Abstract
Chondroprogenitor cells are a subpopulation of multipotent progenitors that are primed for chondrogenesis. They are believed to have the biological repertoire to be ideal for cell-based cartilage therapy. In addition to summarizing recent advances in chondroprogenitor cell characterization, this review discusses the projected pros and cons of utilizing chondroprogenitors in regenerative medicine and compares them to that of preexisting methods, including autologous chondrocyte implantation (ACI) and the utilization of bone marrow derived mesenchymal stem cells (MSCs) for the purpose of cartilage tissue repair.
Keywords: chondroprogenitor, progenitor, stem cell, osteoarthritis, cartilage therapy
Introduction
Chondroprogenitor cells refer to a population of stem/progenitor cells that are capable of chondrogenic differentiation and can be derived from multiple tissue sources including articular cartilage, synovium and adipose tissue (1–4). These cells exhibit different characteristics from articular chondrocytes including high affinity for fibronectin, high colony-forming efficiency and expression of the Notch1 gene (2). Recent evidence, however, suggests that such chondroprogenitor cells exist not only in developing articular cartilage, as expected, but also in fully developed adult articular cartilage tissue (5–7). The existence of such a population of cells within fully developed articular cartilage tissue suggests that chondroprogenitors may have the biological repertoire necessary to be used effectively for the cell-based therapy of cartilage defects and perhaps even degenerative joint diseases. Due to the previous lack of knowledge detailing the specific differences between chondrocytes and chondroprogenitors, it often became commonplace to use interchangeable terminology to describe these two distinct cell populations. However, many recent studies have presented compelling evidence suggesting that several cell surface markers can be utilized to distinguish chondroprogenitors from primary chondrocytes (7–10). In this review, we will summate the current body of knowledge that has come to define the chondroprogenitor cell subpopulation and discuss its potential importance to cell-based cartilage therapy. We will also cover recent advances in chondroprogenitor cell characterization that has helped to establish these cells as a unique subpopulation of progenitors that is markedly distinct from mature articular chondrocytes.
The limited regenerative capabilities of articular cartilage
Hyaline cartilage is infamous for its inability to efficiently self-repair. For this reason, degenerative joint diseases, the most prevalent of which is osteoarthritis (OA), pose a significant clinical challenge that costs the US economy billions of dollars every year. Not only does articular cartilage heal poorly because of its inherent avascularity, but as a tissue that primarily bears load, it is also highly susceptible to injury. Furthermore, tearing of the meniscus and/or ligaments can lead to the destabilization of loadbearing joints thus perpetuating the chronic destruction of cartilage tissue.
Throughout the years, several surgical procedures have been developed with the hope to optimize the local cartilage healing response after injury. Traditional reparative therapies, including the use of engineered alloplastic tissue implants and allogeneic tissue grafts, can lead to complications by the occurrence of donor site morbidity (11). More modern techniques such as autologous chondrocyte implantation (ACI), osteochondral grafting and microfracture surgery are currently used clinically to induce a local tissue repair response in osteochondral defects (12). ACI relies on repopulating the native damaged cartilage with mature chondrocytes whereas osteochondral grafting utilizes cartilage grafts to fill the tissue defect. In both cases, the defect is filled using autologous cells or tissue grafts that are largely similar to the native articular chondrocytes/cartilage. Contrastingly, the minimally invasive microfracture surgery takes a different approach towards stimulating cartilage healing. In pronounced yet contained cartilage defects, exposed regions of subchondral bone are punctured repeatedly to create “microfractures” that promote the migration of stromal cells from the bone marrow into the cartilage defect. In many cases, the penetration of the subchondral bone is enough to cause the formation of a fibrin clot in the site of the cartilage defect. The stem cells contained within the clot then proceed to expand and differentiate thereby filling the cartilage defect of the patient.
