed-dosing schedules applied in the published studies can limit the treatment outcomes of mirodenafil reported in the studies, because a flexible-dosing regimen could result in different treatment outcomes. Thus, head-to-head comparative studies of different PDE5Is as well as well designed clinical studies with larger cohorts or different dosing regimens or long-term follow up are necessary to confirm the favorable efficacy and tolerability profiles reported in the available clinical trials.
Conclusions
Mirodenafil has a high affinity for PDE5 and high selectivity for PDE5 over other PDE isoforms, compared with other existing PDE5Is. Accordingly, most of the available evidence has reported that mirodenafil effectively improves erectile function in a broad range of patient populations with ED that is consistent with results from the published studies on other PDE5Is. Along with other available PDE5Is in clinical use, mirodenafil also appears to offer favorable efficacy and tolerability in the treatment of diabetic ED, a traditional difficult-to-treat population. Mirodenafil has been reported to be effective, safe and well tolerated, even in patients with both ED and hypertension (HTN) who are taking concomitant antihypertensive medications. Furthermore, although mirodenafil has a short T1/2, it might be a helpful option for chronic dosing in the treatment of ED, as supported by favorable outcomes. Mirodenafil, either alone or in combination with an α1-blocker, can improve erectile function, as well as LUTS, without any significant adverse effects, although further randomized, placebo-controlled, comparative studies with different dosing schedules are necessary to confirm it. Due to the pharmacodynamic profiles of mirodenafil, its tolerability is expected to be somewhat better than those of the other PDE5Is.Thus, mirodenafil can be a useful treatment option in a variety of patient populations with ED. Further well designed research is still needed to validate the favorable efficacy and tolerability profiles of mirodenafil and to contribute to determination of the best tailored therapy for ED.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Min Chul Cho and Jae-Seung Paick have no conflicts of interest such as speaker fees. Jae-Seung Paick was one of the authors of the three clinical trials of mirodenafil cited in this article [Paick et al. 2008a, 2008b, 2010].
Contributor Information
Min Chul Cho, Department of Urology, Seoul National University Boramae Medical Center, Seoul, Korea.
Jae-Seung Paick, Department of Urology, Seoul National University College of Medicine, 28, Yongon-Dong, Chongno-Ku, Seoul 110-744, Korea.
References
- Ayta I., McKinlay J., Krane R. (1999) The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 84: 50–56. [DOI] [PubMed] [Google Scholar]
- Bang W., Oh C., Yoo C., Cho J., Yang D., Lee D., et al. (2013) Efficacy and safety of the simultaneous administration of mirodenafil and an α-blocker in men with BPH-LUTS: a multicenter open-label prospective study. Int J Impot Res 25: 149–154. [DOI] [PubMed] [Google Scholar]
- Barazani Y., Stahl P., Nagler H., Stember D. (2015) Is there a rationale for penile rehabilitation following radical prostatectomy? Am J Mens Health 9: 35–40. [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D., Gorny D., Mevel K., Caisey S., Bernabe J., Burgess G., et al. (2005) Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol 47: 87–91. [DOI] [PubMed] [Google Scholar]
- Billups K. (2005) Sexual dysfunction and cardiovascular disease: integrative concepts and strategies. Am J Cardiol 96: 57M–61M. [DOI] [PubMed] [Google Scholar]
- Bischoff E. (2004) Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 16: S11–S14. [DOI] [PubMed] [Google Scholar]
- Blonde L. (2006) Sildenafil citrate for erectile dysfunction in men with diabetes and cardiovascular risk factors: a retrospective analysis of pooled data from placebo-controlled trials. Curr Med Res Opin 22: 2111–2120. [DOI] [PubMed] [Google Scholar]
