Abstract
Background
Aortic stenosis (AS) leads to variable stress for the left ventricle (LV) and consequently a broad range of LV remodeling. Study aim was to describe blood flow patterns in the ascending aorta of AS patients and determine their association with remodeling.
Methods and Results
Thirty-seven patients with AS (14 mild, 8 moderate, 15 severe; age 63±13 years) and 37 healthy controls (age 60±10 years) underwent 4D-flow MRI. Helical and vortical flow formations and flow eccentricity were assessed in the ascending aorta. Normalized flow displacement from the vessel center and peak systolic wall shear stress (WSSpeak) in the ascending aorta were quantified. LV remodeling was assessed based on LV mass index (LVMI-I) and the ratio of LV mass to enddiastolic volume (relative wall mass; RWM). Marked helical and vortical flow formation and eccentricity were more prevalent in patients with AS than in healthy subjects, and AS patients exhibited an asymmetric and elevated distribution of WSSpeak. In AS, aortic orifice area was strongly negatively associated with vortical flow formation (p=0.0274), eccentricity (p=0.0070) and flow displacement (p=0.0021). Bicuspid aortic valve was associated with more intense helical (p=0.0098) and vortical flow formation (p=0.0536), higher flow displacement (p=0.11) and higher WSSpeak (p=0.0926). LVM-I and RWM were significantly associated with aortic orifice area (p=0.0611, p=0.0058) and flow displacement (p=0.0058, p=0.0283).
Conclusions
In this pilot study, AS leads to abnormal blood flow pattern and WSSpeak in the ascending aorta. In addition to aortic orifice area, normalized flow displacement was significantly associated with LV remodeling.
Keywords: magnetic resonance imaging, aortic valve stenosis, remodeling, flow imaging, 4D flow
Aortic orifice area (AOA) and blood flow velocity are often considered insufficient to characterize the severity of aortic stenosis (AS)1,2. The increased stress on the left ventricle (LV) caused by AS can be highly variable and leads to a broad range of LV impairment3,4. As a result, it is challenging to quantitatively determine the burden on the ventricle due to AS in the individual patient. Conversely, unfavorable LV remodeling is an important prognostic factor and contributes significantly to the development of heart failure in patients with AS5,6,7,8.
There are many attempts to better characterize the myocardial stress provoked by AS. Whereas a decline of LV ejection fraction is regarded as a late sign of LV impairment, systolic longitudinal strain assessed by transthoracic echocardiography has been proven to be a valuable marker for early LV dysfunction in AS1,9. ECG signs of LV hypertrophy and LV strain as well as elevated blood markers like troponin and brain natriuretic peptide have also been reported to predict outcome in AS patients10,11. Moreover, the presence of fibrosis assessed by late Gadolinium enhancement cardiovascular magnetic resonance (CMR) is associated with poorer outcome with and without aortic valve replacement12,13.
Altered post-stenotic blood flow in the presence of AS is another potential cause of elevated LV afterload.
Altered helical and vortical blood flow formations have been described to cause power loss due to friction and viscous dissipation and to alter wall shear stress (WSS)14. Integrated over time, these losses can cause an increase in energy required for blood circulation15. The recent use of 4D flow CMR to characterize blood flow patterns and the distribution of WSS is an opportunity to investigate this theory16,17. In this hypothesis-generating study, 4D flow CMR of AS patients was used to characterize the post-stenotic blood flow and to analyze the association of aortic hemodynamics with LV remodeling.
Methods
Study population
Participants were prospectively recruited. The diagnosis of AS was based on the aortic orifice area (AOA) obtained from CMR cine imaging. Severe AS was defined as AOA <1cm2 or AOA indexed by body surface area <0.6cm2/m2, moderate AS 1–1.5cm2, and mild AS >1.5cm2 2. The status “healthy” was based on uneventful medical history, absence of any symptoms indicating cardiovascular dysfunction, normal ECG as well as normal cardiac dimensions and function proven by CMR cine imaging. Subjects with impaired LV ejection fraction <50% or with evidence of coronary artery disease by coronary angiography, non-invasive imaging or clinical assessment were excluded. Patients with arrhythmia, greater than mild valvular disease (other than AS), or general contraindications for CMR were also excluded. For each participant, written informed consent was obtained prior to the study, after due approval by the local ethical committee.
4D flow CMR - Acquisition
All CMR examinations were performed with a 3T system (MAGNETOM Verio, Siemens Healthcare GmbH, Germany). 4D flow CMR data were acquired using a sagittal oblique volume covering the thoracic aorta. Prospective ECG gating was used in combination with a respiratory navigator placed on the lung-liver interface to permit data acquisition during free breathing. The following scan parameters were chosen: echo time [TE] = 2.6 ms, repetition time [TR] = 5.1 ms, bandwith = 450 Hz/pixel, imaging acceleration using PEAK GRAPPA with a reduction factor of R=518, net acceleration 4.17, reference lines = 20, flip angle α = 7–9°, temporal resolution = 40.8 ms, field of view [FOV] (360×270)mm2, matrix (133×118), voxel size = 2.7 × 2.3 × 2.6 mm3, phase encoding direction = anterior-posterior, number of slices = 32, velocity encoding = 1.5 (healthy controls)-2.5m/s (AS).
4D flow CMR - Postprocessing
All 4D flow data were processed as previously described and in concordance with current consensus recommendations19,20. Briefly, data were corrected for Maxwell terms, eddy currents and velocity aliasing (MATLAB; The MathWorks, Natick, MA, USA)21. In a second step, a 3D phase contrast angiogram was calculated based on the flow measurements to position 2D analysis planes and to aid in 3D blood flow visualization (EnSight, CEI, Apex, NC). Three planes were positioned perpendicular to the longitudinal axis of the aortic wall: at the level of the sinotubular junction (S1), in the mid-ascending aorta (S2), and proximal to the brachiocephalic trunk (S3) (Figure 1). These analysis planes were exported into software for the segmentation and calculation of the blood flow parameters (MATLAB)21. A single expert did this analysis with extensive experience in the assessment of 4D flow datasets strictly following standard operating procedures.
