Abstract
Background
Plants produce and emit important volatile organic compounds (VOCs), which have an essential role in biotic and abiotic stress responses and in plant–plant and plant–insect interactions. In order to study the bouquets from plants qualitatively and quantitatively, a comprehensive, analytical method yielding reproducible results is required.
Results
We applied in-tube extraction (ITEX) and solid-phase microextraction (SPME) for studying the emissions of Allium plants. The collected HS samples were analyzed by gas chromatography–time-of-flight–mass spectrometry (GC-TOF–MS), and the results were subjected to multivariate analysis. In case of ITEX-method Allium cultivars released more than 300 VOCs, out of which we provisionally identified 50 volatiles. We also used the VOC profiles of Allium samples to discriminate among groups of A. fistulosum, A. chinense (rakkyo), and A. tuberosum (Oriental garlic). As we found 12 metabolite peaks including dipropyl disulphide with significant changes in A. chinense and A. tuberosum when compared to the control cultivar, these metabolite peaks can be used for chemotaxonomic classification of A. chinense, tuberosum, and A. fistulosum.
Conclusions
Compared to SPME-method our ITEX-based VOC profiling technique contributes to automatic and reproducible analyses. Hence, it can be applied to high-throughput analyses such as metabolite profiling.
Electronic supplementary material
The online version of this article (doi:10.1186/s13104-016-1942-5) contains supplementary material, which is available to authorized users.
Keywords: Volatile organic compounds, GC-TOF–MS, Metabolomics, Headspace, ITEX, Allium
Background
Plants produce various kinds of volatile organic compounds (VOCs) that are a part of the metabolome. By today the total number of identified VOCs is about 1700, and they account for 1 % of secondary metabolites [1, 2]. The major chemical classes of VOCs emitted from plants are terpenoids, phenylpropanoids/benzenoids, and derivatives of fatty acids and amino acids [3]. The genus Allium, is comprised of onions, leeks, and garlic, the total number of species is to 600–750 [4]. Allium plants can produce sulfur-containing VOCs through enzymatic reaction of sulfur-storage compounds [4]. For example, primary “aroma” compounds are thiosulfinates including allicin that are produced from aliphatic cysteine sulfoxides as “aroma” precursors in Genus Allium. Dithiins, ajoenes, and sulfides are known to as secondary “aroma” compounds [5].
We chose Allium fistulosum (Japanese bunching onions), A. chinense (rakkyo), and A. tuberosum (Oriental garlic), because these plants have been cultivated in Japan since the 8th century and are favorites of the Japanese. Allium plants emit VOCs that result in strong odors. The odoriferous compounds whose moiety contains sulfur in their moieties function not only as a defense against pathogens [6] and insects [7], but they also attract special herbivores and insect-eating insects such as moths [8, 9] and bees [10]. The chemical composition of such metabolites is diverse [11]. Since sulfur-containing VOCs produced by Allium plants exhibit anticancer- [12, 13], antithrombotic- [14], and antibacterial activity [15, 16], they are thought to be beneficial to human health.
There are several methods to collect VOCs in various matrices. A traditional way is steam distillation by which oils produced by plants can be collected. Meanwhile headspace (HS) sampling is a non-destructive solvent-free method for collecting VOCs emitted from plants [17, 18] including vegetables [19], humans [20], and microbes [21]. Moreover, the investigation of HS composition is much more meaningful than volatile analysis of samples collected by distillation or extraction methods. In case of high concentration capacity HS (HCC–HS) sampling methods [17, 22] such as solid-phase microextraction (SPME) [23–25], in-tube extraction (ITEX) [26–28], and stir bar sportive extraction (SBSE) [29, 30], VOCs can be easily concentrated. However, there is still a need for developing a comprehensive, reproducible, and high-throughput analysis for detection and quantification of VOCs in biological samples of various cultivars. Less than approximately 20 samples can be analyzed as one batch with one SPME fiber due to capacity of sorbent materials of SPME. Trapping of VOCs depends on SPME fibers’ properties [31]. To date, many types of sorbent materials are commercially available for ITEX-based method. Choosing the appropriate sorbent material of ITEX is important to trap non-polar and/or polar VOCs. Compared to SPME-method sampling according to ITEX procedure is fully-automated at the four steps, i.e., sample conditioning, analyte extraction/sorption, desorption/injection, and trap conditioning. Plus more samples can be analyzed by using ITEX- than SPME-method [32]. As high-throughput analysis is required for VOC profiling, we applied ITEX method in this study.
After HCC-HS sampling, VOCs are directly analyzed by gas chromatography (GC)-based techniques, because target analytes are easily released by heating sorbent materials. Of these methods, GC combined with electron ionization–time-of-flight–mass spectrometry (EI-TOF–MS) may help to identify and estimate the structure of VOCs, because EI-TOF–MS yields comprehensive information on molecular fragments in terms of mass-to-charge ratios [33], and because of well-documented libraries such as NIST/EPA/NIH mass spectral library (NIST-L) [34], Adams library (Ad-L) [35], the terpenoids library (Te-L; http://www.massfinder.com/wiki/Terpenoids_Library), and VocBinbase (Vo) [29], which contain mass spectral and retention index (RI) information of compounds that can be analyzed by GC–MS. Furthermore, several alignment tools such as AMDIS [36], ChromA [37], H-MCR [38], metalign [39], Tagfinder [40], and XCMS [41] have been developed and are freely available for GC–MS data interpretation.
The goal of the study was to develop a comprehensive, reproducible, and high-throughput profiling method for VOC collection from many samples by using fully-automated ITEX procedure and to then provisionally identify the detected VOCs in the HS of plants using the summarized mass spectral libraries. By applying our pipeline, we performed comprehensive HS-VOC profiling of the sheaths and basal plates of 12 Allium cultivars with ITEX-method in this study.
Results and discussion
Optimization of HCC-HS sampling and comparison of HS-VOC profiles in the HS of Allium fistulosum using ITEX and SPME techniques
To achieve the best performance for HCC-HS sampling in HS-GC vials, a method is needed that suits the goal of comprehensive, reproducible analyses. To this end, we modified the method of Tikunov et al. [23] and Kusano et al. [31]. Allium plants produce sulfides such as dipropyl disulfide as the main VOC component [7, 42]. We used ITEX and SPME to conduct HCC-HS collection from the Allium plants and evaluated statistically. The choice of the internal standards (ISs) is also critical for non-targeted metabolite profiling [43, 44]. Several ISs with different physicochemical properties (i.e., RI and chemical structure) are required for a comprehensive VOC analysis to evaluate whether the analytes participate in cross-contribution [44] and whether the RI of each IS peak is reproducible. Therefore we carefully examined dissolving agents for the ISs based on the value of the partition coefficient and the solubility of each IS [45] and selected methanol as the solvent.
We conducted HCC-HS sampling using ITEX and SPME to compare their performance for peak detection and to assess their comprehensiveness and the reproducibility of the results obtained with each technique. First we estimated the lower limit of quantification (LLOQ) of dipropyl disulfide, the major disulfide in A. fistulosum [7] and A. cepa [46], using ITEX- and SPME-GC-TOF–MS (see Additional file 2). The LLOQ of the peak detected by ITEX-GC-TOF–MS analysis was 250 pmol; it was 25 pmol by SPME-GC-TOF–MS analysis (data not shown). Then, using both methods, we analyzed the sheath and the basal part of A. fistulosum (brand name, Mikata spring onion; class01 in Table 1, Fig. 1, Additional file 1). The total ion chromatogram (TIC) of each analyte showed that peak detection was more sensitive with SPME device (Additional file 1). The score scatter plot of samples analyzed with the ITEX device and the SPME fiber showed clear separation of the first principal component (Additional file 1). It may be due to the use of different resins (TGR/CSIII for ITEX and PDMS/DVB for SPME).
