Abstract
Purpose:
The purpose of this study is to assess the outcomes of a consecutive series of patients who underwent revision surgery after humeral head resurfacing (HHR). Our joint registry was queried for all patients who underwent revision arthroplasty for failed HHR at our institution from 2005 to 2010. Eleven consecutive patients (average age 54 years; range 38-69 years) that underwent revision of 11 resurfacing arthroplasties were identified. The primary indication for resurfacing had been osteoarthritis in six, glenoid dysplasia in two, a chondral lesion in two, and postinstability arthropathy in one patient. The indication for revision was pain in 10 and infection in one patient. Seven patients had undergone an average of 1.9 surgeries prior to resurfacing (range 1-3).
Materials and Methods:
All patients were revised to stemmed arthroplasties, including one hemiarthroplasty, two reverse, and eight anatomic total shoulder arthroplasties at a mean 33 months after primary resurfacing (range 10-131 months). A deltopectoral approach was used in seven patients; four patients required an anteromedial approach due to severe scarring. Subscapularis attenuation was found in four cases, two of which required reverse total shoulder arthroplasty. Bone grafting was required in one glenoid and three humeri.
Results:
At a mean follow-up of 3.5 years (range 1.6-6.9 years), modified Neer score was rated as satisfactory in five patients and unsatisfactory in six. Abduction and external rotation improved from 73° to 88° (P = 0.32) and from 23° to 32° (P = 0.28) respectively. Reoperation was required in two patients, including one hematoma and one revision for instability.
Conclusion:
Outcomes of revision of HHR arthroplasty in this cohort did not improve upon those reported for revision of stemmed humeral implants. A comparative study would be required to allow for definitive conclusions to be made.
Keywords: Humeral head resurfacing, revision, shoulder arthroplasty
INTRODUCTION
Humeral head resurfacing (HHR) has gained increasing use over recent years as an alternative to stemmed arthroplasty for the treatment of shoulder arthropathy.[1,2] Complete HHR with or without replacement of the glenoid surface has been shown to lead to satisfactory outcomes and low revision rates for the management of osteoarthritis and rheumatoid arthritis of the shoulder.[2,3] Partial resurfacing has been proposed for the management of focal head defects ranging from chondral lesions to limited head collapse after avascular necrosis.[4,5] Since HHR requires limited bone resection, it is frequently proposed as a preferred option in young, active patients in whom revision surgery is likely to occur at some point in their lives.[6] Besides accurately reproducing the native humeral anatomy by adequately restoring articular retroversion, neck shaft angulation, offset and center of instant rotation,[7,8] revision to a stemmed humeral component is theorized to be facilitated, since the implant used for resurfacing is located within the native humeral head.[1,2,3,6]
While rates of revision after HHR have been found to be similar to those of conventional arthroplasty,[1,2,3] little is known about the outcomes of revision of resurfacing arthroplasty. The purpose of this study was to determine the results, complications, and rate of additional revision surgery of patients who underwent revision surgery after HHR.
MATERIALS AND METHODS
After obtaining IRB approval, our institutional arthroplasty database was queried for all patients that, between January of 2005 and December of 2010, had undergone revision at our institution of a previously performed HHR. A total of 11 consecutive patients were identified and included. Causes for revision were pain believed to be secondary to glenoid erosion in 10 patients, defined as pain during mid-arc of motion and effacement of glenohumeral joint space on standard radiographs. In two of these cases, gross loosening of the humeral component was observed at the time of surgery, suggesting this to have contributed to preoperative pain. One additional patient underwent staged revision for infection. A detailed description of included cases is provided in Tables 1–3. All except one patient had completed at least 2 years of follow-up. One patient was lost to follow-up at 19 months and was included in the study (Case 10). The follow-up included clinical assessment of pain, range of motion, and patient satisfaction allowing calculation of a validated modified Neer score.[9,10] According to this scale, results are graded as excellent, satisfactory or unsatisfactory. An excellent result is defined as no or slight pain, external rotation of at least 45°, active abduction to at least 140°, in a patient who was satisfied with the result. The result is graded satisfactory with no or slight pain or moderate pain only with vigorous activity, external rotation to at least 20°, active abduction to at least 90°, in a patient who was satisfied with the outcome. The result is considered unsatisfactory if any of the criteria for a satisfactory result are not met, or if the patient had had an additional operative procedure.