Figure 1.
Schematic of the analysis planes at the sinotubular level, mid-ascendening and distal-ascending aorta (left). Examples for marked vortical and helical flow formation as well as eccentricity compared to the flow of a healthy volunteer.
4D flow - Parameters
Helical and vortical blood flow formations in the ascending aorta were semi-quantitatively evaluated using pathline movies and graded in: 0 (none), 1 (<360°) and 2 (>360°). A vortical flow formation was defined as revolving particles around a point within the vessel with a rotation direction deviating by more than 90° from the physiological flow directions. A helical flow formation was considered a regional fluid circulation around an axis parallel to bulk fluid motion (i.e. along the longitudinal axis of the vessel), thereby creating a corkscrew-like motion19. To test inter-observer dependency, the analysis was repeated by a second observer for a subset of 25 randomly selected datasets.
Peak velocity blood flow was semi-quantitatively graded as central, mildly eccentric or markedly eccentric in 2D flow profiles at the midascending aorta19,22. Central flow was defined by the high velocity flow occupying the majority of the vessel. A mild eccentric flow occupied one- to two-thirds of the vessel and a marked eccentric flow occupied one-third or less of the vessel. Additionally, normalized flow displacement was calculated to quantitatively describe blood flow eccentricity. Normalized flow displacement is defined as the distance between the center of the lumen and the “center of velocity” of the forward flow, normalized to the lumen diameter23. Finally, a visualization of outflow asymmetry was created by mapping the peak systolic velocity location and the region of the upper 15th percentile of the velocities on a segmental aortic lumen map.
Quantification of peak systolic WSS (WSSpeak in N/m2) was performed for 8 regional segments along the aortic circumference for each plane S1–S317. Regional WSSpeak was averaged with the preceding and 3 subsequent time steps to mitigate measurement noise.
Cine CMR
Steady-state free-precession (SSFP) cine images were obtained to assess wall motion, for cardiac chamber quantification and for planimetry of the AOA24. Imaging parameters were: TR=3.1ms, TE=1.3ms,, FA=45°, FOV (276×340)mm2, matrix (156×192), slice thickness 6mm (chambers) and 5mm (aortic valve), BW 704Hz/px, parallel imaging using GRAPPA reconstruction (R=2), 30 cardiac phases. Axial SSFP still images of the thorax were used to estimate the size of the ascending aorta at the level of the pulmonary bifurcation25. Image analysis was done using CVI42 (Circle Cardiovascular Imaging, Calgary, Canada).
LV remodeling was defined based on LV mass index (LVM-I) and relative wall mass (RWM=LVM divided by EDV in g/ml)3. The categories were: i) normal (LVMI and RWM normal), remodeling (LVMI normal and RWT ≥ 1.16g/ml), hypertrophy (LVMI abnormal26 and RWM ≥ 1.16g/ml). Asymmetric remodeling and hypertrophy were differentiated by wall thickening ≥13mm that was also >1.5-fold the thickness of the opposing myocardial segment. RWM is reported to be more sensitive to detect LV remodeling than global LV mass or 2D diameters of the LV wall, in particular for small concentric remodeled hearts3.
Statistical analysis
Statistical analysis of the data as outlined in this section was performed using SPSS 20 (IBM, Armonk, US) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Graphics were created using PRISM 5 (GraphPad Software Inc, San Diego, California, US) and plug-in software for MATLAB. Categorical data are expressed as percentages, continuous data as mean ± standard deviation (SD). Interobserver variability of helical and vortical flow evaluation was assessed using the intraclass correlation coefficient. Comparisons between the three severity grades of AS were made using the Jonckheere-Terpstra test when a trend across groups was of major interest27, or the Kruskal-Wallis test when looking for any difference between the groups. In case of significance, a Mann-Whitney-U test was added to investigate into the actual effects. Correlation was assessed using the Spearman method.
Modeling of flow parameters
The flow parameters were modeled based on all available data using ordinal logistic regression or linear regression as appropriate for the parameter. The presence of a bicuspid aortic valve, aortic diameter, ejection fraction, age, AOA, AS grade and AS (y/n) we well as the two-way interaction between AS (y/n) and the aforementioned independent variables other than AS grade were independent variables in the models17,28,29. Stepwise selection (p(entry)=0.15, p(stay)=0.15) was used to arrive at sparse models and to avoid spurious effects due to correlations in the independent variables. As for some models, significant interaction terms pointed towards different effect in patients and controls, models were also computed for patients only. The focus of interpretation was put into the main factors, while interaction terms will be subject to further investigation.
Modeling of remodeling parameters
The analysis of associations with the remodeling parameters ‘LVM-I’, ‘RWM’ and ‘presence of LV remodeling’ was based on patients only. Univariate linear or logistic regression models were used to assess univariate relationships between the remodeling parameters and the flow parameters helical and vortical flow formation, eccentricity, normalized flow displacement and WSSpeak. In a first step of multiple modeling, multiple regression models using the influential factors ‘systolic blood pressure’, ‘AOA’ and ‘age’ as independent variables were built to investigate the influence of these more familiar parameters on remodeling parameters3,30. In a second step, the models were extended by the flow parameters helical and vortical flow formations, eccentricity and normalized flow displacement (S1–S3) as well as WSSpeak (S1-S3). Stepwise selection (p(entry=0.15, p(stay)=0.15) was used to identify flow parameters with added value for the explanation of variability in the remodeling parameters.
