Abstract
A typical person infected with the retrovirus human T-lymphotropic virus type 1 (HTLV-1) carries tens of thousands of clones of HTLV-1-infected T lymphocytes, each clone distinguished by a unique integration site of the provirus in the host genome. However, only 5% of infected people develop the malignant disease adult T cell leukaemia/lymphoma, usually more than 50 years after becoming infected. We review the host and viral factors that cause this aggressive disease.
Introduction
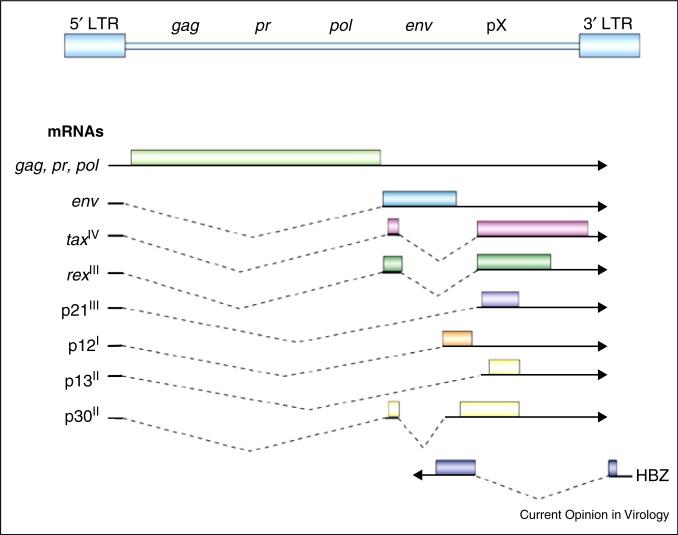
HTLV-1 is a complex deltaretrovirus [1]. Its genome (Figure 1) includes components of simple retroviruses, as well as additional regulatory genes. The gag, pr (Protease), and pol (Polymerase and Integrase) genes are encoded from a single transcript, as a result of ribosome frameshifts. The env gene encodes a glycoprotein that mediates binding to the target cell receptors glucose transporter 1 and neuropilin; viral entry is also promoted by heparan sulphate proteoglycans [2]. The genome also has a unique set of regulatory genes, which encode Tax and Rex proteins, that regulate transcriptional initiation and nuclear RNA export, respectively [3]. Other alternatively spliced RNAs encode the p21, p12, p13, and p30 proteins, important for virus propagation in vivo [4–6]. The p12 protein, or its proteolytic product p8, promotes T cell activation, virus transmission, and escape from CTL and NK cell killing [4,7]. The p30 protein is a latency maintenance factor that cooperates with c-Myc in oncogenesis [8]. Unlike other retroviruses, HTLV-1 also encodes an anti-sense transcript that produces the HBZ, helix-basic loop zipper protein [9].
Figure 1.
Genome structure of the HTLV-1 provirus. The gag, pr, pol, and env genes are shown flanked by long terminal repeat (LTR) sequences. The pX regions includes regulatory genes encoding Tax, Rex, p21, p12, p13, and p30, as well as the antisense gene encoding HBZ.
Modified from [1].
Three to five percent of infected individuals develop HTLV-1-associated myelopathy (HAM), characterized by lower limb spasticity, bowel and bladder disturbances, and progressive neurological decline over several years [10]. HTLV-1 infection is also associated with other inflammatory clinical conditions, including uveitis, arthropathy, myopathy, and pneumopathy [11,12]. These disorders are associated with a high virus load and over-stimulation of dendritic cells, resulting in chronic production of high levels of interferon-stimulated gene products [13]. A further 5% of infected individuals develop a CD4+ T-cell leukaemia or lymphoma, designated adult T-cell leukaemia/lymphoma (ATL), that occurs almost exclusively in individuals who acquired HTLV-1 as a result of breast feeding, although four or five decades generally elapse before development of disease [14]. ATL is characterized by frequent blood, bone marrow, and brain involvement, hypercalcaemia, and lytic bone lesions. The acute forms have a median survival of about one year, despite intensive chemotherapy. Studies of arsenic and interferon treatment show potential clinical benefit, which may be due to inhibition of leukaemia cell initiating activity [15]. This effect may be enhanced by a synthetic retinoid, ST1926 [16•]. Recent data suggest an improved response with the addition to combination chemotherapy of an antibody to CCR4, a chemokine receptor overexpressed on ATL cells responsible for targeting leukocytes to the central nervous system and endothelial cells involved in angiogenesis [17•].
HTLV-1 varies little in sequence compared with HIV-1, and the genotype of HTLV-1 in cases of ATL or HAM/TSP is not distinct from that in asymptomatic carriers of the virus. The different outcomes of HTLV-1 infection must therefore be due to differences in the host. The innate immune response [18] to HTLV-1 has been under-studied until recently. In patients with HAM/TSP, both the frequency and the lytic activity of natural killer (NK) cells are significantly lower than in asymptomatic carriers or uninfected subjects [19–22]. This ill-understood phenomenon deserves further research. Type 1 (beta) inter-feron impairs HTLV-1 replication in vitro [23], but its role in chronic HTLV-1 infection is not known. HTLV-1 Tax protein induces strong expression of Type 2 (gamma) interferon in infected cells [24]. Gene expression micro-array analysis of freshly isolated peripheral blood mono-nuclear cells suggests [21] that chronic expression of interferons — probably both Type 1 and Type 2 — contributes to the inflammatory tissue damage in HAM/TSP. Sustained treatment with type 1 (alpha) interferon in combination with AZT is effective [25] in some cases of ATL, especially chronic ATL. It is believed that this benefit derives from the antiproliferative effects of the two agents, rather than their antiviral actions, but the mechanism remains unclear.
