Researchers studied the mechanism by which the anticonvulsant Retigabine enhances the activity of K+ channels and found that the drug stabilizes on open state at resting membrane potentials.
Abstract
The anticonvulsant Retigabine is a KV7 channel agonist used to treat hyperexcitability disorders in humans. Retigabine shifts the voltage dependence for activation of the heteromeric KV7.2/KV7.3 channel to more negative potentials, thus facilitating activation. Although the molecular mechanism underlying Retigabine’s action remains unknown, previous studies have identified the pore region of KV7 channels as the drug’s target. This suggested that the Retigabine-induced shift in voltage dependence likely derives from the stabilization of the pore domain in an open (conducting) conformation. Testing this idea, we show that the heteromeric KV7.2/KV7.3 channel has at least two open states, which we named O1 and O2, with O2 being more stable. The O1 state was reached after short membrane depolarizations, whereas O2 was reached after prolonged depolarization or during steady state at the typical neuronal resting potentials. We also found that activation and deactivation seem to follow distinct pathways, suggesting that the KV7.2/KV7.3 channel activity displays hysteresis. As for the action of Retigabine, we discovered that this agonist discriminates between open states, preferentially acting on the O2 state and further stabilizing it. Based on these findings, we proposed a novel mechanism for the therapeutic effect of Retigabine whereby this drug reduces excitability by enhancing the resting potential open state stability of KV7.2/KV7.3 channels. To address this hypothesis, we used a model for action potential (AP) in Xenopus laevis oocytes and found that the resting membrane potential became more negative as a function of Retigabine concentration, whereas the threshold potential for AP firing remained unaltered.
INTRODUCTION
The heteromeric KV7.2/KV7.3 channel is the assembly of KV7 channels most commonly found in the central nervous system (Jentsch, 2000; Cooper, 2012). The activity of this heteromeric channel gives rise to the K+ current that is suppressed by muscarinic signaling and is known as the M current (Wang et al., 1998; Jentsch, 2000). M currents are critical in controlling excitability in central nervous system neurons (Brown and Adams, 1980; Wang et al., 1998; Jentsch, 2000; Cooper, 2012; Linley et al., 2012; Soh et al., 2014; Mastrangelo, 2015; Miceli et al., 2015a). Indeed, mutations that detrimentally affect the function of neuronal KV7 channels cause hyperexcitability syndromes such as benign familial neonatal seizures (Charlier et al., 1998; Singh et al., 1998), early onset epileptic encephalopathy (Abidi et al., 2015), and peripheral nerve hyperexcitability (Dedek et al., 2001; Wuttke et al., 2007). Yet, the molecular basis for the role of KV7 channels in the development of these disorders remains unclear.
Epilepsy is a family of encephalopathies characterized by abnormal synchronous and rhythmic neuronal activity in the brain that results in seizures. According to the Centers for Disease Control and Prevention (CDC), ∼1% of children aged 0–17 yr and 1.8% of adults aged 18 yr or older have had a diagnosis of epilepsy or seizure disorder in 2013. This accounts for over five million people in the United States, with an estimate of 56% of these identified cases being active epileptic patients according to CDC estimates. Epileptic disorders are life changing because they can cause learning disabilities and abnormal development in children. Epileptic events can also be life threatening because tonic–clonic (convulsive) seizures can cause out-of-control muscular contractions resulting in injuries.
Epilepsy has a large genetic component (Cooper, 2012; Mastrangelo, 2015). However, some epileptic disorders, such as benign familial neonatal seizures, are monogenic with causative mutations in the KCNQ2 or KCNQ3 genes alone (Charlier et al., 1998; Singh et al., 1998; Wang et al., 1998; Wuttke et al., 2007; Miceli et al., 2011, 2015a,b; Cooper, 2012; Abidi et al., 2015). In these cases, electrical hyperexcitability in neurons is caused by ill-performing M currents (Brown and Passmore, 2009; Maljevic et al., 2010; Cooper, 2012). To compensate for this deficiency, pharmacotherapeutic approaches have been focused on using drugs that boost the activity of KV7 channels (Maljevic et al., 2010). Examples of KV7 agonists are Retigabine (Main et al., 2000; Rundfeldt and Netzer, 2000b), Pyrithione Zinc (Xiong et al., 2007; Linley et al., 2012), ICA-27243 (Wickenden et al., 2008), and Diclofenac (Peretz et al., 2005). Retigabine is a first-in-class anticonvulsant that is currently used in the United States to treat epileptic disorders in adults (Orhan et al., 2012). This drug increases activity in Kv7 channels, except for KV7.1 (Wickenden et al., 2000, 2008; Peretz et al., 2005; Wuttke et al., 2005; Gunthorpe et al., 2012). This latter property makes Retigabine an attractive drug for the treatment of neurological disorders, as no effect on cardiac activity is expected.
Detailed understanding of both the physiological behavior and the molecular basis for the activity of neuronal KV7 channels is needed for further refinement of the available pharmacotherapy for KV7-related encephalopathies, as well as for the design of new ones. In addressing this need, we have discovered that the deactivation kinetics of the heterotetrameric KV7.2/KV7.3 channel slows down as a function of activity. The changes in the deactivation kinetics occurred in at least two stages, revealing the existence of two open states, with the second open state being more stable than the initial one. Further, we found that Retigabine’s action is state dependent, preferentially targeting channels in the more stable open state. Furthermore, we found that channels that are opened at the typical neuronal resting potential also displayed two modes of activity with distinct deactivation kinetics and sensitivity to Retigabine. These combined observations led us to propose that, although Retigabine can change the voltage dependence of activation for KV7 channels, the stabilization of the channels that are already opened at neuronal resting potential levels is the clinically relevant effect of this anticonvulsant.
MATERIALS AND METHODS
Electrophysiology
Defolliculated Xenopus laevis oocytes were injected with in vitro–transcribed cRNA encoding for the human KV7.2 and KV7.3 channels in the vector pTLN. The KV7 constructs were provided by T. Jentsch (Leibniz-Institut für Molekulaire Pharmakologie, Berlin, Germany). These constructs were linearized with MluI and HpaI, respectively (New England Biolabs, Inc.). The linearized DNA was transcribed using SP6 RNA polymerase (mMessage mMachine; Ambion). Potassium currents were recorded using the Xenopus oocyte cut-open voltage-clamp technique using an amplifier (CA-1A; Dagan Corporation). The external recording solution contained (in mM) 12 KOH, 88 N-methly-d-glucamine, 100 methanesulfonic acid, 10 HEPES, and 2 Ca(OH)2. The intracellular solution contained (in mM) 100 KOH, 100 methanesulfonic acid, 10 HEPES, and 2 EGTA. Both solutions were titrated to pH 7.4 with methanesulfonic acid. Retigabine (Alomone Labs) was dissolved at 50 mM in DMSO. The external solution used for control and washouts was added with 0.002–0.02% (vol/vol) of DMSO to account for the potential effect of the solvent on the activity of the channels. Sharp glass electrodes (resistance = 0.2–1 MΩ) were filled with a solution containing (in mM) 1,000 KCl, 10 HEPES, and 10 EGTA, pH 7.4 (KOH).
Electrophysiological data were filtered at 100 kHz, oversampled at 250 kHz to 2 MHz, and stored for off-line analysis at 5–25 kHz. For these recordings, a custom LabVIEW-based package was used to control a multifunction data acquisition board (USB-6221 or USB-6251; National Instruments) for voltage command and electrical signal acquisition. Data were analyzed using a custom Java-based software and Origin2015 (OriginLab).
Oversampling
Oversampling is the process of sampling a signal at a frequency much higher than the natural frequency of the system. Electrophysiological data are typically acquired at frequencies in the kilohertz range. For our study, the acquisition frequency (fa) for currents or potential traces was 250 kHz to 2 MHz, after analogue filtering at 100 kHz. The sampling frequency for traces was 5–25 kHz. To decrease high frequency noise, sampled points were calculated by averaging segments of fa/fs points from the acquired data. The following example illustrates the process. When acquiring data at 1 MHz, we meant to obtain one acquired point every 1 µs; when sampling data at 10 kHz, we stored one data point every 100 µs. To sample at 10 kHz, we averaged 100-point segments from the original trace that was acquired at 1 MHz.