As this was an exploratory study, p-values were considered as descriptive rather than confirmatory throughout. Except for the modeling approaches, p-values below 0.05 were considered to be statistically significant.
Results
Study sample
The study sample comprised of n=37 patients with AS and n=37 healthy control subjects. All were in sinus rhythm. Table 1 and 2 show the characteristics of the study participants.
Table 1.
Patient characteristics. The p-value stems from the Mann-Whitney-U-Test and for sex and valve morphology from the Chi-Square-Test.
| Parameter | AS | Healthy | p-value |
|---|---|---|---|
| n | 37 | 37 | |
| Sex (male | female) | 23 | 14 | 20 | 17 | 0.48 |
| Age (y) | 63 ± 13 | 60 ± 10 | 0.042* |
| Height (cm) | 169 ± 9 | 171 ± 13 | 0.25 |
| Weight (kg) | 83 ± 13 | 79 ± 17 | 0.074 |
| BMI (kg/m2) | 28.8 ± 4.3 | 27.7 ± 10.3 | 0.005* |
| BSA (m2) | 1.97 ± 0.19 | 1.92 ± 0.21 | 0.22 |
| Systolic blood pressure (mmHg) | 140 ± 15 | 134 ± 13 | 0.012* |
| Diastolic blood pressure (mmHg) | 83 ± 14 | 82 ± 10 | 0.84 |
| Heart rate (min−1) | 67 ± 12 | 68 ± 9 | 0.77 |
| Aortic diameter (mm) | 35 ± 7 | 32 ± 3 | 0.016* |
| Aortic orifice area (cm2) | 1.5 ± 0.6 | 3.5 ± 0.5 | <0.001* |
| Aortic orifice area index (cm2/m2) | 0.75 ± 0.33 | 1.85 ± 0.25 | <0.001* |
| Morphology of aortic valve (tricuspid | bicuspid) | 21 | 16 | 37 | 0 | <0.001* |
| LV-EF (%) | 66 ± 6 | 63 ± 5 | 0.062 |
| LV-SV-Index (ml/m2) | 44 ± 11 | 48 ± 8 | 0.044* |
| LV-EDV-Index (ml/cm) | 0.77 ± 0.18 | 0.87 ± 0.18 | 0.020* |
| LV-Mass-Index (g/cm) | 0.94 ± 0.30 | 0.70 ± 0.15 | <0.001* |
| Relative Wall Mass (g/ml) | 1.25 ± 0.38 | 0.81 ± 0.13 | <0.001* |
p<0.05
Table 2.
Patient characteristics separated by aortic stenosis severity grade. The p-value stems from the Jonckheere-Terpstra-Test and for sex and valve morphology from the Chi-Square-Test.
| Parameter | Mild | Moderate | Severe | p-value |
|---|---|---|---|---|
| n | 14 | 8 | 15 | |
| Sex (male | female) | 8 | 6 | 3 | 5 | 12 | 3 | 0.12 |
| Age (y) | 58 ± 17 | 63 ± 13 | 68 ± 8 | 0.13 |
| Height (cm) | 168 ± 9 | 169 ± 11 | 172 ± 8 | 0.18 |
| Weight (kg) | 81 ± 14 | 82 ± 14 | 85 ± 13 | 0.36 |
| BMI (kg/m2) | 28.6 ± 4.0 | 28.8 ± 3.6 | 29.0 ± 4.9 | 0.89 |
| BSA (m2) | 1.93 ± 0.20 | 1.95 ± 0.22 | 2.01 ± 0.15 | 0.121 |
| Systolic blood pressure (mmHg) | 140 ± 14 | 143 ± 18 | 140 ± 16 | 0.93 |
| Diastolic blood pressure (mmHg) | 82 ± 15 | 85 ± 20 | 82 ± 11 | 0.90 |
| Heart rate (min−1) | 67 ± 15 | 68 ± 10 | 69 ± 11 | 0.48 |
| Aortic diameter (mm) | 33 ± 7 | 38 ± 7 | 35 ± 6 | 0.52 |
| Aortic orifice area (cm2) | 2.09 ± 0.39 | 1.28 ± 0.18 | 0.94 ± 0.21 | <0.001* |
| Aortic orifice area index (cm2/m2) | 1.10 ± 0.24 | 0.66 ± 0.06 | 0.47 ± 0.11 | <0.001* |
| Morphology of aortic valve (tricuspid | bicuspid) | 11 | 3 | 5 | 3 | 5 | 10 | 0.10 |
| LV-EF (%) | 64 ± 6 | 66 ± 6 | 67 ± 6 | 0.19 |
| LV-SV-Index (ml/m2) | 45 ± 12 | 42 ± 10 | 44 ± 10 | 0.89 |
| LV-EDV-Index (ml/cm) | 0.80 ± 0.20 | 0.75 ± 0.15 | 0.76 ± 0.18 | 0.63 |
| LV-Mass-Index (g/cm) | 0.79 ± 0.19 | 0.93 ± 0.21 | 1.08 ± 0.36 | 0.036* |
| Relative wall mass (g/ml) | 1.00 ± 0.15 | 1.28 ± 0.39 | 1.43 ± 0.42 | 0.001* |
p<0.05
Patients with AS had a higher body mass index, higher systolic blood pressure and larger aortic diameter, higher LVM-I and higher RWM, lower stroke volume index and lower LVEDV-I than controls. Furthermore, AS patients were slightly older than controls (63 vs. 60 years, p=0.042), but can be regarded as being within in a similar age class. There was no difference regarding the sex-distribution between both groups. With increasing severity of AS, AOA and AOA index decreased (p<0.001). RWM and LVM-I was significantly higher in severe AS compared to mild AS (p<0.001 and p=0.037). The prevalence of LV remodeling increased with increasing AS severity (Figure 2).
