Abstract
Background
Management options for pancreatic neuroendocrine tumors (pNETs) metastatic to the liver include surgical, ablative, cytotoxic, and radioisotope approaches. One potential local treatment option includes selective internal radiotherapy utilizing yttrium-90 (90Y) microspheres. 90Y has also been used in the treatment of hepatocellular carcinoma and tumors metastatic to the liver. It appears to be well tolerated; however, there is no randomized controlled trial reporting long-term toxicities. Previous retrospective reports have described biliary damage as a potential complication of therapy with 90Y and chemoembolization; however, the long-term sequelae of 90Y treatment are poorly understood.
Case Presentation
We present the case of a 65-year-old Caucasian woman who suffered biliary damage following 90Y administration for metastatic pNETs and subsequently developed cirrhosis. Given the timeline of her various treatments and the lack of any other identifiable etiology for her cirrhosis, we believe this to be a potential long-term complication of 90Y therapy.
Conclusion
This case provides pathologic confirmation of cirrhosis as a potential long-term sequela of 90Y treatment. This long-term risk needs to be considered when sequencing therapy for patients with neuroendocrine tumors who have a good prognosis. There are now several other systemic and ablative treatment options available to these patients, and long-term complications must be considered during treatment.
Key Words: Fibrosis, Microspheres, Liver disease, Toxicity, Radiation
Introduction
Pancreatic neuroendocrine tumors (pNETs) represent a relatively uncommon form of malignancy with an incidence of 0.43 cases per 100,000 in the USA [1]. At diagnosis, nearly 70% of patients have metastatic disease, of which 85% will have liver metastases [2]. Management options for pNETs metastatic to the liver include surgical, ablative, cytotoxic, and radioisotope approaches. Unfortunately, due to the scarcity of these tumors there is a paucity of randomized trials to guide optimal therapy sequencing. The North American Neuro-endocrine Tumor Society and European Neuroendocrine Tumor Society both support the use of radioembolization for progressive or symptomatic liver metastasis [3, 4]. To date, yttrium-90 (90Y) therapy has appeared safe; however, there is no randomized controlled trial assessing toxicities [5]. We present the case of a woman undergoing 90Y therapy for metastatic pNET to the liver who developed liver enzyme elevation and subsequent cirrhosis following treatment. There are only 3 other reported de novo cases of cirrhosis following 90Y administration, with only 1 demonstrating confirmatory pathology [6, 7, 8].
Case Report
A 65-year-old woman presented with abdominal discomfort and decreased appetite. Ultrasonography and computed tomography (CT) of the abdomen revealed a 9.5 × 8.6 × 10.5 cm, heterogeneous, hypervascular mass adjacent to the spleen and abutting the stomach wall and tail of the pancreas. Fine-needle aspiration guided by endoscopic ultrasound revealed cytologic evidence of a neuroendocrine tumor. The patient proceeded to a distal pancreatectomy, splenectomy, wedge resection of the stomach, and partial resection of the left adrenal gland. Pathology demonstrated a 13-cm, well-differentiated neuroendocrine tumor of the pancreas with perineural invasion, but no vascular invasion and negative margins. It was found to be adherent to both the spleen and the stomach, but did not invade either. Two lymph nodes were removed, and both were negative for metastases. It had a mitotic rate of 2 mitoses/high-power field and a Ki-67 index of <2%. There were no signs of metastatic disease on staging.
Two months postoperatively, the patient was found to have 4 subcentimeter hypervascular lesions in the liver which were 111In octreotide scan negative. Over the following 9 months, the patient developed 8 new lesions, while the original lesions increased to a maximum size of 1.2 cm. Therapy with octreotide LAR 20 mg intramuscularly once monthly was initiated but discontinued after 9 months due to progressive hepatic disease. The patient subsequently underwent a bland embolization of the right hepatic artery. A CT scan of the liver performed 3 months after embolization demonstrated a mixed tumor response with the overall impression of progressive disease and development of new liver metastasis.
The patient was presented with the option of systemic therapy with everolimus, shown in phase III trials to improve progression-free survival in patients with well-differentiated pNETs [9]. Due to the absence of extrahepatic metastasis, liver-directed therapy with 90Y embolization was also offered, which the patient chose to proceed with. Prior to 90Y treatment, there was no radiologic evidence of cirrhosis and the liver enzymes were within normal ranges (AST 24 U/l [normal range (N) 10–38 U/l], ALT 36 U/l [N <50 U/l], alkaline phosphatase 156 U/l [N 50–200 U/l], total bilirubin 5 μmol/l [N 0–18 μmol/l]). She had a technetium-99 macroaggregate albumin planning SPECT CT demonstrating multiple focal regions of increased activity in the left and right lobes of the liver which corresponded to the patient's known metastases.
