Abstract
Estimates of the numbers and rates of acute decompensated heart failure (ADHF) hospitalization are central to understanding health-care utilization and efforts to improve patient care. We comprehensively estimated the frequency, rate, and trends of ADHF hospitalization in the United States. Based on Atherosclerosis Risk in Communities (ARIC) Study surveillance adjudicating 12,450 eligible hospitalizations during 2005–2010, we developed prediction models for ADHF separately for 3 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 428 discharge diagnosis groups: 428 primary, 428 nonprimary, or 428 absent. We applied the models to data from the National Inpatient Sample (11.5 million hospitalizations of persons aged ≥55 years with eligible ICD-9-CM codes), an all-payer, 20% probability sample of US community hospitals. The average estimated number of ADHF hospitalizations per year was 1.76 million (428 primary, 0.80 million; 428 nonprimary, 0.83 million; 428 absent, 0.13 million). During 1998–2004, the rate of ADHF hospitalization increased by 2.0%/year (95% confidence interval (CI): 1.8, 2.5) versus a 1.4%/year (95% CI: 0.8, 2.1) increase in code 428 primary hospitalizations (P < 0.001). In contrast, during 2005–2011, numbers of ADHF hospitalizations were stable (−0.5%/year; 95% CI: −1.4, 0.3), while the numbers of 428-primary hospitalizations decreased by −1.5%/year (95% CI: −2.2, −0.8) (P for contrast = 0.03). In conclusion, the estimated number of hospitalizations with ADHF is approximately 2 times higher than the number of hospitalizations with ICD-9-CM code 428 in the primary position. The trend increased more steeply prior to 2005 and was relatively flat after 2005.
Keywords: acute decompensated heart failure, adjudicated heart failure, community surveillance, hospitalizations, International Classification of Diseases codes, national inpatient sample, secular trends, United States
Estimates of the numbers and rates of acute decompensated heart failure (ADHF) hospitalization are central to our understanding of health-care utilization and to efforts to improve patient care and reduce cost through policy measures. There are more than 5.7 million Americans living with heart failure, an estimate projected to increase to more than 8 million by the year 2030 (1). Annual mortality following an ADHF hospitalization is about 30% (2).
An ADHF episode is understood as a clinical state of heart failure that requires either initiation or escalation of treatment to relieve acute symptoms or to prevent death. National estimates of ADHF have been hampered by its syndromic nature, with nonspecific signs and symptoms, thus requiring validation of each hospitalization, a costly and time-intensive process (3).
National estimates of the numbers and rates of ADHF hospitalization have focused on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge code 428 in the primary position on the hospital discharge form, the frequency and rates of which have declined over the past decade (4–7). Previous studies from the United States (8, 9) and Europe (10, 11) have found a high specificity but poor sensitivity of ICD-9-CM code 428 in the primary position. Thus, ICD-9-CM code 428 in the primary position probably underestimates numbers of ADHF hospitalizations (9). Indeed, hospitalizations with a nonprimary ICD-9-CM 428 code are 3 times more common than those with a primary 428 code, and they doubled in frequency from 1979 to 2004 (6, 12). It is also known that some ADHF hospitalizations may have ICD-9-CM codes other than 428 (e.g., cardiomyopathy—code 425; pulmonary heart disease including cor pulmonale—code 415/416) (8, 10).
To comprehensively estimate the burden of ADHF in the United States, we derived prediction models for adjudicated ADHF cases based on community-based Atherosclerosis Risk in Communities (ARIC) Study ADHF surveillance data from diverse community hospitals in 4 geographically defined areas. We applied these models to data from the National Inpatient Sample (NIS) to estimate the number and rate of ADHF hospitalization in the United States from 1998 through 2011. We tested the robustness of the estimates using simple versus complex prediction models for ADHF. We compared trends in these new ADHF hospitalization estimates with estimates of ICD-9-CM 428 codes in the primary position.
METHODS
Study samples
Derivation sample
In the Community Surveillance Component of the ARIC Study, epidemiologic surveillance for hospitalized ADHF among the residents of 4 US communities aged ≥55 years began in 2005 (n = 21 hospitals). A sample of hospitalizations with eligible ICD-9-CM codes in any position on the discharge form (code 428.x and non-428 codes 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 415.0, 416.9, 425.4, 518.4, and 786.0x), stratified by ICD-9-CM code, sex, and race, was selected for 2005–2010.
Medical records were abstracted by trained study staff adhering to a common protocol (9). A panel of physician reviewers, assisted by computerized algorithms, adjudicated hospitalizations as definite or possible ADHF (9). Hospitalizations for chronic heart failure were adjudicated and classified as not ADHF. Data for event years 2005–2010 were available and included in this analysis.