The acquired immune response to HTLV-1 has been intensely studied. HTLV-1 infection frequently elicits a very high titre of specific antibody; antibody can confer some protection against infection [26], but its role (if any) in the chronic phase of infection is unclear. HTLV-1 also elicits abundant, chronically activated CD8+ cytotoxic T lymphocytes (CTLs) [27–29]. The efficiency or ‘quality’ of the specific CTL response to HTLV-1 is a major determinant of the proviral load and the risk of inflammatory disease [30]. Since the risk of ATL also increases with proviral load, it is likely that CTL quality also determines ATL risk, but this has not been directly demonstrated. The chief factors that determine a high quality anti-HTLV-1 CTL response [30], and therefore a low risk of associated diseases, are the host genotype in the HLA Class 1 [31,32] and killer immunoglobulin-like receptor (KIR) loci [33] (respectively HLA-A*02 and -Cw*08, and KIR2DL2, in southern Japan), and the ability to present epitopes from the HBZ antigen to CTLs [34].
HTLV-1-specific CD4+ T cells are more abundant in HAM/TSP than in asymptomatic carriers, and may contribute to inflammation [35]. HTLV-1 Tax stimulates CD4+ T cells to produce the chemokine CCL22 [36]. CCL22 in turn maintains a high frequency of regulatory CD4+ T cells (Tregs) that express the characteristic proteins FoxP3, CD25, GITR and the CCL22 receptor, CCR4. Tregs that are not HTLV-1-infected may impair the quality of the CTL response to HTLV-1 [37] and so increase the proviral load and the risk of disease. Clonal primary ATL cells also frequently express these Treg proteins, as do some non-malignant HTLV-1-infected cells. However, in HTLV-1-infected Tregs the expression and actions of FoxP3 in the infected cell are impaired by the products of the tax [38] and HBZ [39] genes, and HTLV-1-infected FoxP3+ cells do not themselves exert regulatory functions [37]. We conclude that, although ATL may arise in a Treg clone, ATL is not necessarily a malignancy of Tregs [40,41].
Mechanisms of oncogenesis
Both Tax and HBZ are implicated in oncogenesis [42]. One model implicates Tax in tumour initiation, and HBZ in maintenance. Although HTLV-1 can infect a wide range of human cells in vitro, the specificity for immortalization of activated T cells appears to be determined at a post-entry step. Recent work suggests that suppression of Tax activity by TCF1 and LEF1, two Wnt transcription factors, is relieved by T-cell activation [43]. Tax has transcriptional effects through CREB/ATF proteins to upregulate virus expression by functioning as a coactivator for CREB-binding proteins, CBP and p300 [44•]. In addition, Tax interactions with CREB binding proteins promotes chemotaxis and transmission of HTLV-1 through enhanced expression of a Ras family member known as Gem [45•]. Tax also promotes the ability of NFAT, AP-1, and NF-κB pathways to upregulate expression of cellular genes that mediate lymphocyte proliferation and resistance to apoptosis [46]. Transgenic expression of Tax results in NK and T cell malignancies, depending on the promoter used. Overexpression of NF-κB pathway genes is a common property of these models [47–49]. Effects of Tax on NF-κB appear to be mediated by direct binding to IKKgamma and p100 as well as alterations of ubiquitination of NF-κB regulatory proteins, such as TRAF6 [46]. Tax-mediated K63-ubiquitination of TRAF6 also promotes interaction with anti-apoptosis protein Mcl-1, and survival of transformed cells [50]. IL-17RB is a NF-κB product expressed in ATL cells, that provides a feed-forward autocrine loop to further activate the NF-κB pathway [51]. Tax can also directly interact with proteins to block cell cycle inhibitors (p16, p15, Rb) and activate cyclins and cyclin-dependent kinases [1]. Moreover, Tax binding to PDZ-containing proteins upregulates PI3KAkt-mTOR signalling. Lastly, Tax induces genetic instability, evidenced by its ability to produce multinucleation, chromatin bridges, aneuploidy, telomere attrition, and clastogenic DNA damage [52]. These effects are mediated by generation of reactive oxygen species and dysfunctional DNA repair processes, as a result of direct interactions with MAD1, PCNA, DNA topoisomerase, DNA polymerase beta, mini-chromosome maintenance MCM2-7 helicase, Chk2, and Ku80 [53–56]. In addition, Tax functionally inactivates p53 [21,57].
