Abstract
Background and Purpose
Comparative studies of exercise interventions for people with Parkinson Disease (PD) rarely considered how one should deliver the intervention. The objective of this study was to compare the success of exercise when administered by 1) home exercise program, 2) individualized physical therapy, or 3) a group class. We examined if common comorbidities associated with PD impacted success of each intervention.
Methods
Fifty-eight people (age 63.9 ± 8) with PD participated. People were randomized into: 1) home exercise program 2) individual physical therapy or 3) group class intervention. All arms were standardized and based on the Agility Boot Camp exercise program for PD, 3 times per week for 4 weeks. The primary outcome measure was the 7-item Physical Performance Test (PPT). Other measures of balance, gait, mobility, quality of life, balance confidence, depressions, apathy, self-efficacy and UPDRS motor and ADL scores were included.
Results
Only the individual group significantly improved in PPT. The individual exercise showed the most improvements in functional and balance measures, while the group class showed the most improvements in gait. The home exercise program improved the least across all outcomes. Several factors effected success, particularly for the home group.
Discussion and Conclusions
An unsupervised, home exercise program is the least effective way to deliver exercise to people with PD and individual and group exercises have differing benefits. Furthermore, people with PD who also have other comorbidities did better in a program directly supervised by a physical therapist. Video Abstract available for additional insights from the authors (See Supplemental Digital Content 1, http://links.lww.com/JNPT/A112).
INTRODUCTION
Parkinson's Disease (PD) is a progressive neurodegenerative disease that results in significant mobility decline that can be partially remediated with exercise.1-13 Although medications and surgery may improve some aspects of movement, exercise is gaining attention as another option that may also improve mobility and other non-motor symptoms related to cognition and emotion over and above the benefits of either medications or surgery.6,14 There is evidence that many types of exercise can improve aspects of mobility in people with PD.14 A recent meta-analysis examined differences between types of exercise by sub-grouping studies according to intervention and found no difference but caution readers about the limited number of studies and the indirect comparison of interventions.9
Often overlooked in exercise trials is how exercise intervention is administered. Most studies use either 1) group intervention or 2) individual physical therapy. However, current standard-of-care is often limited to 3) an unsupervised, home exercise program in which the patient is seen 1-2 times individually and then provided instruction on exercises to be done at home. Insurance coverage of group classes is unreliable and those interested in community group classes often must pay out-of-pocket.
Home exercise programs have reportedly low compliance rates and this could be even worse in a person with greater balance problems and/or other medical complications.15-18 One study found that important barriers such as cognition made execution of a home exercise program difficult for people with PD.19 Nonetheless, home exercise programs remain standard-of-care for mobility deficits in PD.
A second, often-overlooked challenge to exercise rehabilitation is that people with PD have a high number of comorbidities that may impact the success of therapy. People with PD have greater rates of depression, apathy, musculoskeletal problems, and mild cognitive impairment than their peers without PD.20-25 These comorbidities may limit people's ability to participate in exercise programs.26
Our group recently published a paper providing evidence that a sensorimotor-based Agility Boot Camp (ABC) was successful at improving multiple aspects of mobility in people with mild PD when administered in an individual outpatient setting of more frequent time allotment than is considered standard.27 This study prompted the question of whether similar results could be achieved in other settings, such as a group class or home exercise program. The purpose of the current study was to determine if this program would be equally successful when provided as a home exercise program, in a group class, or in individualized physical therapy sessions. We hypothesized that individual therapy would be more successful than a group class or home exercise using the sensorimotor ABC program for PD. Further, we sought to determine if common comorbidities associated with PD impacted the success of each type of exercise intervention.
METHODS
Design Overview
Participants with PD were randomized into either: 1) home exercise 2) individual physical therapy or 3) a group class intervention. All arms were standardized and based on the ABC exercise program for PD.28 The study was designed in waves of 12 participants; 4 people per arm. Each wave occurred over a six-week period; pretesting (week 1), exercise intervention 3x/week 60 minutes per session (weeks 2-5) and post testing (week 6). Testing was performed in the same order and with rest breaks as needed by a blinded research assistant. All participants were tested and all exercise was performed in the ON state as defined subjectively by the participant having recently taken their medications.
