ABSTRACT
Urinary tract infection (UTI) is one of the most common ailments requiring both short-term and prophylactic antibiotic therapies. Progression of infection from the bladder to the kidney is associated with more severe clinical symptoms (e.g., fever and vomiting) as well as with dangerous disease sequelae (e.g., renal scaring and sepsis). Host-pathogen interactions that promote bacterial ascent to the kidney are not completely understood. Prior studies indicate that the magnitude of proinflammatory cytokine elicitation in vitro by clinical isolates of uropathogenic Escherichia coli (UPEC) inversely correlates with the severity of clinical disease. Therefore, we hypothesize that the magnitude of initial proinflammatory responses during infection defines the course and severity of disease. Clinical UPEC isolates obtained from patients with a nonfebrile UTI elicited high systemic proinflammatory responses early during experimental UTI in a murine model and were attenuated in bladder and kidney persistence. Conversely, UPEC isolates obtained from patients with febrile UTI elicited low systemic proinflammatory responses early during experimental UTI and exhibited prolonged persistence in the bladder and kidney. Soluble factors in the supernatant from saturated cultures as well as the lipopolysaccharide (LPS) serotype correlated with the magnitude of proinflammatory responses in vitro. Our data suggest that the structure of the O-antigen sugar moiety of the LPS may determine the strength of cytokine induction by epithelial cells. Moreover, the course and severity of disease appear to be the consequence of the magnitude of initial cytokines produced by the bladder epithelium during infection.
IMPORTANCE The specific host-pathogen interactions that determine the extent and course of disease are not completely understood. Our studies demonstrate that modest changes in the magnitude of cytokine production observed using in vitro models of infection translate into significant ramifications for bacterial persistence and disease severity. While many studies have demonstrated that modifications of the LPS lipid A moiety modulate the extent of Toll-like receptor 4 (TLR4) activation, our studies implicate the O-antigen sugar moiety as another potential rheostat for the modulation of proinflammatory cytokine production.
INTRODUCTION
The plasticity of the Escherichia coli genome has led to the evolution of strains that can serve as keystone commensal organisms in the microbiota as well as to the development of multiple virulent pathotypes that cause both intestinal and extraintestinal infections. Comparison of the genetic content of over 180 strains has revealed that the core E. coli genome is composed of about 1,700 genes, with an additional pangenome of over 16,000 genes (1). It has become increasingly apparent that the plasticity of the E. coli genome allows acquisition of virulence traits from diverse genetic origins and, as such, the potential to attain the same phenotypic trait using different mechanisms (2). Given this vast variation in genetic material available, it has been difficult to assign specific subsets of genes to an individual pathotype, particularly given that many features are shared (1). Within the urinary tract, E. coli can manifest as transient contamination by nonpathogenic commensals, asymptomatic carriage, symptomatic infection of the bladder (cystitis), and severe infection of the kidney (pyelonephritis) that can result in urosepsis and death. While there has been significant interest in identification of genes that are critical for colonization and infection in the urinary tract (3–9), genes that exclusively differentiate strains as commensal, asymptomatic, cystitis, or pyelonephritis are yet to be identified (2, 10–12). For example, flagella appear to be dispensable for cystitis but are important for pyelonephritis (13–15). However, the presence of flagellar genes alone is not discriminatory as motility is a core E. coli function. Our understanding of the molecular events that regulate the progression and severity of disease is limited by the lack of discriminatory bacterial markers associated with each clinical manifestation.
Human and mouse studies have demonstrated that the magnitude of host immune responses has dramatic consequences for the outcome of disease (16–21). For example, human polymorphisms in Toll-like receptor 4 (TLR4) increase susceptibility to asymptomatic carriage (16), while polymorphisms in CXCR1 and interferon regulatory factor 3 (IRF3) increase susceptibility to acute pyelonephritis (17, 18). In addition, cyclooxygenase 2 is critical for establishment of chronic and recurrent urinary tract infections (UTIs) (21). Although there is clear evidence that host immune responses influence disease progression, human genetics is likely not the only factor that directs the disease course, given the statistics that a woman has a 50% chance of having a UTI in her lifetime. A role for bacterial traits in this process is supported by observations that absence of cytotoxic necrotizing factor, α-hemolysin, or the outer membrane chaperone SurA changes the magnitude of immune responses induced by isogenic wild-type strains (22–25). Current evidence supports a model whereby the magnitude of host immune responses, mediated either by host polymorphisms and/or bacterial traits, influences the disease manifestation observed clinically.
Interleukin-6 (IL-6) is present in human urine during acute UTI (26, 27). Prior studies indicate that independent uropathogenic E. coli (UPEC) isolates elicit various degrees of IL-6 produced by cultured human bladder epithelial cells in vitro (25, 28, 29). Moreover, we observe an inverse association between the amount of IL-6 induction in vitro and the clinical severity of UTI (28), suggesting that this phenotype may discriminate between isolates that cause cystitis and pyelonephritis. We extended these observations and, for the first time, demonstrated a correlation between the magnitude of IL-6 elicited from cultured human bladder epithelial cells in vitro and the magnitude of systemic IL-6 production during experimental UTI in the murine model. We further determined that the initial extent of systemic proinflammatory responses was associated with enhanced persistence of UPEC in the kidney during experimental UTI. In addition, the extent of IL-6 elicitation was associated with the lipopolysaccharide (LPS) O-antigen serotype of the strain. Taken together, our experimental evidence provides insight into the molecular mechanisms that demarcate an infection that will be cleared in the bladder from an infection that will progress to a more severe and persistent disease in the kidney.
MATERIALS AND METHODS
Collection of E. coli strains isolated from nonfebrile and febrile UTIs.