Table 1.
Baseline characteristics
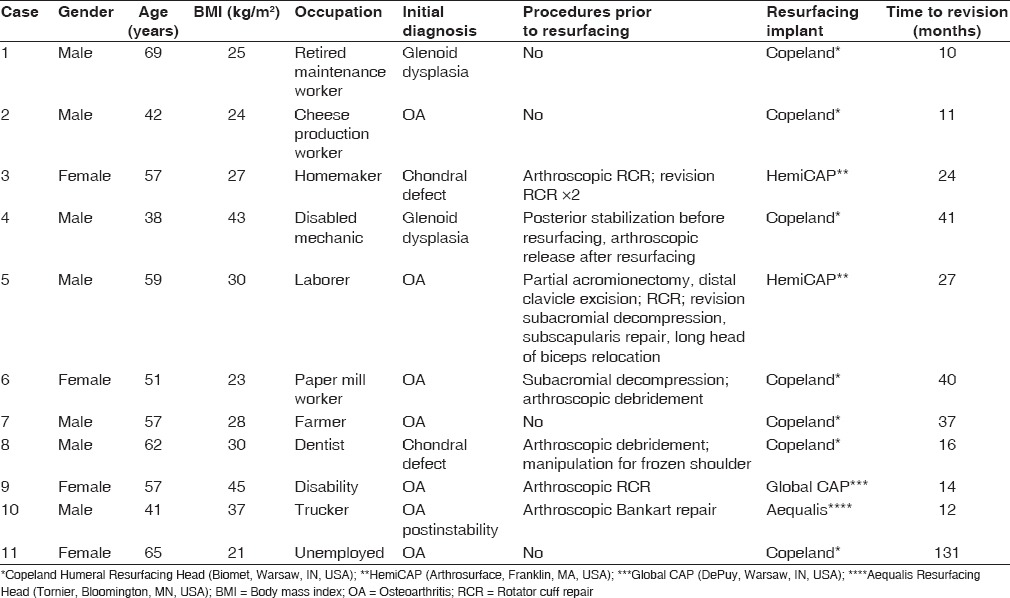
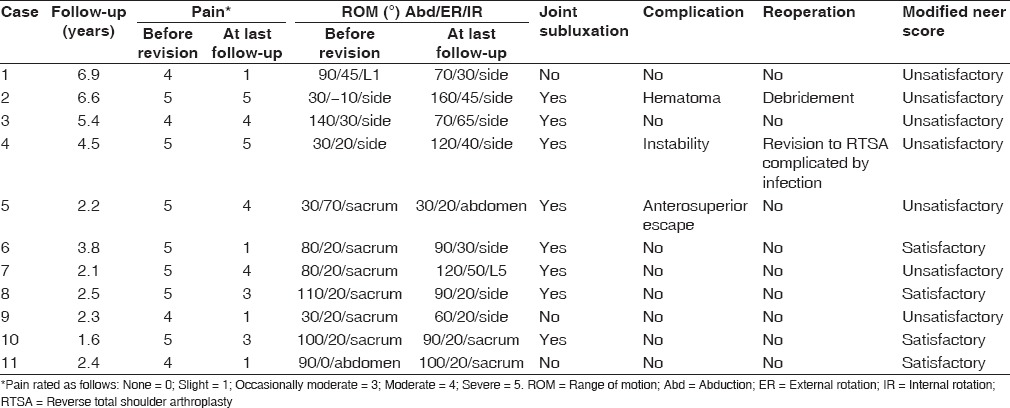
Table 3.
Outcomes
Radiographic assessment was performed using an axillary view and AP views in the plane of the scapula (Grashey) with the humerus in external and internal rotation taken at the time of last follow-up. Periprosthetic lucencies were measured around glenoid and humeral components as described by Sperling et al.[11] A glenoid component at risk for loosening was defined as one that had either shifted in position, or that had a complete lucent line with part of it measuring at least 1.5 mm in width. A humeral component at risk for loosening was defined as one that had either shifted or that had a lucent line 2 mm or greater in width present in 3 of 8 radiographic zones. Glenohumeral subluxation was graded according to a translation of the center of the prosthetic head relative to the center of the glenoid component. Subluxation was graded as absent if the translation was less than 25% and as present if the translation was >25%.[12]
There were seven males and four females with an average age of 54 years (range 38-69 years). Average body mass index was 30.2 kg/m2 (range 21-45 kg/m2). Five patients were heavy laborers, four were either disabled, retired or unemployed, and there were one dentist and one homemaker. The dominant extremity had been affected in seven cases.
