Abstract
The sense of smell is mediated by the olfactory epithelium, which is composed of a mosaic pattern of olfactory sensory cells surrounded by supporting cells. In this issue, Katsunuma et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201509020) show that the differential expression of nectins and cadherins establishes this pattern.
The olfactory epithelium is a pseudo-stratified epithelium composed of epithelial support cells and neuronal olfactory sensory cells (Steinke et al., 2008). The integrity of the epithelial sheet is maintained by adherens and tight junctions that form between support cells as well as between support cells and sensory cells. In adult mammals, sensory cells are not contacting each other and instead are surrounded by support cells. There are more support cells than sensory cells, so the mosaic pattern is closer to a soccer ball than a checkerboard. How is this pattern established during development? When the olfactory epithelium is formed during development, as many as half of the olfactory sensory cells are first attached to each other (Katsunuma et al., 2016). As development progresses in utero and after birth, sensory cells separate from each other and each becomes fully surrounded by support cells. Thus, some adherens junctions, composed of the cell adhesion molecules nectins and cadherins, must be weakened and lost, whereas others are strengthened and preserved.
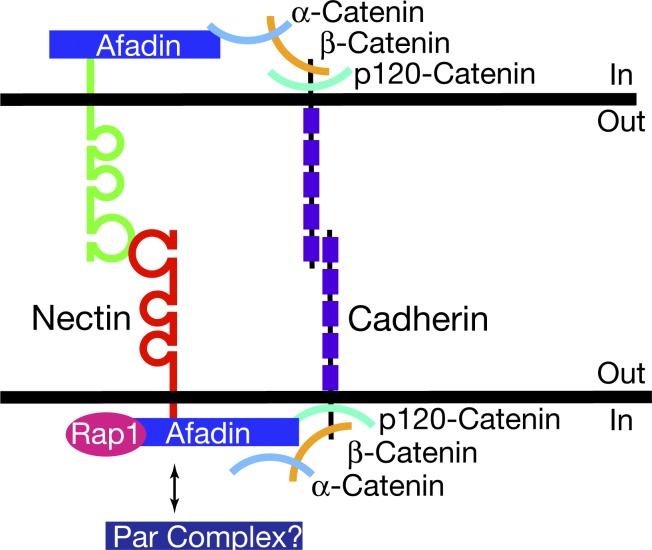
Nectins are immunoglobulin superfamily cell adhesion molecules found at adherens junctions. They are thought to nucleate and regulate junction formation by recruiting cadherins. Whereas cadherins bind in trans between cells nearly exclusively homophilically, nectins have been shown to bind both homophilically and heterophilically (Rikitake et al., 2012). The heterophilic interactions between different members of the nectin family are thought critical for many processes, including axon to dendrite adhesion in neurons and the generation of a checkerboard pattern of neurons and support cells in the auditory epithelium (Togashi et al., 2006, 2011; Fukuda et al., 2014). Intracellulary, nectins have a C-terminal PDZ-binding motif that binds the PDZ domain in afadin. Afadin regulates cadherin function through α-catenin, which binds β-catenin, a direct partner of cadherins (Beaudoin, 2006; Fig. 1). In this issue, Katsunuma et al. show that the formation of the mosaic pattern of sensory and supporting cells in the olfactory epithelium is regulated by the differential adhesion and motility of the cells induced by the expression of nectins and cadherins.
Figure 1.
Molecular interactions linking nectin binding between cells to cadherin recruitment. (top) Nectins bind intracellularly to afadin and recruit α-catenin, which indirectly recruits cadherins by binding β-catenin. (bottom) Afadin may also activate and recruit cadherins by binding p120-catenin in a Rap1-dependent manner. Besides afadin, the PDZ-binding motif of nectins can also recruit the Par complex, as well as other PDZ domain–containing proteins that may affect cadherins.
