Abstract
Despite the success of combined antiretroviral therapy, more than half of HIV-1-infected patients in the USA show HIV-associated neurological and neuropsychiatric deficits. This is accompanied by anatomical and functional alterations in vulnerable brain regions of the mesocorticolimbic and nigrostriatal systems that regulate cognition, mood and motivation-driven behaviors, and could occur at early stages of infection. Neurons are not infected by HIV, but HIV-1 proteins (including but not limited to the HIV-1 trans-activator of transcription, Tat) induce Ca2+ dysregulation, indicated by abnormal and excessive Ca2+ influx and increased intracellular Ca2+ release that consequentially elevate cytosolic free Ca2+ levels ([Ca2+]in). Such alterations in intracellular Ca2+ homeostasis significantly disturb normal functioning of neurons, and induce dysregulation, injury, and death of neurons or non-neuronal cells, and associated tissue loss in HIV-vulnerable brain regions. This review discusses certain unique mechanisms, particularly the over-activation and/or upregulation of the ligand-gated ionotropic glutamatergic NMDA receptor (NMDAR), the voltage-gated L-type Ca2+ channel (L-channel) and the transient receptor potential canonical (TRPC) channel (a non-selective cation channel that is also permeable for Ca2+), which may underlie the deleterious effects of Tat on intracellular Ca2+ homeostasis and neuronal hyper-excitation that could ultimately result in excitotoxicity. This review also seeks to provide summarized information for future studies focusing on comprehensive elucidation of molecular mechanisms underlying the pathophysiological effects of Tat (as well as some other HIV-1 proteins and immunoinflammatory molecules) on neuronal function, particularly in HIV-vulnerable brain regions.
Keywords: neuroAIDS, cognition, medial prefrontal cortex, pyramidal neuron, over-excitation, neurotoxicity, electrophysiology
1. INTRODUCTION
Despite the success of combined antiretroviral therapy (cART), over 50% of the human immunodeficiency virus type 1 (HIV-1)-seropositive patients in the USA are diagnosed with HIV-associated neurocognitive disorders (HAND) manifested by cognitive, psychosocial, and motor deficits, along with some neuropsychiatric deficits [1, 2]. The human brain is profoundly affected by HIV-1 infection [3, 4], even at early stages and young ages [5, 6]. Although neurons are not infected by HIV-1, their function can be altered significantly by HIV-1 proteins and inflammatory molecules released by HIV-infected cells (e.g. leucocytes, astrocytes and microglia cells) that support productive viral replication [7]. HIV-1 Tat, a trans-activator of transcription for viral replication, is one of the toxic HIV-1 proteins that plays a critical role in the development and progression of HAND due in part to its neurotoxicity. Tat is one of the first HIV-1 proteins to be expressed and detected following HIV infection. The deleterious effects of Tat on neurons and other types of cells have been shown to result in dysregulation, injury or death of neurons, which likely contribute to HIV neuropathogenesis [3, 8–11]. Although the cytotoxic or neurotoxic effects of Tat on the central nervous system (CNS) have been studied extensively, the exact mechanisms underlying Tat-induced changes in neuronal function in the brain are not fully understood and require further investigation. This review focuses mainly on summarizing the pathophysiological effects of Tat on dysregulating intracellular Ca2+ homeostasis and functional activity of neurons (rather than causing severe damage or death) in certain brain regions, which may contribute to the progression and severity of HAND.
2. TAT-ASSOCIATED EARLY ALTERATIONS IN HIV-VULNERABLE BRAIN REGIONS
It is reported that certain cortical and subcortical brain regions, which are key regulators of cognition, mood and motivation-driven behaviors, appear to be more vulnerable than other brain regions to the toxic effects of HIV-1 proteins (including Tat), and also are implicated in HIV-induced neurological and neuropsychiatric deficits due to earlier anatomical and functional alterations [3, 8–14]. These brain regions, including but not limited to the hippocampus, prefrontal cortex, basal ganglia (dorsal/ventral striatum; a.k.a. the caudate-putamen and nucleus accumbens, respectively) and midbrain, are part of the mesocorticolimbic and nigrostriatal pathways, suggesting that dysfunction of neurons or their connectivity in these systems play a pivotal role in neuroAIDS. Anatomical and functional alterations in these HIV-vulnerable brain regions are linked in part to the excitotoxic and neurotoxic effects of Tat and other toxic HIV-1 proteins [3, 9, 14]. It is unclear why these brain regions seem to be affected by HIV at earlier stages of infection and associated with more severe changes than other brain regions during the progression of HAND. Nevertheless, such increased susceptibility of these regions to HIV could result from their anatomical location. It is worth noting that all of these HIV-vulnerable brain regions are localized adjacent to, or near by the ventricles [15], which could render them more readily and directly invaded by HIV-infected leukocytes from the peripheral blood system. This situation is illustrated in (Fig. 1), where HIV-infected leukocytes could infiltrate the blood-brain barrier and enter the cerebrospinal fluid (CSF), through which nearby brain regions could be readily invaded. Microglial cells and astrocytes can be infected by HIV following invasion of HIV+ leukocytes in the brain. Tat and other viral proteins secreted by HIV+ cells, as well as immunoinflammatory molecules induced by HIV-1 proteins [16–19], could profoundly alter neuronal function [20, 21]. Further, uptake and axonal transport of Tat could allow spread of Tat throughout the connected hippocampus, prefrontal cortex, basal ganglia and midbrain [22, 23]. Taken together, these findings strongly suggest an integrative structural and functional mechanism that forms a fundamental base for Tat (and other HIV-1 proteins)-induced neuronal dysregulation at earlier stages of HIV infection, and during the progression of HAND, and emphasize the need for further elucidation of the molecular mechanism(s) underlying the neuropathophysiological effects of Tat (and other HIV-1 proteins and immunoinflammatory molecules) in these brain regions.
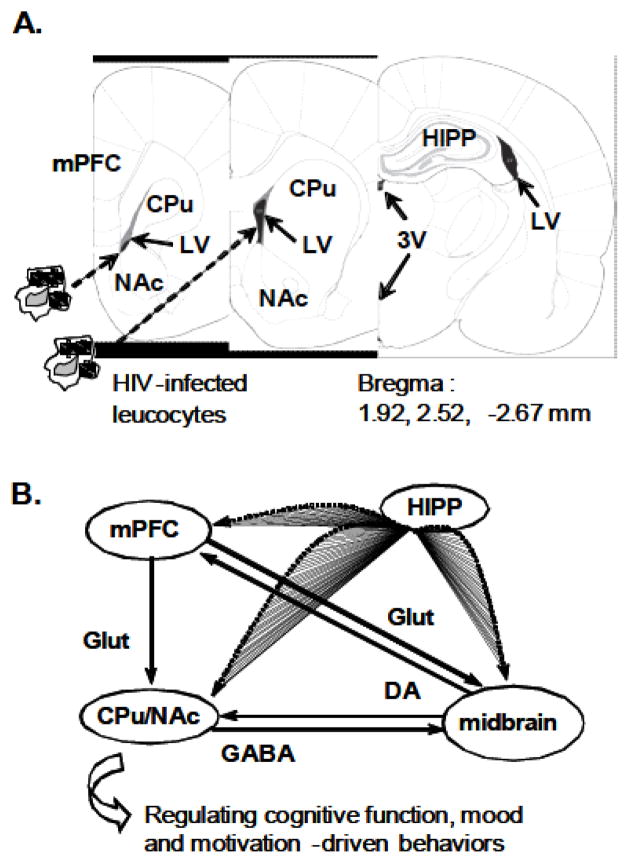
Fig. 1. HIV invades vulnerable brain regions through the ventricles.