Application sample
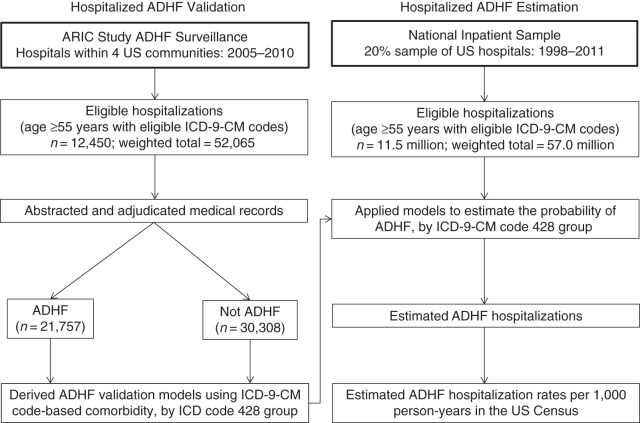
Models predictive of ADHF (definite or possible) developed in the ARIC Community Surveillance Component were applied to data from the NIS, the largest publicly available all-payer hospitalization database. The NIS is a stratified sample of approximately 20% of US community hospitals, and in 2011 it included over 1,000 hospitals sampled from 46 participating states (13). Sampling methods have been consistent since 1998 (13). Among the potential sources of national data, including the National Hospital Discharge Survey, the NIS was selected for these analyses to facilitate comparison with recently published reports that used NIS data (6, 7). The NIS also benefitted from its consistent sampling methods during the study period, larger sampling fractions, and availability of information on health-care utilization. Our analyses included eligible hospitalizations matching ARIC eligibility criteria (Figure 1). From the 57.9 million hospitalizations sampled during 1998–2011, we excluded those without eligible discharge codes (46.1 million), those with patient age <55 years (0.2 million), and those with missing information on sex (n = 781), which left us with 11.5 million hospitalizations.
Figure 1.
Design of a study to estimate numbers and trends of acute decompensated heart failure (ADHF) hospitalization in the United States by applying validation models derived from Atherosclerosis Risk in Communities (ARIC) Study ADHF surveillance (2005–2010) to the National Inpatient Sample (1998–2011). Eligible International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes had the potential to identify ADHF hospitalizations. Three separate models were developed for groups with eligible ICD-9-CM codes: code 428 primary, code 428 nonprimary, and code 428 absent.
Predictors of ADHF
Predictors were considered only if data were available in both the validation and application samples. They included age group (55–<65, 65–<75, or ≥75 years), sex, race (Caucasian vs. other), teaching hospital status, and ICD-9-CM discharge codes. A consistent algorithm was used across the two databases to define the presence and position of the 428 discharge codes, other heart failure codes (see Web Table 1, available at http://aje.oxfordjournals.org/), and comorbidity codes (Web Table 2). The first occurrence of a code among 25 available code positions was used to categorize its position, with the first position used to denote the primary code. Race was unrelated to ADHF validation, and missing information on race in the NIS (23%) was coded as non-Caucasian.
Statistical methods
Derivation of models in the ARIC Study to predict ADHF
We estimated unadjusted positive predictive value (PPV) and developed models of adjusted PPV (“validation models”) for 3 mutually exclusive groups, known a priori to have different average probabilities of ADHF. Groups were defined by the presence of ICD-9-CM code 428 and its position: 428 primary, 428 nonprimary, and 428 absent (non-428 eligible codes). We estimated PPV for the 3 ICD-9-CM 428 code groups while accounting for the stratified sampling. Web Table 3 shows PPVs by 428 group in the presence of additional comorbidity. We then employed a structured approach to build multivariable validation models, using logistic regression to predict ADHF by ICD-9-CM 428 group (Web Appendix, Web Tables 4–6, and Figure 1). The internal validity of the final models was assessed using the area under the receiver operating characteristic curve (AUC) and the calibration slope corrected for optimism (14). We also examined plots of the observed versus expected proportions of ADHF by decile of heart failure risk score, and we fitted models with interaction terms in order to assess consistency of model fit across ARIC study sites and study years.
Application of the models to predict ADHF in the NIS database
We applied unadjusted PPV estimates and the validation models to the NIS data to estimate the probability of ADHF for each hospitalization in the NIS by ICD-9-CM 428 group. The sum of probabilities yielded ADHF estimates. The models accounted for differences in comorbidity and study years between the two studies. The variance in ADHF estimates was obtained via the delta method (Web Appendix). Per NIS analytical guidelines, all analyses were weighted to represent all US community hospitals, and variance estimates accounted for the stratified sampling design (15). To estimate rates, we used yearly US intercensal estimates as denominators (16). Average annual percent change in number or rate of hospitalization was estimated on the basis of regression models of annual log counts or rates, with inverse variance weighting, overall and separately for equal 7-year periods (1998–2004 and 2005–2011). We tested differences in the annual change of ADHF hospitalizations overall versus those with code 428 in the primary position using an interaction term for interaction between a 428-primary indicator term and calendar year. The analysis of trends was repeated in strata defined by age group (55–64, 65–74, and ≥75 years). All analyses were conducted using survey procedures in SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) and SUDAAN (RTI International, Research Triangle Park, North Carolina).