Radioembolization with 3.5 GBq of 90Y TheraSpheres® was administered to the right hepatic artery, delivering a total dose of 140 Gy to the right lobe of the liver, and 1.8 GBq of 90Y TheraSpheres® was delivered through the left hepatic artery with a dosing of 140 Gy to the left lobe of the liver on the same day. Over the following 3 months, the patient developed abdominal pain and nausea and was found to have elevated liver enzymes, in a pattern suggesting damage to the biliary tree (AST 52 U/l, ALT 86 U/l, alkaline phosphatase 405 U/l, GGT 604 U/l, total bilirubin 5 μmol/l). CT scanning of the abdomen demonstrated generalized mottling of the liver, compatible with postembolization edema with a substantial decrease in tumor burden and 2 significant lesions remaining. A repeat 90Y radioembolization focused on the residual lesions was not technically feasible. The patient's liver enzymes remained elevated over the following 6 months until she developed significant right upper quadrant pain. CT 9 months after 90Y treatment revealed a very inhomogeneous liver texture and a large biloma traversing segments 5 and 8 of the liver, measuring 8.5 × 6.5 × 5.5 cm. A radiographic impression of liver surface nodularity was noted. This was initially noted 10 months after 90Y treatment and was slightly progressive on the 14-month scan (fig. 1). Imaging also demonstrated multiple subcentimeter right hepatic lesions raising concern for metastatic disease, some of which had not previously been seen.
Fig. 1.
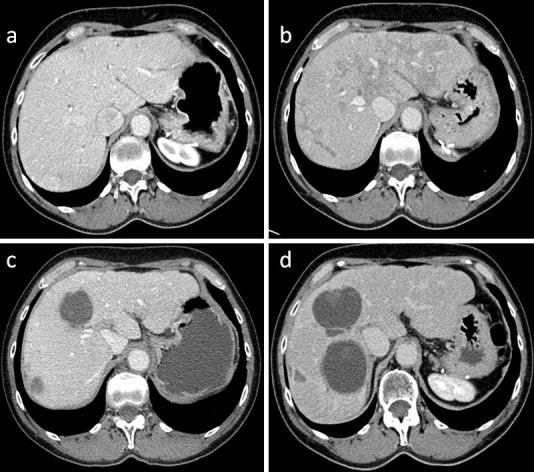
Contrast-enhanced CT imaging demonstrating no cirrhotic changes prior to 90Y administration (a) and generalized mottled changes compatible with postembolization edema 3 months after 90Y administration (b). The patient subsequently developed a large, nonenhancing, complex cystic central hepatic lesion suggestive of a post-90Y biloma 14 months after administration (c) and over the subsequent 45 months developed a progressively irregular texture suggestive of hepatic cirrhosis (d).
Due to radiographic evidence of progressive disease, everolimus 10 mg once daily was initiated, and the patient continued on this for 36 months with stabilization of the disease. Her liver enzymes remained unchanged over this time, with elevated hepatobiliary enzymes and borderline-elevated hepatocellular enzymes. At 25 months after initiation of everolimus therapy, progressive disease was noted in 2 solitary hepatic lesions and was treated with radiofrequency ablation. Due to disease control in the remainder of the hepatic parenchyma, everolimus was resumed after radiofrequency ablation.
Thirty-five months after initiation of everolimus therapy, radiographic note was made of the advanced cirrhotic appearance of the liver (fig. 1) and the increase in size of a left hepatic lesion. There was no evidence of portal hypertension. Due to the radiographic evidence of progressive disease and the cirrhotic appearance of the liver, everolimus therapy was interrupted and a liver biopsy to further characterize the nature of the parenchymal disease was performed. A single 18-gauge core was obtained from the left lobe of the liver in order to avoid the biloma involving the right lobe. Non-tumor-containing tissue was biopsied. The biopsy demonstrated large fibrotic bands in keeping with cirrhosis with mildly active steatohepatitis (approx. 10% macrovesicular steatosis) (fig. 2).
Fig. 2.
