Abstract
The cyclic adenosine monophosphate (cAMP) signaling pathway plays an essential role in immune functions. In this study we examined the role of the cAMP/EPAC1 (exchange protein directly activated by cAMP) axis in regulatory T-cell (Treg)-mediated immune suppression using genetic and pharmacologic approaches. Genetic deletion of EPAC1 in Treg and effector T-cells (Teff) synergistically attenuated Treg-mediated suppression of Teff. Mechanistically, EPAC1 inhibition enhanced activation of the transcription factor STAT3 and up-regulated SMAD7 expression while down-regulating expression of SMAD4. Consequently, CD4+T-cells were desensitized to TGF-β1, a cytokine employed by Treg cells to exert a broad inhibitory function within the immune system. Furthermore, deletion of EPAC1 led to production of significant levels of OVA-IgG antibodies in a low dose oral tolerance mouse mode. These in vivo observations are consistent with the finding that EPAC1 plays an important role in Treg-mediated suppression. More importantly, pharmacological inhibition of EPAC1 using an EPAC specific inhibitor recapitulates the EPAC1 deletion phenotype both in vivo and in vitro. Our results show that EPAC1 boosts Treg-mediated suppression, and identify EPAC1 as a target with broad therapeutic potential since Treg cells are involved in numerous pathologies including autoimmunity, infections, and a wide range of cancers.
Keywords: Regulatory T-cells, cAMP, EPAC1, guanine nucleotide exchange factor (GEF), STAT3, TGF-β1, gap junctions
INTRODUCTION
Regulatory T-cells (Treg) are T-lymphocytes that possess a general suppressor function and play an essential role in both adaptive and innate immunity [1, 2]. They maintain peripheral tolerance to prevent autoimmunity and control the immune response to preclude exaggerated reactions to infections and innocuous antigens [1]. Due to their broad control over the immune system, Treg cells have tremendous therapeutic potential and can be exploited for the treatment of a plethora of diseases. For instance, numerous studies have shown that solid tumors exploit these cells to escape immune surveillance and eliminating the Treg population with antibodies improves prognosis in cancer patients [3–5].
Treg cells employ various mechanisms to inhibit proliferation and activation of other immune cells. Some of these mechanisms are contact-dependent, including suppression through membrane-bound TGF-β1 and the expression of cytotoxic T-lymphocyte protein 4 (CTLA-4), which binds to receptors on the target immune cells, leading to hypo-signaling and anergy [6, 7]. Treg cells also utilize contact-independent mechanisms that rely on secretion of immunosuppressive cytokines like IL-10 and IL-35 [7]. However, the molecular signaling networks underlying the various mechanisms of Treg-mediated suppression remain poorly understood.
Recent studies have shown that cyclic adenosine monophosphate (cAMP), a potent regulator of innate and adaptive immunity [8–10], plays an essential role in Treg-mediated suppression, and this role is transduced by cAMP-dependent protein kinase (PKA)-dependent and -independent pathways [7, 11–13]. These observations potentially suggest the involvement of the cAMP/EPAC (exchange protein activated by cAMP) axis. However, the role of EPAC in modulating Treg function has not been examined and no studies have directly linked EPAC to Treg-mediated suppression. EPAC is a family of cAMP sensor proteins, composed of EPAC1 and EPAC2, which act primarily as guanine exchange factors (GEF) for the small GTPase effector proteins Rap1 and Rap2 [14, 15]. While EPAC2 has not been detected in the immune system, EPAC1 is expressed in T and B-lymphocytes, monocytes, and macrophages [9, 16–18]. In this study, we examined the role of EPAC1 in Treg-mediated suppression using genetic and pharmacologic approaches. Our findings indicate that EPAC1 plays an important role in enhancing Treg suppression of effector T-cells (Teff): it boosts Treg suppressive potency while sensitizing Teff to suppression.
EXPERIMENTAL
Antibodies
The following antibodies were used: EPAC1 (# 4155), EPAC2 (# 4156), phospho-STAT3 (Tyr705) (# 9131), STAT3 (# 4904), SHP-1 (# 3759), SHP-2 (# 3397), SOCS3 (# 2923), LCK (# 2752), p-LCK (Try505) (# 2751), JAK2 (# 3230), SMAD2 (# 5339), p-SMAD2 (Ser465/467) (# 3108), and SMAD4 (# 9515) (Cell Signaling Technology, Inc., Danvers, MA). Actin–Cy3 (C 5838) (Sigma-Aldrich, St. Louis, MO).SMAD7 (sc-11392) (Santa Cruz Biotechnology, Dallas, TX).
Mice
The generation of EPAC1 global knockout mice was described previously [19]. Mice used in this study were backcrossed to the C57BL/6 background for more than 10 generations and derived from wild-type or homozygous littermates. The mice were housed with a 12-h–12-h light-dark cycle, with free access to water and food. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch or the University of Texas Health Science Center at Houston.
In vitro suppression assay
Single-cell suspensions were prepared from spleens of 8–10 week old female mice and Teff (CD4+CD25−) and Treg (CD4+CD25+) cells were isolated using an EasySep™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit (Stemcell Technologies, Vancouver, Canada). The purity of Treg was determined by FACS by staining isolated cells with PerCP-Cy 5.5-conjugated anti-CD4 and PE-conjugated anti-CD25 antibodies (BD Biosciences, San Jose, California). Treg cells used were ≥90% pure. 5 × 104 Teff cells were cultured in complete RPMI (RPMI 1640, 10% FCS, 2 mmol/L L-glutamine, penicillin/streptomycin) alone (positive control), or with Treg (2.5× 104). Wells with Treg cells alone were used as a negative control. Plates were pre-coated with 5 µg/mL anti-CD3 antibody and 2 µg/mL soluble anti-CD28 antibody (Life Technologies, Grand Island, NY) was added to the medium. Plates were incubated at 37°C in 5% CO2 for 96 hrs. Where indicated, ESI-09 (5 µM) and/or stattic (50 ng/mL, Sigma-Aldrich, St. Louis, MO) in DMSO were added to the medium. Cell proliferation was determined based on DNA content using a CyQUANT Cell Proliferation Assay (Life Technologies, Grand Island, NY). Teff proliferation was calculated by subtracting the determined cell number from the negative control wells from that determined for the co-culture wells.
