Abstract
Inflammation is a biologic process that mediates tissue effects including vasodilation, hyperemia, edema, collagenolysis and cell proliferation through complex immunologic pathways. In regards to the ovary, inflammation has key physiologic roles in ovarian folliculogenesis and ovulation. On the other hand inflammatory processes are subject to underlying pathology and if pushed, pro-inflammatory conditions may have a negative impact on ovarian follicular dynamics. Obesity and polycystic ovary syndrome (PCOS) serve as examples of conditions associated with chronic endogenous production of low-grade pro-inflammatory cytokines. Both conditions negatively impact ovarian folliculogenesis and ovulation. The pages that follow summarize the role of inflammation in normal ovarian follicular dynamics and evidence for its role in mediating the negative effects of obesity and PCOS on ovarian follicular dynamics. The review concludes with a summary supporting a role for lifestyle factors that favorably impact inflammatory process involved in obesity and PCOS to improve ovarian function.
Keywords: Inflammation, ovarian follicle, lifestyle, obesity, polycystic ovarian syndrome
Introduction
Inflammation plays a key physiologic role in folliculogenesis and ovulation. However, increasing evidence demonstrates that aberrant inflammation can alter normal ovarian follicular dynamics resulting in impaired oocyte quality, anovulation, and associated infertility. Obesity and polycystic ovarian syndrome (PCOS) are both associated with chronic inflammation. Insulin resistance and hyperandrogenism are important in the pathophysiologic effects of these conditions on the ovary, but the chronic inflammation of these conditions is also important (1–3). In addition to summarizing the role of inflammation in normal ovarian function, this review highlights evidence for the role of inflammation in the aberrant ovarian follicular dynamics of obesity and PCOS (Figure 1). The review concludes by summarizing the data supporting a role for diet and exercise in combating inflammation in PCOS and obesity to improve ovarian health.
Figure 1.
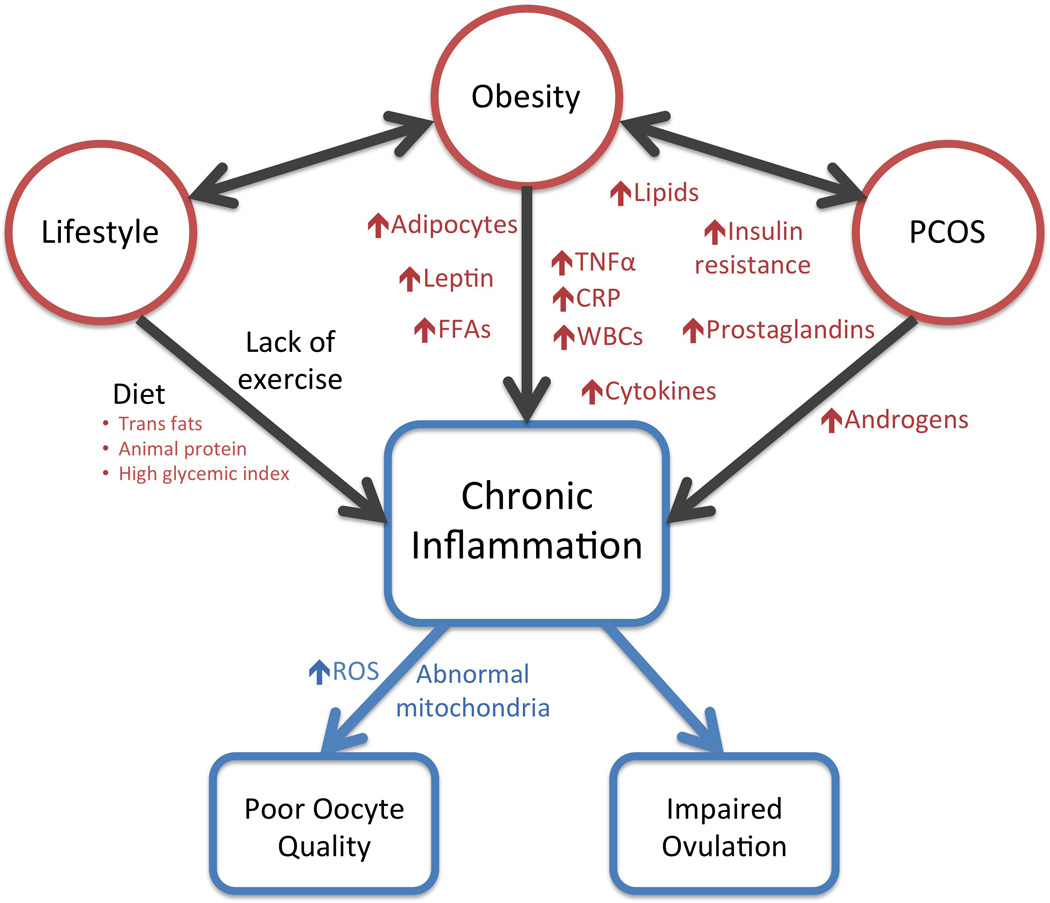
Inflammation and Abnormal Ovarian Physiology
Although inflammation is required for normal folliculogenesis and ovulation, chronic inflammation caused by obesity, PCOS, poor diet or minimal physical activity impairs follicular dynamics and subsequent reproductive potential.