To investigate the nature of the junctions underlying the mosaic cellular pattern of the olfactory epithelium, Katsunuma et al. (2016) first performed in situ hybridizations and immunostainings for nectins and cadherins within the olfactory epithelium. Epithelial support cells expressed nectin-2, nectin-3, N-cadherin, and E-cadherin, whereas neuronal sensory cells only expressed nectin-2 and N-cadherin. Junctions between support cells contained primarily nectin-3 and E-cadherin and to a lesser extent nectin-2 and N-cadherin. In contrast, junctions between sensory cells contained nectin-2 and N-cadherin; and junctions between support cells and sensory cells contained nectin-2, nectin-3, N-cadherin, and to a lesser extent E-cadherin. Katsunuma et al. (2016) also examined the distribution of β-catenin, which is expressed equivalently in both sensory and support cells and acts as a proxy for strength of cadherin recruitment. β-Catenin was more heavily localized to support cell junctions than junctions between sensory cells or between sensory and support cells. The expression of αE-catenin, which is expressed nearly exclusively by support cells, and αN-catenin, which is expressed by sensory cells, was highly expressed at the cell junctions containing support or sensory cells, respectively.
The researchers used various knockout (KO) mice to dissect the roles of nectin-2 and -3 in the process of sensory cell separation. Loss of nectin-3 and to a lesser extent loss of nectin-2 led to the continued presence of sensory cell clusters in which upwards of 20% of sensory cells were not completely surrounded by support cells in juvenile mice. Katsunuma et al. (2016) further tested the requirement of cadherin recruitment by nectins using αN-catenin KO mice in which nectin-dependent recruitment of cadherins is impaired in neuronal sensory cells only. αN-Catenin KO animals showed an increase in clustered sensory cells from 20 to 50%. This data are not entirely comparable to the nectin-null data, because αN-catenin KO animals die within 24 h of birth, allowing for examination of sensory cell distribution only at postnatal day 1, as opposed to day 28 for nectin KO mice. Therefore, although the phenotype is a near complete cessation of separation of sensory cells, it is unclear if the phenotype is more severe than even the nectin-2/nectin-3 double-null phenotype at postnatal day 28, which was similar to the phenotype observed in nectin-3 KO mice. However, these results suggest that the heterophilic interactions between nectin-2 and -3 as well as αN-catenin are involved in the cellular rearrangements of the developing olfactory epithelium.
One of the strengths of this study is the combination of cell culture assays and modeling to delineate the conditions required for separation of sensory cells. By mathematically modeling a polygonal pattern in which polygons made of many polygon edges are packed in a 2D sheet, Katsunuma et al. (2016) found that the strength of adhesion between sensory and support cells had to be greater than the strength between sensory cells and equivalent to the adhesive strength between support cells, or sensory cell junctions would persist. The authors also tested the effects of heterophilic and homophilic interactions between cadherins and nectins on intercalation of cells by culturing two populations of HEK293 (293) cells, which overexpressed cadherins and nectins, separated by a removable barrier. 293 cells are known to express N-cadherin so it was not overexpressed, and the populations of cells overexpressing different proteins were differentiated by overexpression of a specific fluorescent protein, green or red. After removal of the barrier, as the cells spread to interact with each other, intercalation of the different cell populations could be visualized through the fluorescent proteins. Colonies of 293 cells expressing E- and N-cadherins and those expressing N-cadherin never intercalated, as expected from the original description of cadherins as mediating homophilic cell clustering (Nose et al., 1988; Katsamba et al., 2009). This experiment elegantly provided evidence for the requirement of nectins to allow intercalation between populations of cells. Katsunuma et al. (2016) also assessed what happens when different nectins are overexpressed in 293 cells. When two populations of cells expressed the same nectin, either nectin-2 or -3, the cells did not intermingle, a similar result to the cadherin experiment. However, when one population expressed nectin-2 and the other expressed nectin-3, the two colonies mixed. This mosaic cellular patterning requires the nectin PDZ-binding motif, as the intercalation of the two cell types was prevented when using cell lines expressing nectin-2 or -3 constructs truncated for their PDZ-binding motifs. Additionally, the authors explored whether cadherin activity is necessary for nectin-dependent mosaic patterning by turning to a cadherin-deficient neuroblastoma cell line. Overexpression of nectin-2 or -3 in these cells and mixing of the populations did not yield the formation of stable contacts, unless E-cadherin was also overexpressed, indicating that cadherins are required to form an epithelial sheet and obtain the effects of heterophilic nectin interaction.