However, Tax is often repressed once tumours develop [58]. In contrast, HBZ is ubiquitously expressed in ATLL cells and HTLV-1 infected cells in vivo [59]. In mouse models, HBZ also induces inflammatory disorders with elevated CD4+Foxp3+ Treg cells in the intestines, skin, and lung [60]. However, these HBZ-transgenic cells tend to lose FoxP3 expression, resulting in increased interferon gamma expression and a pro-inflammatory phenotype. In addition, HBZ induces T cell neoplasia in vivo, but at a slower rate and lower frequency than that seen in Tax transgenic models. HBZ promotes proliferation of HTLV-1-infected cells [61,62] and minimizes the effect of repeated mitosis on cellular ageing by enhancing expression of the host genes telomerase reverse transcriptase (hTERT) and JunD [63]. Interactions between Tax and HBZ on HTLV-1-infected cells are complex, and in some cases opposite (Figure 3). For example, HBZ represses AP-1, NFAT, and CREB activities [64]. Conversely, Tax inhibits TGFbeta signalling, whereas HBZ activates it. Tax activates both the canonical and non-canonical NF-κB pathways, whereas HBZ inhibits only the canonical NF-κB pathway [65]. Tax promotes cell proliferation but also induces cellular senescence by induction of p21 and p27, whereas HBZ prevents senescence by inhibiting p65 [66]. Lastly, HBZ suppresses the canonical Wnt pathway, but enhances the non-canonical pathway, suggesting that HBZ modulates the intra-cellular environment of peripheral T cells targeted by the virus [67].
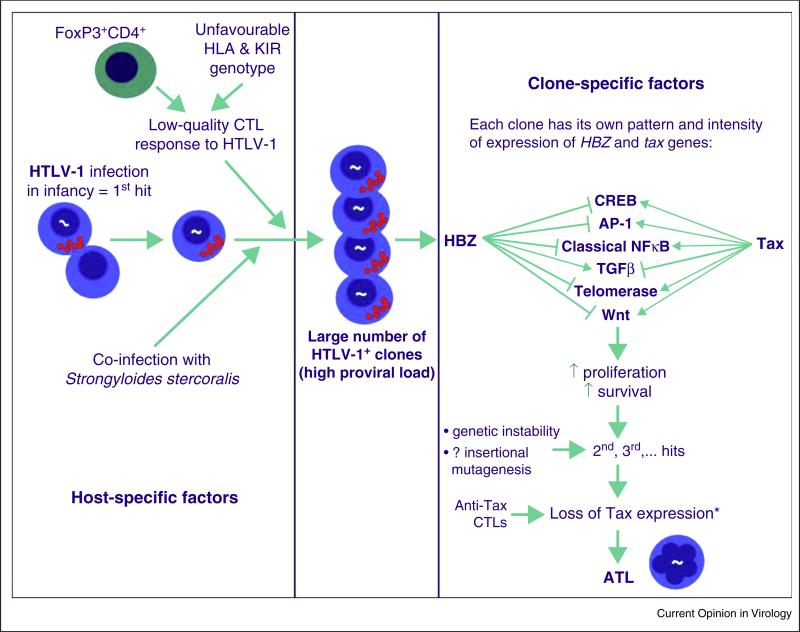
Figure 3.
Factors that determine the risk of ATL. These factors may be divided into three categories: host-specific factors, clone-specific, and stochastic factors. The host-specific factors — host genotype, age at infection, and certain co-infections [84], especially Strongyloides stercoralis — are independent of the proviral integration site. Persistent replication of long-lived infected clones, driven by Tax and HBZ, may lead to the accumulation of replicative mutations [75], whose frequency is strongly correlated with the risk of malignant transformation. This may explain the observed association between ATL and infection with HTLV-1 in early childhood.
The clone-specific oncogenic factors depend on the proviral integration site: spontaneous proviral expression is influenced by the flanking host genome [72]; and the HTLV-1 gene products, especially Tax and HBZ, promote the proliferation and longevity of existing clones and genetic instability. It is possible that insertional mutagenesis contributes to oncogenesis, by activation or disruption of host genes flanking the provirus or via long-range chromatin interactions. Finally, infected T cell clones that are specific to persistent and abundant antigens, such as CMV or Strongyloides antigens, may enjoy an additional proliferative advantage.
It is widely assumed [85] that secondary mutations — the stochastic factors — increase the proliferative or survival advantage of an HTLV-1+ clone, and contribute to malignant transformation. Direct evidence to substantiate this assumption is needed from whole-genome sequencing of ATL clones. Preliminary work along this line has already begun, with evidence that mutations in three signalling pathways — NF-κB, T-cell receptor and CCR4 — are associated with ATL [86••].
* Tax expression is lost in the malignant clone in ~50% of cases of ATL.
Clonality
HTLV-1 stimulates infected T cells to proliferate in vivo, by persistent or intermittent expression of Tax and HBZ. This proliferation results in long-lived, abundant clones of infected T cells in the circulation [68], each clone distinguished by a unique site of integration of the HTLV-1 provirus in the host genome. The majority of infected clones carry a single proviral copy [69]; about 10% of ATL clones contain two copies, some of which may be defective [70•].
The proviral integration site of HTLV-1 in the host genome [71] is not random, but is strongly biased towards certain transcription factor binding sites, notably those that bind STAT1, TP53, and HDAC6 [72]. Integration within 10 kb of a known oncogene confers a survival advantage on the clone in vivo, but does not appear to contribute directly to leukaemogenesis [70•]. Although no hot-spot of integration is associated with ATL, there is evidence that integration within 15 kb upstream of host genes that are frequently dysregulated in other leukaemias occurs significantly more frequently than expected by chance; such clones account for about 6% of ATL cases in southern Japan [70•].