- Brown J., Wessells H., Chancellor M., Howards S., Stamm W., Stapleton A., et al. (2005) Urologic complications of diabetes. Diabetes Care 28: 177–185. [DOI] [PubMed] [Google Scholar]
- Bruzziches R., Francomano D., Gareri P., Lenzi A., Aversa A. (2013) An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother 14: 1333–1344. [DOI] [PubMed] [Google Scholar]
- Cho D., Bae S., Shon J., Bae S. (2013) High-sensitive LC-MS/MS method for the simultaneous determination of mirodenafil and its major metabolite, SK-3541, in human plasma: application to microdose clinical trials of mirodenafil. J Sep Sci 36: 840–848. [DOI] [PubMed] [Google Scholar]
- Cho M., Paick J. (2014) Udenafil for the treatment of erectile dysfunction. Ther Clin Risk Manag 10: 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Lee Y., Bae S., Kim T., Lee B., Lee M. (2009) Dose-dependent pharmacokinetics and first-pass effects of mirodenafil, a new erectogenic, in rats. Biopharm Drug Dispos 30: 305–317. [DOI] [PubMed] [Google Scholar]
- Choi Y., Lee Y., Lee M., Kim T., Lee B. (2010) Pharmacokinetics of mirodenafil, a new erectogenic, and its metabolite, SK3541, in rats: Involvement of CYP1A1/2, 2B1/2, 2D subfamily, and 3A1/2 for the metabolism of both mirodenafil and SK3541. J Pharm Pharmaceut Sci 13: 93–106. [DOI] [PubMed] [Google Scholar]
- Chung J., Kang D., Oh C., Chung J., Lee K., Kim T., et al. (2013) Safety and efficacy of once daily administration of 50 mg mirodenafil in patients with erectile dysfunction: a multicenter, double-blind, placebo controlled trial. J Urol 189: 1006–1013. [DOI] [PubMed] [Google Scholar]
- Corbin J., Francis S. (2002) Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract 56: 453–459. [PubMed] [Google Scholar]
- Corona G., Mondaini N., Ungar A., Razzoli E., Rossi A., Fusco F. (2011) Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: The proper drug for the proper patient. J Sex Med 8: 3418–3432. [DOI] [PubMed] [Google Scholar]
- Del Popolo G., Li Marzi V., Mondaini N., Lombardi G. (2004) Time/duration effectiveness of sildenafil versus tadalafil in the treatment of erectile dysfunction in male spinal cord-injured patients. Spinal Cord 42: 643–648. [DOI] [PubMed] [Google Scholar]
- Du W., Li J., Fan N., Shang P., Wang Z., Ding H. (2014) Efficacy and safety of mirodenafil for patients with erectile dysfunction: a meta-analysis of three multicenter, randomized, double-blind, placebo-controlled clinical trials. Aging Male 17: 107–111. [DOI] [PubMed] [Google Scholar]
- Eardley I., Donatucci C., Corbin J., El-Meliegy A., Hatzimouratidis K., McVary K., et al. (2010) Pharmacotherapy for erectile dysfunction. J Sex Med 7: 524–540. [DOI] [PubMed] [Google Scholar]
- Ergin S., Gunduz B., Ugurlu H., Sivrioglu K., Oncel S., Gok H., et al. (2008) A placebo-controlled, multicenter, randomized, double-blind, flexible-dose, two-way crossover study to evaluate the efficacy and safety of sildenafil in men with traumatic spinal cord injury and erectile dysfunction. J Spinal Cord Med 31: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S., Corbin J. (2003) Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep 4: 457–465. [DOI] [PubMed] [Google Scholar]
- Gacci M., Corona G., Salvi M., Vignozzi L., McVary K., Kaplan S., et al. (2012) A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 61: 994–1003. [DOI] [PubMed] [Google Scholar]
- Giuliano F., Sanchez-Ramos A., Löchner-Ernst D., Del Popolo G., Cruz N., Leriche A., et al. (2007) Efficacy and safety of tadalafil in men with erectile dysfunction following spinal cord injury. Arch Neurol 64: 1584–1592. [DOI] [PubMed] [Google Scholar]
- Goldstein I., Young J., Fischer J., Bangerter K., Segerson T., Taylor T. (2003) Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care 26: 777–783. [DOI] [PubMed] [Google Scholar]
- Gonzalgo M., Brotzman M., Trock B., Geringer A., Burnett A., Jarow J. (2003) Clinical efficacy of sildenafil citrate and predictors of long-term response. J Urol 170: 503–506. [DOI] [PubMed] [Google Scholar]