UPEC isolates were obtained from the urine of patients presenting to the urology service at Nationwide Children's Hospital with UTIs. The ChildLab clinical microbiology laboratory at Nationwide Children's Hospital identified the bacterial species and determined a bacterial burden of at least 105 CFU/ml of urine for each isolate (28, 30). Patient gender, age, and urinary tract diagnosis/etiology are indicated in Table 1. Patients were placed into one of four diagnostic and etiologic categories: (i) neurogenic bladder (NGB) group, (ii) vesicoureteral reflux (VUR) group, (iii) bladder and bowel dysfunction (BBD) group, and (iv) a group with no underlying UTI etiology as previously described (28). Isolates were categorized as causing febrile UTI (clinical pyelonephritis) when patients presented with flank pain, leukocytosis, body temperature of >38.5°C, and nausea and/or vomiting (28). This study was performed with the approval of the Institutional Review Board for human studies (Office of Human Research Protection [OHRP] assurance number FWA00002860) at The Research Institute at Nationwide Children's Hospital (IRB12-00269). UTI89 is a prototypic nonfebrile UPEC isolate obtained from a woman with cystitis (31).
TABLE 1.
Patient and isolate characteristics
| Isolate no. | Patient gendera | Patient age (mo) | Diagnosis | UTI presentation | LPS serotypeb |
|---|---|---|---|---|---|
| PEDUTI173 | M | 10 | NGB | Febrile | O8 |
| PEDUTI175 | M | 11 | VUR | Febrile | O1 |
| PEDUTI177 | F | 96 | BBD | Nonfebrile | ND |
| PEDUTI180 | M | 348 | NUE | Febrile | O4 |
| PEDUTI181 | F | 3 | VUR | Febrile | O6 |
| PEDUTI908 | F | 72 | BBD | Nonfebrile | O21 |
| PEDUTI910 | F | 96 | NUE | Nonfebrile | O7 |
| PEDUTI912 | F | 96 | BBD | Nonfebrile | O25a |
| PEDUTI914 | M | 132 | NBG | Nonfebrile | O25a |
| PEDUTI918 | F | 120 | BBD | Nonfebrile | O16 |
| PEDUTI919 | F | 36 | NGB | Nonfebrile | O2 |
| PEDUTI921 | F | 108 | BBD | Nonfebrile | ND |
| PEDUTI923 | F | 84 | BBD | Febrile | O2 |
| PEDUTI924 | F | 1 | NUE | Febrile | O21 |
| PEDUTI925 | F | 48 | NUE | Nonfebrile | O1 |
| PEDUTI928 | F | 108 | NGB | Nonfebrile | O21 |
| PEDUTI929 | F | 120 | BBD | Nonfebrile | ND |
| PEDUTI932 | F | 60 | VUR | Nonfebrile | O21 |
| PEDUTI933 | F | 96 | NUE | Febrile | O25b |
| PEDUTI934 | F | 48 | VUR | Febrile | O16 |
| PEDUTI935 | F | 132 | BBD | Nonfebrile | O75 |
| PEDUTI939 | F | 1 | NUE | Febrile | O2 |
| PEDUTI944 | F | 72 | NGB | Nonfebrile | O18 |
| PEDUTI950 | F | 204 | NUE | Febrile | O18 |
| PEDUTI953 | M | 24 | VUR | Febrile | ND |
| PEDUTI954 | F | 72 | BBD | Nonfebrile | O25a |
| PEDUTI958 | F | 252 | NGB | Nonfebrile | O18 |
| PEDUTI962 | F | 72 | BBD | Nonfebrile | O18 |
| PEDUTI964 | M | 11 | VUR | Febrile | O16 |
| PEDUTI966 | F | 1 | VUR | Febrile | O18 |
| PEDUTI967 | F | 72 | VUR | Febrile | O18 |
| PEDUTI968 | F | 96 | NUE | Nonfebrile | O75 |
| PEDUTI969 | F | 12 | NUE | Febrile | O18 |
| PEDUTI972 | M | 3 | NUE | Febrile | O2 |
| PEDUTI974 | F | 84 | NUE | Febrile | O25b |
| PEDUTI975 | M | 1 | VUR | Febrile | O2 |
| PEDUTI976 | F | 60 | BBD | Nonfebrile | O18 |
| PEDUTI982 | F | 2 | VUR | Febrile | O18 |
| PEDUTI984 | M | 3 | VUR | Febrile | O16 |
| PEDUTI985 | F | 1 | VUR | Febrile | O25b |
| PEDUTI987 | F | 1 | NUE | Febrile | O2 |
| PEDUTI988 | F | 71 | BBD | Nonfebrile | ND |
F, female; M, male.
ND, not determined.
Murine model of human UTI.
Based upon the magnitude of cytokine elicitation induced by these strains in vitro as part of previous studies (28) and empirical determination that these isolates exhibit similar infection kinetics in vivo as part of this study, PEDUTI177 and PEDUTI914 E. coli isolates were selected as representatives of the nonfebrile (NF-UTI) isolates. PEDUTI175 and PEDUTI939 E. coli isolates were selected as representatives of the febrile (F-UTI) isolates. In addition, the isolates represent each of the four diagnostic and etiologic categories (Table 1). UTI89 and these four pediatric isolates were statically grown in LB broth (Fisher Scientific, Pittsburg, PA) to saturation at 37°C. The presence of type 1 pili was confirmed by mannose-sensitive agglutination using Saccharomyces cerevisiae (32). Female C3H/Hen mice (Harlan Laboratories, Indianapolis, IN), 7 to 9 weeks old, were anesthetized with 3% isoflurane and inoculated transurethrally with 50 μl containing 1 × 107 viable bacteria, as described previously (33). At the indicated time points postinoculation (see below), the mice were humanely sacrificed for aseptic retrieval of bladder and kidney pairs for tissue homogenization and bacterial enumeration. Serum was collected at the time of tissue harvest, and the magnitude of proinflammatory cytokines was determined using a Mouse Inflammation Cytokines Bead Array (BD Biosciences, San Jose, CA) (34). The serum cytokines were determined using two dilutions of the serum. The cytokines were evaluated on three independent occasions. All animal experiments were performed using accredited conditions for animal welfare approved by the Institutional Animal Care and Use Committee (welfare assurance number A3544-01) at The Research Institute at Nationwide Children's Hospital (AR06-00119).