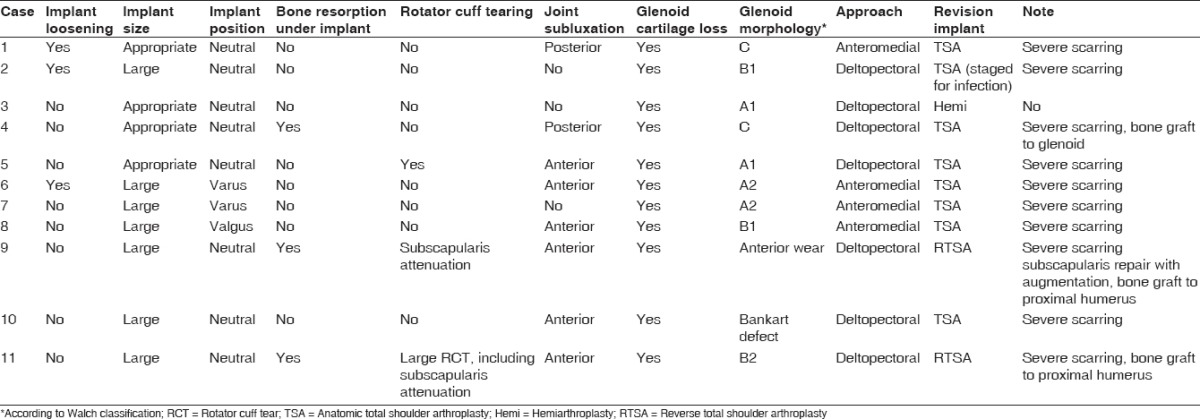
The diagnosis for the initial resurfacing procedure had been osteoarthritis in six, glenoid dysplasia in two, a chondral lesion in two, and postinstability arthropathy in one patient. Seven patients had undergone an average of 1.9 surgeries prior to HHR (range 1-3). Procedures included rotator cuff repair in three patients, diagnostic arthroscopy in one patient, isolated subacromial decompression in two patients, arthroscopically assisted Bankart repair in one patient, posterior stabilization in one patient, and manipulation under anesthesia for stiffness in one patient. One patient (Case 4) underwent posterior stabilization for glenoid dysplasia before and arthroscopic debridement after HHR. The implant used for primary HHR had been Copeland Humeral Resurfacing Head (Biomet, Warsaw, IN, USA) in seven cases [Figure 1], HemiCAP (Arthrosurface, Franklin, MA, USA) in two cases [Figure 2] and Aequalis Resurfacing Head (Tornier, Bloomington, MN, USA) and Global CAP (DePuy, Warsaw, IN, USA) in one case each. None of the patients had undergone glenoid resurfacing at the time of HHR. Nine patients had been referred form elsewhere and two had undergone resurfacing at our institution. At revision, glenoid morphology had been classified according to Walch et al.[13] as A1 in two, A2 in two, B1 in two, B2 in one, and C in two patients. Two patients exhibited anterior glenoid deficiencies, one secondary to a failed Bankart repair and one due to anterior wear.
Figure 1.
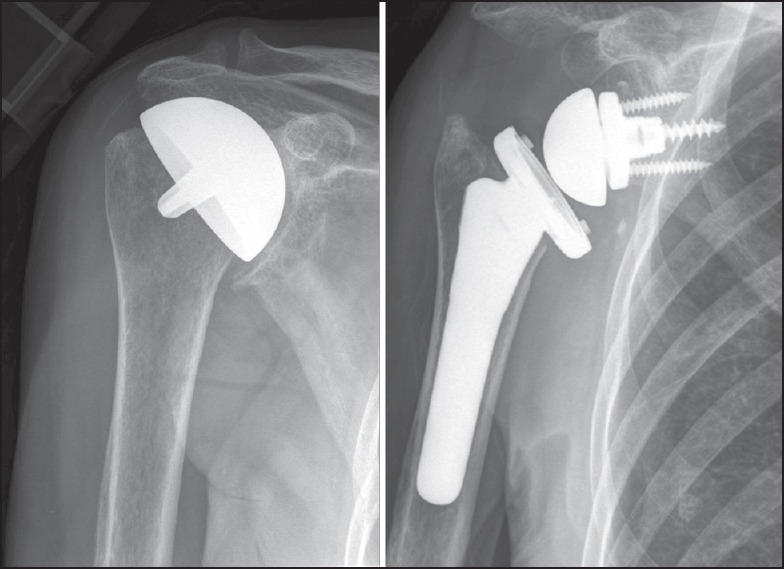
Case 11. (a) Anteroposterior view prior to revision of a complete humeral head resurfacing hemiarthroplasty. An oversized implant had been placed, impinging on the rotator foot print. (b) Due to severe rotator cuff defi ciency, revision with a reverse total shoulder arthroplasty was performed
Figure 2.