Participants
People of either gender with an idiopathic PD diagnosis were recruited from the Movement Disorders Clinic at Oregon Health Sciences University (OHSU) and the local community. To be included, people were required to: 1) have a diagnosis of idiopathic PD, 2) be between 40-80 years old, 3) have a least one co-morbidity associated with PD or aging, 4) walk unassisted. People were excluded if they: 1) needed assistance with ADL's, 2) did not speak or read English, 3) participated in a different exercise study within the year, 4) engaged in >10 hours of exercise/week, 5) participated in a conflicting research study, 6) had a moderate-severe cognitive impairment, 7) lacked transportation to come to OHSU 3X/week.
Eligible subjects completed the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) over the phone to assess medical system comorbidities.29 Based on the results, participants were stratified into either a mild or severe cycle of the study to enhance safety and control the skill level of the exercise class. If a participant scored a 3 (severe) in more than one section, a 3 in the neurologic section, or a 4 in any section, their impairment was deemed severe. If a participant scored a 3 in only one section (not including neurologic) or if they scored lower than 3 their impairment was categorized as mild. This information was used to stratify participants according to severity so that would groups would be equal on the numbers of participants with severe versus mild comorbidities.
Ethical Review
All participants signed informed consent approved by OHSU's Institutional Review Board. All work was conducted in accordance with the declaration of Helsinki (1964). This clinical trial (NCT01361724) was registered on clinical trials.gov and took place between March 2011 and August 2012.
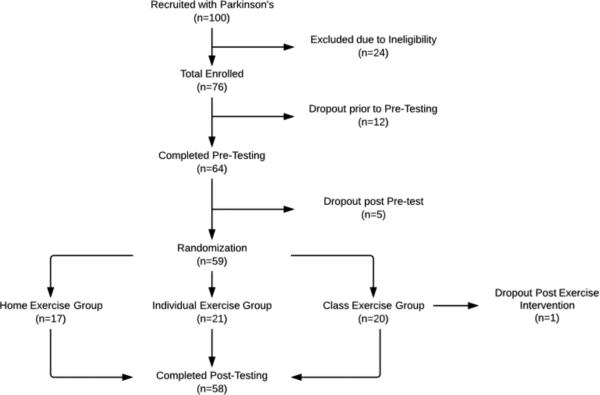
Twenty-four people were ineligible due to lack of transportation, age, cognitive impairment, inability to stand unassisted, conflicting research study, or exercising more than 10 hours a week. Twelve participants dropped out after pre-testing due to inability to commit to exercise 3X/week, and one due to injury. One participant dropped out after completing the exercise intervention due to a family emergency (Fig 1). Patient baseline characteristics are outlined in Table 1.
Figure 1.
Consort Diagram
Table 1.
Characteristics of the Participants
| Characteristic | All (n=58) | Home (n=17) | Individual (n=21) | Class (n=20) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | ||
| Age (y) | 64.2 (7.3) | 63.9 | 64.6 (6.8) | 63.8 | 64.2 (6.7) | 64.7 | 63.9 (8.5) | 64.5 | 0.88 |
| Male (%) | 41.0 | NA | 10.0 | NA | 17.0 | NA | 14.0 | NA | 0.33 |
| H & Y | 2.4 (0.5) | 2.0 | 2.5 (0.5) | 3.0 | 2.4 (0.5) | 2.0 | 2.4 (0.5) | 2.0 | 0.51 |
| Disease Duration (y) | 6.2 (6.1) | 5.3 | 5.2 (5.8) | 1.9 | 7.9 (7.9) | 5.3 | 5.4 (3.6) | 6.0 | 0.36 |
| UPDRS - Motor | 36.8 (12.8) | 37.0 | 35.2 (13.7) | 32.0 | 39.4 (11.1) | 38.0 | 35.4 (14.1) | 37.5 | 0.43 |
| BMI | 27.6 (4.7) | 26.5 | 27.6 (5.0) | 25.9 | 28.0 (4.8) | 25.7 | 27.2 (4.5) | 28.6 | 0.45 |
| MOCA | 26.0 (3.8) | 27.0 | 25.8 (4.0) | 27.0 | 26.1 (2.5) | 26.0 | 25.8 (3.1) | 27.0 | 0.90 |
| Total Cirs-G | 12.4 (4.2) | 12.0 | 12.0 (4.0) | 13.0 | 12.0 (4.0) | 11.0 | 13.2 (4.5) | 11.5 | 0.65 |
This study was powered on data from 9 individuals with PD undergoing individualized ABC training with a physical therapist 4 X/week for 4 weeks. Using the 7-item PPT, we determined that at least 12 subjects per group would be required to show an improvement after exercise with α of 0.05 and Power (1 –β) of 0.80.
