Abstract
Young patients with glenohumeral arthritis are an ongoing treatment challenge. They typically have high demands of their shoulders, require long-term durability due to their young age, and often have altered local anatomy, through their disease process (instability arthropathy, juvenile rheumatoid arthritis, etc.) or from previous surgery (capsulorraphy arthropathy, chondrolysis, etc.). Workup to evaluate underlying causes of early arthritis, and to exclude infectious causes are necessary. When nonoperative management fails, arthroscopic debridement, hemiarthroplasty (isolated, with glenoid reaming, or with biological interposition), and total shoulder arthroplasty are treatment options available to the treating surgeon. Debridement or hemiarthroplasty can provide pain relief for a subset of patients, but results have not been reproducible across the literature and have not been durable over time. Total shoulder arthroplasty provides the most reliable pain relief, but long-term glenoid loosening and wear continue to lead to high revision rates in this patient population.
Keywords: Biological resurfacing, chondrolysis, glenoid dysplasia, hemiarthroplasty, inflammatory arthritis, osteonecrosis, shoulder arthroplasty, young
INTRODUCTION
Shoulder arthritis in the elderly is treated at increasing rates and with increasing effectiveness with total shoulder arthroplasty.[1] In this patient population, excellent pain relief and improved range of motion (ROM) are the expected outcome. In addition, with high implant survivorship rates, revision surgery is uncommon. In young patients, however, surgical options become much more limited. Young patients have higher demands of their arms, they require significantly longer survivorship given their life expectancy, and many have higher postoperative expectations. To confound the problem, young patients with severe glenohumeral (GH) arthritis more commonly have atypical presentations, including previous surgical procedures, posttraumatic altered bony morphology, glenoid dysplasia/hypoplasia, inflammatory arthropathy, and previous infection.[2,3,4,5,6]
Chondrolysis
Chondrolysis is the underlying diagnosis in a growing number of young patients with GH arthritis. While there is an ongoing debate about the specific etiology of chondrolysis, the clinical picture is that of rapid loss of articular cartilage from the humerus and glenoid in the absence of osteophyte formation.[7] Pain pumps, prominent suture anchors, thermal damage, infection, and knot abrasion have been proposed as mechanisms for this rapid cartilage deterioration. Furthermore, there has been a rapid increase in the number of cases reported in recent years, with more than 213 cases reported in the literature.[8] While it is the subject of intense ongoing research, recent data indicate a high likelihood that intraarticular pain pumps are the primary cause of this rapid and catastrophic process.[7,8,9] Modern shoulder arthroplasty solutions have been employed with success in these patients, but ultimately, prevention is the key [Figure 1].
Figure 1.
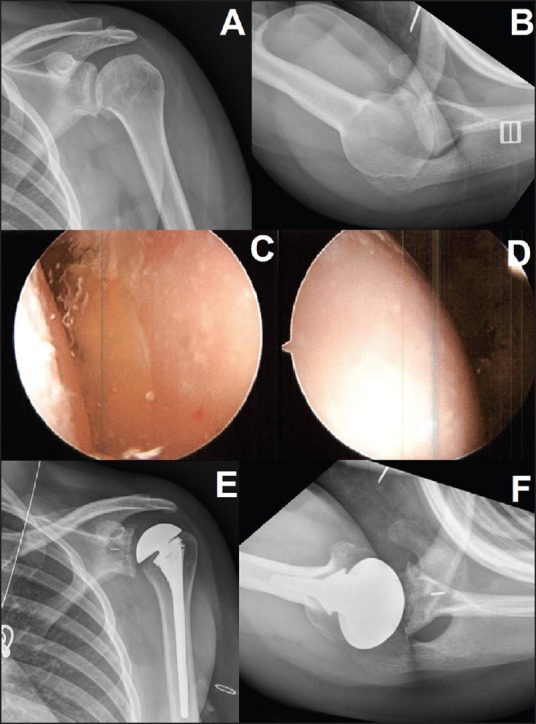
A 24-year-old female with glenohumeral arthritis due to chondrolysis 6 years after arthroscopic labral repair with an intraarticular pain pump. Presenting X-rays are labeled (a and b). Initial management with arthroscopic debridement, capsular release, loose body and suture removal, and biopsy arthroscopic images of the glenoid (c), and humerus (d). One year later she underwent total shoulder arthroplasty for continued pain and dysfunction (e and f)
Inflammatory arthritis
Another cause of GH arthritis in the young patient is inflammatory arthropathy, especially juvenile rheumatoid arthritis.[10] Modern medical management has significantly decreased the burden of these diseases and delayed the necessity of surgical management. In symptomatic patients, however, synovectomy can provide some delayed disease progression and symptomatic relief for many of these patients, especially when some joint space remains.[11] However, a significant proportion of young patients requiring shoulder arthroplasty have inflammatory arthritis.[12,13] Hemiarthroplasty is often less appealing due to concentric joint involvement, with glenoid cartilage loss. Further, bone quality can be considerably compromised, with profound glenoid bone loss/deformity and osteopenia.[12,13] Shoulder arthroplasty, nonetheless, has provided excellent long-term outcomes in patients with end stage joint involvement who fail nonoperative management.[12]
Instability
One challenging group of patients presenting with GH arthritis at a young age are patients with recurrent shoulder instability, with or without instability operations.[14,15] Arthritis is frequently seen in these patients. It is believed to result from repetitive trauma to the articular surfaces of the joint, abnormal loading of the joint surfaces, as well as to “overtightening” of the joint with instability operations. Several historical operations, including the Putti-Platt and Eden Hybbinette, have been shown to have high rates of arthritis in long-term studies.[16,17,18,19,20] More modern stabilizing techniques, including arthroscopic and open Bankart, have been associated with low rates of arthrosis.[2,4,21,22] In addition, there is less alteration of anatomy than with historical options such as the Putti-Platt. Furthermore, arthroscopic Bankart repair allows capsulolabral repair without violating the subscapularis, avoiding potential iatrogenic subscapularis failure. In some cases of instability, especially with glenoid bone loss, the Latarjet procedure is indicated. While long-term follow-up studies have demonstrated modest rates of arthrosis, there is a profound alteration of the surgical planes following this operation.[23,24,25,26] This may complicate subsequent shoulder arthroplasty or other joint preserving options in the setting of subsequent arthritis.