A. HIV-infected leucocytes from the peripheral blood system cross the BBB and from the cerebrospinal fluid circulation, invade vulnerable brain regions in close proximity to the ventricles. Rat brain sections indicate the location of the LV, 3V, mPFC, dorsal/ventral striatum (CPu and NAc, respectively) and HIPP. B. The mesocorticolimbic and nigrostriatal DA pathways, which play a critical role in regulating neurocognitive function, mood and motivation-driven behavior, are anatomically and functionally connected. Abbreviations: BBB, brain-blood barrier; DA, dopamine; GABA, γ-aminobutyric acid; Glut, glutamate; HIV-1, human immunodeficiency virus type 1; LV, the lateral ventricle; 3V, the third ventricle; mPFC, medial prefrontal cortex; CPu, caudate-putamen; NAc, nucleus accumbens; HIPP, hippocampus.
3. TAT-INDUCED NEURONAL DYSFUNCTION AND CA2+ DYSREGULATION
Tat induces neuronal dysfunction through a variety of mechanisms [24–26]. This review focuses on the deleterious effects of Tat on neurons mainly associated with Ca2+ dysregulation, displayed by abnormal and excessive Ca2+ influx/release and consequential increase of intracellular Ca2+ levels ([Ca2+]in), which are mediated by the ionotropic glutamatergic N-methyl-D-aspartate receptor (NMDAR), voltage-gated L-type Ca2+ channel (L-channel) and transient receptor potential canonical (TRPC) channel, along with other possible important players (e.g., a variety of protein kinases and immunoinflammatory molecules, etc.). Although the exact mechanisms underlying HIV-associated neuropathogenesis are poorly understood, extensive studies reveal a pivotal role of Ca2+ dysregulation that induces many neuronal dysfunctions [12, 20, 21, 24]. Cumulating studies demonstrate that the effects of Tat and other HIV-1 proteins (e.g., gp120, Vpr, etc.) are associated with excessive increases of Ca2+ influx and [Ca2+]in in neurons [21, 27–33], and non-neuronal cells [34–38], while some others show that Tat inhibits responses of immune cells by blocking Ca2+ influx [39, 40]. Together, these findings imply cell type-specific effects of Tat on disruption of Ca2+ homeostasis. Tat also induces increased firing and Ca2+ influx in rat hippocampal and cortical neurons [27, 28, 41] and death of striatal neurons. Tat-induced neuronal dysfunction also results in alterations in various neurotransmission. For example, Tat increases glutamate release, but decreases GABA release from cortical neurons [42]. Tat also evokes acetylcholine release from cortical neurons [43], and inhibits dopamine reuptake by striatal neurons [44, 45]. These findings suggest dysfunction or injury of neuronal terminals. In fact, loss of dopamine transporters (normally located in the terminals) is found in the striatum of patients with HIV-associated dementia [46, 47], and in neurons of experimental animals [48]. Given that excessive Ca2+ influx/[Ca2+]in is toxic, which disturbs a variety of intracellular signaling processes and induces injury, tissue loss, and possible death of neurons [21, 29, 31, 32, 49–51] and non-neuronal cells [34–36], HIV-associated neuronal dysfunction could be attributed in part to the neuropathological effects of Tat (and other viral proteins) on Ca2+ dysregulation.
4. TAT-MEDIATED DYSREGULATION OF CA2+ INFLUX AND [CA2+]IN: MECHANISMS AND RELATED INTRACELLULAR SIGNALING
Tat increases [Ca2+]in by inducing Ca2+ influx through Ca2+-permeable ion channels and release of intracellular Ca2+ stores [49, 52–54]. This review seeks to summarize what is currently known about (i) Tat-mediated increases of Ca2+ influx in neurons (mediated mainly by NMDAR, the L-channel and TRPC channel), (ii) Tat-mediated changes in intracellular Ca2+ release, and (iii) the functional interplay of these channels, which could also be altered by Tat.
4.1. Dysregulation of Ionotropic Glutamatergic NMDAR
The NMDAR is a ligand-gated ion channel that is highly permeable for Ca2+ [24–26]. Considerable and highly significant studies have revealed a critical role of NMDAR-mediated increases of Ca2+ influx/[Ca2+]in in HIV-associated excitotoxicity [13, 25, 26, 55], which may contribute to the progression and severity of neuroAIDS. In neurons functional NMDARs are located in the cell membrane and are activated by released glutamate (a major excitatory neurotransmitter in the CNS) or NMDA. Exposure to HIV-1 proteins (and immunoinflammatory molecules) is associated with increased extracellular glutamate levels [56, 57], and significantly-enhanced NMDAR activation [21, 31, 32, 50, 58, 59] and expression [56, 57]. Through such alterations, HIV abnormally increases Ca2+ influx via NMDAR that increases [Ca2+]in. Therefore, NMDAR has been considered to play a critical role in dysregulation of intracellular Ca2+ homeostasis and related neuronal dysfunction. In addition, Tat also inhibits activity of glutamate transporters in astrocytes [60], resulting in decreased glutamate uptake by these cells. This decrease in glutamate uptake could consequently increase extracellular glutamate levels, thereby potentiating Tat (along with other HIV-1 proteins and immunoinflammatory molecules)-induced abnormal Ca2+ influx via NMDAR in neurons surrounded by these dysfunctional astrocytes.
Tat specifically alters NMDAR activity by acting on a NMDAR/low-density lipoprotein receptor-related protein (LRP) complex. In response to transient application of glutamate or NMDA, Ca2+ influx in cultured rat hippocampal neurons is facilitated by Tat pre-exposure [31]. Additionally, Tat-induced excitotoxicity in rat hippocampal pyramidal neurons from cultured slices is reduced by NMDAR blockade [50]. It is reported that the neurotoxic effects of Tat require direct interaction with NMDAR at a polyamine-sensitive site [50], by which Ca2+ influx and [Ca2+]in are elevated in cultured rat hippocampal neurons [51], because this Tat effect is attenuated by selective blockade of the polyamine site and NMDAR [32]. Further, combined exposure to Tat and gp120 at subtoxic concentrations also induces death of neuronal cells, which is associated with prolonged increases of [Ca2+]in, and this combined neurotoxic effect is blocked by selective NMDAR antagonism [21, 49]. Tat can also exert its effects on neuronal activity by entering neurons via the LRP. Previous studies show that LRP mediates uptake of Tat, which is translocated to the nucleus, by cultured human neurons [22]. Tat binding to LRP results in substantial inhibition of neuronal binding, uptake and degradation of various physiological ligands for LRP, suggesting that Tat mediates HIV-induced neuropathology via signaling pathways involving disruption of the metabolic balance of LRP ligands (possibly by competition) and direct activation of harmful gene expression [22]. Tat also induces formation of a macromolecular LRP-postsynaptic density protein-95 (PSD-95)-NMDAR-neuronal nitric oxide synthase complex in the cell membrane, which promotes apoptosis of neurons and astrocytes [61, 62]. In addition, Tat additionally induces synapse loss in neurons that is associated with excessive Ca2+ influx via NMDAR [11].
Tat also produces a time-dependent biphasic change in NMDAR-mediated increases of Ca2+ influx/[Ca2+]in, which is indicated by an initial increase, and then an adapted reduction of Ca2+ influx/[Ca2+]in. The later change is regulated by activation of the Src tyrosine kinase/nitric oxide synthase-mediated signaling pathway, and RhoA/Rho-associated protein kinase (ROCK) signaling, both lead to reduced NMDAR activity and NMDAR-evoked Ca2+ influx in cultured rat hippocampal neurons [63, b, 64]. This adaptation of NMDAR function following excessive increase of Ca2+ is considered a neuronal self-protection mechanism against excitotoxicity induced by over-activation of NMDAR and excessive [Ca2+]in. Together, these findings clearly indicate a NMDAR-mediated mechanism, via which Tat induces abnormal and excessive Ca2+ influx in neurons (Fig. 2).