“Loose” two-electrode voltage-clamp technique
APs were recorded using a modified two-electrode voltage-clamp technique based on the implementation by Shapiro et al. (2012). In brief, the command voltage was set to the resting membrane potential of the oocytes, and the feedback gain was decreased to prevent voltage-clamping. In addition, a 50-MΩ resistor in parallel with a 10-MΩ in series with a diode was placed between the current-injecting microelectrode and the headstage. With the diode anode placed on the headstage side, this arrangement allowed us to inject current to depolarize the membrane and trigger APs, but not to repolarize it.
Exponential fits and weighted average time constant
As described in previous studies (Lacroix et al., 2011; Labro et al., 2012; Priest et al., 2013; Villalba-Galea, 2014), deactivating currents were fitted to a two-exponential function defined as follows:
where A1 and A2 are the current amplitude associated with each component and τ1 and τ2 are the corresponding time constants. The deactivation time constant (τDEACT) was calculated as
Fittings were done using Origin2015.
ITAIL–V plots fit to Boltzmann equations
To quantify the apparent voltage dependence for activation, ITAIL–V plots were fitted to a two-Boltzmann equation defined as
where A1 and A2 are the amplitude associated with each of the components, V1 and V2 are the half-activation potential for each component, z1 and z2 are the corresponding associated sensing charges, k is the Boltzmann constant, and T is absolute temperature. The weighted average half-maximum potential (weighted V1/2) was calculated using the following equation:
Fittings were done using Origin2015.
RESULTS
Stabilization of the open state of the KV7.2/KV7.3 channel
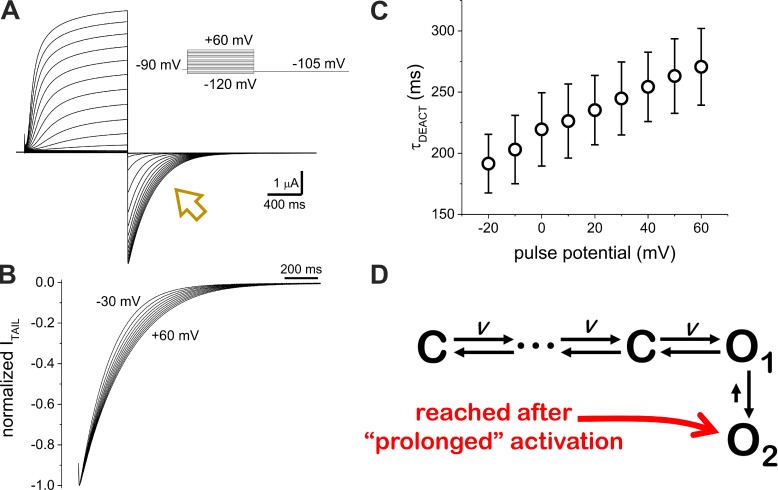
K+ currents were recorded from Xenopus oocytes co-injected with cRNA encoding for human KV7.2 and KV7.3 (Fig. 1 A). The activity of the expressed channels was assessed by applying test pulses ranging from −120 to +60 mV from a holding potential (HP) of −90 mV; K+ current amplitude readily increased when applying pulses of −50 mV and above. After activation, K+ currents were deactivated by applying a pulse to −105 mV (Fig. 1 A, arrow). Using this protocol, we recorded K+ currents that displayed the typical behavior voltage dependence and time-lagged activation of heteromeric KV7.2/KV7.3 channels (Fig. 1). Focusing our attention on the closing of the channels (Fig. 1 A, arrow), we noticed that the deactivation rate of the K+ current decreased as the magnitude of the test pulse increased. This feature was made evident when normalizing the K+ current traces, as we observed that the deactivation was slower when occurring from a stronger activating stimulus (Fig. 1 B). As previously reported in other studies (Lacroix et al., 2011; Labro et al., 2012; Priest et al., 2013; Villalba-Galea, 2014), we quantified the deactivation time constant (τDEACT) of the K+ current by calculating the weighted average time constant from the fitting of a two-exponential function to the deactivating current. The τDEACT versus test pulse potential (τDEACT–VTEST) plot (Fig. 1 C) showed an apparent linear dependence of 1.0 ± 0.2 ms (n = 8) in the deactivation time constant per unit of millivolt imposed during the activation, indicating that the stability of the activated channel increases as a function of the channel activation.
Figure 1.
K+ deactivation rate decreases as a function of activation. (A) K+ currents recorded from Xenopus oocytes coexpressing human KV7.2 and KV7.3 channels using the cut-open voltage-clamp technique. Test pulses ranging from −120 mV to +60 mV were applied from an HP of −90 mV. Deactivation of the current was driven at −105 mV (arrow). (B) Normalized deactivating currents from activating pulses from −30 to +60 mV. Deactivation slowed down after higher activating potentials. (C) Deactivation time constant (τDEACT) versus test pulse potential (τDEACT–VPULSE) plot. Error bars represent standard deviation. (D) Kinetics scheme describing alternative pathways for the deactivation of the heteromeric KV7.2/KV7.3 channel. Voltage-dependent transitions are noted with V.
In terms of stochastic discrete-state modeling of channel activity (Colquhoun and Hawkes, 1977), we discerned more than one τDEACT, which points to the existence of more than one open state. Also, the sigmoidal time course of the K+ current activation indicates that this process involves transitions through multiple closed states preceding channel opening. For this study, we assumed, therefore, that the activity of the heteromeric KV7.2/KV7.3 can be described by a sequential model consisting of a series of closed states leading to two open states, O1 and O2 (Fig. 1 D). In this case, deactivation for the open state O2 will be slower, as this process will involve one additional transition.
Effect of Retigabine on the deactivation of KV7.2/KV7.3
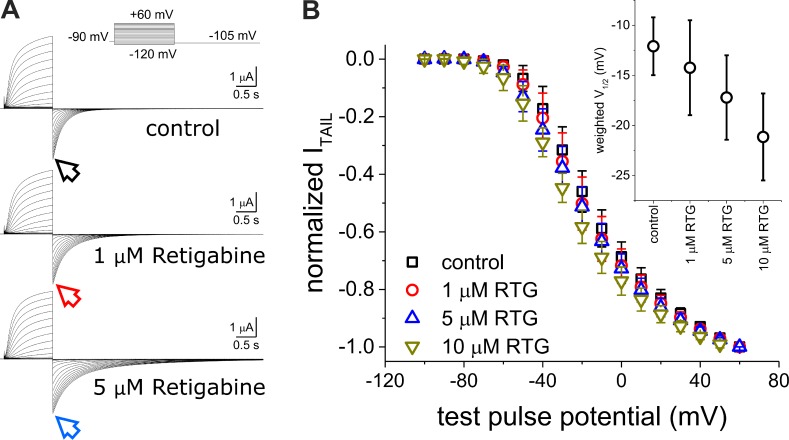
As before, we recorded K+ currents from oocytes co-injected with KV7.2 and KV7.3 from an HP of −90 mV. We applied 1.2-s pulses from −100 to +60 mV followed by a pulse to −105 mV to activate and deactivate the channels, respectively (Fig. 2 A). To assess the effect of Retigabine on the activation of KV7.2/KV7.3 channels, we plotted the normalized amplitude of the deactivating current (ITAIL) as a function of the activating potential (VPULSE). The normalized ITAIL–VPULSE plots showed a modest shift toward negative potential in the voltage dependence of channel activation as a function of the concentration of Retigabine (Fig. 2 B). In spite of this shift, Retigabine seems not to greatly affect activation kinetics (Fig. 2 A). In contrast, we observed that deactivation of the K+ current was unambiguously slower in the presence of the drug (Fig. 2 A, arrows), indicating that Retigabine was preferentially acting on open channels. Because KV7.2/KV7.3 possess at least two open states, we proceeded to investigate whether Retigabine was able to discriminate between them.
Figure 2.