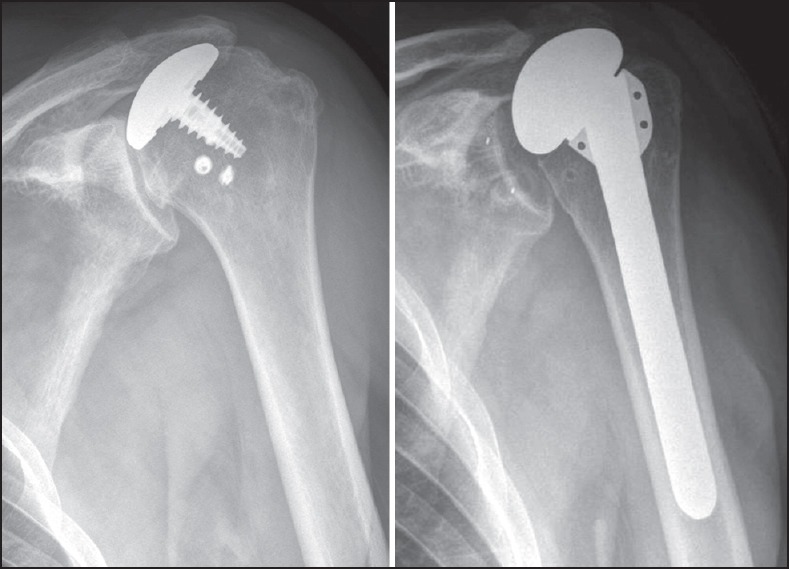
Case 5. (a) Anteroposterior view prior to revision of a partial humeral head resurfacing hemiarthroplasty in a patient with previous history of rotator cuff repair. (b) Radiographs at 26 months of follow-up show an anatomic total shoulder arthroplasty without signs of loosening but with significant superior migration of the humeral component
Mean time from primary HHR to revision surgery was 33 months (range 10-131 months). Patients underwent revision to a stemmed hemiarthroplasty in one patient (Cofield 2, noncemented stem, Smith and Nephew, Memphis, TN, USA) and a reverse total shoulder arthroplasty (RTSA) in two patients (Comprehensive Shoulder, noncemented, Biomet, Warsaw, IN, USA). In addition, a total of eight patients underwent anatomic total shoulder arthroplasty (ATSA): One Cofield 2 cemented, three Cofield 2 noncemented (Smith and Nephew, Memphis, TN, USA), two Aequalis noncemented (Tornier, Bloomington, MN, USA), three Comprehensive Shoulder, noncemented (Biomet, Warsaw, IN, USA); all glenoids were resurfaced using cemented all-polyethylene components. One ATSA was implanted as a staged procedure after placement of an antibiotic-laden cement spacer and 6 weeks of organism-specific intravenous antibiotics in a patient with preoperatively diagnosed infection based on clinical and laboratory findings. Intraoperative cultures were positive for Propionibacterium acnes.