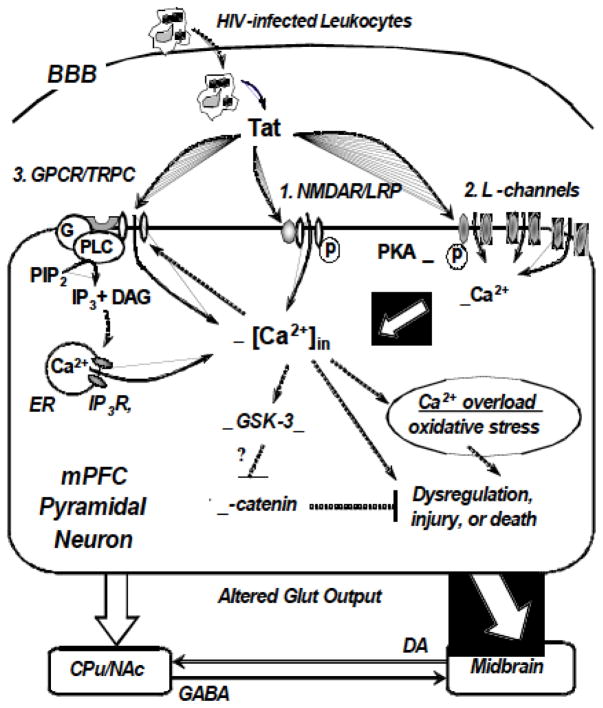
Fig. 2.
Three mechanisms and related signaling that underlie Tat-mediated increases in Ca2+ influx/[Ca2+]in and hyper-excitation of mPFC pyramidal neurons. Tat, which is secreted by HIV-infected leukocyte, microglia and astrocytes in the brain, binds to (1) the ligand-gated NMDAR, (2) voltage-gated L-channel, and (3) GPCR-associated TRPC channel in mPFC pyramidal neurons. The NMDAR and L-channels are highly or selectively permeable for Ca2+, while the TRPC channel is a non-selective cation channel that is also permeable for Ca2+. Activity of TRPC channels is mediated by the GPCR/Gi,q/PLC/IP3 pathway and intracellular Ca2+ release. Tat abnormally increases Ca2+ influx and Ca2+ release in neurons by acting on the NMDAR, L-channel, and GPCR associated with TRPC channel. Enhanced phosphorylation of NMDAR and the L-channel also increases the Ca2+-conducting activity of the channels. Excessive cytosolic free Ca2+ is toxic; and enduring exposure to Tat (and other HIV-1 proteins and immunoinflammatory molecules) in vivo induces overload of Ca2+ that could disturb mitochondrial activity and other neuronal function, causing oxidative stress, dysregulation, over-excitation-associated injury, and death of neurons. Increased [Ca2+]in may also increase GSK-3β activity and reduce β-catenin activity, rendering the neuron more vulnerable to excitotoxicity. In response to co-exposure of HIV-1 proteins (including Tat) and immunoinflammatory molecules, glutamate output from mPFC pyramidal neurons to the basal ganglia (e.g., the CPu and NAc) and midbrain will be altered. DA-containing neurons in the midbrain project to the dorsal/ventral striatum, which also receives local GABA inputs from the NAc and CPu. Integrated dysregulation and neurotoxicity mediated by HIV-1 proteins (and immunoinflammatory molecules) alter the structure and function of the mPFC, and that may ultimately contribute to HIV-associated neuropathogenesis. Blocking or attenuating excessive Ca2+ influx through these pathways may reduce such toxic effects of Tat; combined interventions at early stages and younger ages may be necessary and critical. Abbrivations: BBB, brain-blood barrier; CPu, caudate-putamen; CSF, cerebrospinal fluid; DA, dopamine; DAG, diacyl-glycerol; ER, endoplasmic reticulum; G, G protein; GABA, γ-aminobutyric acid; GPCR, G protein-coupled receptor; Glut, glutamate; GSK-3β, glycogen synthase kinase 3-beta; HAND, HIV-associated neurocognitive disorders; HIV-1, human immunodeficiency virus type 1; IP3, inosotol-1,4,5-trisphosphate; IP3R, IP3 receptor; L-channel, voltage-gated L-type Ca2+ channel; LRP, low-density lipoprotein receptor-related protein; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; NMDAR, N-methyl-D-aspartate receptor; p, phosphorelation; PLC, phospholipase C; PIP2, phosphatidylinositol (4,5)-bisphosphate; PKA, protein kinase A; TRPC, transient receptor potential canonical channels.
4.2. Possible Signaling Pathways that Facilitate Tat-Mediated Over-Activation/Upregulation of NMDAR
Besides the direct effect on NMDAR [50], Tat may also exert an indirect effect on neuronal Ca2+ influx by altering signaling pathways that affect NMDAR activity/expression, including but not limited to those mediated by the tumor necrosis factor alpha (TNF-α), various protein kinases (other than those mentioned above), and glycogen synthase kinase 3-beta (GSK-3β). These mechanisms could also enhance NMDAR activity, and therefore may ultimately contribute to Tat-induced excitotoxicity in neurons.
First, Tat mediates increased cellular production of TNF-α, an inflammatory mediator [16, 35, 36] that facilitates Ca2+ influx via NMDAR in rat cortical neurons [58, 65], leading to additional increase of [Ca2+]in. It is known that TNF-α is increased in neurons in the frontal cortex and basal ganglia of HIV-seropositive patients [66], and is implicated in the pathogenesis of an uncommon and rapidly progressive form of AIDS dementia complex. Thus, this effect of TNF-α on Ca2+ influx could exacerbate Tat-mediated excitotoxicity in neurons.
Second, Tat can also enhance Ca2+ influx by facilitating NMDAR activity via enhancing phosphorylation. Phosphorylation of NMDAR can be mediated by the dopamine D1 receptor (D1R)-coupled cAMP/PKA cascade in neurons. It is established that the functional activity of NMDAR [67, 68, also see 69 for review] in conducting Ca2+ influx is increased in mPFC pyramidal neurons following enhanced phosphorylation mediated by increased PKA activity in the D1R-coupled cAMP/PKA cascade. Therefore, Tat may increase NMDAR activity by enhancing PKA activity [70–72]. Interestingly, a recent study reveals that D1R expression is significantly increased in the brain of HIV-1 Tg rats [73]. Further, death of midbrain dopaminergic neurons induced by Tat, as well as by combined sub-toxic doses of Tat and methamphetamine, is attenuated by inhibition of D1Rs [74, 75]. Because D1R activation facilitates the cAMP/PKA cascade, it is possible that increased D1R expression in HIV-infected brains may lead to enhanced phosphorylation of NMDAR, thereby inducing additional increase of Ca2+ influx in neurons. Further studies are needed to determine whether this is true. In addition, it is also reported that the LRP is a signaling receptor associated directly with activation of the stimulatory G-protein (Gs) and a downstream PKA-dependent pathway [76]. Whether HIV and Tat-induced increases in cAMP levels and PKA activity [70–72] is mediated via this LRP-mediated signaling in neurons also requires further investigation. Nevertheless, a recent study reported inhibition of PKA activity by Tat in vitro [77], but the Tat concentration used was quite high (IC50=1.2 μM), so it is likely that such inhibition may be a non-specific effect. In addition, Tat also potentiates excitotoxicity of glutamate in cultured rat hippocampal neurons via PKC-mediated phosphorylation and activation of NMDAR [31], though an opposite effect of Tat on PKC in cultured HeLa cells is reported [77]. Collectively, these findings show that Tat-induced dysfunction of protein kinases also participates in alteration of NMDAR activity, which could be time-dependent, dose-dependent, and cell type-specific.