There is recent evidence that the HTLV-1 provirus has the potential to alter host chromatin structure (Satou et al., submitted for publication). This observation raises the possibility that HTLV-1 disrupts host gene regulation either in the immediate vicinity of the provirus or by altering long-range chromatin interactions.
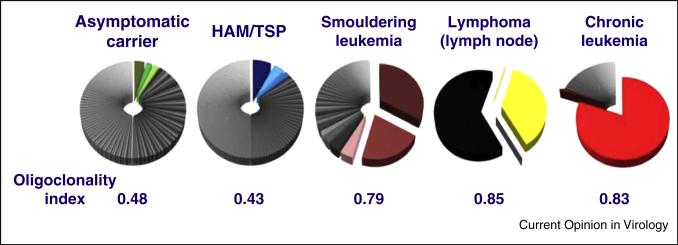
Underlying the small number of highly expanded clones in the circulation, it is now clear that there is a very large number of lower-abundance HTLV-1-infected T cell clones (Figure 2) [71]: the median number of clones in the circulation is around 10 000 in an asymptomatic carrier and around 30 000 in a patient with HAM/TSP, exceeding previous estimates by two orders of magnitude [73•]. The lower-abundance HTLV-1+ clones make up the majority of the HTLV-1 proviral load, and the proviral load — which determines the risk of both inflammatory and malignant disease — correlates with the total number of clones in the individual, not with the degree of oligoclonal proliferation [71].
Figure 2.
Frequency distribution of HTLV-1-infected T-cell clones in different manifestations of HTLV-1 infection. Each pie-chart shows the clones identified in 10 μg of genomic DNA from the peripheral blood mononuclear cells of a single representative host. The size of each sector represents the relative abundance of that clone in the individual's proviral load. The oligoclonality index (OCI) (also known as the Gini index) is a measure of the non-uniformity of the frequency distribution (71). OCI = 0 implies that all clones have the same frequency; OCI = 1 implies that only a single clone is present.
These data were reported in Figure 2A of Gillet et al. [71].
The lower-abundance clones express Tax more frequently than high-abundance clones [72], and Tax-expressing cells turn over faster than non-expressing cells in vivo [74]. The rate of spontaneous Tax expression by a given clone is influenced by the genomic integration site of the provirus in that clone: specifically, the distance and transcriptional orientation of the provirus relative to the nearest host gene [72]. Since the risk of malignant transformation in many cell types is proportional to the total number of cell divisions [75••], persistent HTLV-1-driven proliferation may increase the risk of ATL arising from these low-abundance clones. Several recent lines of evidence support the notion that ATL may arise from low abundance clones rather than the oligoclonally proliferated, high-abundance clones [76]. First, the risk of ATL rises with increased proviral load, and in turn the proviral load correlates with the total number of clones in the circulation, but not with the degree of oligoclonality [71]. Second, the attributes of the genomic integration site in ATL clones resemble those of the low abundance clones, but differ significantly from those of the intermediate-abundance, oligoclonally proliferated clones [70•]. Finally, the closely related virus HTLV-2 produces an oligoclonality profile in peripheral blood similar to that seen in ATL, but with a total number of infected clones about 10-fold lower than seen in HTLV-1 infection [77]. However, despite this strong oligoclonal proliferation, HTLV-2 does not cause malignant disease.
The host and viral factors that contribute to the development of ATL are summarized in Figure 3.
Prospects and suggestions for future work
There remains a gap in our understanding of the events occurring during clinical latency that lead to ATL and HAM from longitudinal clinical studies. In particular, we need to quantify the contributions of infectious HTLV-1 spread (new clone formation) and mitotic spread (proliferation of existing clones) to the maintenance of the proviral load.
The evidence summarized here suggests that every HTLV-1-infected clone has a potential to undergo malignant transformation, and that an individual's risk of ATL is determined primarily by the number of HTLV-1-infected T-cell clones, which in turn is determined by the efficacy of ‘quality’ of that individual's immune response. There are instances of ‘clonal succession’ in ATL, in which the spontaneous disappearance of one malignant clone is followed by the appearance of a second, independent ATL clone [78]. Work is needed on strategies to prevent ATL in high-risk individuals, that is, those with a proviral load > 4%. It may be effective to reduce the total number of HTLV-1-infected clones (and therefore reduce the proviral load), for example with anti-CCR4 monoclonal antibody; to reduce the turnover rate of infected cells; and to boost the innate and acquired immune response.
Recent developments with immunodeficient mice, reconstituted with human hematopoietic stem cells, provide opportunities to study virus replication and tumour initiation in vivo [79,80]. These models will also be useful to test new chemopreventive agents and targeted therapies [81].
Ground-breaking work with immunotherapies has provided novel approaches in a wide range of malignancies. Chimeric antigen receptor-expressing T cells have proven activity in lymphoid leukaemias [82]. Immune checkpoint inhibitors that block costimulatory T cell receptor signalling have provided unexpected activity in a wide range of solid tumours and lymphomas, and may open the door to new clinical trials for ATL [83].