Osteonecrosis
Young patients with osteonecrosis (or avascular necrosis [AVN]) frequently present with GH changes. Furthermore, changes are often bilateral, making treatment even more challenging. While a number of risk factors have been found and studied for AVN, including sickle cell disease, alcohol abuse, clotting disorders, and steroid-induced AVN, many cases are idiopathic.[27,28,29,30] Early treatment, including arthroscopically assisted core decompression, is advocated for “precollapse” patients with symptomatic AVN that fail nonoperative management.[31,32,33,34] A significant number of these patients, however, go on to have a humeral head collapse, and may present with significant degenerative changes. Historical results with shoulder hemiarthroplasty in patients without significant glenoid changes have been encouraging [Figure 2].[5,28,30,35] If, however, significant glenoid cartilage loss is seen, total shoulder arthroplasty may be indicated.[5,28,30,35]
Figure 2.
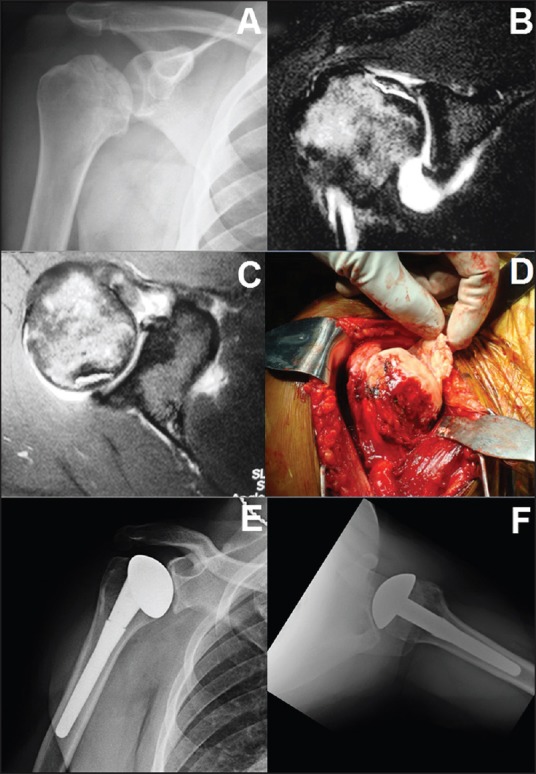
A 40-year-old with right shoulder pain and avascular necrosis secondary to previous steroid use radiograph (a). Magnetic resonance imaging demonstrated subchondral collapse, with moderate glenoid cartilage thinning (b and c). Intraoperatively, a large area of cartilage separation with necrotic subchondral bone was apparent (d). Because of limited glenoid cartilage changes, hemiarthroplasty was completed, with an excellent result at 4 years postoperatively (e and f)
Glenoid dysplasia
A subset of young patients with GH arthritis will be diagnosed with glenoid dysplasia (or glenoid hypoplasia). This disease is defined as dysplasia of the posterior inferior glenoid and scapular neck, often with an enlarged labrum [Figure 3]. While it is rare in the general population (−14%), it has been demonstrated to predispose patients to early osteoarthritis.[36,37,38,39,40] In addition, it makes operative intervention challenging, due to glenoid retroversion and decreased glenoid bone stock. Several studies, however, have documented success with shoulder arthroplasty in this patient population. The largest series, involving 22 patients with an average of 6 years follow-up demonstrated improved pain and elevation with both total shoulder arthroplasty and hemiarthroplasty. Fifty percent of patients with hemiarthroplasty, however, had to be revised to total shoulder arthroplasty, all due to glenoid arthrosis. Glenoid component placement, however, was not without problems, with 3/14 shoulders requiring glenoid component revision during the study period.[36]
Figure 3.
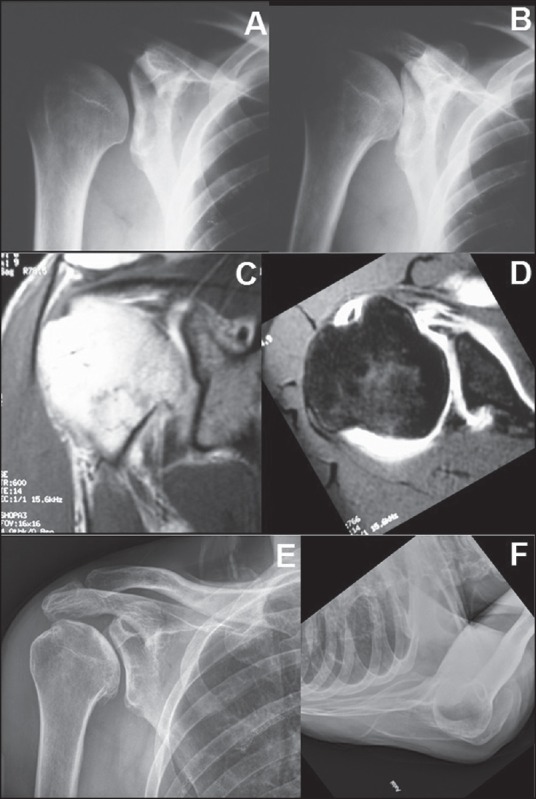
A 42-year-old male with right severe right shoulder pain. Radiographs consistent with glenoid dysplasia/hypoplasia (a and b). Magnetic resonance imaging demonstrated intact rotator cuff (c), with fi ndings characteristic of glenoid dysplasia/hypoplasia (d). Severe glenoid retroversion and posterior labral hypertrophy are evident. The patient was managed with activity modifi cation and occasional steroid injection with excellent result at 7 years (e and f)
Osteoarthritis
While many patients presenting with GH arthritis at a young age have other predisposing factors or causes, many will present with osteoarthritis that parallels that seen in the elderly patient population. This is often found to be associated with significant posterior joint subluxation, with or without posterior glenoid erosion.[41] Many of these patients have a history of repetitive joint loading activities, some with substantial repetitive loading (i.e., weightlifters). This patient population is especially difficult to manage, given their expectation of returning to heavy joint loading activities following return of pain free shoulder motion. Conventional treatment options including arthroscopic debridement, interposition arthroplasty, resurfacing arthroplasty, and standard shoulder arthroplasty will be detailed further.