Effect of Retigabine on the activity of the heteromeric KV7.2/KV7.3 channel. (A) K+ currents recorded in the absence or presence of 1–5-µM Retigabine using the same protocol shown in Fig. 1. (B) Average normalized K+ current amplitude measured at the beginning of the deactivating currents (ITAIL; arrows in A). Normalized ITAIL versus test pulse potential (ITAIL–VPULSE) plots incrementally shift toward negative potentials as the concentration of Retigabine (RTG) was increased. Individual ITAIL–VPULSE plots were fitted to a double Boltzmann distribution, and the weighted average half-maximum potential (weighted V1/2) was plotted as a function of the concentration of Retigabine (inset). Although the weighted V1/2 values were not statistically different, this parameter changed to more negative potentials as a function of the concentration of Retigabine in all our recordings. Error bars represent standard deviation. n = 5–8.
Retigabine preferentially binds to the open state O2
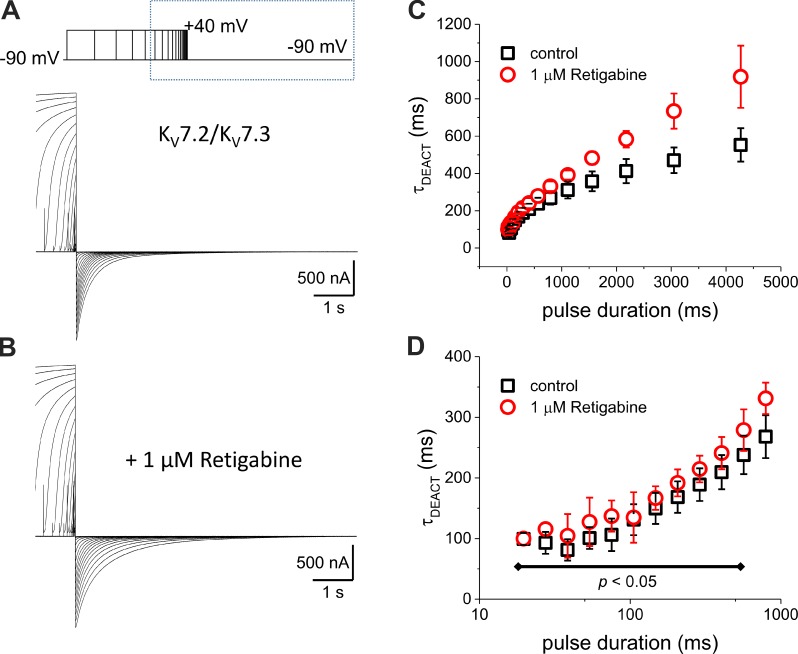
The activation of the KV7.2/KV7.3 channels can be increased by applying pulses of higher voltages, longer duration, or both. Thus, to further explore how deactivation is affected by Retigabine, we studied how τDEACT changes as a function of the duration of the depolarization (τDEACT–tPULSE plot). To do so, we recorded the deactivation of K+ current after activation by 40-mV pulses of variable duration (Fig. 3 A) and analyzed how τDEACT changed as a function of the duration of the activating pulse (tPULSE). We observed that deactivation became slower as the activating pulse duration increased (Fig. 3 C, black squares). We also noticed that τDEACT did not change for activating pulses shorter than 100 ms (Fig. 3 D, black squares), indicating that the stability of the conducting channel increased as a function of activation by depolarization lasting >100 ms. In terms of our initial model (Fig. 1 D), these observations indicated that within the first 100 ms of activation, the channels mainly populate state O1.
Figure 3.
Deactivation time constant increase as a function of the duration of the activating pulse. (A and B) K+ currents were activated by 40-mV pulses with a duration varying from 10 to 4,175 ms in the absence (A) and presence (B) of 1-µM Retigabine. The duration of the pulse increased 1.3-fold for each trace. After activation, the K+ current was deactivated at −90 mV. (C) τDEACT–tPULSE plot shows that τDEACT increased as the activating pulse was longer (black squares). In the presence of Retigabine, τDEACT increased further. (D) The same plot as in C, but the tPULSE is displayed in a logarithmic scale to highlight the τDEACT–tPULSE relationship for shorter activating pulses and showing that τDEACT was unaltered by Retigabine for pulses shorter than 500 ms. Error bars represent standard deviation. Control, n = 7; Retigabine, n = 6.
In the presence of 1-µM Retigabine (Fig. 3 B), deactivation became slower than in the absence of the drug (Fig. 3 C). Remarkably, Retigabine had no significant effect on the deactivation kinetics after activation pulses shorter than 500 ms. Therefore, we concluded that Retigabine stabilizes those channels in state O2.
Retigabine stabilizes channels in the open state at the resting potential
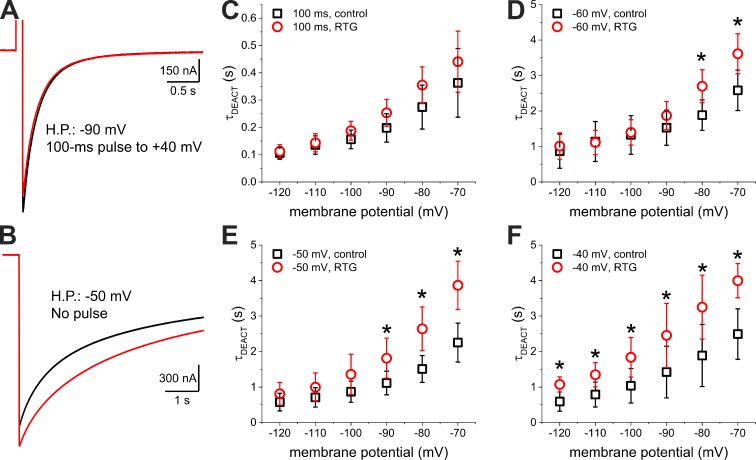
Thus far, we have shown that Retigabine only targets channels that have been activated for a few hundreds of milliseconds. However, the duration of a typical neuronal AP is in the order of a few milliseconds (Bean, 2007). So, we wondered how Retigabine can have any therapeutic effect if this drug is mainly affecting the second open state of the KV7.2/KV7.3 channel, which is reached after a prolonged, nonphysiological depolarization. In addressing this question, we observed that a 1.2-s pulse to −60 mV from an HP of −90 mV was able to activate 2 ± 2% (n = 10) and 6 ± 2% (n = 10) of the maximum KV7.2/KV7.3 conductance observed in our recordings in the absence and presence of 10-µM Retigabine, respectively (Fig. 2 B). Because a membrane potential of −60 mV is within the range of a typical neuronal resting potential (Bean, 2007), we hypothesized that this fraction of channels, which are opened in steady state at the resting potential, may be able to reach the open state O2. If so, these channels could constitute the therapeutically relevant target of Retigabine. To test this idea, we compared the deactivation kinetics of K+ currents at −90 mV from a pulse-activated open state and from a resting potential open state. To assess the first condition, we activated channels by applying a 100-ms pulse to 40 mV for an HP of −90 mV. To assess the second condition, we held the membrane potential at −50 mV. When driving deactivation at −90 mV, we observed that the pulse-activated K+ current deactivated faster (Fig. 4 A, black trace) than the resting potential–activated K+ current (Fig. 4 B, black trace). These observations revealed that the open state reached by the channels at steady-state resting potential was more stable than the open state reached after a 100-ms activating pulse. In light of these results, we then wondered whether this resting potential open state could be further stabilized by Retigabine. To test this, we used the same recording protocol, but in the presence of 1-µM Retigabine. Deactivation from the pulse-activated current was not significantly affected by the drug (Fig. 4 A, red trace). In contrast, the deactivation rate from the resting potential–activated current was unambiguously decreased by the drug (Fig. 4 B, red trace). This observation indicated that the stable open state reached at the typical neuronal resting potential was sensitive to Retigabine.
Figure 4.
Deactivation from the depolarized and resting potential open states. (A) Deactivation at −90 mV of the K+ current elicited by a 100-ms depolarization to 40 mV in the absence (black trace) and presence of 1-µM Retigabine (red trace). (B) Deactivation at −90 mV of the K+ current elicited by holding the membrane potential at −50 mV also in the absence (black trace) and presence of 1-µM Retigabine (red trace). (C–-F) Deactivation time constant (τDEACT) versus deactivating potential (τDEACT–VDEACT) plot of channels activated by a 100-ms depolarization to 40 mV (C) or by holding the membrane potential at −60 mV, −50 mV, and −40 mV (D, E, and F, respectively) in the absence (black squares) and presence of 1-µM Retigabine (RTG; red circles). Error bars represent standard deviation. t test; n = 5–8. *, P < 0.05, t test.