Randomization
The statistician provided a computer-generated randomization list, stratified by comorbidity level, for blocks of 12 people (4 in each group). Sealed envelopes were prepared for each wave. Participants received their randomization letter after pre-testing had been completed. The person responsible for pre/post testing remained blinded to group assignment. Exercise and pre/post testing took place in different buildings to ensure continued blinding.
Intervention
The exercise intervention was based on the sensorimotor ABC Program.27,28 The program targets basic postural systems in a ‘boot camp’ model to target biomechanical constraints, kinesthesia, limits of stability, anticipatory postural adjustments, bradykinesia, and coordination during gait. There were 6 stations: Tai chi, Boxing, Lunges, Kayaking, Agility course and Pilates. Each activity was systematically progressed for 3 levels by (1) challenging sensory integration via alteration of visual and surface conditions, (2) restricting availability of external cues, (3) increasing speed, (4) increasing resistance and (5) adding secondary tasks. Regardless of assignment, the exercise program was designed for 3X/week for 4 weeks with 60 minute sessions (See Appendix [Supplemental Digital Content 2]).
Home Exercise Program: The participants assigned to home exercise met with the physical therapist once to receive their individualized ABC home exercise program. The physical therapist assigned the exercise level based on the participant's ability to safely conduct the exercises in the home. Handouts were provided. Individual Exercise Program: The participants in the individual exercise program met one-on-one with the physical therapist 3X/week for an hour at the outpatient rehabilitation center. The physical therapist progressed the participant through the exercise program based on their ability to complete the exercises safely. Group Exercise Program: The participants randomized to the group class came to the wellness center at the University 3X/week for an hour. The physical therapist leading the class progressed people across the levels as appropriate. Missed sessions were not rescheduled. The 3 physical therapists were highly experienced, had strong backgrounds in PD, and were trained extensively in the ABC Program. Each physical therapist rotated equally with the cycles to avoid bias or effect of therapist.
Exercise progression and compliance
Rate of Perceived Exertion (RPE) was collected after each session on a scale of 0-10. All scores were averaged over the course of the study. Progression level was determined by the physical therapist and recorded by the participants. For those exercising at home, the level of exercise was determined in the beginning and held constant. For those in the group and individual exercise, the level of exercise could change over time, based on the physical therapist's observations. Compliance was calculated by percentage of assigned exercise sessions in which exercise occurred.
