Abstract
CaWRKY40 is a positive regulator of pepper (Capsicum annum) response to Ralstonia solanacearum inoculation (RSI), but the underlying mechanism remains largely unknown. Here, we functionally characterize CaCDPK15 in the defense signaling mediated by CaWRKY40. Pathogen-responsive TGA, W, and ERE boxes were identified in the CaCDPK15 promoter (pCaCDPK15), and pCaCDPK15-driven GUS expression was significantly enhanced in response to RSI and exogenously applied salicylic acid, methyl jasmonate, abscisic acid, and ethephon. Virus-induced gene silencing (VIGS) of CaCDPK15 significantly increased the susceptibility of pepper to RSI and downregulated the immunity-associated markers CaNPR1, CaPR1, and CaDEF1. By contrast, transient CaCDPK15 overexpression significantly activated hypersensitive response associated cell death, upregulated the immunity-associated marker genes, upregulated CaWRKY40 expression, and enriched CaWRKY40 at the promoters of its targets genes. Although CaCDPK15 failed to interact with CaWRKY40, the direct binding of CaWRKY40 to pCaCDPK15 was detected by chromatin immunoprecipitation, which was significantly potentiated by RSI in pepper plants. These combined results suggest that RSI in pepper induces CaCDPK15 and indirectly activates downstream CaWRKY40, which in turn potentiates CaCDPK15 expression. This positive-feedback loop would amplify defense signaling against RSI and efficiently activate strong plant immunity.
Exposure to biotic or abiotic stresses triggers transcription reprogramming of defense-associated gene expression and subsequent biochemical and physiological responses, which leads to plant adaptation1,2. Accumulating evidence indicates that defense-associated transcription reprogramming is tightly regulated by complex signaling networks, which begin with the perception and recognition of stress signals at the plasma membrane, and cumulate with the transcriptional modification of defense-associated gene expression in the nucleus2,3. Genetic modification of some signaling cascades within this transcriptional network can produce significant phenotypic effects4,5. Unraveling this network is one of the most important tasks in plant biology. However, the hierarchical organization, coordination, and fine-tuning of these signaling networks remain to be elucidated.
Calcium (Ca2+) is a ubiquitous second messenger involved in plant responses to abiotic and biotic stresses6. Stress triggers Ca2+ flux across the plasma membrane, leading to rapid increases in the cytoplasmic Ca2+ concentration, with stress-dependent variations in frequency, amplitude, and duration7,8. These changes in Ca2+ concentration are sensed and decoded by different Ca2+ sensors and/or Ca2+ -binding proteins, including calmodulins (CaMs), calmodulin-like proteins (CaMLs), calcineurin B-like proteins (CBLs), and calcium-dependent protein kinases (CDPKs), which subsequently activate different downstream defense responses5,6,9. CDPKs are characterized by an N-variable domain, a protein kinase domain, an autoinhibitory domain, and a CaM-like domain10. These unique features enable CDPKs to function as Ca2+ sensors and effectors, thereby allowing individual CDPK proteins to relay specific Ca2+ signatures to downstream components via CDPK-dependent protein phosphorylation. CDPKs are encoded by a large gene family, including 34 CDPK genes in Arabidopsis10, 31 in rice11, 40 in maize12, 20 in wheat13, 30 in Populus14, 41 in cotton15, and 35 in pepper. Many CDPK family members in Arabidopsis have been reported or predicted to be membrane-associated proteins16. A subset of CDPK genes exhibit inducible expression patterns; have pivotal roles in plant responses to abiotic stresses such as drought17,18,19, cold17,18,19, salinity18,20, heat shock21, dehydration19, and arsenic stress22; function in plant responses to biotic stresses such as pathogen infection23 and herbivore attack24; and have key roles in reactive oxygen species (ROS) signaling23. Currently, the functional identification of CDPKs has focused on a few family members or a few plant species. The roles and functional mechanisms of the majority of CDPK family members in diverse plant species remain to be elucidated.
WRKY proteins constitute one of the largest transcription factor (TF) families, characterized by their highly conserved WRKY domains and indiscriminate binding to their cogent W-box25. WRKY members in different plant species have been implicated in responses to abiotic and biotic stresses, including drought26,27, salt26, cold28, heat27, pathogen28 and virus29 infection, and herbivore attack30. The mechanism underlying WRKY TF-mediated defense responses appears to be very complex. A subset of WRKY genes are frequently induced by single stresses, and WRKY promoters are frequently enriched for W-boxes, which appear to be constitutively occupied by WRKY TFs31. This indicates that multiple WRKY TFs and transcriptional networks are involved in plant stress responses32. Alternatively, one WRKY TF may respond to multiple stresses, which would function as a crucial node in the crosstalk between responses to biotic and abiotic stresses, or responses to different stresses. A WRKY TF might have specific patterns of expression and function in specific contexts, although the underlying mechanisms are unknown. The binding of WRKY TFs to their specific W-box targets is affected by the adjacent DNA sequences outside of the TTGACY-core motif33 and the W-box consensus is degenerate; the majority of analyzed WRKY proteins recognize the TTGACC/T W-box sequences. Therefore, other components are believed to be required by WRKY TFs to mediate stimulus-specific responses33,34. WRKY TFs have protein-protein interactions with MAPKs, CDPKs, 14-3-3 proteins, VQ domain-containing proteins, and NAC (NAM/ATAF/CUC) TFs, which modify WRKY TF activities and modulate important biological processes34,35. However, the molecular linkages between nuclear-localized WRKY TFs and their interactions with upstream cytoplasmic signaling factors remain to be elucidated.
Pepper (Capsicum annuum) is one of the most important vegetables worldwide. Pepper is a typical Solanaceae and is susceptible to various soil-borne diseases such as Ralstonia solanacearum inoculation (RSI) and Phytophthora capsici, which is aggravated by high temperature and high humidity. These diseases cause heavy losses in crop yield and quality. Pepper germplasm evolved under these stresses, and may have developed unique mechanisms for disease tolerance and/or resistance, and tolerance to high temperature and high humidity. Our previous study reported that CaWRKY40 functioned as positive regulator in pepper’s response to Pseudomonas solanacearum attack and heat stress under high humidity36, and CaWRKY40 was transcriptionally modulated by CaWRKY6, another member in the WRKY TF family in pepper37. In the present study, the pepper CDPK protein designated as CaCDPK15 was found to regulate pepper response to Pseudomonas solanacearum attack by indirectly activating CaWRKY40 expression, and CaWRKY40 in turn activated transcriptional expression of CaCDPK15 by binding to the CaCDPK15 promoter, thereby establishing a positive-feedback loop.
