Abstract
Plants have incredible developmental plasticity, enabling them to respond to a wide range of environmental conditions. Among these conditions is the presence of plant growth-promoting rhizobacteria (PGPR) in the soil. Recent studies show that PGPR affect root growth and development within Arabidopsis thaliana root. These effects lead to dramatic changes in root system architecture, that significantly impact aboveground plant growth. Thus, PGPR may promote shoot growth via their effect on root developmental programs. This review focuses on contextualizing root developmental changes elicited by PGPR in light of our understanding of plant-microbe interactions and root developmental biology.
Keywords: Rhizosphere, root development, plant growth-promoting rhizobacteria, Arabidopsis thaliana, hormonal signaling
Beneficial microbes can induce plant growth by modifying root development
In the early 1900s, Hiltner made the key observation that the soil around the plant root contains more micro-organisms than the surrounding soil [1]. Soil has been documented to have exceptional microbial diversity, containing fungi, invertebrates, archaea and bacteria [2]. Among the soil bacteria are plant growth-promoting rhizobacteria (PGPR) (see Glossary) [3]. Unlike obligate symbionts, these bacteria can interact with numerous host plants and improve plant growth and health via a variety of mechanisms that can be direct, such as nitrogen fixation [4,5], or indirect, including competition with pathogens [6,7]. PGPR are capable of modulating the root system architecture (i.e. the spatial configuration of the root system) which is a significant determinant of crop yield [8–10]. The potential of PGPR to affect plant growth and root architecture was excellently addressed in two recent reviews [11,12]. In contrast, the mechanisms by which PGPR influence cell division, and alter the balance between proliferation and differentiation in the primary root and lateral root initiation sites, remain largely unknown.
In this review we focus on the ability of PGPR to affect post-embryonic root development. It has become clear that PGPR affect post-embryonic root development by altering cell division and differentiation within the primary root, as well as affecting root hair formation and lateral root development. We highlight recent findings that suggest that bacteria affect endogenous root developmental mechanisms to establish these effects. This review has three main sections, describing root-bacterial interactions in progressive detail. We begin by describing root-bacterial interactions in the rhizosphere, and the determinants of microbial community structure in association with plant roots. Subsequently, we describe the current knowledge of the effects of soil bacteria on root development at the cellular level. Finally, we discuss current understanding of the effects of bacteria on underlying plant regulatory mechanisms. We have selected a few bacterial species in our discussion that have been described to alter cell division and differentiation in the root of the model plant Arabidopsis thaliana (Arabidopsis). While our focus is on plant growth-promoting bacteria, we conclude our review by addressing how fungi affect root development to draw attention to the similarities between these two plant-microbe interactions.
Future studies integrating the fields of plant-microbe interactions and plant developmental biology will lend insight into how soil microbes affect root development. This work will enhance our understanding of these complex cross-kingdom interactions, and increase our knowledge of root developmental biology and bacterial signaling. Ultimately, this knowledge will foster development of sustainable plant growth-promoting technologies that have the potential to dramatically increase crop yield and food security.
Plant-microbe interactions in the rhizosphere
When Hiltner observed the increased number of micro-organisms around roots compared to bulk soil in the 1900s, he assumed that this increase was due to nutrient secretion by the plant and termed it ‘the rhizosphere effect’ [1]. Since then, the rhizosphere has been defined as the soil around plant roots influenced by the root and its exudates, whereas the rest of the soil is referred to as bulk soil (Figure 1) [13]. Soil properties themselves are exceptionally diverse with abiotic factors that influence the bacteria in the bulk soil including pH and nutrient content. The extent to which plants and soil characteristics influence the microbial communities in the soil has been elegantly reviewed [14–17] Recently, studies using deep-sequencing techniques found that soil type had a more dramatic effect on rhizosphere microbial communities than plant genotype [18–20]. These results suggest that the soil composition plays a pivotal role in shaping the bacterial communities in the soil.
Figure 1. Plants affect the bacterial community composition within the rhizosphere and the root.
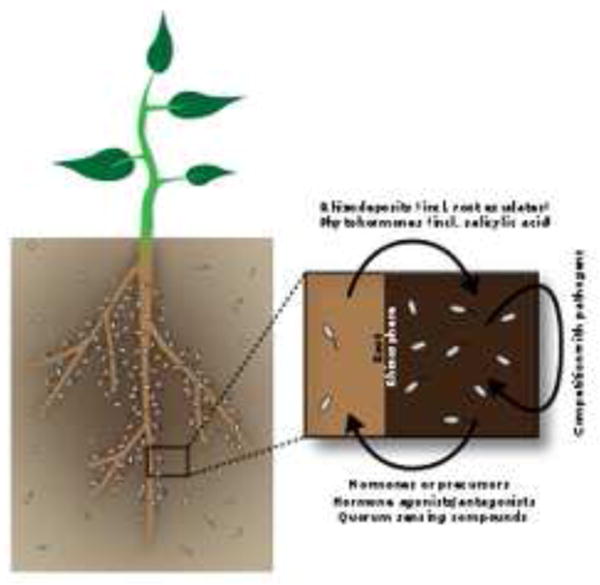
The bacterial community within the rhizosphere, the thin layer of soil around a plant's root system that is affected by the roots and their exudates (dark brown), is more numerous than the community in bulk soil (tan). Plants appear to influence the rhizosphere microbiome composition via their root exudates, although this effect varies between studies. In return, bacteria affect plant growth via many compounds, including hormones and hormone agonists and antagonists and quorum sensing compounds. In addition, they indirectly affect plant growth by competing with pathogens for both space and nutrients. A very select group of bacteria is found within the Arabidopsis root (inset). In the plant-bacteria interface there are a lot of possibilities for communication.