Detection of gap junction formation
Effector T-cells were incubated with carboxyfluorescein succinimidyl ester (CFSE) (1 µM) (Invitrogen) for 5 min in ice cold phosphate buffered saline (PBS). Regulatory T-cells were incubated with Calcein Red-Orange-AM (2 µM) in serum free RPMI-1640 culture medium at 37°C in 5% CO2 for 30 min. The cells were then washed three times and co-cultured as described for the suppression assay at a 1:1 ratio for 24 hrs. GJ formation was determined by the percentage of cells positive for both dyes.
p-STAT3 studies
Single-cell suspensions were prepared from spleens of 10–12 week old female mice and Teff and Treg cells were isolated as described earlier. The isolated cells (3 × 106/well) were then cultured with plate-bound anti-CD3 (5µg/mL) and soluble anti-CD28 (2µg/mL) in complete RPMI 1640 medium at 37°C and 5% CO2. Where indicated, 5 µM ESI-09 was added to the medium. Total cellular proteins were extracted at the indicated time points using SDS lysis buffer (2% SDS, 10% glycerol, 60 mM Tris, pH 6.8). Total protein lysate was separated by SDS-PAGE and transferred to a polyvinylidenedifluoride membrane. The indicated proteins were probed with the appropriate antibodies and protein levels were determined using densitometry.
TGF-β1 studies
Single-cell suspensions were prepared from spleens of 10–12 week old female mice and CD4+ T-cells were isolated as described earlier. Isolated cells were stimulated with plate-bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (2 µg/mL) in the presence or absence of TGF-β1 (2 ng/mL) and/or ESI-09 (5 µM) for 17 hr at 37°C and 5% CO2. Total cellular proteins were extracted and the levels of p-SMAD2, SMAD4, and SMAD7 were determined by Western blotting as described earlier using the appropriate antibodies. Similarly, total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) and used for quantitative real-time PCR analysis. The supernatants were collected and IL-2 levels were determined as described below.
IL-2 ELISA
At the end of their indicated incubation periods, supernatants from the Treg suppression and TGF-β1 sensitivity assays were collected and assayed for IL-2 using a Mouse IL-2 DuoSet Kit (R&D Systems, Minneapolis, MN).
Quantitative real-time PCR
First-strand cDNA synthesis was performed using 1 µg of total RNA with M-MLV MultiScribe™ Reverse Transcriptase (Life Technologies, Grand Island, NY) and quantitative real-time PCR was performed using iTaq™ Universal SYBR®GreenSupermix (Bio-Rad, Hercules, CA) in a LightCycler thermal cycler (Roche,Nutley, NJ). The amplification program included the initial denaturation step at 95 °C for 2 min, and 40 cycles of denaturation at 95 °C for 15 s, annealing at 62 °C for 15 s and extension at 68 °C for 30 s. Gapdh was used as an internal control for normalization of the target gene signal. The primers used were as follows: forward primer 5’-CTCCTCCTTACTCCAGATACC-3’ and reverse primer 5’-TCTTGGACACAGTAGAGCCTC-3’ for Smad7, forward primer 5’-TGGATGGACGACTTCAGGTG-3’ and reverse primer 5’- AGGTGAGACAACCCGCTCAT-3’ for Smad4, and forward primer 5’-TGCACCACCAACTGCTTAG-3’ and reverse primer 5’-GCAGGGATGATGTTCTGG-3’ for Gapdh.
Oral immunization with ovalbumin
Prior to immunization, mice were orally administered 0.2 mL of an isotonic bicarbonate solution (8 parts Hanks' balanced salt solution and 2 parts of 7.5% sodium bicarbonate) to neutralize stomach acidity. Mice were then orally primed with ovalbumin (OVA) (100 µg in 0.2 mL of PBS) on day 1 and boosted with the same dose on day 21. For ESI-09 treatment, mice were orally gavaged with the drug (50 mg/kg in vegetable oil). Drug treatment was initiated 3 days prior to priming and continued daily until 2 days after boosting. Mice were bled on day 28 (one week after boosting). All animals used were 8–12 week old female mice.
Determining total and OVA-specific IgG
For direct ELISA assays, 96-well plates (Corning Life Sciences, Lowell, MA) were coated with ovalbumin (Grade V, Sigma-Aldrich, St. Louis, MO, 3µg/mL, 100 µL/well) overnight at 4 °C. Plates were then incubated with blocking buffer (100 µL 1% BSA in PBS) for 2 hrs at room temperature. After blocking, sera from OVA-immunized mice, diluted in dilution buffer (PBS/0.05% Tween-20), were added (100 µL/well) and incubated for 2 hrs at room temperature. HRP-conjugated secondary anti-mouse IgG antibody (100 µL/well 1:2500 diluted, Cell Signaling Technology, Inc., Danvers, MA) was added and incubated at room temperature for 1 hr. Plates were then incubated with 100 µL per well of o-phenylenediamine (OPD) (Sigma-Aldrich, St. Louis, MO). Optical density was determined at 450 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA). Total serum-IgG levels from naïve 10–12 week old female mice were determined using the same described assay, but the plates were initially coated with anti-mouse IgG (BioLegend, San Diego, CA).
Immunophenotyping of spleen cells
Spleen cells were isolated from 10–12 week old female WT and Epac1−/− mice and stained with the indicated surface markers (BD Biosciences, San Jose, California) using standard flow cytometry staining protocols. Cells were sorted using a BD FACSCalibur Flow Cytometr. Six mice were used for each group.
Statistical Analysis
Student t test was used for data analysis in this study and results were considered as statistically significant if P values were <0.05.