Unfortunately, none of the above mentioned therapeutic approaches can consistently or completely restore the biomechanical properties of the hyaline cartilage tissue that was once lost from the site of the defect. For example, it has been reported that ACI can result in the terminal differentiation of these newly implanted cells into hypertrophy (13,14). Similarly, the microfracture surgery approach may ultimately fill a hyaline cartilage defect with fibrocartilage (15), which is far less effective at bearing load due to its inherently weaker resistance to impact. On the other hand, osteochondral grafts do not seem to lead to chondrocyte hypertrophy nor do they regularly become biomechanically inept at bearing load. Unfortunately, graft size and availability can be limiting factors that dictate the condition and frequency of use.
A compelling reason for seeking out a local progenitor population in cartilage tissue is the potential benefit that it may have for cell-based cartilage therapy. Unlike the mature chondrocytes that are currently utilized for expansion during the ACI procedure, which often become hypertrophic after implantation, chondroprogenitors are a relatively immature and undifferentiated population of cartilage precursors that are less likely to terminally differentiate any sooner than their mature chondrocyte counterparts (7,16). In this sense, it is conceivable that the implantation of chondroprogenitors into cartilage defects may be a superior form of cell-based therapy compared to the use of ACI. This may be a feasible approach to delay or altogether circumvent the terminal differentiation of implanted cells. Conversely, one prominent theory is that chondroprogenitors are further along the chondrogenic lineage than bone marrow derived stem cells, which are utilized in microfracture procedures. Thus, based on this idea, chondroprogenitors are likely to be more committed to becoming chondrocytes, thereby reducing the rate of the incidence of chondrocyte dedifferentiation. More importantly, chondroprogenitors are local to hyaline cartilage tissue and are thus developmentally primed for differentiation into chondrocytes that form their surrounding hyaline cartilage unlike bone marrow derived stem cells, which can form fibrocartilage (15).
Chondroprogenitors: A Committed Subset of Chondrocyte Precursors
Origin and development
During early development, precursor cells of the cellular mesenchyme differentiate down the osteogenic (bone), myogenic (muscle) and chondrogenic (cartilage) lineage and ultimately give rise to the musculoskeletal system. More specifically, limb formation involves the migration and condensation of lateral plate mesoderm cells that are chondrocyte precursors, or chondroprogenitors, which henceforth produce a robust extracellular matrix (ECM) that is rich in fibronectin, hyaluronan, tenascin and type I collagen (17). It has been proposed that these changes in the local pericellular microenvironment are crucial to facilitate the differentiation of these chondroprogenitors into mature articular chondrocytes (18,19) which begin to remodel the existing ECM by producing cartilage specific proteins such as type II, IX, XI collagens as well as aggrecan, matrilins (20) and cartilage oligomeric protein (COMP) (21). Further differentiation of these mature chondrocytes ushers in the process of endochondral ossification as chondrocytes become hypertrophic and begin to express type X collagen. Finally, vascularization by invading blood vessels facilitates the deposition of osteoblasts, which proceed to replace these terminally differentiated chondrocytes and gradually cause mineralization that is necessary for proper long bone development. Several important past studies have demonstrated that a progenitor-like cell population, known today as chondroprogenitor cells, still exists in adult hyaline cartilage tissue (2,7). What is more surprising are the reports that even OA cartilage from older individuals can harbor chondroprogenitors (5,10).
Localization within the normal joint
When Hayes and Hunziker proposed that the development of articular cartilage occurred in an appositional fashion (1,22), this suggested that the articular surface may contain a progenitor population capable of proliferating and expanding the cartilage tissue. Since then, several studies have demonstrated that the superficial zone of cartilage tissue, which includes the articular surface, is a relatively abundant source of progenitor cells within hyaline cartilage (2,6,23,24). Studies have reported that these progenitors make up approximately 0.1% of all cells that can be extracted from the superficial zone of articular cartilage tissue (6,23). Furthermore, a recent study has demonstrated the existence of progenitors within the deep zone of young adult bovine articular cartilage (25). It has also been proposed that a population of bone marrow derived stromal cells with chondrogenic potential can migrate into the articular cartilage in patients with degenerative joint disease; however, this has yet to be demonstrated in normal healthy joints (26).