As Hiltner speculated in the early 1900s, in addition to soil type, the bacterial communities in the rhizosphere likely depends on the type and composition of root exudates secreted by the plant [21–24]. Root exudates include sugars, amino acids, organic acids, fatty acids, phenolics, enzymes and flavonoids [25]. The ability of bacteria to thrive in the rhizosphere depends on their ability to move towards these plant-derived carbon sources (for more on chemotaxis see [26,27]) and to use them and other root-derived rhizodeposits such as sloughed-off root cells or lysates as energy sources [28–30]. The rhizosphere effect has been documented by numerous groups since Hiltner's initial observations (for examples see [31,32]). Correlated with differences in root exudates, Arabidopsis accessions, or natural variants, differ in their root-associated microbial community. Additionally, a single mutation in the gene coding for an ATP-binding cassette transporter in Arabidopsis changes the microbial community around the plant root [22]. A plant-derived compound, rosmarinic acid, was found that affects quorum sensing in soil bacteria [33], suggesting that plants may excrete certain compounds to alter bacteria in the rhizosphere. Microbes within the rhizosphere can in turn modify root exudate composition [40–45], enhance shoot growth [46–48] and induce systemic resistance to subsequent pathogen attack (reviewed in [49]). These examples reveal a rich language of chemical communication between plants and rhizosphere-inhabiting microbes resulting in altered microbial community structure.
Bacteria found within plant roots are referred to as endophytic bacteria and comprise a much less diverse community than what is found in either the rhizosphere or bulk soil [18,19,32]. The decreased bacterial diversity is likely mediated by the plant immune system [34]. Phytohormones used in plant defense, including jasmonic acid, ethylene and salicylic acid, have been shown to influence rhizosphere composition in certain soils [35]. Salicylic acid was recently shown to alter the colonization of certain bacterial families within roots [36]. Additionally, mutant plants, defective in multiple phytohormone signaling pathways, had lower survival rates and distinct endophytic microbial colonization compared to wild-type plants [36]. These results reflect the importance of plant defense mechanisms in regulating bacterial colonization within the root.
Postembryonic root development is affected by PGPR
Postembryonic primary root development in Arabidopsis thaliana
The Arabidopsis root architecture consists of a primary root with iteratively branching lateral roots. The primary root can be divided into three developmental zones: the meristematic, elongation, and differentiation zones (Figure 2a). The root tip, which contains the meristematic zone, is surrounded and protected by a group of gravity-sensing cells called the root cap. The root cap deposits mucilage, cell debris and whole cells into the rhizosphere as the root grows [37]. The meristematic zone contains the stem cell niche, the progenitor cells that give rise to distinct cell types, and the dividing daughter cells [38]. A quiescent center located at the center of the niche consists of four rarely-dividing cells [39] that repress differentiation of the surrounding initials, or stem cells [40]. There are four sets of initials: columella, lateral root cap/epidermis, cortex/endodermis, and stele or vasculature initials (Figure 2c) [39,41]. The transition zone, or basal meristem, is located shootward of the meristematic zone. The cells in this zone do not divide and cell lengthening is slow. In the neighboring elongation zone, cells elongate up to 300% within three hours [38]. Finally, cells acquire their mature characteristics in the differentiation zone, which extends shootward. The differentiation zone can be distinguished by the emergence of root hairs, which emerge from epidermal cells overlying two cortical cells (Figure 2d) [42,43]. Another defining feature of the differentiation zone is the formation of the Casparian strip: a waxy cell wall thickening surrounding endodermal cells that forms a protective barrier [44].
Figure 2. Arabidopsis root structure.

(A) Longitudinal diagram of the root. The root can be divided into three developmental zones: the meristematic zone where the stem cell niche and rapidly dividing cells are located, the elongation zone where cells elongate rapidly and the differentiation zone where cells acquire their unique features. (B) In the root tip, the high concentration of auxin induces division and inhibits differentiation. Scarecrow (SCR) and shortroot (SHR) inhibit differentiation within the quiescent center specifically by inhibiting the cytokinin response via their downstream effector WOX5. More shootward, the decreasing concentration of auxin and the increasing concentration of cytokinin ultimately result in a hormonal balance that shifts in favor of differentiation. The antagonistic action of hydrogen peroxide (H2O2) and superoxide (O2-) is also involved in determining the transition point between division and differentiation, independent of auxin and cytokinin. The mechanism by which reactive oxygen species regulate this developmental transition is unclear. (B) Longitudinal section of the root meristem. The quiescent center (white) is surrounded by the stem cells: the cortex / endodermal initial (green), epidermal / lateral root cap initial (violet) and columella initials (pink). (C) Transverse section of the root in the differentiation zone reveals the radial symmetry of the outer cell types around the pericycle and vasculature (light blue), root hair epidermis (dark purple), non-root hair epidermis (light purple), cortex (blue) and endodermis (yellow). Abbreviations: SCR, SCARECROW; SHR, SHORTROOT; H2O2, hydrogen peroxide; O2-, superoxide
Effect of PGPR on primary root development
Two root phenotypes have been described in the literature after exposure to PGPR. The most common is an inhibition of primary root growth coupled with a proliferation of lateral roots (described below) and root hairs [45]. The second phenotype is an increase in plant biomass without a reduction in primary root growth [46]. These effects are dependent on both the bacterial density and the distance from the plant root at which the bacteria are applied [47–50]. Although growth promotion phenotypes have been well-described [3], few molecular mechanisms underlying these effects are known. Recently, several PGPR have been shown to induce root developmental changes in terms of cell division and differentiation at both the root meristem and at sites of lateral root formation [46–48,51,52]. These cellular level changes alter the root system architecture of the plants [46–48,51,52].
