ABSTRACT
Pseudomonas aeruginosa is an opportunistic human pathogen that causes severe, life-threatening infections in patients with cystic fibrosis (CF), endocarditis, wounds, or artificial implants. During CF pulmonary infections, P. aeruginosa often encounters environments where the levels of calcium (Ca2+) are elevated. Previously, we showed that P. aeruginosa responds to externally added Ca2+ through enhanced biofilm formation, increased production of several secreted virulence factors, and by developing a transient increase in the intracellular Ca2+ level, followed by its removal to the basal submicromolar level. However, the molecular mechanisms responsible for regulating Ca2+-induced virulence factor production and Ca2+ homeostasis are not known. Here, we characterized the genome-wide transcriptional response of P. aeruginosa to elevated [Ca2+] in both planktonic cultures and biofilms. Among the genes induced by CaCl2 in strain PAO1 was an operon containing the two-component regulator PA2656-PA2657 (here called carS and carR), while the closely related two-component regulators phoPQ and pmrAB were repressed by CaCl2 addition. To identify the regulatory targets of CarSR, we constructed a deletion mutant of carR and performed transcriptome analysis of the mutant strain at low and high [Ca2+]. Among the genes regulated by CarSR in response to CaCl2 are the predicted periplasmic OB-fold protein, PA0320 (here called carO), and the inner membrane-anchored five-bladed β-propeller protein, PA0327 (here called carP). Mutations in both carO and carP affected Ca2+ homeostasis, reducing the ability of P. aeruginosa to export excess Ca2+. In addition, a mutation in carP had a pleotropic effect in a Ca2+-dependent manner, altering swarming motility, pyocyanin production, and tobramycin sensitivity. Overall, the results indicate that the two-component system CarSR is responsible for sensing high levels of external Ca2+ and responding through its regulatory targets that modulate Ca2+ homeostasis, surface-associated motility, and the production of the virulence factor pyocyanin.
IMPORTANCE During infectious disease, Pseudomonas aeruginosa encounters environments with high calcium (Ca2+) concentrations, yet the cells maintain intracellular Ca2+ at levels that are orders of magnitude less than that of the external environment. In addition, Ca2+ signals P. aeruginosa to induce the production of several virulence factors. Compared to eukaryotes, little is known about how bacteria maintain Ca2+ homeostasis or how Ca2+ acts as a signal. In this study, we identified a two-component regulatory system in P. aeruginosa PAO1, termed CarRS, that is induced at elevated Ca2+ levels. CarRS modulates Ca2+ signaling and Ca2+ homeostasis through its regulatory targets, CarO and CarP. The results demonstrate that P. aeruginosa uses a two-component regulatory system to sense external Ca2+ and relays that information for Ca2+-dependent cellular processes.
INTRODUCTION
Pseudomonas aeruginosa, a natural inhabitant of soil and water, is able to infect a variety of hosts, including plants and humans. In humans, it causes severe acute and chronic infections by colonizing respiratory and urinary tracts and burned or wounded epithelia, cornea, and muscles (1–3). The versatility of P. aeruginosa pathogenicity is associated with diverse metabolic capabilities, multiple mechanisms of resistance, a large repertoire of virulence factors, and adaptability, due in part to the tightly coordinated regulation of gene expression. A large portion of the P. aeruginosa PAO1 genome, approximately 9.4%, encodes transcriptional regulators (4, 5), including two-component regulators: 89 response regulators, 55 sensor kinases, and 14 sensor-response regulator hybrids (2). The regulatory targets for most of these regulatory systems are unknown.
Calcium plays an important signaling role in both eukaryotic and prokaryotic cells. In prokaryotes, Ca2+ is an essential nutrient, since it is a necessary cofactor for many enzymes. However, Ca2+ can be toxic to cells at high concentrations; therefore, bacteria maintain a low-submicromolar intracellular concentration of Ca2+ (6). P. aeruginosa may encounter environments where external Ca2+ levels are in the millimolar range, varying from 10 mM in soil (7) to 40 mM in hypersaline lakes (8). As a plant and human pathogen, P. aeruginosa may be exposed to lower but also varying Ca2+ levels. For example, in plants, the Ca2+ concentration ranges from 0.01 to 1 mM in extracellular spaces (9) and from 1 to 10 mM in apoplasts (10). In a human body, Ca2+ levels may reach about 1 to 2 mM in extracellular fluids and saliva (11) (12) and 5 mM in blood (13) and human milk (14). In the case of disease, for example, during cystic fibrosis (CF) pulmonary infections, both intracellular and extracellular Ca2+ levels fluctuate in response to inflammation (15, 16), and the overall Ca2+ levels in nasal secretions and sputum increase at least 2-fold (12), reaching up to 3 to 7 mM (17, 18).
In a previous study, we demonstrated that P. aeruginosa maintains a submicromolar intracellular concentration of Ca2+ ([Ca2+]in) (6). However, when the cells are exposed to high levels of extracellular Ca2+, characteristic of the environments described above, the cells undergo a transient increase of [Ca2+]in. The transient increase is followed by a return to submicromolar levels of [Ca2+]in and a maintenance of homeostatic concentrations of internal Ca2+, apparently due to the transport of excess Ca2+ through Ca2+ export pumps. Interestingly, in addition to the maintenance of Ca2+ homeostasis, P. aeruginosa recognizes the external concentration of Ca2+ as a physiological signal and responds through changes in the abundances of intracellular proteins and secreted virulence factors, alginate, pyocyanin, and secreted proteases (19, 20). This Ca2+-triggered change in P. aeruginosa physiology leads to enhanced plant infectivity (21), biofilm formation, and swarming motility (6, 19, 20). Furthermore, Ca2+ alters the abundance of P. aeruginosa proteins involved in iron acquisition, quinolone signaling, nitrogen metabolism, and stress responses (19, 20). These observations suggest that Ca2+ plays an important regulatory role in P. aeruginosa virulence. However, the molecular mechanisms responsible for sensing environmental Ca2+ and regulating the Ca2+-induced responses are not known. Therefore, the goals of this study were to identify and characterize Ca2+-mediated molecular responses.
Bacteria use two-component regulatory systems (TCSs) to sense and respond to diverse and continuously changing environmental stimuli, including changing cation concentrations. TCSs help regulate responses to Na+, Mg2+, and other cations; therefore, they likely are involved in Ca2+-dependent regulation. A typical TCS contains a sensor kinase located partially in the cytoplasmic membrane and a cognate response regulator (22). Upon exposure to a stimulus, the sensor kinase autophosphorylates at histidine residues. The consequent conformation change enables the transfer of a phosphate group to the aspartate residue on the cognate response regulator, which typically results in DNA binding to an activator DNA sequence and changes in gene expression (5, 23). P. aeruginosa has many TCSs, and some of these have been characterized. For example, PhoPQ and PmrAB regulate resistance to polymyxin B and antimicrobial peptides via lipid A modification at a low magnesium (Mg2+) concentration (24–27). PhoPQ also regulates aminoglycoside resistance, twitching and swarming motility, surface attachment, and biofilm formation, ultimately contributing to the regulation of virulence (28, 29). PmrAB is induced by cationic antimicrobial peptides, including polymyxins (25), whereas PhoPQ is induced by polyamines and low [Mg2+] (30). Other TCSs respond to metals, including the CzcRS and CopRS systems that regulate resistance to zinc and copper, respectively (31, 32). CzcRS also regulates the transcription of the CzcrBCA resistance-nodulation-division (RND) efflux pump, which is responsible for carbapenem resistance (31). GacAS and AlgRZ regulate the production of several virulence factors, including pyocyanin, cyanide, lipase, and alginate, as well as systemic virulence and motility (29, 33–37). GacAS also controls the production of the quorum-sensing signaling molecule N-butyryl-homoserine lactone (38) and resistance to diverse antibiotics, including the aminoglycosides (such as gentamicin) and chloramphenicol (29). The transcription of gacS is repressed by subinhibitory concentrations of tobramycin, ciprofloxacin, and tetracycline (39). AlgRZ also regulates early stages of biofilm formation (40) and the expression of quorum-sensing genes (41). Another TCS, FleRS, regulates flagellar synthesis, adhesion (42), motility, and antibiotic resistance (43). Five TCS response regulators, PA1099, PA3702, PA4547, PA4493, and PA5261, are involved in coordinating the interactions of the bacterium with the host lung epithelium (44). However, most other TCSs on the P. aeruginosa genome remain uncharacterized, with their signals and regulatory targets yet to be identified.
In this study, we used microarray analysis to characterize the global transcriptional response of P. aeruginosa to elevated external Ca2+ levels. From these analyses, we identified the TCS PA2656-PA2657 (here referred to as carSR, for calcium regulator sensor and regulator), whose transcription is highly induced by elevated Ca2+ in planktonic cultures of P. aeruginosa PAO1. Using deletion mutations and microarray analysis, we identified the regulatory targets of carSR, which include the hypothetical proteins PA0320 and PA0327. Further characterization of PA0320 and PA0327 indicate that they play roles in maintaining Ca2+ homeostasis. PA0327 also influences the production of the virulence factor pyocyanin and swarming motility in a Ca2+-dependent manner.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Strains and plasmids used in this study are listed in Table S1 in the supplemental material. P. aeruginosa PAO1 is a nonmucoid strain with the complete genome sequence available (2). P. aeruginosa FRD1 is a mucoid cystic fibrosis isolate that also has an available genome (45) sequence. Isogenic mutants were constructed in PAO1. The gene PA2657 (carR) was deleted from PAO1 using allelic exchange as described previously (19). PAO1 mutants with transposon insertion in PA0320 (PA0320-H07::ISlacZ/hah) and PA0327 (PA0327-B11::ISphoA/hah) were provide by the University of Washington two-allele library. The sites of transposon insertions were confirmed by two-step PCR, using the primer sequences available at www.gs.washington.edu. For convenience, the transposon mutants were designated PA::Tn5, where PA is the identifying number of the disrupted gene from the P. aeruginosa PAO1 genome (www.pseudomonas.com). Each mutant gene was complemented by cloning the gene behind the arabinose-inducible PBAD promoter in the Tn7 vector pTJ1 (46) (graciously provided by Joanna Goldberg). For complementing vectors, PA0320 and PA0327 were amplified using PCR with the gene-specific primers listed in Table S1. The PCR products were cloned into TA cloning vectors (Invitrogen). The resulting plasmids were digested with NcoI and HindIII, and the bands containing PA0320 and PA0327 were ligated into pTJ1, producing plasmids pTA56 and pTA57, respectively. A Tn7-based construct containing both PA2657 and PA2656 was used to complement the PA2657 mutant to correct for any possible polar effects due to the disruption of PA2657. PA2656 and PA2657 were amplified separately using Phusion high-fidelity DNA polymerase (NEB). After the addition of a 3′ A overhang by Taq DNA polymerase, PCR products were cloned into TA cloning vectors. The EcoRI-EcoRV fragment containing PA2656 was ligated into pTJ1, followed by ligation of the EcoRI fragment containing PA2657. The resulting plasmid was labeled pTA104. The Tn7-based vectors were integrated into the chromosome of the respective P. aeruginosa mutant strains using electroporation, along with the Tn7 transposase helper plasmid, pTNS1, with selection for trimethoprim resistance. The trimethoprim resistance marker then was removed using pFLP2 (47). pTNS1 and pFLP2 were graciously provided by Herbert Schweizer.