WORKUP
Initial evaluation of the young patient with arthritis should begin with a thorough history and physical examination. The patient should be questioned about the quality, timing, and aggravating factors for shoulder pain, as well as localization of pain. This can often help distinguish GH pain from other pain generators, including the AC joint, the rotator cuff, and labral pathology. Questions about the history of joint trauma, instability, and timing of onset of pain can help distinguish the cause of joint degeneration. Further, a specific medical history to focus on steroid use (systemic, local, and inhaled), sickle cell disease, alcohol use, clotting disorders, history of malignancy, and autoimmune disease should be ascertained. Specific documentation of previous operations, including any postoperative fluid collections, wound healing difficulties, drainage, or repeat operations for infection can help evaluate for low-grade infection.
The physical examination should allow evaluation of several key factors. In patients with previous surgery, wounds should be noted as they may compromise subsequent surgical approaches. ROM should be documented, especially looking at external rotation. In cases of profound external rotation loss, consideration should be given to nerve monitoring if an anterior shoulder dissection is to be completed. Rotator cuff strength should be evaluated, in addition to careful assessment of subscapularis function. While in cases of severe arthritis (and stiffness), this may be difficult to assess, it is essential, especially in the setting of previous anterior shoulder surgery (open Bankart, Latarjet, etc.). Careful documentation of axillary nerve function is also important, as axillary neuropathy may alter treatment options.
The initial evaluation of these patients should include a radiographic evaluation. A preoperative anterior posterior (AP) with internal and external rotation and axillary view are generally sufficient. The AP radiographs should be evaluated for osteophyte formation, any evidence of superior subluxation, consistent with rotator cuff damage, altered proximal humeral anatomy as in the posttraumatic setting, and medialization of the humerus that can be seen with glenoid hypoplasia or glenoid bone loss. An axillary view allows evaluation of glenoid retroversion, dysplasia, GH subluxation or dislocation. Posterior subluxation, which may be accompanied by posterior glenoid erosion, is common in young patients with osteoarthritis. Surgical planning must take this into consideration (through soft tissue balancing, concentric reaming, augmented glenoid components, etc.).
Axial imaging can supplement the evaluation, and is helpful in many young patients, with complex histories and previous operations. Magnetic resonance imaging can allow a detailed evaluation of the rotator cuff (especially important in inflammatory and posttraumatic arthritis), subscapularis integrity (open Bankart repair, Latarjet, etc.), and can assess subchondral bone and/or cartilage changes. In cases involving significant bony deformity, computed tomography scan with three-dimensional reconstruction can allow more precise bony assessment and preoperative planning. This is especially true with posterior glenoid bone loss and glenoid hypoplasia.
In patients with a history of previous surgeries, or with atypical presentations, evaluation of the possibility of infection is critical. This can be initiated with laboratory studies, including complete blood cell count, erythrocyte sedimentation rate, and C-reactive protein. These labs may be normal in many low-grade infections that are common in the shoulder (propionibacterium acnes, staphylococcus epidermidis, etc.). Therefore, aspiration should be considered in patients considered to be high risk. Cultures should be maintained for 14 days to improve the yield of organisms, particularly propionibacterium acnes. In patients with a history of infection, consideration of a two-stage approach (arthroscopic or open irrigation and debridement, followed by oral or parenteral antibiotics) should be considered.
TREATMENT OPTIONS
Nonoperative treatment should be the initial treatment of choice for young patients with GH arthritis. Physical therapy, focusing on rotator cuff strengthening, flexibility, and pain relieving modalities may be beneficial, especially in early arthritis. Anti-inflammatory medications, when indicated, in addition to activity modifications can provide improvement in symptoms, and delay or eliminate the need for surgical treatment in many patients.
Additional, more invasive, nonoperative treatment options may be beneficial when these modalities fail, including corticosteroid injections, hyaluronic acid injections, and platelet rich plasma (PRP) supplementation. Intraarticular corticosteroid injections have been the traditional treatment of choice in this population, and can provide significant relief of shoulder pain in these patients. The efficacy and duration of improvement, however, are patient dependent. Further, many patients experience a decline in efficacy with subsequent injections. In addition, rotator cuff thinning and tearing can result from repeated injections, making this a treatment modality that should be used judiciously. Concerns about corticosteroid injections have led some to advocate for injections of hyaluronic acid and PRP. While these modalities have had some clinical success, further research into the efficacy of these agents, especially when compared with traditional corticosteroid preparations, is necessary before widespread use should be considered. There is currently no compelling evidence in the literature of superiority of these products over steroid injection.
When extensive nonoperative treatment modalities have failed, consideration of surgical intervention is indicated. In the young patient with shoulder arthritis, consideration should be given to arthroscopic debridement, hemiarthroplasty with or without reaming or biological resurfacing, and total shoulder arthroplasty. In extreme circumstances, GH arthrodesis or resection may be salvage options. Each of these options has advantages and disadvantages, and a customized treatment plan should be decided on based on individual patient's history, anatomy, comorbidities, surgeon experience/skill, and expectations.
GLENOHUMERAL DEBRIDEMENT
Patients who fail nonoperative treatment modalities should be considered for surgical intervention. While in the elderly patient with GH arthritis, this would generally be total shoulder arthroplasty, young patients are often considered for joint preserving operations. The most common joint preserving option is GH debridement. This allows the surgeon to have a direct assessment of the joint surfaces and the rotator cuff. In addition, pain generators can be addressed, including biceps tenotomy or tenodesis, removal of suture material or osteophytes and loose bodies. Synovial tissue biopsies can be obtained to rule out infectious causes. Generally, a formal capsular release is added to improve ROM, and potentially improve pathologic posterior subluxation.
Clinical studies of arthroscopic debridement have generally been favorable, with improved ROM and pain scores.[42,43,44] Worse results have been documented in the setting of large osteophytes, complete obliteration of GH joint space, and bipolar disease, each characteristic of later stages of degeneration. In each of these series, a significant number of patients progress to prosthetic arthroplasty. Some of the most recent data on debridement has been published on the “comprehensive arthroscopic management procedure,” which involves GH debridement, capsular release, biceps tenodesis, and axillary neurolysis. With this procedure, the authors were able to demonstrate modest improvement in pain, shoulder scores (American Shoulder and Elbow Surgeons [ASES] 58-83), and high patient satisfaction (median 9/10). They had a survivorship rate (free of arthroplasty) of 92% at 1 year and 85% at 2 years, suggesting that there may be a role for this procedure in delaying prosthetic arthroplasty.[45] A recent meta-analysis was completed, and based on available evidence, suggested that there was level IV evidence to suggest that debridement may improve ROM and patient satisfaction, but that with high levels of conversion to prosthetic arthroplasty there was no clearly defined role for debridement in this patient population.