Colonization of the Arabidopsis root by either PGPR species Pseudomonas simiae WCS417 (formerly P, fluorescens WCS417, [53]) and Bacillus megaterium UMCV1 influences both the maintenance of the root stem cell niche and the transition from proliferation to differentiation in the root [46,52]. In the meristematic zone, P. simiae increases cell division [46], while B. megaterium decreases cell division [52]. These PGPR species, decrease primary root length when applied directly to the roots by decreasing cell elongation in the elongation zone by 40% and 70%, respectively (Figure 3) [46,52]. P. aeruginosa, a pathogenic bacteria, might have the same effect, since it is hypothesized to induce premature differentiation closer to the root tip [47]. Possibly as a result of premature differentiation, root hairs emerge closer to the root tip in colonized plants (Figure 3) [52]. In addition, root hair density increases upon colonization due to a higher number of cortical cells around the radial axis. The higher number of cortical cells increases the number of root-hair forming cells, i.e. epidermal cells overlying two cortical cells [46]. In addition, root hairs grow longer and root hair formation is accelerated by yet undefined mechanisms [46].
Figure 3. Bacteria influence overall plant physiology and primary root development.
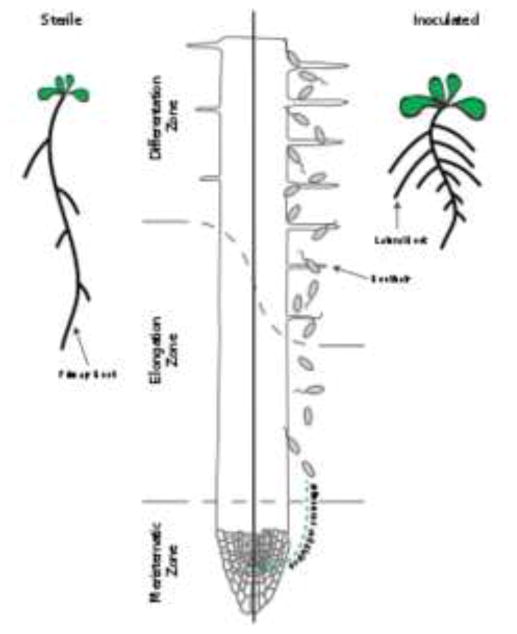
In general, beneficial bacteria enhance lateral root formation and shoot growth and inhibit primary root growth. Within the primary root, cell division in the meristem is affected either positively or negatively, depending on the species and growth conditions. In addition, differentiation is induced closer to the root tip and root hair density and length is increased.
Lateral root development in Arabidopsis thaliana
Apart from affecting primary root development, PGPR also influence lateral root formation. Lateral root development has been elegantly studied by several groups [54–57]. In brief, there are two steps to forming a lateral root. First, pairs of cells in the pericycle, the cell layer in the vasculature neighboring the endodermis, called lateral root founder cells must become competent to form a lateral root. Second, lateral root founder cells are activated, divide multiple times and differentiate to form the lateral root primordia, which ultimately emerges from the primary root in the differentiation zone [58]. Endodermal cells overlying the lateral root primordia separate to accomodate the emerging lateral root [59]. As the lateral root emerges, the endodermal cells surrounding the primordia change shape, reduce their size and accommodate the primordial by forming small holes in the Casparian strip, a waxy protective barrier surrounding endodermal cells [60]. It remains to be seen if these breakpoints in the Casparian strip can account for the abundance of bacteria reported at lateral root emergence sites [18,61]. Further divisions and differentiation of the lateral root cells after emergence ultimately results in a lateral root anatomy identical to the anatomy of the primary root, including a meristematic zone that controls the lateral root's growth rate [58].
Effect of PGPR on lateral root development
P. simiae WCS417 and B. megaterium UMCV1 increase the number of both lateral root primordia and lateral roots [46,52], indicating that the number of lateral root competent sites and lateral root outgrowth are affected by colonization [46]. It is unknown whether PGPR also influence lateral root founder cell specification. Interestingly, the induction of lateral root formation and shoot growth can take place without the aforementioned observed primary root growth inhibition: volatile organic compounds produced by P. simiae WCS417 stimulate lateral root formation, but do not inhibit primary root growth [46].
PGPR affect endogenous root developmental programs
Plant endogenous mechanisms regulating root development
PGPR probably induce the above root development phenotypes by modulating plant endogenous mechanisms regulating root development. As described above, primary root development is controlled in the stem cell niche, which is established during embryogenesis [62]. Postembryonically, positioning and maintenance of the niche requires the plant hormone auxin and its downstream PLETHORA (PLT) transcription factors which form a gradient with a maximum within the niche [63–65]. The transcription factors SHORTROOT (SHR) and SCARECROW (SCR) act in parallel with and independent of the PLTs to maintain stem cell niche identity [64,66,67]. SHR is expressed in the stele and moves to the nuclei of the adjacent cell layers to activate SCR expression [68]. In the quiescent center, SCR inhibits differentiation and maintains the identity of the surrounding stem cells [69] by suppressing cytokinin perception [70]. In the transition zone, the suppression of cytokinin perception is relieved and cytokinin negatively regulates expression of the auxin efflux transport proteins known as PINs. This leads to auxin redistribution and induces differentiation (Figure 2B) [71].
Reactive oxygen species (ROS) also regulate the transition from proliferation to differentiation independent of auxin and cytokinin (reviewed in [72]). Two transcription factors, UPBEAT1 and MYB36, have been found to regulate reactive oxygen homeostasis [73,74]. Repression of certain peroxidases from the elongation zone shootward increases hydrogen peroxide (H2O2) levels and decreases superoxide (O2-) levels, resulting in differentiation. ROS potentially enhances differentiation by stopping the cell cycle and modifying cell walls to allow for cell expansion (Figure 2B) [73].