Like the articular surface, the joint synovium is known to be an abundant source of progenitors capable of successfully differentiating along the chondrogenic lineage (3,27,28). While these synovium derived progenitor cells technically do not reside within cartilage tissue, their presence in the synovium may facilitate their migration into superficial zone of cartilage in response to articular surface injury. It has been shown that a stem-like cell population existing within the synovium, identified by their retention of iododeoxyuridine due to slow doubling time, responds to articular cartilage injury by undergoing rapid proliferation and chondrogenic differentiation (29). Mesenchymal progenitors with chondrogenic capabilities have also been identified in normal joint synovial fluid (30). Although progenitor cells extracted from synovial fluid of patients with degenerative joint disease, such as OA, exhibit lower chondrogenic potential (30), it has been reported that higher quantities of progenitors are present in OA synovial fluid relative to that of normal synovial fluid (31).
Bone marrow is known to harbor a sparse multipotent stromal cell population that lies beneath the subchondral bone. During OA, it has been shown that bone marrow is a viable source of progenitors that can quickly react to articular cartilage damage by locally migrating to the areas of cartilage injury (32). However, this phenomenon has yet to be observed in instances of less severe cartilage tissue damage where the tissue above the subcondral bone surface is still largely intact. Bone marrow derived mesenchymal progenitors are commonly used for their multilineage differentiation potential in tissue engineering and regeneration studies. Like bone marrow, the infrapattelar fat pad of the knee contains a progenitor cell population that is currently being considered for use in cell-based cartilage therapy (33,34).
Molecular characterization and cellular attributes
In the past five years, much progress has been made in the way of distinguishing chondroprogenitors from the vastly more abundant population of chondrocytes that exists in hyaline cartilage. It has been demonstrated that cell surface marker expression analysis is an effective means of discerning chondroprogenitors from chondrocytes (Table 1). Many well-known MSC-associated cell surface markers are abundantly expressed by progenitor populations. Chondroprogenitors are no exception. They have been reported to highly express the hyaluronate receptor (CD44), fibronectin receptor (CD49e), thymocyte antigen-1 (CD90), the type I membrane glycoprotein endoglin (CD105) and activated leukocyte cell adhesion molecule (ALCAM or CD166) (7,35,36). A recent study also reported a correlation between the chondrogenic potential of progenitor cells with the expression of melanoma cell adhesion molecule (MCAM or CD146) (10). Unlike chondroprogenitors, a large percentage of articular chondrocytes do not exhibit cell surface expression of CD49e, CD90, CD105 or CD166 (37) making these markers ideal for discerning these two cell populations.
Table 1. Specific cell surface markers reported to be expressed in chondrocytes and chondroprogenitors from normal and osteoarthritis (OA) cartilage tissue.
CD44 is a common marker between chondrocytes and chondroprogenitor cells in normal cartilage. CD90, CD105 and CD166 are common markers between chondroprogenitor cells in normal and OA cartilage.
In addition to expressing the above mentioned progenitor-specific cell surface markers, chondroprogenitors are also highly clonogenic (7,32) and capable of migration (32,38,39). Interestingly, Williams and colleagues have previously demonstrated that the colony forming efficiency (CFE) of chondroprogenitors have no obvious correlation with the age of the patient from which they were isolated (7) suggesting that these cells exhibit a self-renewal capacity that is altogether unrestricted by the patient’s age. Similar to bone marrow derived progenitors, which have been utilized to repair pulmonary tissue, since they exhibit a remarkable ability to migrate to sites of injury (40), chondroprogenitors can migrate across the articular surface to sites that have suffered trauma (38,39).