Figure 2.
Prevalence of LV remodeling in patients with AS separated for AS severity grades. AS severity grades were found statistically significant for severity of LV remodeling in ordinal logistic regression (p<0.0001). AS = aortic stenosis.
Helical and vortical blood flow formations in the ascending aorta
Interobserver variability to assess helical and vortical flow formations was low with an intraclass correlation coefficient of 0.82 and 0.77, respectively. Examples for helical and vortical blood flow formations are shown in Figure 1. Marked helical and vortical flow formation were more prevalent in patients with AS than in healthy subjects, and with increasing AS severity grade the prevalence generally increased (Figure 3). Table 3 summarizes all models with model estimates and their respective p-values after model selection.
Figure 3.
Qualitative grades for helical and vortical flow formation as well as eccentricity. AS severity grades were found statistically significant for helical and vortical flow formation and eccentricity in ordinal logistic regression (all p<0.0001). AS=aortic stenosis
Table 3.
Multiple regression models regarding the association of flow parameters (helical and vortical flow formation, eccentricity, normalized flow displacement, WSSpeak) with potential influencing factors, parameter estimates and p-values of final models after stepwise selection. Only parameters remaining in the final model are shown.
| Flow parameter (Model) |
Independent Variable |
All subjects (n=74) Estimate β ± SE (p-value) |
Patients (n=37) Estimate β ± SE (p-value) |
|---|---|---|---|
| Helical flow * | Age | −0.04 ± 0.02 (p=0.14) | |
| Bicuspid morphology | 0.98 ± 0.48 (p=0.0406) | 1.13 ± 0.44 (p=0.0098) | |
| Aortic orifice area | −1.81 ± 0.38 (p<0.0001) | ||
| Vortical flow* | Bicuspid morphology | 0.80 ± 0.38 (p=0.0375) | 0.74 ± 0.38 (p=0.0536) |
| Aortic diameter | 0.12 ± 0.06 (p=0.0522) | 0.10 ± 0.07 (p=0.14) | |
| Aortic orifice area | −1.84 ± 0.55 (p=0.0009) | −1.42 ± 0.64 (p=0.0274) | |
| AS (y/n) | −2.71 ± 1.22 (p=0.0258) | - | |
| Eccentricity*† | Aortic orifice area | −2.74 ± 0.47 (p<0.0001) | −2.17 ± 0.81 (p=0.0070) |
| Normalized flow displacement, S1‡ | Bicuspid morphology | 0.032 ± 0.02 (p=0.11) | |
| Ejection fraction | 0.002 ± 0.001 (p=0.0668) | ||
| AS × Aortic diameter | 0.002 ± 0.0003 (p<0.0001) | - | |
| Normalized flow displacement, S2‡ | Age | 0.001 ± 0.0004 (p=0.0788) | |
| Aortic orifice area | −0.039 ± 0.004 (p<0.0001) | −0.041 ± 0.01 (p=0.0021) | |
| Normalized flow displacement, S3‡ | Age | 0.001 ± 0.001 (p=0.0662) | |
| AS × Age | 0.002 ± 0.0002 (p<0.0001) | - | |
| WSSpeak, S1‡ | Ejection fraction | 0.01 ± 0.01 (p=0.0269) | 0.02 ± 0.01 (p=0.0474) |
| AS × Ejection fraction | 0.01 ± 0.001 (p<0.0001) | - | |
| WSSpeak, S2‡ | Age | −0.01 ± 0.003 (p=0.0002) | |
| Bicuspid morphology | 0.22 ± 0.13 (p=0.0926) | ||
| AS × Ejection fraction | 0.01 ± 0.002 (p<0.0001) | - | |
| AS × Aortic orifice area | −0.22 ± 0.07 (p=0.0043) | - | |
| WSSpeak, S3‡ | Age | −0.01 ± 0.003 (p<0.0001) | −0.01 ± 0.004 (p=0.0469) |
| Ejection fraction | 0.02 ± 0.01 (p=0.0592) | ||
| AS × Ejection fraction | 0.01 ± 0.004 (p=0.0004) | - | |
| AS × Aortic diameter | −0.01 ± 0.01 (p=0.0859) | - | |
| AS × Aortic orifice area | −0.13 ± 0.07 (p=0.0540) | - | |
Ordinal logistic regression
Model without AS (y/n) and interaction terms as introduction of these led to quasi-complete separation
Linear regression
- Parameter not presented to selection process
Helical flow formations
Age, ejection fraction, aortic diameter, AS grade and AOA were not relevant with respect to the intensity of helical flow formation within the AS sample. Only when considering both patients and controls, a higher age and a smaller AOA were associated with more intense helical flow formation (p=0.14). Helical flow formation was significantly more intense for AS patients with a bicuspid aortic valve than for AS patients with a tricuspid valve (p=0.0098).
Vortical flow formations
Within the AS sample, the smaller the AOA and the larger the ascending aortic diameter, the more intense the vortical flow formation was (p=0.0274, p=0.14). Vortical flow formations were more pronounced for subjects with a bicuspid aortic valve than for AS patients with a tricuspid valve (p=0.0536).