As mentioned, lateral roots form from pericycle cells in the differentiation zone. Local auxin synthesis has been shown to induce lateral root primordia [75]. Until recently, it was thought that an auxin maximum in the lateral root founder cells dictates lateral root formation (reviewed in [56]). Recently, however, it was shown that competence to form a lateral root is induced by periodic gene oscillations in the transition and the elongation zones and that auxin is not sufficient to generate these gene oscillations [76,77]. However, an auxin response is activated in the pericycle founder cells before the first division [78]. In addition, its downstream targets PLT3, PLT5 and PLT7 prevent clustering of lateral root competence sites and are essential for subsequent lateral root emergence [79]. Thus, auxin is required for lateral root formation but periodic gene oscillations generate lateral root competence sites [57]. As in primary root development, cytokinin functions antagonistically to auxin: cytokinin inhibits lateral root formation [80–82]. Interestingly, cytokinin levels are not decreased in the transition zone. Instead, only the response to cytokinin seems to be down-regulated [82], similar to the inhibition of the cytokinin response in the quiescent center by SCR. As such, the phytohormones auxin and cytokinin are the major opposing players regulating both primary and lateral root growth, with SCR and SHR inhibiting the cytokinin response in the quiescent center specifically. In addition, ROS have emerged as another set of important antagonistic players for controlling the transition from division to differentiation independent of auxin and cytokinin signaling.
Effect of PGPR on endogenous root developmental programs
Several PGPR are known to produce cytokinin [83–85] and inoculation of lettuce plants with cytokinin-producing Bacillus subtilis strains increases plant growth and plant cytokinin content [85]. Moreover, the growth-promoting effects of B. megaterium are dependent on functional cytokinin receptors in Arabidopsis [86]. Similarly, the amount of auxin produced by PGPR has been shown to correlate with the ability to induce plant growth for several bacterial species and isolates from the rhizosphere [87–91]. Moreover, the increased cell division in the Arabidopsis meristem induced by P. simiae as described above is accompanied by increased auxin-responsive gene expression [46] and the decrease in cell division caused by B. megaterium and P. aeruginosa is accompanied by either decreased or stable auxin response in the primary root tip as measured with the auxin-responsive marker construct DR5:uidA [47,52]. Since auxin effects are dose-dependent, these different auxin patterns could explain how bacteria elicit different growth effects or affect growth along the length of the root distinctly. The induction of lateral root outgrowth after exposure to P. simiae is also accompanied by increased auxin-responsive gene expression, with an increase in auxin-response maxima along the root. In fact, the induction of lateral root growth is dependent on the auxin response and on functional auxin efflux machinery [46].
Although a functional auxin response within the plant is essential for several of P. simiae's effects on root development, P. simiae does not make auxin [46]. Instead, it might produce an auxin mimic, as has been shown for P. aeruginosa [47]. Alternatively, bacterial quorum sensing molecules may be involved. Quorum sensing is the process by which bacteria assess the population density in order to coordinate behavior like virulence or biofilm formation. P. aeruginosa produces diketopiperazines (DKPs), compounds produced by many bacterial species that are involved in quorum sensing. DKPs have a planar structure containing a heterocyclic system also found in auxin and activate auxin-inducible gene expression, potentially by binding to the auxin receptor itself [47]. The auxin-responsive gene expression subsequently induces lateral root growth but does not inhibit primary root growth [47].
Indole, a compound used by bacteria for a wide range of functions, such as biofilm formation and virulence, can also affect auxin signaling within a plant. When applied to plants, indole enhances lateral root primordium development. Polar auxin transport is required for this effect, but is not influenced by indole application. Moreover, although indole can be converted into auxin it does not significantly change auxin levels within the plant and impedes the ability of plants to respond to exogenous auxin. This suggests that indole is converted into an auxin antagonist within the plant [92].
Like DKPs and indole, volatiles emitted by P. simiae induce lateral root growth while leaving primary root development unaffected [46], indicating that the effects of PGPR on primary root and lateral root growth are independent. The identities of the volatiles emitted by P. simiae are unknown, but the volatiles do not possess auxin activity [46]. N-acyl-homoserine lactones (AHLs) are another group of quorum sensing molecules produced by P. aeruginosa, which influence root development independent of auxin. AHLs possibly induce lateral root growth and inhibit primary root growth by modifying the cytokinin response instead of the auxin response [51]. Interestingly, the virulence factor pyocyanin (PCN), whose synthesis by P. aeruginosa is regulated by quorum sensing, has been shown to induce the root phenotype independent of either auxin or cytokinin. This molecule may affect root development by manipulating ethylene levels, subsequently leading to changing ROS levels in the primary root tip [48]. Interestingly, Bacillus sp B55 is able to rescue ethylene-insensitive mutants by means of the volatile compound dimethyl disulfide [93]. B. megaterium affects root system architecture independent of either auxin or ethylene by an unknown mechanism [52].
In summary, the cellular effects of PGPR on root system architecture are generally accompanied by changes in plant endogenous responses. While auxin and cytokinin homeostasis are most often affected in the plant [46,47,51], ethylene levels and its downstream effectors the ROS have also been observed to be affected [48]. Since several PGPR have been shown to produce these hormones themselves, it is tempting to suggest that these changes may be induced by these hormones directly. However, so far, most evidence indicates that molecules involved in essential bacterial processes, such as quorum sensing, function as phytohormone mimics or indirectly influence plant phytohormone homeostasis. Interestingly, one bacterial species, P. aeruginosa, produces several compounds, each of which has a distinct mechanism involving different plant hormones by which it modifies root development [47,51]. It has yet to be determined how bacterial-induced root development and growth are beneficial to the bacteria, for example by increasing carbon sources in the form of plant root exudates.