A previous study has shown that elevated expression of the transcription factor Sox-9 during clonal expansion may be a signature characteristic of chondroprogenitors (41). Sox-9 is a well-known marker of cells committed to the chondrogenic lineage. Sox-9 is not highly expressed in mesenchymal stem cells, which are the precursors to chondroprogenitors since they are not yet fully committed to the chondrogenic lineage. Although chondroprogenitors are highly capable of chondrogenic differentiation, it has been demonstrated that they can undergo osteogenesis and, to a certain extent, adipogenesis (7,16). As such, the potential for chondrogenic, osteogenic and adipogenic differentiation has become a defining feature of chondroprogenitors that distinguish them from mature chondrocytes.
Chondroprogenitors in OA cartilage
In OA, the disregulation of anabolic and catabolic factors ultimately leads to cartilage erosion. Furthermore, perichondral ossification results in the formation of bone spurs. These ossified regions diminish the near frictionless properties of the articular surface leading to further joint destruction. Relative to normal cartilage, it has been demonstrated that osteoarthritic cartilage contains roughly twice as many cells that are positive for CD105 and CD166 (8). Moreover, several studies conducted within the past decade have reported the presence of mesenchymal progenitors in osteoarthritic articular cartilage (8,42). Similar to bone marrow-derived mesenchymal stem cells, these cells were reported to be positive for tetraspan (CD9), CD90 and CD166 (5) (Table 1). While one study has reported the reduced capabilities of OA cartilage derived progenitors to differentiate along chondrogenic and adipogenic lineages (43), later studies have confirmed that these cells, like BM-MSCs, exhibit tri-lineage differentiation potential (5,8). It is puzzling why OA cartilage contains more chondroprogenitors that are positive for CD105 and CD166 than normal articular cartilage. One explanation could be that such chondroprogenitors migrate from the synovium or subchondral bone marrow in response to articular cartilage injury (8). However, an alternative explanation is that chondroprogenitor cells residing within adult articular cartilage, which are remnants of developmental cartilage, are activated to undergo replication in response to cartilage injury by giving rise to daughter cells. During this process, one of the daughter cells will remain a progenitor while the rest undergo further replication and differentiation along the chondrogenic lineage. Thus, four potential events may occur in OA cartilage: 1) chondroprogenitor replication; 2) chondroprogenitor replacement; 3) chondroprogenitors differentiate into chondrocytes; and 4) chondrocytes either undergo further differentiation to become hypertrophic chondrocytes or dedifferentiate into fibroblasts (Figure 1). All four events are subject to regulation and, therefore, defects in any of these events may affect cartilage repair. Previously, only the fourth event, which involves articular chondrocytes, was hypothesized to contribute to OA pathogenesis. Because of the identification of chondroprogenitor cells within adult articular cartilage, events 1–3 will also affect cartilage repair.
Figure 1. Diagram of local chondroprogenitor cell activation and differentiation in response to articular cartilage injury in OA.
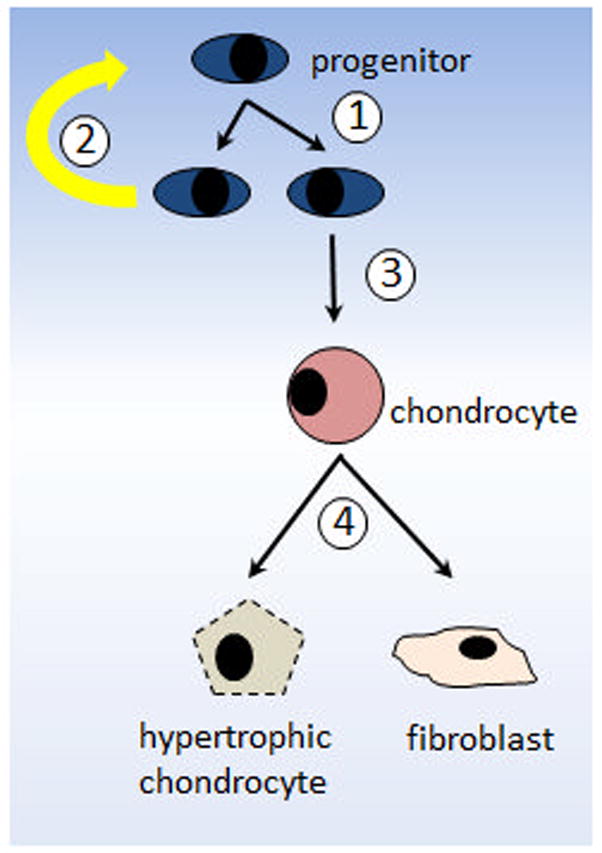
1) Chondroprogenitors replicate. 2) One of the daughter cells keep the progenitor phenotype while 3) others proliferate and undergo chondrogenesis to become mature chondrocytes. 4) Chondrocytes either terminally differentiate becoming hypertrophic cartilage tissue or dedifferentiate into fibrocartilage.
