Abstract
Background:
The proportion of node-negative breast cancer patients has been increasing with improvement of diagnostic modalities and early detection. However, there is a 20–30% recurrence in node-negative breast cancers. Determining who should receive adjuvant therapy is challenging, as the majority are cured by surgery alone. Hence, it requires further stratification using additional prognostic and predictive factors.
Subjects and Methods:
Ours is a single institution retrospective study, on 300 node-negative breast cancer cases, who underwent primary surgery over a period of 7 years (2005–2011). We excluded all cases who took NACT. Prognostic factors of age, size, lymphovascular emboli, estrogen receptor (ER), progesterone receptor (PR), HER2neu Ki-67, grade and molecular classification were analyzed with respect to those with and without early events (recurrence, metastases or second malignancy, death) using-Pearson Chi-square method and logistic regression method for statistical analysis.
Results:
Majority belonged to the age group of 50–70 years. On univariate analysis, size >5 cm (P = 0.03) and ER negativity had significant association (P = 0.05) for early failures; PR negativity and lymphovascular emboli (LVE) had borderline significance (P = 0.07). Multivariate analysis showed size >5 cm to be significant (P = 0.04) and LVE positivity showed borderline significant association (P = 0.07) with early failures. About 62% belonged to luminal category followed by basal-like (25%) in molecular classification.
Conclusions:
ER negativity, PR negativity, LVE/lymphovascular invasion positivity and size >5 cm (T3 and T4) are associated with poor prognosis in node-negative breast cancers.
Keywords: Molecular classification, node-negative breast cancer, prognosis
Introduction
As only relatively few studies have dealt specifically with node-negative disease, especially in Indian scenario, possibly due to small sample size and limited follow-up, we have made an attempt to prognosticate node-negative breast cancer cases with easily available factors like age, size, grade, lymphovascular invasion (LVI), estrogen receptor (ER), PgR, HER2neu and Ki-67 and molecular classification. Aims of our study were (1) to prognosticate 300 node-negative breast cancer cases that had undergone surgery over a 7 years period (2005–2011) (2) to categorize 300 node-negative breast cancer cases according to molecular classification, as per the St. Gallen's International Expert consensus-2011.
Subjects and Methods
Ours is a single institution retrospective study on biopsy proven 300 node-negative breast cancer cases, who underwent surgery in Amrita Institute of Medical Sciences, Kochi, for over past 7 years (2005–2011). Data were collected from the registers maintained in the Pathology Department, Cancer Registry and also from the Electronic Medical Record system in the hospital.
We excluded all cases who took NACT and those with follow-up for <1.5 years. The protocol was approved by the Human Ethics Committee of the Institution. The parameters like age, size, grade, lymphovascular emboli, ER, PgR, HER2neu, molecular classification and Ki-67 were analyzed statistically with respect to those with events within 5 years of treatment (recurrence, metastases or second malignancy, death) by Chi-square method (univariate analysis) and Logistic Regression method (Multivariate analysis) using SPSS format 20. The patients were given uniform and standard treatment either from our center or at their local referral centers.
Results
The total number of 3747 breast cancer cases were treated with surgery or NACT or palliative care, during the period (2005-2011) in our center. Surgery was done in 1570 cases. This includes node positive cases also. We studied 300 node-negative cases who underwent surgery in our institute.
Follow-up status
Among the 300 patients studied, 88% (n = 264) had follow-up, while 12% (n = 36) were lost to follow-up. About 88.6% of the patients with follow-up were free of disease while 11.4% developed events like metastasis, second malignancy, recurrence or death. For convenience in the statistical evaluation, all these events were grouped together and considered as early failures. All of these patients developed the events within 5 years of primary malignancy, and hence we grouped them as early failures.
Median follow-up time was 3.25 years. Minimum and maximum follow-up of 1.5 and 8.5 years was achieved. Survival status at 3.25 years was 93.7%.
Age and node evaluation
Of the 300 node-negative breast cancer cases studied, the majority of the cases (48%) belonged to the age group of 50–70 years. 36% belonged to age group 35–50 years. There were also cases with age less than 35 years (6%) and age more than 75 years (10%).
In 85% of the patients, more than 10 lymph nodes were assessed. Only in <5% of cases, nodes <6 were assessed. Hence, the lymph node yield was considered satisfactory for evaluation.