RESULTS
During 2005–2010, investigators with the Community Surveillance Component of the ARIC Study reviewed 12,450 eligible hospitalizations (weighted to 52,065) representing 4 communities. Patient characteristics in eligible ARIC hospitalizations and 11.5 million NIS hospitalizations (weighted to 57.0 million nationally during 1998–2011) were largely similar (Table 1). The mean age, percent female, proportion of sampled codes, and percent admitted to a teaching hospital were similar across the 2 samples (Web Tables 7 and 8). Eligible hospitalizations in the ARIC Community Surveillance Component had a higher prevalence of uncomplicated hypertension, diabetes, chronic kidney disease, acute kidney injury, and anemia than those in the NIS and a lower prevalence of atrial fibrillation than the NIS (Table 1). Validation models were adjusted for the above variables and other covariates in Table 1 when related to ADHF.
Table 1.
Characteristics of Heart Failure-Eligiblea Hospitalizations (%) in ARIC Study Surveillance for Acute Decompensated Heart Failure (2005–2010) and the National Inpatient Sample (1998–2010), by ICD-9-CM Code 428 Subgroupb
| Position of ICD-9-CM Code 428 Among Discharge Diagnoses |
||||||
|---|---|---|---|---|---|---|
| ARIC Study ADHF Surveillance |
National Inpatient Sample |
|||||
| Primary (n = 10,130)c | Nonprimary (n = 36,410) | Absent (n = 5,525) | Primary (n = 12.6M) | Nonprimary (n = 37.6M) | Absent (n = 6.9M) | |
| Age group, years | ||||||
| 55–64 | 18.6 | 28.5 | 15.5 | 14.3 | 21.9 | 19.7 |
| 65–74 | 24.8 | 27.9 | 25.0 | 24.4 | 28.7 | 25.1 |
| ≥75 | 56.5 | 43.6 | 59.5 | 61.3 | 49.4 | 55.2 |
| Male sex | 43.9 | 46.3 | 44.9 | 44.1 | 47.0 | 47.8 |
| Caucasian raced | 73.3 | 70.7 | 60.2 | 61.3 | 60.2 | 69.3 |
| Heart valve disorder | 12.5 | 7.7 | 14.5 | 13.2 | 11.2 | 19.9 |
| Rheumatic heart failure | 0.1 | 7.4 | 1.6 | 0.1 | 12.6 | 0.0 |
| Hypertension | 43.9 | 50.7 | 39.8 | 40.0 | 35.6 | 45.2 |
| Hypertensive heart failure | 3.6 | 0.6 | 3.4 | 2.4 | 13.6 | 1.1 |
| Diabetes | 46.2 | 35.0 | 36.1 | 35.8 | 29.7 | 46.1 |
| Coronary atherosclerosis | 50.5 | 36.0 | 48.2 | 47.5 | 40.1 | 56.9 |
| Acute myocardial infarction | 8.2 | 4.3 | 8.5 | 10.5 | 6.5 | 3.7 |
| Cardiomyopathy | 10.6 | 43.2 | 14.8 | 9.3 | 40.6 | 15.2 |
| Atrial fibrillation | 36.2 | 26.5 | 33.7 | 33.8 | 28.3 | 39.0 |
| Other arrhythmia (non-AF) | 15.5 | 18.0 | 12.9 | 12.5 | 15.5 | 16.1 |
| Conduction disorder | 9.9 | 8.7 | 14.0 | 12.5 | 13.7 | 12.1 |
| Chronic kidney disease | 30.3 | 19.5 | 15.3 | 15.7 | 9.8 | 31.2 |
| Acute kidney injury | 21.5 | 14.0 | 12.5 | 13.9 | 8.8 | 14.0 |
| Fluid and electrolyte disorder | 11.0 | 10.3 | 6.2 | 6.8 | 6.5 | 6.9 |
| Renal heart failure | 3.4 | 0.4 | 2.0 | 1.9 | 5.3 | 0.8 |
| Dyspnea and respiratory abnormalities | 2.3 | 41.9 | 3.8 | 1.0 | 23.8 | 2.6 |
| COPD exacerbation | 12.0 | 7.3 | 10.0 | 10.6 | 9.3 | 9.7 |
| Pneumonia | 19.8 | 11.9 | 15.8 | 18.7 | 11.5 | 13.3 |
| Acute lung edema | 0.1 | 0.2 | 4.4 | 0.0 | 0.1 | 2.7 |
| Pulmonary heart diseasee | 1.1 | 5.5 | 1.8 | 1.1 | 7.1 | 0.8 |
| Anemia | 26.6 | 18.8 | 15.7 | 15.8 | 13.1 | 24.8 |
| Teaching hospital | 38.8 | 36.7 | 39.6 | 40.3 | 39.0 | 32.3 |
Abbreviations: ADHF, acute decompensated heart failure; AF, atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; COPD, chronic obstructive pulmonary disease; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; M, million; NIS, National Inpatient Sample.