Eccentricity of the blood flow in the ascending aorta
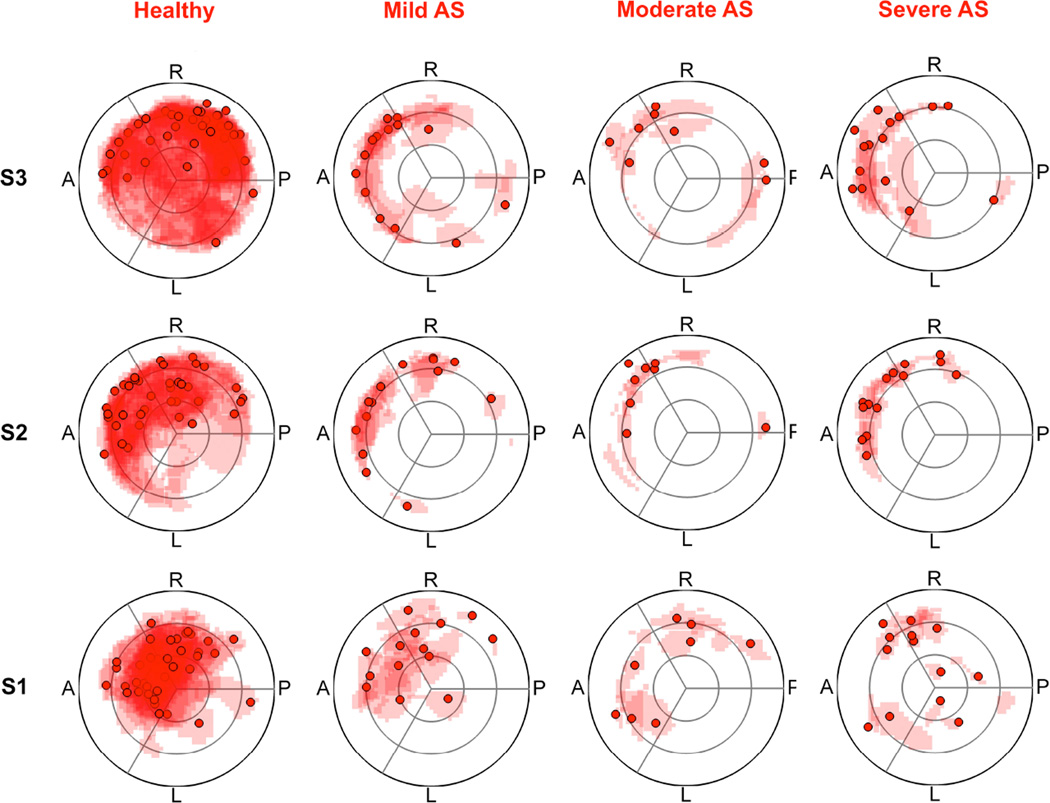
An example for marked eccentricity is shown in Figure 1.The strong eccentricity of the blood flow, which is present in all AS severity grades, is illustrated by the mapping of the peak flow velocity in Figure 4. Marked eccentricity was more prevalent in patients with AS than in healthy subjects, and with increasing AS severity grade the prevalence generally increased (Figure 3). In the regression model, a lower AOA was strongly associated with a higher eccentricity (p<0.0001 in all subjects, p=0.0070 in patients). The results of the normalized flow displacement, which quantitatively describes blood flow eccentricity, are summarized in Table 4. Normalized flow displacement was strongly associated with AOA (pS2=0.0021) and age (pS3=0.0662) among the AS patients, with the smaller the AOA and the higher the age, the larger the flow displacement. The presence of a bicuspid aortic valve was weakly related to normalized flow displacement (pS1=0.11).
Figure 4.
Visualization of the outflow asymmetry by mapping the peak systolic velocity location and the region of the upper 15th percentile of the velocities on a segmental aortic lumen map. S1 = sinotubular junction, S2 = mid-ascending aorta, S3 = distal ascending aorta; AS= aortic stenosis
Table 4.
Normalized flow displacement (mean ± standard deviation).
| Aortic level | Healthy controls |
Mild AS | Moderate AS | Severe AS |
|---|---|---|---|---|
| S1 | 0.11±0.04 | 0.18±0.05 | 0.20±0.05 | 0.19±0.07 |
| S2 | 0.08±0.05 | 0.14±0.04 | 0.17±0.04 | 0.19±0.05 |
| S3 | 0.04±0.01 | 0.12±0.06 | 0.16±0.04 | 0.14±0.07 |
Wall shear stress in the ascending aorta
The distribution of WSSpeak along the aortic circumference is illustrated in Figure 5. Patients with AS exhibited an asymmetric elevation of WSSpeak at the right / right-anterior / anterior side of the aorta at level S1. The magnitude as well as the asymmetry of WSSpeak became more marked in S2. In S3, mild and moderate AS approached the WSSpeak of healthy controls. Severe AS still showed increased and asymmetric WSSpeak results with peaks at the anterior / left-anterior wall (even though there was no statistical difference).
Figure 5.
WSSpeak distribution along the aortic circumference. Comparison of the AS severity grades with healthy controls. S1 = sinotubular junction, S2 = midascending aorta, S3 = distal ascending aorta. A=anterior, LA=left anterior, L=left, LP=left posterior, P=posterior, RP=right posterior, R=right, RA=right anterior. “*” and “**” indicate significant differences between the groups with p<0.05 and p<0.001. Tests were performed per location.
In the regression model, a bicuspid aortic valve was associated with higher WSSpeak in S2 (p=0.0926). In AS patients, ejection fraction correlated positively with WSSpeak in level S1 (p=0.0474) and S3 (p=0.0592), and a higher age was associated with higher WSSpeak in level S3.
Association of flow parameters with parameters of LV remodeling
The results of the regression models are shown in Table 5. In the univariate investigation, stronger vortical flow formations were associated with higher LVM-I, higher RWM and higher probability of LV-remodeling (p=0.0213, p=0.0115, p=0.0285). Higher eccentricity was associated with higher RWM (p=0.0270). A higher normalized flow displacement in level S2 was significantly associated with a higher LVM-I (p=0.0410), a higher RWM (p=0.0023) and with the presence of LV-remodeling (p=0.0459). A higher normalized flow displacement in level S3 was significantly associated with higher LVM-I (p=0.0056) and higher RWM (p=0.0076). A higher WSSpeak at level S1 was significantly associated with higher LVM-I (p=0.0268).