Microscopic evaluation of intracellular communities.
Infected murine bladders were harvested at 6 h postinoculation, bisected, splayed, and fixed in 4% paraformaldehyde (EM Sciences, Hatfield, PA) in phosphate-buffered saline (PBS; Sigma, St. Louis, MO) as described previously (33, 35, 36). For visualization of pediatric E. coli isolates, bladders were treated with 0.1% Triton X-100 (Fisher Scientific, Pittsburg, PA) in PBS to permeabilize the epithelial plasma membranes. Pediatric UPEC strains were visualized with the addition of rabbit anti-E. coli polyclonal antiserum (US Biological, Salem, MA) diluted 1:200 in PBS–0.1% Triton X-100 at 23°C for 1 h. Bladders were washed with PBS three times before the addition of goat anti-rabbit secondary IgG conjugated to Alexa Fluor 594 (Life Technologies, Carlsbad, CA) diluted 1:200 in PBS–0.1% Triton X-100 at 23°C for 1 h. Residual antibodies were removed by washing with PBS three times. Bladders infected with UTI89/pANT4 (37) required no specific staining for visualization of bacteria as this strain constitutively produces the green fluorescent protein. Bacterial and host DNA was visualized by the addition of Hoechst 34580 (Invitrogen, Carlsbad, CA) for 10 min. Bladders were mounted with ProLong Gold antifade reagent (Invitrogen). Images were acquired using an Axiovert 200 M inverted epifluorescence microscope equipped with a motorized stage, an Axiocam MRM charge-coupled-device (CCD) camera, and the Apotome component to improve fluorescence resolution (Carl Zeiss, Inc., Thornwood, NY). The intensity of the fluorescent images was uniformly adjusted to all pixels within the image using the levels function in Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
Preparation of conditioned medium from UPEC isolates.
All UPEC isolates (20 NF-UTI, 22 F-UTI, and UTI89) were grown to saturation in RPMI 1640 medium (HyClone Laboratories, Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO) at 37°C overnight without shaking. Bacteria were removed from the saturated cultures by centrifugation, and the supernatants were further clarified by passage through a 0.22-μm-pore-size filter (EMD Millipore, Billerica, MA) to generate conditioned medium.
To determine the contribution of shed LPS to the magnitude of cytokine elicitation, the conditioned medium obtained from UTI89 and the UPEC isolates was depleted of LPS by two sequential passages over a Detoxi-Gel endotoxin-removing column (Thermo Scientific, Rockford, IL) according to the manufacturer's recommendations. The absence of viable bacteria in the clarified conditioned medium was verified by plating on LB agar (Fisher Scientific).
The contribution of outer membrane vesicles to the magnitude of cytokine elicitation was determined by further clarification of the conditioned medium to remove the outer membrane vesicles by ultracentrifugation at 38,000 × g for 1 h as previously described (38).
Elicitation of interleukin-6 (IL-6) from cultured human bladder epithelial cells in vitro. (i) Use of conditioned medium to elicit cytokine production.
T24 bladder epithelial cells (derived from human bladder carcinoma) (ATCC HTB-4; ATCC, Manassas, VA) were grown in 24-well plates containing RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified atmosphere with 5% carbon dioxide. Confluent epithelial cell monolayers were washed with warm medium and then overlaid with 500 μl of clarified conditioned medium and returned to the incubator for 2 h. Culture supernatants were removed, clarified at 20,000 × g for 5 min, and frozen at −80°C.
(ii) Use of live bacteria to elicit cytokine production.
T24 human bladder epithelial cell monolayers were grown in 24-well plates as described above. After cells were washed with warm medium, they were infected with viable UPEC, grown in RPMI medium as described above at a multiplicity of infection 10 bacteria per epithelial cell, and returned to the incubator for 2 h. Culture supernatants were removed, clarified at 20,000 × g for 5 min, and frozen at −80°C.
The magnitude of IL-6 accumulation within the epithelial cell culture supernatant was determined by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA) as previously described (25, 28). The triplicate values were averaged and plotted as individual points for each isolate tested. Due to daily variation in results, representative studies are depicted. Where indicated (see the legend to Fig. 5), purified E. coli LPS 0111:B4 (Sigma-Aldrich, Saint Louis, MO) was added to a final concentration of 1.25 μg/ml.
FIG 5.

Depletion of LPS and vesicles from conditioned medium abolishes cytokine elicitation by UTI89. (A) IL-6 production in human bladder epithelial cells was quantified following exposure to conditioned medium (CM), conditioned medium following LPS depletion (dCM), LPS-depleted conditioned medium with reconstitution of 1.25 μg/ml commercially available LPS (dCM+LPS), and commercially available LPS alone (LPS only). (B) IL-6 production in UTI89 conditioned medium after removal (−) of vesicles and/or LPS. All samples were normalized using the amounts detected from parallel nontreated cells. Statistical significance was determined using a two-tailed Mann-Whitney U test. (**, P < 0.008; *, P = 0.03).
LPS serotype of strains determined by PCR.
Identification of the O-antigen LPS serotypes was determined by PCR (Table 1) using previously defined primers (39, 40) with genomic DNA purified from each isolate (Qiagen, Carlsbad, CA).
Statistical analysis.
The significance of the results was determined using a two-tailed Mann-Whitney U test, chi-square analysis, or one-way analysis of variance (ANOVA) (GraphPad Software, La Jolla, CA) as indicated in the figure legends.
RESULTS
Magnitude of serum cytokines produced is associated with persistence during experimental UTI.