a Eligible samples of hospitalizations among persons aged ≥55 years were defined by hospital ICD-9-CM discharge code as detailed in the Methods section of the text and Figure 1.
b ICD-9-CM 428 groups: the “primary” group had ICD-9-CM code 428.xx as the primary (first listed) discharge diagnosis; the “nonprimary” group had ICD-9-CM code 428.xx in positions 2–26 of the discharge form; and the “absent” (non-428) group had other eligible codes. The average sampling weight for both the ARIC and NIS samples ranged from 2 to 6.
c Weighted number of hospitalizations.
d Estimated among persons with nonmissing values. Information on race was missing for 23% of eligible hospitalizations in the NIS data.
e Acute/chronic heart disease related to the lungs, such as pulmonary hypertension, pulmonary embolism, or cor pulmonale.
ADHF hospitalization validation models and burden
The ADHF validation PPV was 90.2% (standard error (SE), 0.8%) for code 428 primary, 32.2% (SE, 0.6%) for code 428 nonprimary, and 16.5% (SE, 0.8%) for code 428 absent (Table 2). In the multivariable ADHF validation models, comorbid conditions such as acute myocardial infarction, chronic kidney disease, and atrial fibrillation were statistically significant predictors in each of the ICD-9-CM code 428 categories, while age group, race, and sex were not significant in any of the 3 categories (Web Tables 9–11). Prediction of ADHF improved for each of the ICD-9-CM 428 groups after patient characteristics were taken into account (Web Figure 1). Predictive ability was better in the group with low PPV (optimism-corrected AUCs were 0.73 (95% confidence interval (CI): 0.72, 0.75) for 428 nonprimary and 0.81 (95% CI: 0.79, 0.83) for 428 absent) than in the group with high PPV (AUC = 0.63 (95% CI: 0.59, 0.67) for 428 primary).
Table 2.
Hospitalization Burden of Acute Decompensated Heart Failure and Temporal Trends in Acute Decompensated Heart Failure for Persons Aged 55 Years or Older (Validated NIS Data Using ARIC Adjudication), United States, 1998–2011
| Position of ICD-9-CM Code 428 Among Discharge Diagnoses |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|
| Primary |
Nonprimary |
Absent |
||||||
| No., %, or Rate | 95% CI or IQR | No., %, or Rate | 95% CI or IQR | No., %, or Rate | 95% CI or IQR | No., %, or Rate | 95% CI or IQR | |
| Eligiblea hospitalizations | ||||||||
| ARIC Study ADHF surveillance, 2005–2010 | ||||||||
| Sample size (no. of hospitalizations) | 2,083 (10,130)b | 6,936 (36,410) | 3,431 (5,525) | 12,931 (54,851) | ||||
| Validated ADHF positive predictive value, % | 90.2 | 88.7, 91.6 | 32.2 | 31.0, 33.4 | 16.5 | 15.1, 18.0 | ||
| Probability of validated ADHF based on the prediction models, %c | 90.1 | 81.0–97.5 | 26.9 | 9.5–83.6 | 8.1 | 3.5–86.6 | ||
| National Inpatient Sample, 1998–2011 | ||||||||
| Sample size (weighted no. of hospitalizations), in millions | 12.55 | 37.57 | 6.92 | 57.04 | ||||
| No. of eligible hospitalizations/year, in millions | 0.90 | 0.87, 0.92 | 2.68 | 2.51, 2.86 | 0.49 | 0.46, 0.53 | 4.07 | 3.94, 4.20 |
| No. of ADHF hospitalizations/year, in millionsd | 0.80 | 0.79, 0.82 | 0.83 | 0.75, 0.90 | 0.13 | 0.09, 0.16 | 1.76 | 1.71, 1.80 |
| Hospitalization rate per 1,000 person-years | ||||||||
| Eligible hospitalizations | 13.5 | 12.5, 14.5 | 40.0 | 38.7, 41.3 | 7.5 | 6.6, 8.4 | 61.0 | 59.2, 62.9 |
| ADHF hospitalizationsd | 12.1 | 11.3, 2.9 | 12.3 | 11.7, 12.8 | 2.0 | 1.4, 2.6 | 26.3 | 25.1, 27.6 |
Abbreviations: ADHF, acute decompensated heart failure; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IQR, interquartile range; NIS, National Inpatient Sample.
a Eligible hospitalizations in both ARIC and the NIS were hospitalizations with index codes for identifying potential heart failure hospitalization among persons aged ≥55 years.
b Numbers in parentheses, weighted number of hospitalizations.