ARTHROSCOPIC RESURFACING ARTHROPLASTY
An intermediate option for the young patient with shoulder arthritis between arthroscopic debridement and hemiarthroplasty, which requires an open approach and violation of the subscapularis, is arthroscopic resurfacing arthroplasty. There have been several variations in this technique that have been reported in the literature. Arthroscopic resurfacing with meniscal allograft was first described by Pennington and Bartz.[46] Other techniques have involved a modification of graft material (Graftjacket, Restore patch).[47,48,49] More recently, arthroscopic partial humeral head resurfacing with metallic implants has been described in the literature, with promising early results.[50] While these options are appealing, in terms of preserving soft tissue and bone stock in young patients, data are limited to small, designer case series of these procedures, which makes decision making about their success challenging. At this point, there is not enough data to support or reject these techniques.
HEMIARTHROPLASTY/RESURFACING HEMIARTHROPLASTY
While the popularity of hemiarthroplasty has waned in recent years due to accumulating evidence of improved pain relief and functional outcomes with total shoulder arthroplasty, there remain certain indications in which it is a reasonable option. In patients with AVN, with minimal or no glenoid sided arthritic changes, hemiarthroplasty may be the treatment of choice. While some series have documented improved ROM and pain relief with hemiarthroplasty for AVN, others have demonstrated less consistent results, with up to 45% unsatisfactory results.[28,30,51] Hemiarthroplasty has also been used with some success in inflammatory arthritis, with one series demonstrating 90% 5-year survival rates in a large series of patients with rheumatoid arthritis.[12] This is especially advantageous given the profound glenoid bone loss is often seen in these patients, as well as the propensity for rotator cuff tears. No series in the literature, however, has clearly documented the effectiveness of hemiarthroplasty for young patients with rheumatoid arthritis, who may have higher demands of their shoulders.
In addition to these specific groups of patients, hemiarthroplasty is an attractive option for the young patient with advanced osteoarthritis. Glenoid component loosening is a frequent cause of failure of total shoulder arthroplasty, occurring in up to 38% of total shoulder arthroplasties in young patients at 10 years.[52] Avoiding placement of a glenoid component avoids this complication. Frequently surgeons will allow unrestricted activity following hemiarthroplasty, which is appealing to the young, active patient population. Clinical results have demonstrated consistently improved ROM and pain, although less so than expected with total shoulder arthroplasty. One large series demonstrated 92% survivorship at 5 years; however 47% of patients were described as having “unsatisfactory” results.[53] This has been corroborated in other series, which document early improvements in pain and function, with higher intermediate and long-term failure rates due to glenoid arthrosis.[54,55,56] In addition, many young patients have posterior subluxation and posterior bone loss, with Walch B2 glenoid morphology.[57] Hemiarthroplasty alone will not correct this bony deformity. In patients with a centered humeral head and posterior bone loss, who are unwilling to accept postoperative activity restrictions, hemiarthroplasty may be considered as a reasonable treatment option, preserving bone stock in case of subsequent operations.
Humeral resurfacing has been advocated as an alternative to stemmed humeral hemiarthroplasty. It has the potential advantage of preserving proximal humeral metaphyseal bone stock. Overall, these implants have had low rates of loosening, with clinical results that mirror those of stemmed hemiarthroplasty.[19,58,59] They may be especially valuable in cases of distorted proximal humeral anatomy such as in developmental abnormalities or posttraumatic settings. Accurate recreation of anatomy with resurfacing arthroplasty has proved challenging, leading to concern that many implants are too large, leaving the joint “overstuffed.” This was confirmed in one study.[60] Another study refuted this but found that many components were placed in varus, indicating the technical nature of this procedure.[61] Given the lack of compelling evidence of the benefit of resurfacing arthroplasty in the literature and the technical nature of the component placement, their role in the treatment of young patients with GH arthritis is not clear.
HEMIARTHROPLASTY WITH GLENOID REAMING (REAM AND RUN)
Saltzman et al. have popularized a combination of hemiarthroplasty with concentric reaming of the glenoid as a potential option for patients with GH arthritis.[62] This allows centering of the humeral component on the glenoid, and putatively creates a smooth fibrocartilaginous surface on which the component sits. Encouraging results have been documented, especially in the motivated, older patient population.[62,63] Results, however, have been less encouraging in the young patient population, with up to 14% revision in relatively short-term follow-up.[62]
HEMIARTHROPLASTY WITH BIOLOGICAL INTERPOSITION
Another option that has generated considerable research interest in treating young patients with shoulder arthritis is hemiarthroplasty with biological interposition. A number of different biological tissues have been used, including anterior capsule autograft, fascia lata autograft, lateral meniscal allograft, and Achilles tendon allograft, among others. The goal of the procedure is to preserve and maintain glenoid bone stock while creating a smooth cartilage-like surface on the glenoid. This may hold the promise of decreasing glenoid sided joint pain after shoulder hemiarthroplasty. Krishnan et al. published a series of young patients (<60-year-old) treated with this operation. They demonstrated encouraging results, with ASES scores improving from 39 to 91, and 50% excellent, 36% satisfactory, and 14% unsatisfactory results.[64] Other series, however, have not been able to replicate the initial success seen with this operation, documenting 44-77% failure rates.[65,67,68,69] In addition, a recent series of two patients demonstrated significant foreign body reaction to interposition material.[70] Furthermore, a recent review of 23 patients with hemiarthroplasty alone and 21 patients with hemiarthroplasty with biological resurfacing demonstrated worse outcomes in the biological resurfacing group, with a 57% revision rate (compared to 26% in hemiarthroplasty alone).[55] Therefore, at present, the role for hemiarthroplasty with biological resurfacing remains to be demonstrated.
NEWER RESURFACING DESIGNS
There continues to be innovation in the design of hemiarthroplasty components. More research into resurfacing and stemless designs continues. Stemless designs have the advantage of removing less humeral bone, as well as the ability to be placed independently of shaft deformity [Figure 4]. In the young patient with posttraumatic arthritis, this may be an advantage. Early results have showed similar results to stemmed arthroplasty choices, although data are still limited.[71] In addition to stemless implants, there has been ongoing work on investigation of different materials for arthroplasty. Pyrolytic carbon and ceramic materials hold promise, but require substantially more investigation in large clinical series [Figure 5].