Plant-fungi interactions show great similarity to plant-bacteria interactions
The effects of PGPR on plant development are not specific to plant-bacteria interactions since similar phenotypes are induced within roots upon exposure to fungi. The ectomycorrhizal fungus Laccaria bicolor S238N, the truffles Tuber borchii strains ATCC 96540 and 43BO and Tuber melanopsorum strains Bal1 and Rey_t, Trichoderma virens Gv. 29-8 and Trichoderma atroviride (IMI 206040) all increase lateral root growth [94–97]. Trichoderma atroviride also increases root hair length and density [97]. In addition, the truffles inhibit primary root growth [95]. Therefore it is tempting to speculate on the conserved responses to biotic factors.
Like PGPR, fungi affect endogenous plant mechanisms to influence root development. The increased development of lateral roots in response to T. virens is accompanied by increased expression of the auxin-inducible marker DR5:uidA in the primary root tip and the developing lateral roots and is dependent on functional auxin transport and responses in the plant [94]. Since T. virens produces the auxin precursor IAAd [94], this suggests that fungal auxin leads to a change in auxin signaling in the plant, ultimately leading to the observed root phenotype. Plants also respond to contact with L. bicolor with an increased auxin response. However, although L. bicolor produces auxin, auxin produced by the fungus is not the only trigger to induce LR formation in Arabidopsis. Instead, a volatile compound produced by the fungus induces the phenotype, which potentially leads to enhanced auxin biosynthesis within the plant resulting in the observed root phenotype [96]. This volatile might be ethylene, which is produced by the truffles and, together with fungal auxin, induces the mentioned root phenotype when applied to the roots directly [95]. The possible interplay between auxin and ethylene in response to beneficial fungi is supported by work on T, atroviride. T, atroviride produces both auxins and ethylene. Ethylene signaling within the plant is important for the fungal-induced root hair phenotype while lateral root induction is ethylene-independent [97]. Lateral root induction might instead be dependent on auxin signaling in the plant, which is affected by the bioactive metabolite 6-pentyl-2H -pyran-2-one (6-PP) produced by T. atroviride [98]. 6-PP increases auxin responsiveness in the lateral root primordia, as shown by increased DR5:GFP expression in the primordia, possibly by changing expression of PIN proteins, which are auxin transporters [98]. The signals from the two hormones might be integrated in a positive feedback loop by MPK6, although the precise interactions have not been uncovered [97].
Together, the research on fungi presents another example of the complex mechanisms underlying the effect of soil microorganisms on root development. In addition, it exemplifies the similarities between the interaction of both bacteria and fungi with plants. Like PGPR, soil fungi appear to change lateral root and primary root growth primarily by affecting plant endogenous phytohormone signaling. As seen for PGPR-induced effects, while microorganism-produced auxin might induce these effects, there is evidence that other compounds, such as the volatile ethylene, are the causal agents of the effect on root development and that intricate crosstalk mechanisms are involved. These clear similarities between fungal and bacterial effects on root development indicate that results obtained on plant-bacteria interactions are of general interest in the study of other plant-microbe interactions as well and might help shed light on general mechanisms of plant – microorganism communication.
Concluding remarks and future perspectives
Bacteria influence post-embryonic development in many organisms. Examples can be found across the Eukaryotic kingdom. Bacteria induce the transition from a floating larva to stationary juvenile in a tubeworm [99], stimulate single celled choanoflagellate to form colonies [100], and regulate the development of epithelial brush border cells in the zebrafish gut [101–103]. It is thus not surprising that beneficial microbes in the soil affect root development. Here we review recent evidence regarding the effects of PGPR on Arabidopsis root development. One clear trend emerges: bacteria manipulate endogenous host mechanisms regulating postembryonic root development to exert their effects on root growth. These findings open up exciting new paths for future research (see outstanding questions). Research harnessing natural variation in Arabidopsis populations [104,105], and novel technologies such as emerging root imaging techniques [106–109] and high throughput sequencing technologies [110,111] will be useful for addressing these questions. Ultimately, a more thorough understanding of the interaction between beneficial soil bacteria and plants leading to enhanced plant growth will prove valuable for several research disciplines, including root development, plant-microbe interactions and bacterial signaling. Moreover, it will nurture the development of sustainable agricultural techniques that use naturally occurring soil microbes to promote plant growth and health that may reduce herbicides and synthetic fertilizers used in the field.
Trends box.
-
-
Plant roots and the beneficial bacteria within their rhizosphere interact extensively, as a result plants influence the composition of the microbial community and microbes enhance plant growth and induce plant pathogen defense.
-
-
Plant growth-promoting rhizobacteria (PGPR) modify root system architecture, which contributes to the enhanced shoot growth phenotype seen upon colonization by the bacteria. These modifications are established by changing plant endogenous signaling pathways.
-
-
While several PGPR can produce phytohormones, most effects on plant developmental pathways are exerted by other molecules. Often, these molecules are produced as part of a bacteria-specific process, such as quorum sensing.
-
-
Several fungi have the same effects on root system architecture as PGPR, indicating that interaction mechanisms might be conserved across kingdoms.
Outstanding questions.
How do beneficial bacteria evade the plant immune system?
What is the mechanism for entry of beneficial bacteria into the root? Do the cell wall and Casparian strip play an active or passive role in regulating bacterial (endophytic) colonization?
What bacterially-produced compounds affect root development and how does the plant respond to these compounds at a cellular level?
Do bacteria influence the gene oscillations that appear to prime lateral root founder cells and do they thus influence founder cell specification in addition to lateral root development?