RESULTS
EPAC1 regulates Treg-mediated suppression
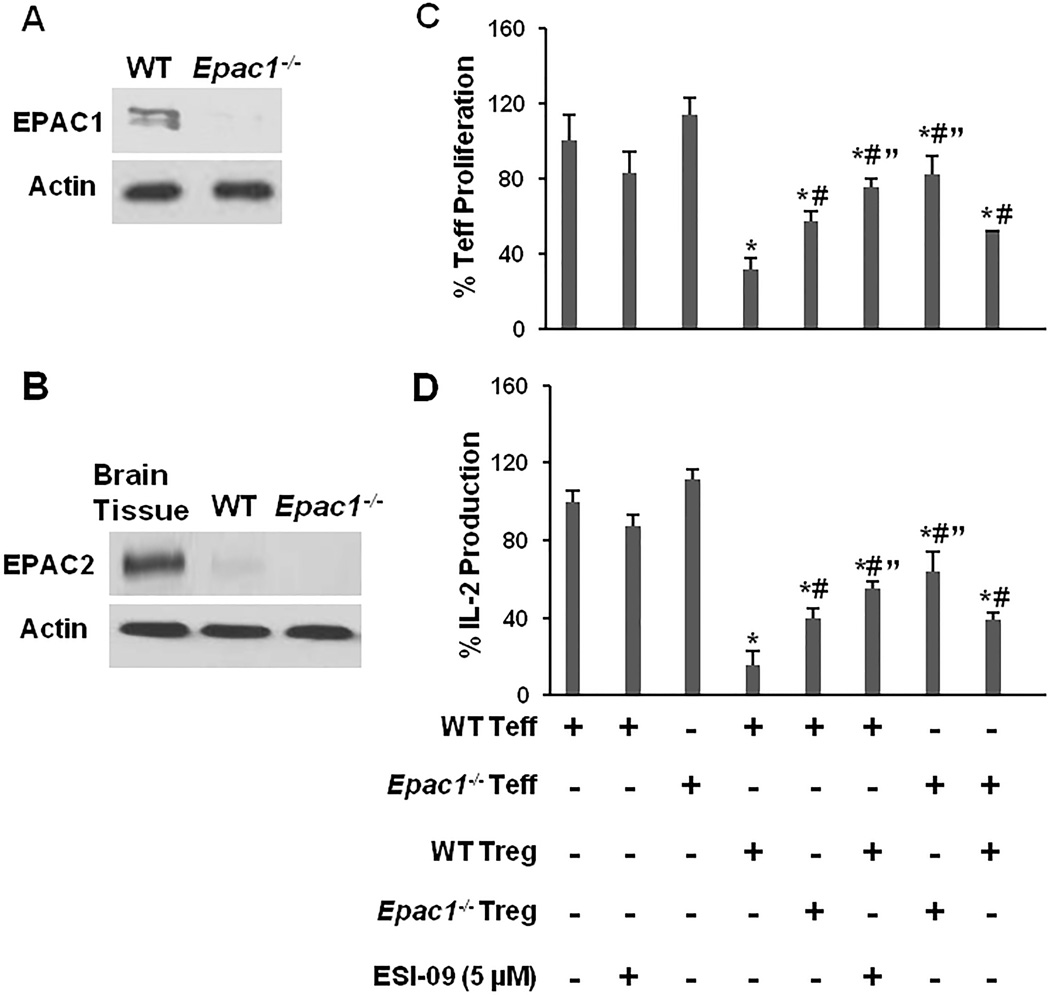
To address whether EPAC1 modulates Treg cell function, we generated Epac1−/− mice [19], confirmed the deletion of EPAC1 in CD4+ T-cells (Fig. 1A) and verified that EPAC2 level was low and not up-regulated in T-cells as a result of EPAC1 deletion (Fig. 1B). We examined the suppressive potency of WT and Epac1−/− Treg using a standard in vitro assay, in which proliferation of CD4+CD25− T cells (Teff) was monitored in the presence or absence of CD4+CD25+Treg cells (Fig. 1C). Epac1−/− and WT Teff proliferated at the same rate when stimulated with anti-CD3/CD28 alone. As expected, the addition of WT Treg suppressed the proliferation of WT Teff significantly (~75% suppression). However, the addition of Epac1−/− Treg only suppressed WT Teff by ~45%, indicating an attenuated suppressive potency for Epac1-deficient cells. On the other hand, the ability of WT Treg cells to suppress Epac1−/− Teff was also compromised (~50% suppression), and combining Epac1−/− Treg and Epac1−/− Teff resulted in the least amount of Teff suppression (~20%). To confirm our observations of EPAC1’s impact on Treg-mediated suppression based on genetic manipulation, we repeated the suppression assay using the EPAC-specific inhibitor ESI-09 [20]. Pharmacologic inhibition recapitulated the effect of EPAC1 deletion and reduced Treg-mediated suppression significantly (~25% suppression).
Figure 1. Deletion or inhibition of EPAC1 diminishes Treg-mediated suppression.
(A) and (B) CD4+ T-cells were isolated from 10–12 week old female WT and Epac1−/− mice and probed for EPAC1 and EPAC2 expression. Brain tissue was used as a positive control for detection of EPAC2. (C) Teff cells (5 × 104) were stimulated with plate-bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (2 µg/mL) in the presence or absence of Treg cells (2.5 × 104) and/or ESI-09 (5 µM). Proliferation was measured by determining DNA content 96 hrs later. Samples containing only Treg were used as negative controls. (D) At the end of the proliferation assay, the concentration of IL-2 in the supernatants was measured by ELISA. Data from the vehicle treated Teff only samples (positive controls) was normalized to the WT Teff positive control. Data from the co-culture assays was normalized to the positive control matching the genotype of the Teff cells in the assay. Bars represent mean ± SD of at least 3 experiments. * Significantly lower than the corresponding positive control samples (P < 0.05). # Significantly higher than WT Teff/WT Treg co-culture samples (P < 0.05). " Significantly higher than mixed WT/Epac1−/− co-culture samples (P < 0.05).
Since Treg are also known to inhibit Teff’s production of IL-2 [1], an important cytokine for T-cell proliferation, we examined the concentration of this cytokine in the co-culture assays. Treg suppression of IL-2 production by Teff followed a pattern similar to that described for suppression of Teff proliferation (Fig. 1D). Together, these results indicate that EPAC1 plays a role in regulating both Treg suppressive potency and Teff susceptibility to Treg-mediated suppression.