Figure 4.
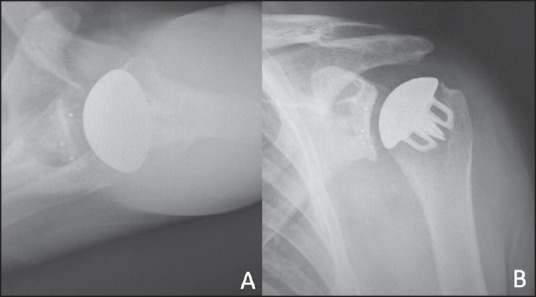
Axillary (a) and anterior posterior (b) radiographs of a total shoulder arthroplasty with a stemless humeral component design
Figure 5.
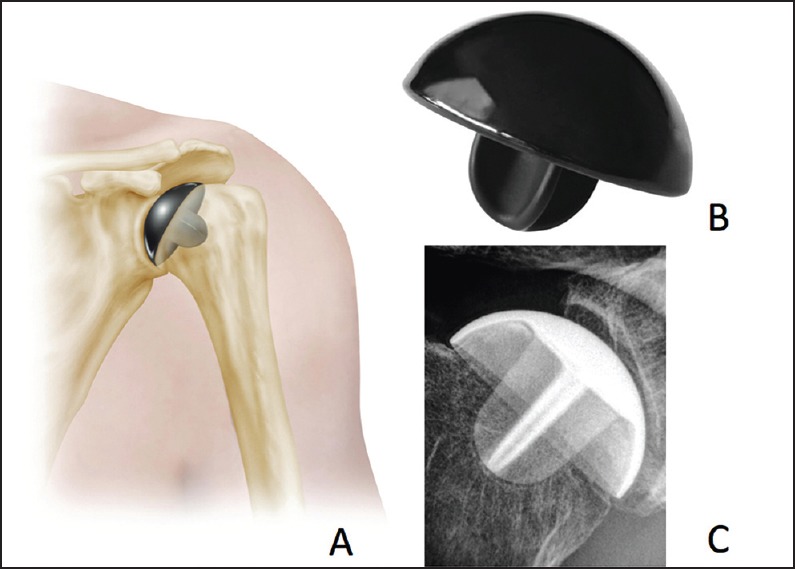
Pyrolytic carbon resurfacing hemiarthroplasty, shown in cartoon (a), actual image of the component (b), and with a radiograph of an implanted component (c)
BIPOLAR BIOLOGICAL RESURFACING
With continued advances in cartilage transplantation techniques, interest has developed in the possibility of bipolar biological resurfacing. Gobezie et al. published a case series of arthroscopic bipolar resurfacing. This utilized fresh osteochondral allograft transplantation to the humeral head and tibial plateau allografting to the glenoid. While they were able to demonstrate the technical feasibility of the operation, and encouraging short-term results, the long-term outcome of this technique are unknown and deserve further investigation.[72]
Total shoulder arthroplasty
While operations that preserve glenoid bone stock are appealing, total shoulder arthroplasty, with glenoid component insertion, remains the most reliable operation in terms of pain relief and ROM. This trend holds true in the younger patient population as well. Bartelt et al. demonstrated improved pain relief, motion, and implant survival with total shoulder arthroplasty when compared to hemiarthroplasty in patients younger than 55-year-old.[53] Another large study demonstrated that hemiarthroplasty was significantly more likely to be revised in this patient population (under 59) than total shoulder arthroplasty, most commonly for painful glenoid arthrosis.[54] This comes with the caveat that at final time points, there was a significant number of patients with glenoid components that were “at risk” in the Bartelt et al. series (approximately 1/3).[53] With long-term follow-up, this number is bound to increase, as seen in a study with 63% glenoid component survival at 10 years with a keeled component in young patients.[52] Therefore, individual decision making is necessary based on patient age, activity level, expectations, priorities, and glenoid bone loss when offering a total shoulder arthroplasty to a young patient. Nevertheless, total shoulder arthroplasty remains the treatment of choice in the informed, compliant young patient with GH arthritis failing nonoperative management.
Reverse total shoulder arthroplasty
Reverse total shoulder arthroplasty should be reserved as a salvage operation for the young patient with GH arthritis. Extenuating circumstances may be present including malignancy, limited life expectancy, an irreparable rotator cuff, severe bone loss, revision of anatomic total shoulder arthroplasty, and subscapularis deficiency. Recent work has demonstrated that reliable pain relief and ROM can be expected in young patients. However, there is a high complication rate with this operation.[73,74] Further, satisfaction with the postoperative outcome is lower in young patients than in elderly patients who have traditionally been treated with reverse total shoulder arthroplasty.[73,74] Therefore, reverse total shoulder arthroplasty should be reserved as a salvage procedure for patients not eligible for hemiarthroplasty or total shoulder arthroplasty in this patient population. Also, the patient needs to be extensively counseled on the risk-benefit ratio of this operation and limited experience in this patient population.
Arthrodesis/resection arthroplasty
There are limited indications for arthrodesis or resection arthroplasty in the young patient population. Arthrodesis, while providing reliable pain relief, has limited functional outcomes. This operation is traditionally reserved for young patients with significant brachial plexus or other nerve injury (e.g., axillary).[75,76,77,78] One advantage of arthrodesis is that it allows the patient to return to activities as tolerated following healing. This may be advantageous for manual laborers who work primarily below shoulder height. Certainly the role of arthrodesis has diminished with continued advances in arthroplasty.
Resection arthroplasty is another salvage option for some young patients with difficult shoulder problems. This is generally considered for patients with recalcitrant shoulder infections, Charcot arthropathy with pain, and severe posttraumatic bony deformity with compromised soft tissue envelope. While pain relief can be expected with this option, functional shoulder motion can be variable and at times fairly limited. Therefore, it should only be considered in salvage situations.[79,80]
CONCLUSION
Young patients with GH arthritis present unique diagnostic and treatment challenges. Thorough history, physical, and radiographic analysis is paramount to distinguish possible secondary causes of arthritis, as well as to prepare for potential surgical management (ruling out rotator cuff disease, infection, subscapularis insufficiency, etc.). Nonoperative management is the mainstay of treatment of shoulder arthritis in this population.