To further investigate the differential effect of Retigabine on channel closing, we carried out a detailed analysis of the effect of the membrane potential on the deactivation of the channel. For this, we recorded K+ currents deactivating at different potentials from either the pulse-activated open state (Fig. 4 C) or the resting potential open state (Fig. 4, D–F). In all cases, we observed that τDEACT decreased as the deactivating potential was more negative (Fig. 4, C–F). Deactivation of the K+ currents activated at the resting potential was ∼10-fold slower than deactivation of the pulse-activated currents at all deactivating potentials. As before, in the presence of Retigabine, deactivation of the pulse-activated currents showed no significant changes in τDEACT with respect to control (Fig. 4 C). In contrast, Retigabine significantly increased τDEACT for resting potential–activated currents depending on the deactivating potentials and the HP imposed to activate the currents. Particularly, when channels were activated at an HP of −60 mV, τDEACT increased only when deactivation was driven at potentials above −90 mV (Fig. 4 D). Holding the membrane potential at −50 mV (Fig. 4 E) enhanced the effect of Retigabine, making τDEACT significantly larger when deactivating potentials were above −100 mV. Activation at −40 mV (Fig. 4 F) resulted in a further enhancement of the drug’s effect, increasing τDEACT at all the deactivating potentials that we tested (Fig. 4 F). These observations suggested that stabilization of the open pore by Retigabine increases the energy barrier for deactivation, which has to be overcome by the electrical work the electrical field does on the voltage-sensing domains (VSDs). Therefore, making the membrane potential more negative provides more energy for the VSD to overcome the Retigabine-induced stabilization of the open pore.
Retigabine modulates AP triggering in Xenopus oocytes
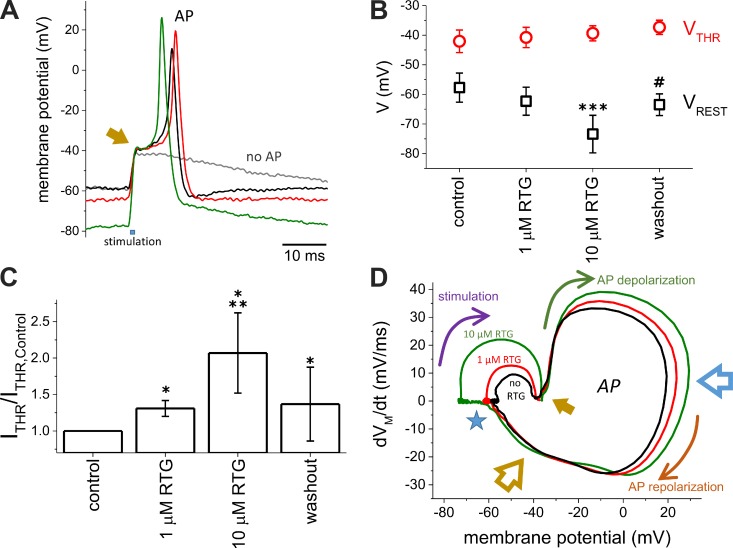
Thus far, we have shown that, on one hand, Retigabine has no significant effect on deactivation of the heteromeric KV7.2/KV7.3 channels that are activated by short depolarizing pulses. In contrast, Retigabine stabilizes channels that are already opened at the resting potential. These observations are consonant with the notion that Retigabine induces hyperpolarization of the plasma membrane (Main et al., 2000; Tatulian et al., 2001; Wainger et al., 2014). On the other hand, the activation of KV7.2/KV7.3 seems to be too slow to significantly contribute to the increase of the K+ conductance during a typical neuronal AP. Therefore, we hypothesized that Retigabine would have little or no effect on AP repolarization. To further test this view, we decided to evaluate the effect of this anticonvulsant on the generation of AP in a system with a defined background of channel expression. To do so, we co-injected Xenopus oocytes with cRNAs encoding for the sodium-selective, voltage-gated channel NaV1.4 (α and β subunits) and the KV channels ShakerIR, KV7.2, and KV7.3. From such oocytes, we were able to evoke AP-like electrical activity using a modified (“loose”) two-electrode voltage-clamp technique (see Materials and methods for details; Shapiro et al., 2012). Using this approach, we were able to depolarize the membrane to evoke APs while being able to poorly voltage-clamp the oocytes (Fig. 5 A). From a resting potential (VREST) near −60 mV, a brief (0.25–0.75 ms) depolarization of the membrane was induced by current injection. If the depolarization failed to evoke an AP, the membrane potential retuned to its resting value within the next 100 ms (Fig. 5 A, gray trace). However, if an AP was successfully triggered, repolarization was achieved within a few milliseconds after the peak of the AP (Fig. 5 A, black trace). Under our experimental conditions, the minimum amplitude of the current required to evoke an AP (ITHR) ranged between 1 and 5 μA. In the presence of 1-µM Retigabine (Fig. 5 A, red trace), VREST tended to become more negative (Fig. 5 B), requiring an increase of 30% in the magnitude of ITHR to reach the threshold potential for evoking an AP (VTHR; Fig. 5 C). For 10-µM Retigabine, the resting membrane potential became more negative with respect to control (Fig. 5 B), requiring a further increase in ITHR, ∼86%, to reach VTHR (Fig. 5 C). In spite of the unambiguous effect of 10-µM Retigabine on VREST, Retigabine seemed not to affect VTHR, as this parameter remained fairly constant in the presence of the drug (Fig. 5 B). Because VTHR is the potential at which the inwardly rectifying Na+ cancels the outwardly rectifying K+ current, we reasoned that the slow response of the heteromeric KV7.2/KV7.3 channel to changes in membrane potential results in a negligible increase in the contribution of these channels to the K+ conductance during a single AP. Therefore, the VTHR was mainly determined by the activation of NaV1.4 and ShakerIR, both of which are not sensitive to Retigabine.
Figure 5.
The resting potential, but not the threshold potential for triggering AP, was affected by Retigabine. (A) APs were recorded from Xenopus oocytes coexpressing both the α and β subunits of NaV1.4, ShakerIR, KV7.2, and KV7.3. The APs were recorded using the loose two-electrode voltage-clamp technique (see Materials and methods for details) in the absence (black trace) or presence of 1-µM (red trace) or 10-µM (green trace) Retigabine. The gray trace shows the response of VM when a subthreshold was applied. The arrow indicates the moment at which AP threshold (VTHR) is reached. (B) Effect of Retigabine (RTG) on the resting membrane potential (VREST) and AP threshold (VTHR). (C) Minimum current injection needed to evoke an AP (ITHR). The values of ITHR were normalized by ITHR in the absence of Retigabine (n = 4). (D) Example of a phase plot calculated from the APs shown on A. VREST was strongly affected by Retigabine (blue star), whereas VTHR remained fairly unaltered. Although the depolarization phase was affected by Retigabine (blue arrow), the late phase of repolarization (yellow arrow) remained unaltered. Arrows indicate the progress of the AP in time during the stimulation phase (purple arrow), depolarization phase (green arrow), and repolarization phase (orange arrow). The open blue arrow points at the times when APs reached their maximum voltage. The open yellow arrow points at the late repolarization phase. Error bars represent standard deviation. t test; n = 5. *, P < 0.002 with respect to control; **, P < 0.07 with respect to 1-µM Retigabine; ***, P < 0.0025 with respect to control; #, P < 0.072 with respect to control.