- Hanson-Divers C., Jackson S., Lue T., Crawford S., Rosen R. (1998) Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol 159: 1541–1547. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Amar E., Eardley I., Giuliano F., Hatzichristou D., Montorsi F., et al. (2010) Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 57: 804–814. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Hatzichristou D. (2009) Erectile dysfunction and diabetes mellitus. Insulin 4: 114–122. [Google Scholar]
- Hatzimouratidis K., Hatzichristou D. (2014) How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep 14: 545–554. [DOI] [PubMed] [Google Scholar]
- Jung J., Kim S., Kim B., Lee S., Park Y., Kim S., et al. (2008) The penile erection efficacy of a new phosphodiesterase type 5 inhibitor, mirodenafil (SK3530), in rabbits with acute spinal cord injury. J Vet Med Sci 70: 1199–1204. [DOI] [PubMed] [Google Scholar]
- Kim B., Yi S., Kim J., Lim K., Kim K., Lee B., et al. (2009) Influence of alcohol on the hemodynamic effects and pharmacokinetic properties of mirodenafil: a single-dose, randomized-sequence, open-label, crossover study in healthy male volunteers in Korea. Clin Ther 31: 1234–1243. [DOI] [PubMed] [Google Scholar]
- Kim H., Sohn D., Kim S., Hong S., Suh H., Lee C., et al. (2010) The effect of mirodenafil on the penile erection and corpus cavernosum in the rat model of cavernosal nerve injury. Int J Impot Res 22: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Han D., Lim S., Kim T., Chae M., Chung K., et al. (2011) Effects of Ginkgo biloba extracts with mirodenafil on the relaxation of corpus cavernosal smooth muscle and the potassium channel activity of corporal smooth muscle cells. Asian J Androl 13: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner R., Brown M., Prisant L., Collins M. (2001) Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Sildenafil Study Group. Am J Hypertens 14: 70–73. [DOI] [PubMed] [Google Scholar]
- Kloner R., Sadovsky R., Johnson E., Mo D., Ahuja S. (2005) Efficacy of tadalafil in the treatment of erectile dysfunction in hypertensive men on concomitant thiazide diuretic therapy. Int J Impot Res 17: 450–454. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos A., Giannitsas K., Athanasopoulos A., Spathas D., Perimenis P. (2009) The impact of daily sildenafil on levels of soluble molecular markers of endothelial function in plasma in patients with erectile dysfunction. Expert Opin Pharmacother 10: 155–160. [DOI] [PubMed] [Google Scholar]
- Kubin M., Wagner G., Fugl-Meyer A. (2003) Epidemiology of erectile dysfunction. Int J Impot Res 15: 63–71. [DOI] [PubMed] [Google Scholar]
- Kupelian V., Shabsigh R., Araujo A., O’Donnell A., McKinlay J. (2006) Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study.J Urol 176: 222–226. [DOI] [PubMed] [Google Scholar]
- Lee H., Park E., Ji H., Kim S., Im G., Lee S., et al. (2008) Identification of cytochrome P450 enzymes responsible for N-dealkylation of a new oral erectogenic, mirodenafil. Xenobiotica 38: 21–33. [DOI] [PubMed] [Google Scholar]
- Lee J., Chae M., Park J., Jeon J., Lee S. (2012) The effects of the combined use of a PDE5 inhibitor and medications for hypertension, lower urinary tract symptoms and dyslipidemia on corporal tissue tone. Int J Impot Res 24: 221–227. [DOI] [PubMed] [Google Scholar]
- Lee J., Cho S., Oh C., Ha U., Lee S., Park S., et al. (2011a) Efficacy and safety of combination therapy with mirodenafil and α1-blocker for benign prostatic hyperplasia-induced lower urinary tract symptoms accompanied by erectile dysfunction: a multicenter, open-label, prospective study. Int J Impot Res 23: 249–256. [DOI] [PubMed] [Google Scholar]
- Lee J., Yoo H., Rhim K., Sohn D., Kim D. (2007) Metabolism and excretion of 5-ethyl-2-{5-[4-(2-hydroxy-ethyl)-piperazine-1-sulfonyl]-2-propoxy-phenyl}-7-propyl-3,5-dihydro-pyrrolo[3,2-d]pyrimidin-4-one (SK3530) in rats. Rapid Communications in Mass Spectrometry 21: 1139–1149. [DOI] [PubMed] [Google Scholar]