Histopathological categorization
About 75% were invasive ductal carcinoma cases, followed by mixed carcinoma (5%), and Invasive papillary carcinoma and invasive lobular carcinoma forming 3% each. Almost all the varieties described by WHO were seen.
Size status
Tumor size is defined by measuring the tumor in at least two dimensions, with the greatest dimension used for tumor staging (TNM staging). Size was assessed during the pathological assessment after the routine formalin fixation. After excluding cases without follow-up, 264 cases were included for analysis. As the No. of patients in the groups with T3 and T4 size tumors were less, we clubbed them together for the ease of statistical analysis. Majority of the cases (23%) were of stage T2 (2 cm<x<5 cm) followed by stage T1 (<2 cm) which formed 23% of cases and T3 and T4 stage cases formed 11% of cases. After analysis, P = 0.039 for univariate analysis; P = 0.04 for multivariate analysis.
Lymphovascular emboli/invasion
Lymphovascular emboli/invasion (LVE/LVI) was assessed microscopically on the H and E stained histopathological tissue sections from the tumor. Among the patients with follow-up, LVE status was not available in 21 patients. Hence, the cases with follow-up and LVE status are 243. The details are shown in Table 1.
Table 1.
Distribution of cases as per the LVE status

Hormone receptor status
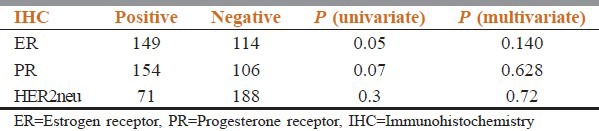
ER, PgR, HER2neu status was assessed on tumor tissue by standard immunohistochemistry (IHC) protocol. HER2neu positivity status of 2+ and more only was considered positive ER and PgR positivity of ≥1% was considered positive. Among the patients with follow-up, the hormonal status was available in 264 patients. The details are given in the Table 2.
Table 2.
Distribution of cases according to hormone receptor status
Ki-67 status
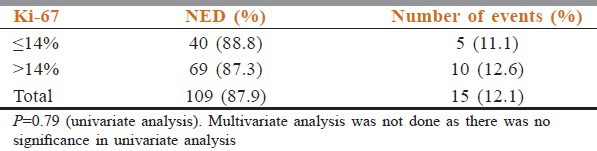
Ki-67 is identified to have prognostic value ie. increased levels of Ki-67 for decreased survival, as it is a cell proliferation-associated antigen, expressed in all stages of the cell cycle except G0. A cut-off of 14% was taken in our study. Ki-67 expression of ≥14% was considered high as per the recommendations put forward by St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011.[1] Among the patients with follow-up (n = 264), Ki-67 was available only in 124 cases. The details are given in Table 3.
Table 3.
Distribution of cases according to Ki-67 status
Modified Bloom Richardson grading
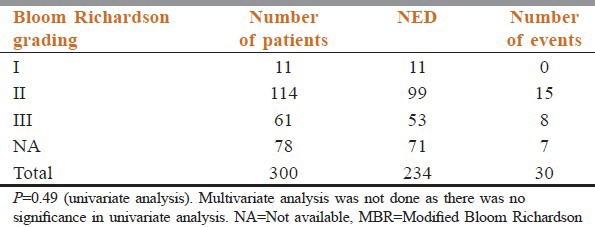
Tumor grade is a classification based on the degree of differentiation of the tumor tissue. We have used Modified Bloom Richardson (MBR) grading in our study. Among the patients with follow-up, MBR grading was available in 222 patients. The details are given in Table 4.
Table 4.
Distribution of cases according to MBR grading status
Molecular classification
Among the patients with follow-up, molecular classification was available in 261 cases. Totally, 170 belonged to Luminal Category. A total of 26 cases were HER2neu enriched group and 65 cases were Basal Like. The Luminal category could be further classified on the basis of availability of Ki-67. Of 170 patients in Luminal category, 22 were Luminal A, 66 were Luminal B and 82 were either Luminal A or B, as they were ER and PgR positive, in whom Ki-67 was not available. The results are given in Table 5.
Table 5.