Results
pCaCDPK15-driven GUS expression was upregulated by RSI and exogenously applied signaling mediators in pepper plants
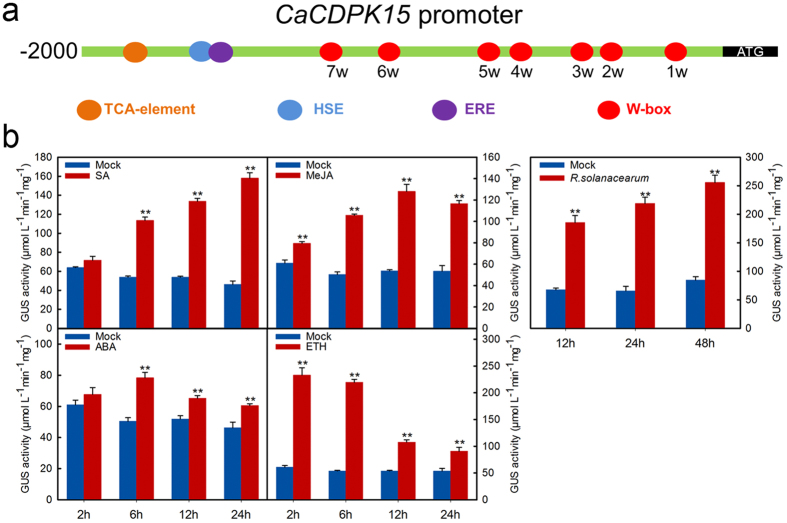
Our previous study performed a genome-wide identification of pepper CDPK members. A total of 35 CDPK genes were identified in the genome of the pepper variety CM334 using genome sequence data38 (DOI:10.3389/fpls.2015.00737). The CaCDPK15 gene contained an N-variable domain, a protein kinase domain, an autoinhibitory domain, and a CaM-like domain, which are unique features of CDPK proteins10 (Supplementary Fig. S1). CaCDPK15 exhibited inducible transcriptional expression in response to RSI (DOI:10.3389/fpls.2015.00737), suggesting a possible role in pepper immunity to R. solanacearum. In the present study, the CaCDPK15 promoter (pCaCDPK15) was identified, along with the cis-elements within the promoter (1 TCA element, 1 HSE, 1 ERE, and 7 W-boxes) (Fig. 1a). A pCaCDPK15-driven GUS reporter was expressed in pepper leaves by agroinfiltration, and GUS expression in pepper leaves was measured in response to RSI and exogenous application of salicylic acid (SA), methyl jasmonate (MeJA), abscisic acid (ABA), and ethephon (ETH). The results showed that GUS expression was upregulated by RSI, SA, MeJA, ABA, and ETH, with different temporal expression patterns (Fig. 1b).
Figure 1. The expression of pCaCDPK15-driven GUS was induced by hormones and RSI.
(a) The main cis-elements including one TCA-element, one HSE, one ERE and seven W-boxes in pCaCDPK15. (b) The pCaCDPK15 driven GUS expression was promoted by exogenous application of SA, MeJA, ETH and ABA and RSI. The leaves of pepper plants at eighty-leaf stage ware infiltrated with GV3101 cells (OD600 = 0.6) containing pCabZIP63:GUS, and 24 hours later the plants were treated with 1 mm SA, 100 μm MeJA, 100 μm ETH, 100 μm ABA, or inoculated with the R. solanacearum (OD600 = 0.6). The leaves were harvested at different time points for GUS activity assay by microplate reader using pepper leaves treated with ddH2O as mock. Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with Mock (treated with ddH2O). (t-test, **P < 0.01).
CaCDPK15 is localized to the plasma membrane and nucleus
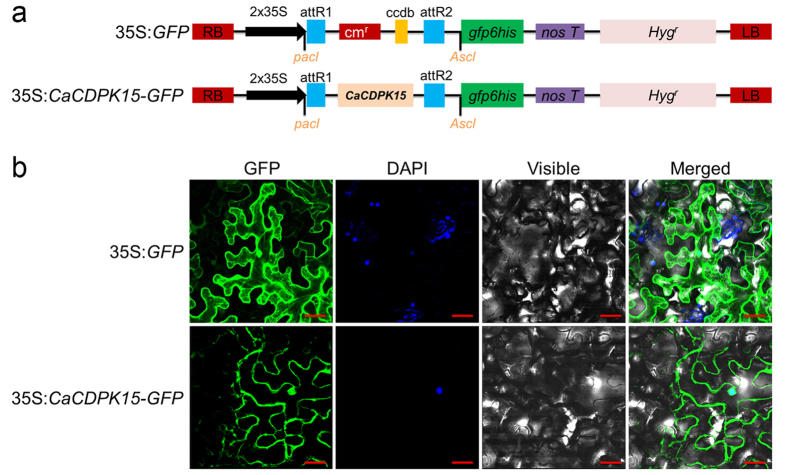
The subcellular localization of a protein can determine or influence its function. To determine the subcellular localization of CaCDPK15, we generated a CaCDPK15-GFP fusion construct driven by the constitutive CaMV35S promoter, and expressed the construct in Nicotiana benthamiana leaves by agroinfiltration. The subcellular locations of CaCDPK15-GFP and GFP control were visualized with laser scanning confocal microscopy. The results revealed that CaCDPK15-GFP was localized in both the plasma membrane and the nucleus, whereas the GFP control was localized in multiple subcellular compartments including the cytoplasm and the nucleus (Fig. 2).
Figure 2. Subcellular location of CaCDPK15.
(a) Schematic representations of the vector constructs of 35S:GFP and 35S:CaCDPK15-GFP. (b) GFP fusion protein of CaCDPK15 was localized in the cytomembrane and nucleus of N. benthamiana cells. The plant nuclei were stained with DAPI. Images were taken by using Leica confocal microscopy at 48 hours post agroinfiltration (GFP fluorescence, green; DAPI fluorescence, blue; visible, visible light image; merged, merged images of above three images). Bars = 200 μm.
Effect of CaCDPK15 silencing on pepper resistance to RSI
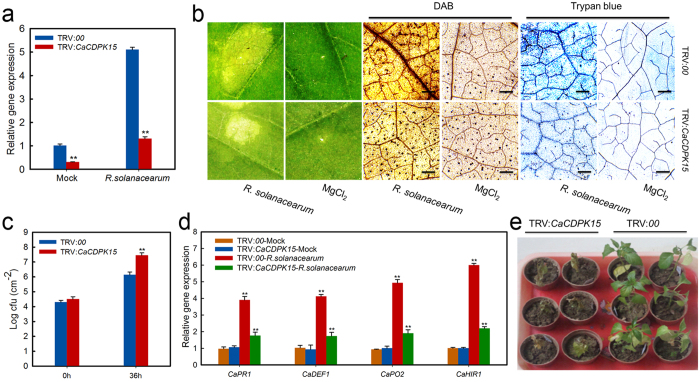
To test the role of CaCDPK15 in pepper immunity, we evaluated CaCDPK15 loss-of-function in pepper seedlings by performing virus-induced gene silencing (VIGS). The vectors TRV1 (PYL192) and TRV2:CaCDPK15 (PYL279) were separately transformed separately into Agrobacterium tumefaciens GV3101, and the two resulting GV3101 strains were mixed and co-infiltrated into pepper seedling leaves. The infiltrated seedlings were incubated at 16 °C for 56 h without light, and then were kept at 25 °C. Six independent experiments were performed, and we obtained approximately 100 plants of TRV:00 and 100 plants of TRV:CaCDPK15. A TRV:PDS control construct was used in the same way to monitor gene silencing by the resulting photobleaching phenotype36. Six plants were randomly selected to check the gene silencing efficiency. In TRV:CaCDPK15 pepper plants, CaCDPK15 transcript levels were reduced to ~30% of those in TRV:00 plants (Fig. 3a). The R. solanacearum strain FJC100301 was used to inoculate six individual TRV:CaCDPK15 plants and six individual TRV:00 empty vector control plants. We stained R. solanacearum-infected CaCDPK15-silenced and control leaves with DAB (indicator of H2O2 accumulation) and trypan blue (indicator of cell death or necrosis). Strongly polymerized DAB (dark brown) and hypersensitive response (HR)-mimicking cell death were detected in control leaves at 48 h post inoculation (hpi), whereas the intensities of DAB and trypan blue staining were distinctly reduced in CaCDPK15-silenced leaves (Fig. 3b). Our data also showed that R. solanacearum growth was significantly increased in CaCDPK15-silenced pepper plants, manifested by higher colony-forming units (cfu) compared with that in control plants (Fig. 3c). The expression of known pepper defense genes involved in the response to pathogen infection was analyzed by quantitative real-time PCR (qPCR) analysis. The results showed that transcript levels of the defense-related pepper genes CaPR139, CaDEF140, CaPO241, and CaHIR142 were lower in CaCDPK15-silenced leaves than in leaves of control pepper plants at 24 hpi (Fig. 3d). At 14 days post inoculation (dpi), we observed definite wilting symptoms on TRV:CaCDPK15 pepper leaves, but the TRV:00 empty-vector control leaves exhibited only faint wilting symptoms (Fig. 3e).