Although the heterophilic interactions between nectin-2 and -3 were shown to support this invasive intercalation of cells, the results were obtained in the presence of only N-cadherin or only E-cadherin in both cell populations, which does not exactly mirror the in vivo situation. When two populations of 293 cells expressing either nectin-2 or -3, in addition to the constitutive expression of N-cadherin, are put in contact, they intermingle equally, suggesting symmetrical invasion of both colonies. What happens when E-cadherin is overexpressed with N-cadherin? Surprisingly, intermingling of a population of 293 cells expressing nectin-2 with a population expressing E-cadherin and nectin-3 displayed directionality. Katsunuma et al. (2016) examined the cell boundaries in the mixed cultures of 293 cells expressing nectin-2 or expressing nectin-3 and E-cadherin to understand the origin of this directionality. The junctions between cells expressing exogenous nectin-2 and endogenous N-cadherin no longer localized β-catenin to the membrane apposition, suggesting loss of the adherens junction. These results suggest that nectin-2–expressing 293 cells invaded the other colony and lost their homotypic adhesion, whereas cells overexpressing nectin-3 and E-cadherin maintained their homotypic contacts and did not invade the other colony. Besides adhesive strength, cells expressing only a nectin protein and N-cadherin migrated faster than cells expressing nectin and E-cadherin and, similarly, sensory cells migrated faster than supporting cells in the mouse olfactory epithelium, as shown by time-lapse microscopy of organ cultures.
Overall, the data establish the importance of the heterophilic binding between nectin-2–expressing olfactory cells and nectin-3–expressing supporting cells as well as of the selective recruitment of E-cadherin to homophilic interactions between nectin-3–expressing supporting cells. The combination of these two mechanisms leads to the weakening and loss of homophilic junctions between olfactory cells expressing nectin-2 and N-cadherin. As a result, olfactory cells migrate away from one another. These are the conditions necessary for creating the cellular mosaic (soccer ball–like) configuration seen in the olfactory epithelium. There are other instances in which different cell types are intercalated in an epithelium, e.g., dendritic cells form junctions with cells in the epithelium of the lung or gut (Hammad and Lambrecht, 2008). Nectins are known to be expressed by immune cells and may play a similar role in this context as in sensory cells of the olfactory epithelium. Thus, the model proposed by Katsunuma et al. (2016) likely reflects a common mechanism regulating the dispersion of cells in an epithelium.
The data of Katsunuma et al. (2016) is very convincing, but as always some mysteries remain. What are the cellular changes that orchestrate the separation of the sensory cells? The authors’ expression data suggests possible cues regulating the separation of the cell types, including an increase in the expression of nectin-2 in sensory cells and a change in the expression level of N-cadherin in supporting and/or sensory cells. Whether these fluctuations are caused by changes in protein expression or by differential regulation of protein trafficking is unclear. For instance, the Notch signaling components Numb and Numb-like are known to regulate surface expression of N-cadherin and cell polarity during neurogenesis (Rašin et al., 2007). Further work will be needed to investigate the possible involvement of similar mechanisms or pathways in the control of adhesion molecule trafficking and expression in the developing olfactory epithelium.
The intracellular mechanisms leading to cadherin recruitment and activation are also unknown. The data presented by Katsunuma et al. (2016) implicate αN-catenin and the PDZ-binding motif of nectin-2 and -3 in the regulation of the activity of cadherins. Nectins bind afadin through their PDZ motif, and this interaction is required for the recruitment of the cadherin–catenin complex via α-catenin to nectin-based adhesion sites. However, α-catenin is not the only protein involved in afadin-mediated regulation of cadherins. Afadin regulates p120-catenin recruitment to cadherins in a Rap1-dependent manner, suggesting an alternative mode of cadherin recruitment (Beaudoin, 2006). Additionally, the PDZ-binding motif of nectins recruits the Par complex as well as other PDZ domain–containing proteins (Rikitake et al., 2012). It will be interesting to delineate the involvement of these proteins in the recruitment of cadherins to nectin-based junctions.
Finally, in vivo data suggests that nectins mainly regulate cadherin function in the context of heterophilic interactions (Togashi et al., 2006, 2011; Rikitake et al., 2012; Fukuda et al., 2014). In vitro evidence suggests that heterophilic interactions are favored over homophilic interactions because of the kinetic and thermodynamic properties of this interaction type (Samanta and Almo, 2015). An alternative model to explore is whether heterophilic interactions trigger cellular events that homophilic interactions do not. The importance of homophilic nectin interactions in regulating cadherin function may be obscured by residual nectin expression in single nectin KO mice. In support of this hypothesis, most nectin isoforms bind afadin, and the KO of afadin is embryonically lethal (Ikeda et al., 1999; Zhadanov et al., 1999). Additionally, as shown by Katsunuma et al. (2016), without expression of nectin, cadherins only mediate homophilic interactions without dynamic reorganization of the epithelial sheet. These findings suggest that nectins are critical for regulation of cadherin function and that nectins may confer properties that cadherin-based junctions between cells do not. Further, although nectins are required to modulate the cadherin complex and allow cells to migrate within the epithelial sheet, Katsunuma et al. (2016) showed that expression of an additional cadherin leads to further modulation of the epithelial sheet. Deciphering the intracellular signaling mechanisms underlying these observations would provide insights into how the combinatorial expression of nectins and cadherins contributes to the production of complex cell patterns in epithelia.