For the estimation of free intracellular calcium ([Ca2+]in), PAO1 and mutant derivatives were transformed with pMMB66EH-AEQ (graciously provided by D. Dominguez and A. Campbell), which carries the gene for aequorin (48), and was selected for by carbenicillin resistance (49). The presence of the aequorin gene in the resulting strains was verified by PCR using the aequorin-specific primers (see Table S1 in the supplemental material).
Biofilm and planktonic cultures were cultivated in biofilm minimal medium (BMM) (19) which contained (per liter) 9.0 mM sodium glutamate, 50 mM glycerol, 0.02 mM MgSO4, 0.15 mM NaH2PO4, 0.34 mM K2HPO4, 145 mM NaCl, 20 μl trace metals, and 1 ml vitamin solution. The trace-metal solution consisted of (per liter of 0.83 M HCl) 5.0 g CuSO4 · 5H2O, 5.0 g ZnSO4 · 7H2O, 5.0 g FeSO4 · 7H2O, and 2.0 g MnCl2 · 4H2O. Vitamins solution contained (per liter) 0.5 g thiamine and 1 mg biotin. The pH of the medium was adjusted to 7.0. When required, CaCl2 · 2H2O was added to a final concentration of 10 mM.
Growth of planktonic cultures and biofilms.
P. aeruginosa strains PAO1 and FRD1 were cultured planktonically for 18 h at 37°C, with shaking at 250 rpm, in BMM with 10 mM added CaCl2 or with no added CaCl2. Biofilms were cultured on the surface of silicone tubing at 37°C in a single flowthrough system for 72 h with and without 10 mM added CaCl2 as described previously (19). Biofilms were detached from the interior surface of the silicone tubing with the plunger from a 3-ml syringe. For FRD1, the cells were plunged into 150 ml of 110 mM sodium citrate. All resulting cell suspensions were collected by centrifugation at 10,000 rpm at 4°C for 3 min.
For physiological studies of PAO1 and its mutant derivatives, cells were cultured by inoculating 100 μl of a mid-exponential-phase culture into 100 ml of BMM alone or with 10 mM CaCl2. Cultures were incubated at 37°C at 200 rpm with absorbance sampling every 2 to 4 h in a Biomate 3 spectrophotometer (Thermo Scientific).
RNA extraction, processing, and microarray analysis.
RNA was isolated from cells using a hot phenol extraction method (50). Briefly, pelleted cells were suspended in 1.5 ml lysis buffer (0.15 M sucrose, 0.01 M sodium acetate, pH 4.5) and 1.5 ml 2% sodium dodecyl sulfate. Following the addition of 3 ml of water-saturated phenol, the mixture was incubated for 5 min at 65°C with frequent vortexing. Three milliliters of chloroform was added, and the mixture was centrifuged for 30 min at 4°C. The aqueous phase was precipitated overnight, washed, and resuspended in RNase-free water. The RNA was cleaned on an RNeasy column (Qiagen) by following the manufacturer's mini cleanup protocol and then subjected to two 30-min Turbo DNase (Ambion) treatments before precipitation and resuspension. RNA quality was assessed using an RNA6000 nano assay (Bioanalyzer 2100; Agilent Technologies, Palo Alto, CA). Labeled cDNA was synthesized from 8 μg of total RNA according to SOP#M007 (from the J. Craig Venter Institute [JCVI], formerly The Institute for Genomic Research [TIGR]), using a 2:1 ratio of amino-allyl dUTP to dTTP and Superscript II. cDNA quality and dye incorporation were assessed with a NanoDrop 1000.
Pseudomonas aeruginosa version 1 glass slide DNA microarrays were obtained from the JCVI and prepared according to SOP#M008, with minor modifications. For each condition (PAO1 planktonic, PAO1 biofilm, FRD1 planktonic, and FRD1 biofilm), labeled cDNA from cells grown with 10 mM added CaCl2 and cells grown with no added CaCl2 were cohybridized to the same slide, and a dye swap was performed. In this manner, for each of the four growth conditions, a minimum of four biological samples was hybridized to four microarrays.
Microarray slides were scanned using a GenePix 4000B scanner (Molecular Devices), and emissions at 532 and 650 nm were recorded. GenePix Pro software v 6.0 (Molecular Devices) was used to obtain median pixel intensity values for each spot on the array and generate GenePix Results (gpr) files. gpr files were imported into Flexarray v 1.6.1 for normalization and analysis. Background correction was performed using the normexp algorithm. The LOESS algorithm was used for within-array normalization, followed by scaling for between-array normalization. The Limma TREAT (t tests relative to a threshold) method was used to generate symmetrical fold changes to conservatively assess differential expression due to calcium addition. Empty and reserved spots were not included in the data set. Replicate spots (three per gene) were averaged, and genes with a greater than 2-fold change at P < 0.05 were selected for further analysis.
For validation of the JCVI microarray data, two additional biological replicates of PAO1 were grown planktonically with and without 10 mM added CaCl2 as described above. Affymetrix microarrays were performed with these samples as described previously (51). Transcripts identified as upregulated in two-color arrays (JCVI) also were found to be upregulated on the Affymetrix platform, with 92% (33/36) being greater than 2-fold upregulated (P <10−14). Transcripts identified as downregulated in two-color arrays also were downregulated on the Affymetrix platform, with 90% (26/29) being greater than 2-fold (P < 10−14).
RT-qPCR.
At 6, 12, and 24 h, 1-ml aliquots of wild-type and ΔcarR mutant cells were removed from a 25-ml volume of BMM (with or without 10 mM CaCl2 added), pelleted, and frozen at −80°C. RNA was extracted with the hot phenol method, cleaned on RNA Clean & Concentrator-25 columns (Zymo Research), and turbo DNase treated (Ambion). One-step reverse transcription-quantitative PCR (RT-qPCR) was performed with the Rotor-Gene SYBR green RT-PCR kit (Qiagen) as described previously (52). Three biological replicates for each strain, time point, and Ca2+ level were assayed in triplicate using primers designed for the acpP, PA0320, and PA0327 transcripts (see Table S1 in the supplemental material). Negative controls lacking reverse transcriptase were performed with each of the 36 samples and revealed that samples were free from DNA contamination. RT-qPCR efficiencies, calculated from the slope of standard curves using Rotor-Gene software, were similar for acpP (1.06), PA0320 (0.97), and PAO327 (1.03), with r2 > 0.98 for each. The Relative Expression Software Tool (REST) (53) was used to calculate mean fold changes of PA0320 (carO) and PA0327 (carP) transcripts due to the addition of 10 mM Ca2+ (compared to no added Ca2+) using acpP as a normalizer. To determine if transcripts were significantly upregulated by Ca2+, a nonparametric one-tailed Mann-Whitney test, assuming unequal variation, was performed using GraphPad Prism version 6.04 for Windows. This test also was used to determine if there was a significant increase in fold change due to Ca2+ addition between the wild-type strain PAO1 and the ΔcarR mutant strain.
Measurement of [Ca2+]in.
Luminescence measurements and the estimation of free cellular calcium concentrations ([Ca2+]in) were done as described previously (6), with slight modifications. Briefly, mid-log-phase cells were induced with IPTG (1 mM) for 2 h for apoaequorin production and then harvested by centrifugation at 6,000 × g for 5 min at 4°C. Aequorin was reconstituted by incubating the cells in the presence of 2.5 μM coelenterazine for 30 min. One hundred microliters of cells with reconstituted aequorin were equilibrated for 10 min in the dark at room temperature. Luminescence was measured using a Synergy Mx multimode microplate reader (Biotek). To estimate the basal level of [Ca2+]in, the measurements were recorded for 1 min at 5-s intervals, the cells were challenged with 1 mM Ca2+ and mixed for 1 s, and then the luminescence was recorded for 20 min at 5-s intervals. The injection of buffer alone was used as a negative control and did not cause any significant fluctuations in [Ca2+]in. [Ca2+]in was calculated by using the formula pCa = 0.612 (−log10k) + 3.745, where k is a rate constant for luminescence decay (per second) (54). The results were normalized against the total amount of available aequorin as described previously (6). The discharge was performed by permeabilizing cells with 2% Nonidet 40 (NP-40) in the presence of 12.5 mM CaCl2. The luminescence released during the discharge was monitored for 10 min at 5-s intervals. The estimated remaining available aequorin was at least 10% of the total amount of aequorin. The experimental conditions reported here were optimized to prevent any significant cell lysis.
Swarming motility assay.
Swarming motility was assayed as described in reference 6. PAO1 and mutants were grown in 5 ml BMM with no added or 10 mM CaCl2. Two microliters of the mid-log cultures normalized to an optical density at 600 nm (OD600) of 0.3 were spot inoculated onto the surface of BM2 agar plates (62 mM potassium phosphate buffer [pH 7], 0.02 mM MgSO4, 10 μM FeSO4, 0.4% [wt/vol] glucose, 0.5% [wt/vol] Casamino Acids, and 0.5% [wt/vol] Difco agar) (34). After inoculation, the plates were incubated for 24 h, and then the colony diameters and morphology were recorded.
Pyocyanin analysis.
Pyocyanin production was assayed by using chloroform extraction as described previously (21), with modifications. Briefly, P. aeruginosa cultures grown in BMM until late log phase were normalized to an OD600 of 0.3. Two microliters of the normalized culture was inoculated in the middle of a BM2 agar plate (34) and incubated for 24 h at 37°C. Since swarming colonies spread within agar matrix, they were excised from the agar and split in halves, one to be used for pyocyanin extraction and the other for total cellular protein quantification. Agar slices of the same size were used as negative controls. The samples were mashed into fine pieces. Pyocyanin was extracted with 30 ml chloroform followed by 15 ml of 0.2N HCl. The absorbance of the extract was measured at 520 nm, and the amount of pyocyanin was calculated by using a coefficient of extinction of 17.1 M−1 cm−1 (55) and normalized per milligram of total cell protein. The latter was determined by using the Bradford assay (EMD) by following the manufacturer's protocol. The data shown represent one of three independent experiments, each including three biological replicates. Statistical significance was calculated using Student's t test.