c Values are presented as median (50th percentile) and IQR (25th–75th percentiles).
d ADHF validation models (Web Tables 9–11) included age, race, sex, and teaching hospital for all models and the following additional variables, by model: code 428 in primary position—heart valve disease, chronic kidney disease, acute myocardial infarction, and atrial fibrillation; code 428 in nonprimary position—acute myocardial infarction, other heart failure codes, pneumonia, code 428 in second position, acute kidney injury, atrial fibrillation, chronic obstructive pulmonary disease, heart valve disorder, cardiomyopathy, fluid-electrolyte disorder, dyspnea-respiratory abnormalities, hypertension, conduction disorder, and other cardiac arrhythmias; code 428 absent—other heart failure codes, acute myocardial infarction, chronic kidney disease, fluid-electrolyte disorder, atrial fibrillation, and cardiomyopathy.
During 1998–2011, the average annual number of hospitalizations for persons aged ≥55 years with 428 primary, 428 nonprimary, and 428 absent discharge codes was 0.90 million, 2.68 million, and 0.49 million, respectively (Table 2). The estimated number of ADHF hospitalizations per year among the groups with 428 primary, nonprimary, and absent codes was 0.80 million, 0.83 million, and 0.13 million, respectively. Estimated numbers of ADHF hospitalizations across groups summed to 1.76 million (95% CI: 1.71, 1.80) per year. The estimated rates of ADHF hospitalization for persons aged ≥55 years were 12.12, 12.26, and 1.96 per 1,000 person-years among those with 428 primary, nonprimary, and absent discharge codes, respectively, summing to an estimated rate of ADHF of 26.3 per 1,000 person-years.
National temporal trends in heart failure codes and estimated ADHF: 1998–2011
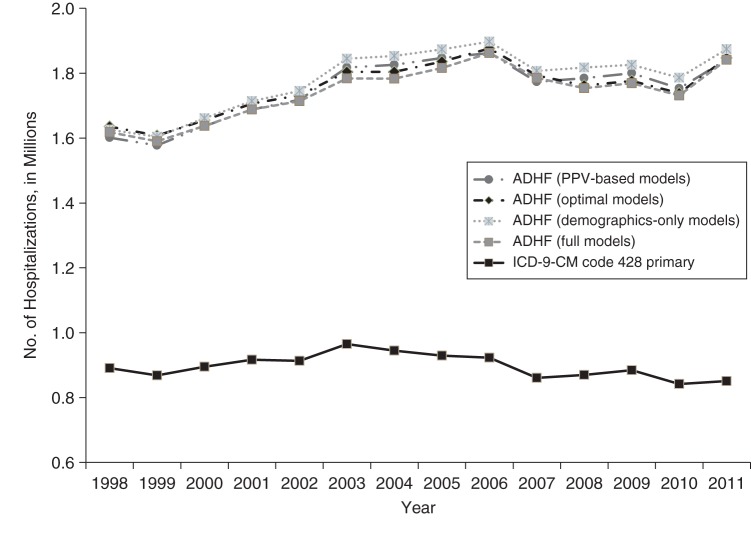
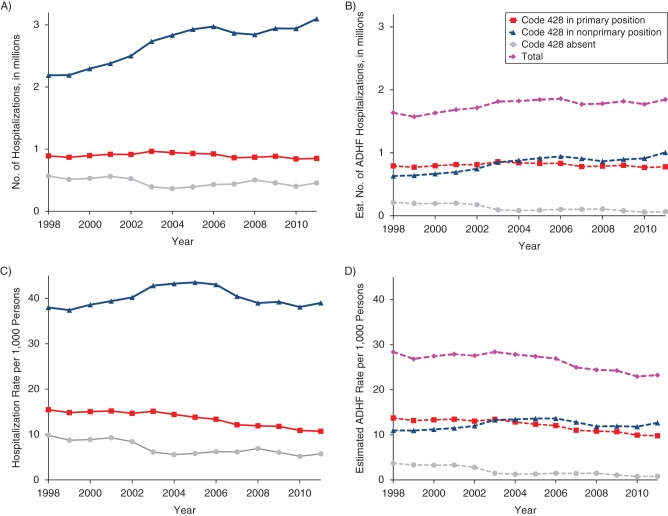
The estimates of total ADHF hospitalizations were similar when different prediction models were used (unadjusted PPV, demographics only, optimal, and full) and approximately 2 times higher than estimates of hospitalizations with 428 in the primary position (Figure 2). The overall estimates of ADHF hospitalizations obtained using a weighted sum of number of hospitalizations in each ICD-9-CM group multiplied by respective PPV were similar to those obtained with our modeling approach (Figure 2). Numbers of hospitalizations with ICD-9-CM code 428 in the primary position increased by 1.4% annually during 1998–2004 and then declined by 1.5% annually from 2005 through 2011 (Figure 3A, Web Tables 12 and 13). In contrast, numbers of hospitalizations with code 428 in a nonprimary position increased at a much higher rate of 4.6% during 1998–2004, followed by a statistically nonsignificant rise of 0.3% annually during 2005–2011. Starting in 2004, the percentage of estimated ADHF hospitalizations from the ICD-9-CM 428 nonprimary group was higher than the percentage from the 428 primary group (55% vs. 42% in 2011, with only 3% from the 428-absent group; Figure 3B, Web Tables 13 and 14). Compared with ADHF hospitalizations among 428-primary hospitalizations, total estimated numbers of ADHF hospitalizations increased at a higher rate during 1998–2004 (2.0% vs. 1.5%, P < 0.001) and declined at a lower rate during 2005–2011 (−0.5% vs. −1.2%, P = 0.03). Allowing for growth of the US population, rates of hospitalization for ADHF decreased for all ICD-9-CM 428 groups throughout the study period, except for the 428 nonprimary group during 1998–2004 (Figure 3C and 3D). The decline in the total estimated rate of ADHF hospitalization occurred at a slower pace than the decline in ADHF among 428-primary hospitalizations during both periods (i.e., 1998–2004 (−0.1% vs. −0.7%, P < 0.001) and 2005–2011 (−3.2% vs. −3.9%, P = 0.03)).