Genetic differences between independent UPEC isolates as well as the multiplicity of infection can influence the kinetics of infection (25, 29, 41–43). Therefore, we sought to identify independent UPEC isolates from our library that exhibit similar infection kinetics during the first few hours of infection. These isolates would provide a means to determine the concordance of the cytokine profiles observed in vitro with those produced in response to experimental UTI. To this end, the kinetics of the initial stages of infection was first evaluated in the well-established mouse model of human UTI (33) using four pediatric UPEC isolates. Two representatives for each of the nonfebrile and febrile isolates were chosen to increase the reliability of the results. There was no significant difference in the bacterial burdens of either the urinary bladder or the kidney pairs at 6 h after introduction of any of the four representative UPEC isolates into the bladder (Fig. 1A and B). Furthermore, the development of intracellular bacterial communities within superficial bladder epithelial cells was similar among these four representative UPEC isolates and comparable to that with the prototypical cystitis isolate, UTI89 (Fig. 1C, D, and E) (37, 44, 45). Therefore, as evidenced by the similarity in intracellular community development and bacterial burdens, these isolates are appropriate for comparison of the immunomodulation capacity of each strain on initial proinflammatory responses during infection in vivo.
FIG 1.
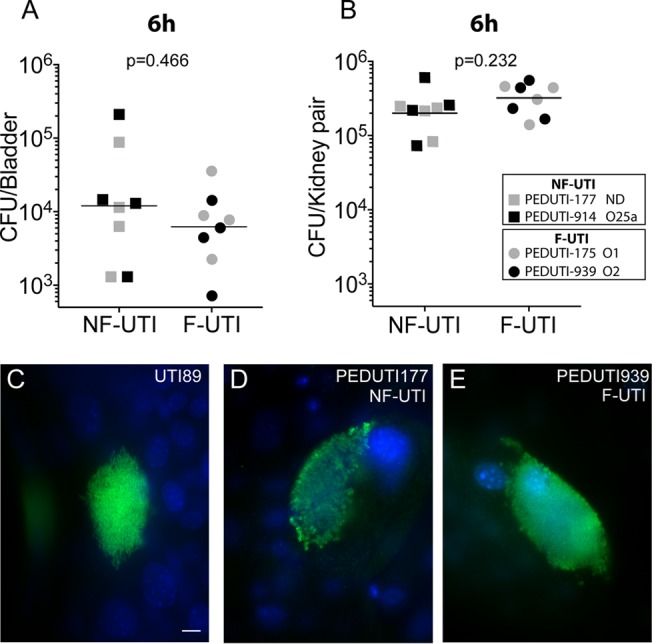
Colonization and microscopic examination of UPEC clinical isolates associated with febrile and nonfebrile UTI. (A and B) Female mice (n = 4 per cohort) were inoculated with 107 CFU of either a UPEC nonfebrile isolate (NF-UTI; PEDUTI177 or PEDUTI914) or febrile isolate (F-UTI; PEDUTI175 or PEDUTI939). The serotype of each strain is indicated in the legend. Organs indicated were harvested at 6 h postinfection for homogenization and enumeration of bacteria as the total number of CFU per organ. Statistical significance was determined using a two-tailed Mann-Whitney U test. ND, not determined. (C to E) Additional bladders were bisected, splayed, fixed, and stained for microscopic examination of intracellular bacterial communities (green) within the epithelial cells (blue nuclei). Representative intracellular communities of UTI89, PEDUTI177, and PEDUTI939 are presented. Scale bar, 10 μm.
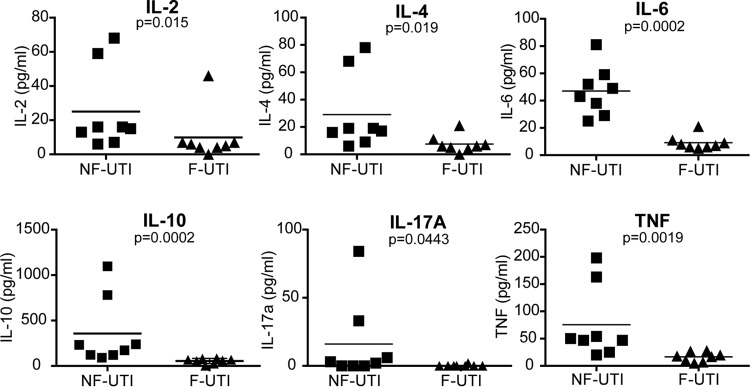
The magnitude of systemic proinflammatory responses induced by each of the independent UPEC isolates during experimental UTI was evaluated. Consistent with the in vitro observations (28), mice infected with the two representative nonfebrile UPEC isolates (high elicitation of IL-6 in vitro) displayed a statistically significant increase in the magnitude of systemic IL-6 produced compared with the level in mice infected with the two representative febrile UPEC isolates (low elicitation of IL-6 in vitro) (Fig. 2). Although not as pronounced as in the case of IL-6, statistically significant increases in IL-2, IL-4, IL-10, IL-17A, and tumor necrosis factor (TNF) were also observed in the sera of mice infected with the two representative nonfebrile UPEC isolates compared with the levels for mice infected with the two representative febrile UPEC isolates (Fig. 2). Therefore, the immunomodulation of cytokine production observed in vitro is recapitulated during infection in the host.
FIG 2.
Nonfebrile UTI isolates elicit a higher magnitude of systemic cytokine production. The magnitude of serum cytokines at 6 h after intraurethral introduction of UPEC clinical isolates is associated with febrile and nonfebrile UTI. The serum obtained from each individual mouse was measured independently on two separate occasions. The averages were plotted. Statistical significance was determined using a two-tailed Mann-Whitney U test.