Antibiotic susceptibility assays.
Antibiotic susceptibility assays were performed using tobramycin and polymyxin B E-strips (bioMérieux). In brief, strains were cultured in BMM with no added CaCl2 or 10 mM CaCl2 for 18 h and normalized to an OD600 of 0.1. One hundred microliters of the normalized cultures then was spread on BMM agar plates with or without added CaCl2. E-strips with tobramycin and polymyxin B gradients were placed onto the inoculated plates. After 24 h of incubation at 37°C, the MICs were recorded by determining the concentration of antibiotics on the strip at which no bacterial growth was detected. At least three replicates were tested in at least two independent experiments; the reported MICs are the mean values of the collected measurements. The coefficient of variation between biological replicates was less than 25%.
Bioinformatics analyses.
Sequence homology searches were performed using the NCBI nr database (GenBank release 160.1). Sequence alignments and phylogenetic analysis of histidine kinases and response regulators were performed using MEGA software (56). Homologous proteins were selected based on at least 25% identity over the full length of amino acid sequence. Functional domains were predicted using Pfam (57). Protein subcellular localization was predicted using pSORTb v3.0 (58) and Loc tree (59) analysis. Predictions of transmembrane helices and signal peptides were performed using TMHMM (60) and SignalP 4.0, respectively (61). Protein three-dimensional (3D) structure was predicted using HHpred (62) and iTASSER (63) and visualized using PyMOL (version 1.7.6; Schrödinger, LLC).
Microarray data accession number.
The microarray data have been deposited in NCBI's Gene Expression Omnibus (64) and are accessible through GEO Series accession number GSE74491.
RESULTS
Transcriptome response of P. aeruginosa to Ca2+.
We performed whole-genome transcriptome analysis of P. aeruginosa strain PAO1 and the mucoid cystic fibrosis strain FRD1 in order to identify P. aeruginosa genes whose transcription is positively or negatively regulated by Ca2+. The strains were cultured under low and high CaCl2 concentrations (no added or 10 mM added CaCl2) in both planktonic cultures and in biofilms. As with our prior proteomics results (20), the transcriptomics data showed both strain- and condition-specific effects on gene expression due to added Ca2+. Table 1 shows genes differentially regulated by Ca2+ (at least 2-fold change in expression and P < 0.05) for strain PAO1 and the same genes in strain FRD1. Among the genes upregulated by Ca2+ in PAO1 is the operon PA0102-PA0104, encoding a carbonic anhydrase and permease that likely are involved in CO2-mediated calcification (M. A. Patrauchan, unpublished data). The upregulated genes also included PA0320 and PA0327, encoding hypothetical proteins, whose role in calcium-dependent processes is described below. Also significantly upregulated by Ca2+ are the genes involved in quorum sensing, lasI and rhlR, as well as several genes that are regulated by the quorum-sensing systems. The latter include hcnA, encoding hydrogen cyanide production, and many of the pvd genes responsible for pyoverdine biosynthesis. Pyoverdine biosynthetic proteins were shown to increase in abundance in response to Ca2+ in our prior proteomics study (20).
TABLE 1.
P. aeruginosa PAO1 genes affected by high calcium levels
| PA no. and regulation status | Gene or domain | Change in growth between 0 and 10 mM CaCl2 conditions for strain: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PAO1 |
FRD1 |
||||||||
| Planktonic |
Biofilm |
Planktonic |
Biofilm |
||||||
| Fold change | P value | Fold change | P value | Fold change | P value | Fold change | P value | ||
| Upregulated by high Ca during growth in planktonic culture | |||||||||
| PA0102 | Carbonic anhydrase | 7.1 | <0.01 | 1.0 | 1.00 | −1.1 | 1.00 | −1.3 | 0.91 |
| PA0103 | Permease | 5.6 | <0.01 | 1.1 | 0.98 | 1.1 | 0.98 | −1.5 | 0.83 |
| PA0104 | Hypothetical | 3.5 | 0.03 | 1.3 | 0.91 | −1.3 | 1.00 | −1.0 | 1.00 |
| PA0122 | rahU | 6.0 | 0.02 | 1.6 | 0.74 | 2.1 | 0.28 | −2.6 | 0.26 |
| PA0320 | OB-fold | 17.1 | <0.01 | 1.0 | 1.00 | 1.3 | 0.96 | 1.8 | 0.69 |
| PAO327 | Ca2+-binding β-propeller | 13.0 | <0.01 | 1.0 | 1.00 | −1.0 | 1.00 | 1.0 | 1.00 |
| PA0940 | DUF2024 | 10.6 | <0.01 | 1.2 | 0.97 | −1.3 | 1.00 | 1.1 | 1.00 |
| PA0941 | Thioredoxin | 10.3 | <0.01 | 1.4 | 0.89 | −1.3 | 1.00 | 1.3 | 0.99 |
| PA0942 | DNA binding | 2.9 | 0.04 | 1.0 | 1.00 | −1.3 | 0.99 | −1.2 | 1.00 |
| PA1134 | DUF393 | 3.0 | 0.02 | 1.9 | 0.58 | −1.2 | 1.00 | 1.2 | 0.99 |
| PA1432 | lasI | 3.1 | 0.01 | 1.0 | 1.00 | −1.9 | 0.54 | −1.2 | 0.97 |
| PA2081 | kynB | 3.3 | 0.02 | 1.0 | 1.00 | −2.1 | 0.34 | 1.1 | 1.00 |
| PA2193 | hcnA | 2.4 | 0.04 | 1.0 | 1.00 | −1.8 | 0.72 | −1.4 | 0.89 |
| PA2384 | Fur-like | 3.1 | 0.02 | 2.1 | 0.32 | 1.0 | 1.00 | 1.1 | 1.00 |
| PA2386 | pvdA | 4.1 | <0.01 | 2.0 | 0.49 | −1.3 | 0.99 | 1.1 | 1.00 |
| PA2397 | pvdE | 2.6 | 0.02 | 2.2 | 0.29 | 1.1 | 1.00 | −1.2 | 1.00 |
| PA2412 | Pyoverdine synthesis | 7.6 | <0.01 | 1.9 | 0.54 | −1.1 | 1.00 | 1.4 | 1.00 |
| PA2413 | pvdH | 3.4 | <0.01 | 1.4 | 0.98 | −1.0 | 1.00 | 1.1 | 1.00 |
| PA2424 | pvdL | 2.6 | 0.03 | 2.2 | 0.35 | −1.3 | 0.94 | 1.8 | 0.74 |
| PA2425 | pvdG | 4.0 | <0.01 | 2.3 | 0.21 | −1.5 | 0.92 | 1.5 | 0.99 |
| PA2427 | Hypothetical | 3.8 | <0.01 | 1.4 | 0.89 | −1.1 | 1.00 | 1.7 | 0.77 |
| PA2656 | Histidine kinase | 9.7 | <0.01 | −1.1 | 1.00 | 1.1 | 1.00 | 1.1 | 1.00 |
| PA2657 | Response regulator | 12.6 | <0.01 | −1.5 | 0.89 | 1.0 | 1.00 | 1.1 | 1.00 |
| PA2658 | PepSY domain | 12.1 | <0.01 | −1.0 | 1.00 | 1.1 | 1.00 | 1.4 | 0.98 |
| PA2659 | PepSY domain | 12.4 | <0.01 | −1.1 | 1.00 | −1.2 | 1.00 | −1.1 | 1.00 |
| PA3322 | DNA binding | 2.8 | 0.03 | 1.0 | 1.00 | −1.0 | 1.00 | 1.0 | 1.00 |
| PA3407 | hasAp | 4.2 | <0.01 | 1.1 | 1.00 | 1.1 | 1.00 | −1.2 | 0.99 |
| PA3477 | rhlR | 4.4 | 0.04 | 2.0 | 0.50 | 1.3 | 1.00 | −1.8 | 0.79 |
| PA3478 | rhlB | 6.9 | 0.04 | 2.1 | 0.47 | 1.2 | 1.00 | −1.7 | 0.81 |
| PA3885 | tpbA | 7.1 | <0.01 | −1.0 | 1.00 | 4.3 | 0.07 | 2.4 | 0.16 |
| PA4141 | Hypothetical | 3.7 | 0.03 | 2.4 | 0.30 | 2.1 | 0.41 | −1.4 | 0.94 |
| PA4378 | inaA | 3.1 | 0.02 | −1.3 | 0.99 | 2.7 | 0.05 | 1.4 | 0.93 |
| PA4379 | Hypothetical | 5.1 | <0.01 | −1.6 | 0.83 | 3.8 | <0.01 | 1.9 | 0.65 |