Third, Tat mediates increase of GSK-3β activity in rat cerebellar granule neurons [78] and midbrain primary neurons [54], and decrease of β-catenin activity in astrocytes [79, 80]. These changes are also associated with abnormal increases of [Ca2+]in. Given that β-catenin plays a critical role in neuroprotection and other neuronal functions, and GSK-3β decreases β-catenin activity [81, 82], these effects of Tat on astrocytes could impair the function of astrocytes to uptake glutamate [60], and therefore result in dysregulation of extracellular glutamate levels and dysfunction of neurons surrounded by these astrocytes.
4.3. Dysregulation of the Voltage-Gated L-Channel, Independent of NMDAR
HIV-1 protein-induced neuronal dysfunction and Ca2+ dysregulation do not depend solely on over-activation and expression of NMDAR. Previous studies also suggest a critical role for the L-channel. For example, blockade of L-channels reduces Tat-induced neuronal death by decreasing excessive Ca2+ influx [83]. Other studies also show that Tat-mediated Ca2+ influx is regulated in part by the L-channels [34], although activation of β-chemokine receptors [84] and glutamatergic NMDAR [31, 32, 51] are involved. Moreover, low (femtomolar and nanomolar) concentrations of Tat dose-dependently induce membrane depolarization, increase evoked firing, and elicit a fast transient increase of [Ca2+]in in rat CA1 hippocampal neurons in culture or from rat brain slices [28, 41], but this increased [Ca2+]in is not affected by sodium channel blockade, and is not completely blocked by antagonists for NMDAR or AMPA receptor (AMPAR, another ionotropic glutamatergic receptor that can also conduct Ca2+ currents). Tat effects on increasing Ca2+ influx through over-activation of the L-channels appear to be consistent in neurons. Previous studies indicate that Tat injection into the rat striatum induces brain tissue loss, including loss of striatal neurons and glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes, by seven days after injection [29], which is similarly observed after combined injection of subtoxic concentrations of Tat and gp120. Such toxic effects of Tat (and gp120) are mediated, at least in part, by over-activating the L-channels because L-channel blockade significantly reduces Tat/gp120-induced tissue loss and cell death [21, 49, 83]. Together, these studies strongly suggest a critical role of the L-channel as another major player in Tat-mediated excessive Ca2+ influx.
Involvement of the L-channel in Tat-induced Ca2+ influx has also been observed in immune cells. Direct binding of Tat to the L-channels in immune cells has been reported [39, 40], though which has not been reported in neurons yet. The Tat effects on altering Ca2+ influx via the L-channels seem to be cell type-specific in immune cells. For example, Tat impairs function of human natural killer cells and dendritic cells by blocking Ca2+ influx through the L-channels [39, 40], but increase L-channel-mediated [Ca2+]in in human monocytes [36] and human microglia [34]. Further investigation is required to determine the mechanisms underlying these differences in L-channel activity in different cell types.
Our recent studies extend these Tat/L-channel findings by separating Ca2+ influx in mPFC pyramidal neurons through voltage-gated L-channels, independent of NMDAR. While recognizing the significance of the NMDAR in HIV-associated neuropathogenesis, we have demonstrated an additional important and novel pathway for Tat dysregulation of neuronal excitability, which is functional with blockade of NMDAR (and other ionotropic glutamate receptors): specifically, over-activation and upregulation of the L-channels [85–87]. We have demonstrated that the L-channels mediate Tat (and other HIV-1 protein)-induced Ca2+ influx and neuronal hyper-excitation in rat mPFC pyramidal neurons: (1) Under blockade of NMDAR (and AMPAR), Tat exposure of mPFC pyramidal neurons in rat brain slices ex vivo significantly increases Ca2+ influx by over-activating the L-channels, rendering these cortical neurons more susceptible and vulnerable to relatively “normal” excitatory stimuli (mimicked by moderate depolarizing currents injected into neurons) [86, 87]. (2) The Tat effects on neuronal excitability are concentration-dependent, via which lower nanomolar concentrations of Tat increase Ca2+ influx, while higher concentrations of Tat decrease Ca2+ influx [86, 87]. The later finding suggests acute excitotoxic injury, or a self-protective mechanism that could be mediated by internalization of the L-channels in response to over-activation [88]. (3) When injected into the rat lateral ventricle [85], Tat induces a region-specific increase of L-channel expression in the rat mPFC, which is not found in the dorsal/ventral striatum or motor cortex. (4) Increased NMDAR-independent Ca2+ influx is also found in mPFC pyramidal neurons from non-infectious HIV-1 transgenic (Tg) rats, which express 7 of the 9 HIV-1 proteins with deletion of gag and pol [89], which is partially abolished by selective blockade of the L-channels (our unpublished findings). Because the Tat effects on Ca2+ influx in cortical neurons are detected with blockade of NMDAR/AMPAR, our findings, in combination with others discussed above, demonstrate that the Tat action on cortical neurons results not only from dysregulation of the ligand-gated NMDAR, but also from over-activation and upregulation of the voltage-gated L-channels (Fig. 2). Collectively, these studies reveal a unique mechanism via which Tat and other HIV-1 proteins induce hyper-excitation of mPFC pyramidal neurons. Therefore, we propose that Tat-induced dysfunction of cortical and striatal neurons (associated with injury of dopaminergic terminals in the basal ganglia) could be attributed in part to L-channel over-activation and upregulation in mPFC pyramidal neurons. Because these cortical neurons are glutamatergic and provide excitatory inputs to the striatum and midbrain, such changes could ultimately contribute to dysfunction of the mesocorticolimbic and nigrostriatal systems in patients with neuroAIDS.
4.4. Possible Signaling Pathways that Promote Tat-Mediated Over-Activation/Upregulation of the L-Channel
The exact mechanism(s) of Tat-mediated L-channel over-activation and upregulation remains unknown, but increasing evidence suggests certain molecular mechanism(s) or signaling pathways that could underlie or modify Tat-induced L-channel dysregulation. These mechanisms/signaling pathways may include, but not limited to, dysfunction of the α1 subunit (a ‘pore’-forming protein) and other auxiliary subunits of the L-channel, dysregulation of protein expression of the L-channel mediated by micro RNA, and/or changes in a variety of protein kinases, etc. Abnormal alterations in these mechanism(s) and/or related signaling pathways could lead to various dysfunctions of the L-channel, including over-activation and upregulation.
Structural alterations in the α1 subunit and/or other auxiliary subunits of voltage-gated ion channels may underlie Tat-induced changes in functional activity of these channels. For example, splice variant alterations in ion channel subunits could result in over-activation of the channels. Previous studies have reported an alternative splice variant of the Cav1.2 α1c subunit, the ‘pore’-forming protein of the L-channel, which includes an alternative exon of 75 base pair in the sequence between exons 9 and 10 (a.k.a. the exon 9*) [90, 91]. This variant is associated with functional changes in the gating mechanism and kinetics of the L-channel, with a significant shift of the current-voltage (I–V) relationship to the more hyperpolarized membrane potential levels [90], revealing an altered voltage-dependence that leads to an increase in L-channel activity. Consequently, such an abnormal increase in L-channel activity mediated by this variant renders the cell membrane more susceptible to depolarizing stimuli, potentially causing increased neuronal excitability in response to excitatory inputs, particularly to abnormally-increased extracellular glutamate levels and HIV-1 proteins (e.g., Tat, gp120 and Vpr, etc.). These studies suggest that over-expression of the exon 9* splice variant could mediate increased L-channel activity. Due to limited information regarding the biophysiological properties of the 9*-containing Cav1.2 L-channels [90, 92], it is difficult to precisely predict whether and how (1) Tat may bind to this variant, and (2) increase of the 9*-containing L-channels is implicated in our observation regarding Tat-induced L-channel over-activation in mPFC pyramidal neurons [85–87]. Further investigation is required to identify a possible effect of this or other splice variants on L-channel activity.