To evaluate the consequences of modulation of proinflammatory responses on the manifestation of disease, the persistence of each of the four representative UPEC isolates was followed throughout the course of an acute infection. The bacterial burdens of bladders infected with either of the representative febrile UPEC isolates were significantly higher than those of bladders infected with either of the nonfebrile isolates at 16 h postinoculation (Fig. 3A). This time point coincides with the maximal influx of polymorphonuclear leukocytes (PMNs) and macrophages into the bladder (46). By 24 h, bacteria were not recovered from either the bladder or the kidneys of mice infected with the two representative nonfebrile isolates (Fig. 3B and C, solid lines). The time to clearance of UPEC from both the bladders and the kidneys infected with either of the representative febrile isolates (Fig. 3B and C, dashed lines) significantly increased compared with times for tissues infected with either of the representative nonfebrile isolates. Therefore, the magnitude of early systemic cytokines produced inversely correlated with persistence in the urinary tract.
FIG 3.
Persistence of UPEC clinical isolates associated with febrile and nonfebrile UTI. (A) Female mice (n = 8 per cohort) were inoculated with 107 CFU of either a UPEC nonfebrile isolate (NF-UTI; PEDUTI177 or PEDUTI914) or a febrile isolate (F-UTI; PEDUTI175 or PEDUTI939). The serotype of each strain is indicated in the legend. Organs indicated were harvested at 16 h postinfection for homogenization and enumeration of bacteria. Statistical significance was determined using a two-tailed Mann-Whitney U test. Bacterial persistence in the bladders (B) and the kidneys (C) of mice infected with nonfebrile (solid line; n = 9 mice each for PEDUTI177 and PEDUTI914 per time point) and febrile (dotted line; n = 8 mice each for PEDUTI175 and PEDUTI175 per time point) UPEC isolates is presented as a percentage of the values at 48 h after inoculation. Statistical significance was determined by chi-square analysis.
Magnitude of IL-6 elicitation by conditioned medium is associated with febrile and nonfebrile UTI.
The concordance of the in vitro and in vivo cytokine profiles provided support for the use of the in vitro system as an appropriate high-throughput first approach for the identification of bacterial factors that modulate the proinflammatory responses. The immunosuppression of cytokine responses by UPEC was abolished when LPS O-antigen synthesis was disrupted (25, 43). In addition, the binding of LPS by the TLR4 receptor is the predominant signal for proinflammatory responses to bacteria in the urinary tract (42, 47–49). LPS and other bacterial metabolites accumulate in the culture medium during exponential growth. The clarified culture supernatant is termed conditioned medium and has been used previously with mutants defective in assembly of LPS O-antigen (25, 43). Conditioned medium was obtained from each of the 42 isolates within our library to stimulate cultured human bladder epithelial cells in vitro. The inverse correlation of the magnitude of IL-6 proinflammatory responses and clinical disease severity observed with the complete panel of viable bacteria (28) was recapitulated with the conditioned medium (Fig. 4) (P > 0.0001). Moreover, the magnitude of IL-6 elicited from all nonfebrile isolates was indistinguishable from that in the conditioned medium of the prototypical and well-characterized isolate obtained from a woman with nonfebrile cystitis (UTI89) (Fig. 4, circle). Therefore, this evidence suggests that one or more constituents of the culture medium contribute to the immunomodulation associated with UPEC isolates.
FIG 4.

Magnitude of IL-6 production elicited by conditioned medium is associated with disease presentation. Immortalized human bladder carcinoma cells were stimulated with conditioned medium produced by 42 independent isolates. The IL-6 accumulated in the culture supernatant was quantified by ELISA after a 2-h incubation. Each data point is the average value for the triplicate quantification of each independent isolate. UPEC strains were categorized as nonfebrile (NF-UTI) or febrile (F-UTI) according to the clinical symptoms at the time of isolation from the urine. The 95% confidence intervals and median values are represented for each group. Statistical significance was determined using a two-tailed Mann-Whitney U test.
Contribution of LPS to cytokine elicitation in conditioned medium.
To begin to define the bacterial factors that contribute to modulation of epithelial proinflammatory responses, we used two approaches to deplete constituents from the conditioned medium. Elicitation of IL-6 in human bladder epithelial cells with conditioned medium from UTI89 was reduced to baseline following a two-stage depletion of LPS (P = 0.008) (Fig. 5A) with the use of Detoxi-Gel endotoxin resin. Additional passages over the Detoxi-Gel endotoxin-removing column did not have any additional effect on the magnitude of cytokine elicitation (data not shown). Reconstitution of the depleted conditioned medium with commercially available LPS resulted in similar levels of IL-6 elicitation compared to those with the commercial LPS alone (Fig. 5A), suggesting that the depletion of the LPS abolishes immune stimulation. Bacterial LPS is shed in the form of micelles and outer membrane vesicles. Under these experimental conditions, outer membrane vesicles (and any associated proteins) could have been depleted due to the content of LPS. The magnitude of cytokine elicitation was significantly reduced when outer membrane vesicles were removed by centrifugation (P = 0.03) (Fig. 5B). Extraction of residual LPS following removal of outer membrane vesicles resulted in IL-6 production that was indistinguishable from that in uninfected cells (Fig. 5B). Centrifugation of the conditioned medium after LPS depletion did not significantly affect the extent of IL-6 elicitation (Fig. 5B), suggesting that outer membrane vesicles were removed during depletion of the LPS.
Since the depletion of LPS by column chromatography was sufficient to remove the factors that stimulate the immune response from the conditioned medium of UTI89, we used this methodology to evaluate the conditioned medium of all the UPEC isolates. As observed with UTI89, the conditioned medium from the clinical isolates failed to elicit IL-6 production following depletion with the Detoxi-Gel resin (results of four representative febrile and four nonfebrile isolates are depicted in Fig. 6), suggesting that the molecules that modulate the immune response in the conditioned medium from the clinical isolates are associated with LPS and/or outer membrane vesicles.
FIG 6.