Table 1.
Allium species used in this study
| Class in PCA | Binomial name | Species name | Bland name | Harvested field in Japan |
|---|---|---|---|---|
| 08 | Allium chinense | Rakkyo | Young rakkyo | Namegata, Ibaraki |
| 01 | Allium fistulosum | Spring onion | Mikata spring onion | Hamamatsu, Shizuoka |
| 02 | Allium fistulosum | Green spring onion | Aoi-chan green spring onion | Akitakata, Hiroshima |
| 03 | Allium fistulosum | Scallion | Hakata scallion | Hakata, Fukuoka |
| 04 | Allium fistulosum | Green spring onion | Green spring onion from Nagareyama | Nagareyama, Chiba |
| 05 | Allium fistulosum | White spring onion | White spring onion from Nagano | Nagano |
| 06 | Allium fistulosum | Leek | Shimonita leek | Gunma |
| 07 | Allium fistulosum | Leek | Shirakami leek | Noshiro, Akita |
| 09 | Allium fistulosum | Scallion | Kujo scallion | Nagahama, Shiga |
| 11 | Allium fistulosum | Spring onion | Goudo spring onion | Anpachi, Gifu |
| 10 | Allium tuberosum | Oriental garlic | Oriental garlic | Nagahama, Shiga |
| 12 | Allium fistulosum | Red spring onion | Red spring onion | Tsuruoka, Yamagata |
All samples were harvested in October 2012
We used the same class names in the PCA score scatter plot (see Fig. 4)
Fig. 1.

Visual phenotypes of the Allium samples used in this study. a A. chinense (rakkyo, class08 in Table 1 and Fig. 4). b A. fistulosum (spring onion, class01), c A. fistulosum (green spring onion, class 02), d A. fistulosum (scallion, class03), e A. fistulosum (green spring onion, class04), f A. fistulosum (white spring onion, class05), g A. fistulosum (leek, class06), h A. fistulosum (leek, class07), i A. fistulosum (scallion, class09), j A. fistulosum (spring onion, class11), k A. tuberosum (Oriental garlic, class10), l A. fistulosum (red spring onion, class12). The red and white areas of the scale bar are 5 cm
An advantage of the use of ITEX lies in its use of a stainless-steel needle and a special purge-, trap-, and trap-cleaning system [27, 47, 48]. This makes it possible to run more samples while maintaining the high reproducibility of data. On the other hand, the SPME method is appropriate for semi-targeted analysis because it features a wide variety of fibers rather than the ITEX sorbent materials. Although the SPME showed more sensitivity than ITEX to detect VOCs, less than approximately 20 samples can be analyzed as one batch due to capacity of sorbent materials of SPME. Thus, we applied the ITEX method for non-targeted HS-VOC profiling of the Allium samples.
Comparison of the libraries for the tentative identification of HS-VOCs
We estimated how many volatile peaks in the mass spectra overlapped in NIST-L, Ad-L, Te-L, and in Vo before provisional identification of the detected peaks. Non-processed MS data from the HS-ITEX-GC-TOF–MS analysis can be exported and then processed using our method for metabolite profiling (Fig. 2). However, the putative identification of the detected VOCs is limited because few libraries show the EI mass spectra and RI and because it is very difficult to obtain authentic standards for VOCs. Despite this limitation, we estimated how many mass spectra overlapped among Vo and the three commercially-available libraries for volatiles (Ad-L, Te-L, NIST05). The estimation procedure is clarified in details in the Materials and Methods section. Instead of complete matching of the compounds using CAS numbers and/or compound names, we used the similarity of each mass spectrum and the RI difference of the corresponding peak in the query library (Ad-L, Te-L, Vo) and the NIST05 reference library (Tables 2 and 3). Approximately 35 % of the mass spectra in Ad-L (3rd edition, 555/1607; 4th edition, 765/2205) exhibited high similarity against NIST05. On the other hand, only four compounds (β-maaliene, methyl tridecanoate, methyl undecanoate, and methyleugenol) showed a similarity value greater than 900 in Te-L; the SD of the RI differences of the four compounds was 5.6 (Table 3). Using their chemical structures in NIST05 we compared these compounds and found that they were identical in Ad-L and NIST05. The mass spectra in Te-L tend to be unique. Consequently, the difference shown in Table 3 may increase the number of compounds that can be annotated.
Fig. 2.
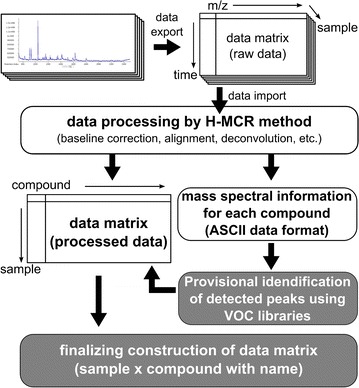
Schema of the workflow for data processing and peak annotation to obtain the data matrix. Non-processed data for GC-TOF–MS analysis of each sample were exported as NetCDF files. These files were imported in MATLAB for baseline correction, peak alignment, and deconvolution by the H-MCR method. Libraries were prepared for the provisional identification of the extracted mass spectra of the VOC peaks (gray box). After merging the information into a data matrix, we obtained a data matrix comprised of the compound name, sample name, and the sum of the peak area of each extracted mass
Table 2.
Libraries used for the provisional identification of VOCs
| Library name | RI | Phase composition of the GC column(s) |
|---|---|---|
| Adams library (3rd ed.) | Available | 5 % diphenyl, 95 % dimethyl polysiloxane |
| Adams library (4th ed.) | Available | 5 % diphenyl, 95 % dimethyl polysiloxane |
| Terpenoids library | Available | 100 % dimethyl polysiloxane |
| VocBinBase | Available | 5 % diphenyl, 95 % dimethyl polysiloxane |
| NIST05 | Available | Various types (polar and non-polar) |
Table 3.