Figure 2.
Temporal trends in estimated numbers of US hospitalizations with acute decompensated heart failure (ADHF) and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge code 428 in the primary position among persons aged ≥55 years, 1998–2011. Data were obtained from the Atherosclerosis Risk in Communities Study and the National Inpatient Sample. Estimates of ADHF hospitalization were obtained using various models (Web Table 14). PPV, positive predictive value.
Figure 3.
A) Temporal trends in numbers of US hospitalizations with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge code 428 primary, nonprimary, and absent among persons aged ≥55 years, 1998–2011. B) Estimated (Est.) number of acute decompensated heart failure (ADHF) hospitalizations contributed by each ICD-9-CM code group and their total. C) Rates of hospitalization per 1,000 persons based on panel A and the US Census. D) Estimated rates of ADHF hospitalization based on panel B. Data were obtained from the Atherosclerosis Risk in Communities Study and the National Inpatient Sample. Corresponding annual percentage change in estimates is shown in Web Table 12.
Sensitivity analyses
Sensitivity analyses were used to explore the robustness of the findings by study community, study year, and model assumptions. The goodness of fit was appropriate for each model according to ICD-9-CM code 428 group (Web Figure 2) and was consistent by ARIC study center and study year (data not shown).
When results were stratified by age group, the estimated frequency and rate of ADHF hospitalizations were approximately twice those of code 428 primary hospitalizations in all age groups. Comparing 428-primary and ADHF hospitalizations, similar trends were observed in the number of hospitalizations, with the exception of persons aged 55–64 years after 2005 (−0.6% (95% CI: −1.3, 0) vs. 1.5% (95% CI: 0.6, 2.4)) and persons aged 65–74 years after 2005 (−2.2% (95% CI: −3.1, −1.2) vs. −0.3% (95% CI: −1.3, 0.7)) (Web Figure 3).
DISCUSSION
Our study provides comprehensive estimates of the number and rate of ADHF hospitalizations during 1998–2011 in US adults aged 55 years or older, extending previous reports that relied on ICD-9-CM code 428 in the primary position on the hospital discharge form (4–7). Average annual estimates of the number of ADHF hospitalizations (1.76 million) were about 2 times higher than those based on 428 codes in the primary position (0.90 million, including 0.80 million ADHF cases based on our prediction models). Hospitalization trends differed by study period. Specifically, total numbers of ADHF hospitalizations increased during 1998–2004, along with a rise in 428-primary hospitalizations. In contrast, numbers of ADHF hospitalizations were stable during 2005–2011 despite a decrease in 428-primary hospitalizations, due to an increase in ADHF cases without code 428 in the primary position. Notably, a decrease in 428-primary hospitalizations has been reported for Medicare-eligible patients, with a similar yearly rate of change as observed by us (5), as well as nationally when estimated using NIS survey data (6, 7). Due to a parallel increase in the US population, the rates of total ADHF were stable during 1998–2004 and then declined slowly during 2005–2011, whereas the rates of 428-primary hospitalizations decreased throughout the study period.