Abbreviations: CRP, c-reactive protein; FFAs, free fatty acids; PCOS, polycystic ovarian syndrome; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; WBCs, white blood cells
Inflammation and Normal Ovarian Physiology
Ovulation is an essential component to mammalian reproduction and separates the menstrual cycle into two parts: folliculogenesis and luteinization. In order for ovulation to occur, normal ovarian tissue must rupture to allow expulsion of the mature oocyte. Early theories speculated that increasing intra-follicular pressure caused this rupture (4). However, we now know that inflammation, induced by gonadotropin stimulation, has a physiologic role creating a weakening in the follicle wall and eventual rupture (5, 6).
The ovary contains five layers overlying each oocyte. The most external layer is the epithelium, followed by the tunica albuginea, the theca externa, the theca interna, and finally, the granulosa. Ovulation is associated with significant tissue remodeling as the follicle increases in size, and the thecal layers fuse with the tunica albuginea and subsequently, thin to allow for ovarian rupture. In humans, this ovulatory process takes approximately forty hours. Inflammation has been theorized to induce both ovulation and its associated tissue remodeling. The inflammatory process includes direct and indirect actions to cause vasodilation, hyperemia, edema, collagenolysis, and cell proliferation; all of which, are mirrored in the process of ovulation.
The luteinizing hormone (LH) surge induces cyclic adenosine monophosphate (cAMP) production, steroidogenesis and the release of histamines as well as other inflammatory mediators. Prostaglandins, predominantly E2, and eicosanoids also increase in response to LH and concentrations peak at ovulation. Prostaglandins enhance the inflammatory reaction and activate thecal fibroblasts. Proteolytic enzymes released by prostaglandins and eicosanoids promote angiogenesis and hyperemia and activate collagenases and other proteolytic enzymes to degrade the follicle’s connective tissue and cause ovulation. In addition, bradykinin, which induces vasodilation, has been shown to increase 10-fold during the ovulatory process (6).
C-reactive protein (CRP) is an acute phase reactant produced by hepatocytes and is a marker for systemic inflammation. Serum CRP levels rise in response to increased production of tumor necrosis factor α (TNFα) and interleukin (IL) 6 from macrophages and adipocytes, which then activates an inflammatory response through the complement system (7). It has been hypothesized that CRP levels fluctuate in the menstrual cycle with a peak near ovulation. Clancy et al. demonstrated an elevated CRP concentration in women with three follicular waves rather than the more common, two follicular waves, suggesting that systemic CRP concentrations are altered with changes in follicular dynamics (8).
Proinflammatory cytokines are produced throughout folliculogenesis and participate in ovulation induction. Studies evaluating the follicular fluid of women undergoing assisted reproductive technologies (ART) describe differences in cytokine levels that correspond to their infertility diagnosis and their stimulation protocol (9, 10). For example, IL-8 has chemotactic activity that encourages migration of neutrophils. This cytokine was present in the follicular fluid of all samples in one study. IL-18 induces cytokines, IL-1β and TNF-α, essential to follicular growth and oocyte maturation. The role of IL-18 was supported by a positive correlation between its follicular levels and the number of oocytes retrieved, successful implantation and parity, whereas women with unexplained infertility had lower levels of IL-18. These findings suggest that an unperturbed inflammatory response is necessary for appropriate folliculogenesis and that impaired inflammation maybe contribute to infertility.