Estimation of the number of similar compounds in the Adams (Ad-L) and the Terpenoids library (Te-L), and in VocBinBase (Vo) against NIST05
| Library name | Number of EI spectra | Number of identified compounds | ≥850a | SD of RI diff (≥850) | ≥900a | SD of RI diff (≥900) |
|---|---|---|---|---|---|---|
| Ad-L (3rd ed.) | 1607 | 1607 | 794 | 8.36 | 555 | 8.29 |
| Ad-L (4th ed.) | 2205 | 2205 | 1077 | 8.73 | 765 | 8.69 |
| Te-L | 1982 | 1982 | 91 | 8.48 | 4 | 5.56 |
| Vob | 1632 | 212 | 258 | 8.67 | 143 | 8.09 |
| NIST05 | 190,825 | 163,198 | – | – | – | – |
The RI difference (diff) was calculated by subtracting the RI of a compound peak in the query library from that in the reference library (NIST05). The values were transformed into absolute values
SD standard deviation; RI diff absolute RI difference
aThe value represents similarity defined as described in “Methods”
bVocBinBase contains 1420 unidentified EI spectra
HS-VOC profiling of the 12 Allium plants using the ITEX technique
We conducted VOC profiling in the HS of 10 A. fistulosum-, one A. chinense-, and one A. tuberosum cultivars with the ITEX technique. The visual phenotypes of each Allium plant are presented in Fig. 1. We focused on the sheaths and basal plates to analyze the VOCs. The entire aerial parts of the other Allium cultivars used in this study are eaten in Japan. We obtained VOC profile data on 35 samples [three biological replicates except for A. fistulosum (class05, n = 2)] and 354 extracted mass spectral peaks as a data matrix. The detected peaks were identified or provisionally identified using our fully-automated annotation pipeline. Of these, 52 peaks, including two artifacts (Si-containing peaks derived from column breeding) were tentatively identified by comparing their mass spectra and the RI corresponding to those in the four libraries (Table 2), or identified using authentic standards (Additional file 3). The molecular formula of each annotated peak was investigated and the proportion of sulfur-containing peaks in the annotated peaks was calculated (Fig. 3). Approximately half of the annotated peaks contained sulfur atom(s) in their moieties. According to Pino et al. [49], sulfur-containing compounds account for approximately 90 % of the total volatile content in diethyl ether extracts of A. chinense and A. tuberosum. Our findings suggest that ITEX-based VOC profiling could detect not only sulfur-containing peaks but also other types of VOCs.
Fig. 3.
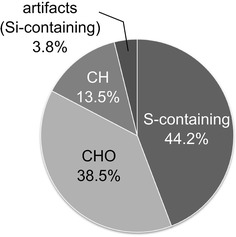
Proportion of sulfur-containing peaks in the 52 annotated peaks, including two artifacts (Si-containing peaks), in the HS of Allium plants. The proportion was calculated by counting the number of annotated compounds that consisted of CHOS, CHO, CH, or CHOSi
We conducted principal component analysis (PCA) to visualize the similarities/differences in the VOC composition of each Allium cultivar (Fig. 4). The score scatter plot of the VOC profile data showed subspecies-dependent separations among A. chinense, A. fistulosum, and A. tuberosum (Fig. 4a). Next, we investigated the distribution of tentatively identified peaks in the profiles of the Allium cultivars. The PCA loading plot showed that, some peaks tended to be abundant in A. fistulosum cultivars [e.g. 3,4-dimethylthiophene (ID026)], while the levels of the two sulfur-containing compounds [2,5-thiophenedicarboxaldehyde (ID154) and diallyl disulphide (ID091)] were more abundant in A. tuberosum than in A. fistulosum cultivars (Fig. 4b).
Fig. 4.
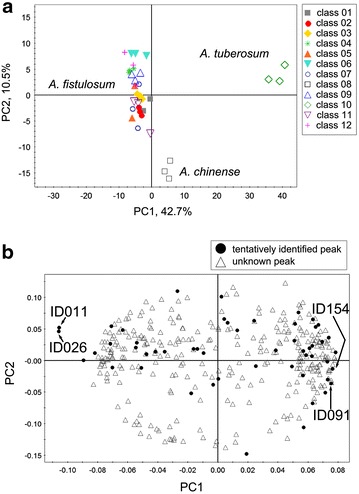
Score (a) and loading (b) plots of PCA of the VOC profiles of the Allium samples. Principal components one and two (PC1, PC2) represent the first two principal components that account for a total of 53.2 % of the variance. Each plot represents an independent plant. In the loading plot, black dots and white triangles represent tentatively identified- and unknown peaks, respectively. All compound names and IDs are listed in the Additional file 3. ID011 2-butenal, 2-ethyl; ID026 3,4-dimethylthiophene; ID091 diallyl disulphide; ID154 2,5-thiophenedicarboxaldehyde
Discriminative VOCs among the Allium cultivars
We compared the VOC profiles of each Allium cultivar to determine whether the VOC composition in the HS can be used in their differentiation. VOC changes in the HS of Allium samples were recorded by subtracting the average of the normalized responses of the annotated peaks (log2-transformed value) in each Allium cultivar from those of the control, Mikata spring onion (class01, Fig. 1b). The extent of the VOC changes tended to be similar to that shown by PCA (Fig. 4, Additional file 3). For example, the visual phenotype of the control cultivar Mikata spring onion (class01), and of Aoi-chan green spring onion (class02) was very similar (Fig. 1b, c). There was no significant difference in the level of the annotated VOCs between these cultivars (Additional file 3). In the VOC profiles of other cultivars of A. fistulosum, there were a few differences in the VOC levels when compared to the control (data not shown). Thus, we focused on the subspecies-dependent differences.
We compared changes in the level of the 50 annotated VOC peaks in the profiles of A. chinense and A. tuberosum against the control (class01) (false discovery rate, FDR < 0.05). Of these, The 15 compound peaks showed significant changes in the profiles of A. chinense, while the level of 36 peaks was changed in A. tuberosum (Fig. 5).
Fig. 5.
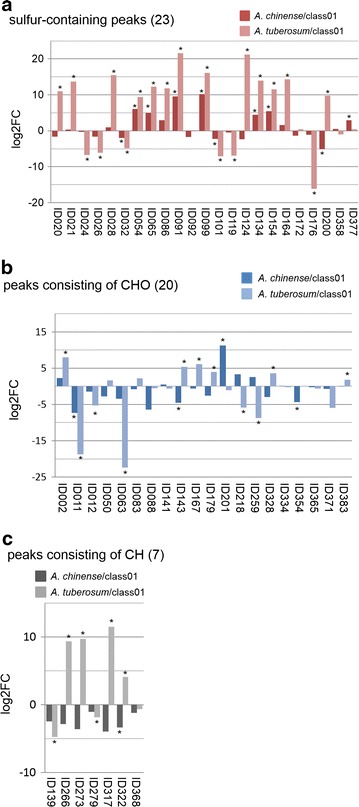