Figure 3. Enhanced susceptivity of CaCDPK15-silenced pepper plants to R. solanacearum inoculation.
(a) Relative transcriptional expression of CaCDPK15 in CaCDPK15-silenced pepper plants by real-time RT-PCR 24 h after R. solanacearum inoculation or the MgCl2 treatment (Mock). (b) Trypan blue staining and DAB staining in R. solanacearum inoculated CaCDPK15-silenced (TRV:CaCDPK15) and empty vector (TRV:00) pepper leaves at 2 days post inoculation. Bars = 50 μm. (c) Detection of R. solanacearum growth in CaCDPK15-silenced or the control pepper plants inoculated by R. solanacearum at 36 hours post inoculation. (d) Relative transcriptional expression of the defense marker genes by real-time RT-PCR in CaCDPK15-silenced pepper plants inoculated by R. solanacearum and the mock at 24 hpi. (e) Susceptivity analysis of CaCDPK15-silenced (TRV:CaCDPK15) and empty vector (TRV:00) pepper plants to R. solanacearum inoculation at 10 days post inoculation. CaPR1, pepper basic PR-1; CaPO2, peroxidase; CaDEF1, defensin; CaHIR1, hypersensitive induced reaction protein. Expression values are normalized by the expression levels of CaACTIN and 18s rRNA. (a,c,d) Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with TRV:00 and the treatment of MgCl2 (Mock). (t-test, **P < 0.01).
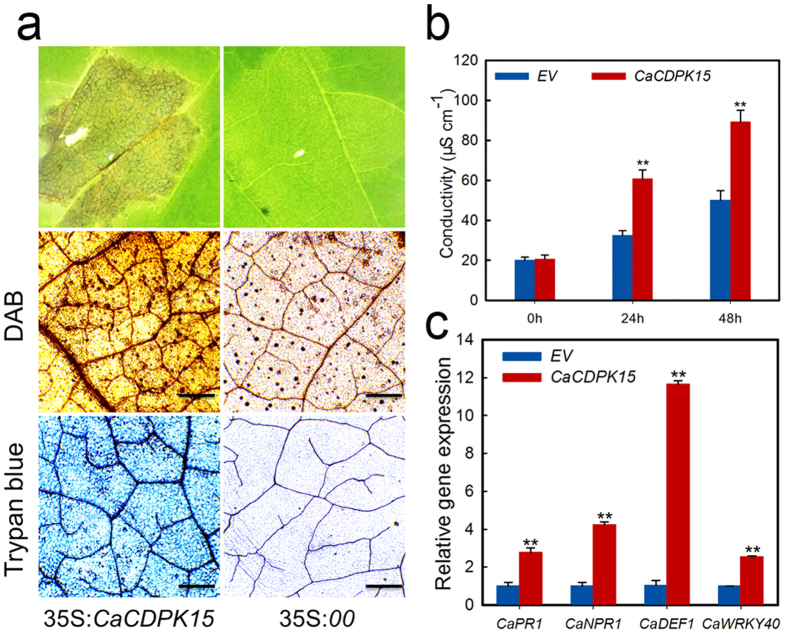
Transient CaCDPK15 expression induces the hypersensitive response, cell death, and H2O2 accumulation in pepper leaves
We attempted to generate transgenic CaCDPK15-overexpressing tobacco plants, but found that CaCDPK15 overexpression was lethal in transgenic tobacco. Therefore, a transient overexpression system for CaCDPK15 was generated by agroinfiltration of 35S:CaCDPK15 or 35S:00 (empty vector) into pepper leaves (Fig. 4a). HR-mediated cell death and H2O2 accumulation were assessed by staining with trypan blue to identify necrotic cells and DAB, respectively. The 35S:CDPK15 construct distinctly induced a necrotic response in pepper leaves and H2O2 accumulation, whereas the empty-vector control did not induce a necrotic response and resulted in only weak DAB staining. We also performed an ion leakage test to analyze the severity of plasma membrane damage and thereby the severity of cell necrosis in cells transiently expressing CaCDPK15. Pepper leaves transiently overexpressing CaCDPK15 exhibited more ion leakage at 24 and 48 h after agroinfiltration than that in leaves expressing the empty vector control (Fig. 4b). Real-time RT-PCR analysis of CaCDPK15 transcripts in the transient expression system showed that transcripts were higher in leaves expressing 35 S:CaCDPK15 than in empty-vector control leaves (Fig. 4c). We also examined changes in the expression of defense-related genes including SA-responsive CaPR1 and CaNPR143, JA-responsive CaDEF1, and CaWRKY40 in the transient expression system. The results showed that the relative transcript levels of CaPR1, CaNPR1, CaDEF1, and CaWRKY40 increased continuously during transient expression of CaCDPK15.
Figure 4. HR cell death and immunity was significantly triggered by transient overexpression of CaCDPK15 in pepper leaves.
(a) Cell death was found in pepper leaves at 4 days after infiltration with Agrobacterium GV3101 cells carrying 35S:CaCDPK15, GV3101 cells carrying an empty vector (35S:00) was used as a control. Trypan blue staining and DAB staining of pepper leaves transiently overexpressing 35S:CaCDPK15 or 35S:00 at 4 days post agro-infiltration. Bars = 50 μm. (b) Electrolyte leakages of leaf discs of pepper leaves infiltrated with GV3101 cells carrying 35S:CaCDPK15 or 35S:00 at 0, 24 or 48 hpi. (c) Relative expression of immunity associated genes and CaWRKY40 in the pepper leaves transiently overexpressing 35S:CaCDPK15 or empty vector at 24 hpi. CaNPR1, non-expresser of pathogenesis-related gene. Expression values are normalized by the expression levels of CaACTIN and 18s rRNA. (b,c) Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with 35S:00. (t-test, **P < 0.01).