RESULTS
Operative findings and techniques
All original HHR components were removed at the time of revision surgery; two components (complete resurfacing components) were grossly loose. Bone loss was noted in three proximal humeri at the time of revision, requiring cancellous bone allograft at the time of revision in two cases to improve press fitting of noncemented humeral stemmed implants. Bone graft to the humeral head included particulate bone graft in one case, and severe scarring of the subdeltoid space was present in all, except one case. In seven instances, the shoulder could be safely exposed using a deltopectoral approach. However, in four cases, due to advanced scarring and attenuation of the underlying rotator cuff, an anteromedial approach with anterior deltoid detachment from the anterior clavicle and acromion was required.[14] Subscapularis attenuation, defined as a nonrepairable subscapularis was found in two cases (Cases 9 and 11). In both cases a RTSA was performed. In one of these, a porcine dermis xenograft (Conexa, Tornier, Bloomington, MN, USA) was used to bridge the defect between the subscapularis and lesser tuberosity. In one case, a rotator cuff was repaired through bone tunnels after implantation of ATSA (Case 5). Glenoid bone grafting was required in one patient with underlying glenoid dysplasia who underwent revision to an ATSA.
Statistical analysis
Descriptive statistics included absolute counts and percentages for categorical data and means and ranges for continuous data. Continuous variables for pre- and post-revision values were compared with paired two-tailed Student's t-test. Statistical significance was set at P < 0.05. Statistical analysis was performed with SPSS® Version 16.0 for Windows® (SPSS Inc., Chicago, IL, USA).
At a mean follow-up of 3.5 years (range 1.6-6.9 years), two patients required reoperation. Surgical incision and drainage of a wound hematoma were required 1 week after final component implantation in the patient that had undergone staged revision for infection (Case 2). One patient who was revised to ATSA with glenoid bone graft due to glenoid dysplasia had recurrent posterior instability 2 years after revision, requiring repeat revision to RTSA. This procedure was further complicated by deep infection by P. acnes and S. epidermidis (Case 4). One patient presented with clinically frank anterosuperior escape with pseudoparalysis of the shoulder 2 years after revision to an ATSA (Case 5).
At final follow-up, average abduction and external rotation were 88° (range 30° to 160°) and 32° (range 20° to 65°) respectively. This had improved from a mean 73° (range 30° to 140°) of abduction and 23° (range −10° to 70°) of external rotation preoperatively. However, this difference was not statistically significant (P = 0.32 for abduction and P = 0.28 for external rotation). There was a significant improvement in pain from a mean 4.6 points (range 4-5) before revision to 2.9 (range 1-5) after revision (P = 0.005). Based on the modified Neer score, results were rated as satisfactory in four patients and unsatisfactory in seven. Unsatisfactory results were due to repeat surgery in two cases, abduction of less than 90° in four cases, and a pain score of 4 or higher in 5 in five patients [Table 3].
Radiographic assessment of retained implants at the time of last follow-up did not show any loose implant or at risk of loosening. Of the nine patients revised to an ATSA, eight had some level of anterior subluxation with associated superior migration of the humeral head, including one patient that presented with frank anterosuperior escape during final follow-up. In only one patient was head migration controlled using an anatomic component [Table 3].
DISCUSSION
The purpose of this study was to describe the results, complications, and rate of additional revision surgery of patients who underwent revision surgery after HHR. Our study found that at an average of 3.5 years, unsatisfactory results are achieved in over half of patients. This shorter follow-up seemed worthwhile to report as so many outcomes were rather poor, and we doubted improvement would occur with time. An extensile approach due to soft tissue scarring was required in one-third of cases and humeral head bone grafting in one-fifth of cases. Reoperation was required in two patients, one of which required repeat revision arthroplasty. One single de novo infection occurred.
Rotator cuff insufficiency at the time of revision required a RTSA in two cases and preceded delayed superior migration after revision ATSA in eight cases. Of these, several had HHR implants that were too large as shown in Table 2. While a causative relationship cannot be established with the current study, it appears logical that, as for stemmed arthroplasties, having a large overstuffed resurfacing implant is likely to cause a rotator cuff deficient shoulder.[11,15,16]
Table 2.
Intraoperative findings
There are no other series of revision resurfacing procedures we can compare with this study. Several studies have assessed the outcomes of revision surgery after stemmed arthroplasty with most showing consistent improvement in pain and range of motion with low complication rates.[11,15,17,18,19,20,21] However, unsatisfactory functional outcomes occur in up to one-half of these patients,[11,15] and were even more prevalent in our study.