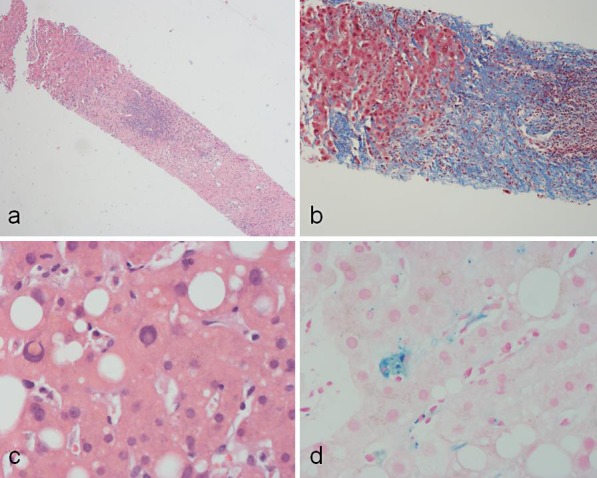
Needle core biopsy of the liver taken 47 months after 90Y administration. a There is a large band of chronically inflamed fibrous tissue, highly suggestive of cirrhosis. HE. ×10. b This is highlighted by Masson trichrome staining, demonstrating chronically inflamed fibrous tissue (blue), highly suggestive of cirrhosis. ×10. c The nonfibrotic liver showed macrovesicular steatosis with mildly active steatohepatitis. HE. ×40. d On the other hand, sinusoidal Kupffer cells showed a mild degree of hemosiderosis. Perls iron stain. ×40.
The patient had not been started on any other new medications, did not drink alcohol excessively, performed no high-risk activities for viral hepatitis, and had no family history of liver disease. Serology was nonreactive for HBSAg, HBCAb, and hepatitis C virus, and the patient had immunity to hepatitis B virus. Her antinuclear antibody was equivocal, and her anti-smooth muscle antibody and antimitochondrial antibody were negative. Given the chronologic association of 90Y administration with liver enzyme elevation, with the exclusion of other causes of cirrhosis, the diagnosis of 90Y-induced cirrhosis was made. She has not suffered any cirrhosis-associated complications and is currently Child-Turcotte-Pugh class A.
Discussion
To date, 90Y-related cirrhosis has only been reported in 3 cases [6, 7, 8]. A prior systematic review assessing outcomes following 90Y radioembolization identified grade 3 and higher adverse events in 0–12.9% of patients. In their review, the authors were able to identify 1 episode of cirrhosis and 3 episodes of hepatic failure [5]. The 3 cases of hepatic failure were reported with no mention of cirrhosis. The 1 cirrhotic patient identified was seen by Saxena et al. [6], who described a patient with cirrhosis and ascites 6 months after treatment with grade 3 elevation of serum alkaline phosphatase and grade 2 elevations of ALT and bilirubin. It is unclear whether the patient had tissue biopsy confirmation of cirrhosis.
Other reported cases of cirrhosis come from case reports by Brock et al. [7] and Ayav et al. [8]. Brock et al. [7] described a patient with metastatic breast cancer with bone and liver metastases who received 90Y. The patient had a good response to treatment at 4 weeks, but 3 months later developed CT imaging findings consistent with cirrhosis and portal hypertension. The patient subsequently died. There was no tissue biopsy. Ayav et al. [8] described a 39-year-old man who received 90Y for colon carcinoma metastatic to the liver. Four months after administration, the patient was found to have signs of portal hypertension and had a biopsy consistent with cirrhosis. The timeline of initial hepatic changes seen with our patient closely resembles these previous reports, with a subacute onset of irregular echotexture and cirrhosis occurring several months following treatment with 90Y [6, 7, 8]. 90Y microspheres deliver focused radiotherapy to metastases in the liver while sparing viable hepatic tissue, due to the predominant arterial supply of hepatic metastases as compared to the predominantly portal venous blood supply of normal liver parenchyma. An assessment of explanted livers following 90Y treatment showed no fibrosis >1 cm from tumor borders [10]. The case of our patient adds to 3 previously reported cases that demonstrate cirrhosis as a potential long-term complication of 90Y treatment. This is clinically relevant as it may influence therapy sequencing. Multiple ablative and systemic treatment options exist for patients with metastatic, unresectable pNETs. An increasingly long median life expectancy is observed with pNETs, with a 5-year overall survival of patients with low-grade tumors of 75% [3]. Therapies with a significant rate of long-term toxicities should therefore be avoided early in the course of therapy among patients with advanced disease.