To further evaluate the effect of Retigabine on our oocyte AP model, we generated phase plots from records of the membrane potential (VM) during an AP. Phase plots are built by plotting the first derivative of VM with respect to time (dVM/dt) as a function of the VM itself (Bean, 2007). Starting at VREST (Fig. 5 D, star), injecting current depolarized the membrane. This was seen as a transient positive deflection in the phase plot (Fig. 5 D, stimulation [purple arrow]). At the end of the current injection, VM was more positive, but dVM/dt went back to zero. At this point, if VTHR was reached during current injection (Fig. 5, A and D, solid yellow arrow), an AP was evoked. As NaV channels were activated, depolarization appeared as a rapid increase in dVM/dt, driving the VM to more positive voltages (Fig. 5 D, AP depolarization [green arrow]). Then, the combination of inactivation of NaV channels and activation of KV channels resulted in the decrease of dVM/dt to zero, at which the peak voltage of the AP is reached (Fig. 5 D, open blue arrow). After the peak, dVM/dt became negative as the repolarization phase of the AP started. This late phase was driven by the activity of KV channels. Remarkably, we observed that the late phase of the repolarization was unaltered by Retigabine (Fig. 5 D, open yellow arrow). These observations were consistent with the idea that KV7 channel activation was too slow to be increased within the time of a single AP. Furthermore, Retigabine caused the peak of the AP to be more positive. We interpreted this to be the result of the recovery of NaV channels from steady-state inactivation as a consequence of the VREST hyperpolarization (Fig. 5 C). Based on these results, we concluded that the effect of Retigabine on excitability was restricted to the modulation of the resting potential open state of the heteromeric KV7.2/KV7.3.
DISCUSSION
In agreement with previous studies, our data show that Retigabine enhanced the activity of KV7.2/KV7.3 channels. Previous publications have reported that Retigabine boosts channel activity by shifting the voltage dependence for K+ current activation toward more negative values (Rundfeldt and Netzer, 2000a,b; Wickenden et al., 2000; Schenzer et al., 2005; Wuttke et al., 2005; Gunthorpe et al., 2012; Orhan et al., 2012; Kim et al., 2015). Although our observations are consonant with these previous studies (Fig. 2 B), we argue that the effect of Retigabine on the deactivation kinetics have a deeper impact on excitability than the change in voltage dependence that facilitates channel activation. On this topic, we would like to point out that the relative slow activation kinetics of KV7.2/KV7.3 channels with respect to the lifetime of an AP strongly suggests that the number of activated KV7 channels remains constant during this type of electrical event. Hence, slowing down the deactivation of channels that are already opened at the resting potential seems to have a higher impact on the activity of KV7.2/KV7.3 channels during neuronal activity.
We observed that, along with a slight shift in the voltage dependence for activation, Retigabine mainly stabilized KV7.2/KV7.3 channels that were already opened. Further, we also observed that these channels deactivate in two modalities, revealing the existence of at least two types of open states, with deactivation from the second (O2) open state being slower (Figs. 3 and 4). Furthermore, we also discovered that Retigabine discriminated between these two types of open states, decreasing the deactivation rate from the open state O2 (Fig. 1 D). This latter finding confirmed the existence of at least two open states, as they were distinguished not only by their deactivation kinetics, but also by their distinct sensitivities to Retigabine. Combined, these observations cast doubts on the notion that the clinically relevant effect of Retigabine is to facilitate channel activation. Instead, our findings indicate that Retigabine stabilizes those channels that have been already activated.
Inquiring about the mechanism of KV7.2/KV7.3 channel activity, we wondered whether the two open states or modes were the consequence of the channels being formed by two different types of subunits, KV7.2 and KV7.3. This idea requires that the stabilization of the conductance is caused by differential contributions of both subunit types to the conductive states of the channels. If so, this would imply that Retigabine is acting only on one type of subunit, as only the deactivation of the more stable open state is affected by the drug. However, both KV7.2 and KV7.3 are sensitive to Retigabine (Main et al., 2000; Rundfeldt and Netzer, 2000b; Wuttke et al., 2005, 2008; Kim et al., 2015). Thus, this later possibility is likely not probable. Therefore, we argue that the two different open states are not caused by the heteromeric nature of the channel.
The lack of effect of Retigabine on the stabilization of O1 indicates that these modes likely represent distinct sets of conformations of the activated channel. Thus, the emerging question is, which of the available KV7 channel models corresponds to the state O2? Addressing this question highlights that a detailed understanding of the atomic interactions underlying the effect of anticonvulsants is needed to expedite the development of new drugs. A few residues in the intracellular side of the pore have been identified as critical for the interaction of KV7 channels and Retigabine. Among them, a Tryptophan on the channel’s fifth transmembrane (S5) segments, namely W236 in KV7.2 (Schenzer et al., 2005; Wuttke et al., 2005) and the corresponding W265 in KV7.3 (Schenzer et al., 2005; Kim et al., 2015), is a key player for the atomic interaction underlying the action of Retigabine, whereby removing a single hydrogen bond impairs drug action (Kim et al., 2015). Thus, the structural differences associated with the open states O1 and O2 may depend on subtle rearrangements within the pore domain.
One outstanding question is why the changes in voltage dependence of activity that we observed were much lower than reported by other laboratories. We assessed voltage dependence of the KV7.2/KV7.3 channel using a pulse protocol like the one shown in Fig. 2 A. During the initial stages of this project, we observed that Retigabine changed the voltage dependence for current activation over −20 mV. However, we also noticed that increasing the duration of the period of time between each trace (intertrace intervals) from 1–2 s to 10–25 s decreased the shift in voltage dependence. So, we modified our protocols and, regretfully, did not systematically follow up on this matter at that time; we are currently addressing this issue, as further experiments are needed. Nevertheless, as shown in Fig. 4, τDEACT at −90 mV can reach >2 s. This implies that, assuming a single exponential process for closing, it will take at least 10 s to close 99% of the channels. Based on these observations, we concluded that short intertrace intervals may lead to overestimating the shift in the voltage dependence for activation because using short intertrace intervals does not allow channels to fully deactivate.
One intriguing observation made in this study is that the VTHR for triggering AP remained unaltered by the drug. Although we did not directly address this issue, we argue that the increased robustness of the K+ conductance caused by the stabilization of the opened KV7 channels by Retigabine could be compensated for by the increase of the number of available Na+ channels rescued from steady-state inactivation. Favoring this idea, we found that the amplitude of the AP tended to increase as VREST hyperpolarized (Fig. 5 A). Furthermore, we noticed that the delay between stimulation and the peak of APs shortened in the presence of 10-µM Retigabine, which is also consistent with an increase in the number of NaV channels driving depolarization (Fig. 5 A). In addition to these indirect effects of Retigabine on the availability of NaV channels, the closing of KV7.2/KV7.3 channels by hyperpolarization may have relieved the channels from the action of Retigabine, as this drug only acts on channels dwelling in the open state O2. Therefore, in an apparent paradox, we propose that Retigabine action constitutes a negative feedback loop. As stabilization of the open KV7 channels drives hyperpolarization of the plasma membrane, channels tend to close. In turn, channels in closed states are less sensitive to Retigabine. Therefore, the overall effect of the drug decreases.
Another outstanding question is why the effect of Retigabine diminished when deactivation was driven at more negative potentials (Fig. 4). On one hand, here, we have shown that Retigabine stabilizes those channels that are already opened either by a prolonged depolarizing pulse or at steady state at the typical neuronal resting potential. On the other hand, other laboratories have shown that the putative binding site for Retigabine is located at the intracellular side of the central pore domain of KV7 channels (Schenzer et al., 2005; Wuttke et al., 2005; Lange et al., 2009; Kim et al., 2015). Thus, we concluded that our results could be readily explained by a kinetic scheme like the one described in Fig. 1 D. However, we argue that this scheme may not be consonant with the current understanding of the mechanism of activity of KV channels. The canonical KV channel is a tetrameric protein with a central pore domain controlled by four VSDs surrounding it (Long et al., 2005, 2007). Activation of KV channels occurs as membrane depolarization drives conformational changes in the VSDs (Bezanilla, 2008) that lead to the opening of the central pore by a coupling mechanism that is not fully understood (Vargas et al., 2012). Likewise, deactivation of KV channels is caused by the repolarization of the plasma membrane that drives VSDs toward their initial resting conformation, which, in turn, closes the central pore. As already mentioned, one interpretation for these results is that, at more negative potential, the increased driving force for the movement of the VSD provides enough energy to overcome the effect of Retigabine.