Chondroprogenitors and Prospective Cell-based Cartilage Therapies
For many years, researchers have tried to identify an abundant and suitable cell population with which to replenish injured or diseased hyaline cartilage. So far, several promising avenues of cell-based cartilage therapy have been explored including the use of autologous mature chondrocytes (i.e. ACI), bone marrow-derived as well as adipose-derived stem cells and, recently, induced pluripotent stem cells (iPSCs). Unfortunately, many of the preexisting methods as well as those methods that have yet to be fully realized clinically, have clear and/or foreseeable limitations that may hinder their utility in the field of cell-based cartilage repair (Table 2). The use of chondroprogenitors for cell-based therapy may indeed represent a novel and more effective approach towards cartilage tissue repair that addresses at least some of these concerns.
Table 2.
Potential benefits and limitations of utilizing different cell sources for autologous cell implantation therapy in cartilage defect repair.
| Cell type | Benefits | Limitations |
|---|---|---|
| Chondrocyte |
|
|
| Bone marrow- derived progenitors |
|
|
| Adipose-derived progenitors |
|
|
| iPSCs |
|
|
| Cartilage-derived progenitors |
|
|
Limitations of preexisting autologous cell implantation approaches
As previously mentioned, while autologous chondrocyte implantation is a widely used clinical method of cartilage repair, it can result in hypertrophic differentiation of the implanted chondrocytes (13). Excessive hypertrophy of implanted chondrocytes can lead to the need for debridement of the resulting tissue. Equally problematic is the potential dedifferentiation of these chondrocytes (44) during the crucial in vitro expansion stage that is used to produce a sufficient number of cells for implantation (45). Autologous implantation of excessively dedifferentiated chondrocytes can greatly favor the formation of fibrocartilage (46,47). In both circumstances, the resulting tissue is not a very effective substitute for weight-bearing hyaline cartilage. One way to approach the problem of chondrocyte dedifferentiation during in vitro expansion is to try and redifferentiate them by 2D or 3D culture in media supplemented with chondrogenic growth factors (48,49). Previous studies have focused on optimizing the redifferentiation process as to allow for the implanted cells to produce a better cartilage phenotype. Many of these studies involve using a time consuming culturing process to expand chondrocytes in an expensive cocktail of mitogens including TGFβ1, FGF-2, EGF and various prostaglandins (48,50,51) in order to prevent further dedifferentiation.
Bone marrow-derived MSCs are a pluripotent stem cell population that can fully differentiate along bone, cartilage and adipose tissue lineages. MSCs can be isolated from bone marrow and expanded for therapeutic use. While various preclinical (52–55) and clinical studies (56,57) have demonstrated the efficacy of using MSCs as a cell-based therapy for cartilage defects and OA, a potential concern lies in the multilineage potential of MSCs that are prone to hypertrophy or differentiate along the osteogenic lineage altogether. MSCs are highly influenced by their local microenvironment therefore conferring a lack of stability in their commitment to a desired tissue lineage (in this case cartilage) (58). This is especially a risk worth addressing when treating an OA joint where there is a prominent disregulation of cytokines, chemokines and growth factors underlying the disrupted tissue homeostasis. To avoid this risk, it may be advantageous to instead utilize a population of progenitors that is lineage restricted to achieve the same end.