When nonoperative management fails, several surgical options may be considered. Arthroscopic debridement is a reasonable first avenue of treatment, providing diagnostic and therapeutic value. Hemiarthroplasty with or without glenoid reaming preserves glenoid bone stock when compared to total shoulder arthroplasty, but provides inferior pain relief and ROM. Hemiarthroplasty with biological resurfacing has limited role presently in treatment and requires further investigation. Total shoulder arthroplasty generally provides better pain relief, ROM, and lower risk of revision in this patient population. Glenoid component loosening, however, is a frequent finding. Therefore, careful preoperative counseling and patient selection is critical. Reverse total shoulder arthroplasty is a salvage operation, with worse clinical outcomes than in the elderly population, and high complication rates. Arthrodesis or resection arthroplasty provides pain relief, but limited functional outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19:1115–20. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Boileau P, Fourati E, Bicknell R. Neer modification of open Bankart procedure: What are the rates of recurrent instability, functional outcome, and arthritis? Clin Orthop Relat Res. 2012;470:2554–60. doi: 10.1007/s11999-012-2296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busfield BT, Romero DM. Pain pump use after shoulder arthroscopy as a cause of glenohumeral chondrolysis. Arthroscopy. 2009;25:647–52. doi: 10.1016/j.arthro.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi F, Papalia R, Del Buono A, Vasta S, Maffulli N, Denaro V. Glenohumeral osteoarthritis after arthroscopic Bankart repair for anterior instability. Am J Sports Med. 2011;39:1653–9. doi: 10.1177/0363546511404207. [DOI] [PubMed] [Google Scholar]
- 5.Hattrup SJ, Cofield RH. Osteonecrosis of the humeral head: Results of replacement. J Shoulder Elbow Surg. 2000;9:177–82. [PubMed] [Google Scholar]
- 6.McNickle AG, L’Heureux DR, Provencher MT, Romeo AA, Cole BJ. Postsurgical glenohumeral arthritis in young adults. Am J Sports Med. 2009;37:1784–91. doi: 10.1177/0363546509333481. [DOI] [PubMed] [Google Scholar]
- 7.Hasan SS, Fleckenstein CM. Glenohumeral chondrolysis: Part I — Clinical presentation and predictors of disease progression. Arthroscopy. 2013;29:1135–41. doi: 10.1016/j.arthro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Matsen FA, 3rd, Papadonikolakis A. Published evidence demonstrating the causation of glenohumeral chondrolysis by postoperative infusion of local anesthetic via a pain pump. J Bone Joint Surg Am. 2013;95:1126–34. doi: 10.2106/JBJS.L.01104. [DOI] [PubMed] [Google Scholar]
- 9.Yeh PC, Kharrazi FD. Postarthroscopic glenohumeral chondrolysis. J Am Acad Orthop Surg. 2012;20:102–12. doi: 10.5435/JAAOS-20-02-102. [DOI] [PubMed] [Google Scholar]
- 10.Glueck D, Gellman H. Management of the upper extremity in juvenile rheumatoid arthritis. J Am Acad Orthop Surg. 2005;13:254–66. doi: 10.5435/00124635-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Smith AM, Sperling JW, O’Driscoll SW, Cofield RH. Arthroscopic shoulder synovectomy in patients with rheumatoid arthritis. Arthroscopy. 2006;22:50–6. doi: 10.1016/j.arthro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23:791–9. doi: 10.1016/j.jse.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: A 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91:1197–200. doi: 10.1302/0301-620X.91B9.22035. [DOI] [PubMed] [Google Scholar]
- 14.Buscayret F, Edwards TB, Szabo I, Adeleine P, Coudane H, Walch G. Glenohumeral arthrosis in anterior instability before and after surgical treatment: Incidence and contributing factors. Am J Sports Med. 2004;32:1165–72. doi: 10.1177/0363546503262686. [DOI] [PubMed] [Google Scholar]
- 15.Cameron ML, Kocher MS, Briggs KK, Horan MP, Hawkins RJ. The prevalence of glenohumeral osteoarthrosis in unstable shoulders. Am J Sports Med. 2003;31:53–5. doi: 10.1177/03635465030310012001. [DOI] [PubMed] [Google Scholar]
- 16.Green A, Norris TR. Shoulder arthroplasty for advanced glenohumeral arthritis after anterior instability repair. J Shoulder Elbow Surg. 2001;10:539–45. doi: 10.1067/mse.2001.118007. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins RJ, Angelo RL. Glenohumeral osteoarthrosis. A late complication of the Putti-Platt repair. J Bone Joint Surg Am. 1990;72:1193–7. [PubMed] [Google Scholar]
- 18.Hovelius L. "Glenohumeral osteoarthrosis after Putti-Platt repair". J Shoulder Elbow Surg. 2000;9:257. [PubMed] [Google Scholar]
- 19.Rachbauer F, Ogon M, Wimmer C, Sterzinger W, Huter B. Glenohumeral osteoarthrosis after the Eden-Hybbinette procedure. Clin Orthop Relat Res. 2000;373:135–40. doi: 10.1097/00003086-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 20.van der Zwaag HM, Brand R, Obermann WR, Rozing PM. Glenohumeral osteoarthrosis after Putti-Platt repair. J Shoulder Elbow Surg. 1999;8:252–8. doi: 10.1016/s1058-2746(99)90138-6. [DOI] [PubMed] [Google Scholar]
- 21.Elmlund AO, Ejerhed L, Sernert N, Rostgård LC, Kartus J. Dislocation arthropathy and drill hole appearance in a mid- to long-term follow-up study after arthroscopic Bankart repair. Knee Surg Sports Traumatol Arthrosc. 2012;20:2156–62. doi: 10.1007/s00167-012-2076-5. [DOI] [PubMed] [Google Scholar]