| PA4469 | Hypothetical | 2.9 | 0.02 | 2.8 | 0.08 | 1.1 | 0.89 | 1.3 | 1.00 |
| PA4517 | DUF1705 | 5.0 | 0.01 | 1.1 | 1.00 | 15.0 | <0.01 | 1.1 | 1.00 |
| PA5530 | Permease | 5.7 | <0.01 | −1.4 | 0.79 | 4.6 | <0.01 | 1.3 | 1.00 |
| Upregulated by calcium during growth in biofilms, PA4570 | Hypothetical | 1.0 | 1.00 | 4.3 | 0.01 | −2.0 | 0.37 | −1.0 | 1.00 |
| Downregulated by high Ca during planktonic growth | |||||||||
| PA0391 | Hypothetical | −2.6 | 0.02 | −1.2 | 0.95 | −1.3 | 0.96 | −3.7 | 0.01 |
| PA0603 | Transporter | −2.5 | 0.03 | −1.0 | 1.00 | −2.6 | 0.05 | −1.1 | 1.00 |
| PA0610 | ptrN | −4.3 | 0.03 | −1.9 | 0.56 | −1.3 | 1.00 | −2.0 | 0.48 |
| PA0733 | Pseudouridine synthase | −3.5 | 0.01 | 1.0 | 1.00 | 1.1 | 1.00 | 1.1 | 1.00 |
| PA0865 | hpd | −6.5 | <0.01 | −1.4 | 0.86 | 1.6 | 0.89 | −1.2 | 0.98 |
| PA1336 | Histidine kinase | −4.2 | <0.01 | 1.2 | 1.00 | 1.1 | 1.00 | 1.1 | 1.00 |
| PA1541 | Efflux transporter | −2.6 | 0.03 | 1.1 | 1.00 | −1.1 | 1.00 | 1.3 | 0.98 |
| PA1559 | Epimerase | −3.0 | 0.01 | −2.0 | 0.45 | −16.6 | <0.01 | −13.1 | <0.01 |
| PA2780 | DNA binding | −2.7 | 0.01 | 1.1 | 0.99 | 1.2 | 0.99 | −1.4 | 0.94 |
| PA3217 | cyaB | −2.6 | 0.03 | −1.4 | 0.76 | 1.0 | 1.00 | −1.0 | 1.00 |
| PA4555 | pilY2 | −4.8 | 0.02 | 1.3 | 0.98 | 1.0 | 1.00 | −1.6 | 0.90 |
| PA4556 | pilE | −3.8 | 0.01 | 1.2 | 0.95 | 1.1 | 1.00 | −1.5 | 0.92 |
| PA4568 | rpmA | −2.6 | 0.04 | −1.1 | 1.00 | 1.3 | 1.00 | 1.3 | 0.99 |
| PA4699 | TPR domain | −2.5 | 0.03 | −1.1 | 1.00 | −1.0 | 1.00 | 1.3 | 0.99 |
| PA4823 | Hypothetical | −3.3 | 0.02 | −1.2 | 1.00 | −1.4 | 0.90 | 1.2 | 0.99 |
| PA5024 | Permease | −2.5 | 0.02 | 1.3 | 0.98 | 1.0 | 1.00 | 1.2 | 0.99 |
| Downregulated by high Ca during biofilm growth | |||||||||
| PA0316 | serA | 1.3 | 1.00 | −3.1 | <0.01 | 1.0 | 1.00 | −1.4 | 0.97 |
| PA0890 | aotM | 1.5 | 0.98 | −4.0 | 0.01 | −5.2 | <0.01 | −5.6 | <0.01 |
| PA1053 | Hypothetical | −1.4 | 0.90 | −3.2 | <0.01 | 6.7 | 0.12 | −3.0 | 0.02 |
| PA1927 | metE | −1.1 | 1.00 | −10.6 | <0.01 | −23.0 | <0.01 | −1.5 | 0.73 |
| PA4453 | ABC transporter | 1.1 | 1.00 | −2.6 | 0.03 | −1.1 | 1.00 | −1.9 | 0.65 |
| PA4455 | ABC transporter | −1.1 | 1.00 | −3.0 | 0.03 | −1.1 | 1.00 | −1.9 | 0.65 |
| PA4773 | decarboxylase | −1.8 | 0.72 | −9.1 | 0.01 | −27.6 | <0.01 | −23.1 | <0.01 |
| PA4774 | Spermidine biosynthesis | −2.3 | 0.28 | −12.3 | <0.01 | −42.9 | <0.01 | −37.4 | <0.01 |
| PA4775 | Hypothetical | −1.2 | 1.00 | −4.9 | 0.01 | −10.0 | <0.01 | −13.8 | <0.01 |
| PA4776 | pmrA | −1.3 | 1.00 | −4.3 | 0.02 | −7.4 | <0.01 | −9.4 | <0.01 |
| PA4777 | pmrB | 1.1 | 1.00 | −6.9 | <0.01 | −5.6 | <0.01 | −12.5 | <0.01 |
| PA4781 | Response regulator | −1.9 | 1.00 | −3.0 | 0.03 | −1.5 | 0.98 | −2.2 | 0.14 |
| PA4782 | Hypothetical | −2.7 | 0.19 | −12.0 | <0.01 | −11.6 | <0.01 | −23.4 | <0.01 |
| PA4826 | Hypothetical | −1.1 | 0.94 | −4.7 | 0.01 | −4.9 | 0.01 | −1.3 | 0.99 |
| Downregulated by high Ca during both planktonic and biofilm growth | |||||||||
| PA1178 | oprH | −58.8 | <0.01 | −28.2 | <0.01 | −1.5 | 0.89 | −12.8 | 0.04 |
| PA1179 | phoP | −15.1 | <0.01 | −18.1 | <0.01 | −1.3 | 0.98 | −11.6 | <0.01 |
| PA1180 | phoQ | −12.5 | <0.01 | −14.7 | <0.01 | −1.4 | 0.96 | −11.2 | <0.01 |
| PA1343 | Hypothetical | −12.2 | <0.01 | −13.5 | <0.01 | 5.5 | <0.01 | −6.3 | 0.07 |
| PA3552 | arnB | −12.5 | <0.01 | −4.8 | <0.01 | −5.2 | <0.01 | −20.3 | <0.01 |
| PA3553 | arnC | −11.1 | <0.01 | −4.7 | <0.01 | −8.3 | <0.01 | −29.4 | <0.01 |
| PA3554 | arnA | −11.5 | <0.01 | −6.5 | 0.02 | −27.5 | <0.01 | −43.5 | <0.01 |
| PA3556 | arnT | −5.6 | 0.01 | −5.1 | 0.01 | −22.8 | <0.01 | −37.1 | <0.01 |
| PA3559 | arnG | −5.2 | 0.02 | −5.9 | 0.01 | −5.9 | 0.04 | −14.4 | 0.01 |
| PA4010 | Glycosylase | −4.7 | <0.01 | −4.3 | 0.01 | −2.9 | 0.10 | −4.7 | <0.01 |
| PA4011 | Hypothetical | −3.5 | 0.02 | −4.1 | 0.01 | −3.2 | 0.11 | −5.1 | <0.01 |
| PA4359 | Transport | −4.2 | <0.01 | −3.8 | 0.01 | −4.1 | 0.01 | −11.5 | <0.01 |
| PA4825 | mgtA | −4.0 | 0.05 | −3.5 | 0.12 | −3.7 | 0.06 | −1.2 | 0.95 |
Ca2+ influences the expression of three TCSs, carSR, phoPQ, and pmrAB.
The expression of the novel TCS PA2656-PA2657 (here termed carSR) increased 10- to 12-fold in response to Ca2+, while the TCSs phoPQ and pmrAB had significantly reduced expression (Table 1). The carSR operon contains four genes, which include the histidine kinase, carS, and the response regulator, carR, as well as two small periplasmic lipoproteins (PA2658 and PA2659), that both contain peptidase propeptide domains with possible protease inhibitory function. The increased expression of this operon was observed during the growth of PAO1 in planktonic culture but not in biofilms or in FRD1 cultures. The expression of both phoPQ and pmrAB TCSs was downregulated by Ca2+ in planktonic and biofilm cultures of both PAO1 and FRD1 (Table 1). Other studies have shown that PhoPQ affects the expression of the arn operon (PA3552-PA3559), which is involved in the modification of lipopolysaccharide (LPS) and enhanced resistance to cationic peptide antibiotics (28, 65). The transcriptomics results here are consistent with those results and indicate that Ca2+ also caused the reduced expression of the arn operon in biofilm and planktonic cultures of both PAO1 and FRD1 (Table 1).
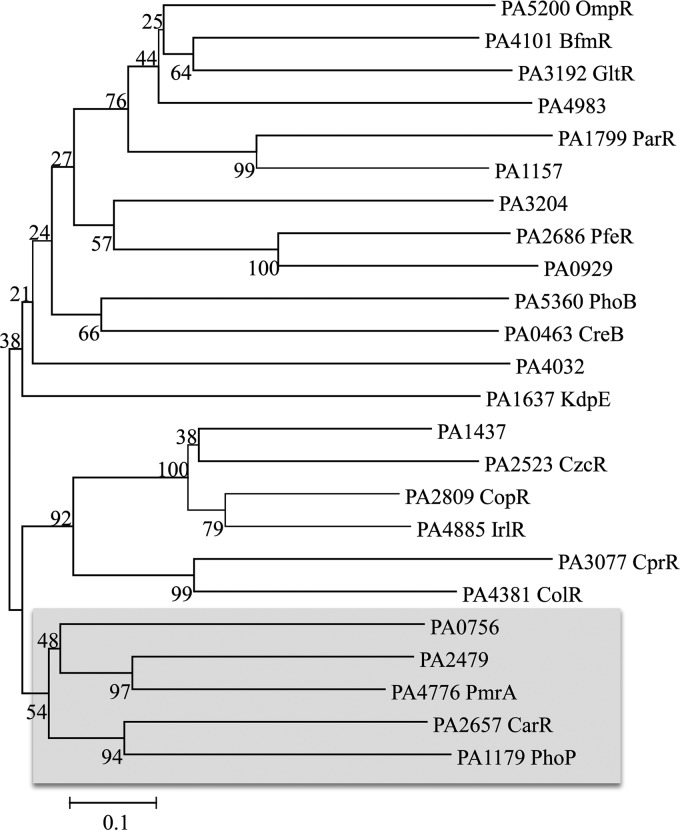
P. aeruginosa encodes multiple TCSs from each of the OmpR, NarL, and NtrC subgroups. We performed a sequence alignment of paralogs from each of the TCS clades for both the histidine kinases and the response regulators. The results of the OmpR subclade for the response regulators are shown in Fig. 1. Interestingly, although having regulatory responses opposite those of Ca2+, CarSR, PhoPQ, and PmrAB are closely related paralogs within the OmpR group, and all are involved in divalent cation sensing and osmoregulation (24).
FIG 1.
Phylogenetic analysis of P. aeruginosa response regulators in the OmpR clade of two-component systems. The OmpR response regulator protein sequences were aligned using MEGA software (56). Phylogenetic relationships were constructed by using neighbor-joining and bootstrapping analysis within the MEGA program. The shaded region shows the subclade that includes PhoP, PmrA, and CarR.
CarSR regulates PA0320 and PA0327 in a Ca2+-dependent manner.