Another aspect to be considered is that the sequence of events inherent to the closing of channels is not exactly the reverse sequence of events observed during the opening; changes in the conductive status is always initiated by electrically driven changes in the VSD. In this view, activation and deactivation follow distinct pathways that may display distinct voltage sensitivities. Indeed, a commonly overlooked feature of KV channels and other VSD proteins is that their activity displays hysteresis (Bezanilla et al., 1982; Shirokov et al., 1992; Olcese et al., 1997; Pennefather et al., 1998; Piper et al., 2003; Kuzmenkin et al., 2004; Männikkö et al., 2005; Villalba-Galea et al., 2008; Akemann et al., 2009; Xiao et al., 2010; Lacroix et al., 2011; Benndorf et al., 2012; Labro et al., 2012; Priest et al., 2013; Villalba-Galea, 2014; Li et al., 2015). Hysteresis is a property of many physical and chemical systems whereby their response to a stimulus depends on whether the magnitude of the stimulus is increasing or decreasing. In KV channels, hysteresis is manifested as a shift in the voltage dependence of their activity, a decrease in the deactivation rate, or both (Olcese et al., 1997; Pennefather et al., 1998; Piper et al., 2003; Lacroix et al., 2011; Labro et al., 2012; Priest et al., 2013). In the case of ShakerIR and KV1.2, the relationship between τDEACT and duration of the activation pulse (τDEACT–-tPULSE plot) increases in three stages: the first one in which τDEACT is small and remains constant, a second stage in which τDEACT increases, and a third stage in which τDEACT reaches a plateau (Labro et al., 2012). The first stage is associated with stabilization of the open pore, and the last stage is the result of VSD relaxation. This latter process is a hysteretic mechanism that is intrinsic of the VSD (Villalba-Galea et al., 2008, 2009, 2013; Akemann et al., 2009; Lacroix et al., 2011; Villalba-Galea, 2012, 2014). Here, we show that the τDEACT–tPULSE plot for KV7.2/KV7.3 also displays three stages, with the third one, however, not reaching a plateau within the time frame used in these studies. This observation suggests that the deactivation for state O2 follows a distinct voltage-dependent pathway, implying that the activity of KV7.2/KV7.3 is hysteretic. In this view, stabilizing the pore domain in an open conformation can cause a shift in voltage dependence, as channel closing will require more energy.
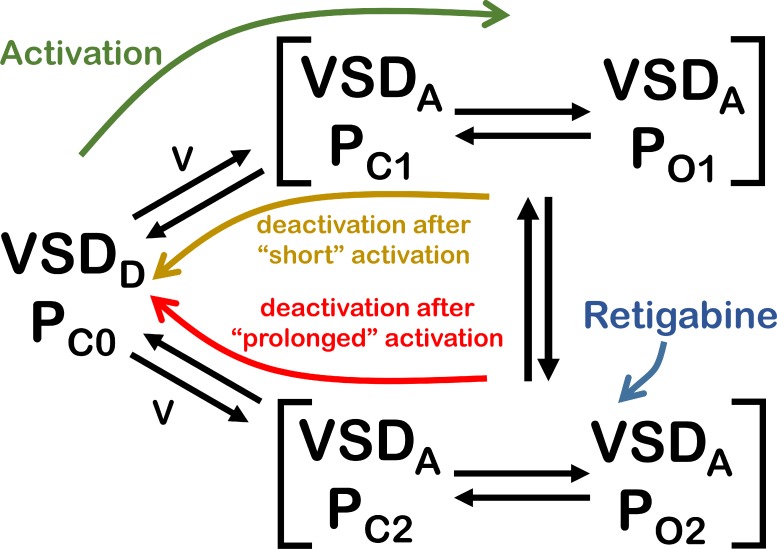
According to the sequential model in Fig. 1 D, depolarization drives the VSDs from their deactivated conformations to the activated ones. In doing so, the energy gained by this electrical work drives the opening of the pore to the first open state (O1). Eventually, the second open state (O2) is reached from O1. In the presence of Retigabine, the stability of O2 is enhanced. According to the scheme in Fig. 1 D, deactivation only occurs from O1, which implies that channels need to be relieved from the action of the drug before closing. Although this is a possible scenario, in terms of the current understanding of structure-function of channels, there is no reason to believe that the VSD will not deactivate from state O2. Based on this idea, we argue that the initial scheme depicted in Fig. 1 D seems to be insufficient to describe our findings. To address this issue, we have modified our original kinetic scheme to explicitly define the VSDs and pore as intrinsically independent modules that are coupled (Fig. 6). For simplicity, we represented the activation of four VSDs to occur in a single step instead of the explicit 16 states emerging from all the possible combinations of activated and deactivated VSDs and pore domain. Also, another simplification implemented is that we did not make any explicit distinction between KV7.2 and KV7.3 subunits. The purpose of this new scheme (Fig. 6) is to illustrate a basic framework that allows us to describe our findings in terms of what we know on the dynamics of VSD proteins. According to the new scheme, hyperpolarization of the membrane drives the four VSDs into their deactivated conformation (VSDD), which in turn keeps the pore closed (PC0; Fig. 6, state VSDD/PC0). As the membrane potential becomes more positive (e.g., at the membrane resting potential), VSD sojourns between the VSDD and the activated (VSDA) state, allowing the pore to also sojourn between the nonconductive (PC1) and the conductive (PO1) states (Fig. 6). If the membrane is immediately hyperpolarized, VSD deactivates, driving the pore domain back to state PC0. In contrast, holding the membrane at the resting potential will allow the pore to eventually change conformation into a second set of open (PO2) and closed (PC2) states. From these new states, deactivation is slower, as the pore has stabilized. In addition, the channels become Retigabine sensitive, so that in the presence of this drug, the pore is further stabilized and deactivation becomes slower.
Figure 6.
Proposed kinetic scheme for the activity of KV7.2/KV7.3 and the effect of Retigabine. The scheme contains five global states, which are different with respect to each other depending on the activation status of the VSD and the pore domain (P). In the scheme, VSD represents the four voltage sensors of the channel. At negative potentials, the VSDs are in the deactivated state (VSDD) and the pore is, therefore, closed (PC0). Upon depolarization, the VSDs are activated (VSDA), allowing the pore to sojourn between a conductive and a nonconductive state (PO1 and PC1, respectively). Holding the membrane depolarized potentials keeps the VSDs in the VSDA state while allowing a second transition in the pore. As a result, the pore sojourns between a second pair of conductive and nonconductive states (PO2 and PC2, respectively) and the channel becomes Retigabine sensitive. Deactivation from these states is slower than from the pair PC1–PO1. Retigabine further decreases the rate of deactivation from the pair PC2–PO2 while leaving deactivation unaffected from the other pair.
Open pore stabilization provides a straightforward mechanism for hysteresis in KV channels. However, the charge movement of an isolated VSD can also show hysteresis through an intrinsic process known as VSD relaxation (Villalba-Galea et al., 2008, 2009). After relaxation, the voltage dependence for charge movement (sensing/gating currents) can shift to more negative potentials as well as to become slower. This implies that hysteresis in KV7 channels could also originate from VSD relaxation (Lacroix et al., 2011; Labro et al., 2012). Our observations, alas, do not allow us to evaluate this possibility, as we have not been able to record gating currents from KV7.2/KV7.3.
In spite of the findings reported here, the overall impact that hysteresis can have on electrical signaling and its biological role remain elusive. Here, for the first time, we provide an example of hysteresis in the activity of a KV channel that directly affects the channel’s pharmacology under physiological conditions. In line with recent findings on the action of Retigabine on the isolated pore of KV7 channels (Syeda et al., 2015), our observations clearly show that the stabilized open state of the KV7.2/KV7.3 channel is the target for the action of the anticonvulsant Retigabine. These findings have several implications. First, using the effect on the activation of the KV7 channels is an important but not sufficient parameter when evaluating new drugs targeting the activity of these channels. Second, the observation of a hysteretic behavior in the activity of KV7 channels implies the existence of two or more distinct sets of open states. These sets of states are likely associated with also structurally distinct sets of conformations. Our results showed that Retigabine can differentially affect the stability of these states, confirming that the channel adopts a different set of conformations that can be pharmacologically discriminated. It remains to be established, however, whether the stabilization of the second open state (O2) is driven by conformational changes in the pore, VSD relaxation, or both. For this, future studies will need to directly study the dynamics of the VSD and determine whether the VSD of KV7 channels relaxes. On this issue, no study showing gating currents from these channels has been reported to our knowledge. Recording gating currents from both KV7.2 and KV7.3 has proven technically challenging, as we also failed to record gating currents during the present study. At this point, we can conclude that, although it seems that Retigabine is acting on the pore domain, we cannot rule out the possibility that VSD relaxation is involved in the stabilization of the open state.