Depletion of LPS abolishes cytokine elicitation of UPEC isolates. Four representative isolates of the nonfebrile and febrile isolates evaluated for elicitation of IL-6 in the presence (black bars) and absence (gray bars) of LPS in the conditioned medium are depicted. The serotype and strain name are indicated on the x axis. Statistical significance was determined using a two-tailed Mann-Whitney U test. (*, P < 0.05).
LPS serotype and disease severity.
The common bacterial factor depleted from the conditioned medium using both approaches is LPS. Therefore, a potential correlation between the LPS O-antigen serotype and clinical disease severity was evaluated. The LPS serotype was successfully determined for 37 of the 42 UPEC isolates (Table 1). We observed a bias for the presence of certain serotypes with disease presentation (Fig. 7A). Serotypes O2, O16, and 025b were primarily obtained from children with febrile UTI. Conversely, serotypes O21, O25a, and O75 were primarily obtained from children with nonfebrile infection. Our results are consistent with a prior study of 343 UPEC strains that demonstrated an enrichment for serotypes O2 and O16 with pyelonephritis and a lack of association for disease severity with O18 (50).
FIG 7.

Association of LPS serotype with clinical disease severity and cytokine responses. (A) Distribution of LPS serotypes from patients that present with nonfebrile or febrile UTI. The LPS serotype could not be determined (ND) by this method in five of the isolates (11%). (B) IL-6 elicitation was determined following infection of cultured bladder epithelial cells with intact viable E. coli. Each data point represents the average of three independent replicates. Statistical significance was determined using a one-way ANOVA (P = 0.0001).
LPS serotype and magnitude of cytokine elicitation.
To further investigate the association of LPS serotype with the magnitude of cytokine elicitation, IL-6 was quantified from cultured human bladder epithelial cell viable bacteria. Within our collection, certain serotypes were excluded from further evaluation due to statistical limitations (O1, O4, O6, O7, and O8) (Fig. 7A). Therefore, this analysis included 33 of the isolates from the library. With the exception of serotypes O18 and O25b, the magnitudes of cytokines elicited for each independent clinical isolate were very similar within the same serotype (Fig. 7B). The association of the degree of the cytokine elicitation with the LPS serotype of the strain was statistically significant (P = 0.0001) (Fig. 7B). Taken together, serotypes O21, O25a, and O75 elicit high IL-6 secretion and represent a low risk for progression to febrile infection. In contrast, serotypes O2, O16, and O25b elicit low IL-6 secretion and represent a high risk for progression to a febrile infection. This association was further explored through the evaluation of combinatorial exposure of human bladder epithelial cells to outer membrane vesicles displaying similar protein profiles but exhibiting different LPS serotypes (see Fig. S1 in the supplemental material). Consistent with our observations with whole cells (Fig. 7B), we observed differences in the magnitude of IL-6 elicitation when bladder epithelial cells were exposed to outer membrane vesicles from two representative nonfebrile isolates (PEDUTI177 and PEDUTI914) and two representative febrile isolates (PEDUTI175 and PEDUTI939) (see Fig. S2 in the supplemental material). When the vesicles were mixed, the IL-6 elicitation was representative of one of the two isolates, suggesting that the cells respond to the vesicles produced by only one of the LPS serotypes tested. Therefore, the LPS serotype appears to correlate with the magnitude of IL-6 responses and bacterial persistence, as well as the severity of clinical disease.
DISCUSSION
The specific host-bacterial interactions that transform the disease course leading to enhanced bacterial persistence and disease severity are not completely understood. In our study, we use the same genetic host background (e.g., T24 bladder cells and C3H/HeN mice) to focus only on bacterial attributes that modulate the immune response to elucidate the molecular events that determine disease outcomes. During experimental UTI, we demonstrated that mice infected with UPEC isolates obtained from patients with febrile UTI displayed decreased systemic immune responses and increased bacterial persistence in the urinary tract compared with mice infected with UPEC isolates obtained from patients with nonfebrile UTI. This observation suggests that the rapid clearance of bacteria during experimental UTI is likely a consequence of the high cytokine elicitation that has been observed in vitro (25, 29, 43, 51). Prior studies indicate that bacterial persistence is promoted by two sequential instillations of the prototypical cystitis strain, UTI89, into the bladder 1 to 6 h apart (52). However, increasing the time between the two instillations (>24 h) nullifies the benefit of superinfection. In light of our data, we suggest that immunosuppression of epithelial responses from the first inoculum could protect the second inoculum from early innate immune responses. We further propose that the enhanced survival of the febrile isolates correlates with a reduction in the recruitment of immune cells combined with a decrease in epithelial production of extracellular and intracellular antibacterial agents (3, 32, 53). Thus, suppression of the TLR4 cascade provides multiple mechanisms to ensure a more hospitable environment for the expansion of UPEC strains in the urinary tract. Our data not only support the use of in vitro systems to investigate epithelial proinflammatory responses to UPEC (25, 28, 29, 42, 43) but also demonstrate that modulation of the initial inflammatory responses, mediated at the urothelium, has a direct impact on disease progression. As such, these studies provide mechanistic insight into the molecular events that contribute to disease severity.
Bacterial LPS is a very diverse class of molecules. LPS is readily remodeled through modifications of the lipid A moiety (54), the portion that directly interacts with the TLR4 receptor (55). The ability of lipid A modifications to alter the magnitude of inflammatory responses has been exploited to identify optimal vaccine adjuvants (54, 56). Moreover, lipid A mimics are being investigated as potential therapeutics to enhance or antagonize TLR4 signaling (57–59). Although each strain appears to produce only one O antigen, as a species, E. coli can assemble ∼158 different sugar moieties onto the lipid A/core complex. Although modifications to the O antigen within a particular strain participate in pathogenesis (54), a specific role for the structure of the sugar moiety in pathogenesis is unclear. Given the diversity in LPS O antigens, the use of isogenic strains is not easily amenable to the direct evaluation of the variations in O-antigen sugar structure during disease. Therefore, our use of a panel of E. coli isolates provided a first line of experimental evidence that the LPS O-antigen moiety is associated with the magnitude of cytokine production. Although our in vitro study focused on the induction of only IL-6, our in vivo evidence suggests that multiple cytokines and chemokines may be modulated by UPEC during UTI. Future studies will include evaluation of an increased repertoire of LPS serotypes to further elucidate the molecular interactions that determine the magnitude of TLR4 responses.