Table 5.
Univariate and multiple regression models to assess relationships between parameters of LV remodelling and flow parameters. In univariate models only significant associations (p<0.05) are shown. LVMI-I = Left ventricular mass index, RWM = Relative wall mass, WSS = wall shear stress
| Model | Remodeling parameter |
Independent variable | Estimate | p-value |
|---|---|---|---|---|
| Univariate models: | ||||
| linear regression | LVM-I | vortical flow formation | 10.94 ± 4.54 | 0.0213 |
| normalized flow displacement, S2 | 172.00 ± 81.04 | 0.0410 | ||
| normalized flow displacement, S3 | 185.61 ± 62.94 | 0.0056 | ||
| WSSpeak, S2 | 23.47 ± 10.15 | 0.0268 | ||
| linear regression | RWM | vortical flow formation | 0.18 ± 0.07 | 0.0115 |
| eccentricity | 0.29 ± 0.13 | 0.0270 | ||
| normalized flow displacement, S2 | 3.65 ± 1.11 | 0.0023 | ||
| normalized flow displacement, S3 | 2.65 ± 0.94 | 0.0076 | ||
| logistic regression | LV-remodeling | vortical flow formation | 0.92 ± 0.42 | 0.0285 |
| normalized flow displacement, S2 | 16.53 ± 8.28 | 0.0459 | ||
| Final multiple models, after stepwise selection: | ||||
| linear regression | LVM-I | age* | −0.48 ± 0.33 | 0.15 |
| systolic blood pressure* | −0.24 ± 0.26 | 0.36 | ||
| aortic orifice area* | −13.84 ± 7.11 | 0.0611 | ||
| normalized flow displacement, S3 | 195.40 ± 65.82 | 0.0058 | ||
| linear regression | RWM | age* | −0.003 ± 0.005 | 0.50 |
| systolic blood pressure* | 0.002 ± 0.003 | 0.55 | ||
| aortic orifice area* | −0.28 ± 0.10 | 0.0058 | ||
| normalized flow displacement, S3 | 2.01 ± 0.87 | 0.0283 | ||
| WSSpeak, S2 | −0.29 ± 0.15 | 0.0691 | ||
| logistic regression | LV-remodeling | age* | 0.05 ± 0.04 | 0.27 |
| systolic blood pressure* | 0.004 ± 0.03 | 0.87 | ||
| aortic orifice area* | −2.27 ± 0.97 | 0.0195 | ||
parameter forced into the model
In multiple regression, LVM-I, RWM and ‘presence of LV remodeling’ were found to be significantly negatively associated with AOA. The covariates age and systolic blood pressure were not found to be significant for the remodeling parameters. The only parameter in addition to AOA that was linked to parameters of LV remodeling was the normalized flow displacement. Its addition improved the model for LVMI to R2=0.3108 as compared to R2=0.1083 (p=0.0058), and for RWM to R2=0.3351 (from 0.2575; p=0.0713), indicating that a larger normalized flow displacement was associated with a higher LVMI and RWM.
Discussion
Patients with AS exhibited a high prevalence of abnormal flow patterns with regard to helical and vortical flow formations and eccentricity. Vortical flow formation, eccentricity and normalized flow displacement were strongly associated with AOA, indicating that the obstruction influences boundary layer separation and leads to significant destabilization of the antegrade blood flow. As there was no difference in the ascending aortic size between the AS severity grades as well as only a weak general association between aortic size and vortical flow formation, these flow abnormalities can be mainly attributed to the abnormal AOA. In addition to the mere flow obstruction, the bicuspid morphology of the valve was a relevant contributor to abnormal blood flow. This finding is in agreement with previous studies that demonstrated altered blood flow in bicuspid valve disease even in the absence of a stenosis17,31.
Abnormal blood flow may reflect an elevated afterload for the LV, as energy is dissipated by frictional losses associated with the various flow phenomena and with the aortic wall14. The latter hypothesis is strengthened by the observation of the univariate analysis, where vortical flow formation, eccentricity and normalized flow displacement were associated with signs of LV remodeling. In the multiple model, however, normalized flow displacement was the only parameter that had impact on LV remodeling in addition to AOA. The relatively small impact of flow parameters on remodeling parameters in multiple models as compared to univariate models might be explained by their quite high correlation to AOA (helix: r=−0.31, vortex: r=−0.52, eccentricity=−0.48, normalized flow displacement r=−0.56). Thus, the added value of flow parameters to models containing AOA is limited, but it was present at least for normalized flow displacement. The association of LV remodeling with AOA underlines that remodeling is a feature of AS severity. Whether it is also a feature of disease progression needs confirmation in a longitudinal study.
Hypertension is another contributor to LV remodeling32. The mean systolic blood pressure was slightly elevated in the AS group compared to the healthy controls, while the various severity grades of AS did not differ regarding the blood pressure. Nevertheless, a comparable blood pressure in a patient with severe vs. mild AS may have varying degree of LV impact, as it is the combination of vascular and valvular LV hemodynamic load that is decisive. In this study, the multiple regression model on LV remodeling accounted for blood pressure and did not find a significant influence among the AS patients.
Blood flow abnormalities are also thought to contribute to post-stenotic aortic dilatation by the chronic vascular wall strain33. Patients with AS revealed significantly elevated WSSpeak at the level of the sinotubular junction and mid-ascending aorta. This was observed similarly in all severity grades of AS, underlining that the aortic blood flow changes as soon as the morphology of the aortic valve changes, even in the absence of a clinically relevant obstruction. Higher WSSpeak was not linked to markers of LV remodeling, but adds knowledge to the development of post-stenotic dilatation in AS34.