- Lee S., Kim Y., Kim T., Im G., Lee B., Kim D., et al. (2009) Determination of mirodenafil and sildenafil in the plasma and corpus cavernous of SD male rats. J Pharm Biomed Anal 49: 513–518. [DOI] [PubMed] [Google Scholar]
- Lee Y., Choi Y., Kim T., Ryu K., Lee B., Lee M. (2010) Pharmacokinetics of mirodenafil and its two metabolites, SK3541 and SK3544, after intravenous and oral administration of mirodenafil to streptozotocin-induced diabetes mellitus rats. Xenobiotica 40: 129–137. [DOI] [PubMed] [Google Scholar]
- Lee Y., Choi Y., Yoon I., Kim T., Ryu K., Lee B., et al. (2011b) Pharmacokinetics of mirodenafil and its two metabolites, SK3541 and SK3544, in spontaneously or DOCA-salt-induced hypertensive rats. Biopharm Drug Dispos 32: 38–49. [DOI] [PubMed] [Google Scholar]
- McVary K. (2007) Clinical practice. Erectile dysfunction. N Engl J Med 357: 2472–2481. [DOI] [PubMed] [Google Scholar]
- McVary K., Carrier S., Wessells H. (2001) Subcommittee on Smoking and Erectile Dysfunction Socioeconomic Committee, Sexual Medicine Society of North America. Smoking and erectile dysfunction: evidence-based analysis. J Urol 166: 1624–1632. [PubMed] [Google Scholar]
- McVary K., Monnig W., Camps J., Jr, Young J., Tseng L., van den Ende G. (2007) Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 177: 1071–1077. [DOI] [PubMed] [Google Scholar]
- Montague D., Jarow J., Broderick G., Dmochowski R., Heaton J., Lue T., et al. (2005) Erectile Dysfunction Guideline Update Panel. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol 174: 230–239. [DOI] [PubMed] [Google Scholar]
- Moon D., Yang D., Lee C., Ahn T., Min K., Park K., et al. (2011) A therapeutic confirmatory study to assess the safety and efficacy of Zydena (udenafil) for the treatment of erectile dysfunction in male patients with diabetes mellitus. J Sex Med 8: 2048–2061. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (1993) Consensus development conference statement. Impotence. December 7–9, 1992. Int J Impot Res 5: 181–284. [PubMed] [Google Scholar]
- Noh Y., Lim H., Cho S., Ghim J., Choe S., Jung J., et al. (2012) Assessment of the influence of severe renal impairment on the pharmacokinetics of mirodenafil in Korean male volunteers. Int J Clin Pharmacol Ther 50: 880–888. [DOI] [PubMed] [Google Scholar]
- Paick J., Ahn T., Choi H., Chung W., Kim J., Kim S., et al. (2008a) Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med 5: 2672–2680. [DOI] [PubMed] [Google Scholar]
- Paick J., Choi H., Kim S., Ahn T., Kim J., Park J., et al. (2008b) Efficacy and safety of oral SK3530 for the treatment of erectile dysfunction in Korean men: a multicenter, randomized, double-blind, placebo-controlled, fixed dose, parallel group clinical trial. Asian J Androl 10: 791–798. [DOI] [PubMed] [Google Scholar]
- Paick J., Kim J., Kim S., Moon K., Min K., Park K., et al. (2010) Efficacy and safety of mirodenafil in men taking antihypertensive medications. J Sex Med 7: 3143–3152. [DOI] [PubMed] [Google Scholar]
- Paick J., Kim S., Park Y., Hyun J., Park N., Lee S., et al. (2009) The efficacy and safety of udenafil [Zydena] for the treatment of erectile dysfunction in hypertensive men taking concomitant antihypertensive agents. J Sex Med 6: 3166–3176. [DOI] [PubMed] [Google Scholar]
- Palit V., Eardley I. (2010) An update on new oral PDE5 inhibitors for the treatment of erectile dysfunction. Nat Rev Urol 7: 603–609. [DOI] [PubMed] [Google Scholar]
- Park H., Choi H., Ahn T., Park J., Chung W., Lee S., et al. (2010) Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Sex Med 7: 2842–2850. [DOI] [PubMed] [Google Scholar]
- Park K., Cho S., Kim S. (2011) Erectile response to type 5 phosphodiesterase inhibitor could be preserved with the addition of simvastatin to conventional insulin treatment in rat model of diabetes. Int J Androl 34: e468–e474. [DOI] [PubMed] [Google Scholar]