Acknowledgements
CB is a Wellcome Trust Senior Investigator (WT100291MA) and is supported by the Medical Research Council (UK) (MR K019090). LR is supported by grants P01 CA100730-11A1, R01 CA63417-15, P50 CA094056-12, and LLS 6067-10 from the National Institutes of Health (USA).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Matsuoka M, Jeang K-T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 2.Jones KS, Lambert S, Bouttieer M, Benit L, Ruscetti FW, Hermine O, Pique C. Molecular aspects of HTLV-1 entry: functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors. Viruses. 2011;3:794–810. doi: 10.3390/v3060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lairmore MD, Anupam R, Bowden N, Haines R, Haynes RA, Ratner L, Green PL. Molecular determinants of human T-lymphotropic virus type 1 transmission and spread. Viruses. 2011;3:1131–1165. doi: 10.3390/v3071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pise-Masison CA, Castro-Amarante MFd, Enose-Akahata Y, Buchmann RC, Fenizia C, Washington PR, Edwards D, Fiocchi M, Alcantara LC, Bialuk I, et al. Co-dependent of HTLV-1 p12 and p8 function in virus persistence. PLoS Pathog. 2014;10:e1004454. doi: 10.1371/journal.ppat.1004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valeri VW, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, Chung HK, Fukumoto R, Parks RW, Ferrari MG, et al. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood. 2013;116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai XT, Nicot C. Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis. Front Microbiol. 2012;3:400. doi: 10.3389/fmicb.2012.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prooyen NV, Gold H, Andresen V, Schwartz O, Jones K, Ruscetti F, et al. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc Natl Acad Sci U S A. 2010;107:20738–20743. doi: 10.1073/pnas.1009635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romeo MM, Koa B, Kim J, Bradyu R, Healtley HC, He J, Harrod CK, Barnett B, Ratner L, Lairmore MD, et al. Acetylation of the c-MYC oncoprotein is required for cooperation with the HTLV-1 p30II accessory protein and the induction of oncogenic cellular transformation by p30II/c-MYC. Virology. 2015;475:271–288. doi: 10.1016/j.virol.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao T, Matsuoka M. HBZ and its roles in HTLV-1 oncogenesis. Front Microbiol. 2012;3:247. doi: 10.3389/fmicb.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKendall RR. Neurologic disease due to HTLV-1 infection. Handb Clin Neurol. 2014;123:507–530. doi: 10.1016/B978-0-444-53488-0.00024-9. [DOI] [PubMed] [Google Scholar]
- 11.Kamoi K, Mochizuki M. HTLV infection and the eye. Curr Opin Ophthalmol. 2012;23:557–561. doi: 10.1097/ICU.0b013e328358b9ec. [DOI] [PubMed] [Google Scholar]
- 12.Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci. 2007;98:772–776. doi: 10.1111/j.1349-7006.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook LB, Elemans M, Rowan AG, Asquith B. HTLV-1: persistence and pathogenesis. Virology. 2013;435:131–140. doi: 10.1016/j.virol.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia–lymphoma. Lancet Oncol. 2014;15:e517–e526. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 15.ElHajj H, El-Sabban M, Hasegawa H, Zaatari G, Ablain J, Saab ST, Janin A, Mahfouz R, Nasr R, Kfoury Y, et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J Exp Med. 2010;207:2785–2792. doi: 10.1084/jem.20101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.ElHajj H, Khalili K, Ghandour B, Nasr R, Shahine S, Ghantous A, Abdel-Samad R, Sinjab A, Hasegawa H, Jabbour M, et al. Preclinical efficacy of the synthetic retinoid ST1926 for treating adult T-cell leukemia/lymphoma. Blood. 2014;124:2072–2080. doi: 10.1182/blood-2014-03-560060. [This paper and the following paper [17•] test the efficacy of two new agents of potential therapeutic value in the treatment of ATL. They are particularly notable because each agent attacks a new therapeutic pathway in the disease; combination therapy, combining agents against more than one pathway, may offer greater efficacy in treatment.] [DOI] [PubMed] [Google Scholar]
- 17•.Tatsuro J, Ishida T, Takemoto S, et al. Randomized Phase II Study of Mogamulizumab (KW-0761) Plus VCAP-AMP-VECP (mLSG15) versus mLSG15 Alone for Newly Diagnosed Aggressive Adult T-cell Leukemia–Lymphoma (ATL) ASCO; Chicago: 2013. [See annotation to Ref. [16•.] [Google Scholar]
- 18.Journo C, Mahieux R. HTLV-1 and innate immunity. Viruses. 2011;3:1374–1394. doi: 10.3390/v3081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujihara K, Itoyama Y, Yu F, Kubo C, Goto I. Cellular immune surveillance against HTLV-I infected T lymphocytes in HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). J Neurol Sci. 1991;105:99–107. doi: 10.1016/0022-510x(91)90125-q. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Braud VM, Goon P, Hanon E, Taylor GP, Saito A, Eiraku N, Tanaka Y, Usuku K, Weber JN, et al. Low frequency of CD94/NKG2A+T lymphocytes in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis, but not in asymptomatic carriers. Blood. 2003;102:577–584. doi: 10.1182/blood-2002-09-2855. [DOI] [PubMed] [Google Scholar]