In conclusion, this hypothesis-generating study using 4D flow CMR provided new insights into the aortic blood flow in the presence of AS. Blood flow was abnormal in AS, with increasing intensity as the AOA decreased, and enhanced by the presence of a bicuspid aortic valve. In addition to AOA, the flow parameter ‘normalized flow displacement’ was significantly linked to signs of LV remodeling and might therefore serve as surrogate of unfavorable blood transport and myocardial stress. WSS was elevated and asymmetrically distributed in the aorta in all severity grades of AS. Whereas this parameter was not associated with LV remodeling, the findings suggested that mechanotransduction risks for poststenotic dilatation are present already in the early stages of AS. At that time, the results are mainly descriptive and add to the knowledge base surrounding flow patterns in the proximity of AS. The added clinical value of this new information still has to be proven. Yet we speculate that the fluid dynamics information might enable a better characterization of the disease stage in the future.
Limitations of the study
There are several limitations in this work that make it a hypothesis-generating study. i) Further studies with larger samples, more quantitative flow information and integration of multimodality information are needed to test the generated hypothesis that flow pattern and remodeling are linked and to allow adequate subgroup-analysis. ii) Helical and vortical flow patterns were only assessed qualitatively. Absolute quantification of flow and energy loss might be superior and more objective; furthermore, volumetric assessments might overcome limitatons of 2D analyses. However, validation using MRI, CFD, and particle image velocity data has only recently emerged, with mixed results relating to segmentation and resolution related errors35,36,37,38,39. The computation of these parameters requires specialized algorithms and/or volumetric segmentations, neither of which were available for this study.
Additional development and validation needs to be performed before the quantitative approaches can be integrated in future clinically oriented studies. After having overcome these challenges, extending the definitions of the tested flow parameters from 2D to 3D and achieving a volumetric description will certainly be an important future direction. iii) Age is a known influencing factor for ascending aortic hemodynamics29. The groups were statistically not perfectly age-matched, but they were in the same age category (middle-aged adults). To account for this age difference, age was included in the regression models.
Clinical Perspective.
Aortic orifice area and blood flow velocity are sometimes considered incomplete to characterize the stress for the left ventricle caused by aortic stenosis, which is highly variable and leads to a broad range of ventricular impairment. Unfavorable left ventricular remodeling is an important prognostic factor and contributes significantly to the development of heart failure in patients with aortic stenosis. There are many attempts to better characterize the myocardial stress provoked by aortic stenosis. In this study, 4D flow magnetic resonance imaging was used to characterize the post-stenotic blood flow in the ascending aorta, which is suspected to influence ventricular afterload and therefore ventricular remodeling. The aortic blood flow pattern in aortic stenosis was completely different than in healthy controls. Vortical and helical flow formations were more prevalent, the lower the orifice area was. Also, the peak blood flow velocity was more eccentric and the flow displacement higher in patients with aortic stenosis, and the wall shear stress elevated and asymmetrically distributed. In addition to aortic orifice area, flow displacement, which quantitatively describes the blood flow eccentricity, was associated with markers of left ventricular remodeling. These results are preliminary, but hypothesis generating and stimulating future trials about the interplay of valvular disease, myocardial impairment and blood flow in order to comprehensively characterize aortic valve disease.
Acknowledgments
The authors wish to acknowledge the technician Kerstin Kretschel, Evelyn Polzin, Denise Kleindienst and Franziska Neumann acquiring the CMR data and the study nurses Elke Nickel-Szczech and Antje Els for assisting in the organization of the CMR scans. We thank Mrs. Susanne Schwenke (scossis, Berlin) for her valuable support of the statistical analyses.
Sources of Funding
Stiftung für Herzforschung (Frankfurt, Germany) (F/37/12) (FvKB), NIH K25HL119608 (AJB), and NIH R01HL115828 (MM)
Footnotes
Disclosures
None.
References
- 1.Pibarot P, Dumesnil JG. Improving assessment of aortic stenosis. J Am Coll Cardiol. 2012;60:169–180. doi: 10.1016/j.jacc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 3.Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour A, Maceira A, Roussin I, Northridge DB, Kilner PJ, Cook SA, Boon NA, Pepper J, Mohiaddin RH, Newby DE, Pennell DJ, Prasad SK. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: Insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:50. doi: 10.1186/1532-429X-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: A disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 5.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 6.Gerdts E, Rossebo AB, Pedersen TR, Cioffi G, Lonnebakken MT, Cramariuc D, Rogge BP, Devereux RB. Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circ Cardiovasc Imaging. 2015;8:e003644. doi: 10.1161/CIRCIMAGING.115.003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess OM, Villari B, Krayenbuehl HP. Diastolic dysfunction in aortic stenosis. Circulation. 1993;87:IV73–IV76. [PubMed] [Google Scholar]
- 8.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: An inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Ng AC, Delgado V, Bertini M, Antoni ML, van Bommel RJ, van Rijnsoever EP, van der Kley F, Ewe SH, Witkowski T, Auger D, Nucifora G, Schuijf JD, Poldermans D, Leung DY, Schalij MJ, Bax JJ. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: A two-dimensional speckle tracking analysis. Eur Heart J. 2011;32:1542–1550. doi: 10.1093/eurheartj/ehr084. [DOI] [PubMed] [Google Scholar]