The effect of transient overexpression of CaCDPK15 on the binding of CaWRKY40 to its target genes
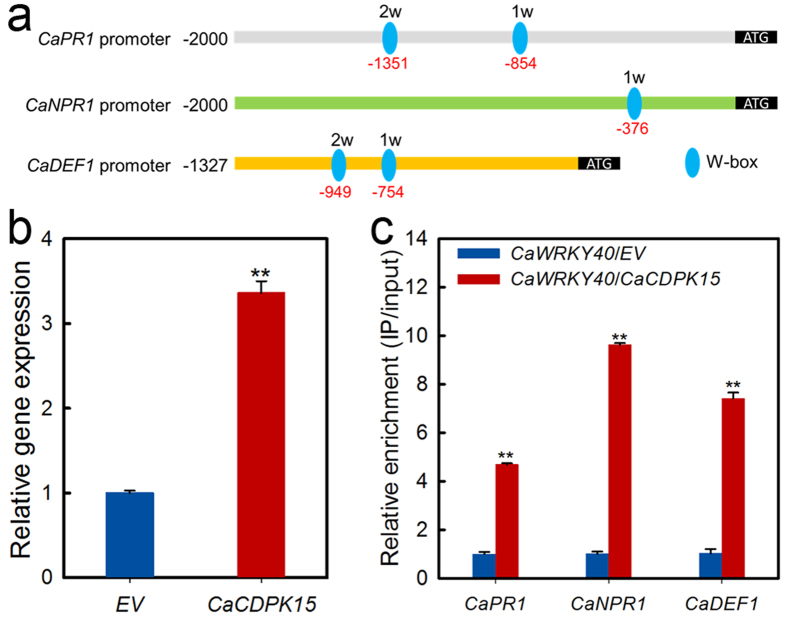
CaCDPK15 may modify CaWRKY40 transcriptional activity by altering its binding to the promoters of target genes. We tested this hypothesis by performing chromatin immunoprecipitation (ChIP) experiments. A specific primer pair was designed based on the flanking sequences of each typical W-box in the promoters of CaPR1, CaNPR1, and CaDEF1 (Fig. 5a). For promoters with more than one W-box, the primer pairs were screened for product amplification and used in the real-time RT-PCR measurements of specific CaWRKY40 binding to the promoter. The results showed that CaWRKY40 binding to the promoters of CaPR1, CaNPR1, and CaDEF1 was significantly enhanced by transient CaCDPK15 overexpression (Fig. 5b,c).
Figure 5. The transcriptional expression of CaWRKY40 and the bindings of CaWRKY40 to the promoters of its target genes were enhanced by transient expression of CaCDPK15.
(a) Schematic representation of the typical W-boxes in the promoters of the target genes of CaWRKY40. (b) The transcript level of CaCDPK15 by transient overexpression itself in pepper leaves. GV3101 cells containing the construct of 35S:CaCDPK15 was infiltrated into pepper leaves, which were harvest at 24 hpi to isolate the total RNA for transcriptional expressional assay of CaCDPK15 by real-time RT-PCR. Expression values are normalized by the expression levels of CaACTIN and 18s rRNA. (c) The bindings of CaWRKY40 to the promoters of its target genes were potentiated by transient expression of 35S:CaCDPK15 in pepper plants. The GV3101 cells carrying the construct of 35S:CaWRKY40-HA and that containing 35S:CaCDPK15-Flag were mixed at a ratio of 1:1 and were co-infiltrated into pepper leaves, with GV3101 cells containing 35S:00 as mock. The leaves were harvested at 48 hpi for chromatin preparation, the isolated chromatins were digested with micrococcal nuclease and the acquired DNA collections with 300–500 bp in length were used as templates for real-time RT-PCR to assay the bindings of CaWRKY40 to the promoters of its target genes, for each target gene of CaWRKY40, a specific primer pair flanking each typical W-box was designed and the one (primer pair based on 1 W in CaPR1 promoter, 1 W in CaNPR1 promoter and 2 W in CaDEF1 promoter, respectively) that amplified product was used in the real-time RT-PCR analysis. Relative enrichment levels of samples of the CaWRKY40 transient overexpression were set to 1 after normalization by input. (a,b) Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with 35S:00 (EV) and 35S:CaWRKY40/35S:00 (EV). (t-test, **P < 0.01).
Detection of potential interactions between CaCDPK15 and CaWRKY40 by co-immunoprecipitation analysis
If CaCDPK15 acts as an upstream modifier of CaWRKY40 signaling, one possibility might be that CaWRKY40 is a target of CaCDPK15. We tested this hypothesis by performing co-immunoprecipitation (co-IP) analyses to evaluate possible interactions between the two proteins. These experiments employed a transient coexpression system in N. benthamiana leaves with the tagged constructs 35S:CaCDPK15-HA and 35S:CaWRKY40-Flag, and the positive control constructs 35S:CaPIK1-Flag and 35S:CaSGT1-HA44. The results showed that CaWRKY40 does not interact with CaCDPK15, indicating that CaWRKY40 is not a direct target of CaCDPK15 (Supplementary Fig. S2).
Effect of transient CaWRKY40 expression on CaCDPK15 transcriptional expression
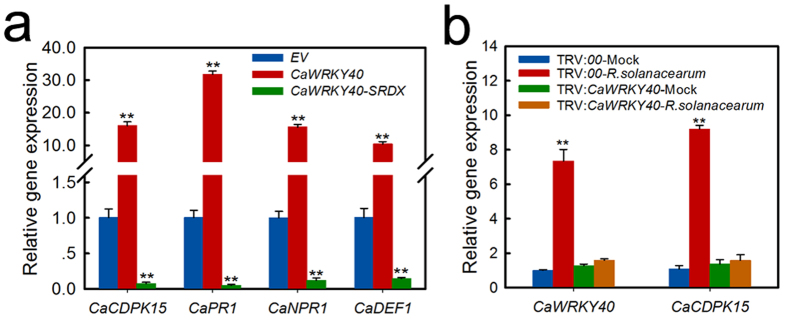
More than 7 W-boxes were identified in the CaCDPK15 promoter, suggesting that CaWRKY40 might act as a regulator of CaCDPK15. To test this hypothesis, the transcriptional expression of CaCDPK15 was determined in pepper leaves transiently overexpressing CaWRKY40 or its repressor version CaWRKY40-SRDX by performing real-time RT-PCR analysis. The results showed that transient CaWRKY40 overexpression significantly activated the expression of CaCDPK15 and the immunity-associated marker genes CaNPR1, CaPR1, and CaDEF1. By contrast, the expression of CaCDPK15, CaNPR1, CaPR1, and CaDEF1 was significantly reduced by CaWRKY40-SRDX overexpression (Fig. 6a). Consistently, VIGS-mediated CaWRKY40 silencing in pepper plants significantly downregulated CaCDPK15 transcriptional expression in response to RSI (Fig. 6b).
Figure 6. The transcriptional expression of CaCDPK15 was upregulated by transient overexpression of CaWRKY40 but downregulated by CaWRKY40-SRDX.