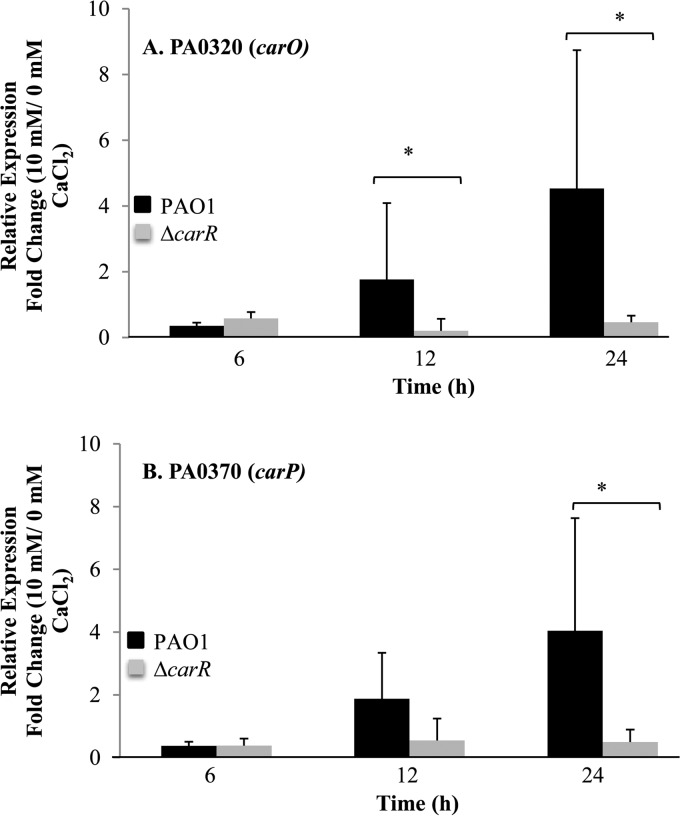
To identify the regulatory targets of CarSR, we constructed a deletion mutation of the carR response regulator in strain PAO1 and performed transcriptome analysis of the mutant strain grown at low and high CaCl2 concentrations. The results were compared to the responses of wild-type strain PAO1 to Ca2+. The expression of PA0320 and PA0327, which was highly upregulated by Ca2+ in the PAO1 strain, was unaffected by Ca2+ in the ΔcarR mutant strain, suggesting that CarSR directly (or indirectly) regulates the transcription of PA0320 and PA0327 in a Ca2+-dependent manner. To confirm the role of CarSR in the regulation of PA0320 and PA0327, we performed RT-qPCR on PAO1 and on the PAO1 ΔcarR mutant using PA0320- and PA0327-specific primers. The results show that at the 6-h time point, PA0320 and PA0327 were slightly repressed by Ca2+ in both strains (Fig. 2A and B). However, after 12 and 24 h of growth, the expression of both PA0320 and PA0327 was induced by Ca2+ in strain PAO1 but not in the ΔcarR mutant. PA0320 had a 4.5-fold increase in mRNA abundance due to Ca2+ at 24 h (P = 0.05) and PA0327 had a 4.1-fold increase due to Ca2+ (P = 0.05), as determined by a Mann-Whitney test. In the ΔcarR mutant strain, PA0320 and PA0327 mRNA was slightly reduced by Ca2+, confirming the role of carR in the Ca2+-dependent transcription of PA0320 and PA0327.
FIG 2.
RT-qPCR analysis of PA0320 (carO) and PA0327 (carP) in P. aeruginosa PAO1 and in the P. aeruginosa ΔcarR mutant. (A) Relative expression of carO in BMM with 10 mM CaCl2 compared to that with 0 mM CaCl2 for P. aeruginosa PAO1 and the P. aeruginosa ΔcarR mutant. (B) Relative expression of carP in BMM with 10 mM CaCl2 compared to that with 0 mM CaCl2 for P. aeruginosa PAO1 and the P. aeruginosa ΔcarR mutant. Data show the means and standard deviations for three biological replicates (three technical replicates for each biological replicate) at each time point. Asterisks represent a significant difference (at P = 0.05) in fold change, as determined by a Mann-Whitney test.
Predicted roles of PA0320 and PA0327 based on protein structural models.
Structural modeling of PA0320 using HHpred and iTASSER indicates that this protein contains an OB-fold (oligonucleotide/oligosaccharide binding motif) (see Fig. S1A and B in the supplemental material) with similarity to YgiW of Escherichia coli (66), which is required for cell survival in hydrogen peroxide and cadmium (67). PA0320 is predicted to have a signal peptide for transport of the protein to the periplasm. The 3D structure of PA0327, predicted by HHPred and I-Tasser, forms a 5-bladed β-propeller (see Fig. S1C and D). Structural homologs of PA0327 included YjiK, hemagglutinin-neuraminidase, α-l-arabinofuranosidases, and levansucrase, the latter two of which require Ca2+ for stability (reviewed in reference 68). The predicted SdiA-regulated domain is commonly found in TolB proteins, which include Ca2+-dependent phosphotriesterases. Sequence analysis predicts that the N terminus of PA0327 has an uncleaved transmembrane domain, suggesting that PA0327 is anchored in the cytoplasmic membrane with the rest of the protein facing the periplasm. Overall, the predicted structure of PA0327 suggests that it binds Ca2+ in a central pocket, and that Ca2+ binding is required for protein stability. PAO1 possesses two paralogs of PA0327 (PA0319 and PA2017) with 38 to 47% amino acid sequence identity. However, the expression of PA0319 and PA2017 is not affected by Ca2+. Therefore, although PA0319 is adjacent to and in the same orientation as PA0320, these two genes have different regulatory mechanisms. From the structural predictions, we refer to PA0320 as CarO (for calcium-regulated OB-fold protein) and PA0327 as CarP (for calcium-regulated β-propeller protein).
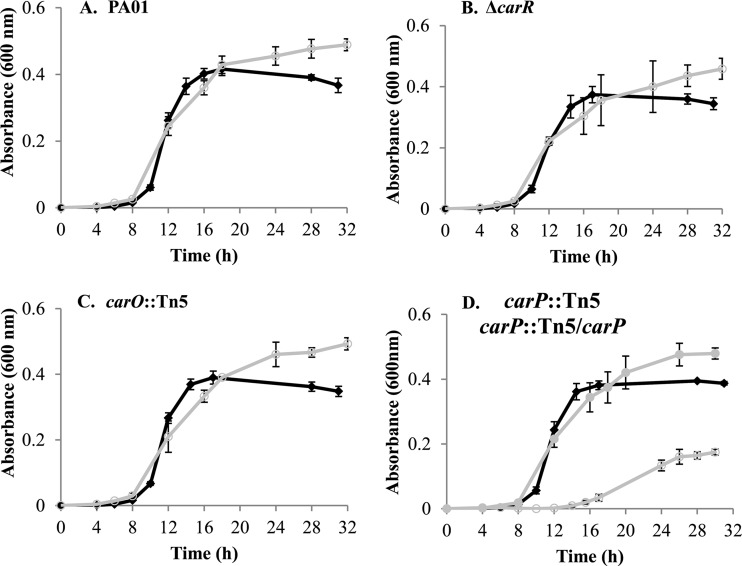
CarP is required for optimal growth of PAO1 in high [Ca2+] medium.
In order to characterize the functional roles of carO and carP in response to Ca2+, we obtained transposon mutants of each gene from the University of Washington two-allele library (69). Each mutation was complemented by cloning the respective gene into the single-copy Tn7 expression vector pTJ1 (46). To determine the effect of Ca2+ on growth, each mutant strain and its respective complemented counterpart was cultured in BMM with or without 10 mM CaCl2. The mutations in carR and carO did not affect growth at either Ca2+ level (Fig. 3). However, the carP mutation caused a defect in growth at high [Ca2+]. The lag phase of the carP::Tn5 mutant increased by 4 h, and the strain had a 2-fold decrease in growth rate. The maximum growth yield of the carP::Tn5 mutant also was reduced in the presence of 10 mM CaCl2. No effect on growth was observed for the carP::Tn5 mutant when no Ca2+ was added to the medium. Complementation of carP restored the wild-type growth characteristics (Fig. 3).
FIG 3.
Growth of P. aeruginosa PAO1 and mutant strains in BMM containing 0 mM CaCl2 (black diamonds) or 10 mM CaCl2 (open gray circles). (A) Growth of P. aeruginosa PAO1 in BMM. (B) Growth of P. aeruginosa ΔcarR mutant. (C) Growth of P. aeruginosa carO::Tn5 mutant. (D) Growth of P. aeruginosa carP::Tn5 mutant. Filled gray circles show the P. aeruginosa carP::Tn5 mutant complemented with carP. Data show the means and standard deviations for three biological replicates.
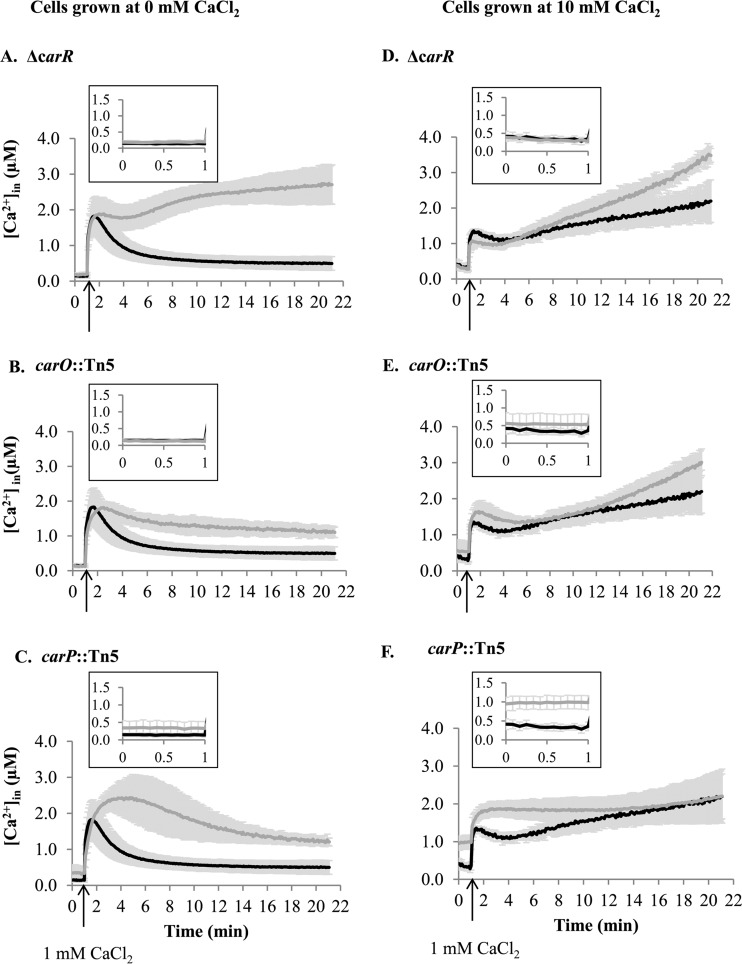
CarSR regulates Ca2+ homeostasis in PAO1 through the activities of CarO and CarP.
To characterize further the functional roles of CarRS and its regulatory targets, carO and carP, we measured the intracellular concentration of Ca2+ ([Ca2+]in) and monitored its changes in response to a rapid increase in extracellular CaCl2. For this, we expressed the recombinant Ca2+-binding luminescence protein, aequorin, in PAO1 and in its corresponding mutant strains. We assayed each strain when first cultured without CaCl2 or with 10 mM CaCl2. In the former case, PAO1 maintained [Ca2+]in at 0.14 ± 0.05 μM, which transiently increased in response to the addition of 1 mM CaCl2 (Fig. 4A to C, black lines) (6). Mutation in carR or carO did not affect the basal level of [Ca2+]in. The increase in the level of [Ca2+]in following the addition of 1 mM CaCl2 also was unaffected by the mutations (Fig. 4A and B, gray lines). However, the ensuing recovery to the resting [Ca2+]in was impaired, and the remaining level of [Ca2+]in was 6-fold higher in the ΔcarR strain (Fig. 4A) and 2-fold higher in the carO:Tn5 strain (Fig. 4B) than in wild-type PAO1, suggesting that CarR and CarO play roles in maintaining [Ca2+]in homeostasis. Unlike the ΔcarR and carO::Tn5 mutants, the carP::Tn5 strain had a 2.5-fold higher basal level of [Ca2+]in (0.34 ± 0.17 μM) than PAO1 (Fig. 4C, inset). Recovery to the resting [Ca2+]in from an immediate increase in response to external Ca2+ also was impaired, and the level of Ca2+in remained 2.5-fold higher in the carP::Tn5 strain (Fig. 4C, gray lines) than in PAO1 (Fig. 4C, black lines).