- Park K., Ryu K., Li W., Kim S., Paick J. (2008) Chronic treatment with a type 5 phosphodiesterase inhibitor suppresses apoptosis of corporal smooth muscle by potentiating Akt signalling in a rat model of diabetic erectile dysfunction. Eur Urol 53: 1282–1288. [DOI] [PubMed] [Google Scholar]
- Porst H., Giuliano F., Glina S., Ralph D., Casabé A., Elion-Mboussa A., et al. (2006) Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5 mg and 10 mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol 50: 351–359. [DOI] [PubMed] [Google Scholar]
- Porst H., Rajfer J., Casabé A., Feldman R., Ralph D., Vieiralves L., et al. (2008) Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med 5: 2160–2169. [DOI] [PubMed] [Google Scholar]
- Rizio N., Tran C., Sorenson M. (2012) Efficacy and satisfaction rates of oral PDE5is in the treatment of erectile dysfunction secondary to spinal cord injury: a review of literature. J Spinal Cord Med 35: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz de Tejada I., Anglin G., Knight J., Emmick J. (2002) Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care 25: 2159–2164. [DOI] [PubMed] [Google Scholar]
- Shabsigh R., Duval S., Shah M., Regan T., Juhasz M., Veltry L. (2007) Efficacy of vardenafil for the treatment of erectile dysfunction in men with hypertension: a meta-analysis of clinical trial data. Curr Med Res Opin 23: 2453–2460. [DOI] [PubMed] [Google Scholar]
- Shamloul R., Ghanem H. (2013) Erectile dysfunction. Lancet 381: 153–165. [DOI] [PubMed] [Google Scholar]
- Shin B., Hu S., Kim J., Oh J., Youn W., Lee B., et al. (2007) Development of LC/MS/MS assay for the determination of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one (SK3530) in human plasma: application to a clinical pharmacokinetic study. J Pharm Biomed Anal 45: 176–184. [DOI] [PubMed] [Google Scholar]
- Shin H., Lee J., Kim D. (2006) Synthesis of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazin-1-ylsulfonyl]-2-npropoxyphenyl}-7-n-propyl-3,5-dihydro-4Hpyrrolo[3,2-d]-[2–14C]pyrimidin-4-one·2HCl (14C-SK3530.2 HCl). J Labelled Comp Radiopharm 49: 1141–1149. [Google Scholar]
- Shin K., Kim B., Kim T., Kim J., Yi S., Yoon S., et al. (2009) The effects of ketoconazole and rifampicin on the pharmacokinetics of mirodenafil in healthy Korean male volunteers: an open-label, one-sequence, three-period, three-treatment crossover study. Clin Ther 31: 3009–3020. [DOI] [PubMed] [Google Scholar]
- Soler J., Previnaire J., Denys P., Chartier-Kastler E. (2007) Phosphodiesterase inhibitors in the treatment of erectile dysfunction in spinal cord-injured men. Spinal Cord 45: 169–173. [DOI] [PubMed] [Google Scholar]
- Souverein P., Egberts A., Meuleman E., Urquhart J., Leufkens H. (2002) Incidence and determinants of sildenafil (dis)continuation: The Dutch cohort of sildenafil users. Int J Impot Res 14: 259–265. [DOI] [PubMed] [Google Scholar]
- Tsertsvadze A., Fink H., Yazdi F., MacDonald R., Bella A., Ansari M., et al. (2009) Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med 151: 650–661. [DOI] [PubMed] [Google Scholar]
- van Ahlen H., Wahle K., Kupper W., Yassin A., Reblin T., Neureither M. (2005) Safety and efficacy of vardenafil, a selective phosphodiesterase 5 inhibitor, in patients with erectile dysfunction and arterial hypertension treated with multiple antihypertensives. J Sex Med 2: 856–864. [DOI] [PubMed] [Google Scholar]
- Vardi M., Nini A. (2007) Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst Rev: CD002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H., Kim N., Im G., Kim D. (2007) Pharmacokinetics and tissue distribution of a novel PDE5 inhibitor, SK-3530, in rats. Acta Pharmacol Sin 28: 1247–1253. [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhang R., Yang Z., Lee J., Liu Y., Tian J., et al. (2013) Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 63: 902–912. [DOI] [PubMed] [Google Scholar]
- Zhao C., Kim S., Lee S., Jeon J., Kang K., Choi S., et al. (2011) Activity of phosphodiesterase type 5 inhibitors in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int 107: 1943–1947. [DOI] [PubMed] [Google Scholar]