(a) The effect of transient overexpression of CaWRKY40 and CaWRKY40-SRDX on the transcript level of CaCDPK15 and immunity associated marker genes CaPR1, CaNPR1 and CaDEF1 in pepper leaves at 24 hpi. (b) The transcriptional expression of CaCDPK15 was downregulated significantly in CaWRKY40-silenced pepper plants inoculated by R. solanacearum after 24 hours. Expression values are normalized by the expression levels of CaACTIN and 18s rRNA. (a,b) Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with EV ([a]) and the treatment of MgCl2 (Mock, [b]). (t-test, **P < 0.01).
ChIP analysis of CaWRKY40 binding to the CaCDPK15 promoter
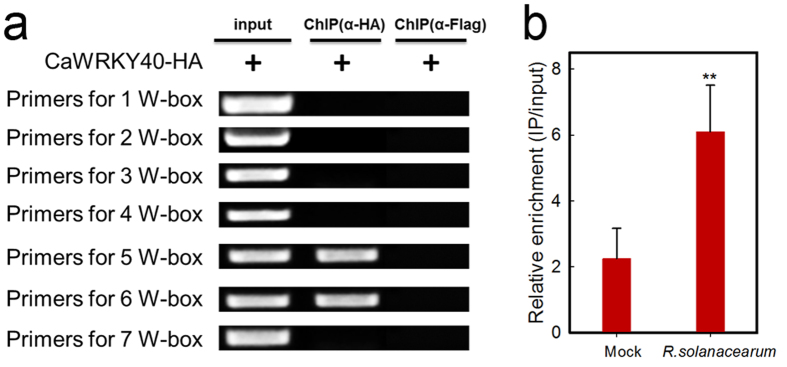
As CaWRKY40 significantly activated CaCDPK15 expression, we speculated that CaWRKY40 might act as a TF in directly modulating CaCDPK15 expression. We tested this hypothesis by performing ChIP to determine if CaWRKY40 binds to the CaCDPK15 promoter. For this experiment, GV3101 cells containing the p35S:CaWRKY40-HA construct or the empty vector were infiltrated into pepper (GZ03) leaves, which were harvested at 48 hpi for chromatin isolation. The isolated chromatin was randomly sheared into fragments with lengths of 300−500 base pairs, and chromatin fragments that bound to CaWRKY40 were immunoprecipitated using the HA antibody. The resulting DNA fragments were isolated and used as templates for PCR analysis with specific primer pairs. The results showed that only the primer pairs flanking the fifth and sixth W-boxes produced amplified products, suggesting that CaWRKY40 directly binds to the CaCDPK15 promoter (Fig. 7a). To test the effect of RSI on CaWRKY40 binding to the CaCDPK15 promoter, ChIP analysis was performed during RSI. The real-time RT-PCR results showed that the infected pepper leaves had higher enrichment of CaWKRY40 in the CaCDPK15 promoter compared with that of the mock control (Fig. 7b).
Figure 7. The binding of CaWRKY40 to the promoter of CaCDPK15 by Chromatin immunoprecipitation (ChIP).
(a) Binding of CaWRKY40 to the promoter of CaCDPK15 by ChIP with different pairs of primers according to flanking sequences of different W-boxes. GV3101 cells containing the construct of 35S:CaWRKY40-HA was inoculated to the pepper leaves, which are harvested at 48 hpi for preparation of chromatin for ChIP assay, the immunoprecipitated DNA was used as template for PCR with specific primer pairs designed according to the seven W-boxes. Lanes 1, input (total DNA-protein complex); lanes 2, (DNA-protein complex) immunoprecipitated with anti-HA antibody (α-HA), the anti-Flag antibody (α-Flag) was used as a negative control to discriminate the possible unspecific IP in HA-IgG. (b) The binding of CaWRKY40 to the promoter of CaCDPK15 was enhanced by RSI. GV3101 cells containing the construct of 35S:CaWRKY40-HA was inoculated to the pepper leaves, 24 hours later, the leaves were further inoculated with R. solanacearum, 24 hours later, the leaves were harvested for preparation of chromatin for ChIP assay, and a specific primer pair was used in the real-time RT-PCR. Data are the means ± SD from at least three independent experiments. Asterisks indicate statistically significant differences compared with the treatment of MgCl2 (Mock, [b]). (t-test, **P < 0.01).
Discussion
Although CDPKs and WRKYs have both been implicated in pathogen attack45, the molecular linkage between these two proteins has not been established. We provide strong evidence that CaCDPK15 forms a positive-feedback loop with CaWRKY40 during RSI in pepper, and previously established that CaWRKY40 is a positive regulator of pepper’s response to RSI36.
Accumulating evidence indicates that upregulated genes responding to plant defense signaling can have important roles in disease resistance46. The CaCDPK15 promoter contains the following cis-elements: 1 SA-responsive TCA element, 1 ethylene-responsive ERE box, and 7W-boxes. These cis elements are frequently involved in plant immunity responses47. Transgenic tobacco expressing pCaCDPK15:GUS consistently exhibited significantly inducible GUS expression in response to RSI, suggesting that CaCDPK15 might act as a positive regulator in pepper’s response to RSI. This possibility was confirmed by loss-of-function experiments, which showed that CaCDPK15 silencing significantly reduced pepper resistance to RSI, and significantly down-regulated the expression of the immunity marker genes CaNPR1, CaPR1, and CaDEF1. By contrast, transient CaCDPK15 overexpression in pepper plants triggered HR-mimicking cell death, enhanced electrolyte leakage, and enhanced accumulation of H2O2. Ca2+ is a ubiquitous signal in plant defense responses to biotic and abiotic stresses48,49, and CDPKs are one of the Ca2+ sensors that relay Ca2+ signatures to downstream components via protein phosphorylation and transcriptional reprogramming45. These combined results suggest that CaCDPK15 acts as a positive regulator of pepper’s response to RSI.
Our previous work showed that CaWRKY40 was upregulated in response to RSI and high temperature/high humidity, and functioned as a positive regulator in pepper’s response to these two stresses36. CaWRKY40 and CaCDPK15 have similar expression patterns and functions in pepper’s response to RSI, which suggests a close relationship between these two genes and their encoded proteins. This was corroborated by data showing that the transcriptional expression of CaWRKY40 was upregulated by transient CaCDPK15 overexpression in pepper plants, and was significantly down-regulated by VIGS-mediated CaCDPK15 silencing. Ca2+ influx occurs very early during stress challenge. CDPK proteins localized in the plasma membrane and/or cytoplasm are expected to be involved in early signaling pathways that respond to biotic and abiotic stresses49. CaCDPK15 is believed to act as an upstream regulator of CaWRKY40. Similar molecular linkages between CDPKs and WRKYs have been reported previously50,51. For example, the overexpression of wheat (Triticum aestivum) TaCPK2-A in rice (Oryza sativa) promoted OsWRKY45-1 expression, which is a TF involved in resistance to fungi and bacteria, by regulating genes involved in JA and SA signaling50. In Arabidopsis, the CDPK4/5/6/11 isoforms phosphorylate a specific subgroup of WRKY TFs (WRKY8/28/48) that regulate crucial transcriptional reprogramming that restricts pathogen growth51. However, unlike these CDPK and WRKY proteins, this study provided evidence that CaCDPK15 and CaWRKY40 do not directly interact with each other, and CaCDPK15 does not phosphorylate CaWRKY40; co-IP detected no interaction between CaCDPK15 and CaWRKY40, and CaCDPK15 localized to nuclei. Therefore, CaCDPK15 might phosphorylate and activate other TFs that target CaWRKY40. Further identification of possible CaCDPK15 interactors that subsequently target CaWRKY40 might provide new insights into the mechanism of pepper immunity mediated by CaCDPK15 and CaWRKY40.