EPAC1 has no impact on GJ mediated communication between Treg and Teff
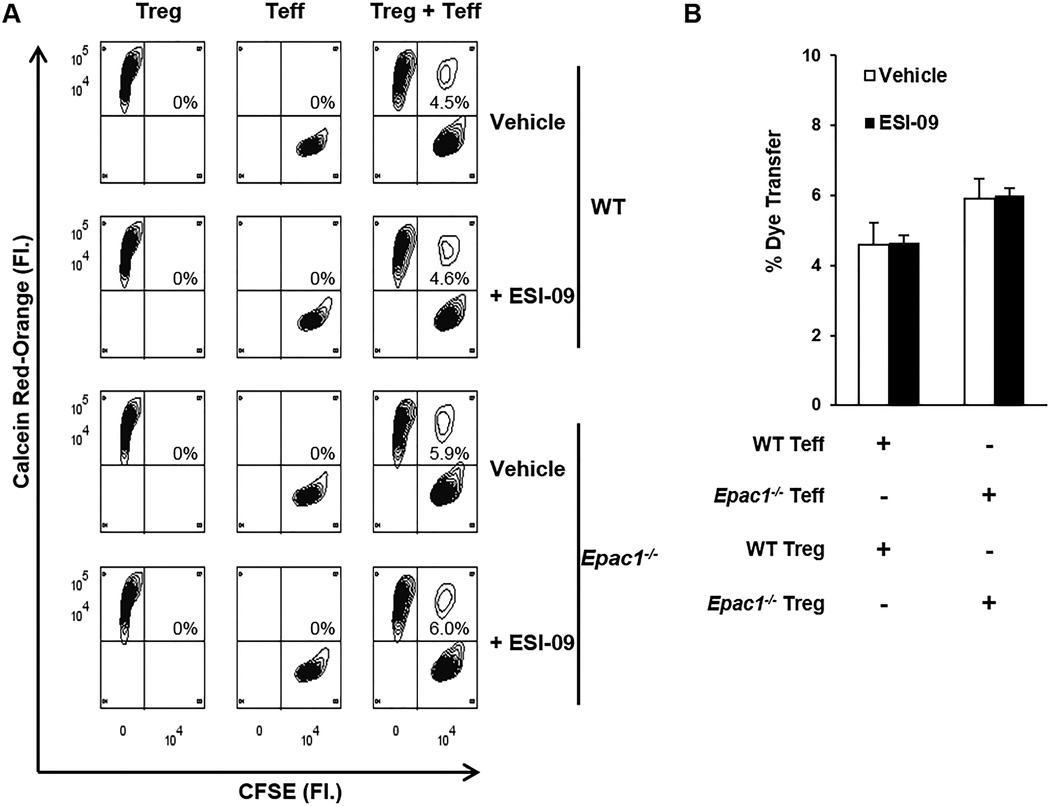
Treg cells are known to suppress Teff cells by direct transfer of cAMP through gap junctions (GJ) [21–23], and EPAC1 has been shown to play a role in the formation of GJ by facilitating the trafficking of their subunits [24]. We hypothesized that EPAC1 might regulate the formation of GJ between Treg and Teff cells. Effective GJ-mediated communication between Treg and Teff cells, as determined by the appearance of cell populations double positive for CFSE and Calcein Red-Orange, was observed. However, transfer of the membrane-impermeable dye was not affected by inhibition or deletion of EPAC1 (Fig. 2). These results suggest it is unlikely that cAMP transfer between Treg and Teff is affected by EPAC1.
Figure 2. Deletion or inhibition of EPAC1 has no impact on gap junction (GJ) mediated intercellular communication between Teff and Treg cells.
Treg and Teff cells were loaded with Calcein Red-Orange-AM and CFSE, respectively, and co-cultured for 24 hrs at a 1:1 ratio under plate-bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (2 µg/mL) stimulation. Cellular transfer between Treg and Teff cells was determined by the percentage of cells positive for both dyes. (A) Representative plots showing dye transfer between Treg and Teff. (B) Quantification of dye transfer from three experiments. Bars represent mean ± SD.
EPAC1 modulates T-cell sensitivity to suppression by attenuating STAT3 activation
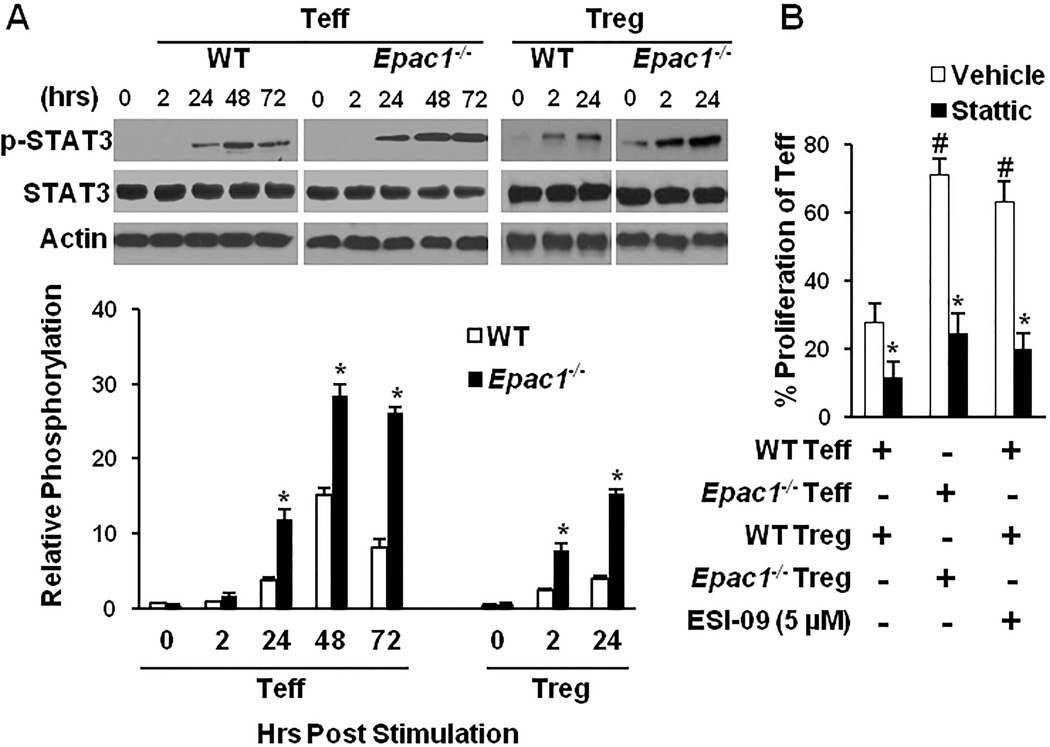
Our group and others have previously shown that EPAC1 weakens activation (phosphorylation) of signal transducer and activator of transcription 3 (STAT3) in non-immune cells [19, 25]. STAT3 is a known downstream signaling target of the T-cell receptor (TCR) [26, 27], and its activation in Treg and Teff has been shown to decrease suppression of the latter by the former [28]. Hence, we reasoned that EPAC1 could affect Treg-mediated suppression by attenuating STAT3 signaling. We examined STAT3 activation levels by measuring phospho-STAT3 (p-STAT3-Tyr705) in Epac1−/− Treg and Teff to determine if EPAC1 alters STAT3 signaling in these cells. Upon activation of the T-cell receptor, p-STAT3 levels increased to a significantly higher level in both Epac1−/− Treg and Teff cells, and were sustained longer when compared to their WT counterparts (Fig. 3A). Also, pharmacologic inhibition of EPAC1 by ESI-09 recapitulated the impact of genetic knockdown and increased STAT3 phosphorylation in WT Teff cells; while addition of the EPAC-activator 007-AM reduced ESI-09’s impact p-STAT3 levels (Supplemental Fig. S1A). Furthermore, addition of stattic, an inhibitor of STAT3 activation, enhanced Treg suppressive potency and abolished Epac1−/− Teff’s resistance to Treg suppression (Fig. 3B). Additionally, stattic treatment negated the effect of the EPAC-specific inhibitor ESI-09 on Treg activity (Fig. 3B). Taken together, these findings suggest that EPAC1 increases Treg suppressive potency and enhances Teff sensitivity to suppression by reducing the level of STAT3 phosphorylation in these cells.