- 22.Kavaja L, Pajarinen J, Sinisaari I, Savolainen V, Björkenheim JM, Haapamäki V, et al. Arthrosis of glenohumeral joint after arthroscopic Bankart repair: A long-term follow-up of 13 years. J Shoulder Elbow Surg. 2012;21:350–5. doi: 10.1016/j.jse.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Allain J, Goutallier D, Glorion C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am. 1998;80:841–52. doi: 10.2106/00004623-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Freehill MT, Srikumaran U, Archer KR, McFarland EG, Petersen SA. The Latarjet coracoid process transfer procedure: Alterations in the neurovascular structures. J Shoulder Elbow Surg. 2013;22:695–700. doi: 10.1016/j.jse.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: Study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15:279–89. doi: 10.1016/j.jse.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Spoor AB, de Waal Malefijt J. Long-term results and arthropathy following the modified Bristow-Latarjet procedure. Int Orthop. 2005;29:265–7. doi: 10.1007/s00264-005-0634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau MW, Blinder MA, Williams K, Galatz LM. Shoulder arthroplasty in sickle cell patients with humeral head avascular necrosis. J Shoulder Elbow Surg. 2007;16:129–34. doi: 10.1016/j.jse.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Orfaly RM, Rockwood CA, Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 Suppl):S27–32. doi: 10.1016/j.jse.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Poignard A, Flouzat-Lachaniette CH, Amzallag J, Galacteros F, Hernigou P. The natural progression of symptomatic humeral head osteonecrosis in adults with sickle cell disease. J Bone Joint Surg Am. 2012;94:156–62. doi: 10.2106/JBJS.J.00919. [DOI] [PubMed] [Google Scholar]
- 30.Smith RG, Sperling JW, Cofield RH, Hattrup SJ, Schleck CD. Shoulder hemiarthroplasty for steroid-associated osteonecrosis. J Shoulder Elbow Surg. 2008;17:685–8. doi: 10.1016/j.jse.2008.01.149. [DOI] [PubMed] [Google Scholar]
- 31.Chapman C, Mattern C, Levine WN. Arthroscopically assisted core decompression of the proximal humerus for avascular necrosis. Arthroscopy. 2004;20:1003–6. doi: 10.1016/j.arthro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Dines JS, Strauss EJ, Fealy S, Craig EV. Arthroscopic-assisted core decompression of the humeral head. Arthroscopy. 2007;23:103e.1–4. doi: 10.1016/j.arthro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Harreld KL, Marulanda GA, Ulrich SD, Marker DR, Seyler TM, Mont MA. Small-diameter percutaneous decompression for osteonecrosis of the shoulder. Am J Orthop (Belle Mead NJ) 2009;38:348–54. [PubMed] [Google Scholar]
- 34.Sahajpal DT, Zuckerman JD. Core decompression for nontraumatic osteonecrosis of the humeral head: A technique article. Bull NYU Hosp Jt Dis. 2008;66:118–9. [PubMed] [Google Scholar]
- 35.Feeley BT, Fealy S, Dines DM, Warren RF, Craig EV. Hemiarthroplasty and total shoulder arthroplasty for avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2008;17:689–94. doi: 10.1016/j.jse.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Allen B, Schoch B, Sperling JW, Cofield RH. Shoulder arthroplasty for osteoarthritis secondary to glenoid dysplasia: An update. J Shoulder Elbow Surg. 2014;23:214–20. doi: 10.1016/j.jse.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Harper KW, Helms CA, Haystead CM, Higgins LD. Glenoid dysplasia: Incidence and association with posterior labral tears as evaluated on MRI. AJR Am J Roentgenol. 2005;184:984–8. doi: 10.2214/ajr.184.3.01840984. [DOI] [PubMed] [Google Scholar]
- 38.Smith SP, Bunker TD. Primary glenoid dysplasia. A review of 12 patients. J Bone Joint Surg Br. 2001;83:868–72. doi: 10.1302/0301-620x.83b6.11649. [DOI] [PubMed] [Google Scholar]
- 39.Sperling JW, Cofield RH, Steinmann SP. Shoulder arthroplasty for osteoarthritis secondary to glenoid dysplasia. J Bone Joint Surg Am. 2002;84-A:541–6. doi: 10.2106/00004623-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Wirth MA, Lyons FR, Rockwood CA., Jr Hypoplasia of the glenoid. A review of sixteen patients. J Bone Joint Surg Am. 1993;75:1175–84. doi: 10.2106/00004623-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Walch G, Ascani C, Boulahia A, Nové-Josserand L, Edwards TB. Static posterior subluxation of the humeral head: An unrecognized entity responsible for glenohumeral osteoarthritis in the young adult. J Shoulder Elbow Surg. 2002;11:309–14. doi: 10.1067/mse.2002.124547. [DOI] [PubMed] [Google Scholar]
- 42.Kerr BJ, McCarty EC. Outcome of arthroscopic débridement is worse for patients with glenohumeral arthritis of both sides of the joint. Clin Orthop Relat Res. 2008;466:634–8. doi: 10.1007/s11999-007-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards DP, Burkhart SS. Arthroscopic debridement and capsular release for glenohumeral osteoarthritis. Arthroscopy. 2007;23:1019–22. doi: 10.1016/j.arthro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Van Thiel GS, Sheehan S, Frank RM, Slabaugh M, Cole BJ, Nicholson GP, et al. Retrospective analysis of arthroscopic management of glenohumeral degenerative disease. Arthroscopy. 2010;26:1451–5. doi: 10.1016/j.arthro.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Millett PJ, Horan MP, Pennock AT, Rios D. Comprehensive arthroscopic management (CAM) procedure: Clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29:440–8. doi: 10.1016/j.arthro.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Pennington WT, Bartz BA. Arthroscopic glenoid resurfacing with meniscal allograft: A minimally invasive alternative for treating glenohumeral arthritis. Arthroscopy. 2005;21:1517–20. doi: 10.1016/j.arthro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Arthroscopic technique of interposition arthroplasty of the glenohumeral joint. Arthroscopy. 2006;22:570.e1–5. doi: 10.1016/j.arthro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Savoie FH, 3rd, Brislin KJ, Argo D. Arthroscopic glenoid resurfacing as a surgical treatment for glenohumeral arthritis in the young patient: Midterm results. Arthroscopy. 2009;25:864–71. doi: 10.1016/j.arthro.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 49.de Beer JF, Bhatia DN, van Rooyen KS, Du Toit DF. Arthroscopic debridement and biological resurfacing of the glenoid in glenohumeral arthritis. Knee Surg Sports Traumatol Arthrosc. 2010;18:1767–73. doi: 10.1007/s00167-010-1155-8. [DOI] [PubMed] [Google Scholar]