Outcomes Measures
Our primary outcome measure was the 7-item PPT.30 The PPT is designed to simulate common tasks including writing, simulated eating, putting a book on a shelf, donning and doffing a jacket, picking up a penny from the floor, performing a 360 degree turn, and walking 50 feet. This instrument has been well studied and validated for PD and does not have a floor or ceiling effect.30,31
Other Clinical Outcomes
The Mini-BESTest is a sensitive measure of balance in the PD population and includes 14 balance items.32,33,34 Timed up and go (TUG) test is a test of mobility where the person is asked to stand, walk 3 meters at a comfortable pace, turn around, come back and sit down.35 This test has excellent reliability for assessing people with PD.36 Timed up and go with dual task (TUG-D) is performed as the TUG but simultaneously the person is asked to perform a secondary task, such as counting backwards by threes.37,38 The test is timed and compared to the standard TUG. Slowing under the addition of a secondary task of greater than 10% is considered abnormal.32 Parkinson Disease Questionnaire-39 (PDQ-39) is a questionnaire for measuring quality of life for individuals with PD. Activities-Specific Balance Confidence Scale (ABC) is a reliable 16-item questionnaire for detecting loss of balance confidence.39,40 The Exercise Self-Efficacy Scale (SES) is an 18-item test that measures an individual's self-efficacy to participate in exercise when various barriers, social and physical are present.41 Lille Apathy Rating Scale (LARS) is a 33-item test that measures apathy in persons with PD.42 Unified Parkinson's Disease Rating Scale Activities of Daily Living (UPDRS-ADL Part II) is a 13-item questionnaire focused on symptomatic effects of PD on a variety of ADL's.43 Unified Parkinson's Disease Rating Scale (UPDRS-Motor- Part III) is the most commonly used test for evaluating motor deficits in PD.13,43,44
All gait measures were derived using APDM system and software.45 Participants wore 6 Opal sensors on the posterior trunk at L5, ankles, wrists and sternum. The sensors record 3D accelerations and angular velocity and wirelessly stream data to a laptop. The sensors on the ankles are used to detect basic gait events and temporal gait measures are calculated based on the time of gait events. Spatial gait measures are estimated using a biomechanical model.46,47 All gait parameters were derived from a 2-minute walk. We calculated the following metrics based on previous studies suggesting sensitivity to early PD, good reliability and a comprehensive characterization of commonly impaired aspects of PD: 1) stride velocity, 2) arm swing velocity, 3) trunk velocity 4) stride time variability 5) turn duration. Turns were averaged out of the 2-minute walk. Freezing of gait was measured using the Freezing of gait questionnaire, a six item questionnaire to assess severity of freeing of gait.48
Comorbidities, possible confounders and effect modifiers
The Cumulative Illness Rating Score—Geriatric (CIRS-G) measures comorbidity in the geriatric population and measures medical problem severity on a scale from 0 to 4 (0=no problem; 4=extremely severe) for each organ-specific category (heart, vascular, hematopoietic, respiratory, eyes/ears/nose/throat/larynx, upper gastrointestinal, lower gastrointestinal, liver, renal, genitourinary, musculoskeletal, neurological, endocrine/metabolic/breast and psychiatric illness).49 The CIRS-G has good inter-rater reliability, face validity and been validated for use over the phone.49 Montreal Cognitive Assessment (MOCA) assesses mild cognitive impairment by measuring attention and concentration, executive function, memory, language, visoconstructional skills, conceptual thinking, calculations and orientation.50-52 It is valid and reliable for persons with PD.51,53 Geriatric Depression Scale (GDS) is a self-reporting questionnaire for depression in the community-dwelling elderly and is both reliability and validity.54 Body Mass Index (BMI) was calculated at their pretest visit.
Statistical analysis methods
Baseline characteristics among the three groups were compared using Kruskal-Wallis tests (for continuous variables) or Chi-square test (for categorical variables). Wilcoxon signed-rank tests were conducted to determine whether the outcome measures improved from baseline for each group. Standardized Response Mean (SRM) (= d/SDdiff, the mean score change divided by the standard deviation of change) was calculated for each outcome and a value of 0.20 represents a small change, of 0.50 a moderate, and 0.80 represents a large change.55 Linear regression models were fitted to compare the changes in outcome measures from baseline among the three groups, after controlling for potential confounders and/or effect modifiers. For identified effect modifiers that interact with group, we assessed the association between the effect modifiers with the outcome variables within each group. Instead of the traditionally used 5% significance level, we set alpha to 10% for statistical significance in testing interaction terms. SAS 9.2 (Cary, NY) was used for data analysis.56-58
Results
There were no differences in exercise difficulty level among the groups at the end of the study (Home: 2.4 ± 0.61; Individual 2.5 ± 0.40; Group class: 2.4 ± 0.25). All 3 groups reported between moderate and somewhat heavy RPE (Home: 4.1 ±1.5; Individual: 4.1 ± 1.1; Group class: 3.4 ±1.2). The home group recorded 85% compliance, individual 97% and the group class 95%. The groups had roughly equivalent people who had freezing of gait as defined by a positive response to item 3 on the FOG questionnaire; home group had 60% people with FOG and both individual and group had 46% of people.59
The individual group was the only group to improve in our primary outcome measure, the PPT, on which the study was powered. Further, this group (individual) showed the most improvements in functional measures such as the PPT, UPDRS-ADL's, apathy, self-efficacy, depression, and balance. The group class showed the most improvements in gait measures such as freezing of gait, stride velocity, arm swing, trunk movement, gait variability and gait under dual task. The home exercise program improved the least across all outcomes. Table 2 reports statistics on outcomes for each group. The last column reports p-values of direct comparison of the pre/post changes among the 3 groups, while the individual p-value columns compare pre/post-values for each group separately.