Figure 3. EPAC1 modulates Treg-mediated suppression through the STAT3 pathway.
(A) Teff or Treg cells were stimulated with plate bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (2 µg/mL) and p-STAT3 (Tyr705) was probed by Western blotting at the indicated time points (one representative blot is shown). p-STAT3 levels were determined by densitometry and expressed as a percentage of total STAT3. * Significantly higher than WT counterpart (P < 0.01). (B) The suppression assay was carried out as described in Fig. 1 in the presence or absence of the STAT3 inhibitor stattic (50 ng/mL) and/or ESI-09 (5 µM). # Significantly higher than vehicle treated WT cells (P < 0.01). * Significantly lower than vehicle treated counterpart (P < 0.05). Bars represent mean ± SD of at least three experiments.
EPAC1 regulates STAT3 activation by an atypical mechanism
Phosphorylation of STAT3 in response to cytokine and TCR stimulation is typically attenuated by suppressor of cytokine signaling 3 (SOCS3) [29, 30] and the protein tyrosine phosphatases (PTP) SHP-1 and SHP-2 [31–33]. We hypothesized that the lack of EPAC1 might potentially alter the induction of these proteins. However, the levels of SOCS3 induction were similar in WT and Epac1−/− CD4+ T-cells, while SHP-1 and SHP-2 were unexpectedly higher in Epac1−/− cells (Supplemental Fig. S2A). We further examined the levels of JAK2, the main activator of STAT3 in response to cytokine stimulation, and activation of LCK (p-LCK-Try505), the main kinase triggering STAT3 phosphorylation downstream of TCR stimulation [33, 34]. However, there was no difference between WT and Epac1−/− T cells (Supplemental Fig. S2B). These results suggest that EPAC1 most likely exerts its impact on STAT3 independently of the canonical regulatory pathways of STAT3 activation.
EPAC1 sensitizes CD4+ T-cells to TGF-β1 signaling
Several recent studies have shown that TGF-β1 plays a significant role in Treg-mediated suppression [35–37]. Not only does TGF-β1 serve to suppress Teff, but it also maintains the suppressive capacity of Treg through autocrine signaling [38]. Given the well documented cross-talk between STAT3 and TGF-β1 signaling [39–41] and that Epac1−/− have elevated levels of p-STAT3 upon T-cell activation, we hypothesized that EPAC1 might modulate TGF-β1 sensitivity in T-cells.
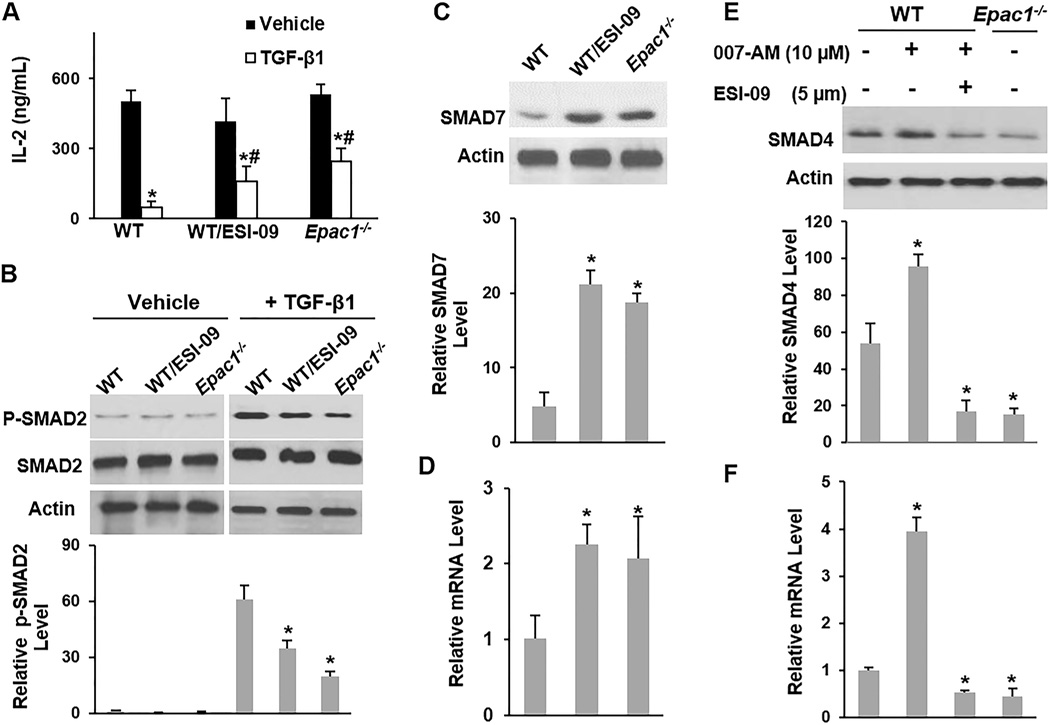
TGF-β1 is known to suppress IL-2 production by T-cells [42], so we measured WT and Epac1−/− Teff’s sensitivity to TGF-β1 by determining their IL-2 production in the presence or absence of TGF-β1. While TGF-β1 suppressed IL-2 production by ~90% in WT Teff, it only suppressed production by ~50% and ~60% in Epac1−/− Teff and ESI-09 treated WT Teff, respectively (Fig. 4A). TGF-β1 induced phosphorylation of SMAD2 was also significantly inhibited in WT CD4+ T-cells after the addition of ESI-09 and in Epac1−/− CD4+ T-cells (Fig. 4B), indicating a reduced TGF-β1 response. Furthermore, upon TCR stimulation, SMAD7, which inhibits TGF-β1 signaling [43], rose to significantly higher levels at the mRNA and protein levels in Epac1−/−CD4+ T-cells and ESI-09-treated WT CD4+ T-cells compared to their vehicle-treated WT counterparts (Fig. 4C & D). To further confirm the specificity of the impact of EPAC1 on the TGF-β1 pathway, we also utilized the EPAC-activator 007-AM. Addition of 007-AM enhanced TGF-β1 induced phosphorylation of SMAD2 (Supplemental Fig. S1B) and reduced the protein and mRNA levels of SMAD7 (Supplementary Fig. S1C & D).
Figure 4. Inhibition of EPAC1 leads to resistance to TGF-β1 signaling.