Our results confirm the poor sensitivity of ICD-9-CM 428 codes in the primary position and indicate that only approximately half of ADHF hospitalizations are tracked by this code. We have shown that in 2011, more than half (55%) of hospitalizations were identified by ICD-9-CM code 428 in nonprimary positions and a small proportion (3%) were identified by non-428 eligible codes. The high proportion of ADHF cases identified in the 428 nonprimary group reflects a high proportion of hospitalizations with this code and about one-third of them being adjudicated as ADHF. It is likely that decompensation of heart failure may be precipitated by another acute condition, like arrhythmia, myocardial ischemia, or exacerbation of chronic obstructive pulmonary disease, and discharge codes for these conditions may take the primary position. The observed temporal decrease in rates of hospitalization for ADHF for selected ICD-9-CM groupings could be due to changing patterns in the placement of ICD-9-CM code 428 in the secondary position versus the primary position, possibly reflecting temporal changes in comorbidity associated with ADHF. Whether any role was played by improvements in the management of acute coronary syndrome and care of chronic heart failure patients, as well as hospital discharge planning and postdischarge follow-up, introduced or emphasized by the heart failure management guideline update published in 2005 (17) remains unclear. Further work is needed to determine potential reasons for the observed trends.
Heart failure affects approximately 5.7 million Americans, and the associated costs of care are vast, driven primarily by hospitalizations and frequent readmissions (25% are readmitted within 1 month) (1, 18). The Hospital Readmissions Reduction Program of the Centers for Medicare and Medicaid Services reduces payments to hospitals with excess readmissions within 30 days of discharge (19). Both the rehospitalization reduction program and performance measures target only hospitalizations with ICD-9-CM code 428 in the primary position. Our findings suggest that reliance on primary 428 codes may limit these efforts to fewer than half of ADHF hospitalizations. Strategies to appropriately include nonprimary 428 hospitalizations are needed. Algorithms which include a broader range of codes should be more robust to changes in billing and coding practices for continued surveillance. Of interest, performance measures were poorer for hospitalizations with 428 in a nonprimary position than for those with 428 in the primary position, and associated 1-year mortality was higher (20). Thus, appropriate algorithms focusing on ADHF hospitalizations and not 428 primary hospitalizations may allow targeted patient education programs to improve patient care and outcomes and to reduce the number of heart failure hospitalizations. Such algorithms may move the current focus away from hospitalizations with 428 in the primary position and toward ADHF hospitalizations in the Hospital Readmissions Reduction Program, and could aid in attempts to remove or reduce penalties for rehospitalization in more complex cases. ADHF could develop during hospitalization, but in the ARIC surveillance program, this occurred in only 7% of the ADHF hospitalizations (21).
The impact of the transition to the International Classification of Diseases, Tenth Revision, which offers higher granularity for heart failure classification, will need to be evaluated, but the position of the heart failure discharge codes (primary vs. nonprimary) is likely to remain an important distinction. Further, 40%–50% of patients with ADHF hospitalizations have preserved ejection fraction, a subtype of heart failure associated with a similar morbidity profile and costs of care as heart failure with reduced ejection fraction but probably more favorable survival (2). The data presented for our study do not differentiate among these subtypes, since most heart failure hospitalizations are assigned the nonspecific ICD-9-CM code of 428.00.
Our results also suggest that the inpatient cost of care for ADHF is considerably higher than that estimated for heart failure hospitalizations identified merely from primary 428 codes. Further, although the rates of ADHF are declining, the numbers of ADHF hospitalizations have not declined and may impact future health-care costs.
Strengths
Our ADHF validation models were based on a large and diverse sample of hospitalizations from more than 20 hospitals in 4 regions of the United States, with rigorously standardized record abstraction and adjudication by a panel of physician reviewers. The derivation estimates were stable across time and geographic locales. These estimates were applied to the largest sample of US community hospitals available and account for differences in comorbidity across hospitals and settings, as well as adjust for any differences between ARIC and NIS in the variables examined. Estimates based on PPV alone were similar to model-based estimates, indicating robustness at the population level if much simpler models are used to estimate overall ADHF burden.
Limitations
Our ADHF estimates only apply to community-based hospitals, excluding federal hospitals such as those in the Veterans Affairs Health System, institutions such as nursing homes, short observational stays, or emergency room discharges without hospitalization. With the introduction of the International Classification of Diseases, Tenth Revision, and penalties for early readmissions, the observed validity of the heart failure discharge codes and the performance of the ADHF prediction models may change and may need recalibration in future years. Despite the large size of the derivation sample, the Community Surveillance Component of the ARIC Study draws on only 4 regions of the country, extends only from 2005 to 2010, and is limited to persons aged 55 years or older. Information on race was missing for a substantial proportion of hospitalizations in the NIS data, but race was not associated with the probability of ADHF in derivation data and thus should not have influenced overall estimates. Reassuringly, the weighted proportions of eligible ICD-9-CM codes, age group, and sex were similar in the derivation sample to those in the national sample of hospitals.