- 50.Anderl W, Kriegleder B, Neumaier M, Laky B, Heuberer P. Arthroscopic partial shoulder resurfacing. Knee Surg Sports Traumatol Arthrosc. 2015;23:1563–70. doi: 10.1007/s00167-014-2981-x. [DOI] [PubMed] [Google Scholar]
- 51.Harreld KL, Marker DR, Wiesler ER, Shafiq B, Mont MA. Osteonecrosis of the humeral head. J Am Acad Orthop Surg. 2009;17:345–55. doi: 10.5435/00124635-200906000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22:894–900. doi: 10.1016/j.jse.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20:123–30. doi: 10.1016/j.jse.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Dillon MT, Inacio MC, Burke MF, Navarro RA, Yian EH. Shoulder arthroplasty in patients 59 years of age and younger. J Shoulder Elbow Surg. 2013;22:1338–44. doi: 10.1016/j.jse.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Hammond LC, Lin EC, Harwood DP, Juhan TW, Gochanour E, Klosterman EL, et al. Clinical outcomes of hemiarthroplasty and biological resurfacing in patients aged younger than 50 years. J Shoulder Elbow Surg. 2013;22:1345–51. doi: 10.1016/j.jse.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80:464–73. doi: 10.2106/00004623-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–60. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 58.Alizadehkhaiyat O, Kyriakos A, Singer MS, Frostick SP. Outcome of Copeland shoulder resurfacing arthroplasty with a 4-year mean follow-up. J Shoulder Elbow Surg. 2013;22:1352–8. doi: 10.1016/j.jse.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 59.Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90:110–7. doi: 10.2106/JBJS.F.01552. [DOI] [PubMed] [Google Scholar]
- 60.Mechlenburg I, Amstrup A, Klebe T, Jacobsen SS, Teichert G, Stilling M. The Copeland resurfacing humeral head implant does not restore humeral head anatomy. A retrospective study. Arch Orthop Trauma Surg. 2013;133:615–9. doi: 10.1007/s00402-013-1715-8. [DOI] [PubMed] [Google Scholar]
- 61.Mansat P, Coutié AS, Bonnevialle N, Rongières M, Mansat M, Bonnevialle P. Resurfacing humeral prosthesis: Do we really reconstruct the anatomy? J Shoulder Elbow Surg. 2013;22:612–9. doi: 10.1016/j.jse.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA., 3rd Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20:609–15. doi: 10.1016/j.jse.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 63.Lynch JR, Franta AK, Montgomery WH, Jr, Lenters TR, Mounce D, Matsen FA., 3rd Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89:1284–92. doi: 10.2106/JBJS.E.00942. [DOI] [PubMed] [Google Scholar]
- 64.Krishnan SG, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis. Two to fifteen-year outcomes. J Bone Joint Surg Am. 2007;89:727–34. doi: 10.2106/JBJS.E.01291. [DOI] [PubMed] [Google Scholar]
- 65.Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91:419–24. doi: 10.2106/JBJS.H.00318. [DOI] [PubMed] [Google Scholar]
- 66.Muh SJ, Streit JJ, Shishani Y, Dubrow S, Nowinski RJ, Gobezie R. Biologic resurfacing of the glenoid with humeral head resurfacing for glenohumeral arthritis in the young patient. J Shoulder Elbow Surg. 2014;23:e185–90. doi: 10.1016/j.jse.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Namdari S, Alosh H, Baldwin K, Glaser D, Kelly JD. Biological glenoid resurfacing for glenohumeral osteoarthritis: A systematic review. J Shoulder Elbow Surg. 2011;20:1184–90. doi: 10.1016/j.jse.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 68.Strauss EJ, Verma NN, Salata MJ, McGill KC, Klifto C, Nicholson GP, et al. The high failure rate of biologic resurfacing of the glenoid in young patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2014;23:409–19. doi: 10.1016/j.jse.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Wirth MA. Humeral head arthroplasty and meniscal allograft resurfacing of the glenoid. J Bone Joint Surg Am. 2009;91:1109–19. doi: 10.2106/JBJS.H.00677. [DOI] [PubMed] [Google Scholar]
- 70.Namdari S, Melnic C, Huffman GR. Foreign body reaction to acellular dermal matrix allograft in biologic glenoid resurfacing. Clin Orthop Relat Res. 2013;471:2455–8. doi: 10.1007/s11999-013-2904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Churchill RS. Stemless shoulder arthroplasty: Current status. J Shoulder Elbow Surg. 2014;23:1409–14. doi: 10.1016/j.jse.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Gobezie R, Lenarz CJ, Wanner JP, Streit JJ. All-arthroscopic biologic total shoulder resurfacing. Arthroscopy. 2011;27:1588–93. doi: 10.1016/j.arthro.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199–208. doi: 10.1016/j.jse.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 74.Muh SJ, Streit JJ, Wanner JP, Lenarz CJ, Shishani Y, Rowland DY, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95:1877–83. doi: 10.2106/JBJS.L.10005. [DOI] [PubMed] [Google Scholar]
- 75.Clare DJ, Wirth MA, Groh GI, Rockwood CA., Jr Shoulder arthrodesis. J Bone Joint Surg Am. 2001;83-A:593–600. doi: 10.2106/00004623-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Richards RR, Beaton D, Hudson AR. Shoulder arthrodesis with plate fixation: Functional outcome analysis. J Shoulder Elbow Surg. 1993;2:225–39. doi: 10.1016/S1058-2746(09)80081-5. [DOI] [PubMed] [Google Scholar]
- 77.Safran O, Iannotti JP. Arthrodesis of the shoulder. J Am Acad Orthop Surg. 2006;14:145–53. doi: 10.5435/00124635-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Sousa R, Pereira A, Massada M, Trigueiros M, Lemos R, Silva C. Shoulder arthrodesis in adult brachial plexus injury: What is the optimal position? J Hand Surg Eur Vol. 2011;36:541–7. doi: 10.1177/1753193411405742. [DOI] [PubMed] [Google Scholar]
- 79.Muh SJ, Streit JJ, Lenarz CJ, McCrum C, Wanner JP, Shishani Y, et al. Resection arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:247–52. doi: 10.1016/j.jse.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 80.Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. J Bone Joint Surg Br. 2007;89:1184–7. doi: 10.1302/0301-620X.89B9.19464. [DOI] [PubMed] [Google Scholar]