On a final note, knowing that the activation of KV7.2/KV7.3 takes several tens of milliseconds to occur (Fig. 1 A), whereas a typical neuronal AP lasts less than a couple of milliseconds (Fig. 5; Bean, 2007), highlights the apparent disconnect between the kinetics of both processes. This upholds the idea that, during the lifetime of an AP, the number of activated KV7.2/KV7.3 channels likely remains constant. In our view, this is consistent with the notion that neuronal KV7 channels function as regulators of excitability by contributing the control of the resting potential (Jentsch, 2000; Peters et al., 2005; Miceli et al., 2008; Cooper, 2012). Thus, our observations indicate that the voltage-dependent activation of KV7.2/KV7.3 channels may constitute a mechanism that is physiologically relevant only at transmembrane voltages near the resting potential. Therefore, the use of activation by depolarization to experimentally evaluate the effect of drugs or mutations on these channels may not be suitable.
Acknowledgments
We would like to thank Drs. Diomedes E. Logothetis and Louis J. De Felice for their invaluable support to this project. We also thank Dr. Frank Bosmans for his insightful comments on the manuscript. Also, thanks to Dr. Thomas Jentsch for providing the constructs of KCNQ2 (KV7.2) and KCNQ3 (KV7.3) in the vector pTLN.
This work was funded by the Clinical and Translational Science Award grant KL2TR000057 and the Center for Clinical and Translational Research (CCTR) Endowment Fund grant to C.A. Villalba-Galea through the CCTR at Virginia Commonwealth University, which is supported by the National Institutes of Health grant UL1TR000058.
The authors declare no competing financial interests.
Author contributions: C.A. Villalba-Galea designed the research. A. Corbin-Leftwich, S.M. Mossadeq, J. Ha, I. Ruchala, A.H.N. Le, and C.A. Villalba-Galea performed the research. A. Corbin-Leftwich, S.M. Mossadeq, and C.A. Villalba-Galea analyzed the data. C.A. Villalba-Galea wrote the paper.
Kenton J. Swartz served as editor.
Footnotes
Abbreviations used in this paper:
- AP
- action potential
- HP
- holding potential
- VSD
- voltage-sensing domain
References
- Abidi A., Devaux J.J., Molinari F., Alcaraz G., Michon F.X., Sutera-Sardo J., Becq H., Lacoste C., Altuzarra C., Afenjar A., et al. 2015. A recurrent KCNQ2 pore mutation causing early onset epileptic encephalopathy has a moderate effect on M current but alters subcellular localization of Kv7 channels. Neurobiol. Dis. 80:80–92. 10.1016/j.nbd.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Akemann W., Lundby A., Mutoh H., and Knöpfel T.. 2009. Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophys. J. 96:3959–3976. 10.1016/j.bpj.2009.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P. 2007. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8:451–465. 10.1038/nrn2148 [DOI] [PubMed] [Google Scholar]
- Benndorf K., Kusch J., and Schulz E.. 2012. Probability fluxes and transition paths in a Markovian model describing complex subunit cooperativity in HCN2 channels. PLOS Comput. Biol. 8:e1002721 10.1371/journal.pcbi.1002721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. 2008. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9:323–332. 10.1038/nrm2376 [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R.E., and Fernández J.M.. 1982. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J. Gen. Physiol. 79:21–40. 10.1085/jgp.79.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A., and Adams P.R.. 1980. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 283:673–676. 10.1038/283673a0 [DOI] [PubMed] [Google Scholar]
- Brown D.A., and Passmore G.M.. 2009. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156:1185–1195. 10.1111/j.1476-5381.2009.00111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C., Singh N.A., Ryan S.G., Lewis T.B., Reus B.E., Leach R.J., and Leppert M.. 1998. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 18:53–55. 10.1038/ng0198-53 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., and Hawkes A.G.. 1977. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc. R. Soc. Lond. B Biol. Sci. 199:231–262. 10.1098/rspb.1977.0137 [DOI] [PubMed] [Google Scholar]
- Cooper E.C. 2012. Potassium channels (including KCNQ) and epilepsy. Jasper’s Basic Mechanisms of the Epilepsies. Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., and Delgado-Escueta A.V., Fourth edition Oxford University Press, Bethesda, MD: 10.1093/med/9780199746545.003.0005 [DOI] [PubMed] [Google Scholar]
- Dedek K., Kunath B., Kananura C., Reuner U., Jentsch T.J., and Steinlein O.K.. 2001. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc. Natl. Acad. Sci. USA. 98:12272–12277. 10.1073/pnas.211431298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe M.J., Large C.H., and Sankar R.. 2012. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 53:412–424. 10.1111/j.1528-1167.2011.03365.x [DOI] [PubMed] [Google Scholar]
- Jentsch T.J. 2000. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 1:21–30. 10.1038/35036198 [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Yau M.C., Galpin J.D., Seebohm G., Ahern C.A., Pless S.A., and Kurata H.T.. 2015. Atomic basis for therapeutic activation of neuronal potassium channels. Nat. Commun. 6:8116 10.1038/ncomms9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmenkin A., Bezanilla F., and Correa A.M.. 2004. Gating of the bacterial sodium channel, NaChBac: voltage-dependent charge movement and gating currents. J. Gen. Physiol. 124:349–356. 10.1085/jgp.200409139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labro A.J., Lacroix J.J., Villalba-Galea C.A., Snyders D.J., and Bezanilla F.. 2012. Molecular mechanism for depolarization-induced modulation of Kv channel closure. J. Gen. Physiol. 140:481–493. 10.1085/jgp.201210817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J.J., Labro A.J., and Bezanilla F.. 2011. Properties of deactivation gating currents in Shaker channels. Biophys. J. 100:L28–L30. 10.1016/j.bpj.2011.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W., Geissendörfer J., Schenzer A., Grötzinger J., Seebohm G., Friedrich T., and Schwake M.. 2009. Refinement of the binding site and mode of action of the anticonvulsant Retigabine on KCNQ K+ channels. Mol. Pharmacol. 75:272–280. 10.1124/mol.108.052282 [DOI] [PubMed] [Google Scholar]
- Li X., Anishkin A., Liu H., van Rossum D.B., Chintapalli S.V., Sassic J.K., Gallegos D., Pivaroff-Ward K., and Jegla T.. 2015. Bimodal regulation of an Elk subfamily K+ channel by phosphatidylinositol 4,5-bisphosphate. J. Gen. Physiol. 146:357–374. 10.1085/jgp.201511491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley J.E., Pettinger L., Huang D., and Gamper N.. 2012. M channel enhancers and physiological M channel block. J. Physiol. 590:793–807. 10.1113/jphysiol.2011.223404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., and Mackinnon R.. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903. 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., and MacKinnon R.. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382. 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Main M.J., Cryan J.E., Dupere J.R., Cox B., Clare J.J., and Burbidge S.A.. 2000. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol. Pharmacol. 58:253–262. [DOI] [PubMed] [Google Scholar]
- Maljevic S., Wuttke T.V., Seebohm G., and Lerche H.. 2010. KV7 channelopathies. Pflugers Arch. 460:277–288. 10.1007/s00424-010-0831-3 [DOI] [PubMed] [Google Scholar]