- 21.Tattermusch S, Skinner JA, Chaussabel D, Banchereau J, Berry MP, McNab FW, O'Garra A, Taylor GP, Bangham CRM. Systems biology approaches reveal a specific IFN-inducible signature in HTLV-1 associated myelopathy. PLoS Pathog. 2012;8:e1002480. doi: 10.1371/journal.ppat.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu F, Itoyama Y, Fujihara K, Goto I. Natural killer (NK) cells in HTLV-I-associated myelopathy/tropical spastic paraparesis-decrease in NK cell subset populations and activity in HTLV-I seropositive individuals. J Neuroimmunol. 1991;33:121–128. doi: 10.1016/0165-5728(91)90056-d. [DOI] [PubMed] [Google Scholar]
- 23.Kinpara S, Hasegawa A, Utsunomiya A, Nishitsuji H, Furukawa H, Masuda T, Kannagi M. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. J Virol. 2009;83:5101–5108. doi: 10.1128/JVI.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanon E, Goon P, Taylor GP, Hasegawa H, Tanaka Y, Weber JN, Bangham CR. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood. 2001;98:721–726. doi: 10.1182/blood.v98.3.721. [DOI] [PubMed] [Google Scholar]
- 25.Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti G, Hermine O. Meta-analysis on the use of zidovudine and interferon-alpha in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 26.Hisada M, Maloney EM, Sawada T, Miley WJ, Palmer P, Hanchard B, Goedert JJ, Manns A. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin Infect Dis. 2002;34:1551–1557. doi: 10.1086/340537. [DOI] [PubMed] [Google Scholar]
- 27.Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24:6035–6046. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J Infect Dis. 2002;186(Suppl. 2):S187–S192. doi: 10.1086/344269. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 30.Bangham CR. CTL quality and the control of human retroviral infections. Eur J Immunol. 2009;39:1700–1712. doi: 10.1002/eji.200939451. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery KJ, Siddiqui AA, Bunce M, Lloyd AL, Vine AM, Witkover AD, Izumo S, Usuku K, Welsh KI, Osame M, et al. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J Immunol. 2000;165:7278–7284. doi: 10.4049/jimmunol.165.12.7278. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci U S A. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seich al Basatena NK, MacNamara A, Vine AM, Thio CL, Astemborski J, Usuku K, Osame M, Kirk GD, Donfield SM, Goedert JJ, et al. KIR2DL2 enhances protective and detrimental HLA class I-mediated immunity in chronic viral infection. PLoS Pathog. 2011;7:e1002270. doi: 10.1371/journal.ppat.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacNamara A, Rowan A, Hilburn S, Kadolsky U, Fujiwara H, Suemori K, Yasukawa M, Taylor G, Bangham CR, Asquith B. HLA class I binding of HBZ determines outcome in HTLV-1 infection. PLoS Pathog. 2010;6:e1001117. doi: 10.1371/journal.ppat.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goon PK, Hanon E, Igakura T, Tanaka Y, Weber JN, Taylor GP, Bangham CR. High frequencies of Th1-type CD4(+) T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood. 2002;99:3335–3341. doi: 10.1182/blood.v99.9.3335. [DOI] [PubMed] [Google Scholar]
- 36.Toulza F, Nosaka K, Tanaka Y, Schioppa T, Balkwill F, Taylor GP, Bangham CR. Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3 + regulatory T cells. J Immunol. 2010;185:183–189. doi: 10.4049/jimmunol.0903846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111:5047–5053. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamano Y, Takenouchi N, Li HC, Tomaru U, Yao K, Grant CW, Maric DA, Jacobson S. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I-associated neuroimmunological disease. J Clin Invest. 2005;115:1361–1368. doi: 10.1172/JCI23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto-Taguchi N, Satou Y, Miyazato P, Ohshima K, Nakagawa M, Katagiri K, Kinashi T, Matsuoka M. HTLV-1 bZIP factor induces inflammation through labile Foxp3 expression. PLoS Pathog. 2013;9:e1003630. doi: 10.1371/journal.ppat.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangham CR, Toulza F, Adult T. Cell leukemia/lymphoma: FoxP3(+) cells and the cell-mediated immune response to HTLV-1. Adv Cancer Res. 2011;111:163–182. doi: 10.1016/B978-0-12-385524-4.00004-0. [DOI] [PubMed] [Google Scholar]
- 41.Toulza F, Nosaka K, Takiguchi M, Pagliuca T, Mitsuya H, Tanaka Y, Taylor GP, Bangham CR. FoxP3+ regulatory T cells are distinct from leukemia cells in HTLV-1-associated adult T-cell leukemia. Int J Cancer. 2009;125:2375–2382. doi: 10.1002/ijc.24664. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka M, Yasunaga J. Human T-cell leukemia virus type 1: replication, proliferation and propagation by Tax and HTLV-1 bZIP factor. Curr Opin Virol. 