What are the differences between volatile-induced and direct contact-induced bacterial effects on root development and how are these differences established?
What is the spatial distribution of bacteria along the root and how are roots colonized in space and time?
Are their universal beneficial microbial partners that contribute to plant health?
Are similar genes and cellular mechanisms involved in these interactions in different plant species?
What is the microbial variation between diverse natural isolates?
Acknowledgments
We thank Philip Benfey for mentorship and helpful critique of the manuscript, Corné Pieterse (Utrecht), Colleen Drapek (Duke), Jazz Dickinson (Duke), Guy Wachsmann (Duke), Cara Winter (Duke) and Jeff Dangl (UNC) for insightful comments on the manuscript. L.M.L. was supported in part by a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research. Eline received funding from the ‘Jo Kolk Studiefonds’, ‘Hugo de Vries-Fonds’ and ‘Hendrik Muller Fonds’ for her internship in the Benfey lab at Duke and funding from the Netherlands Organization for Scientific Research (NWO) for her PhD in Utrecht.
Glossary
- Casparian strip
a cell wall thickening in the root endodermis that restricts the flow of solutes and water into and out of the central vasculature. This barrier also restricts bacteria and fungi from entering these cells. In addition, the Casparian strip is a hallmark of differentiated endodermis
- Endophytes
microorganisms living within plant tissue without causing harm to the plant
- PGPR
plant growth-promoting rhizobacteria. Bacteria found in the rhizosphere that promote plant growth or health either directly or indirectly
- Phytohormone
Signaling molecule produced by the plant that regulates a broad range of cellular processes from cell division and plant defense to aging. Some examples include
- Auxin
Phytohormone that, among other plant processes, is involved in cell division and specification in the root meristem as well as formation of lateral root primordia
- Cytokinin
Phytohormone that often functions antagonistically of auxin. In root development, cytokinin induces differentiation of cells as the move shootward
- Ethylene
Phytohormone involved in aging and fruit ripening
- Quorum sensing
a process by which bacteria measure the bacterial density in their surroundings and consequently adapt their behavior accordingly
- Rhizosphere
the thin layer of soil around plant roots that is influenced by the root and its exudates. The rhizosphere harbors a more numerous but less diverse group of microorganisms than the surrounding bulk soil
- Stem cell niche
the group of cells near the root tip that contain the initials, or stem cells, and quiescent cells. Together, these cells supply cells that enable primary root elongation and root topology. A new stem cell niche is established in the tip of lateral roots during lateral root formation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Hiltner L. Uber neure Erfahrungen und probleme auf dem gebeit der bodenbackteriologie und unter besonderer berucksichtigung der grundungung und brache. Arb Deut Landwirsch Ges. 1904;98:59–78. [Google Scholar]
- 2.Tringe SG, et al. Comparative metagenomics of microbial communities. Science (80- ) 2005;308:554–557. doi: 10.1126/science.1107851. [DOI] [PubMed] [Google Scholar]
- 3.Kloepper JW, Schroth MN. Plant growth-promoting rhizobacteria on radishes. Proc IVth Int Conf Plant Pathog Bact 2 Stn Pathol Veg Phyto-Bacteriologie 1987 [Google Scholar]
- 4.Soyano T, et al. Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci U S A. 2014;111:14607–12. doi: 10.1073/pnas.1412716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson BJ, Mathesius U. Phytohormone regulation of legume-rhizobia interactions. J Chem Ecol. 2014;40:770–90. doi: 10.1007/s10886-014-0472-7. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Montaño F, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169:325–36. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Lugtenberg B, Kamilova F. Plant-Growth-Promoting Rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 8.Uga Y, et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet. 2013;45:1097–102. doi: 10.1038/ng.2725. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa S, et al. Root system architecture variation in response to different NH4 + concentrations and its association with nitrogen-deficient tolerance traits in rice. Acta Physiol Plant. 2014;36:2361–2372. [Google Scholar]
- 10.Ning P, et al. New maize hybrids had larger and deeper post-silking root than old ones. F Crop Res. 2014;166:66–71. [Google Scholar]
- 11.Vacheron J, et al. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukumar P, et al. Involvement of auxin pathways in modulating root architecture during beneficial plant-microorganism interactions. Plant Cell Environ. 2013;36:909–19. doi: 10.1111/pce.12036. [DOI] [PubMed] [Google Scholar]
- 13.Prashar P, et al. Rhizosphere: its structure, bacterial diversity and significance. Rev Environ Sci Bio/Technology. 2013;13:63–77. [Google Scholar]
- 14.Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 15.Philippot L, et al. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–99. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 16.Bulgarelli D, et al. Structure and Functions of the Bacterial Microbiota of Plants. Annu Rev Plant Biol. 2013;64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 17.Lareen A, et al. Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol. 2016 doi: 10.1007/s11103-015-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundberg DS, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulgarelli D, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 20.Schlaeppi K, et al. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc Natl Acad Sci U S A. 2014;111:585–92. doi: 10.1073/pnas.1321597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haichar F, el Z, et al. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008;2:1221–30. doi: 10.1038/ismej.2008.80. [DOI] [PubMed] [Google Scholar]
- 22.Badri DV, et al. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol. 2009;151:2006–2017. doi: 10.1104/pp.109.147462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauregard PB, et al. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci. 2013;110:E1621–30. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaparro JM, et al. Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions. PLoS One. 2013;8:e55731. doi: 10.1371/journal.pone.0055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weert S de, et al. Flagella-Driven Chemotaxis Towards Exudate Components Is an Important Trait for Tomato Root Colonization by Pseudomonas fluorescens. 2007 doi: 10.1094/MPMI.2002.15.11.1173. [DOI] [PubMed] [Google Scholar]