- Männikkö R., Pandey S., Larsson H.P., and Elinder F.. 2005. Hysteresis in the voltage dependence of HCN channels: conversion between two modes affects pacemaker properties. J. Gen. Physiol. 125:305–326. 10.1085/jgp.200409130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo M. 2015. Novel genes of early-onset epileptic encephalopathies: from genotype to phenotypes. Pediatr. Neurol. 53:119–129. 10.1016/j.pediatrneurol.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Miceli F., Soldovieri M.V., Martire M., and Taglialatela M.. 2008. Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr. Opin. Pharmacol. 8:65–74. 10.1016/j.coph.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Miceli F., Soldovieri M.V., Iannotti F.A., Barrese V., Ambrosino P., Martire M., Cilio M.R., and Taglialatela M.. 2011. The voltage-sensing domain of Kv7.2 channels as a molecular target for epilepsy-causing mutations and anticonvulsants. Front. Pharmacol. 2:2 10.3389/fphar.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli F., Soldovieri M.V., Ambrosino P., De Maria M., Migliore M., Migliore R., and Taglialatela M.. 2015a. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J. Neurosci. 35:3782–3793. 10.1523/JNEUROSCI.4423-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli F., Striano P., Soldovieri M.V., Fontana A., Nardello R., Robbiano A., Bellini G., Elia M., Zara F., Taglialatela M., and Mangano S.. 2015b. A novel KCNQ3 mutation in familial epilepsy with focal seizures and intellectual disability. Epilepsia. 56:e15–e20. 10.1111/epi.12887 [DOI] [PubMed] [Google Scholar]
- Olcese R., Latorre R., Toro L., Bezanilla F., and Stefani E.. 1997. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J. Gen. Physiol. 110:579–589. 10.1085/jgp.110.5.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan G., Wuttke T.V., Nies A.T., Schwab M., and Lerche H.. 2012. Retigabine/Ezogabine, a KCNQ/KV7 channel opener: pharmacological and clinical data. Expert Opin. Pharmacother. 13:1807–1816. 10.1517/14656566.2012.706278 [DOI] [PubMed] [Google Scholar]
- Pennefather P.S., Zhou W., and DeCoursey T.E.. 1998. Idiosyncratic gating of HERG-like K+ channels in microglia. J. Gen. Physiol. 111:795–805. 10.1085/jgp.111.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A., Degani N., Nachman R., Uziyel Y., Gibor G., Shabat D., and Attali B.. 2005. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol. Pharmacol. 67:1053–1066. 10.1124/mol.104.007112 [DOI] [PubMed] [Google Scholar]
- Peters H.C., Hu H., Pongs O., Storm J.F., and Isbrandt D.. 2005. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8:51–60. 10.1038/nn1375 [DOI] [PubMed] [Google Scholar]
- Piper D.R., Varghese A., Sanguinetti M.C., and Tristani-Firouzi M.. 2003. Gating currents associated with intramembrane charge displacement in HERG potassium channels. Proc. Natl. Acad. Sci. USA. 100:10534–10539. 10.1073/pnas.1832721100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest M.F., Lacroix J.J., Villalba-Galea C.A., and Bezanilla F.. 2013. S3-S4 linker length modulates the relaxed state of a voltage-gated potassium channel. Biophys. J. 105:2312–2322. 10.1016/j.bpj.2013.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundfeldt C., and Netzer R.. 2000a. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 50:1063–1070. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C., and Netzer R.. 2000b. The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary-cells tranfected with human KCNQ2/3 subunits. Neurosci. Lett. 282:73–76. 10.1016/S0304-3940(00)00866-1 [DOI] [PubMed] [Google Scholar]
- Schenzer A., Friedrich T., Pusch M., Saftig P., Jentsch T.J., Grötzinger J., and Schwake M.. 2005. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J. Neurosci. 25:5051–5060. 10.1523/JNEUROSCI.0128-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M.G., Homma K., Villarreal S., Richter C.P., and Bezanilla F.. 2012. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 3:736 10.1038/ncomms1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokov R., Levis R., Shirokova N., and Ríos E.. 1992. Two classes of gating current from L-type Ca channels in guinea pig ventricular myocytes. J. Gen. Physiol. 99:863–895. 10.1085/jgp.99.6.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.A., Charlier C., Stauffer D., DuPont B.R., Leach R.J., Melis R., Ronen G.M., Bjerre I., Quattlebaum T., Murphy J.V., et al. 1998. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 18:25–29. 10.1038/ng0198-25 [DOI] [PubMed] [Google Scholar]
- Soh H., Pant R., LoTurco J.J., and Tzingounis A.V.. 2014. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J. Neurosci. 34:5311–5321. 10.1523/JNEUROSCI.3919-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R., Santos J.S., and Montal M.. 2015. The sensorless pore module of Kv7 channels embodies the target site for the anticonvulsant Retigabine. J. Biol. Chem. 10.1074/jbc.M115.683185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L., Delmas P., Abogadie F.C., and Brown D.A.. 2001. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 21:5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas E., Yarov-Yarovoy V., Khalili-Araghi F., Catterall W.A., Klein M.L., Tarek M., Lindahl E., Schulten K., Perozo E., Bezanilla F., and Roux B.. 2012. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 140:587–594. 10.1085/jgp.201210873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A. 2012. Voltage-controlled enzymes: The new JanusBifrons. Front. Pharmacol. 3:161 10.3389/fphar.2012.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A. 2014. Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 107:1564–1572. 10.1016/j.bpj.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A., Sandtner W., Starace D.M., and Bezanilla F.. 2008. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA. 105:17600–17607. 10.1073/pnas.0807387105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A., Sandtner W., Dimitrov D., Mutoh H., Knöpfel T., and Bezanilla F.. 2009. Charge movement of a voltage-sensitive fluorescent protein. Biophys. J. 96:L19–L21. 10.1016/j.bpj.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A., Frezza L., Sandtner W., and Bezanilla F.. 2013. Sensing charges of the Ciona intestinalis voltage-sensing phosphatase. J. Gen. Physiol. 142:543–555. 10.1085/jgp.201310993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger B.J., Kiskinis E., Mellin C., Wiskow O., Han S.S., Sandoe J., Perez N.P., Williams L.A., Lee S., Boulting G., et al. 2014. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Reports. 7:1–11. 10.1016/j.celrep.2014.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., Dixon J.E., and McKinnon D.. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893. 10.1126/science.282.5395.1890 [DOI] [PubMed] [Google Scholar]
- Wickenden A.D., Yu W., Zou A., Jegla T., and Wagoner P.K.. 2000. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol. Pharmacol. 58:591–600. [DOI] [PubMed] [Google Scholar]
- Wickenden A.D., Krajewski J.L., London B., Wagoner P.K., Wilson W.A., Clark S., Roeloffs R., McNaughton-Smith G., and Rigdon G.C.. 2008. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol. Pharmacol. 73:977–986. 10.1124/mol.107.043216 [DOI] [PubMed] [Google Scholar]
- Wuttke T.V., Seebohm G., Bail S., Maljevic S., and Lerche H.. 2005. The new anticonvulsant retigabine favors voltage-dependent opening of the KV7.2 (KCNQ2) channel by binding to its activation gate. Mol. Pharmacol. 67:1009–1017. 10.1124/mol.104.010793 [DOI] [PubMed] [Google Scholar]
- Wuttke T.V., Jurkat-Rott K., Paulus W., Garncarek M., Lehmann-Horn F., and Lerche H.. 2007. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 69:2045–2053. 10.1212/01.wnl.0000275523.95103.36 [DOI] [PubMed] [Google Scholar]
- Wuttke T.V., Penzien J., Fauler M., Seebohm G., Lehmann-Horn F., Lerche H., and Jurkat-Rott K.. 2008. Neutralization of a negative charge in the S1-S2 region of the KV7.2 (KCNQ2) channel affects voltage-dependent activation in neonatal epilepsy. J. Physiol. 586:545–555. 10.1113/jphysiol.2007.143826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.F., Chandler N., Dobrzynski H., Richardson E.S., Tenbroek E.M., Wilhelm J.J., Sharma V., Varghese A., Boyett M.R., Iaizzo P.A., and Sigg D.C.. 2010. Hysteresis in human HCN4 channels: a crucial feature potentially affecting sinoatrial node pacemaking. Sheng Li Xue Bao. 62:1–13. [PubMed] [Google Scholar]
- Xiong Q., Sun H., and Li M.. 2007. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat. Chem. Biol. 3:287–296. 10.1038/nchembio874 [DOI] [PubMed] [Google Scholar]