Conclusions
In the United States, there are 1.76 million hospitalizations with ADHF each year on average, as compared with estimates of 0.90 million per year based on ICD-9-CM code 428 in the primary position. During 1998–2004, the total number of estimated ADHF hospitalizations increased at a higher rate than hospitalizations with ICD-9-CM code 428 in the primary position (2.0%/year vs. 1.5%/year). During 2005–2011, the number of ADHF hospitalizations was stable, while previous reports, as well as our study, demonstrated declines when ADHF hospitalizations were defined by ICD-9-CM code 428 in the primary position. Given population growth, hospitalization rates for ADHF were initially stable (1998–2004) and have slightly declined recently (2005–2011). Our comprehensive estimates of ADHF hospitalizations provide a better basis for estimating inpatient heart failure costs and will be less sensitive to the order of International Classification of Diseases codes to be used to monitor and improve patient care.
Supplementary Material
ACKNOWLEDGMENTS
Authors affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland; (Sunil K. Agarwal, Kunihiro Matsushita, Josef Coresh); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland; (Sunil K. Agarwal, Kunihiro Matsushita, Josef Coresh); Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Lisa Wruck, Miguel Quibrera); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Laura R. Loehr, Wayne D. Rosamond, Gerardo Heiss); Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Laura R. Loehr, Patricia P. Chang); and National Heart, Lung, and Blood Institute, Bethesda, Maryland (Jacqueline Wright).
The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. S.K.A. was supported by Cardiovascular Epidemiology Training Grant T32HL007024 from the National Heart, Lung, and Blood Institute (Principal Investigator: Prof. Josef Coresh).
We thank the staff of the ARIC Study for their important contributions.
This research was presented in poster form at the American College of Cardiology's 64th Annual Scientific Session & Expo (San Diego, California, March 14–16, 2015) and also received the “best poster” award at the 6th annual Johns Hopkins 2015 Cardiovascular Research Retreat (Baltimore, Maryland, May 22, 2015).
Conflict of interest: none declared.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;1314:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Dhingra A, Garg A, Kaur S et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;114:354–365. [DOI] [PubMed] [Google Scholar]
- 3.Loehr LR, Agarwal SK, Baggett C et al. Classification of acute decompensated heart failure: an automated algorithm compared with a physician reviewer panel. The Atherosclerosis Risk in Communities Study. Circ Heart Fail. 2013;64:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;1293:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Normand SL, Wang Y et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;30615:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blecker S, Paul M, Taksler G et al. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;6112:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Dharmarajan K, Wang Y et al. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;6110:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC Jr, Pandey DK, Chan FA et al. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;1602:197–202. [DOI] [PubMed] [Google Scholar]
- 9.Rosamond WD, Chang PP, Baggett C et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) Study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;52:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khand AU, Shaw M, Gemmel I et al. Do discharge codes underestimate hospitalisation due to heart failure? Validation study of hospital discharge coding for heart failure. Eur J Heart Fail. 2005;75:792–797. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca C, Sarmento PM, Marques F et al. Validity of a discharge diagnosis of heart failure: implications of misdiagnosing. Congest Heart Fail. 2008;144:187–191. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Mensah GA, Croft JB et al. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;526:428–434. [DOI] [PubMed] [Google Scholar]
- 13.Houchens R, Ross D, Elixhauser A et al. Nationwide Inpatient Sample (NIS) Redesign Final Report. HCUP Methods Series, report no. 2014-04 Rockville, MD: Agency for Healthcare Research and Quality, US Department of Health and Human Services; 2014. http://www.hcup-us.ahrq.gov/reports/methods/2014-04.pdf Accessed April 4, 2014. [Google Scholar]
- 14.Steyerberg EW, Harrell FE Jr, Borsboom GJ et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;548:774–781. [DOI] [PubMed] [Google Scholar]
- 15.Houchens R, Elixhauser A. Calculating National Nationwide Inpatient (NIS) Variances for Data Years 2011 and Earlier. HCUP Methods Series, report no. 2003-02 Rockville, MD: Agency for Healthcare Research and Quality, US Department of Health and Human Services; 2014. http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp Revised December 11, 2015. Accessed December 18, 2014. [Google Scholar]
- 16.United States Census Bureau. National Intercensal Estimates (2000–2010). Intercensal estimates of the resident population by sex and age for the United States: April 1, 2000 to July 1, 2010 [data table] Washington, DC: Bureau of the Census, US Department of Commerce; 2011. http://www.census.gov/popest/data/intercensal/national/nat2010.html Updated October 10, 2014. Accessed December 8, 2015. [Google Scholar]
- 17.Hunt SA, Abraham WT, Chin MH et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;11212:e154–e235. [DOI] [PubMed] [Google Scholar]
- 18.Dharmarajan K, Hsieh AF, Lin Z et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;3094:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services. Readmissions Reduction Program. 2013. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html Accessed April 22, 2014.
- 20.Blecker S, Agarwal SK, Chang PP et al. Quality of care for heart failure patients hospitalized for any cause. J Am Coll Cardiol. 2014;632:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel MD, Kalbaugh CA, Chang PP et al. Characteristics and outcomes of patients with acute decompensated heart failure developing after hospital admission. Am J Cardiol. 2014;11410:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.