The log2-fold changes in the VOCs of 50 annotated peaks in the VOC profiles of sulfur-containing peaks (a), peaks consisting of CHO (b), and peaks consisting of CH (c). The log2-fold changes (log2FC) in the normalized response of peaks of each cultivar (A. chinense or A. tuberosum) against that of the control cultivar class01 (A. fistulosum) are shown in the Additional file 3. We analyzed three biological replicates of each Allium plant except the white spring onion (class05, n = 2) cultivar. Asterisks on the top of the bars present that the level of VOCs in A. chinense and/or A. tuberosum was significantly changed using the LIMMA package (see the “Methods” section) when compared to the control cultivar (class01 in Table 1). The significance level was set at FDR <0.05 (asterisk *). Sulfur-containing peaks were: ID020 1-propene, 3,3’-thiobis, ID021 methanesulfonic acid, methyl ester, ID024 thiophene, 2,5-dimethyl, ID026 3,4-dimethylthiophene, ID028 thiophene, 2-ethenyl, ID032 thiophene, 2,4-dimethyl, ID054 dimethyl trisulfide, ID065 3-thiophenecarboxaldehyde, ID086 S-methyl methanethiosulphonate, ID091 diallyl disulphide, ID092 5-methyl-2-thiophenecarboxaldehyde, ID099 1,2,4-trithiolane, ID101 dipropyl disulfide, ID119 prop-1-enyl dithiopropanonate, ID124 thiophene, 2-propyl, ID134 3-vinyl-1,2-dithiacyclohex-5-ene, ID154 2,5-thiophenedicarboxaldehyde, ID164 trisulfide, di-2-propenyl, ID172 trisulfide, dipropyl, ID176 1,2,4-trithiolane, 3,5-diethyl, ID200 thieno[2,3-b]thiophene, 2-methyl, ID358 cyclic octaatomic sulfur, ID377 disulfide, methyl 1-propenyl, Peaks consisting of CHO: ID002 hexanal, ID011 2-butenal, 2-ethyl, ID012 2-pentenal, 2-methyl, ID050 heptenal, (2E), ID063 2-furanone, 2,5-dihydro-3,5-dimethyl; ID083 octen-1-al, (2E); ID088 2-octen-1-ol, (E); ID141 nonanoic acid; ID143 decenal, (2E); ID167 decadienal, (2E,4E); ID179 2-dodecenal, (E); ID201 3(2H)-furanone, 2-hexyl-5-methyl; ID218 2-tridecanone; ID259 3(2H)-furanone, 5-methyl-2-octyl; ID328 hexadecanoic acid methyl ester, n; ID334 1,2-benzenedicarboxylic acid, butyl octyl ester; ID354 9,12-octadecadienoic acid, methyl ester, (E,E); ID365 [1,1′,3′,1′-terphenyl]-2′-ol; ID371 2,5-cyclohexadiene-1,4-dione, 2,5-diphenyl; ID383 benzaldehyde; Peaks consisting of CH: ID139 benzene, 1,3-bis(1,1-dimethylethyl); ID266 8-heptadecene; ID273 heptadecane, n; ID279 1,1′-biphenyl, 2,2′,5,5′-tetramethyl; ID317 5-octadecene, (E); ID322 nonadecane; ID368 tricosane
Among 23 sulfur-containing peaks, 10 peaks showed significant changes in A. chinense, while 19 peaks were significantly changed in A. tuberosum (Fig. 5a). Of these, there were nine discriminant peaks in both subspecies. Thiosulfinates are the initial compounds in the HS of Allium species when their tissues are chopped or homogenated [50]; they decompose immediately and then sulfides are emitted as major aroma compounds [11, 42]. HS-ITEX VOC profiling detected a monosulfide (ID020), two disulfides (ID091 and ID101), and two trisulfides (ID054 and ID164). Their level was higher in A. tuberosum than in the control except for dipropyl disulfide (ID101) that is the major disulfide in Allium plants. The level of this compound was significantly lower in A. chinense and A. tuberosum than in the control (Fig. 5a). Interestingly, the level of this compound differed among the cultivars of A. fistulosum (Additional file 3). It suggests that this compound can be used for the chemotaxonomic classification of A.fistulosum cultivars. For bunching onions like A.fistulosum, DNA markers such as simple sequence repeats (SSRs), amplified fragment length- and single-nucleotide polymorphisms (AFLPs, SNPs) are available (http://www.vegmarks.nivot.affrc.go.jp/VegMarks/jsp/index.jsp). However, its high cost hampers the data collection of many cultivars. As a first step, our VOC profiling is useful for choosing representative cultivars in Allium plants for further analyses.
We detected nine peaks that are considered to be thiophene compounds [thiophene, 2,5-dimethyl, 3,4-dimethylthiophene, thiophene, 2,4-dimethyl, thiophene, 2,4-dimethyl, 3-thiophenecarboxaldehyde, thiophene, 2-propyl, 2,5-thiophenedicarboxaldehyde, 1,2,4-trithiolane, 3,5-diethyl, and thieno(2,3-b)thiophene, 2-methyl] in the Allium samples. Of these, two dimethylthiophenes (thiophene, 2,5-dimethyl, and 3,4-dimethylthiophene) were found to be thermal decomposition products from dialkyl disulfides in the distilled oils of Allium species [42, 51]. In the HS of Allium cultivars next to sulfur-containing volatiles there were VOCs, which consisted of CHO atoms. Out of those VOCs five compounds (hexanal, 2-pentenal, 2-methyl, heptenal, (2E), 2-tridecanone, and 3(2H)-furanone, 5-methyl-2-octyl) are previously found in distilled oils of A. fistulosum cultivars [52]. The level of four compound peaks such as hexanal, 2-butenal, 2-ethyl-, 2-tridecanone, and 3(2H)-furanone, 5-methyl-2-octyl was significantly different in the profiles of A. chinense, while 11 VOCs with asterisk (*) on the top of the bars were significantly changed in the profiles of A. tuberosum (Fig. 5b).
Annotated or identified compounds whose moieties included only CH atoms were categorized as alkanes or alkenes (Fig. 5c), out of which odd-numbered alkanes (heptadecane and nonadecane) are previously found in the methanol extract of garlic (A. sativum) [53] and in the HS of flowers of heliotrope and mandarin [54, 55]. The function(s) and biosynthetic pathway(s) of such compounds remain largely limited, except for dipropyl trisulphide in A. fistulosum and diallyl disulphide in A. tuberosum as described in [31]
Among 50 annotated peaks, 12 metabolite peaks showed significant changes in A. chinense and A. tuberosum when compared to the control cultivar, A. fistulosum (class 01). Dipropyl disulphide (ID101) known as the main VOC component in Allium plants was included in the 12 metabolite peaks. The 12 compounds as well as previously reported compounds were listed in Table 4 including thiosulfinates produced from S-alk(en)yl cysteine sulfoxides which are sulfur-storage compounds. These peaks are like to be used as discriminative compounds in VOC profiles of A. chinense, A. tuberosum, and A. fistulosum.
Table 4.