Another possible mechanism that may also underlie L-channel over-activation may result from dysfunction of auxiliary L-channel (e.g., α2, β, γ and δ) subunits, which modulate activation, inactivation and trafficking of the channel. For example, the β and α2/δ subunit of voltage-gated Ca2+ channels modulate channel gating properties [93], including the inactivation kinetics of the L-channel and other subtypes of Ca2+ channels (e.g., Cav2.2 and Cav2.3; a.k.a. N- and R-type, respectively) [94, 95]. Thus, if the functional activity and/or expression of the β and α2/δ1 subunits is reduced after chronic exposure to Tat (or other HIV-1 proteins and inflammatory molecules), the ability of these auxiliary subunits to regulate inactivation of the L-channels could be impaired. Under such circumstances, inactivation of the L-channels will be reduced or lost, and consequentially Ca2+ influx through the L-channels may be prolonged. This could also induce over-excitation of neurons, or eventually cause injury or death of neurons. The γ subunit usually has smaller effect than other subunits on modulating ion channel activity [93], and whether it is involved in HIV-associated dysfunction of L-channels remains to be determined.
Tat may also exert an indirect effect to increase Ca2+ influx by over-activating the L-channels via altering functional activity in some pathways, including but not limited to TNF-α production and PKA-mediated phosphorylation, as is seen with NMDAR dysregulation (and was described above). For example, Tat increases TNF-α production which facilitates Ca2+ influx via the L-channels in hippocampal neurons [96, 97]. Although the exact mechanism underlying the TNF-α-induced increase of Ca2+ influx remains to be determined, this increase could exacerbate Tat-mediated neurotoxicity. In addition, increased L-channel activity also is associated with enhanced phosphorylation by PKA in mPFC pyramidal neurons [98, 99], suggesting that Tat can increase L-channel activity by facilitating PKA activation [70–72]. Moreover, increased D1R expression found in the brain of HIV-1 Tg rats [73] could also facilitate PKA activity, which would enhance PKA-mediated phosphorylation of the L-channels thereby increasing Ca2+ influx. Collectively, these findings suggest that Tat-induced dysregulation of TNF-α and protein kinases in neurons may also contribute to L-channel over-activation.
4.5. Interplay of the NMDAR and the L-Channel in Dys-regulation of Ca2+ Influx Mediated by Tat
Although both the NMDAR and the L-channel are highly permeable for Ca2+, they are activated through different mechanisms: the ligand-gated, ionotropic glutamatergic NMDAR requires binding of selective agonists (e.g., glutamate or NMDA) and co-agonists as well as removal of Mg2+ blockade, while the voltage-gated L-channel is sensitive to, and activated by cell membrane depolarization. Ionotropic glutamate receptors regulate synaptic excitability, and the majority of voltage-gated Ca2+ channels regulate intrinsic excitability and firing at more depolarized Vm levels [93]. It is unknown whether a functional interplay between over-activated/upregulated NMDAR and L-channel alter neuronal Ca2+ dysregulation, and whether and how such interplay contributes to Tat-induced neuropathogenesis. Previous studies have shown that NMDAR and the low voltage-activated (LVA) Ca2+ channels, specifically, the T-type Ca2+ channel that is activated at Vm near the resting membrane potential, are interacting to regulate the subthreshold excitability of pyramidal neurons [100–102]. However, it is unknown whether the LVA-Cav1.3 Ca2+ channel, another subtype of the L-channel which is also activated by small membrane depolarization similar to the T-type Ca2+ channel [103], also participates in such interplay to integrate, amplify, and relay Vm changes mediated by NMDAR to other high-voltage activated (HVA)-ion channels in the cell membrane, ultimately controlling neuronal firing. Encouragingly, evidence from our recent electrophysiological studies suggest that Tat-induced increases of Ca2+ influx in mPFC pyramidal neurons is mediated by over-activation of both LVA and HVA L-type Ca2+ channels [86, 87]. Similarly, increased Ca2+ influx is also found in mPFC pyramidal neurons from HIV-1 Tg rats that express multiple viral proteins, which is partly abolished by L-channel blockade (our unpublished data). Because the function of both NMDAR and L-channel is altered by Tat, these studies support the need to further elucidate alterations in the interplay of NMDAR and L-channels, and their possible contribution to HIV-associated neuropathogenesis. Outcomes from such future studies will not only aid in a better understanding of the underlying mechanisms of HIV-induced neurotoxicity, but may also be informative in the development of novel therapeutic strategies for treating neuroAIDS.
4.6. Dysregulation of Intracellular Ca2+ Release and Related Signaling
Besides altering NMDAR and L-channel activity, Tat also alters [Ca2+]in by facilitating intracellular Ca2+ release from the endoplasmic reticulum (ER) via the inosotol-1,4,5-trisphosphate (IP3) receptor (IP3R) and ryanodine receptor (RyR)-mediated signaling pathways. Notably, Tat-induced increases of [Ca2+]in and death of human fetal neurons in vitro are associated with a 3-fold elevation in IP3 levels, which are blocked by inhibition of IP3R [49]. Associated with increased Ca2+ influx, Tat-induced dysregulation of IP3 signaling (mediated by G proteins and G protein-coupled phospholipase C) could lead to mitochondrial Ca2+ overload and oxidative stress [104], which could induce neurodegeneration. Furthermore, Tat-induced dysregulation of IP3R activity and elevated Ca2+ release are associated with increased production of tumor necrosis factor-alpha (TNF-α, a cytopathic cytokine that is linked to the neuropathogenesis of HIV-associated dementia) in human macrophages [35], and likely in monocytes [36]. Whether this also occurs in neurons remains unknown and needs to be determined in future studies. In addition, Tat also induces rapid intracellular Ca2+ release from the ER and mitochondria in cortical neurons, which appears to be mediated by RyR, independent of IP3R [52–54]. Abnormal RyR signaling induces the unfolded protein response (UPR) that leads to failure of mitochondrial energy metabolism (subsequent neuronal respiratory decline), which could cause death of neurons. It is also worth noting that both IP3R and RyR are involved in TRPC channel-mediated Ca2+ influx (see below). Together, these studies indicate that although much emphasis has been placed on NMDAR, the L-channel and intracellular Ca2+ stores also play a very important role in Tat-induced Ca2+ dysregulation that contributes to neuronal dysfunction.
4.7. Dysregulation of the TRPC Channel and Related Signaling
In contrast to NMDAR and L-channel that highly or selectively conducts Ca2+ influx, the TRPC channel is a non-selective cation channel permeable for Ca2+ [105–107]. This channel has a variety of physiological functions in neurons and other types of cells [105, 106], likely due to diversity of TRPC subtypes and coupled signaling pathways, which could either be neuroprotective and pro-survival [108–111], or deleterious and pro-apoptotic [112, 113]. The complicated couplings of these channels and downstream effects are in need of more research.