Interestingly, it has been demonstrated that the use of adipose stem cells, typically derived from the infrapatellar fat pad, may be a viable source of chondrogenic cells for cartilage repair (4,59). However, these cells appear to maintain limited chondrogenic potential during established differentiation protocols compared to MSCs (60,61).
The potential utility of iPSCs in the field of tissue engineering is undeniable. Since iPSCs can be abundantly generated and patient matched, it is not surprising that they are currently being considered for usage in cartilage defect repair. A recent study by Diekman et al. has demonstrated that iPSCs can be induced into differentiation along the chondrogenic lineage to form a cartilaginous tissue expressing high levels of type II collagen and glycosaminoglycans while maintaining low expression of type I and type X collagens (61). A daunting limitation of the utilization of iPSCs for autologous cell/tissue implantation was demonstrated by Uto et al. who reported that in some cases, transplanted iPSCs proceeded to form teratoma (62). Thus the tumorigenic potential of iPSCs is an alarming hurdle that must be cleared before their serious consideration for cell-based cartilage therapy.
Chondroprogenitors for cell-based cartilage tissue repair
Similar to many of the previously discussed approaches towards cell-based cartilage therapy, using autologous chondroprogenitors to fill cartilage defects has foreseeable limitations (Table 2). For one thing, chondroprogenitors make up significantly less than even 1% of all cells found in adult articular cartilage making them a very rare population of cells (6,23). The search of alternative sources of progenitors with chondrogenic potential has brought much attention to pluripotent progenitors that can be derived from various tissue types including that of the joint synovium (3,28) and infrapatellar fat pads (27). Nonetheless, the major challenge of identifying a practical and abundant source of expandable chondroprogenitors is still a limiting factor for their utilization for cartilage repair.
Despite this nagging limitation, there are clear advantages associated with the use of chondroprogenitors in cell-based cartilage therapy. Firstly, cartilage tissue derived chondroprogenitors can be derived from local cartilage and they have sufficient clonability for expansion without losing their capacity for chondrogenic differentiation. The benefit of obtaining a progenitor population from native cartilage for the purpose of cartilage repair is that these cells contain the biological repertoire necessary to differentiate accordingly; thus they are likely to be primed to become native cartilage tissue. Relative to immature stromal cells from bone marrow and adipose derived progenitors, chondroprogenitors from native cartilage are believed to be further along in their commitment to the chondrogenic lineage making them a logical choice for cell implantation into cartilage. Furthermore, in contrast to the unpredictable differentiation capacity of iPSCs, chondroprogenitors are lineage restricted making them a safer option for consideration as a cell-based cartilage repair modality.
Modulating chondrogenesis of chondroprogenitors
The key to successful cartilage repair is chondrogenesis of cells that give rise to cartilage, which include bone-marrow-derived MSCs, chondroprogenitors and chondrocytes. This is a common feature of all cell-based cartilage repair processes including ACI and microfracture. Tissue microenvironment plays a vital role in cellular development. Growth factors and extracellular matrix molecules are crucial components of the tissue microenvironment that helps to regulate progenitor cell differentiation. While chondroprogenitors are more committed to the chondrocyte lineage than mesenchymal stromal cells, a highly chondrogenic microenvironment is key to ensure proper chondrogenesis (63,64). In addition to commonly used chondrogenesis inducing growth factors, such as IGF and the TGF-β family, cartilage specific ECM molecules like matrilin-3 have been shown to promote chondrogenesis of chondroprogenitors (64). Moreover, a recent study shows that matrilin-3 can also inhibit chondrocyte hypertrophy by sequestering BMP2 thereby competitively inhibiting its activation of the BMP signaling pathway (65). Since premature hypertrophy and terminal differentiation of autologously implanted chondrocytes can be a clinical problem in cell-based cartilage therapy, such challenges can be overcome using chondroprogenitors in combination with anti-hypertrophic molecules like matrilin-3 in a scaffold to promote chondrogenesis and prevent further hypertrophy.