Acknowledgments
The author declares no competing financial interests.
References
- Beaudoin G.M., III 2006. Con-nectin axons and dendrites. J. Cell Biol. 174:7–9. 10.1083/jcb.200606024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T., Kominami K., Wang S., Togashi H., Hirata K., Mizoguchi A., Rikitake Y., and Takai Y.. 2014. Aberrant cochlear hair cell attachments caused by Nectin-3 deficiency result in hair bundle abnormalities. Development. 141:399–409. 10.1242/dev.094995 [DOI] [PubMed] [Google Scholar]
- Hammad H., and Lambrecht B.N.. 2008. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8:193–204. 10.1038/nri2275 [DOI] [PubMed] [Google Scholar]
- Ikeda W., Nakanishi H., Miyoshi J., Mandai K., Ishizaki H., Tanaka M., Togawa A., Takahashi K., Nishioka H., Yoshida H., et al. 1999. Afadin: A key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 146:1117–1132. 10.1083/jcb.146.5.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsamba P., Carroll K., Ahlsen G., Bahna F., Vendome J., Posy S., Rajebhosale M., Price S., Jessell T.M., Ben-Shaul A., et al. 2009. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc. Natl. Acad. Sci. USA. 106:11594–11599. 10.1073/pnas.0905349106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsunuma S., Honda H., Shinoda T., Ishimoto Y., Miyata T., Kiyonari H., Abe T., Nibu K.-I., Takai Y., and Togashi H.. 2016. Synergistic action of nectins and cadherins generates the mosaic cellular pattern of the olfactory epithelium. J. Cell Biol. 10.1083/jcb.201509020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., and Takeichi M.. 1988. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 54:993–1001. 10.1016/0092-8674(88)90114-6 [DOI] [PubMed] [Google Scholar]
- Rašin M.R., Gazula V.R., Breunig J.J., Kwan K.Y., Johnson M.B., Liu-Chen S., Li H.S., Jan L.Y., Jan Y.N., Rakic P., and Sestan N.. 2007. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat. Neurosci. 10:819–827. 10.1038/nn1924 [DOI] [PubMed] [Google Scholar]
- Rikitake Y., Mandai K., and Takai Y.. 2012. The role of nectins in different types of cell–cell adhesion. J. Cell Sci. 125:3713–3722. 10.1242/jcs.099572 [DOI] [PubMed] [Google Scholar]
- Samanta D., and Almo S.C.. 2015. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell. Mol. Life Sci. 72:645–658. 10.1007/s00018-014-1763-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke A., Meier-Stiegen S., Drenckhahn D., and Asan E.. 2008. Molecular composition of tight and adherens junctions in the rat olfactory epithelium and fila. Histochem. Cell Biol. 130:339–361. 10.1007/s00418-008-0441-8 [DOI] [PubMed] [Google Scholar]
- Togashi H., Miyoshi J., Honda T., Sakisaka T., Takai Y., and Takeichi M.. 2006. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J. Cell Biol. 174:141–151. 10.1083/jcb.200601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H., Kominami K., Waseda M., Komura H., Miyoshi J., Takeichi M., and Takai Y.. 2011. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science. 333:1144–1147. 10.1126/science.1208467 [DOI] [PubMed] [Google Scholar]
- Zhadanov A.B., Provance D.W. Jr., Speer C.A., Coffin J.D., Goss D., Blixt J.A., Reichert C.M., and Mercer J.A.. 1999. Absence of the tight junctional protein AF-6 disrupts epithelial cell–cell junctions and cell polarity during mouse development. Curr. Biol. 9:880–888. 10.1016/S0960-9822(99)80392-3 [DOI] [PubMed] [Google Scholar]