Previous studies demonstrate that activation of the TRPC channel and consequential Ca2+ influx via the channel are regulated by the G protein-coupled receptor (GPCR)/Gq,i/phospholipase C (PLC)/IP3/IP3R signaling pathway and intracellular Ca2+ release [106], which is referred to as either receptor-operated or store-operated Ca2+ entry [105]. As discussed above, Tat dose-dependently increases intracellular IP3 levels and cytosolic Ca2+ release/[Ca2+]in in cultured human fetal neurons and astrocytes [31, 35, 49]. Inhibition of Gi, PLC, or IP3R reduces the Tat effects on neurons and astrocytes, suggesting that Tat alters TRPC activity by affecting the GPCR/Gq,i/PLC/IP3/IP3R pathway. Tat-induced increase in IP3 levels and [Ca2+]in in neurons is followed by a secondary increase of Ca2+ influx via NMDAR, which is blocked by selective NMDAR antagonist [49]. On the other hand, Tat-induced intracellular Ca2+ release in neurons is also mediated by RyR, which in turn could also alter activity of TRPC channels [52, 53]. These findings indicate that Tat can enhance TRPC channel activity by facilitating the GPCR/Gi/PLC/IP3/IP3R signaling and RyR-mediated signaling, and elicit interplay of TRPC channel, IP3R, and RyR to alter intracellular Ca2+ release and mitochondrial function, which could contribute to Tat-associated neuropathogenesis (Fig. 2).
Intriguingly, recent studies have also reported that the TRPC channel plays a unique and important role in neuroprotection against Tat-induced neurotoxicity. It has been demonstrated that the TRPC channels are involved in mediating the neuroprotective effects of platelet-derived growth factor (PDGF) in the chemokine (C-C motif) ligand 2 (CCL2)/Gq/PLC/IP3 and Pyk2/extracellular signal-regulated kinase (ERK)/cAMP response element-binding protein (CREB) pathways in rodent midbrain dopaminergic neurons. Under such circumstances, blockade of TRPC channels results in suppression of CCL2-induced ERK/CREB activation and reduction of PDGF-mediated neuroprotection [111, b, 114]. Further, PDGF-mediated neuroprotection against Tat is related to inactivation of GSK-3β mediated by the TRPC channel [115]. Based on these studies, the authors propose that their findings underscore a novel role of the TRPC channel in neuroprotection mediated by PDGF. Although the exact mechanism(s) underlying such differences between the function of TRPC channels in mediating neurotoxicity vs. neuroprotection is not fully understood, it is likely that such differences could result from functional involvement of various signaling pathways and subtypes of TRPC channels, depending upon which one has greater effect. More studies are required to elucidate related mechanisms regarding this issue.
5. LESSONS LEARNED FROM CLINICAL STUDIES TARGETING HIV-ASSOCIATED CA2+ DYSREGULATION MEDIATED BY NMDAR AND THE L-CHANNEL
Clarifying the mechanisms of NMDAR and L-channel dysregulation of intracellular Ca2+ homeostasis is highly relevant for a better understanding of HIV-associated neuropathogenesis, as well as for the development of novel therapeutic strategies for treating HAND. NMDAR is known to play a critical role in HIV-mediated increases of Ca2+ influx in neurons, which has been related to over-excitation-associated dysfunction, injury or death of neurons [25, 26]. The L-channel has also been implicated as another major player in such Ca2+ dysregulation [12, 85–87]. Based upon findings regarding the role of NMDAR and L-channel in neurotoxicity, extensive studies have been conducted to determine whether a reduction of abnormally-increased Ca2+ influx via over-activated/upregulated NMDAR or L-channels could stop, or slow-down, the progression of HAND. Unfortunately, previous Clinical trial studies have reported that, although there are some improvements, e.g., amelioration of neuronal metabolism by antagonizing NMDAR, or a trend toward stabilization in peripheral neuropathy by blocking L-channels (likely in patients with mild impairments), repeated treatments with either a selective NMDAR antagonist, memantine (up to 40 mg/day, or to the maximum tolerated dose, for 16 weeks followed by a 4-week washout period) [116], or a specific L-channel blocker, nimodipine (30 or 60 mg/day for 16 weeks as adjuvant treatment to anti-retroviral therapy) [117], failed to stop or significantly slow-down the progression of HIV-associated dementia or other impairments in HIV+ patients.
Despite the unexpected ‘failure’ of these clinical studies, these findings (in combination with others reviewed above) send us three clear messages. First, the NMDAR is not the only target of HIV-1 proteins (and immunoinflammatory molecules), and therefore is not solely responsible for inducing excessive Ca2+ influx in neurons and consequential neuropathogenesis associated with excitotoxicity. Second, the L-channel undoubtedly plays a critical role in HIV-induced dysregulation of intracellular Ca2+ homeostasis and neuronal hyper-excitation, particularly in mPFC pyramidal neurons, which could exacerbate neurotoxicity mediated by NMDAR. Third, novel therapeutic strategies, e.g., combined NMDAR/L-channel antagonism at early stages of HAND that actually begin in adolescence [5,6], may be required to effectively slow-down the progression, or alleviate the severity of HAND. Possible interplay of NMDAR and the L-channel (which jointly regulate the membrane excitability of pyramidal neurons) provides further support for such a combined therapeutic strategy.
Collectively, the studies discussed in this review not only highlight the clinical relevance of Tat-mediated alterations in NMDAR and L-channels, but also suggest the role of other players, including but not limited to the TRPC channel and a variety of related signaling pathways, which alter intracellular Ca2+ homeostasis in neurons. Such dysregulation may contribute to HIV-associated neuronal hyper-excitation in response to deleterious or even normal excitatory stimuli in the mesocorticolimbic and nigrostriatal dopamine pathways (Fig. 2). Future studies focusing on the underlying mechanisms of HIV-induced over-activation and/or upregulation (or other types of dysregulation) of the NMDAR, L-channel and TRPC channel, as well as their interplay in regulating abnormal, excessive Ca2+ influx and neuronal hyper-excitation, will help to identify targets for development of novel therapeutic strategies. Outcomes from such studies will likely provide further support for combined treatments using selective L-channel blocker and NMDAR antagonist (or other agents for a variety of related signaling pathways) to (1) combat HIV-induced Ca2+ dysregulation as a more effective and efficient therapeutic strategy than either one along, (2) restore normal neuronal activity, and (3) ameliorate HIV-induced brain dysfunction in patients with HAND, especially at early stages and young ages.
SUMMARY
HIV-1 induces early anatomical and functional alterations in vulnerable brain regions (e.g., the prefrontal cortex, hippocampus and basal ganglia) that regulate cognition, mood and motivation-driven behaviors. Such changes are likely related to anatomical location near the cerebrospinal fluid circulation in the brain. Tat-induced Ca2+ dysregulation in neurons in these vulnerable brain regions is mainly indicated by excessive increases of Ca2+ influx through over-activated and/or upregulated NMDAR and L-type Ca2+ channels, and abnormal elevation of Ca2+ release from intra-cellular stores. Tat-induced dysregulation of TRPC channels is also implicated in Ca2+ dysregulation via release from intracellular stores in neurons, though less is known about its role in this dysregulation. Furthermore, Tat-induced increases of Ca2+ influx can also be mediated by altered interplay of these Ca2+ permeable channels. Tat-mediated increases of Ca2+ influx and [Ca2+]in, followed by over-excitation and excitotoxicity in neurons, also could be worsened by other HIV-1 proteins and inflammatory molecules (e.g., gp120, Vpr, TNFα, etc.) via disturbing functional activity of these Ca2+-permeable ion channels. Excessive increases of Ca2+ influx and [Ca2+]in is toxic, and could lead to dysregulation, injury and death of neurons, as well as interruption of neuronal connectivity. Such dysfunction in mPFC pyramidal neurons could significantly alter glutamate output to the basal ganglia and midbrain, which may ultimately contribute to the progression and severity of neuroAIDS.