Distribution of cases according to molecular classification

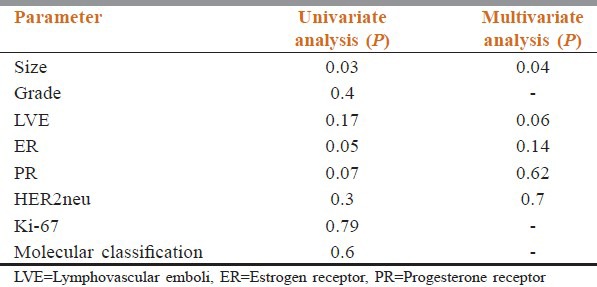
The results of final statistical analysis are given in Table 6.
Table 6.
Statistical analysis results of the cases
Discussion
The proportion of node-negative breast cancer patients has been increasing with improvement of diagnostic modalities and early detection. However, there is a 20-30% risk of recurrence in node-negative breast cancers[2]. Determining who should receive adjuvant therapy is challenging, as the majority are cured by surgery alone. Hence, it requires further stratification using additional prognostic and predictive factors.
An extensive list of potential prognostic factors was compiled from review articles identified through an initial Medline and PubMed search. We then searched the Medline and PubMed database from 1990 to 2013, using “prognosis,” “node-negative breast cancer” and the name of the specific prognostic factor as key words. This window of time was selected because many of the biochemical/molecular factors involve rapidly evolving technologies, and it was believed that studies might be more homogeneous within a more recent time frame. The results of our study were comparable with several other studies.
The data from the present study after univariate and multivariate analysis showed that increased tumor size of more than 5 cm, ER negative status, PgR negative status and LVE/LVI positivity has either significant association or borderline significant association with decreased survival in node-negative patients with breast cancer. The other variables assessed like MBR grade, HER2neu receptor status, Ki-67 and molecular classification did not show an association with bad prognosis in the node-negative breast cancer cases. The majority of cases belonged to the age group of 50–70 years, which remains the same for node positive cases as well.
In our study, tumor size more than 5 cm showed significant association with bad prognosis, both after univariate (P = 0.03) and multivariate analysis (P = 0.04). Hence in node-negative cases, increase in size can be considered as an indicator for bad prognosis. Studies by Reed et al.,[3] Saimura et al.[4] as well as Thor et al.[5] concludes the same. ER negative status also showed significant association after univariate analysis (P = 0.05). However, multivariate analysis did not show any significant association. This variation could be due to the small follow-up period. However, the literature review showed that studies by Fischer et al.[6] concludes the same. However, the detection method used was dextran-coated charcoal absorption assay with titrated ligands. Hence, the comparability is less. In studies using IHC techniques as in studies by Railo et al.[7] and Reed et al.,[3] there was no association with bad prognosis. This variation could be due to difference in the cut-off used in these studies.
PgR negative status and LVE/LVI positivity status showed borderline significance after univariate (P = 0.07) and multivariate (P = 0.06) analysis respectively. The work by Sigurdsson et al.[8] also concluded the same. This borderline significance could be explained by the small follow-up period as well as the variation in the technique and the cut off used in the work by Sigurdsson et al. two studies by Lee et al.[9] and Pinder’ et al.,[10] after multivariate analysis showed that LVI have an association with bad prognosis. However, we can see that various studies show mixed results in assessing the significance of LVI/LVE as bad prognostic factor. This difference in results may be overcome by including IHC marker CD34 for confirming the vascular channel status. The small follow-up time might also have been a cause for a negative association, after univariate analysis, in our study.
The HER2neu oncogene codes for a transmembrane tyrosine kinase and has been suggested as an etiologic factor in several kinds of cancer. HER2neu amplification/overexpression can be assessed with IHC or fluorescence in situ hybridization. Studies by Reed et al.[3] also supports our results after both univariate and multivariate analysis that HER2neu positivity does not have a significant association with bad prognosis.
Ki-67 is identified to have prognostic value ie. increased levels of Ki-67 for decreased survival, as it is a cell proliferation-associated antigen, expressed in all stages of the cell cycle except G0. A cut-off of 14% was taken in our study. Railo et al.[7] and Brown et al.,[11] after univariate and multivariate analysis showed no association of Ki-67 with bad prognosis like our study which also did not show a significant association of high (>14%) Ki-67 index with bad prognosis.