Table 2.
Estimated means, standard deviation (SD), Standardized Response Mean (SRM), and P-Value differences before and after exercise for each group.
| Home | Individual | Class | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Outcome |
Pre Mean (sd) |
Post Mean (sd) |
Diff (mean, med; 95% CI) |
SRM | P- Value |
Pre Mean (sd) |
Post Mean (sd) |
Diff (mean, med; 95% CI) |
SRM | P- Value |
Pre Mean (sd) |
Post Mean (sd) |
Diff (mean, med; 95% CI) |
SRM | P- Value |
P- Value ALL |
||||
| PPT | 21.4 (05.0) | 22.6 (3.6) | 0.71, 0.0; | −0.7, 2.2 | 0.28 | 0.371 | 20.0 (4.2) | 21.9 (4.0) | 1.81, 1.0; | 0.69, 2.9 | 0.74 | 0.004 | 21.4 (3.5) | 22.0 (2.9) | 0.55, 0.5; | −0.4, 1.5 | 0.27 | 0.156 | 0.265 | |
| Secondary Outcome | ||||||||||||||||||||
| Functional | UPDRS- ADL | 11.6 (05.1) | 11.0 (7.10) | −0.65, −1.0; | −2.7, 1.4 | 0.00 | 0.489 | 13.5 (6.3) | 11.9 (5.1) | −1.67, −1.0; | −2.9, −4.3 | 0.61 | 0.011 | 11.7 (5.0) | 9.8 (4.9) | −1.90, −2.0; | −4.0, 0.2 | 0.43 | 0.061 | 0.691 |
| UPDRS- Motor | 35.2 (13.7) | 33.8 (14.0) | −1.47, −2.0; | −4.1, 1.2 | 0.29 | 0.308 | 39.4 (11.1) | 38.0 (10.4) | −1.30, −1.0; | −4.3, 1.6 | 0.21 | 0.212 | 35.4 (14.1) | 33.5 (12.7) | −1.90, −1.5; | −5.3, 1.5 | 0.26 | 0.191 | 0.990 | |
| PDQ | 33.1 (18.4) | 26.5 (18.2) | −6.65, −9.0; | 11.6, −1.7 | 0.69 | 0.015 | 40.7 (23.5) | 33.8 (16.2) | −6.30, −5.5; | 13.1, 0.5 | 0.43 | 0.068 | 34.8 (21.8) | 21.1 (11.9) | −10.4, −9.0; | 16.8, −4.0 | 0.81 | 0.002 | 0.448 | |
| LARS | −24.1 (05.3) | −24.5 (3.8) | −0.41, 0.0; | −2.4, 1.6 | 0.11 | 0.683 | −22.1 (5.3) | −24.3 (4.8) | −2.24, −2.0; | −4.4, −0.1 | 0.47 | 0.048 | −23.9 (5.3) | −24.2 (7.0) | −0.25, −1.0; | −2.7, 2.2 | 0.05 | 0.745 | 0.377 | |
| SES | 60.4 (15.4) | 62.8 (17.4) | 2.41, 2.0; | −5.2, 10.0 | 0.16 | 0.524 | 68.0 (15.4) | 73.2 (12.6) | 5.24, 5.0; | 0.8, 9.7 | 0.54 | 0.017 | 67.9 (15.1) | 70.5 (14.1) | 2.65, 0.5; | −1.2, 6.5 | 0.32 | 0.237 | 0.655 | |
| GDS | 7.4 (04.8) | 6.8 (5.1) | 0.07, 1.0; | −2.0, 2.1 | 0.01 | 0.669 | 9.5 (4.9) | 8.1 (5.2) | −1.43, −2.0; | −2.5, −0.4 | 0.64 | 0.014 | 7.0 (5.9) | 5.4 (5.8) | −1.11, 0.0; | −2.4, 0.2 | 0.40 | 0.138 | 0.142 | |
| Mini- BESTest | 20.5 (04.2) | 22.3 (3.9) | 1.82, 2.0; | 0.4, 3.2 | 0.67 | 0.013 | 19.8 (5.0) | 21.6 (5.2) | 1.81, 2.0; | 0.9, 2.8 | 0.86 | 0.001 | 21.7 (3.8) | 22.3 (4.0) | 0.06, (0.5); | −0.7, 1.9 | 0.21 | 0.347 | 0.293 | |