Given the fact that (1) the pathological effects of HIV-1 Tat on neurons result in significant alterations in functional activities of the mesocorticolimbic systems (as that observed in the mPFC, HIPP, and dorsal/ventral striatum), and (2) these brain regions play critical roles in regulating cognition, mood and motivation-driven behaviors that are disturbed in the context of HIV infection or by HIV-1 proteins, such HIV-1 Tat-induced pathology may contribute, at least in part, to the mechanism(s) that underlying HIV-1 neuropathogenesis. Development of novel therapeutic strategies, including but not limited to combined treatment against over-activation/upregulation of NMDAR and the L-channels that lead to neuronal hyper-excitation, may be needed at early stages of infection to combat HIV-1 induced neuropathogenesis.
Acknowledgments
This work is supported by grants from the National Institute of Health (NIH): DA026746, DA033882, DA033206, NS084817, and from Perter McManus Charitable Trust. The author thanks Wesley Wayman and Drs. Lihua Chen, Jingli Zhang, Sonya Dave and Christina Khodr for conducting some related studies discussed in this review, Dr. Christina Khodr for reading and providingvaluable and constructive inputs for the manuscript, and Dr. T. Celeste Napier for research collaboration.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 3.Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: Current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20(1):25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 5.Lyon ME, McCarter R, D’Angelo LJ. Detecting HIV associated neurocognitive disorders in adolescents: what is the best screening tool? J Adolesc Health. 2009;44(2):133–5. doi: 10.1016/j.jadohealth.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Benton TD. Treatment of Psychiatric disorders in children and adolescents with HIV/AIDS. Current Psychiatry Rep. 2010;12:104–10. doi: 10.1007/s11920-010-0092-z. [DOI] [PubMed] [Google Scholar]
- 7.Bagashev A, Sawaya BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 9.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 10.Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 2004;107(2):97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci. 2008;28(48):12604–13. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005 Aug;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 14.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–8. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- 16.New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273(28):17852–8. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- 17.Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4(3):281–90. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- 18.McManus CM, Weidenheim K, Woodman SE, et al. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000;156(4):1441–53. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Bovenkamp M, Nottet HS, Pereira CF. Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia. Eur J Clin Invest. 2002;32(8):619–27. doi: 10.1046/j.1365-2362.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 20.Nath A, Anderson C, Jones M, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14(3):222–7. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- 21.Nath A, Haughey N, Jones M, Anderson C, Bell J, Geiger J. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–94. [PubMed] [Google Scholar]
- 22.Liu Y, Jones M, Hingtgen CM, et al. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6(12):1380–7. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan A, Turchan J, Pocernich C, et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278(15):13512–9. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- 24.Lipton SA. AIDS-related dementia and calcium homeostasis. Ann N Y Acad Sci. 1994;747:205–24. doi: 10.1111/j.1749-6632.1994.tb44411.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 26.King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8(5):1347–57. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Nath A, Psooy K, Martin C, et al. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475–80. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Nath A, Knudsen B, et al. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82(1):97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- 29.Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879(1–2):42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 30.Aksenov MY, Hasselrot U, Bansal AK, et al. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305(1):5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- 31.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78(3):457–67. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 32.Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2003;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 33.Rom I, Deshmane SL, Mukerjee R, Khalili K, Amini S, Sawaya BE. HIV-1 Vpr deregulates calcium secretion in neural cells. Brain Res. 2009;1275:81–6. doi: 10.1016/j.brainres.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegg CC, Hu S, Peterson PK, Thayer SA. Beta-chemokines and human immunodeficiency virus type-1 proteins evoke intracellular calcium increases in human microglia. Neuroscience. 2000;98(1):191–9. doi: 10.1016/s0306-4522(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 35.Mayne M, Holden CP, Nath A, Geiger JD. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol. 2000;164(12):6538–42. doi: 10.4049/jimmunol.164.12.6538. [DOI] [PubMed] [Google Scholar]
- 36.Contreras X, Bennasser Y, Chazal N, et al. Human immunodeficiency virus type 1 Tat protein induces an intracellular calcium increase in human monocytes that requires DHP receptors: involvement in TNF-alpha production. Virology. 2005;332(1):316–28. doi: 10.1016/j.virol.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Minghetti L, Visentin S, Patrizio M, Franchini L, jmone-Cat MA, Levi G. Multiple actions of the human immunodeficiency virus type-1 Tat protein on microglial cell functions. Neurochem Res. 2004;29(5):965–78. doi: 10.1023/b:nere.0000021241.90133.89. [DOI] [PubMed] [Google Scholar]
- 38.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50(2):91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poggi A, Rubartelli A, Zocchi MR. Involvement of dihydropyridine-sensitive calcium channels in human dendritic cell function. Competition by HIV-1 Tat. J Biol Chem. 1998;273(13):7205–9. doi: 10.1074/jbc.273.13.7205. [DOI] [PubMed] [Google Scholar]
- 40.Zocchi MR, Rubartelli A, Morgavi P, Poggi A. HIV-1 Tat inhibits human natural killer cell function by blocking L-type calcium channels. J Immunol. 1998;161(6):2938–43. [PubMed] [Google Scholar]
- 41.Brailoiu GC, Brailoiu E, Chang JK, Dun NJ. Excitatory effects of human immunodeficiency virus 1 Tat on cultured rat cerebral cortical neurons. Neuroscience. 2008;151(3):701–10. doi: 10.1016/j.neuroscience.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musante V, Summa M, Neri E, et al. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex. 2010;20(8):1974–84. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- 43.Feligioni M, Raiteri L, Pattarini R, et al. The human immunodeficiency virus-1 protein Tat and its discrete fragments evoke selective release of acetylcholine from human and rat cerebrocortical terminals through species-specific mechanisms. J Neurosci. 2003;23(17):6810–8. doi: 10.1523/JNEUROSCI.23-17-06810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329(3):1071–83. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115(4):885–96. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang GJ, Chang L, Volkow ND, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(Pt 11):2452–8. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- 47.Chang L, Wang GJ, Volkow ND, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42(2):869–78. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aksenova MV, Silvers JM, Aksenov MY, et al. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395(3):235–9. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- 49.Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73(4):1363–74. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- 50.Prendergast MA, Rogers DT, Mulholland PJ, et al. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;954(2):300–7. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- 51.Chandra T, Maier W, Konig HG, et al. Molecular interactions of the type 1 human immunodeficiency virus transregulatory protein Tat with N-methyl-d-aspartate receptor subunits. Neuroscience. 2005;134(1):145–53. doi: 10.1016/j.neuroscience.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 52.Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol. 2007;178(2):869–76. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 53.Norman JP, Perry SW, Reynolds HM, et al. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS ONE. 2008;3(11):e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry SW, Barbieri J, Tong N, et al. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci. 2010;30(42):14153–64. doi: 10.1523/JNEUROSCI.1042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010 Apr;11(4):227–38. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 56.Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85(5):1299–311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 57.Eugenin EA, King JE, Hazleton JE, et al. Differences in NMDA receptor expression during human development determine the response of neurons to HIV-tat-mediated neurotoxicity. Neurotox Res. 2011;19(1):138–48. doi: 10.1007/s12640-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem. 2007 Mar;100(5):1407–20. doi: 10.1111/j.1471-4159.2006.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye L, Huang Y, Zhao L, et al. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 2013;125(6):897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci. 2004;27(3):296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Eugenin EA, King JE, Nath A, et al. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007 Feb 27;104(9):3438–43. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27(47):12844–50. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krogh KA, Lyddon E, Thayer SA. HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem. 2015;132(3):354–66. doi: 10.1111/jnc.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krogh KA, Lyddon E, Thayer SA. HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem. 2014 Aug 25; doi: 10.1111/jnc.12936. [DOI] [PMC free article] [PubMed]