We showed that CaCDPK15 modulates CaWRKY40 expression. Unexpectedly, we found that CaCDPK15 expression in pepper plants was transcriptionally upregulated by transient CaWRKY40 overexpression, whereas it was downregulated by transient overexpression of CaWRKY40-SRDX (repressor) and by CaWRKY40 silencing. This suggests that there is a positive-feedback loop between CaCDPK15 and CaWRKY40. The presence of 7W-boxes in the CaCDPK15 promoter indicated that WRKY TFs might directly transcriptionally regulate CaCDPK15 expression. Our ChIP analysis data revealed that CaWRKY40 binds to the CaCDPK15 promoter, which was significantly enhanced by RSI. These results strongly suggest that CaWRKY40 acts as a direct TF in the transcriptional modulation of CaCDPK15 expression. Similar positive-feedback loops in plant responses to stresses including pathogen attack have been reported52,53,54. For example, SA was reported to act in a positive-feedback loop with ACCELERATED CELL DEATH6 (ACD6) to potentiate plant responsiveness to pathogen-associated molecular patterns (PAMPs)52; HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN form a positive-feedback loop that modulates long-term acquired thermotolerance53; and positive-feedback regulation by ABA on LOS6/ABA1 expression provides a quick adaptation strategy for plants under osmotic stress54. In general, plant defense systems tend to focus on early stress-mediated events, and these positive-feedback loops may be important for amplifying defense signaling55. Our data also showed that exogenous application of SA, MeJA, ABA, and ETH synergistically upregulated CaCDPK15 expression, which is consistent with their effects on CaWRKY40 expression36. These results strongly suggest a molecular linkage between CaCDPK15 and CaWRKY40.
In our previous study, CaWRKY40 positively regulated pepper response to RSI and plant thermotolerance under high humidity, which is important for plant adaption to conditions that promote the invasion and growth of soil-borne pathogens. Although the present study focused on the role of CaCDPK15 in pepper immunity, there were indications that CaCDPK15 might be involved in thermotolerance under high humidity. For example, we identified an HSE element in the CaCDPK15 promoter, determined that pCaCDPK15-driven GUS expression also was activated by heat stress treatment. and found that CaCDPK15 silencing impaired plant thermotolerance and downregulated the expression of the thermotolerance-associated marker gene CaHSP24. CaHSP24 expression was consistently upregulated by CaCDPK15 expression (data not shown).
Collectively, our data indicate that CaCDPK15 expression is upregulated by RSI, which indirectly activates the transcriptional expression of downstream CaWRKY40. Likewise, transcriptional expression of CaWRKY40 directly activates the transcriptional expression of CaCDPK15. This generates a positive-feedback loop that would rapidly amplify plant signaling in response to RSI and efficiently activate plant defense responses.
Methods
Plant materials and growth conditions
Seeds of the pepper (Capsicum annuum) cultivar GZ03 and Nicotiana benthamiana were provided by the pepper breeding group at Fujian Agriculture and Forestry University. The seeds were sown in a soil mix [peat moss:perlite, 2:1 (v/v)] in plastic pots, and were placed in a greenhouse at 25 °C, 60–70 μmol photons m−2 s−1, 70% relative humidity, and a 16-h light/8-h dark photoperiod.
Pathogens and inoculation procedures
R. solanacearum strain FJC100301 was isolated previously in our lab and amplified according to the method of Dang et al.36. The bacterial cell culture was diluted to 108 cfu ml−1 (OD600 = 0.8) with 10 mM MgCl2. Pepper plants were inoculated by infiltrating 10 ml of the resulting R. solanacearum suspension into the third leaves from the apical meristem using a syringe with a needle. The leaves were harvested at the indicated time points for the analysis of GUS activity, ChIP, or RNA.
Treatment of plants with exogenous phytohormones and RSI
Pepper plants at the four-leaf stage were sprayed with 1 mM salicylic acid, 100 μM methyl jasmonate, 100 μM abscisic acid, or 100 μM ethephon. Mock-treated plants were sprayed with a corresponding solvent or sterile ddH2O. To study the relative CaCDPK15 transcript levels in response to R. solanacearum infection, pepper plants were inoculated at the eight-leaf stage by injecting 10 ml of bacterial suspension (108 cfu ml−1) or distilled sterile 10 mM MgCl2, and then harvested at different time points.
Analysis of CaCDPK15 subcellular localization
The full-length cDNA of CaCDPK15 was cloned into the plant expression vector pMDC83 downstream of the two CaMV35S promoters and in-frame with GFP using the Gateway cloning system (Invitrogen). The 35S:CaCDPK15-GFP and 35S:GFP (used as a control) constructs were transformed into Agrobacterium tumefaciens strain GV3101, which was cultured in induction medium (10 mM ethanesulfonic acid, pH 5.4, 10 mM MgCl2, and 200 mM acetosyringone), harvested when the OD600 was approximately 1.0, and diluted to OD600 = 0.8. Bacterial suspensions expressing p35S:CaCDPK15-GFP and p35S:GFP were injected into Nicotiana benthamiana leaves using a syringe without a needle. GFP fluorescence was imaged using laser confocal fluorescence microscopy (Leica TCS SP8) with an excitation wavelength of 488 nm and a 505–530 nm band-pass emission filter.
Histochemical staining
Staining with trypan blue and DAB was performed according to the previously published method of Choi et al.56. For trypan blue staining, pepper leaves were boiled in trypan blue staining solution for 2 min, left at room temperature for 8 h, transferred into a chloral hydrate solution (2.5 g of chloral hydrate dissolved in 1 mL of distilled water), and boiled for 20 min to destain. After multiple changes of chloral hydrate solution to reduce the background, samples were mounted in 70% glycerol. For DAB staining, the leaves were stained overnight in 1 mg ml−1 DAB. The stained leaves were cleared by boiling in lactic acid:glycerol:absolute ethanol [1:1:3 (v/v/v)], and then destained overnight in absolute ethanol. Representative images of DAB and trypan blue staining were photographed with a light microscope (Leica, Wetzlar, Germany).
Virus-induced gene silencing (VIGS) of CaCDPK15 in pepper plants
The tobacco rattle virus (TRV)-based VIGS system was employed to silence CaCDPK15. The PYL192 and PYL279 VIGS vectors were described previously57. A fragment of the transcribed region of CaCDPK15 was amplified using gene-specific primers (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCTTTTCTTTTCGC CCTTTA-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCAATGAACT CCATCCAGCA-3′), and cloned into the PYL279 VIGS vector using the Gateway cloning system (Invitrogen). The PYL192 and PYL279 vectors were with or without CaCDPK15, respectively. PYL279 contained a 250- or 500-bp PDS fragment. The PYL192 and PYL279 vectors were transformed into A. tumefaciens strain GV3101. Agrobacterium harboring PYL192 with PYL279 (PYL192 vector with PYL279 as TRV:00), PYL279-CaCDPK15 (PYL192 with PYL279-CaCDPK15 as TRV:CaCDPK15), or PYL279-PDS (OD600 = 1.0) were mixed at a 1:1 ratio, and the mixture was infiltrated into cotyledons of 2-week-old pepper plants using a 1 ml sterile syringe without a needle. The Agrobacterium-inoculated pepper plants were grown for 2–3 weeks in a growth chamber at 16 °C (in darkness for the first 56 h) with 45% relative humidity, and then transferred into a growth room at 25 ± 2 °C, 60–70 μmol photons m−2 s−1, 70% relative humidity, and a 16-h light/8-h dark photoperiod.