CD4+ T-cells were stimulated with plate bound anti-CD3 (5 µg/mL) and soluble anti-CD28 (2 µg/mL) for 17 hrs in the presence or absence of TGF-β1 (2 ng/mL), ESI-09 (5 µM), and 007-AM (10 µM) as indicated. (A) Supernatants were assayed for IL-2 concentration by ELISA. * Significantly lower than vehicle treated counterpart (P <0.03). # Significantly higher than TGF-β1-treated WT cells (P < 0.05). (B) The level of p-SMAD2 was determined by Western blotting (one representative blot is shown) and expressed as a percentage of total SMAD2. * Significantly lower than TGF-β1-treated WT cells (P <0.02). (C–F) The protein and mRNA levels of SMAD7 or SMAD4 were determined by Western blotting (one representative blot is shown) and qRT-PCR, respectively. Protein levels were expressed as percentage of the loading control actin. mRNA levels were expressed relative to Gapdh RNA levels. * Significantly higher or lower than vehicle treated WT cells (P < 0.03). Bars represent mean ± SD of at least three experiments.
We also examined the level of SMAD4, which is essential for nuclear translocation of activated SMAD2 in response to TGF-β1 stimulation. We found that EPAC1 regulates the mRNA and protein levels of SMAD4. Stimulation of CD4+ T-cells in the presence of the EPAC agonist 007-AM significantly increased the mRNA and protein levels of SMAD4, while pharmacologic inhibition or genetic deletion of EPAC1 significantly reduced SMAD4 levels (Fig. 4E & F). Together, the results presented here suggest that EPAC1 potentially regulates TGF-β1 signaling in CD4+ T-cells by multiple mechanisms including regulation of p-STAT3 and SMAD4 levels.
Epac1−/− mice are resistant to developing oral tolerance
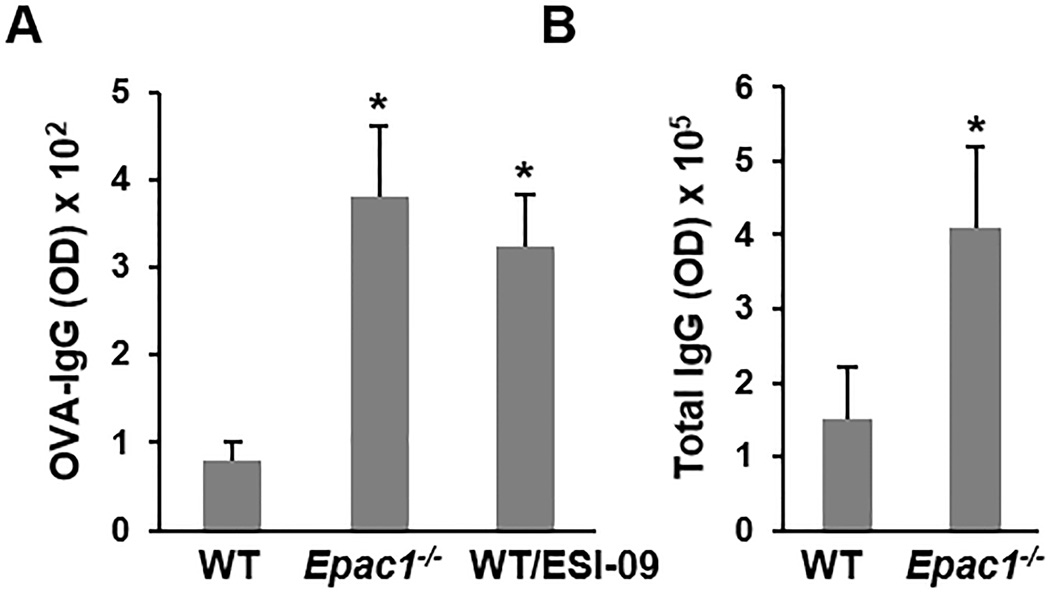
To test whether EPAC1 might regulate Treg-mediated suppression in vivo, we utilized a mouse model in which mice are administered a low dose of ovalbumin (OVA) orally. This model has been shown to induce activation and expansion of antigen-specific Treg, which actively suppress the immune response to OVA and lead to lower levels of IgG production [36, 44–46]. As expected, intragastric administration of low dose OVA (100 µg) led to a weak response in WT mice as determined by serum OVA-IgG levels (Fig. 5A). Conversely, a significantly higher level of OVA-IgG was detected in Epac1−/− mice (Fig. 5A). To confirm that the altered immune response was a direct consequence of EPAC1 suppression, rather than a compensatory mechanism as a result of global knockdown of Epac1, we inhibited EPAC pharmacologically by feeding WT mice with an EPAC-specific inhibitor, ESI-09 [20, 47]. Similarly to genetic deletion of EPAC1, pharmacologic inhibition also resulted in a higher anti-OVA IgG titer (Fig. 5A). Furthermore, the basal level of total serum-IgG was higher in naïve unchallenged Epac1−/− mice than in their WT counterparts (Fig. 6B), indicating a generally heightened immune response in the absence of EPAC1. Taken together, these findings support a potential role for EPAC1 in modulation of Treg activity in vivo. While we cannot rule out other possible roles for EPAC1 in controlling the immune response, we found EPAC1 to be dispensable for immune cell development as similar populations of various immune cells were detected in WT and Epac1−/− mice (Supplemental Table S1).
Figure 5. Deletion or inhibition of EPAC1 heightens the immune response.
(A) 10–12 week old female mice were administered 100 µg OVA orally on days 1 and 21. ESI-09 treated mice were administered the drug daily (50 mg/kg orally). Serum was collected on day 28 and anti-OVA IgG levels were determined by ELISA (n=5). (B) Serum was collected from naive 10–12 week old female mice and total IgG levels were determined by ELISA (n=8). Data is shown as means ± SD. * Significantly higher than WT group (P < 0.05).
DISCUSSION
The cAMP signaling axis exerts broad control over the immune system through mediating the activity of Treg cells, but the pathways regulated by this axis have not been fully elucidated. In the current study, we examined the previously unexplored role of the cAMP sensor protein EPAC1 in Treg-mediated suppression using genetic and pharmacologic approaches. Our results revealed that EPAC1 plays an essential role in Treg-mediated suppression. The suppressive capacity of Treg cells was reduced in the absence of EPAC1. Furthermore, the removal of EPAC1 in Teff rendered them resistant to suppression by Treg, and inhibition/deletion of this protein in both cell populations had an additive effect on compromising Treg-mediated suppression. Our findings are in agreement with the facts that stimulation of human CD4+CD25+ Treg cells leads to significant elevation of the main EPAC1 effector Rap1 [48], and transgenic mice expressing a constitutively active Rap1 have lower levels of pro-inflammatory cytokines [49].