- 27.Vande Broek A, et al. Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology 144 ( Pt. 1998;9:2599–606. doi: 10.1099/00221287-144-9-2599. [DOI] [PubMed] [Google Scholar]
- 28.Jones DL, et al. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004;163:459–480. doi: 10.1111/j.1469-8137.2004.01130.x. [DOI] [PubMed] [Google Scholar]
- 29.Singh BK, et al. Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends Microbiol. 2004;12:386–93. doi: 10.1016/j.tim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Dennis PG, et al. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol. 2010;72:313–27. doi: 10.1111/j.1574-6941.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 31.Bakker PAHM, et al. The rhizosphere revisited: root microbiomics. Front Plant Sci. 2013;4:165. doi: 10.3389/fpls.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micallef SA, et al. Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J Exp Bot. 2009;60:1729–1742. doi: 10.1093/jxb/erp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corral-Lugo A, et al. Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci Signal. 2016;9:ra1. doi: 10.1126/scisignal.aaa8271. [DOI] [PubMed] [Google Scholar]
- 34.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 35.Doornbos RF, et al. Effects of Jasmonic Acid , Ethylene , and Salicylic Acid Signaling on the Rhizosphere Bacterial Community of Arabidopsis thaliana. 2011;24:395–407. doi: 10.1094/MPMI-05-10-0115. [DOI] [PubMed] [Google Scholar]
- 36.Lebeis SL, et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Sci. 2015 doi: 10.1007/s11103-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 37.Kumpf RP, Nowack MK. The root cap: a short story of life and death. J Exp Bot. 2015 doi: 10.1007/s11103-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 38.Verbelen JP, et al. Root apex of Arabidopsis thaliana Consists of Four Distinct Zones of Growth Activities: Meristematic Zone, Transition Zone, Fast Elongation Zone, and Growth Terminating Zone. Plant Signal Behav. 2006;1:296–304. doi: 10.4161/psb.1.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolan L, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 40.van den Berg C, et al. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–9. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- 41.van den Berg C, et al. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature. 1995;378:62–5. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- 42.Dolan L, et al. CLONAL RELATIONSHIPS AND CELL PATTERNING IN THE ROOT EPIDERMIS OF ARABIDOPSIS. DEVELOPMENT. 1994;120:2465–2474. [Google Scholar]
- 43.Galway ME, et al. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–54. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- 44.Caspary R. Bemerkungen über die Schutzscheide und die Bildung des Stammes und der Wurzel. Methods Mol Biol 1865 [Google Scholar]
- 45.Ryu CM, et al. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil. 2005;268:285–292. [Google Scholar]
- 46.Zamioudis C, et al. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria Plant Physiol. 2013;162:304–318. doi: 10.1104/pp.112.212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz-Castro R, et al. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Natl Acad Sci U S A. 2011;108:7253–8. doi: 10.1073/pnas.1006740108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortiz-Castro R, et al. Pyocyanin, a virulence factor produced by Pseudomonas aeruginosa, alters root development through reactive oxygen species and ethylene signaling in Arabidopsis. Mol Plant Microbe Interact. 2014;27:364–78. doi: 10.1094/MPMI-08-13-0219-R. [DOI] [PubMed] [Google Scholar]
- 49.Kapulnik Y, et al. Changes in root morphology of wheat caused by Azospirillum inoculation. Can J Microbiol. 1985;31:881–887. [Google Scholar]
- 50.Persello-Cartieaux F, et al. Utilization of mutants to analyze the interaction between Arabidopsis thaliana and its naturally root-associated Pseudomonas. Planta. 2001;212:190–8. doi: 10.1007/s004250000384. [DOI] [PubMed] [Google Scholar]
- 51.Ortíz-Castro R, et al. N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Env. 2008;31:1497–1509. doi: 10.1111/j.1365-3040.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- 52.López-Bucio J, et al. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact. 2007;20:207–217. doi: 10.1094/MPMI-20-2-0207. [DOI] [PubMed] [Google Scholar]
- 53.Berendsen RL, et al. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genomics. 2015;16:539. doi: 10.1186/s12864-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubrovsky JG, et al. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Ann Bot. 2006;97:903–15. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benková E, Bielach A. Lateral root organogenesis - from cell to organ. Curr Opin Plant Biol. 2010;13:677–683. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Péret B, et al. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Van Norman JM, et al. To branch or not to branch: the role of pre-patterning in lateral root formation. Development. 2013;140:4301–10. doi: 10.1242/dev.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- 59.Laskowski M, et al. Expression profiling of auxin-treated Arabidopsis roots: Toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 2006;47:788–792. doi: 10.1093/pcp/pcj043. [DOI] [PubMed] [Google Scholar]
- 60.Vermeer JEM, et al. A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science. 2014;343:178–83. doi: 10.1126/science.1245871. [DOI] [PubMed] [Google Scholar]
- 61.Dong Y, et al. Quantitative assessments of the host range and strain specificity of endophytic colonization by Klebsiella pneumoniae 342. Plant Soil. 2003;257:49–59. [Google Scholar]
- 62.Scheres B, et al. Embryonic origin of the Arabidopsis primary root and root-meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- 63.Grieneisen VA, et al. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–13. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 64.Aida M, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–20. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 65.Galinha C, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–7. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 66.Benfey PN, et al. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- 67.Scheres B, et al. MUTATIONS AFFECTING THE RADIAL ORGANIZATION OF THE ARABIDOPSIS ROOT DISPLAY SPECIFIC DEFECTS THROUGHOUT THE EMBRYONIC AXIS. Development. 1995;121:53–62. [Google Scholar]