The notion that 90Y therapy may lead to cirrhosis is supported by radiographic studies assessing volume changes and signs of portal hypertension following 90Y treatment. In a series of 17 patients treated with 90Y, a mean decrease in liver volume of 11.8% and a mean increase in splenic volume of 27.9% were noted, accompanied by increases in portal vein diameter [11]. In another series of 45 patients, liver volumes were shown to decrease in lobes treated with radioembolization by up to 45% at 12 months after treatment [12]. The difficulty with utilizing volume changes and liver texture to define outcomes following 90Y administration lies in differentiating normal radiographic changes following radioembolization from true cirrhosis. In the current case report, imaging was reported as being consistent with prior radioembolization throughout many of the reports, with a progressive worsening noted over time. While differentiating this from cirrhosis is very difficult, the differential for these radiographic changes needs to include cirrhosis.
The only other agent that could be chronologically associated with cirrhosis in our patient is everolimus; however, this was started after radiographic changes had already been seen in the liver. In addition, early preclinical work suggests that inhibition of the mTOR pathway with mTOR inhibitors may be antifibrotic [13]. There is, however, 1 case report of steatohepatitis following administration of everolimus [14]. While our patient did not have steatosis severe enough to be considered solely responsible for the observed cirrhosis, it is possible that she suffered an initial insult from 90Y administration and had impaired hepatic regeneration subsequently due to everolimus-induced steatosis, thus causing accelerated progression to cirrhosis.
In addition to cirrhosis, our patient suffered a second complication of 90Y administration. She developed a large biloma 10 months following 90Y treatment. In a 327-patient retrospective review by Atassi et al. [15], 10.1% of patients had developed some form of biliary complication following 90Y administration. In contrast to normal hepatocytes, intrahepatic bile ducts lack a dual blood supply and are fed by intrahepatic arteries. Biloma development is thought to develop following peripheral bile duct necrosis, resulting in biliary drainage into surrounding tissue. The damage can range from mild bile duct dilation to severe strictures. Most of these lesions remain stable, with minimal intervention required.
Conclusions
This case report adds to a growing body of evidence that 90Y treatment may cause serious damage to the biliary tree, and it is clinically important because it demonstrates pathology confirming that a long-term outcome of 90Y administration may be cirrhosis. This long-term risk needs to be considered when sequencing therapy for patients who have a high rate of 5-year survival and who have other systemic and ablative treatment options.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report.
Disclosure Statement
All authors report no conflicts of interest.
References
- 1.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. vii. [DOI] [PubMed] [Google Scholar]
- 2.Pape U-F, Bernd U, Müller-Nordhorn J, Böhmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 3.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 4.Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang TX, Chua TC, Morris DL. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases – a systematic review. Surg Oncol. 2012;21:299–308. doi: 10.1016/j.suronc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251:910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 7.Brock H, Günther RW, Haage P. Liver cirrhosis as sequela of selective hepatic radio-embolization with yttrium (Y-90) microspheres (in German) Rofo. 2006;178:546–548. doi: 10.1055/s-2005-858926. [DOI] [PubMed] [Google Scholar]
- 8.Ayav A, Habib N, Jiao LR. Portal hypertension secondary to 90Yttrium microspheres: an unknown complication. J Clin Oncol. 2005;23:8275–8276. doi: 10.1200/JCO.2005.03.7820. [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Jakobs TF, Saleem S, Atassi B, Reda E, Lewandowski RJ, Yaghmai V, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90Yttrium microspheres. Dig Dis Sci. 2008;53:2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 12.Theysohn JM, Ertle J, Müller S, Schlaak JF, Nensa F, Sipilae S, et al. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014;69:172–178. doi: 10.1016/j.crad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786–796. doi: 10.1016/j.jhep.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Schieren G, Bölke E, Scherer A, Raffel A, Gerber PA, Kröpil P, et al. Severe everolimus-induced steatohepatis [sic!]: a case report. Eur J Med Res. 2013;18:22. doi: 10.1186/2047-783X-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atassi B, Bangash AK, Lewandowski RJ, Ibrahim S, Kulik L, Mulcahy MF, et al. Biliary sequelae following radioembolization with yttrium-90 microspheres. J Vasc Interv Radiol. 2008;19:691–697. doi: 10.1016/j.jvir.2008.01.003. [DOI] [PubMed] [Google Scholar]