2013;3:684–691. doi: 10.1016/j.coviro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Ma G, Yasunaga J-i, Akari H, Matsuoka M. TCF1 and LEF1 act as T-cell intrinsic HTLV-1 antagonists by targeting Tax. Proc Natl Acad Sci U S A. 2015;112:2216–2221. doi: 10.1073/pnas.1419198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Szerlong HJ, Prenni JE, Nyborg JK, Hansen JC. Acitvator-dependent p300 acetylation of chromatin in vitro: Enhancement of transcription by disruption of repressive nucleosome–nucleosome interactions. J Biol Chem. 2010;285:31954–31964. doi: 10.1074/jbc.M110.148718. [TCF-1 and LEF-1 are two WNT transcription factors that are specifically expressed in T cells. Marr et al. demonstrate that TCF1 and LEF1 inhibit HTLV-1 replication by antagonising the functions of Tax protein. In activated T cells, however, TCF-1 and LEF-1 expression is inhibited, enabling re-expression of HTLV-1. This identifies a new mechanism of regulation of HTLV-1 latency in vivo..] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Chevalier SA, Turpin J, Cachat A, Afonso PV, Gessain A, Brady JN, Pise-Masison CA, Hahieux R. Gem-induced cytoskeleton remodeling increases cellular migration of HTLV-1-infected cells, formation of infected-to-target T-cell conjugates and viral transmission. PLoS Pathog. 2014;10:e1003917. doi: 10.1371/journal.ppat.1003917. [These authors demonstrate that HTLV-1 Tax induces expression of a small Ras superfamily GTP binding protein, Gem, which regulates spontaneous T cell migration, chemotaxis, and plays a role in cell-to-cell transmission of HTLV-1 via the virological synapse.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuoka M, Jeang K-T. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ, and therapy. Oncogene. 2011;30:1379–1389. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grossman WJ, Kimata JT, Wong FH, Zutter M, Ley TJ, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, et al. Thymus-derived leukemia–lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- 49.Ohsugi T. A transgenic mouse model of human T cell leukemia virus type 1-associated diseases. Front Microbiol. 2013;449 doi: 10.3389/fmicb.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi YB, Harhaj EW. HTLV-1 Tax stabilizes MCL-1 via TRAF6-dependent K63-linked polyubiquitination to promote cell survival and transformation. PLoS Pathog. 2014;10:e1004458. doi: 10.1371/journal.ppat.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavorgna A, Matsuoka M, Harhaj EW. A critical role for IL-17RB signaling in HTLV-1 Tax-induced NFkB activation and T cell transformation. PLoS Pathog. 2014;10:e1004418. doi: 10.1371/journal.ppat.1004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baydoun HH, Bai XT, Shelton S, Nicot C. HTLV-1 tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS ONE. 2012;7:e42226. doi: 10.1371/journal.pone.0042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin DY, Spencer F, Jeang KT. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 54.Durkin SS, Guo X, Fryrear KA, Mihaylova VT, Gupta SK, Belgnaoui SM, Haoudi A, Kupfer GM, Semmes OJ. HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J Biol Chem. 2008;283:36311–36320. doi: 10.1074/jbc.M804931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kao SY, Lemonie FJ, Marriott SJ. Suppression of DNA repair by HTLV-I Tax is rescued by a functional p53 signaling pathway. J Biol Chem. 2000 doi: 10.1074/jbc.M004397200. [DOI] [PubMed] [Google Scholar]
- 56.Boxus M, Twizere JC, Legros S, Kettmann R, Willems L. Interaction of HTLV-1 Tax with minichromosome maintenance proteins accelerates the replication timing program. Blood. 2012;119:151–160. doi: 10.1182/blood-2011-05-356790. [DOI] [PubMed] [Google Scholar]
- 57.Zane L, Yasunaga J, Miagami Y, Yedavalli V, Tang SW, Chen CY, Ratner L, Lu X, Jeang KT. Wip1 and p53 contribute to HTLV-1 Tax-induced tumorigenesis. Retrovirology. 2012;9:114. doi: 10.1186/1742-4690-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinoshita T, Shimoyama M, Tobinai K, et al. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86:5620–5630.. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satou Y, Yasunaga J, Zhao T, Yoshida M, Miyazato P, Takai K, Shimizu K, Ohshima K, Green PL, Ohkura N, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7:e1001117. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhlmann AS, Villaudy J, Gazzolo L, Castellazzi M, Mesnard JM, Duc Dodon M. HTLV-1 HBZ cooperates with JunD to enhance transcription of the human telomerase reverse transcriptase gene (hTERT). Retrovirology. 2007;4:92. doi: 10.1186/1742-4690-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mesnard J-M, Barbeau B, Devaux C. HBZ, a new important player in the mystery of adult T-cell leukemia. Blood. 2006;108:3979–3982. doi: 10.1182/blood-2006-03-007732. [DOI] [PubMed] [Google Scholar]
- 65.Zhao T, Yasunaga J, Satou Y, Nakao M, Takahashi M, Fujii M, Matsuoka M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood. 2009;113:2755–2764. doi: 10.1182/blood-2008-06-161729. [DOI] [PubMed] [Google Scholar]
- 66.Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-kB hyperactivation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011;7:e1002480. doi: 10.1371/journal.ppat.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma G, Yasunaga J, Fan J, Yanagawa S, Matsuoka M. HTLV-1 bZIP factor dysregulates the Wnt pathways to support proliferation and migration of adult T-cell leukemia cells. Oncogene. 2013;32:4222–4230. doi: 10.1038/onc.2012.450. [DOI] [PubMed] [Google Scholar]