List of previously reported compounds in Allium species and 12 VOCs with significant changes in A. chinense and A. tuberosum against A. fistulosum in this study
| Name | ID | Detected Allim subspecies | Reference |
|---|---|---|---|
| Sulfur-containing peaks | |||
| Thiophene, 2,4-dimethyl | ID032 | A. chinense, A. tuberosum and A. fistulosum | |
| Dimethyl trisulfide | ID054 | A. chinense, A. tuberosum and A. fistulosum | [42] |
| 3-Thiophenecarboxaldehyde | ID065 | A. chinense, A. tuberosum and A. fistulosum | |
| Diallyl disulphide | ID091 | A. chinense, A. tuberosum and A. fistulosum | |
| 1,2,4-Trithiolane | ID099 | A. chinense, A. tuberosum and A. fistulosum | |
| Dipropyl disulfide | ID101 | A. chinense, A. tuberosum and A. fistulosum | |
| 3-Vinyl-1,2-dithiacyclohex-5-ene | ID134 | A. chinense, A. tuberosum and A. fistulosum | |
| 2,5-Thiophenedicarboxaldehyde | ID154 | A. chinense, A. tuberosum and A. fistulosum | |
| Thieno[2,3-b]thiophene, 2-methyl | ID200 | A. chinense, A. tuberosum and A. fistulosum | |
| Fully saturated thiosulfinates | ND | A. cepa, A. sativum, A. ursinum, A. porrum, A. fistulosum, A. ascalonicum, A. ampeloprasum, A. schoenoprasum and A. tuberosum | [10] |
| Mono-S-b,c-unsaturated thiosulfinates | ND | A. cepa, A. sativum, A. ursinum, A. porrum, A. fi A. m, A, A. ascalonicum, A. ampeloprasum, A. schoenoprasum and A. tuberosum | [10] |
| Di-S-b, c-unsaturated thiosulfinates | ND | A. cepa, A. sativum, A. ursinum, A. porrum, A. fistulosum, A. ascalonicum, A. ampeloprasum, A. schoenoprasum and A. tuberosum | [10] |
| Mono-a, b-unsaturated thiosulfinates | ND | A. cepa, A. sativum, A. ursinum, A. porrum, A. fistulosum, A. ascalonicum, A. ampeloprasum, A. schoenoprasum and A. tuberosum | [10] |
| Mixed a, b- and c-unsaturated thiosulfinates. | ND | A. cepa, A. sativum, A. ursinum, A. porrum, A. fistulosum, A. ascalonicum, A. ampeloprasum, A. schoenoprasum and A. tuberosum | [10] |
| Allicin (diallylthiosulphinate) | ND | A. sativum | [5] |
| Alliin (S-allyl-l-cysteine sulphoxide) | ND | A. sativum,A. ursinum, A. ampeloprasum and A. longicuspis | [5, 10] |
| Dipropyl disulphide | ID101 | A. fistulosum and A. tuberosum | [6, 42] |
| Dipropyl trisulphide | ND | A. fistulosum and A. tuberosum | [6] |
| 1-Propenyl propyl disulphide | ND | A. fistulosum and A. tuberosum | [6] |
| Methiin (S-methyl-l-cysteine sulfoxide) | ND | A. cepa, A. sativum,A. chinens and A. longicuspis | [10] |
| Propiin (S-propyl-l-cysteine sulfoxide) | ND | A. cepa, A. porrum, A. porrum, A. altaicum and A. fistulosum | [10] |
| Isoalliin (S-propenyl-l-cysteine sulfoxide) | ND | A. cepa, A. nutans,A. ascalonicum and A. schoenoprasum | [10] |
| Ethiin (S-ethyl-l-cysteine sulfoxide) | ND | A. aflatunens, A. ampeloprasum, A. ochotense and A. victorialis | [10] |
| Butiin (S–n-butyl-l-cysteine sulfoxide) | ND | A. siculum | [10] |
| 1-Propenyl-containing disulfides | ND | A. uictorialis | [13] |
| Thiopropanal S-oxide | ND | A. cepa | [42] |
| Propenyl propyl disulphide | ND | A. cepa | [42] |
| 1-Propenyl propyl disulphide | ND | A. cepa | [42] |
| Di-1-propenyl disulphide | ND | A. cepa | [42] |
| Methyl propyl trisulphide | ND | A. cepa | [42] |
| Propenyl propyl trisulphide | ND | A. cepa | [42] |
| Peaks consisting of CHO | |||
| 2-Butenal, 2-ethyl | ID011 | A. chinense, A. tuberosum and A. fistulosum | [13] |
| Decenal, (2E) | ID143 | A. chinense, A. tuberosum and A. fistulosum | [13] |
| 2-Methyl-2-pentenal | ND | A. uictorialis and A. cepa | [13, 42] |
| Prop(en)yl aldehydes | ND | A. cepa | [42] |
| 2-Methyl-2-pentenal | ND | A. cepa | |
| Peaks consisting of CH | |||
| Nonadecane | ID322 | A. chinense, A. tuberosum and A. fistulosum | [53–55] |
ND not detected in this study
Conclusions
Since Allium plants emit various types of sulfur-containing compounds and other VOCs, comprehensive profiling techniques are needed. We developed a VOC profiling method for the HS of Allium samples that is based on an ITEX technique and our metabolomics pipeline by using GC-TOF–MS [56, 57]. The amount of sample material needed for HS collection was much lower with our ITEX-method than the traditional methods such as solvent extraction and steam distillation. Our findings suggest, that ITEX-based VOC profiling yields good reproducibility for the detection of various types of VOC in Allium plants. As ITEX-based VOC profiling captures differences in the composition of VOCs in the HS of Allium plants, it is probably appropriate for chemotaxonomic classification of these plants. For odor analysis of samples with strong odors, for example Allium plants, GC–olfactometry (GC–O) coupled with MS is useful because it facilitates the evaluation of odor compounds and yields MS spectral information. However, as the concentration of odor compound is often very low and odor-related VOCs can interact synergistically or additively, the identification of actual “odor” peaks remains difficult. Taken together, we think that the HS sampling- and the ITEX-based VOC profiling methods presented here help to improve the detection of odor compounds in Allium plants.
Methods
Chemicals
All chemicals and reagents used for this study were of spectrometric grade. The n-alkane standard solution C8–C20 for determination of RI was purchased from Fluka Chemical (Tokyo, Japan), deuterium-labeled alkanes used to distinguish natural alkanes collected from Allium samples were obtained from Cambridge Isotope Laboratories (Andver, USA), and dipropyl disulfide (98 %) and surrogate standard mixture (EPA524.2) from Sigma-Aldrich Japan (Tokyo, Japan). The other chemicals were purchased from Nacalai Tesque (Kyoto, Japan) or Wako Pure Chemical Industries (Osaka, Japan).
Plant material and sample preparation procedure
Metadata for this study are provided in Additional file 2.
Ten Allium (A.) fistulosum species, six spring onion cultivars, two scallions, and two Japanese-leek cultivars, rakkyo (A. chinense) and Oriental garlic (A. tuberosum), were purchased from a grocer in Kawasaki, Japan or harvested in a Japanese field (see Table 1 and Additional file 2). After removing the roots, a 10-cm length of the sheath and the basal plate of each plant sample were collected and chopped with stainless steel surgical blades (Feather, Tokyo, Japan). Out of the A. fistulosum cultivars, four were grown by applying a method (hilling) similar to that used for growing the leek A. ampeloprasum var. porrum to obtain longer white stems for consumption in Japan (Fig. 1f, g, h, l). Each sample was immediately frozen in liquid nitrogen and kept at −80 °C until use. As the group of samples of Mikata spring onion (class01) was gathered center of the PCA score scatter plot (Fig. 4), this cultivar was chosen as the control.
The samples were crushed into powder (2 min at 4 °C) in a Mixer Mill MM 311 instrument featuring a grinding jar with a stainless steel screw cap (Restech, Tokyo, Japan) and the frozen powder from each sample (flesh weight, 1 g) was weighed in a 20-ml HS vial (Supelco, MO, USA). For VOC profiling of Allium plants we used a modified method of Tikunov et al. [23] and Kusano et al. [31]. Briefly, the 20-ml HS-GC vial (Supelco) containing the frozen powder was closed with a magnetic screw cap (AMR, Tokyo, Japan) for ITEX- and SPME-analysis. Then, 1 ml of 100 mM 2,2′,2′’,2′’’-(ethane-1,2-diyldinitrilo) tetraacetic acid (EDTA)- NaOH water solution (pH 7.5) was added to each vial; the water derived from an Allium sample was considered to be equal to 1 ml. After vortexing, 10 μl of solution containing n-decane (d22, 99 %; 50 μM), n-pentadecane (d32, 98 %; 50 μM), n-eicosane (d42, 98 %; 50 μM) for definition of RI and EPA524.2 fortification solution (20 μg/ml of fluorobenzene, 4-bromofluorobenzene, and 1,2-dichlorobenzene-d4) as ISs was mixed in methanol, then solution was added to each vial as IS. Solid CaCl2 was added to obtain a final concentration of 5 M and the samples were stored overnight at 22 °C.