Transient expression assay of CaCDPK15
Agrobacterium tumefaciens strain GV3101 harboring the pK7WG2-CaCDPK15 vector was cultured to OD600 = 1.0 in induction medium (10 mM ethanesulfonic acid, pH 5.7, 10 mM MgCl2, and 200 mM acetosyringone) and diluted to OD600 = 0.8. The diluted culture was injected into pepper or Nicotiana benthamiana leaves using a syringe without a needle. The plants were kept in a growth room for 2 days, and then the injected leaves were harvested for further use.
Co-immunoprecipitation assay
To construct vectors for co-IP analysis, CaCDPK15 or CaWRKY40 in the pDONR vector was directly introduced into the destination vectors p35S:HA (pEarleyGate 201) and p35S:Flag (pEarleyGate 202) to generate p35S:CaCDPK15-HA and p35S:CaWRKY40-Flag by the LR reaction (Invitrogen). Plasmids were transformed into Agrobacterium strain GV3101, and cells harboring p35S:CaCDPK15-HA and p35S:CaWRKY40-Flag were simultaneously infiltrated into leaves of N. benthamiana plants. Leaves were harvested at 48 h after infiltration (hai), and total proteins were extracted using protein extraction buffer [10% glycerol, 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 2% Triton X-100, 10 mM DTT, 1× complete protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), and 2% (w/v) polyvinylpolypyrrolidone]58. Extracted proteins were incubated with monoclonal anti-HA magnetic beads at 4 °C overnight. Beads were then collected with a magnet and washed three times with protein extraction buffer. Eluted proteins were immunoblotted using anti-HA-peroxidase antibody.
Quantitative real-time PCR
To determine the relative transcript levels of selected genes, real-time PCR was performed with specific primers (Supplementary Table S1 and Table S2) according to the manufacturer’s instructions for the BIO-RAD Real-time PCR system (Foster City, CA, USA) and the SYBR Premix Ex Taq II system (TaKaRa Perfect Real Time). Total RNA was isolated from pepper plants using TRIzol reagent (Invitrogen), and was reverse-transcribed using the PrimeScript RT-PCR kit (TaKaRa, Dalian, China)59. A 10-fold dilution of the resulting cDNA was amplified using the SYBR Premix Ex Taq II kit and the BIO-RAD Real-time PCR system in a 10 μl volume with the following program: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 34 s; and 95 °C for 15 s. Amplification of the target genes was monitored every cycle by SYBR green fluorescence. The Ct (threshold cycle) value, which is defined as the real-time PCR cycle at which a statistically significant increase of reporter fluorescence was first detected, was used as a measure for the starting target gene copy number. Three replicates of each experiment were performed. Data were analyzed by the Livak method and expressed as a normalized relative expression level (2−ΔΔCT) of the respective genes. The relative transcript levels were normalized with respect to the transcript levels of CaActin and 18 s rRNA. In each case, three technical replicates were performed for at least three independent biological replicates.
Chromatin immunoprecipitation analysis
The 35S:CaWRKY40-HA and 35S:CaCDPK15-Flag constructs were generated by Gateway cloning (Invitrogen)60, and were transformed into Agrobacterium strain GV3101. GV3101 cells containing 35S:CaWRKY40-HA and 35S:CaCDPK15-Flag were co-infiltrated at a ratio of 1:1 or infiltrated individually into pepper leaves, which were harvested at 48 hpi for chromatin preparation. ChIP was performed according to standard protocols. Briefly, approximately 2 g of pepper leaves was treated with either 10 mM bithionol sulfoxide or DMSO (solvent control) for 16 h and subsequently fixed with 1.0% formaldehyde for 5 min. Antibody against HA or FLAG (Santa Cruz Biotechnology) were used for immunoprecipitation. Protein-A-agarose beads were blocked with salmon sperm DNA and used to pull down the protein-DNA complex. Equal amounts of starting plant material and the ChIP products were used for PCR or real-time PCR with specific primers for the promoters of CaCDPK15, CaPR1, CaNPR1, and CaDEF1 (Supplementary Table S3).
Additional Information
How to cite this article: Shen, L. et al. CaCDPK15 positively regulates pepper responses to Ralstonia solanacearum inoculation and forms a positive-feedback loop with CaWRKY40 to amplify defense signaling. Sci. Rep. 6, 22439; doi: 10.1038/srep22439 (2016).
Supplementary Material
Acknowledgments
We thank Mark D. Curtis for kindly providing the Gateway destination vectors, Bernd Weisshaar for pBT10-GUS, and Dr. S. P. Dinesh-Kumar (Yale University) for the pTRV1 and pTRV2 vectors. This work was supported by grants from the National Natural Science Foundation of China (31372061, 31401890, 31401312, 31301254, 31260482 and 31060263).
Footnotes
Author Contributions L.S. and S.Y. performed most of the experiments and drafted the manuscript. T.Y., J.Q.L., J.Y.W., Y.Y.L., J.Z.L., L.P.S. and Q.T. participated in vector construction. W.S., H.Y.C., J.H., C.L.L. and Y.W.Z. contributed reagents, materials, analysis tools. W.C., S.L.M., Z.Q.L., L.H., Y.W. and D.Y.G. analyzed the data. S.H. conceived and designed the study and revised the manuscript.