One of the main contact-dependent suppression mechanisms employed by Treg cells is the direct transfer of cAMP into Teff cells through gap junctions [21–23], EPAC1 facilitates the accumulation of the ubiquitous subunit Cx43 at the site of GJ formation in cardiac cells [24]. Cx43 is the main connexin in the immune system [50, 51], and thus it possible that EPAC1 mediates cAMP transfer between Treg and Teff cells by maintaining GJ permeability. Since formation of GJ requires trafficking of connexins in both cell types, we hypothesized that a loss of EPAC1 in Treg or Teff would partially hinder their communication, while a lack of EPAC1 in both Treg and Teff would have an additive negative impact. However, this possibility is unlikely since neither genetic deletion, nor pharmacologic inhibition of EPAC1 reduced gap junction communication between these cells as determined by membrane impermeable dye transfer.
On the other hand, our results show that EPAC1 regulates Treg-mediated suppression through the STAT3 and TGF-β1 pathways. In the absence of EPAC1, both Treg and Teff cells had higher levels of p-STAT3, and inhibition of STAT3 activation restored the potency of Epac1−/− Treg-mediated suppression. These results are consistent with previous reports showing that activation of STAT3 in Treg and Teff synergistically leads to desensitization to suppression of the latter by the former [28, 52, 53]. The levels of SMAD7, which inhibits TGF-β1 signaling, were elevated after suppression/deletion of EPAC1, in concordance with previous studies showing hyper-activation of STAT3 leads to desensitization to TGF-β1 signaling by inducing SMAD7 [39, 40]. Consequently, in the absence of EPAC1 CD4+ T-cells were less sensitive to TGF-β1 as demonstrated by the lower activation levels of SMAD2 and reduced IL-2 production in response to stimulation by TGF-β1.
Interestingly, we found that even in the absence of TGF-β1 stimulation, activation of EPAC1 induced expression of SMAD4, while inhibition/deletion of EPAC1 blunted its expression. These results suggest the presence of a previously unidentified connection between EPAC1 and SMAD4. The lower levels of SMAD4 in EPAC1 deficient CD4+ T-cells most likely contributed to their resistance to TGF-β1 stimulation.
Treg cells utilize membrane-bound TGF-β1 as one of their main mechanisms of suppressing Teff cells. Not only does this membrane-bound form suppress activation and proliferation of target cells, but it also maintains the suppressor function of Treg cells through autocrine signaling and activation of the SMAD2/SMAD3 cascade as has been shown by several studies [35, 38, 54]. In the absence of TGF-β1 signaling, CD4+CD25+ T-cells had diminished suppressive potency in vivo and in vitro [35]. Therefore, our data are consistent with a model in which EPAC1, through attenuation of p-STAT3, a transcriptional regulator of SMAD7, and promotion of SMAD4 expression, plays an essential role in sensitizing Treg and Teff cells to TGF-β1 signaling. As such, its inhibition in both cells has an additive impact on compromising Treg-mediated suppression.
Our results show that EPAC1 regulates STAT3 activation independently of the canonical regulatory loops involving SOCS3 and SHP-1/2. A recent study showed that SMAD4 inhibits STAT3 phosphorylation in non-immune cells [55]. Hence, it is feasible that by inducing SMAD4, EPAC1 blunts STAT3 phosphorylation. Additionally, studies have shown that TGF-β1 signaling, through SMAD2, inhibits STAT3 activation and nuclear translocation [41]. Therefore, EPAC1 may mediate a regulatory loop in which it promotes expression of SMAD4, which maintains low levels of p-STAT3 and consequently reduces SMAD7 levels; leading to additional up-regulation of TGF-β1 signaling and-SMAD2 activation. The latter in turn further suppresses p-STAT3. However, more studies are needed to confirm and fully elucidate the details of this potential pathway.
Consistent with our findings based on the in vitro suppression assay, genetic deletion and pharmacological inhibition of EPAC1 led to an enhanced antibody production in an active oral tolerance model. Intragastric administration of low dose ovalbumin induces the production of antigen-specific Treg cells, which in turn suppress the immune response to the administered protein in an antigen nonspecific manner [36, 44–46]. Furthermore, while the composition of immune cells was similar between Epac1−/− and WT mice, the former had significantly higher basal IgG levels even in the absence of an antigen challenge. These findings suggest that Treg-mediated suppression is attenuated in the absence of EPAC1 in vivo. Nonetheless, our results cannot rule out other possible roles for EPAC1 in the function of other immune cells that might affect oral tolerance and antibody production, including antigen presenting cells, B-cells, or myeloid-derived suppressor cells (MDSC).
In conclusion, our study shows that EPAC1 facilitates cAMP signaling during Treg-mediated suppression. Inhibition of EPAC1 leads to resistance of Teff to Treg suppression and concurrently diminishes the suppressive potency of the latter. Such knowledge significantly expands our understanding of the mechanism of cAMP-mediated Treg suppression and suggests that PKA and EPAC1 differentially and/or synergistically regulate T cell functions. The impact of EPAC1 on these cells seems to be mediated by regulation of STAT3 activation and TGF-β1 signaling. These findings have significant potential clinical implications as they validate EPAC1 as a potential target for fine tuning Treg cell activity and TGF-β1-mediated immune suppression. One of the biggest hurdles facing the clinical application of Treg-modulating therapies is the significant potential for severe side effects. Treatments that significantly reduce the number of Treg cells can cause autoimmunity. In fact, Ipilimumab, one of the few cancer immunotherapies currently approved by the FDA, is an anti-CTLA-4 antibody that eliminates Treg cells, but despite its efficacy, it led to potentially fatal immune-related gastrointestinal side effects in some cases [56]. On the other hand, treatments that boost the number of Tregs in patients, such as transplant recipients in whom attenuation of the immune response is desired, might compromise the immune system and increase the risk of serious infections [57]. Therefore, it is of paramount importance that we elucidate the intricate signaling networks that regulate the potency of Treg cells to identify targets that can be utilized to fine tune Treg activity in a controlled manner. Neither genetic deletion, nor long term pharmacologic inhibition of EPAC1 had observable side effects in mice as we have shown previously [19]. Hence, pharmacologic modulators of this protein might have excellent therapeutic potential in vaccine adjuvant development, infection control, autoimmune disease, and cancer immunity, among other fields.
Supplementary Material
Summary statement.