- 65.Wheeler D, Knapp E, Bandaru VV, et al. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109(5):1237–49. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rostasy K, Monti L, Lipton SA, Hedreen JC, Gonzalez RG, Navia BA. HIV leucoencephalopathy and TNFalpha expression in neurones. J Neurol Neurosurg Psychiatry. 2005;76(7):960–4. doi: 10.1136/jnnp.2004.036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24(22):5131–9. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15(1):49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 69.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53(3):362–8. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zidovetzki R, Wang JL, Chen P, Jeyaseelan R, Hofman F. Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C- and cAMP-dependent protein kinase pathways. AIDS Res Hum Retroviruses. 1998;14(10):825–33. doi: 10.1089/aid.1998.14.825. [DOI] [PubMed] [Google Scholar]
- 71.Hofmann B, Nishanian P, Nguyen T, Insixiengmay P, Fahey JL. Human immunodeficiency virus proteins induce the inhibitory cAMP/protein kinase A pathway in normal lymphocytes. Proc Natl Acad Sci USA. 1993;90(14):6676–80. doi: 10.1073/pnas.90.14.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofmann B, Nishanian P, Nguyen T, Liu M, Fahey JL. Restoration of T-cell function in HIV infection by reduction of intracellular cAMP levels with adenosine analogues. AIDS. 1993;7(5):659–64. doi: 10.1097/00002030-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4(3):309–16. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- 74.Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28(6):1184–90. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. D1/NMDA receptors and concurrent methamphetamine+ HIV-1 Tat neurotoxicity. J Neuroimmune Pharmacol. 2012;7(3):599–608. doi: 10.1007/s11481-012-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goretzki L, Mueller BM. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem J. 1998;336(Pt 2):381–6. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekokoski E, Aitio O, Tornquist K, Yli-Kauhaluoma J, Tuominen RK. HIV-1 Tat-peptide inhibits protein kinase C and protein kinase A through substrate competition. Eur J Pharm Sci. 2010;40(5):404–11. doi: 10.1016/j.ejps.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73(2):578–86. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 79.Sharma A, Hu XT, Napier TC, Al-Harthi L. Methamphetamine and HIV-1 Tat down regulate beta-catenin signaling: implications for methampetamine abuse and HIV-1 co-morbidity. J Neuroimmune Pharmacol. 2011;6(4):597–607. doi: 10.1007/s11481-011-9295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson L, Sharma A, Monaco MCG, Major EO, Al-Harthi L. Human immunodeficiency virus type 1 (HIV-1) Tansactivator of transcription through its intact core and cysteine-rich domains inhibits Wnt/beta-catenin signaling in astrocytes: relevance to HIV neuropathogenesis. J Neuroscie. 2012;32(46):16306–13. doi: 10.1523/JNEUROSCI.3145-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Harthi L. Interplay between Wnt/beta-catenin signaling and HIV: virologic and biologic consequences in the CNS. J Neuroimmune Pharmacol. 2012;7(4):731–9. doi: 10.1007/s11481-012-9411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Harthi L. Wnt/beta-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders. J Neuroimmune Pharmacol. 2012;7(4):725–30. doi: 10.1007/s11481-012-9412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strijbos PJ, Zamani MR, Rothwell NJ, Arbuthnott G, Harkiss G. Neurotoxic mechanisms of transactivating protein Tat of Maedi-Visna virus. Neurosci Lett. 1995;197(3):215–8. doi: 10.1016/0304-3940(95)11940-x. [DOI] [PubMed] [Google Scholar]
- 84.Albini A, Ferrini S, Benelli R, et al. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA. 1998;95(22):13153–8. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wayman WN, Dodiya HB, Persons AL, et al. Enduring cortical alterations after a single in vivo treatment of HIV-1 Tat. Neuroreport. 2012;23:825–9. doi: 10.1097/WNR.0b013e3283578050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wayman W, Chen L, Napier TC, Hu X-T. Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to HIV-1 Tat. Eur J Neurosci. 2015;41(9):1195–206. doi: 10.1111/ejn.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Napier TC, Chen L, Kashanchi F, Hu X-T. Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels. J Neuroimmune Pharmacol. 2014;9(3):354–68. doi: 10.1007/s11481-014-9524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1. 2. Neuron. 2007;55(4):615–32. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98(16):9271–6. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao P, Yu D, Lu S, et al. Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1. 2 calcium channels. J Biol Chem. 2004;279(48):50329–35. doi: 10.1074/jbc.M409436200. [DOI] [PubMed] [Google Scholar]
- 91.Graf EM, Bock M, Heubach JF, et al. Tissue distribution of a human Ca v 1. 2 alpha1 subunit splice variant with a 75 bp insertion. Cell Calcium. 2005;38(1):11–21. doi: 10.1016/j.ceca.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Biel M, Ruth P, Bosse E, et al. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990;269(2):409–12. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- 93.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8):1–23. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yasuda T, Chen L, Barr W, et al. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20(1):1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 95.Yasuda T, Lewis RJ, Adams DJ. Overexpressed Ca(v)beta3 inhibits N-type (Cav2. 2) calcium channel currents through a hyperpolarizing shift of ultra-slow and closed-state inactivation. J Gen Physiol. 2004;123(4):401–16. doi: 10.1085/jgp.200308967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currrents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70(5):1876–86. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- 97.Pollock J, McFarlane SM, Connell MC, et al. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42(1):93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- 98.Ford KA, Wolf ME, Hu XT. Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse. 2009;63(8):690–7. doi: 10.1002/syn.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heng L, Markham JA, Hu XT, Tseng KY. Concurrent upregulation of postynaptic L-type Ca2+ channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca2+ plateau potentials in the prefrontal cortex. Neuropharmacology. 2011;60(6):953–62. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA. 1994;91(11):5207–11. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 102.Wang Z, Kai L, Day M, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50(3):443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 103.Lipscombe D. L-type calcium channels - Highs and new lows. Circ Res. 2002;90(9):933–5. doi: 10.1161/01.res.0000019740.52306.92. [DOI] [PubMed] [Google Scholar]
- 104.Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154(2):276–88. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 105.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23(2):297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 107.Zholos AV. TRPC5. Handb Exp Pharmacol. 2014;222:129–56. doi: 10.1007/978-3-642-54215-2_6. [DOI] [PubMed] [Google Scholar]
- 108.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2005;280(3):2132–40. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bollimuntha S, Ebadi M, Singh BB. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006;1099(1):141–9. doi: 10.1016/j.brainres.2006.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10(5):559–67. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- 111.Yao H, Peng F, Dhillon N, et al. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29(6):1657–69. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Satoh S, Tanaka H, Ueda Y, et al. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem. 2007;294(1–2):205–15. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- 113.Foller M, Kasinathan RS, Duranton C, Wieder T, Huber SM, Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell Physiol Biochem. 2006;17(5–6):201–10. doi: 10.1159/000094125. [DOI] [PubMed] [Google Scholar]
- 114.Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 2009;16(12):1681–93. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peng F, Yao H, Akturk HK, Buch S. Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta. PLoS ONE. 2012;7(10):e47572. doi: 10.1371/journal.pone.0047572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schifitto G, Navia BA, Yiannoutsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21(14):1877–86. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 117.Navia BA, Dafni U, Simpson D, et al. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology. 1998;51(1):221–8. doi: 10.1212/wnl.51.1.221. [DOI] [PubMed] [Google Scholar]