Inhibition of cyclooxygenase-2 (COX-2) prevents follicle rupture but not the other aspects of luteinization. Data regarding nonsteroidal anti-inflammatory agents, such as indomethacin, suggest that administration during the first 80% of the ovulatory process will inhibit ovulation (6). Overall, patients, particularly those with demonstrated infertility, should be advised to avoid the use of drugs that inhibit prostaglandin synthesis (11–13).
Inflammation and Abnormal Ovarian Physiology
Obesity
It is well known that maternal body weight influences reproductive function (1). Obesity has established associations with anovulation, infertility, miscarriage and pregnancy complications. The mechanisms behind these associations are likely multifactorial, but growing evidence supports a correlation between obesity’s associated low-grade, chronic inflammation and impaired folliculogenesis (14). Although inflammation as the definitive, direct mechanism linking obesity with impaired reproductive performance cannot be concluded, the literature posits that inflammation likely plays at least some role in the pathophysiologic mechanism.
Several clinical studies support the impact of obesity on oocyte quality. Marquard et al. showed that oocytes from women with obesity were significantly smaller than normal weight controls (15). In 2011, Shah et al. demonstrated a connection between morbid obesity and decreased fertilization rates (16) as well as decreased pregnancy rates. Luke et al. showed that using donor oocytes normalized the pregnancy rates in obese women, providing further evidence that oocyte quality is affected by BMI. Although obesity has also been linked to increased miscarriage rates, obesity has not been correlated with embryo aneuploidy (17–20).
More than the storage of triglycerides, adipose tissue is an endocrine organ generating cytokines and free fatty acids (FFAs)—mostly in the form of palmitic acid, a long-chain saturated fatty acid. The inflamed adipose tissue induces a systemic chronic inflammatory response, which makes other tissue susceptible to conditions such as insulin resistance, hypertension and cardiovascular disease. A strong correlation exists between body mass index (BMI) and CRP levels, but perhaps of more importance than BMI is the amount of visceral adiposity, which correlates with CRP concentrations independent of total adiposity. Visceral adipocytes are likely potently inducing the inflammatory pathway creating a chronic, low-grade inflammatory state.
Follicular fluid in obese women has also shown elevated levels of CRP as well as leptin (14). Leptin is a protein that acts as a signaling factor from adipose tissue to the central nervous system, serves as a metabolic indicator of energy stores, and interacts with the reproductive axis at multiple sites. As an acute phase reactant, leptin modulates hematopoietic and immunomodulatory activity.
Understanding the mechanism by which obesity affects oocyte quality is imperative to developing therapeutic interventions (21). Murine studies utilizing a diet high in saturated fat to induce obesity have shown that oocytes from obese mice have increased lipid deposits (22). Exposure to high levels of lipids and saturated fatty acids damage mitochondria as evidenced by altered mitochondrial morphology, a compensatory increase in mitochondrial deoxynucleic acid (DNA) copy number and impaired mitochondrial function (23, 24). Specifically, the quantity of reactive oxygen species (ROS) seen in oocytes was significantly increased. ROS are important to ovulation, but in excess they are cytotoxic to the cell and organelles, and without a responsive increase in antioxidants, oocytes are subsequently damaged or of poorer quality through the phenomenon known as lipotoxicity (22, 25). The combination of lipotoxicity and chronic inflammation inducing oxidative stress on the oocyte likely impairs oocyte quality and therefore, reduces obese women’s reproductive potential. This may explain why women who are obese but ovulatory experience a longer time to pregnancy than non-obese, ovulatory women (26).
Finally, it is important to consider the cohort of obese women who are without metabolic changes (27). Is it the adipose tissue itself or downstream effects of insulin resistance and hyperglycemia that generate inflammation? Data suggests that obesity without symptoms of metabolic syndrome does not increase the risk for cardiovascular disease compared to normal weight individuals (28). In a cross-sectional analysis of postmenopausal women in the Women’s Heath Initiative Observational Study, obese women with and without metabolic features were compared to normal weight women also with and without metabolic symptoms (29). Obese women without a clustering of cardiovascular risk factors still possess abnormal levels of inflammatory markers. Whether there is something about metabolically abnormal obese women versus those who do not have metabolic effects that impact their follicular development, oocyte quality and subsequent pregnancy rates requires further research.