FIG 4.
Free [Ca2+]in profiles of P. aeruginosa wild-type strain PAO1 (black lines) and ΔcarR (A and D), carO::Tn5 (B and E), and carP::Tn5 (C and F) mutants (gray lines). Cells were grown in BMM with 0 mM CaCl2 (A, B, and C) or 10 mM CaCl2 (D, E, and F). CaCl2 (1 mM) was added at the time indicated by the arrow. Changes in free [Ca2+]in were calculated as described in Materials and Methods. Insets show the basal level of [Ca2+]in monitored for 1 min before 1 mM Ca2+ was added. Data show the means and standard deviations for at least three biological replicates.
The intracellular Ca2+ levels also were measured when the strains first were cultured with 10 mM CaCl2. For these experiments, cells first were cultured in BMM with 10 mM CaCl2, washed to remove excess CaCl2, and assayed for response to the rapid addition of 1 mM Ca2+. Under these conditions, the basal [Ca2+]in for the wild-type strain was 0.35 ± 0.09 μM, or 2.5-fold higher than that when cultured in BMM without Ca2+ (Fig. 4D to F, black lines). The addition of 1 mM external Ca2+ resulted in increased [Ca2+]in to 1.38 ± 0.03 μM and only partial recovery of resting [Ca2+]in after 4 min, followed by a slow increase to 2.17 ± 0.48 μM after 20 min (Fig. 4D, black line). Mutation in carR or carO did not significantly change the [Ca2+]in profile compared to that of the wild-type strain, with both strains showing partial recovery of the resting [Ca2+]in at 4 min, followed by a slow increase in [Ca2+]in (Fig. 4D and E, gray lines). However, the carP::Tn5 mutant strain had a very different response to externally added Ca2+ (Fig. 4F, gray lines). First, the basal level of [Ca2+]in was 3-fold higher than that in PAO1, at 0.98 ± 0.16 μM (Fig. 4F, inset). Second, recovery to basal levels following the addition of 1 mM CaCl2 was impaired compared to that of the wild type, and the [Ca2+]in remained elevated. The results indicate that [Ca2+]in homeostasis was impaired in all three mutant strains when grown at low [Ca2+] and was impaired in the carP::Tn5 mutant strain when first cultured with high [Ca2+].
CarP modulates Ca2+-induced swarming motility and pyocyanin production.
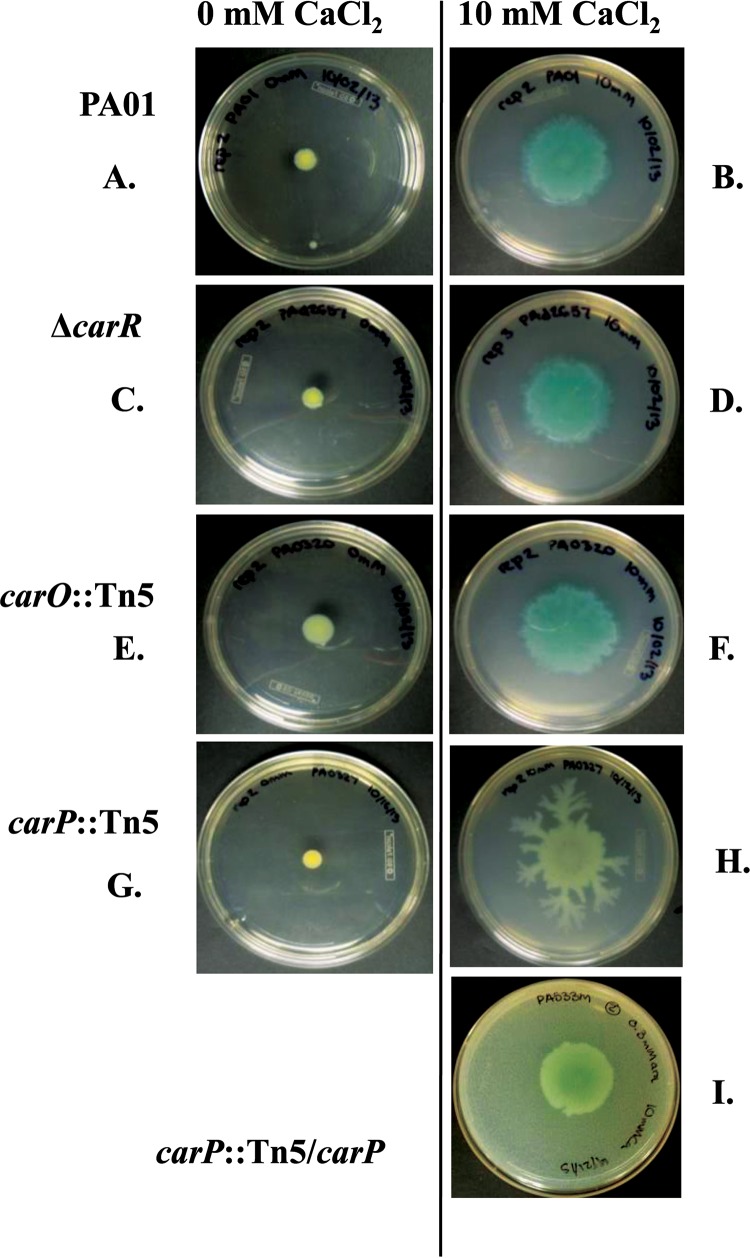
In previous work, we noted that Ca2+ addition results in phenotypic changes to P. aeruginosa (6). In particular, growth at elevated Ca2+ results in changes in swarming motility and pyocyanin production in the wild-type strain. The transcriptome data presented here show that elevated Ca2+ also causes an increase in the expression of genes for pyocyanin production (Table 1). Therefore, to determine if carR, carO, or carP plays a role in either of these Ca2+-dependent phenotypes, we assayed the mutant and complemented strains for swarming motility and pyocyanin production at low and high Ca2+ levels. As seen in the prior study (6), 10 mM Ca2+ caused a 5-fold increase in PAO1 swarming (Fig. 5A and B). Mutation of carR or carO did not have a significant effect on swarming motility at any Ca2+ level (Fig. 5C to F). In contrast, the mutation in carP significantly altered the morphology of swarming colonies at elevated [Ca2+], showing multiple branches that extended from the swarming colonies (Fig. 5G and H). The complementation of carP restored the wild-type swarming morphology (Fig. 5I).
FIG 5.
Swarming colonies of P. aeruginosa strains growing on BM2 agar with 0 or 10 mM CaCl2. PAO1, ΔcarR, carO::Tn5, carP::Tn5, and carP::Tn5/carP strains were tested. The complemented mutant was grown on BM2 swarm agar containing 10 mM CaCl2 and 0.3 mM arabinose. The diameter and morphology of the colonies were reported after 24 h of incubation. The pictures show representative photographs selected from at least three biological replicates collected from five independent experiments.
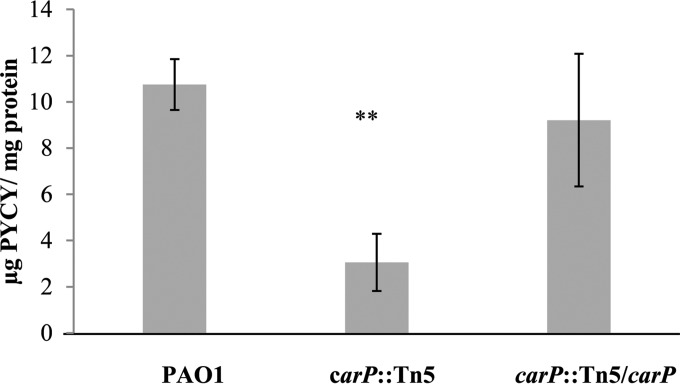
While the ΔcarR and carO::Tn5 mutants had pigment production similar to that of PAO1 at both low and high Ca2+ levels, the carP::Tn5 mutant appeared impaired for pigment production in swarming colonies. We extracted and quantified pyocyanin produced by the swarming colonies. Figure 6 shows that the Tn5 disruption of carP reduced the pyocyanin amount by 72%. The carP complemented strain had levels of pyocyanin production that were similar to those of the wild type.
FIG 6.
Pyocyanin production by P. aeruginosa PAO1, carP::Tn5, and carP::Tn5/carP strains. Pyocyanin was extracted from swarming colonies grown on BM2 plates in the presence of 10 mM CaCl2. The excised agar plugs containing the colonies were cut into halves. Pyocyanin amounts were quantified from half of the agar plug and then normalized to the total cellular protein extracted from the other half of the plug. The data represent the means and standard deviations for at least three biological replicates in three independent experiments. Statistical significance of the differences was calculated using Student's t test. **, P < 0.005.
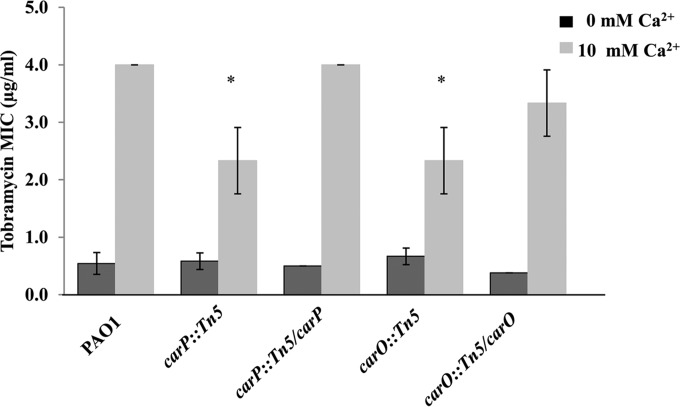
carO and carP mutations cause increased sensitivity to tobramycin at high [Ca2+].