- 68.Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cook LB, Rowan AG, Melamed A, Taylor GP, Bangham CR. HTLV-1-infected T cells contain a single integrated provirus in natural infection. Blood. 2012;120:3488–3490. doi: 10.1182/blood-2012-07-445593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Cook LB, Melamed A, Niederer H, Valganon M, Laydon D, Foroni L, Taylor GP, Matsuoka M, Bangham CR. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123:3925–3931. doi: 10.1182/blood-2014-02-553602. [Using a quantitative, high-throughput protocol to map proviral integration sites (described in reference [71]), these authors quantified the oligoclonality in ATL and identified the contribution of proviral structure and the role of the genomic integration site in the disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillet NA, Malani N, Melamed A, Gormley N, Carter R, Bentley D, Berry C, Bushman FD, Taylor GP, Bangham CR. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood. 2011;117:3113–3122. doi: 10.1182/blood-2010-10-312926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melamed A, Laydon DJ, Gillet NA, Tanaka Y, Taylor GP, Bangham CR. Genome-wide determinants of proviral targeting clonal abundance and expression in natural HTLV-1 infection. PLoS Pathog. 2013;9:e1003271. doi: 10.1371/journal.ppat.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Laydon DJ, Melamed A, Sim A, Gillet NA, Sim K, Darko S, Kroll JS, Douek DC, Price DA, Bangham CR, et al. Quantification of HTLV-1 clonality and TCR diversity. PLoS Comput Biol. 2014;10:e1003646. doi: 10.1371/journal.pcbi.1003646. [The authors report a mathematical analysis of HTLV-1 clonality that makes it possible to estimate the total number of HTLV-1-infected clones in the circulation. The same model was applied to estimate T cell receptor diversity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asquith B, Zhang Y, Mosley AJ, de Lara CM, Wallace DL, Worth A, Kaftantzi L, Meekings K, Griffin GE, Tanaka Y, et al. In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc Natl Acad Sci U S A. 2007;104:8035–8040. doi: 10.1073/pnas.0608832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75••.Tomasetti C, Vogelstein B. Cancer etiology Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [This seminal paper demonstrated a remarkable linear correlation, over five orders of magnitude, between the risk of malignant transformation and the total number of cell cycles in many different types of mammalian cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bangham CR, Cook LB, Melamed A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin Cancer Biol. 2014;26C:89–98. doi: 10.1016/j.semcancer.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melamed A, Witkover AD, Laydon DJ, Brown R, Ladell K, Miners K, Rowan AG, Gormley N, Price DA, Taylor GP, et al. Clonality of HTLV-2 in natural infection. PLoS Pathog. 2014;10:e1004006. doi: 10.1371/journal.ppat.1004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsukasaki K, Tsushima H, Yamamura M, Hata T, Murata K, Maeda T, Atogami S, Sohda H, Momita S, Ideda S, et al. Integration patterns of HTLV-I provirus in relation to the clinical course of ATL: frequent clonal change at crisis from indolent disease. Blood. 1997;89:948–956. [PubMed] [Google Scholar]
- 79.Tezuka K, Xun R, Tei M, Ueno T, Tanaka M, Takenouchi N, Fujisawa J. An animal model of adult T-cell leukemia humanized mice with HTLV-1 specific immunity. Blood. 2014;123:255–346. doi: 10.1182/blood-2013-06-508861. [DOI] [PubMed] [Google Scholar]
- 80.Villaudy J, Wencker M, Gadot N, Scoazec J-Y, Gazzolo L, Manz MG, Bangham CR, DucDodon M. HTLV-1 propels thymic human T cell development in ‘human immune system' Rag2S/SIL-2RgammacS/S mice. PLoS Pathog. 2011;7:e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saito M, Tanaka R, Fujii H, Kodama A, Takahashi Y, Matsuzaki T, Takashima H, Tanaka Y. The neutralizing function of the anti-HTLV-1 antibody is essential in preventing in vivo transmission of HTLV-1 to human T cells in NOD-SCID/inverted question markcnull (NOG) mice. Retrovirology. 2014;11:74. doi: 10.1186/s12977-014-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maude SL, Shpall EJ, Grupp SA. Chimeric antigen receptor T-cell therapy for ALL. Hematology. 2014;559-564 doi: 10.1182/asheducation-2014.1.559. [DOI] [PubMed] [Google Scholar]
- 83.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillet NA, Cook L, Laydon DJ, Hlela C, Verdonck K, Alvarez C, Gotuzzo E, Clark D, Farre L, Bittencourt A, et al. Strongyloidiasis and infective dermatitis alter human T lymphotropic virus-1 clonality in vivo. PLoS Pathog. 2013;9:e1003263. doi: 10.1371/journal.ppat.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Kataoka K, Nagata Y, Kitanaka A, Totoki Y, Yasunaga J-i, Kotani S, Sato-Otsubo A, Sanada M, Shiraishi Y, Shimamamura T, et al. Landscape of Genetic Alterations in Adult T-cell Leukemia/ Lymphoma. Vol. 75. American Society for Hematology; San Francisco: 2014. [This preliminary report from a large consortium clusters of somatic mutations in three key pathways in T cells in adult T cell leukaemia/lymphoma.] [Google Scholar]