Polycystic Ovarian Syndrome
Polycystic ovarian syndrome (PCOS) is the most common reproductive disorder in the United States (2) and is characterized by three key features: clinical or laboratory evidence of excess androgen activity, oligo- or anovulation, and polycystic appearing ovaries. The Androgen Excess Society stresses the importance of hyperandrogenism as the most consistent and obvious diagnostic feature of the syndrome. However, a broad range of phenotypes identify with this syndrome varying by age, BMI, ethnicity and associated co-morbidities. Despite the diversity in presentation, inflammation has been proposed as unifying feature among women with PCOS. Inflammation is also associated with other aspects of the syndrome, such as obesity, type 1 diabetes, hyperandrogenemia, insulin resistance and an increased risk for cardiovascular disease.
PCOS ovaries are characterized by an increased number of antral follicles and an increased stromal volume. This alteration in architecture is reflected by variations in function as well. Theca cells produce increased amounts of androgens and granulosa cells produce elevated levels of anti-Müllerian hormone. Additionally, several proinflammatory markers and mediators have been demonstrated to be elevated in women with PCOS, including CRP, leukocytes, cytokines, and ROS (30).
Several studies have shown changes in the expression of inflammatory genes. Schmidt et al. demonstrated up regulation of IL8, IL1B, nitric oxide synthase 2 (NOS2), and prostaglandin-endoperoxide synthase 2 (PTGS2) in granulosa cells and hypothesized that early expression of these inflammatory markers may induce a premature influx of leukocytes and as a result, impair maturation and subsequent ovulation (31). Leukocytosis is more prevalent in women with PCOS than normal cycling controls and importantly, is also predictive of cardiovascular risks, such as obesity and hyperlipidiemia (32–34).
Interestingly, IL1 was under expressed in the ovarian stroma as compared to normal controls. Because of the proposed role of IL1 in the inhibition of gonadotropin-stimulated androstenedione production, the authors suggest that the down regulation of IL1 may be contributing to the elevated androgens levels that characterize this syndrome (35).
In 2011, a meta-analysis evaluating the association between inflammation and PCOS demonstrated a 96% increase in serum CRP levels in women with PCOS when compared to controls, independent of BMI (36). Kelly et al. also showed that women with PCOS have significantly higher levels of CRP (2.12 versus 0.67 mg/L, P=0.016) when compared to controls (37). Age and BMI were positively correlated with CRP levels as well, but after adjusting for these variables, the association with PCOS remained significant. However, this significance was lost after adjustment for insulin sensitivity, suggesting that inflammation is highly correlated with the level of insulin resistance rather than hyperandrogenemia. These data are relevant as even subtle increases in serum CRP levels have been strongly predictive of cardiovascular risks (38).
Additionally, inflammation has been associated with oxidative stress, and oxidative stress has been shown to induce inflammation, thus suggesting a perpetuating cycle. Data demonstrate that women with PCOS have an increase in oxidative stress as well as a decrease in antioxidant capacity (39). Blair et al. showed that even when women with PCOS are matched for age, BMI and insulin resistance, oxidative stress was significantly elevated in the PCOS cohort (40). Gonzalez et al. has suggested that women with PCOS are particularly susceptible to the effects of hyperglycemia on mononuclear cells in the induction of ROS (41). ROS then trigger a chain reaction of inflammatory markers, such as nuclear factor-κB and TNFα, which subsequently mediate insulin resistance. ROS have also been correlated with mitochondrial dysfunction, impaired spindle formation and abnormal chromosome arrangement, which in turn, increase the risk for oocyte aneuploidy (23). One could hypothesize that both chronic inflammation and ROS generation contribute to the increased rate of miscarriage in women with PCOS (42).
Although evidence demonstrates a correlation between PCOS and chronic low-grade inflammation, controlling for strong confounders, such BMI and insulin resistance, has not elucidated a direct link. It is imperative that future research clearly defines PCOS by society-recognized criteria, that data are stratified for BMI and adjusted for co-morbidities. Finally, while the diagnostic criteria for PCOS are essential for generalizability and reproducibility in research, patient care should be individualized with focus on treating symptoms rather than the diagnosis.
Lifestyle Factors and Ovarian Physiology
Diet
Beyond the effects of BMI and the diagnosis of PCOS, diet may be independently and/or synergistically influencing systemic inflammation and as a result, ovarian function and follicular development. High inflammatory foods are those with saturated fats, high glycemic indices, and animal protein other than fish. Diet interventions, such as the Mediterranean diet, have been shown to decrease features of metabolic syndrome. In 2004, a randomized controlled trial compared 180 patients with metabolic syndrome who were assigned to either a Mediterranean diet or a control, calorie-prudent diet. The intervention group had significantly lower levels of serum CRP, IL-6, and decreased insulin resistance.