Our earlier studies showed that growth at elevated Ca2+ increases the MIC of PAO1 to tobramycin and polymyxin B (S. Khanam, D. L. Lenaburg, R. Kubat, and M. A. Patrauchan, unpublished data). To assess the potential role of carR, carO, and carP in Ca2+-dependent resistance, we assayed each mutant and complemented strain for MIC using Etest strips (Fig. 7). In wild-type cells, the MIC for tobramycin was 8-fold greater in cells cultured at elevated Ca2+ levels. Both carO::Tn5 and carP::Tn5 strains had 2-fold reductions in tobramycin MIC when grown at elevated Ca2+ levels. The MIC for tobramycin was restored in these strains to the wild-type levels when the mutant genes were complemented. No difference in tobramycin susceptibility was detected in the carO::Tn5 and carP::Tn5 mutant cells cultured at low Ca2+ levels. The ΔcarR mutation did not affect tobramycin resistance at any Ca2+ level. The MIC of polymyxin B is 32-fold greater for the wild-type strain PAO1 when cells are cultured in the presence of 10 mM CaCl2 than when no CaCl2 is added to the medium. The mutations in carR, carO, or carP had no effect on polymyxin B resistance compared to that of the wild type at either Ca2+ concentration.
FIG 7.
MICs of tobramycin for P. aeruginosa PAO1, carO::Tn5, and carP::Tn5 mutants and their carO::Tn5-carO and carP::Tn5-carP complemented counterparts grown on BMM with 0 mM CaCl2 (dark gray bars) or 10 mM CaCl2 (light gray bars). Cells were grown in BMM without adding CaCl2 until mid-log phase, their OD600s were normalized to 0.1, and aliquots of 100 μl were plated onto BMM agar for MIC measurements. E-strips with tobramycin gradient were placed on the bacterial lawns, and the MICs were recorded after 24 h of incubation. The data represent the means and standard deviations from at least three biological replicates from two independent experiments. The statistical significance of the differences was calculated using Student's t test. *, P < 0.05.
DISCUSSION
Our earlier studies showed that Ca2+ regulates a number of physiological processes, including virulence, in the opportunistic pathogen P. aeruginosa (6, 19–21). However, the molecular mechanisms responsible for sensing extracellular Ca2+ and orchestrating the cellular responses are not known. Here, we applied a genome-wide transcriptome analysis of P. aeruginosa to identify genes whose transcription is differentially regulated by Ca2+. Among the genes identified as being induced by Ca2+ is an operon (PA2656-2659) containing the two-component regulatory system carSR. The CarSR proteins are closely related to other P. aeruginosa TCSs, PhoPQ and PmrAB, which are involved in Mg2+ sensing (24). Other Gram-negative bacteria have been shown to recognize or respond to extracellular Ca2+ through TCSs. In Salmonella enterica serovar Typhimurium, the PhoQ kinase of the PhoPQ system binds Ca2+, Mg2+, and Mn2+ and regulates the transcription of genes, including virulence factors (reviewed in reference 70). PhoQ has distinct Ca2+ and Mg2+ binding sites (71), suggesting an intricate nature of PhoPQ regulation by divalent cations. In Vibrio cholerae, the expression of the CarSR TCS negatively regulates polysaccharide production and biofilm formation in response to elevated Ca2+ (72). In Escherichia coli, AtoSC is induced by Ca2+ and regulates the biosynthesis and the intracellular distribution of cPHB [complexed poly-(R)-3-hydroxybutyrate], building the nonproteinaceous complexes that act as voltage-gated Ca2+ channels (73–75). Here, we demonstrate that the expression of the P. aeruginosa TCS CarSR increases in response to high levels of external Ca2+. CarSR in turn regulates the transcription of carO and carP genes, which are involved in Ca2+-dependent responses. Based on these results, we propose that CarSR is a Ca2+-regulated TCS in P. aeruginosa PAO1.
In addition to inducing the transcription of carSR, elevated Ca2+ represses the transcription of two closely related TCSs, phoPQ and pmrAB. McPhee showed that phoPQ and pmrAB also are downregulated by elevated Mg2+ (24, 25). Changes in Mg2+ concentrations in the media did not affect the transcription of carSR (24). The transcriptional response of the TCSs due to Ca2+ addition is affected by the mode of growth: expression of carSR is induced only in planktonic cultures, whereas expression of pmrAB and phoPQ is reduced in biofilms and in planktonic cultures. The results suggest an additional level of regulation coordinating responses to Ca2+ during a switch from planktonic to biofilm growth.
Comparison of the Ca2+-induced regulon reported here and the Mg2+-induced regulon reported previously (24) revealed that 36 PAO1 genes are positively regulated by elevated Ca2+ but not by elevated Mg2+. These genes include PA0102-0104, encoding carbonic anhydrase and permease, hypothetical proteins PA0320 (here named CarO) and PA0327 (here named CarP), which are regulated by carSR, and quorum-sensing systems las and rhl, together with 14 genes regulated by these quorum-sensing proteins (76–78). The latter include rahU, involved in modulating biofilm formation and interaction with host innate immunity, hcnA, involved in hydrogen cyanide production, kynB, involved in the anthranilate pathway, rhlB, essential for rhamnolipid biosynthesis, and pvd genes, involved in pyoverdine biosynthesis. This comparison found no genes that were significantly induced by elevated levels of both Ca2+ and Mg2+. However, 20 genes were downregulated by both cations. Most of these downregulated genes belong to the regulons of PhoPQ and PmrAB, including the arn operon (25, 28), which is involved in the modification of LPS with arabinose groups. The arn-mediated LPS modifications were shown to increase P. aeruginosa resistance to polymyxin B (27). However, here we observed a decreased transcription of the arn operon and an increase in resistance to polymyxin B in response to elevated Ca2+. This result suggests that P. aeruginosa possesses an alternative mechanism for resistance to polymyxin B that is positively regulated by Ca2+ and is independent of Arn modification of LPS.
The Ca2+-induced TCS CarSR regulates the expression of carO and carP in a Ca2+-dependent manner. CarSR, CarO, and CarP all are required for P. aeruginosa PAO1 response to elevated Ca2+ levels. In particular, CarSR, CarO, and CarP all are required to maintain intracellular [Ca2+] at a low level when cells are grown at low [Ca2+]. In addition, CarP is required for maintaining low cytosolic [Ca2+] and for optimal growth when cells are exposed to high [Ca2+]. CarP also is involved in other processes that are influenced by high [Ca2+], including modulating the amount of pyocyanin production and affecting swarming motility. Both CarO and CarP also contribute to Ca2+-induced resistance to tobramycin.
The molecular roles of CarO and CarP in Ca2+-dependent processes presently are unknown. Since CarO and CarP are located primarily in the periplasm, they likely are not involved in the direct biosynthesis of pyocyanin, flagella, flagellum motor, or chemotaxis system. However, they may allow the cells to sense or modulate the periplasmic level of Ca2+, which itself plays a role in regulating the production of these factors either directly or via reducing the intracellular concentration of Ca2+. Intracellular [Ca2+] may in turn affect the expression of genes required for pyocyanin production or swarming motility. Whether their activities are direct or indirect, CarO and CarP are important for P. aeruginosa responses to high levels of Ca2+, since the phenotypes observed for the carO and carP mutants occur primarily at high [Ca2+].
The results presented here indicate that the two-component system CarSR is responsible for P. aeruginosa sensing elevated levels of external Ca2+, and it is responding through the induction of its regulatory targets, carO and carP. CarO and CarP affect intracellular Ca2+ homeostasis, surface-associated motility, resistance to tobramycin, and the production of the virulence factor pyocyanin.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jessica Richards for her help with the bioinformatics analysis. We also thank Anthony Campbell from the School of Pharmacy and Pharmaceutical Sciences, Cardiff University, United Kingdom, for sharing his expertise and the templates for calculating [Ca2+]in, and Delfina Dominguez from The University of Texas at El Paso for sharing the E. coli strain carrying pMMB66EH.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00963-15.