| ABC | 81.4 (15.5) | 82.9 (15.3) | 1.55, 1.9; | −2.8, 5.9 | 0.18 | 0.571 | 71.1 (14.5) | 82.2 (12.2) | 3.16, 3.1; | −0.1, 6.5 | 0.44 | 0.061 | 83.5 (17.6) | 84.5 (17.9) | 4.62, 3.0; | 1.5, 7.7 | 0.75 | 0.001 | 0.429 | |
| Gait | Freezing of Gait | 5.1 (04.8) | 5.4 (4.8) | 0.35, 0.0; | −0.5, 1.2 | 0.20 | 0.413 | 6.3 (6.3) | 5.7 (5.0) | −0.62, 2.0; | −1.9, 0.6 | 0.23 | 0.308 | 4.6 (4.4) | 3.4 (3.7) | −1.20, −1.0; | −1.9, −0.5 | 0.83 | 0.001 | 0.038 |
| Stride Velocity (height/s%) | 76.4 (11.4) | 76.7 (12.7) | 0.37, 1.6; | −2.5, 3.2 | 0.06 | 0.790 | 72.3 (10.7) | 73.1 (12.6) | 0.81, 1.8; | −1.5, 3.1 | 0.16 | 0.476 | 74.6 (11.8) | 78.4 (11.9) | 3.80, 3.1; | 1.6, 6.0 | 0.80 | 0.002 | 0.124 | |
| Arm Velocity (degrees/s) | 139.7 (64.5) | 148.0 (75.1) | 8.39, 18.4; | −3.1, 19.9 | 0.37 | 0.142 | 141.3 (67.2) | 159.0 (79.9) | 17.7, 13.9; | 2.7, 32.8 | 0.54 | 0.024 | 134.2 (67.8) | 166.3 (80.6) | 32.1, 18.9; | 15.6, 48.6 | 0.91 | 0.001 | 0.232 | |
| Trunk Velocity (degrees/s) | 23.2 (10.5) | 23.4 (9.8) | 0.18, −0.2; | −1.5, 1.9 | 0.05 | 0.824 | 22.1 (7.7) | 25.0 (7.7) | 3.0, 3.3; | −0.5, 5.5 | 0.54 | 0.023 | 19.7 (6.0) | 23.1 (9.1) | 3.50, 2.3; | 1.2, 5.8 | 0.70 | 0.005 | 0.028 | |
| Stride Time Variability (%) | 0.02 (0.01) | 0.03 (0.02) | −0.004, −0.002; | 0.02, 0.03 | 0.25 | 0.318 | 0.03 (0.01) | 0.03 (0.01) | 0.001, 0.001; | 0.02, 0.03 | 0.02 | 0.937 | 0.03 (0.01) | 0.02 (0.01) | 0.005, 0.001; | 0.02, 0.03 | 0.50 | 0.049 | 0.051 | |
| Turn Duration (s) | 2.6 (0.8) | 2.7 (1.3) | 0.04, −0.09; | −0.3, 0.4 | 0.06 | 0.306 | 2.6 (0.7) | 2.6 (0.8) | −0.01, −0.05; | −0.2, 0.2 | 0.04 | 0.567 | 2.6 (0.7) | 2.4 (0.6) | −0.13, −0.01; | −0.3, 0.1 | 0.30 | 0.316 | 0.935 | |
| TUG (s) | 11.3 (0.9) | 11.0 (1.3) | −0.35, 0.3; | −1.4, 0.5 | 0.23 | 0.487 | 10.9 (3.5) | 10.2 (2.9) | −0.64, −0.05; | −1.5, 0.7 | 0.16 | 0.389 | 13.2 (8.9) | 13.1 (7.7) | −0.16, −0.2; | −0.9, 0.02 | 0.29 | 0.234 | 0.979 | |
| TUG-D (s) | 13.7 (02.5) | 13.7 (3.4) | 0.05, 0.3; | −1.8, 0.9 | 0.20 | 0.890 | 14.9 (6.1) | 13.2 (3.9) | 1.90, −0.8; | −2.5, 2.0 | 0.05 | 0.547 | 15.6 (6.5) | 16.2 (9.6) | −0.40, −0.9; | −3.3, −0.04 | 0.52 | 0.012 | 0.392 | |