Conclusions
Much progress has been made in recent years in the way of characterizing the distinguishing chondroprogenitors from the more abundant population of chondrocytes that reside in articular cartilage. The discovery and ongoing understanding of this minor population of progenitors that are local to cartilage tissue has led us to the fundamental question of whether they are suitable for use in cartilage repair and even degenerative joint disease therapy. While there is so much more to learn about these remarkable cells, including more about their definitive cell surface markers and cell specific genes, if the past five years is any indication of our projected rate of progress, we should have an answer sooner than later.
Acknowledgments
This study is supported by NIH grant P20GM104937.
References
- 1.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001 Jun;203(6):469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 2.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004 Feb 29;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 3.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001 Aug;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 5.Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6(5):R422–32. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010 Oct 14;5(10):e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004 May;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 9.Campbell DD, Pei M. Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells. 2012 Nov 16;1(4):1107–1120. doi: 10.3390/cells1041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J Orthop Res. 2015 Jan;33(1):84–91. doi: 10.1002/jor.22731. [DOI] [PubMed] [Google Scholar]
- 11.Cancedda R, Dozin B, Giannoni P, Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003 Mar;22(1):81–91. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 12.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998 Aug;41(8):1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Henderson I, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006 Dec;22(12):1318–1324. e1. doi: 10.1016/j.arthro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 14.Kreuz PC, Steinwachs M, Erggelet C, Krause SJ, Ossendorf C, Maier D, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007 Dec;15(12):1339–1347. doi: 10.1016/j.joca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instr Course Lect. 2007;56:419–428. [PubMed] [Google Scholar]
- 16.McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012 Jun;192(3):345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Sandell LJ, Nalin AM, Reife RA. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994 Feb;199(2):129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- 18.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000 Sep;8(5):309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 19.Tuan RS. Biology of developmental and regenerative skeletogenesis. Clin Orthop Relat Res. 2004 Oct;(427 Suppl):S105–17. doi: 10.1097/01.blo.0000143560.41767.ee. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen Q. Changes of matrilin forms during endochondral ossification. Molecular basis of oligomeric assembly. J Biol Chem. 2000 Oct 20;275(42):32628–32634. doi: 10.1074/jbc.M002594200. [DOI] [PubMed] [Google Scholar]
- 21.Geng H, Carlsen S, Nandakumar KS, Holmdahl R, Aspberg A, Oldberg A, et al. Cartilage oligomeric matrix protein deficiency promotes early onset and the chronic development of collagen-induced arthritis. Arthritis Res Ther. 2008;10(6):R134. doi: 10.1186/ar2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007 Apr;15(4):403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007 Jun 22;358(1):99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, et al. Identification of a Prg4-positive articular cartilage progenitor cell population. Arthritis Rheumatol. 2015 Jan 20; doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Zheng H, Buckwalter JA, Martin JA. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthritis Cartilage. 2014 Sep;22(9):1318–1326. doi: 10.1016/j.joca.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Makarov SS. An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17432–17437. doi: 10.1073/pnas.0607939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008 Aug;17(4):761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura K, Solchaga LA, Caplan AI, Yoo JU, Goldberg VM, Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999 Dec;42(12):2631–2637. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011 May;63(5):1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 30.Krawetz RJ, Wu YE, Martin L, Rattner JB, Matyas JR, Hart DA. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One. 2012;7(8):e43616. doi: 10.1371/journal.pone.0043616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008 Jun;58(6):1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- 32.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009 Apr 3;4(4):324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003 Jul;(412):196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 34.Jurgens WJ, van Dijk A, Doulabi BZ, Niessen FB, Ritt MJ, van Milligen FJ, et al. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11(8):1052–1064. doi: 10.3109/14653240903219122. [DOI] [PubMed] [Google Scholar]