Our study after univariate and multivariate analysis also conveys that tumor grade has no association with bad prognosis. Our results correlate well with the studies by Saimura et al.[4] and Thor et al.[5]
The results of molecular categorization were Luminal A (8%), Luminal B (25%), HER2neu enriched (9%), Basal-like (25%), Luminal A/B (31%). In some cases, Luminal category could not be categorized further as Luminal A or B, as Ki-67 could not be done. Hence, they were clubbed as Luminal A/B on the basis of ER positivity and HER2neu negativity. Only few studies have studied the molecular classification in node-negative breast cancers. Hence, comparative data is minimal. However, molecular categorization also did not show any significant association with bad prognosis.
Limitations of the study
When we critically evaluated our study, we realized that our study deals with only early failures, because of short follow-up period compared to other similar studies. Hence, more follow-up is required to assess the long-term prognosis in these patients. About 36/300 patients in our study were lost to follow-up. The data could have been improved if we could include those cases too. Also, as the no. of events were less, all of them such as second malignancy, death, recurrence, and metastasis were clubbed together as a group, though their molecular basis is different. A detailed study including the more no. of cases and a longer follow-up of at least 10 years will be ideal.
Conclusions
In node-negative breast cancer cases,
T3, T4 tumor size and ER negativity is associated with bad prognosis
PgR negativity and LVE positivity has a borderline significance in the assessment of bad prognosis
Ki-67 status, HER2neu positivity, tumor grade and molecular classification do not have a significant association with bad prognosis.
Thus, we have been able to point out the factors/markers thatcurrently have broad clinical usefulness in this patient group of node-negative breast cancers, which could be responsible for early failures.
Acknowledgment
Cancer Registry, AIMS, Kochi
Technicians of Histopathology Lab, Department of Pathology, AIMS, Kochi.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes – dealing with the diversity of breast cancer: Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park BW, Kim JH, Kim KS, Lee KS. Prognostic factors for the node-negative breast cancers. Yonsei Univ Coll Med Seoul Korea Breast Cancer Res. 2007;9(Suppl 1):1. [Google Scholar]
- 3.Reed W, Hannisdal E, Boehler PJ, Gundersen S, Host H, Marthin J. The prognostic value of p53 and c-erb B-2 immunostaining is overrated for patients with lymph node negative breast carcinoma: A multivariate analysis of prognostic factors in 613 patients with a follow-up of 14-30 years. Cancer. 2000;88:804–13. doi: 10.1002/(sici)1097-0142(20000215)88:4<804::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Saimura M, Fukutomi T, Tsuda H, Sato H, Miyamoto K, Akashi-Tanaka S, et al. Prognosis of a series of 763 consecutive node-negative invasive breast cancer patients without adjuvant therapy: Analysis of clinicopathological prognostic factor. J Surg Oncol. 1999;71:101–5. doi: 10.1002/(sici)1096-9098(199906)71:2<101::aid-jso8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Thor AD, Liu S, Moore DH, 2nd, Edgerton SM. Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol. 1999;17:470–7. doi: 10.1200/JCO.1999.17.2.470. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: Findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol. 1988;6:1076–87. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- 7.Railo M, Lundin J, Haglund C, von Smitten K, von Boguslawsky K, Nordling S. Ki-67, p53, Er-receptors, ploidy and S-phase as prognostic factors in T1 node negative breast cancer. Acta Oncol. 1997;36:369–74. doi: 10.3109/02841869709001282. [DOI] [PubMed] [Google Scholar]
- 8.Sigurdsson H, Baldetorp B, Borg A, Dalberg M, Fernö M, Killander D, et al. Indicators of prognosis in node-negative breast cancer. N Engl J Med. 1990;322:1045–53. doi: 10.1056/NEJM199004123221505. [DOI] [PubMed] [Google Scholar]
- 9.Lee AK, DeLellis RA, Silverman ML, Heatley GJ, Wolfe HJ. Prognostic significance of peritumoral lymphatic and blood vessel invasion in node-negative carcinoma of the breast. J Clin Oncol. 1990;8:1457–65. doi: 10.1200/JCO.1990.8.9.1457. [DOI] [PubMed] [Google Scholar]
- 10.Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. III. Vascular invasion: Relationship with recurrence and survival in a large study with long-term follow-up. Histopathology. 1994;24:41–7. doi: 10.1111/j.1365-2559.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown RW, Allred CD, Clark GM, Osborne CK, Hilsenbeck SG. Prognostic value of Ki-67 compared to S-phase fraction in axillary node-negative breast cancer. Clin Cancer Res. 1996;2:585–92. [PubMed] [Google Scholar]