Data published from the Nurses’ Health Study II (NHS II) revealed a correlation between dietary intake and the presence of ovulatory disorder infertility (3). The authors assigned a “fertility diet” score according to the quantity and quality of dietary components that had previously been shown to predict infertility associated with ovulatory disorders. When comparing the highest quintile score to the lowest, women with the highest “fertility diet” score had a 66% lower risk of ovulatory disorder infertility and a 27% lower risk of infertility due to other causes. Analyses were adjusted for known confounders, including age, parity, and BMI. Proteins from vegetable and nut sources rather than animal sources, monounsaturated fats rather than trans-fats, high fiber intake, and carbohydrates lower in glycemic index were all associated with a decreased risk of ovulatory disorder infertility. Although systemic markers for inflammation were not evaluated, a low “fertility diet” score was composed of foods considered as pro-inflammatory. One could conjecture that dietary intake of certain micronutrients may alter inflammatory pathways and impair folliculogenesis and subsequent ovulation.
FFAs are important for normal physiologic function in the body as well as granulosa cell function and oocyte maturation. Maturing oocytes generate energy through mitochondrial oxidation of FFAs generated through lipolysis (43). The LH surge converts cortisone to cortisol, which promotes cumulus cell lipid metabolism and successful ovulation and pregnancy (44). However, elevated total levels of FFAs have been correlated with pro-inflammatory states, such as obesity, PCOS, metabolic syndrome, and cardiovascular disease (45, 46).
After adjusting for age, BMI and other confounders, Jungheim, et al. revealed that elevated levels of follicular FFAs correlated with abnormal cumulus oocyte complex morphology (47). The authors proposed that excess FFAs adversely influence ovarian follicular function via effects on peroxisome proliferator-activated receptor γ with subsequent impairments in fatty acid metabolism or via altered lipogenic pathways essential to granulosa cell function and steroidogenesis. On the other hand, specific FFAs serve specific purposes.
While some FFAs serve as important substrates for energy production or steroidogenesis, others play important roles in the synthesis of prostaglandins. Polyunsaturated fatty acids (PUFAs) such as omega-6 and omega-3, are derived from the diet. The end products of omega-6 fatty acid metabolism are generally pro-inflammatory and include arachidonic acid and leukotrienes, whereas the end products of omega-3 fatty acid metabolism include the anti-inflammatory eicosapentaenoic acid and docosahexanoic acid. Looking specifically at PUFAs, Jungheim, et al. went on to show that serum PUFA levels influence pregnancy rate in women undergoing ART (48). However, in this work it was the ratio rather than the independent quantity of PUFAs was particularly pertinent. Specifically, the ratio of linoleic acid (an omega-6 fatty acid) to α-linolenic acid (an omega-3 fatty acid) was critical as they are metabolized by the same enzyme and as a result, excess of one influences the availability of the other. Women with elevated linoleic to α-linolenic acid ratios had higher pregnancy rates than women with lower ratios. The extent of the effects of PUFAs on follicular development and resultant oocyte quality in addition to implantation is not known. However, murine models have shown that increased dietary intake of omega-3 fatty acids led to altered oocyte mitochondrial distribution, increased ROS, and impaired embryonic competence (49).
Exercise
In addition to diet, exercise is an important, modifiable lifestyle factor that may favorably impact systemic inflammation. In the case of obesity and PCOS, this may result in improved ovarian function. Importantly, data from NHS II demonstrated that for every one hour per week of vigorous activity, women have a 5% reduction in the risk of ovulatory infertility—independent of body mass index and dietary factors (3, 50). Other work in women with PCOS demonstrates that exercise results in a reduction in circulating white blood cells and inflammatory markers, including CRP and TNFα (51, 52). Whether or not improvements in inflammatory profiles associated with exercise in women with PCOS are important to improved ovulatory function is yet to be determined.
Conclusion
Successful folliculogenesis, oocyte maturation and ovulation require a healthy inflammatory response. Obesity is well documented to be associated with chronic, low-grade inflammation and has also been shown to increase the time to conception, the risk for anovulation, and infertility. The definition of PCOS includes ovulatory dysfunction and although the phenotype is diverse, inflammation has been proposed as the unifying feature. In addition, a diet rich in foods considered to be proinflammatory, such as trans-fats, animal protein, foods high on the glycemic index and low in fiber, have been associated with ovulatory disorder infertility. The literature documents elevated serum and follicular levels of CRP, leptin and multiple cytokines in both obesity and PCOS, and alterations in these levels have been correlated with differing diagnoses of infertility as well as ART stimulation protocols.