- 10.Shah AS, Chin CW, Vassiliou V, Cowell SJ, Doris M, Kwok TC, Semple S, Zamvar V, White AC, McKillop G, Boon NA, Prasad SK, Mills NL, Newby DE, Dweck MR. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. 2014;130:1607–1616. doi: 10.1161/CIRCULATIONAHA.114.011085. [DOI] [PubMed] [Google Scholar]
- 11.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin i concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 13.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 14.Barker AJ, van Ooij P, Bandi K, Garcia J, Albaghdadi M, McCarthy P, Bonow RO, Carr J, Collins J, Malaisrie SC, Markl M. Viscous energy loss in the presence of abnormal aortic flow. Magn Reson Med. 2014;72:620–628. doi: 10.1002/mrm.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kheradvar A, Pedrizzetti G. Vortex formation in the cardiovascular system. Springer-Verlag; 2012. [Google Scholar]
- 16.Markl M, Kilner PJ, Ebbers T. Comprehensive 4d velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:7. doi: 10.1186/1532-429X-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circulation. Cardiovascular imaging. 2012;5:457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 18.Bauer S, Markl M, Foll D, Russe M, Stankovic Z, Jung B. K-t grappa accelerated phase contrast mri: Improved assessment of blood flow and 3-directional myocardial motion during breath-hold. J Magn Reson Imaging. 2013;38:1054–1062. doi: 10.1002/jmri.24077. [DOI] [PubMed] [Google Scholar]
- 19.von Knobelsdorff-Brenkenhoff F, Trauzeddel RF, Barker AJ, Gruettner H, Markl M, Schulz-Menger J. Blood flow characteristics in the ascending aorta after aortic valve replacement-a pilot study using 4d-flow mri. Int J Cardiol. 2014;170:426–433. doi: 10.1016/j.ijcard.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhall CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M. 4d flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson. 2015;17:72. doi: 10.1186/s12968-015-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2d and 3d phase contrast mri: Optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60:1218–1231. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 22.Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, Higgins CB. 4d flow cmr in assessment of valve-related ascending aortic disease. JACC. Cardiovascular imaging. 2011;4:781–787. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Sigovan M, Hope MD, Dyverfeldt P, Saloner D. Comparison of four-dimensional flow parameters for quantification of flow eccentricity in the ascending aorta. J Magn Reson Imag. 2011;34:1226–1230. doi: 10.1002/jmri.22800. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich MG, Schulz-Menger J, Poetsch T, Pilz B, Uhlich F, Dietz R. Quantification of valvular aortic stenosis by magnetic resonance imaging. Am Heart J. 2002;144:329–334. doi: 10.1067/mhj.2002.124057. [DOI] [PubMed] [Google Scholar]
- 25.von Knobelsdorff-Brenkenhoff FRA, Wassmuth R, Abdel-Aty H, Schulz-Menger J. Aortic dilatation in patients with prosthetic aortic valve: Comparison of mri and echocardiography. J Heart Valve Dis. 2010;19:349–356. [PubMed] [Google Scholar]
- 26.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 27.Jonckheere AR. A distribution-free kappa-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 28.Burk J, Blanke P, Stankovic Z, Barker A, Russe M, Geiger J, Frydrychowicz A, Langer M, Markl M. Evaluation of 3d blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4d cmr. J Cardiovasc Magn Reson. 2012;14:84. doi: 10.1186/1532-429X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ooij P, Garcia J, Potters WV, Malaisrie SC, Collins JD, Carr JC, Markl M, Barker AJ. Age-related changes in aortic 3d blood flow velocities and wall shear stress: Implications for the identification of altered hemodynamics in patients with aortic valve disease. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25081. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 31.Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J, Markl M. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129:673–682. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 33.Hope MD, Dyverfeldt P, Acevedo-Bolton G, Wrenn J, Foster E, Tseng E, Saloner D. Post-stenotic dilation: Evaluation of ascending aortic dilation with 4d flow mr imaging. Int J Cardiol. 2012;156:e40–e42. doi: 10.1016/j.ijcard.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, Mewhort HE, Svystonyuk DA, Kang S, Verma S, Collins J, Carr J, Bonow RO, Markl M, Thomas JD, McCarthy PM, Fedak PW. Valve-related hemodynamics mediate human bicuspid aortopathy: Insights from wall shear stress mapping. J Am Coll Cardiol. 2015;66:892–900. doi: 10.1016/j.jacc.2015.06.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz R, Bock J, Barker AJ, von Knobelsdorff-Brenkenhoff F, Wallis W, Korvink JG, Bissell MM, Schulz-Menger J, Markl M. 4d flow magnetic resonance imaging in bicuspid aortic valve disease demonstrates altered distribution of aortic blood flow helicity. Magn Reson Med. 2014;71:1542–1553. doi: 10.1002/mrm.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morbiducci U, Ponzini R, Rizzo G, Cadioli M, Esposito A, De Cobelli F, Del Maschio A, Montevecchi FM, Redaelli A. In vivo quantification of helical blood flow in human aorta by time-resolved three-dimensional cine phase contrast magnetic resonance imaging. Ann Biomed Eng. 2009;37:516–531. doi: 10.1007/s10439-008-9609-6. [DOI] [PubMed] [Google Scholar]
- 37.Binter C, Gulan U, Holzner M, Kozerke S. On the accuracy of viscous and turbulent loss quantification in stenotic aortic flow using phase-contrast mri. Magn Reson Med. 2015 doi: 10.1002/mrm.25862. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Casas B, Lantz J, Dyverfeldt P, Ebbers T. 4d flow mri-based pressure loss estimation in stenotic flows: Evaluation using numerical simulations. Magn Reson Med. 2015 doi: 10.1002/mrm.25772. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Cibis M, Jarvis K, Markl M, Rose M, Rigsby C, Barker AJ, Wentzel JJ. The effect of resolution on viscous dissipation measured with 4d flow mri in patients with fontan circulation: Evaluation using computational fluid dynamics. J Biomech. 2015;48:2984–2989. doi: 10.1016/j.jbiomech.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]