Using pharmacologic and genetic approaches, our study identifies EPAC1 (exchange protein directly activated by cAMP) as a key modulator of regulatory T-cell (Treg)-mediated suppression. Our data suggest that EPAC1’s alters Treg activity through the STAT3 and TGF-β1 pathways.
Acknowledgments
Funding
This study was supported by NIH grants R01GM066170. M. A. is a recipient of training fellowships from the Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia funded by the NIH NIGM grant T32GM89657-3, and the Biodefense Training Program at The University of Texas Medical Brach funded by the NIH grant T32AI60549-10.
Footnotes
The authors declare no conflict of interest.
Author contribution
Participated in research design: Almahariq, Mei, Soong, Sun, Cong and Cheng.
Conducted experiments: Almahariq, Mei, Wang, Cao, and Yao.
Contributed new reagents or analytic tools: Chen
Performed data analysis: Almahariq, Cong, and Cheng.
Wrote or contributed to the writing of the manuscript: Almahariq and Cheng.
References
- 1.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 5.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011;3:517–537. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirshev SV. Role of Epac proteins in mechanisms of cAMP-dependent immunoregulation. Biochemistry (Mosc) 2011;76:981–998. doi: 10.1134/S000629791109001X. [DOI] [PubMed] [Google Scholar]
- 10.Mosenden R, Taskén K. Cyclic AMP-mediated immune regulation--overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Vang AG, Housley W, Dong H, Basole C, Ben-Sasson SZ, Kream BE, Epstein PM, Clark RB, Brocke S. Regulatory T-cells and cAMP suppress effector T-cells independently of PKA-CREM/ICER: a potential role for Epac. Biochem J. 2013;456:463–473. doi: 10.1042/BJ20130064. [DOI] [PubMed] [Google Scholar]
- 12.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taskén K. Waking up regulatory T cells. Blood. 2009;114:1136–1137. doi: 10.1182/blood-2009-06-223222. [DOI] [PubMed] [Google Scholar]
- 14.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 16.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–284. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosenden R, Taskén K. Cyclic AMP-mediated immune regulation--overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Bryce PJ, Dascombe MJ, Hutchinson IV. Immunomodulatory effects of pharmacological elevation of cyclic AMP in T lymphocytes proceed via a protein kinase A independent mechanism. Immunopharmacology. 1999;41:139–146. doi: 10.1016/s0162-3109(98)00060-5. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I, Hao D, Stutz SJ, Dineley KT, Motamedi M, Hommel JD, Cunningham KA, Chen J, Cheng X. Enhanced Leptin Sensitivity, Reduced Adiposity, and Improved Glucose Homeostasis in Mice Lacking Exchange Protein Directly Activated by Cyclic AMP Isoform 1. Mol Cell Biol. 2013;33:918–926. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A Novel EPAC-Specific Inhibitor Suppresses Pancreatic Cancer Cell Migration and Invasion. Mol Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fassbender M, Gerlitzki B, Ullrich N, Lupp C, Klein M, Radsak MP, Schmitt E, Bopp T, Schild H. Cyclic adenosine monophosphate and IL-10 coordinately contribute to nTreg cell-mediated suppression of dendritic cell activation. Cell Immunol. 2010;265:91–96. doi: 10.1016/j.cellimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 25.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson P, Sundstedt A, Li L, O'Neill EJ, Li S, Wraith DC, Wang P. Differential activation of signal transducer and activator of transcription (STAT)3 and STAT5 and induction of suppressors of cytokine signalling in T(h)1 and T(h)2 cells. Int Immunol. 2003;15:1309–1317. doi: 10.1093/intimm/dxg130. [DOI] [PubMed] [Google Scholar]
- 27.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 28.Goodman WA, Young AB, McCormick TS, Cooper KD, Levine AD. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol. 2011;186:3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chueh FY, Yu CL. Engagement of T-cell antigen receptor and CD4/CD8 co-receptors induces prolonged STAT activation through autocrine/paracrine stimulation in human primary T cells. Biochem Biophys Res Commun. 2012;426:242–246. doi: 10.1016/j.bbrc.2012.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, Lucet IS, Norton RS, Nicola NA. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–250. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Qu CK. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund TC, Coleman C, Horvath E, Sefton BM, Jove R, Medveczky MM, Medveczky PG. The Src-family kinase Lck can induce STAT3 phosphorylation and DNA binding activity. Cell Signal. 1999;11:789–796. doi: 10.1016/s0898-6568(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 35.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 39.Luwor RB, Baradaran B, Taylor LE, Iaria J, Nheu TV, Amiry N, Hovens CM, Wang B, Kaye AH, Zhu HJ. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene. 2013;32:2433–2441. doi: 10.1038/onc.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 41.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das L, Levine AD. TGF-beta inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J Immunol. 2008;180:1490–1498. doi: 10.4049/jimmunol.180.3.1490. [DOI] [PubMed] [Google Scholar]
- 43.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 44.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung Y, Lee SH, Kim DH, Kang CY. Complementary role of CD4+CD25+ regulatory T cells and TGF-beta in oral tolerance. J Leukoc Biol. 2005;77:906–913. doi: 10.1189/jlb.1004599. [DOI] [PubMed] [Google Scholar]
- 46.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL, Cheng X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci U S A. 2012;109:18613–18618. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Greenwald RJ, Lafuente EM, Tzachanis D, Berezovskaya A, Freeman GJ, Sharpe AH, Boussiotis VA. Rap1-GTP is a negative regulator of Th cell function and promotes the generation of CD4+CD103+ regulatory T cells in vivo. J Immunol. 2005;175:3133–3139. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- 50.Mendoza-Naranjo A, Bouma G, Pereda C, Ramírez M, Webb KF, Tittarelli A, López MN, Kalergis AM, Thrasher AJ, Becker DL, Salazar-Onfray F. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121–3132. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oviedo-Orta E, Hoy T, Evans WH. Intercellular communication in the immune system: differential expression of connexin40 and 43, and perturbation of gap junction channel functions in peripheral blood and tonsil human lymphocyte subpopulations. Immunology. 2000;99:578–590. doi: 10.1046/j.1365-2567.2000.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 54.Stockis J, Fink W, François V, Connerotte T, de Smet C, Knoops L, van der Bruggen P, Boon T, Coulie PG, Lucas S. Comparison of stable human Treg and Th clones by transcriptional profiling. Eur J Immunol. 2009;39:869–882. doi: 10.1002/eji.200838807. [DOI] [PubMed] [Google Scholar]
- 55.Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, Freeman JW. Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor beta-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]
- 56.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spoerl S, Li XC. Regulatory T cells and the quest for transplant tolerance. Discov Med. 2011;11:25–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.