While the association between inflammation and follicular development is well supported in the literature, further research is needed to elucidate a direct link. Multiple confounders, including BMI, visceral adiposity, insulin resistance, hyperlipidemia, etc., independently increase the risk for inflammation and continue to cloud the relationship. Future research must clearly define obesity, using the World Health Organization’s recommended categorization, and also clearly define PCOS, using society recommended guidelines. Finally, stratifying or adjusting by metabolic confounders is essential to identifying the pathophysiologic etiology of impaired folliculogenesis and oocyte quality associated with inflammatory conditions, such as obesity and PCOS.
Acknowledgments
C.E.B. received support from the National Research Training Program in Reproductive Medicine sponsored by the National Institute of Health (T32 HD040135-13) and the Scientific Advisory Board of Vivere Health. E.S.J. received support from the Women’s Reproductive Health Research Program sponsored by the National Institute of Health (K12 HD063086), the Institute of Clinical and Translational Sciences at Washington University (UL1 TR000448), the Barnes Jewish Hospital Foundation, and the March of Dimes.
Abbreviations
- PCOS
Polycystic ovarian syndrome
- FFA
free fatty acids
- CRP
c-reactive protein
- IL
interleukin
- cAMP
cyclic adenosine monophosphate
- LH
luteinizing hormone
- TNFα
tumor necrosis factor α
- ART
assisted reproductive technologies
- COX-2
cyclooxengenase-2
- BMI
body mass index
- DNA
deoxynucleic acid
- ROS
reactive oxygen species
- NOS2
nitric oxide synthase 2
- PTGS2
prostaglandin-endoperoxide synthase 2
Footnotes
There are no conflicts of interest to declare.
Contributor Information
Christina E. Boots, Clinical Fellow of Reproductive Endocrinology & Infertility, Obstetrics & Gynecology, Washington University, 4444 Forest Park, Suite 3100, St. Louis, MO 63108 USA, bootsc@wudosis.wustl.edu, Phone: 314.286.2400, Fax: 314.286.2455.
Emily S. Jungheim, Obstetrics & Gynecology, Washington University, 4444 Forest Park, Suite 3100, St. Louis, MO 63108 USA, jungheime@wudosis.wustl.edu, Phone: 314.286.2400, Fax: 314.286.2455.
References
- 1.Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203:525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and Lifestyle in the Prevention of Ovulatory Disorder Infertility. Obstet Gynecol. 2007;110:1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 4.Asdell SA. In: The Ovary. Zuckerman S, editor. Academic Press; London: 1962. pp. 435–449. [Google Scholar]
- 5.Espey LL. Ovulation as an Inflammatory Reaction-A Hypothesis. Biol Reprod. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Espey LL. Current Status of the Hypothesis That Mammalian Ovulation Is Comparable to an Inflammatory Reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 8.Clancy KB, Baerwald AR, Pierson RA. Systemic inflammation is associated with ovarian follicular dynamics during the human menstrual cycle. PLoS One. 2013;8:e64807. doi: 10.1371/journal.pone.0064807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Bene MC, de Carvalho Bittencourt M, et al. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol. 2012;2012:606459. doi: 10.1155/2012/606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büscher U, Chen FCK, Kentenich H, Schmiady H. Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries: is ovulation a suppressed inflammatory reaction? Hum Reprod. 1999;14:162–166. doi: 10.1093/humrep/14.1.162. [DOI] [PubMed] [Google Scholar]
- 11.Priddy AR, Killick SR, Elstein M, Morris J, Sullivan M, Patel L, et al. The effect of prostaglandin synthetase inhibitors on human preovulatory follicular fluid prostaglandin, thromboxane, and leukotriene concentrations. J Clin Endocrinol Metab. 1990;71:235–242. doi: 10.1210/jcem-71-1-235. [DOI] [PubMed] [Google Scholar]
- 12.Pall M, Fridén BE, Brännström M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001;16:1323–1328. doi: 10.1093/humrep/16.7.1323. [DOI] [PubMed] [Google Scholar]
- 13.Smith G, Roberts R, Hall C, Nuki G. Reversible ovulatory failure associated with the development of luteinized unruptured follicles in women with inflammatory arthritis taking non-steroidal anti-inflammatory drugs. Br J Rheumatol. 1996;35:458–462. doi: 10.1093/rheumatology/35.5.458. [DOI] [PubMed] [Google Scholar]
- 14.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88:142–148. doi: 10.1016/j.jri.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:2146–2149. doi: 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. 2011;118:63–70. doi: 10.1097/AOG.0b013e31821fd360. [DOI] [PubMed] [Google Scholar]