HS collection using the SPME fiber
The SPME device for a CTC CombiPAL auto-sampler (CTC Analytics, Zwingen, Switzerland) was purchased from AMR (Tokyo, Japan). We used an SPME fiber comprised of a 65-μm-thick layer of polydimethylsiloxane (PDMS)/divinylbenzene (DVB)-fused silica (FS) fiber/stainless-steel (SS) tube. Before analysis, the fiber was conditioned at 250 °C for 30 s in the injection port of an Agilent 6890 N gas chromatograph (Agilent Technologies, Wilmington, USA) equipped with a 30 m × 0.25 mm inner diameter fused-silica capillary column with a chemically bound 0.25-μl film Rtx-5 Sil MS stationary phase (RESTEK, Bellefonte, USA). Collection of volatiles was carried out by inserting the SPME-fiber to the vial and by trapping the VOCs for 20 min at 80 °C under continuous agitation. After HS collection it was placed in the injection port of the gas chromatograph that was coupled to a Pegasus III TOF mass spectrometer (LECO, St. Joseph, USA). The thermodesorption of VOCs occurred for 15 s at 250 °C.
HS collection using the ITEX device
We used a CTC CombiPAL auto-sampler (PAL COMBI-xt) featuring the ITEX device PAL ITEX-2 option (CTC Analytics). The ITEX procedure was controlled with a PAL Cycle Composer (CTC analytics). We conducted preliminary experiments to choose an appropriate sorbent material from the four materials, Tenax TA, Tenax GR (TGR), Carbosieve SIII (CSIII) and mixed TGR and CSIII (TGR/CSIII), that are commercially available (data not shown). Then, we chose that the sorbent material for the ITEX-2 portion was TGR (80/100 mesh)/CSIII (60/80 mesh). The parameters for HS collection were as described in the Additional file 2. After HS collection, 500 μl of the HS sample were injected into the injection port of the gas chromatograph coupled to the mass spectrometer used for HS collection by ITEX.
GC-TOF–MS analysis
GC-TOF–MS conditions were as described in the Additional file 2. Data acquisition was on a Pegasus III TOF mass spectrometer (LECO); the acquisition rate was 30 spectra/s in the mass range of a mass-to-charge ratio of m/z = 30–550. Five ISs were used for data normalization.
Data analysis
Raw data were exported in the network common data form (NetCDF) file format using LECO ChromaTOF software (version 2.32) and then processed with the hierarchical multi-curve resolution (H-MCR) method [38]. We obtained the normalized response for calculating the signal intensity of each metabolite from the mass-detector response by using the cross-contribution compensating multiple standard normalization (CCMN) method [44]. The resolved mass spectra were matched against reference mass spectra in the NIST-L (version NIST05) using NIST MS search program (version 2.0, http://www.chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:ms-search). Peaks were tentatively identified according to the guidelines for metabolite identification [58]. When mass spectra exhibited a match value greater than 799 and the corresponding peaks had RIs with small differences upon comparison of their resolved mass spectra and RIs against those in the reference libraries (Ad-L, 3rd and 4th edition, and Te-L) and against Vo and NIST-L (see Table 2 and “Results and discussion” section), the peaks were considered to be putatively annotated compounds. We compared the RIs of sulfur-containing metabolites and compounds we detected with those reported in the literature [51, 52, 59].
To estimate the number of compounds that overlapped with each reference library, we first exported the mass spectral information, including the compound name, RI, synonyms, and m/z value, with relative peak intensity (maximum, 999; minimum, one) from each library in ASCII text format (.MSP) automatically. Then we compared the similarity of the mass spectra in each library using MassBank [60]. The similarity (≥850 or 900) and the RI difference (<|30 unit|) were used to extract the same or very similar compounds from the query library and NIST05. It should be noted that the standard deviation (SD) of the absolute RI difference of these compounds is less than 8.8 when we applied similarity of ≥850 (Table 3).
The lower limit of quantification (LLOQ) and the limit of detection (LOD) of dipropyl disulfide obtained from ITEX-GC-TOF–MS- and SPME-GC-TOF–MS analyses were estimated as described in the Additional file 2.
Statistical analysis
Multivariate analysis was performed with SIMCA-P + 12.0 software (Umetrics AB, Umeå, Sweden). For our analysis, profile data were log10-transformed, centered, and scaled to unit variance.
Log2-transformed profile data were statistically analyzed using the LIMMA package [61]. It includes FDR correction for multiple testing [62] in the R environment for statistical computing (version 2.14.1 for 64-bit).
Authors’ contributions
Conceived and designed the experiments: MK. Performed the experiments: MK, MKo, and YI. Analyzed the data: MK, MKo, and AF. Contributed reagents/materials/analysis tools: MK, MKo, YI, and AF. Wrote the paper: MK, AF, and KS. All authors read and approved the final manuscript.
Acknowledgements
We thank Mr. Takano and Ms. Nishizawa for preparing the standard compounds and for taking pictures of the Allium samples. We thank Mrs. Ursula Petralia for editorial assistance. The work was also supported, in part, by Japan Advanced Plant Science Network and a project using supplementary budget by Ministry of Agriculture, Forestry and Fisheries.
Competing interests
The authors declare that they have no competing interests.
Additional files
10.1186/s13104-016-1942-5 Comparison of the peaks collected with ITEX and SPME devices. a. Total ion chromatograms (TICs) of VOCs in the HS of A. fistulosum (class01) obtained by ITEX-GC-TOF–MS (left) and SPME-GC-TOF–MS (right). b. VOC peak annotation with RI information obtained by ITEX-GC-TOF–MS (left) and SPME-GC-TOF–MS (right). c. The PCA score scatter plot of VOC profiles of A. fistulosum (class01) obtained by using ITEX-GC-TOF–MS and SPME-GC-TOF–MS. We used three biological replicates (six individual plants per replicate). AU arbitrary unit. Fluorobenzene, 4-bromofluorobenzene, and 1,2-dichlorobenzene-d 4 were used as ISs. Of these, peaks of luorobenzene and 4-bromofluorobenzene were detected (black arrow in Additional file 1).
10.1186/s13104-016-1942-5 Metabolomic methods.
10.1186/s13104-016-1942-5 Tentatively annotated VOCs in the HS of Allium plants collected with the ITEX device. The log2-fold change (log2FC) was calculated using the LIMMA package (see the “Methods” section). We analyzed three biological replicates of each Allium plant except for the white spring onion cultivar (class05, n = 2). The FDR was <0.05. A2 tentatively identified by referring to the libraries (match value >799); I identified by comparing the authentic standards.
Contributor Information
Miyako Kusano, Email: kusano.miyako.fp@u.tsukuba.ac.jp.
Makoto Kobayashi, Email: kobamako@riken.jp.
Yumiko Iizuka, Email: yiizuka5@gmail.com.
Atsushi Fukushima, Email: atsushi.fukushima@riken.jp.
Kazuki Saito, Email: kazuki.saito@riken.jp.