References
- Moore J. W., Loake G. J. & Spoel S. H. Transcription dynamics in plant immunity. Plant Cell 23, 2809–2820 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K. & Somssich I. E. Transcriptional networks in plant immunity. New Phytol 206, 932–947 (2015). [DOI] [PubMed] [Google Scholar]
- Caarls L., Pieterse C. M. & Van Wees S. C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 6, 170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkostefanakis S., Roth S., Schleiff E. & Scharf K. D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ (2014). [DOI] [PubMed] [Google Scholar]
- Tang N., Zhang H., Li X., Xiao J. & Xiong L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol 158, 1755–1768 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Smigel A., Tsai Y. C., Braam J. & Berkowitz G. A. Innate immunity signaling: cytosolic Ca2+.elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol 148, 818–828 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. G., Toyota M., Kim S. H., Hilleary R. & Gilroy S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111, 6497–6502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M. C., Stancombe M. A. & Webb A. A. Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol 163, 625–634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkostefanakis S., Roth S., Schleiff E. & Scharf K. D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ 38, 1881–1895 (2015). [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Willmann M. R., Chen H. C. & Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129, 469–485 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Tanaka N., Yang G., Hayashi N. & Komatsu S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol 46, 356–366 (2005). [DOI] [PubMed] [Google Scholar]
- Kong X. et al. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genomics 14, 433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. L. et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol Biol 66, 429–443 (2008). [DOI] [PubMed] [Google Scholar]
- Huang Y., Deng T. & Zuo K. Cotton annexin proteins participate in the establishment of fiber cell elongation scaffold. Plant Signal Behav 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. et al. Genome-Wide Survey and Expression Analysis of Calcium-Dependent Protein Kinase in Gossypium raimondii. PLoS One 9, e98189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T. & Herde M. From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Curr Opin Plant Biol 20, 1–10 (2014). [DOI] [PubMed] [Google Scholar]
- Chen F. et al. The evolutionary history and diverse physiological roles of the grapevine calcium-dependent protein kinase gene family. PLoS One 8, e80818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Hata S., Kyozuka J., Shimamoto K. & Izui K. Over-expression of a single Ca2+ -dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23, 319–327 (2000). [DOI] [PubMed] [Google Scholar]
- Ludwig A. A., Romeis T. & Jones J. D. CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot 55, 181–188 (2004). [DOI] [PubMed] [Google Scholar]
- Vivek P. J., Tuteja N. & Soniya E. V. CDPK1 from ginger promotes salinity and drought stress tolerance without yield penalty by improving growth and photosynthesis in Nicotiana tabacum. PLoS One 8, e76392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan B., Lin Y. & Mou T. Expression of rice Ca(2+)-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett 581, 1179–1189 (2007). [DOI] [PubMed] [Google Scholar]
- Huang T. L. et al. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol Biol 80, 587–608 (2012). [DOI] [PubMed] [Google Scholar]
- Kobayashi M. et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19, 1065–1080 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T. & Herde M. From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Curr Opin Plant Biol 20C, 1–10 (2014). [DOI] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Robatzek S. & Somssich I. E. The WRKY superfamily of plant transcription factors. Trends Plant Sci 5, 199–206 (2000). [DOI] [PubMed] [Google Scholar]
- Ding Z. J. et al. Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. Plant J 79, 13–27 (2014). [DOI] [PubMed] [Google Scholar]
- Rizhsky L., Liang H. & Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130, 1143–1151 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N. et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot 64, 5085–5097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. H. et al. Chimeric repressor of PtSND2 severely affects wood formation in transgenic Populus. Tree Physiol 33, 878–886 (2013). [DOI] [PubMed] [Google Scholar]
- Skibbe M., Qu N., Galis I. & Baldwin I. T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 20, 1984–2000 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F., Zhou A. & Somssich I. E. Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to Its native promoter and the defense-related gene PcPR1-1 in Parsley. Plant Cell 16, 2573–2585 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T. Dissecting the WRKY web of plant defense regulators. PLoS Pathog 2, e126 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolkowski I., Wanke D., Birkenbihl R. P. & Somssich I. E. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68, 81–92 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y. et al. Protein-protein interactions in the regulation of WRKY transcription factors. Mol Plant 6, 287–300 (2013). [DOI] [PubMed] [Google Scholar]
- Shan W., Chen J. Y., Kuang J. F. & Lu W. J. Banana fruit NAC transcription factor MaNAC5 cooperates with MaWRKYs to enhance the expression of pathogenesis-related genes against Colletotrichum musae. Mol Plant Pathol (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang F. F. et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ 36, 757–774 (2013). [DOI] [PubMed] [Google Scholar]
- Cai H. et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J Exp Bot 66, 3163–3174 (2015). [DOI] [PubMed] [Google Scholar]
- Kim S. et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46, 270–278 (2014). [DOI] [PubMed] [Google Scholar]
- Kim H. Pepper gene encoding a basic pathogenesis-related 1 protein is pathogen and ethylene inducible. Physiologia Plantarum (2000). [Google Scholar]
- Mee Do H., Chul Lee S., Won Jung H., Hoon Sohn K. & Kook Hwang B. Differential expression and in situ localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Plant Science 166, 1297–1305 (2004). [Google Scholar]
- Choi H. W., Kim Y. J., Lee S. C., Hong J. K. & Hwang B. K. Hydrogen Peroxide Generation by the Pepper Extracellular Peroxidase CaPO2 Activates Local and Systemic Cell Death and Defense Response to Bacterial Pathogens. Plant Physiology 145, 890–904 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi The Hypersensitive Induced Reaction and Leucine-Rich Repeat Proteins Regulate Plant Cell Death Associated with Disease and Plant Immunity-CaHIR1. Molecular Plant-Microbe Interactions (2011). [DOI] [PubMed]
- Arthikala M.-K., Nanjareddy K., Lara M. & Sreevathsa R. Utility of a tissue culture-independent Agrobacterium-mediated in planta transformation strategy in bell pepper to develop fungal disease resistant plants-CaNPR1. Scientia Horticulturae 170, 61–69 (2014). [Google Scholar]
- Kim N. H., Kim D. S., Chung E. H. & Hwang B. K. Pepper suppressor of the G2 allele of skp1 interacts with the receptor-like cytoplasmic kinase1 and type III effector AvrBsT and promotes the hypersensitive cell death response in a phosphorylation-dependent manner. Plant Physiol 165, 76–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M. & Sheen J. CDPKs in immune and stress signaling. Trends Plant Sci 18, 30–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M. et al. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18, 1038–1051 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Schmelzer E., Hahlbrock K. & Somssich I. E. Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18, 4689–4699 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Pennell R. I., Alvarez M. E., Palmer R. & Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol 6, 427–437 (1996). [DOI] [PubMed] [Google Scholar]
- Seybold H. et al. Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 204, 782–790 (2014). [DOI] [PubMed] [Google Scholar]
- Geng S. et al. TaCPK2-A, a calcium-dependent protein kinase gene that is required for wheat powdery mildew resistance enhances bacterial blight resistance in transgenic rice. J Exp Bot 64, 3125–3136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. & He P. Nuclear dynamics of Arabidopsis calcium-dependent protein kinases in effector-triggered immunity. Plant Signal Behav 8, e23868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda C. et al. Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signaling. Plant Cell 26, 4171–4187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. Y. et al. A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties. Plant Physiol 164, 2045–2053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Lee H., Ishitani M. & Zhu J. K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277, 8588–8596 (2002). [DOI] [PubMed] [Google Scholar]
- Cecchini N. M., Jung H. W., Engle N., Tschaplinski T. J. & Greenberg J. ALD1 regulates basal immune components and early inducible defense responses in Arabidopsis. Mol Plant Microbe Interact (2014). [DOI] [PubMed] [Google Scholar]
- Choi D. S., Hwang I. S. & Hwang B. K. Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 24, 1675–1690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Schiff M. & Dinesh-Kumar S. P. Virus-induced gene silencing in tomato. Plant J 31, 777–786 (2002). [DOI] [PubMed] [Google Scholar]
- Choi D. S. & Hwang B. K. Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell 23, 823–842 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Choi W. G., Lee I. T. & Yun S. J. Cloning and characterization of a rice cDNA encoding glutamate decarboxylase. J Biochem Mol Biol 38, 595–601 (2005). [DOI] [PubMed] [Google Scholar]
- Earley K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45, 616–629 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.