- 17.Boots C, Stephenson MD. Does obesity increase the rate of miscarriage in spontaneous conception? A systematic review. Sem Rep Med. 2011;29:507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- 18.Boots CE, Bernardi L, Stephenson MD. Does obesity increase the risk of euploid miscarriage in women with recurrent pregnancy loss? Fertil Steril. 2014;102:455–459. doi: 10.1016/j.fertnstert.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644–1646. doi: 10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- 20.Goldman KN, Hodes-Wertz B, McCulloh DH, Flom JD, Grifo JA. Association of body mass index with embryonic aneuploidy. Fertil Steril. 2015;103:744–748. doi: 10.1016/j.fertnstert.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 2015;72:251–271. doi: 10.1007/s00018-014-1739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu LL, Norman RJ, Robker RL. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Reprod Fertil Dev. 2011;24:29–34. doi: 10.1071/RD11904. [DOI] [PubMed] [Google Scholar]
- 23.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carman GM. Thematic minireview series on the lipid droplet, a dynamic organelle of biomedical and commercial importance. J Biol Chem. 2012;287:2272. doi: 10.1074/jbc.R111.323931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 29.Wildman RP, Kaplan R, Manson JE, Rajkovic A, Connelly SA, Mackey RH, et al. Body size phenotypes and inflammation in the Women's Health Initiative Observational Study. Obesity (Silver Spring) 2011;19:1482–1491. doi: 10.1038/oby.2010.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97:7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20:49–58. doi: 10.1093/molehr/gat051. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T, Negishi T, Deguchi M. WBC count, atherosclerosis and coro- nary risk factors. J Atheroscler Thromb. 2002;9:219–223. doi: 10.5551/jat.9.219. [DOI] [PubMed] [Google Scholar]
- 33.Herlihy AC, Kelly RE, Hogan JL, O’Connor N, Farah N, Turner MJ. Polycystic ovary syndrome and the peripheral blood white cell count. J Obstet Gynaecol. 2011;31:242–244. doi: 10.3109/01443615.2011.553693. [DOI] [PubMed] [Google Scholar]
- 34.Orio FJ, Palomba S, Cascella T, Di Biase S, Manguso F, Tauchmanova L, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 35.Hurwitz A, Payne DW, Packman JN, Andrean CL, Resnick CE, Hernandez ER, et al. Cytokine-mediated regulation of ovarian function: interleukin-1 inhibits gonadotropin-induced androgen biosynthesis. Endocrinology. 1991;129:1250–1256. doi: 10.1210/endo-129-3-1250. [DOI] [PubMed] [Google Scholar]
- 36.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058. e1–e2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly CCJ, Lyall H, Petrie JR, Gould GW, Connell JMC, Sattar N. Low Grade Chronic Inflammation in Women with Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. 2001;86:2453–2455. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 39.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syn- drome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–127. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 40.Blair SA, Kyaw-Tun T, Young IS, Phelan NA, Gibney J, McEneny J. Oxidative stress and inflammation in lean and obese subjects with polycystic ovary syndrome. J Reprod Med. 2012;58:107–114. [PubMed] [Google Scholar]
- 41.Gonzalez F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab. 2012;302:E297–E306. doi: 10.1152/ajpendo.00416.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97:1492–1500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 43.Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction. 2014;148:R15–R27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- 44.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–316. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol. 1994;41:463–471. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 47.Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95:1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab. 2013;98:E1364–E1368. doi: 10.1210/jc.2012-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294 doi: 10.1152/ajpendo.00409.2007. E425-E34. [DOI] [PubMed] [Google Scholar]
- 50.Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi G, et al. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS) Clin Endocrinol. 2008;69:792–798. doi: 10.1111/j.1365-2265.2008.03305.x. [DOI] [PubMed] [Google Scholar]
- 52.Covington JD, Tam CS, Pasarica M, Redman LM. Higher circulating leukocytes in women with PCOS is reversed by aerobic exercise. Biochimie. 2014 doi: 10.1016/j.biochi.2014.10.028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