References
- 1.Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci. 2006;25(5):417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 2.Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Bot Rev. 2006;72(1):1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- 3.D’Alessandro M, Turlings TCJ. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst. 2006;131(1):24–32. doi: 10.1039/B507589K. [DOI] [PubMed] [Google Scholar]
- 4.Block E. Garlic and other Alliums: The lore and the science: royal society of chemistry. 2009.
- 5.Michael K. Volatile compounds of the genus Allium L. (Onions). In: Volatile sulfur compounds in food. Am Chem Soc. 2011;1068:183–214.
- 6.Slusarenko AJ, Patel A, Portz D. Control of plant diseases by natural products: allicin from garlic as a case study. Eur J Plant Pathol. 2008;121(3):313–322. doi: 10.1007/s10658-007-9232-7. [DOI] [Google Scholar]
- 7.Hori M. Onion aphid (Neotoxoptera formosana) attractants, in the headspace of Alliumfistulosum and A. tuberosum leaves. J Appl Entomol. 2007;131(1):8–12. doi: 10.1111/j.1439-0418.2006.01130.x. [DOI] [Google Scholar]
- 8.Landry JF. Taxonomic review of the leek moth genus Acrolepiopsis (Lepidoptera: acrolepiidae) in North America. Can Entomol. 2007;139(3):319–353. doi: 10.4039/n06-098. [DOI] [Google Scholar]
- 9.Dugravot S, Thibout E. Consequences for a specialist insect and its parasitoid of the response of Allium porrum to conspecific herbivore attack. Physiol Entomol. 2006;31(1):73–79. doi: 10.1111/j.1365-3032.2005.00489.x. [DOI] [Google Scholar]
- 10.Thibout E, Guillot JF, Auger J. Microorganisms are involved in the production of volatile kairomones affecting the host-seeking behavior of Diadromus–Pulchellus, a parasitoid of Acrolepiopsis–Assectella. Physiol Entomol. 1993;18(2):176–182. doi: 10.1111/j.1365-3032.1993.tb00465.x. [DOI] [Google Scholar]
- 11.Rose P, Whiteman M, Moore PK, Zhu YZ. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22(3):351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 12.Scherer C, Jacob C, Dicato M, Diederich M. Potential role of organic sulfur compounds from Allium species in cancer prevention and therapy. Phytochem Rev. 2009;8(2):349–368. doi: 10.1007/s11101-009-9122-z. [DOI] [Google Scholar]
- 13.Azadi HG, Riazi GH, Ghaffari SM, Ahmadian S, Khaife TJ. Antitumor activity of Allium hirtifolium (Iranian shallot) and allicin: microtubule-interaction properties and effects on cancer cell lines. FEBS J. 2008;275:379. [Google Scholar]
- 14.Nishimura H, Wijaya CH, Mizutani J. Volatile flavor components and antithrombotic agents: vinyldithiins from Allium victorialis L. J Agr Food Chem. 1988;36(3):563–566. doi: 10.1021/jf00081a039. [DOI] [Google Scholar]
- 15.Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum I Isolation, physical properties, and antibacterial action. J Am Chem Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. [DOI] [PubMed] [Google Scholar]
- 16.Kyung KH. Antimicrobial properties of Allium species. Curr Opin Biotech. 2012;23(2):142–147. doi: 10.1016/j.copbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Bicchi C, Cordero C, Liberto E, Sgorbini B, Rubiolo P. Headspace sampling of the volatile fraction of vegetable matrices. J Chromatogr A. 2008;1184(1–2):220–233. doi: 10.1016/j.chroma.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Palma-Harris C, McFeeters RF, Fleming HP. Solid-phase microextraction (SPME) technique for measurement of generation of fresh cucumber flavor compounds. J Agr Food Chem. 2001;49(9):4203–4207. doi: 10.1021/jf010182w. [DOI] [PubMed] [Google Scholar]
- 19.Bicchi C, Cordero C, Liberto E, Rubiolo P, Sgorbini B. Automated headspace solid-phase dynamic extraction to analyse the volatile fraction of food matrices. J Chromatogr A. 2004;1024(1–2):217–226. doi: 10.1016/j.chroma.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Farquar G, Jones AD, Frank M. Human breath analysis: methods for sample collection and reduction of localized background effects. Anal Bioanal Chem. 2010;396(2):739–750. doi: 10.1007/s00216-009-3217-7. [DOI] [PubMed] [Google Scholar]
- 21.Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J, Amann A. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol. 2012;12:113. doi: 10.1186/1471-2180-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bicchi C, Ruosi MR, Cagliero C, Cordero C, Liberto E, Rubiolo P, Sgorbini B. Quantitative analysis of volatiles from solid matrices of vegetable origin by high concentration capacity headspace techniques: determination of furan in roasted coffee. J Chromatogr A. 2011;1218(6):753–762. doi: 10.1016/j.chroma.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Tikunov Y, Lommen A, de Vos CHR, Verhoeven HA, Bino RJ, Hall RD, Bovy AG. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005;139(3):1125–1137. doi: 10.1104/pp.105.068130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaulieu JC, Grimm CC. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J Agr Food Chem. 2001;49(3):1345–1352. doi: 10.1021/jf0005768. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira RCS, Oliveira LS, Franca AS, Augusti R. Evaluation of the potential of SPME-GC-MS and chemometrics to detect adulteration of ground roasted coffee with roasted barley. J Food Compos Anal. 2009;22(3):257–261. doi: 10.1016/j.jfca.2008.10.015. [DOI] [Google Scholar]
- 26.Salanţă L, Tofană M, Socaci S, Lazar C, Michiu D, Fărcas A. Determination of the volatile compounds from hop and hop products using ITEX/GC-MS technique. J Agroaliment Process Technol. 2012;18:2. [Google Scholar]
- 27.Laaks J, Jochmann MA, Schilling B, Schmidt TC. In-tube extraction of volatile organic compounds from aqueous samples: an economical alternative to purge and trap enrichment. Anal Chem. 2010;82(18):7641–7648. doi: 10.1021/ac101414t. [DOI] [PubMed] [Google Scholar]
- 28.Jochmann MA, Yuan X, Schilling B, Schmidt TC. In-tube extraction for enrichment of volatile organic hydrocarbons from aqueous samples. J Chromatogr A. 2008;1179(2):96–105. doi: 10.1016/j.chroma.2007.11.100. [DOI] [PubMed] [Google Scholar]
- 29.Skogerson K, Wohlgemuth G, Barupal DK, Fiehn O. The volatile compound BinBase mass spectral database. BMC Bioinformatics. 2011;12:321. doi: 10.1186/1471-2105-12-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malowicki SM, Martin R, Qian MC. Volatile composition in raspberry cultivars grown in the Pacific Northwest determined by stir bar sorptive extraction-gas chromatography-mass spectrometry. J Agric Food Chem. 2008;56(11):4128–4133. doi: 10.1021/jf073489p. [DOI] [PubMed] [Google Scholar]
- 31.Kusano M, Iizuka Y, Kobayashi M, Fukushima A, Saito K. Development of a direct headspace collection method from arabidopsis seedlings using HS-SPME-GC-TOF-MS analysis. Metabolites. 2013;3(2):223–242. doi: 10.3390/metabo3020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laaks J, Jochmann MA, Schilling B, Schmidt TC. Optimization strategies of in-tube extraction (ITEX) methods. Anal Bioanal Chem. 2015;407(22):6827–6838. doi: 10.1007/s00216-015-8854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veriotti T, Sacks R. High speed GC/MS of gasoline-range hydrocarbon compounds using a pressure-tunable column ensemble and time-of-flight detection. Anal Chem. 2000;72(14):3063–3069. doi: 10.1021/ac000081h. [DOI] [PubMed] [Google Scholar]
- 34.Stein SE, Ausloos P, Clifton CL, Klassen JK, Lias SG, Mikaya AI, Sparkman OD, Tchekhovskoi DV, Zaikin V, Zhu D. Evaluation of the NIST/EPA/NIH mass spectral library. Abstr Pap Am Chem S. 1999;218:U368. doi: 10.1016/S1044-0305(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 35.Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy: Allured Pub. Corporation. 2001.