- 68.Nakajima K, et al. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 69.Sabatini S, et al. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–8. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moubayidin L, et al. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev Cell. 2013;26:405–15. doi: 10.1016/j.devcel.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dello Ioio R, et al. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008;322:1380–4. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt R, Schippers JHM. ROS-mediated redox signaling during cell differentiation in plants. Biochim Biophys Acta. 2015;1850:1497–1508. doi: 10.1016/j.bbagen.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 73.Tsukagoshi H, et al. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 74.Liberman LM, et al. MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proc Natl Acad Sci. 2015;112:12099–12104. doi: 10.1073/pnas.1515576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubrovsky JG, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreno-Risueno MA, et al. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science. 2010;329:1306–11. doi: 10.1126/science.1191937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xuan W, et al. Root Cap-Derived Auxin Pre-patterns the Longitudinal Axis of the Arabidopsis Root. Curr Biol. 2015;25:1381–1388. doi: 10.1016/j.cub.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 78.Benková E, et al. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 79.Hofhuis H, et al. Phyllotaxis and rhizotaxis in Arabidopsis are modified by three PLETHORA transcription factors. Curr Biol. 2013;23:956–62. doi: 10.1016/j.cub.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 80.Laplaze L, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007;19:3889–900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, et al. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol. 2006;47:1112–23. doi: 10.1093/pcp/pcj082. [DOI] [PubMed] [Google Scholar]
- 82.Bielach A, et al. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell. 2012;24:3967–81. doi: 10.1105/tpc.112.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salamone IEG de, et al. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol. 2001;47:404–411. doi: 10.1139/w01-029. [DOI] [PubMed] [Google Scholar]
- 84.Timmusk S, et al. Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem. 1999;31:1847–1852. [Google Scholar]
- 85.Arkhipova TN, et al. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil. 2005;272:201–209. [Google Scholar]
- 86.Ortíz-Castro R, et al. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 2008;3:263–265. doi: 10.4161/psb.3.4.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalid A, et al. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol. 2004;96:473–480. doi: 10.1046/j.1365-2672.2003.02161.x. [DOI] [PubMed] [Google Scholar]
- 88.H A, et al. Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biol. Fertil Soils. 2002;35:231–237. [Google Scholar]
- 89.Lim JH, Kim SD. Synergistic Plant Growth Promotion by the Indigenous Auxins-producing PGPR Bacillus subtilis AH18 and Bacillus licheniforims K11. J Korean Soc Appl Biol Chem. 2009;52:531–538. [Google Scholar]
- 90.Remans R, et al. Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.) Plant Soil. 2007;302:149–161. [Google Scholar]
- 91.Spaepen S, et al. Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014;201:850–861. doi: 10.1111/nph.12590. [DOI] [PubMed] [Google Scholar]
- 92.Groenhagen U, et al. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014;80:758–71. doi: 10.1111/tpj.12666. [DOI] [PubMed] [Google Scholar]
- 93.Meldau DG, et al. Dimethyl disulfide produced by the naturally associated bacterium bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell. 2013;25:2731–47. doi: 10.1105/tpc.113.114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Contreras-Cornejo HA, et al. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009;149:1579–92. doi: 10.1104/pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Splivallo R, et al. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009;150:2018–29. doi: 10.1104/pp.109.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Felten J, et al. Lateral root stimulation in the early interaction between Arabidopsis thaliana and the ectomycorrhizal fungus Laccaria bicolor: is fungal auxin the trigger? Plant Signal Behav. 2009;5:864–7. doi: 10.4161/psb.5.7.11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Contreras-Cornejo HA, et al. Mitogen-Activated Protein Kinase 6 and Ethylene and Auxin Signaling Pathways Are Involved in Arabidopsis Root-System Architecture Alterations by Trichoderma atroviride. Mol Plant-Microbe Interact. 2015;28:701–710. doi: 10.1094/MPMI-01-15-0005-R. [DOI] [PubMed] [Google Scholar]
- 98.Garnica-Vergara A, et al. The volatile 6-pentyl-2 H -pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2015 doi: 10.1007/s11103-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 99.Shikuma NJ, et al. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science. 2014;343:529–33. doi: 10.1126/science.1246794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alegado RA, et al. A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals. Elife. 2012;2012:e00013. doi: 10.7554/eLife.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rawls JF, et al. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 2004;101:4596–601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bates JM, et al. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Cheesman SE, et al. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci U S A. 2011;108(Suppl):4570–4577. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao J, et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet. 2011;43:956–963. doi: 10.1038/ng.911. [DOI] [PubMed] [Google Scholar]
- 105.Schmitz RJ, et al. Patterns of population epigenomic diversity. Nature. 2013;495:193–8. doi: 10.1038/nature11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bucksch A, et al. Image-Based High-Throughput Field Phenotyping of Crop Roots. Plant Physiol. 2014;166:470–486. doi: 10.1104/pp.114.243519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Topp CN, et al. 3D phenotyping and quantitative trait locus mapping identify core regions of the rice genome controlling root architecture. Proc Natl Acad Sci U S A. 2013;110:E1695–704. doi: 10.1073/pnas.1304354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mairhofer S, et al. Recovering complete plant root system architectures from soil via X-ray mu-Computed Tomography. Plant. 2013 doi: 10.1186/1746-4811-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keyes SD, et al. High resolution synchrotron imaging of wheat root hairs growing in soil and image based modelling of phosphate uptake. New Phytol. 2013;198:1023–1029. doi: 10.1111/nph.12294. [DOI] [PubMed] [Google Scholar]
- 110.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loman NJ, et al. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol. 2012;10:599–606. doi: 10.1038/nrmicro2850. [DOI] [PubMed] [Google Scholar]