We examined potential confounding variables (i.e. comorbidity scores, disease severity, age, BMI, number of medications, cognition, depression) and did not find any potential confounders related to both outcome variable and group assignment (i.e. no difference in results when controlling for each potential confounder). However, we found significant effect modifiers when examining the same comorbidities (i.e., several variables had a significant effect on certain outcome measures after exercise). Table 3 summarizes effect modifiers by providing p-values for comorbidities that had an effect on each outcome measure. In Table 3, statistically significant effect modifiers are bolded, and presented with the p-value for the interaction term in the linear regression model. The p-values represent significant interaction effects between the potential effect modifier and the outcome variable after exercise while “NS” means non-significant effect modifier.
Table 3.
Summary of interaction effects (represented as P-values) between potential effect modifiers and outcome variables after exercise.
| Effect Modifiers | ||||||||
|---|---|---|---|---|---|---|---|---|
| UPDRS | AGE | BMI | MEDICATION | MOCA | COMORBIDITY | DEPRESSION | ||
| Outcome Measures | UPDRS-ADL | 0.093 | NS | NS | NS | NS | 0.02 | NS |
| Physical Performance Test | NS | 0.086 | NS | NS | NS | NS | NS | |
| UPDRS - Motor | NS | NS | NS | NS | NS | NS | 0.019 | |
| PDQ-39 | NS | NS | NS | NS | NS | NS | NS | |
| Apathy | NS | 0.009 | 0.086 | NS | NS | NS | NS | |
| Self Efficacy | NS | 0.07 | NS | NS | NS | NS | NS | |
| Mini Best | NS | NS | NS | NS | NS | NS | NS | |
| ABC | NS | NS | NS | NS | NS | NS | NS | |
| Freezing of Gait | NS | NS | NS | 0.04 | 0.057 | NS | NS | |
| Stride Velocity | NS | NS | NS | 0.09 | 0.072 | NS | NS | |
| Arm Velocity | 0.07 | NS | NS | NS | NS | NS | NS | |
| Trunk Velocity | NS | NS | NS | NS | NS | NS | NS | |
| Stride Time Variability | 0.0004 | NS | 0.001 | 0.028 | 0.016 | 0.008 | NS | |
| Turn Duration | NS | NS | 0.001 | NS | 0.039 | NS | NS | |
| Tug Time | NS | NS | 0.026 | 0.038 | NS | NS | NS | |
| Tug Dual Task Time | NS | NS | NS | 0.032 | NS | NS | NS | |
Of the 7 effect modifiers, all, except age, were significantly associated with exercise effectiveness for the home group while only a few had significant associations with exercise effectiveness for the individual and group class. For example, the presence of depression, high comorbidities status and mild cognitive impairment only impacted success of people in the home program. In contrast, the number of medications, disease severity and BMI impacted success for all 3 groups (Table 4).
Table 4.