Outer membrane vesicles are produced by a wide variety of bacteria and contribute to pathogenesis and immune modulation (38, 60–62). We observed that outer membrane vesicles retained the ability to modulate the production of IL-6 from cultured bladder epithelial cells. In addition to LPS, proteins and nucleic acids are constituents of outer membrane vesicles. Proteins that are known to regulate proinflammatory responses (e.g., hemolysin and SurA substrates) (23–25) are packaged into UPEC outer membrane vesicles (63). Therefore, further biochemical characterization of outer membrane vesicles could identify additional bacterial traits that contribute to the diversity of cytokine responses observed for some LPS serotypes (i.e., O18 and 25b).
In summary, we provide experimental evidence that bacterial persistence correlates with the extent of immunosuppression during the initial stages of infection. Moreover, the LPS serotype, by virtue of the association with the magnitude of cytokine elicitation, is associated with the severity of disease.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00664-15.
REFERENCES
- 1.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo Y, Zhang L, Foxman B, Zollner S. 2015. Whole-genome sequencing of uropathogenic Escherichia coli reveals long evolutionary history of diversity and virulence. Infect Genet Evol 34:244–250. doi: 10.1016/j.meegid.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunstad DA, Justice SS. 2010. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol 64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 4.Ejrnaes K. 2011. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 58:B4187. [PubMed] [Google Scholar]
- 5.Norinder BS, Koves B, Yadav M, Brauner A, Svanborg C. 2012. Do Escherichia coli strains causing acute cystitis have a distinct virulence repertoire? Microb Pathog 52:10–16. doi: 10.1016/j.micpath.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Subashchandrabose S, Mobley HL. 2015. Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics 7:935–942. doi: 10.1039/C4MT00329B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JR. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4:80–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JR, Orskov I, Orskov F, Goullet P, Picard B, Moseley SL, Roberts PL, Stamm WE. 1994. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J Infect Dis 169:119–126. doi: 10.1093/infdis/169.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Blanco M, Blanco JE, Alonso MP, Blanco J. 1996. Virulence factors and O groups of Escherichia coli isolates from patients with acute pyelonephritis, cystitis and asymptomatic bacteriuria. Eur J Epidemiol 12:191–198. doi: 10.1007/BF00145506. [DOI] [PubMed] [Google Scholar]
- 10.Tarchouna M, Ferjani A, Ben-Selma W, Boukadida J. 2013. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int J Infect Dis 17:e450–453. doi: 10.1016/j.ijid.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Yun KW, Kim HY, Park HK, Kim W, Lim IS. 2014. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J Microbiol Immunol Infect 47:455–461. doi: 10.1016/j.jmii.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Firoozeh F, Saffari M, Neamati F, Zibaei M. 2014. Detection of virulence genes in Escherichia coli isolated from patients with cystitis and pyelonephritis. Int J Infect Dis 29:219–222. doi: 10.1016/j.ijid.2014.03.1393. [DOI] [PubMed] [Google Scholar]
- 13.Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A 104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane MC, Lockatell V, Monterosso G, Lamphier D, Weinert J, Hebel JR, Johnson DE, Mobley HL. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect Immun 73:7644–7656. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright KJ, Seed PC, Hultgren SJ. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun 73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragnarsdottir B, Jonsson K, Urbano A, Gronberg-Hernandez J, Lutay N, Tammi M, Gustafsson M, Lundstedt AC, Leijonhufvud I, Karpman D, Wullt B, Truedsson L, Jodal U, Andersson B, Svanborg C. 2010. Toll-like receptor 4 promoter polymorphisms: common TLR4 variants may protect against severe urinary tract infection. PLoS One 5:e10734. doi: 10.1371/journal.pone.0010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundstedt AC, McCarthy S, Gustafsson MC, Godaly G, Jodal U, Karpman D, Leijonhufvud I, Linden C, Martinell J, Ragnarsdottir B, Samuelsson M, Truedsson L, Andersson B, Svanborg C. 2007. A genetic basis of susceptibility to acute pyelonephritis. PLoS One 2:e825. doi: 10.1371/journal.pone.0000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer H, Lutay N, Ragnarsdottir B, Yadav M, Jonsson K, Urbano A, Al Hadad A, Ramisch S, Storm P, Dobrindt U, Salvador E, Karpman D, Jodal U, Svanborg C. 2010. Pathogen specific, IRF3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog 6:e1001109. doi: 10.1371/journal.ppat.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudick CN, Jiang M, Yaggie RE, Pavlov VI, Done J, Heckman CJ, Whitfield C, Schaeffer AJ, Klumpp DJ. 2012. O-antigen modulates infection-induced pain states. PLoS One 7:e41273. doi: 10.1371/journal.pone.0041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, Miyoshi H, Mack M, Schwendener RA, Hooton TM, Stappenbeck TS, Hansson GC, Stenson WF, Colonna M, Stapleton AE, Hultgren SJ. 2014. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1:46–57. doi: 10.1016/j.ebiom.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. 2015. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 112:E871–E880. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia TA, Ventura CL, Smith MA, Merrell DS, O'Brien AD. 2013. Cytotoxic necrotizing factor 1 and hemolysin from uropathogenic Escherichia coli elicit different host responses in the murine bladder. Infect Immun 81:99–109. doi: 10.1128/IAI.00605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhakal BK, Mulvey MA. 2012. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11:58–69. doi: 10.1016/j.chom.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. 2005. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun 73:3999–4006. doi: 10.1128/IAI.73.7.3999-4006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheu JN, Chen MC, Chen SM, Chen SL, Chiou SY, Lue KH. 2009. Relationship between serum and urine interleukin-6 elevations and renal scarring in children with acute pyelonephritis. Scand J Urol Nephrol 43:133–137. doi: 10.1080/00365590802478742. [DOI] [PubMed] [Google Scholar]