Glenoid component implantation is a key aspect of revision surgery for painful stemmed hemiarthroplasty. In most instances, modular humeral components can be retained if adequately implanted, as shown by Groh and Wirth In their study on revision arthroplasty of 15 painful stemmed hemiarthroplasties, humeral component revision was required in only two cases. Based on the University of California-Los Angeles shoulder score, good or excellent results were achieved in 14 of 15 patients. However, humeral component revision in the setting of painful stemmed hemiarthroplasty is required for implant malposition and component loosening.[11] Furthermore, humeral stem revision is required for glenoid exposure in the presence of monoblock humeral components and for older implants when revision to a RTSA is required, a scenario that is analogous to that of HHR.
As reported by other studies on revision shoulder arthroplasty, the majority of cases of our series had undergone primary arthroplasty at a different institution.[20] This may suggest that primary surgeries are often performed at institutions with lower procedure specific volumes and revised at higher volume centers. Research on both lower and upper extremity arthroplasty has shown patient outcomes to be influenced by the surgeon and institutional experience. For shoulder arthroplasty specifically, surgeons with higher caseloads of shoulder arthroplasties have decreased complication rates.[22] While no such data exists to our knowledge for HHR, this phenomenon likely plays a role in outcomes.[7,8] It is the authors' perception that HHR likely represents a more complex procedure compared to stemmed humeral head replacement since adequate exposure is more difficult to obtain, contributing to inaccurate identification and sizing of the anatomic neck and placement of an implant that is too large or in malaligned. Furthermore, glenoid exposure to allow for implantation of a glenoid component requires extensive experience and skill. As has been demonstrated in multiple studies, persistent pain after hemiarthroplasty is the main cause for revision surgery, significantly exceeding that of revision for loose glenoid components after ATSA.[11,15,16]
One of the main difficulties we found with regards to revision surgery was surgical exposure to safely preserve the rotator cuff. Inadequate surgical release during primary HHR and suboptimal implantation of the humeral implant may have led to higher than expected scarring of the soft tissues. In four instances, an extensile anteromedial approach was required to allow accurate identification of the subacromial space, with the goal of preserving the rotator cuff tendons. In two instances, rotator cuff pathology required implantation of a RTSA. Furthermore, Cases 3, 4, 5 and 7 had joint subluxation on follow-up radiographs after undergoing revision ATSA, suggesting that rotator cuff function may not be predictable at the time of revision. At the present time, these cases would potentially have been revised with a RTSA. However, this study spans a period of time during which RTSA was being introduced to our practice under narrow indications, including cuff tear arthropathy and nonrepairable rotator cuff disruption at the time of revision arthroplasty.
Cil et al. observed 71% satisfactory results based on the modified Neer rating system after 35 stemmed humeral component revisions after aseptic loosening. Reoperations were required in four patients at a mean 7 years of follow-up. Similar to our study, bone grafting was required in less than one-third of cases. While these results compare favorably to our outcomes, the authors reported several intraoperative complications, including cement extrusion in eight, fracture of the shaft of the humerus in two and of the tuberosity in four cases.[17] In our series, no intraoperative fractures occurred and due to the implantation of cementless humeral components, no cement related complications occurred.
Two patients in our series underwent revision to a RTSA. In both instances, the primary diagnosis for resurfacing had been osteoarthritis, in one instance with a previous history of an arthroscopically assisted rotator cuff repair. While the main complaint to undergo revision arthroplasty had been pain, revision to a reversed implant was elected intraoperatively due to an incompetent rotator cuff. Levy et al. showed that RTSA significantly improves function and pain for revision of stemmed hemiarthroplasties performed in the setting of glenohumeral arthritis with rotator cuff deficiency. However, 32% prosthesis and 16% nonprosthesis related complication occurred in their study.[21] Reoperation rates ranging between 20% and 40% have been reported in this setting.[21,23,24]
This study is the first to specifically analyze a consecutive series of patients with prior HHR who underwent revision surgery at a single institution. While the overall results suggest that revision surgery after HHR may yield less favorable outcomes to those of revision of stemmed shoulder hemiarthroplasty, a control group for direct comparison was not available. However, the large proportion of revised implants that was either in suboptimal placement or inadequately sized, and the presence of several confounding variables, including prior surgery, mixed underlying pathology and the use of different implant designs make reaching a conclusion difficult. Furthermore, due to the limited sample size, risk factors for poor outcome or failure could not be established.