- 36.Stein SE. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J Am Soc Mass Spectr. 1999;10(8):770–781. doi: 10.1016/S1044-0305(99)00047-1. [DOI] [Google Scholar]
- 37.Hoffmann N, Stoye J. ChromA: signal-based retention time alignment for chromatography–mass spectrometry data. Bioinformatics. 2009;25(16):2080–2081. doi: 10.1093/bioinformatics/btp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonsson P, Johansson ES, Wuolikainen A, Lindberg J, Schuppe-Koistinen I, Kusano M, Sjostrom M, Trygg J, Moritz T, Antti H. Predictive metabolite profiling applying hierarchical multivariate curve resolution to GC–MS data—a potential tool for multi-parametric diagnosis. J Proteome Res. 2006;5(6):1407–1414. doi: 10.1021/pr0600071. [DOI] [PubMed] [Google Scholar]
- 39.Lommen A. MetAlign: interface-driven, Versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal Chem. 2009;81(8):3079–3086. doi: 10.1021/ac900036d. [DOI] [PubMed] [Google Scholar]
- 40.Luedemann A, Strassburg K, Erban A, Kopka J. TagFinder for the quantitative analysis of gas chromatography–mass spectrometry (GC-MS)-based metabolite profiling experiments. Bioinformatics. 2008;24(5):732–737. doi: 10.1093/bioinformatics/btn023. [DOI] [PubMed] [Google Scholar]
- 41.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using Nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 42.Jarvenpaa EP, Zhang ZY, Huopalahti R, King JW. Determination of fresh onion (Allium cepa L.) volatiles by solid phase microextraction combined with gas chromatography mass spectrometry. Z Lebensm Unters F A. 1998;207(1):39–43. doi: 10.1007/s002170050292. [DOI] [Google Scholar]
- 43.Gullberg J, Jonsson P, Nordstrom A, Sjostrom M, Moritz T. Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal Biochem. 2004;331(2):283–295. doi: 10.1016/j.ab.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Redestig H, Fukushima A, Stenlund H, Moritz T, Arita M, Saito K, Kusano M. Compensation for systematic cross-contribution improves normalization of mass spectrometry based metabolomics data. Anal Chem. 2009;81(19):7974–7980. doi: 10.1021/ac901143w. [DOI] [PubMed] [Google Scholar]
- 45.Kolb B, Ettre LS. Static headspace–gas chromatography: theory and practice: John Wiley and Sons; 2006.
- 46.Eady CC, Kamoi T, Kato M, Porter NG, Davis S, Shaw M, Kamoi A, Imai S. Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol. 2008;147(4):2096–2106. doi: 10.1104/pp.108.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laaks J, Jochmann MA, Schmidt TC. Solvent-free microextraction techniques in gas chromatography. Anal Bioanal Chem. 2012;402(2):565–571. doi: 10.1007/s00216-011-5511-4. [DOI] [PubMed] [Google Scholar]
- 48.Zapata J, Mateo-Vivaracho L, Lopez R, Ferreira V. Automated and quantitative headspace in-tube extraction for the accurate determination of highly volatile compounds from wines and beers. J Chromatogr A. 2012;1230:1–7. doi: 10.1016/j.chroma.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 49.Pino JA, Fuentes V, Correa MT. Volatile constituents of Chinese chive (Alliumtuberosum Rottl. ex Sprengel) and rakkyo (Alliumchinense G. Don) J Agric Food Chem. 2001;49(3):1328–1330. doi: 10.1021/jf9907034. [DOI] [PubMed] [Google Scholar]
- 50.Shen CX, Xiao H, Parkin KL. In vitro stability and chemical reactivity of thiosulfinates. J Agr Food Chem. 2002;50(9):2644–2651. doi: 10.1021/jf011013e. [DOI] [PubMed] [Google Scholar]
- 51.Block E, Putman D, Zhao SH. Allium chemistry–Gc Ms analysis of thiosulfinates and related-compounds from onion, leek, scallion, shallot, chive, and Chinese chive. J Agr Food Chem. 1992;40(12):2431–2438. doi: 10.1021/jf00024a018. [DOI] [Google Scholar]
- 52.Kuo MC, Ho CT. Volatile constituents of the distilled oils of Welsh onions (Alliumfistulosum L. variety maichuon) and scallions (Alliumfistulosum L. variety caespitosum) J Agr Food Chem. 1992;40(1):111–117. doi: 10.1021/jf00013a021. [DOI] [Google Scholar]
- 53.Yusuf OK, Bewaji CO. Evaluation of essential oils composition of methanolic Alliumsativum extract on Trypanosoma brucei infected rats. Res Pharm Biotech. 2011;3(2):17–21. [Google Scholar]
- 54.Kays SJ, Hatch J, Yang DS. Volatile floral chemistry of Heliotropium arborescens L. ‘Marine’. HortScience. 2005;40(5):1237–1238. [Google Scholar]
- 55.Flamini G, Cioni PL, Morelli I. Use of solid-phase micro-extraction as a sampling technique in the determination of volatiles emitted by flowers, isolated flower parts and pollen. J Chromatogr A. 2003;998(1–2):229–233. doi: 10.1016/S0021-9673(03)00641-1. [DOI] [PubMed] [Google Scholar]
- 56.Kusano M, Fukushima A, Kobayashi M, Hayashi N, Jonsson P, Moritz T, Ebana K, Saito K. Application of a metabolomic method combining one-dimensional and two-dimensional gas chromatography-time-of-flight/mass spectrometry to metabolic phenotyping of natural variants in rice. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855(1):71–79. doi: 10.1016/j.jchromb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Kusano M, Fukushima A, Arita M, Jonsson P, Moritz T, Kobayashi M, Hayashi N, Tohge T, Saito K. Unbiased characterization of genotype-dependent metabolic regulations by metabolomic approach in Arabidopsis thaliana. BMC Syst Biol. 2007;1:53. doi: 10.1186/1752-0509-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, et al. Proposed minimum reporting standards for chemical analysis. Metab Off J Metab Soc. 2007;3(3):211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo MC, Ho CT. Volatile constituents of the solvent extracts of Welsh onions (Alliumfistulosum L. variety maichuon) and scallions (A. fistulosum L. variety caespitosum) J Agr Food Chem. 1992;40(10):1906–1910. doi: 10.1021/jf00022a036. [DOI] [Google Scholar]
- 60.Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, et al. MassBank: a public repository for sharing mass spectral data for life sciences. JMS. 2010;45(7):703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- 61.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical applications in genetics and molecular biology. 2004;3:Article3. [DOI] [PubMed]
- 62.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc. 1995;B57:289–300. [Google Scholar]