- 35.Fickert S, Fiedler J, Brenner RE. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage. 2003 Nov;11(11):790–800. doi: 10.1016/s1063-4584(03)00167-5. [DOI] [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Choi BH, Min BH, Park SR. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis Rheum. 2009 Aug;60(8):2325–2332. doi: 10.1002/art.24786. [DOI] [PubMed] [Google Scholar]
- 38.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012 Nov;64(11):3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Brouillette MJ, Seol D, Zheng H, Buckwalter JA, Martin JA. Functional full-thickness articular cartilage repair by rhSDF-1alpha loaded fibrin/ha hydrogel network via chondrogenic progenitor cells homing. Arthritis Rheumatol. 2015 Jan 26; doi: 10.1002/art.39049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008 Apr;22(4):1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 41.Khan IM, Bishop JC, Gilbert S, Archer CW. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009 Apr;17(4):518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011 Apr 15;13(2):R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002 Mar;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 44.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 45.Schulze-Tanzil G, Mobasheri A, de Souza P, John T, Shakibaei M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis Cartilage. 2004 Jun;12(6):448–458. doi: 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003 Feb;85-A(2):185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Roberts S, McCall IW, Darby AJ, Menage J, Evans H, Harrison PE, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5(1):R60–73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997 Dec 15;237(2):318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 49.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012 Oct;20(10):1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 50.van Osch GJ, van der Veen SW, Verwoerd-Verhoef HL. In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plast Reconstr Surg. 2001 Feb;107(2):433–440. doi: 10.1097/00006534-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001 Mar 26;81(2):368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 52.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003 Dec;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 53.Alfaqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia. 2008 Jul;63( Suppl A):37–38. [PubMed] [Google Scholar]
- 54.Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007 Feb;23(2):178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009 Dec;15(4):647–658. doi: 10.1089/ten.TEC.2008.0569. [DOI] [PubMed] [Google Scholar]
- 56.Roberts S, Genever P, McCaskie A, De Bari C. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011 May;6(3):351–366. doi: 10.2217/rme.11.21. [DOI] [PubMed] [Google Scholar]
- 57.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002 Mar;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 58.Richter W. Cell-based cartilage repair: illusion or solution for osteoarthritis. Curr Opin Rheumatol. 2007 Sep;19(5):451–456. doi: 10.1097/BOR.0b013e3282a95e4c. [DOI] [PubMed] [Google Scholar]
- 59.Boeuf S, Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther. 2010 Oct 13;1(4):31. doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010 Feb;16(2):523–533. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19172–19177. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uto S, Nishizawa S, Takasawa Y, Asawa Y, Fujihara Y, Takato T, et al. Bone and cartilage repair by transplantation of induced pluripotent stem cells in murine joint defect model. Biomed Res. 2013;34(6):281–288. doi: 10.2220/biomedres.34.281. [DOI] [PubMed] [Google Scholar]
- 63.Pei M, Luo J, Chen Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthritis Cartilage. 2008 Sep;16(9):1110–1117. doi: 10.1016/j.joca.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayasuriya CT, Zhou FH, Pei M, Wang Z, Lemme NJ, Haines P, et al. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-beta in chondroprogenitor cells. Int J Mol Sci. 2014 Aug 21;15(8):14555–14573. doi: 10.3390/ijms150814555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X, Trehan SK, Guan Y, Sun C, Moore DC, Jayasuriya CT, et al. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J Biol Chem. 2014 Dec 12;289(50):34768–34779. doi: 10.1074/jbc.M114.583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005 Mar;202(3):731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]



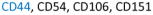 . (
. ( . (
. (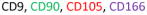 . (
. (