REFERENCES
- 1.Zhao GJ, Hong GL, Liu JQ, Lu Y, Lu ZQ. 2014. Septic shock due to community-acquired Pseudomonas aeruginosa necrotizing fasciitis: a case report and literature review. Exp Ther Med 7:1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 3.Lakkis C, Fleiszig SM. 2001. Resistance of Pseudomonas aeruginosa isolates to hydrogel contact lens disinfection correlates with cytotoxic activity. J Clin Microbiol 39:1477–1486. doi: 10.1128/JCM.39.4.1477-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Gooderham WJ, Hancock RE. 2009. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol Rev 33:279–294. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- 6.Guragain M, Lenaburg DL, Moore FS, Reutlinger I, Patrauchan MA. 2013. Calcium homeostasis in Pseudomonas aeruginosa requires multiple transporters and modulates swarming motility. Cell Calcium 54:350–361. doi: 10.1016/j.ceca.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson S, Appanna VD, Huang J, Viswanatha T. 1992. A novel role for calcite in calcium homeostasis. FEBS Lett 308:94–96. doi: 10.1016/0014-5793(92)81059-U. [DOI] [PubMed] [Google Scholar]
- 8.Glunk C, Dupraz C, Braissant O, Gallagher KL, Verrecchia EP, Visscher PT. 2011. Microbially mediated carbonate precipitation in a hypersaline lake, Big Pond (Eleuthera, Bahamas). Sedimentology 58:720–738. doi: 10.1111/j.1365-3091.2010.01180.x. [DOI] [Google Scholar]
- 9.Steinhorst L, Kudla J. 2013. Calcium–a central regulator of pollen germination and tube growth. Biochim Biophys Acta 1833:1573–1581. doi: 10.1016/j.bbamcr.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. 2011. Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson WG, Marshall RW, Bowers GN. 1981. Ionized calcium in body-fluids. Crit Rev Clin Lab Sci 15:85–125. doi: 10.3109/10408368109105869. [DOI] [PubMed] [Google Scholar]
- 12.Blomfiel J, Warton KL, Brown JM. 1973. Flow-rate and inorganic components of submandibular saliva in cystic-fibrosis. Arch Dis Childhood 48:267–274. doi: 10.1136/adc.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg JM, Tymoczko JL, Stryer L. 2002. Biochemistry, vol 5 WH Freeman & Co, New York, NY. [Google Scholar]
- 14.Vitolo MR, Valente Soares LM, Carvalho EB, Cardoso CB. 2004. Calcium and magnesium concentrations in mature human milk: influence of calcium intake, age and socioeconomic level. Arch Latinoam Nutr 54:118–122. [PubMed] [Google Scholar]
- 15.Halmerbauer G, Arri S, Schierl M, Strauch E, Koller DY. 2000. The relationship of eosinophil granule proteins to ions in the sputum of patients with cystic fibrosis. Clin Exp Allergy 30:1771–1776. doi: 10.1046/j.1365-2222.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- 16.Lorin MI, Gaerlan PF, Mandel ID, Denning CR. 1976. Composition of nasal secretion in patients with cystic-fibrosis. J Lab Clin Med 88:114–117. [PubMed] [Google Scholar]
- 17.Sanders NN, Franckx H, De Boeck K, Haustraete J, De Smedt SC, Demeester J. 2006. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 61:962–968. doi: 10.1136/thx.2006.060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DJ, Anderson GJ, Bell SC, Reid DW. 2014. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J Cyst Fibros 13:289–295. doi: 10.1016/j.jcf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ. 2005. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J Bacteriol 187:4327–4337. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrauchan MA, Sarkisova SA, Franklin MJ. 2007. Strain-specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology 153:3838–3851. doi: 10.1099/mic.0.2007/010371-0. [DOI] [PubMed] [Google Scholar]
- 21.Sarkisova SA, Lotlikar SR, Guragain M, Kubat R, Cloud J, Franklin MJ, Patrauchan MA. 2014. A Pseudomonas aeruginosa EF-hand protein, EfhP (PA4107), modulates stress responses and virulence at high calcium concentration. PLoS One 9:e98985. doi: 10.1371/journal.pone.0098985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Hoch JA. 2000. Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3:165–170. doi: 10.1016/S1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 24.McPhee JB, Bains M, Winsor G, Lewenza S, Kwasnicka A, Brazas MD, Brinkman FS, Hancock RE. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J Bacteriol 188:3995–4006. doi: 10.1128/JB.00053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPhee JB, Lewenza S, Hancock REW. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol 50:205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Ernst RK, Miller SI. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol 186:575–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, Miller SI, Hoiby N, Moskowitz SM. 2011. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooderham WJ, Gellatly SL, Sanschagrin F, McPhee JB, Bains M, Cosseau C, Levesque RC, Hancock RE. 2009. The sensor kinase PhoQ mediates virulence in Pseudomonas aeruginosa. Microbiology 155:699–711. doi: 10.1099/mic.0.024554-0. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman FSL, MacFarlane ELA, Warrener P, Hancock REW. 2001. Evolutionary relationships among virulence-associated histidine kinases. Infect Immun 69:5207–5211. doi: 10.1128/IAI.69.8.5207-5211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon DH, Lu CD. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother 50:1615–1622. doi: 10.1128/AAC.50.5.1615-1622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perron K, Caille O, Rossier C, van Delden C, Dumas JL, Kohler T. 2004. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J Biol Chem 279:8761–8768. doi: 10.1074/jbc.M312080200. [DOI] [PubMed] [Google Scholar]
- 32.Teitzel GM, Geddie A, De Long SK, Kirisits MJ, Whiteley M, Parsek MR. 2006. Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J Bacteriol 188:7242–7256. doi: 10.1128/JB.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahme LG, Ausubel FM, Cao H, Drenkard E, Goumnerov BC, Lau GW, Mahajan-Miklos S, Plotnikova J, Tan MW, Tsongalis J, Walendziewicz CL, Tompkins RG. 2000. Plants and animals share functionally common bacterial virulence factors. Proc Natl Acad Sci U S A 97:8815–8821. doi: 10.1073/pnas.97.16.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overhage J, Lewenza S, Marr AK, Hancock RE. 2007. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J Bacteriol 189:2164–2169. doi: 10.1128/JB.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitchurch CB, Alm RA, Mattick JS. 1996. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 93:9839–9843. doi: 10.1073/pnas.93.18.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carterson AJ, Morici LA, Jackson DW, Frisk A, Lizewski SE, Jupiter R, Simpson K, Kunz DA, Davis SH, Schurr JR, Hassett DJ, Schurr MJ. 2004. The transcriptional regulator AlgR controls cyanide production in Pseudomonas aeruginosa. J Bacteriol 186:6837–6844. doi: 10.1128/JB.186.20.6837-6844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lizewski SE, Lundberg DS, Schurr MJ. 2002. The transcriptional regulator AlgR is essential for Pseudomonas aeruginosa pathogenesis. Infect Immun 70:6083–6093. doi: 10.1128/IAI.70.11.6083-6093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol 24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 39.Linares JF, Gustafsson I, Baquero F, Martinez JL. 2006. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci U S A 103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitchurch CB, Erova TE, Emery JA, Sargent JL, Harris JM, Semmler ABT, Young MD, Mattick JS, Wozniak DJ. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J Bacteriol 184:4544–4554. doi: 10.1128/JB.184.16.4544-4554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morici LA, Carterson AJ, Wagner VE, Frisk A, Schurr JR, Bentrup KHZ, Hassett DJ, Iglewski BH, Sauer K, Schurr MJ. 2007. Pseudomonas aeruginosa AlgR represses the Rhl quorum-sensing system in a biofilm-specific manner. J Bacteriol 189:7752–7764. doi: 10.1128/JB.01797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchings BW, Almira EC, Lory S, Ramphal R. 1995. Cloning and phenotypic characterization of Fles and Fler, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect Immun 63:4868–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breidenstein EBM, Khaira BK, Wiegand I, Overhage J, Hancock REW. 2008. Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob Agents Chemother 52:4486–4491. doi: 10.1128/AAC.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurley BP, Goodman AL, Mumy KL, Murphy P, Lory S, McCormick BA. 2010. The two-component sensor response regulator RoxS/RoxR plays a role in Pseudomonas aeruginosa interactions with airway epithelial cells. Microbes Infect 12:190–198. doi: 10.1016/j.micinf.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silo-Suh LA, Suh SJ, Ohman DE, Wozniak DJ, Pridgeon JW. 2015. Complete genome sequence of Pseudomonas aeruginosa mucoid strain FRD1, isolated from a cystic fibrosis patient. Genome Announc 3:e00153-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damron FH, McKenney ES, Schweizer HP, Goldberg JB. 2013. Construction of a broad-host-range Tn7-based vector for single-copy PBAD-controlled gene expression in Gram-negative bacteria. Appl Environ Microbiol 79:718–721. doi: 10.1128/AEM.02926-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 48.Watkins NJ, Knight MR, Trewavas AJ, Campbell AK. 1995. Free calcium transients in chemotactic and non-chemotactic strains of Escherichia coli determined by using recombinant aequorin. Biochem J 306:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irani VR, Rowe JJ. 1997. Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. Biotechniques 22:54–56. [DOI] [PubMed] [Google Scholar]
- 50.Lenz AP, Williamson KS, Pitts B, Stewart PS, Franklin MJ. 2008. Localized gene expression in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 74:4463–4471. doi: 10.1128/AEM.00710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart PS, Franklin MJ, Williamson KS, Folsom JP, Boegli L, James GA. 2015. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 59:3838–3847. doi: 10.1128/AAC.00433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson KS, Richards LA, Perez-Osorio AC, Pitts B, McInnerney K, Stewart PS, Franklin MJ. 2012. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J Bacteriol 194:2062–2073. doi: 10.1128/JB.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones HE, Holland IB, Baker HL, Campbell AK. 1999. Slow changes in cytosolic free Ca2+ in Escherichia coli highlight two putative influx mechanisms in response to changes in extracellular calcium. Cell Calcium 25:265–274. doi: 10.1054/ceca.1999.0028. [DOI] [PubMed] [Google Scholar]
- 55.Kurachi M. 1958. Studies on the biosynthesis of pyocyanine. (II) Isolation and determination of pyocyanin. Bull Inst Chem Res Kyoto Univ 36:13. [Google Scholar]
- 56.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 57.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD. 2012. The Pfam protein families database. Nucleic Acids Res 40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair R, Rost B. 2005. Mimicking cellular sorting improves prediction of subcellular localization. J Mol Biol 348:85–100. doi: 10.1016/j.jmb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Moller S, Croning MD, Apweiler R. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 61.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 62.Biegert A, Mayer C, Remmert M, Söding J, Lupas AN. 2006. The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res 34:W335–W339. doi: 10.1093/nar/gkl217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. 2015. The I-TASSER Suite: protein structure and function prediction. Nat Methods 12:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macfarlane EL, Kwasnicka A, Ochs MM, Hancock RE. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol Microbiol 34:305–316. doi: 10.1046/j.1365-2958.1999.01600.x. [DOI] [PubMed] [Google Scholar]
- 66.Fukushima K, Kumar SD, Suzuki S. 2012. YgiW homologous gene from Pseudomonas aeruginosa 25W is responsible for tributyltin resistance. J Gen Appl Microbiol 58:283–289. doi: 10.2323/jgam.58.283. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Hiibel SR, Reardon KF, Wood TK. 2010. Identification of stress-related proteins in Escherichia coli using the pollutant cis-dichloroethylene. J Appl Microbiol 108:2088–2102. [DOI] [PubMed] [Google Scholar]
- 68.Chen CK, Chan NL, Wang AH. 2011. The many blades of the beta-propeller proteins: conserved but versatile. Trends Biochem Sci 36:553–561. doi: 10.1016/j.tibs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prost LR, Miller SI. 2008. The Salmonellae PhoQ sensor: mechanisms of detection of phagosome signals. Cell Microbiol 10:576–582. doi: 10.1111/j.1462-5822.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 71.Vescovi EG, Ayala YM, Di Cera E, Groisman EA. 1997. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+. J Biol Chem 272:1440–1443. [DOI] [PubMed] [Google Scholar]
- 72.Bilecen K, Yildiz FH. 2009. Identification of a calcium-controlled negative regulatory system affecting Vibrio cholerae biofilm formation. Environ Microbiol 11:2015–2029. doi: 10.1111/j.1462-2920.2009.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theodorou MC, Panagiotidis CA, Panagiotidis CH, Pantazaki AA, Kyriakidis DA. 2006. Involvement of the AtoS-AtoC signal transduction system in poly-(R)-3-hydroxybutyrate biosynthesis in Escherichia coli. Biochim Biophys Acta 1760:896–906. doi: 10.1016/j.bbagen.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Reusch RN, Huang R, Bramble LL. 1995. Poly-3-hydroxybutyrate/polyphosphate complexes form voltage-activated Ca2+ channels in the plasma membranes of Escherichia coli. Biophys J 69:754–766. doi: 10.1016/S0006-3495(95)79958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theodorou MC, Tiligada E, Kyriakidis DA. 2009. Extracellular Ca2+ transients affect poly-(R)-3-hydroxybutyrate regulation by the AtoS-AtoC system in Escherichia coli. Biochem J 417:667–672. doi: 10.1042/BJ20081169. [DOI] [PubMed] [Google Scholar]
- 76.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuster M, Lostroh CP, Ogi T, Greenberg E. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bredenbruch F, Geffers R, Nimtz M, Buer J, Häussler S. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ Microbiol 8:1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.