CONCLUSION
In summary, our study showed a high rate of unsatisfactory outcomes after revision shoulder arthroplasty for failed HHR. Outcomes are influenced by the need of repeat surgery, poor range of motion and pain. Furthermore, rotator cuff insufficiency may either require RTSA or may be related to a high rate of radiographic proximal humeral migration despite the theoretical benefits of bone preservation after HHR. Despite the benefit of preserving bone stock and the absence of greater tuberosity and humeral shaft fractures, clinical outcomes were generally unsatisfactory, suggesting that in this setting soft tissues significantly influence the outcomes of revision shoulder arthroplasty.
Based on the findings of our study, it cannot be concluded that humeral head arthroplasty provides any tangible benefit over stemmed humeral implants at the time of revision surgery. A comparative study would be required to allow for definitive conclusions to be made.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Levy O, Copeland SA. Cementless surface replacement arthroplasty of the shoulder 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br. 2001;83:213–21. doi: 10.1302/0301-620x.83b2.11238. [DOI] [PubMed] [Google Scholar]
- 2.Levy O, Funk L, Sforza G, Copeland SA. Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surg Am. 2004;86-A:512–8. doi: 10.2106/00004623-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg. 2004;13:266–71. doi: 10.1016/j.jse.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Delaney RA, Freehill MT, Higgins LD, Warner JJ. Durability of partial humeral head resurfacing. J Shoulder Elbow Surg. 2014;23:e14–22. doi: 10.1016/j.jse.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Uribe JW, Botto-van Bemden A. Partial humeral head resurfacing for osteonecrosis. J Shoulder Elbow Surg. 2009;18:711–6. doi: 10.1016/j.jse.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90:110–7. doi: 10.2106/JBJS.F.01552. [DOI] [PubMed] [Google Scholar]
- 7.Burgess DL, McGrath MS, Bonutti PM, Marker DR, Delanois RE, Mont MA. Shoulder resurfacing. J Bone Joint Surg Am. 2009;91:1228–38. doi: 10.2106/JBJS.H.01082. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SR, Sforza G, Levy O, Copeland SA. Geometrical analysis of Copeland surface replacement shoulder arthroplasty in relation to normal anatomy. J Shoulder Elbow Surg. 2005;14:186–92. doi: 10.1016/j.jse.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66:899–906. doi: 10.2106/00004623-198466060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–37. [PubMed] [Google Scholar]
- 11.Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9:507–13. doi: 10.1067/mse.2000.109384. [DOI] [PubMed] [Google Scholar]
- 12.Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13:604–13. doi: 10.1016/S1058274604001296. [DOI] [PubMed] [Google Scholar]
- 13.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–60. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 14.Foruria AM, Oh LS, Sperling JW, Cofield RH. Anteromedial approach for shoulder arthroplasty: Current indications, complications, and results. J Shoulder Elbow Surg. 2010;19:734–8. doi: 10.1016/j.jse.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Carroll RM, Izquierdo R, Vazquez M, Blaine TA, Levine WN, Bigliani LU. Conversion of painful hemiarthroplasty to total shoulder arthroplasty: Long-term results. J Shoulder Elbow Surg. 2004;13:599–603. doi: 10.1016/j.jse.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen JV, Jakobsen J, Brorson S, Olsen BS. The danish shoulder arthroplasty registry: Clinical outcome and short-term survival of 2,137 primary shoulder replacements. Acta Orthop. 2012;83:171–3. doi: 10.3109/17453674.2012.665327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91:75–81. doi: 10.1302/0301-620X.91B1.21094. [DOI] [PubMed] [Google Scholar]
- 18.Deutsch A, Abboud JA, Kelly J, Mody M, Norris T, Ramsey ML, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16:706–16. doi: 10.1016/j.jse.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Dines JS, Fealy S, Strauss EJ, Allen A, Craig EV, Warren RF, et al. Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg Am. 2006;88:1494–500. doi: 10.2106/JBJS.D.02946. [DOI] [PubMed] [Google Scholar]
- 20.Groh GI, Wirth MA. Results of revision from hemiarthroplasty to total shoulder arthroplasty utilizing modular component systems. J Shoulder Elbow Surg. 2011;20:778–82. doi: 10.1016/j.jse.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89:189–95. doi: 10.1302/0301-620X.89B2.18161. [DOI] [PubMed] [Google Scholar]
- 22.Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A:2318–24. doi: 10.2106/00004623-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: Design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–61. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–86. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]