- 27.Sheu JN, Chen MC, Lue KH, Cheng SL, Lee IC, Chen SM, Tsay GJ. 2006. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine 36:276–282. doi: 10.1016/j.cyto.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Storm DW, Patel AS, Horvath DJ Jr, Li B, Koff SA, Justice SS. 2012. Relationship among bacterial virulence, bladder dysfunction, vesicoureteral reflux and patterns of urinary tract infection in children. J Urol 188:236–241. doi: 10.1016/j.juro.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Billips BK, Forrestal SG, Rycyk MT, Johnson JR, Klumpp DJ, Schaeffer AJ. 2007. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect Immun 75:5353–5360. doi: 10.1128/IAI.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storm DW, Koff SA, Horvath DJ Jr, Li B, Justice SS. 2011. In vitro analysis of the bactericidal activity of Escherichia coli Nissle 1917 against pediatric uropathogens. J Urol 186:1678–1683. doi: 10.1016/j.juro.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Smith P, Horvath DJ Jr, Romesberg FE, Justice SS. 2010. SOS regulatory elements are essential for UPEC pathogenesis. Microbes Infect 12:662–668. doi: 10.1016/j.micinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat Protoc 4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolton M, Horvath DJ Jr, Li B, Cortado H, Newsom D, White P, Partida-Sanchez S, Justice SS. 2012. Intrauterine growth restriction is a direct consequence of localized maternal uropathogenic Escherichia coli cystitis. PLoS One 7:e33897. doi: 10.1371/journal.pone.0033897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. 2008. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol 6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 36.Justice SS, Li B, Downey JS, Dabdoub SM, Brockson ME, Probst GD, Ray WC, Goodman SD. 2012. Aberrant community architecture and attenuated persistence of uropathogenic Escherichia coli in the absence of individual IHF subunits. PLoS One 7:e48349. doi: 10.1371/journal.pone.0048349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A 101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe SW, Kuehn MJ, Mason KM. 2011. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect Immun 79:4361–4369. doi: 10.1128/IAI.05332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Liu B, Chen M, Guo D, Guo X, Liu F, Feng L, Wang L. 2010. A multiplex PCR method to detect 14 Escherichia coli serogroups associated with urinary tract infections. J Microbiol Methods 82:71–77. doi: 10.1016/j.mimet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother 61:1024–1028. doi: 10.1093/jac/dkn084. [DOI] [PubMed] [Google Scholar]
- 41.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. 2007. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun 75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schilling JD, Martin SM, Hunstad DA, Patel KP, Mulvey MA, Justice SS, Lorenz RG, Hultgren SJ. 2003. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun 71:1470–1480. doi: 10.1128/IAI.71.3.1470-1480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billips BK, Schaeffer AJ, Klumpp DJ. 2008. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun 76:3891–3900. doi: 10.1128/IAI.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 45.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 46.Horvath DJ Jr, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, Justice SS. 2011. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect 13:426–437. doi: 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Eden C. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. 2004. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun 72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindberg U, Hanson A, Jodal G, Lidin-Janson G, Lincoln K, Olling S. 1975. Asymptomatic bacteriuria in schoolgirls: II. Differences in Escherichia coli causing asymptomatic and symptomatic bacteriuria. Acta Paediatr Scand 64:432–436. [DOI] [PubMed] [Google Scholar]
- 51.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. 2006. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun 74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz DJ, Conover MS, Hannan TJ, Hultgren SJ. 2015. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog 11:e1004599. doi: 10.1371/journal.ppat.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heimlich DR, Harrison A, Mason KM. 2014. Host antimicrobial peptides in bacterial homeostasis and pathogenesis of disease. Antibiotics 3:645–676. doi: 10.3390/antibiotics3040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 56.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A 110:1464–1469. doi: 10.1073/pnas.1218080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stark R, Choi H, Koch S, Lamb F, Sherwood E. 2015. Monophosphoryl lipid A inhibits the cytokine response of endothelial cells challenged with LPS. Innate Immun 21:565–574. doi: 10.1177/1753425914564172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewicky JD, Ulanova M, Jiang ZH. 2013. Improving the immunostimulatory potency of diethanolamine-containing lipid A mimics. Bioorg Med Chem 21:2199–2209. doi: 10.1016/j.bmc.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Dowling QM, Sivananthan SJ, Guderian JA, Moutaftsi M, Chesko JD, Fox CB, Vedvick TS, Kramer RM. 2014. Modulating potency: physicochemical characteristics are a determining factor of TLR4-agonist nanosuspension activity. J Pharm Sci 103:879–889. doi: 10.1002/jps.23868. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Lee J, Park J, Gho YS. 2015. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Ferrand J, Ferrero RL. 2013. Recognition of extracellular bacteria by NLRs and its role in the development of adaptive immunity. Front Immunol 4:344. doi: 10.3389/fimmu.2013.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones HE, Copland A, Hamstra HJ, Cohen J, Brown J, Klein N, van der Ley P, Dixon G. 2014. LOS oligosaccharide modification enhances dendritic cell responses to meningococcal native outer membrane vesicles expressing a non-toxic lipid A. Cell Microbiol 16:519–534. doi: 10.1111/cmi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wurpel DJ, Moriel DG, Totsika M, Easton DM, Schembri MA. 2015. Comparative analysis of the uropathogenic Escherichia coli surface proteome by tandem mass-spectrometry of artificially induced outer membrane vesicles. J Proteomics 115:93–106. doi: 10.1016/j.jprot.2014.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.