Interaction effects separated by interventions to highlight group-specific interaction effects between effect modifiers and outcomes.
| Effect Modifiers | Outcomes | Home | Group | Individual |
|---|---|---|---|---|
| Depression | UPDRS - Motor | 0.49 | 0.56 | |
| Co-morbidity Score | UPDRS - ADL | 0.04 | 0.28 | 0.12 |
| Stride Time Variability | 0.02 | 0.29 | 0.59 | |
| Mild Cognitive Impairment | Freezing of Gait | 0.006 | 0.49 | 0.04 |
| Stride Time Variability | 0.02 | 0.37 | 0.86 | |
| Number of Medications | Freezing of Gait | 0.55 | 0.37 | 0.01 |
| Stride Time Variability | 0.04 | 0.35 | 0.46 | |
| Stride Velocity | 0.02 | 0.86 | 0.37 | |
| Tug Time | 0.32 | 0.4 | 0.02 | |
| Dual Tug Time | 0.0009 | 0.88 | 0.12 | |
| Disease Severity (UPDRS - Motor) | UPDRS - ADL | 0.032 | 0.69 | 0.63 |
| Arm Velocity | 0.13 | 0.45 | 0.05 | |
| Stride Time Variability | 0.02 | 0.008 | 0.58 | |
| Age | PPT | 0.12 | 0.45 | 0.2 |
| LARS | 0.55 | 0.07 | 0.02 | |
| BMI | Stride Time Variability | 0.0012 | 0.6 | 0.93 |
| Turn Duration | 0.0013 | 0.05 | 0.51 | |
| Tug Time | 0.0004 | 0.78 | 0.99 | |
| LARS | 0.16 | 0.73 | 0.04 | |
| MOCA | Freezing of Gait | 0.0063 | 0.41 | 0.48 |
| Stride Velocity | 0.04 | 0.35 | 0.83 | |
| Stride Time Variability | 0.07 | 0.2 | 0.25 | |
| Turn Duration | 0.05 | 0.68 | 0.52 |
Discussion
Delivery method of rehabilitation and the presence of common comorbidities critically impact the success of rehabilitation for people with PD. Our main findings were that 1) home exercise – the standard-of-care for PD- is the least effective method to improve mobility, 2) individually-treated participants improved the most in balance and functional measures, 3) group class participants improved mainly in gait measures, and 4) the presence of certain comorbidities limited success of the therapeutic intervention primarily for participants in the home exercise assignment.
Only those receiving individual physical therapy improved significantly in the PPT. The average change was 1.8 points, close to the 2.5 Minimal Detectable Change (MDC) for people with PD and the 2 point improvement after exercise found after exercise in people without PD.31,60 It should be noted that we used the 7-item PPT while MDC is based on the 9-item test. Although the people who received individual therapy improved the most in balance measures, the group class had the largest improvement in balance confidence. It has been reported that balance confidence changes do not always correlate with balance ability in people with PD and that balance ability can be improved without associated increase in balance confidence.61,62 In our study, both the home and individual exercise (but not the group class) improved significantly in balance as measured by the Mini-BESTest. Both mean changes were below the published MDC but roughly one quarter of the people in each group achieved at or above the level of clinically important change (24% home, 19% individual and 25% class) suggesting that the ABC program is helpful for balance in a subset of people regardless of delivery. In a group class, the instructor may not be able to safely challenge balance, but the overall movement and interaction involved in a class may improve perception of balance control. The UPDRS-ADL subscore measures the impact of PD on ADL's and function and had been suggested to be a stable measure of disease progression since it is less affected by drug and motor fluctuations.63 ADL's changed only in the individual group and this change averaged 1.6, similar to changes found in other exercise interventions for PD and approximated the MDC of 2 points.64-66 Participants averaged 1.4 to 1.9 points of changed improvement in UPDRS Motor subscore, lower than the published MDC of 3.5 to 5.64,65 The UPDRS-Motor change was not significant in any group.
Surprisingly, the group class improved the most in gait. While the ABC program does not specifically target gait, many exercises emphasize big movements, trunk flexibility and arm swing, all of which may improve gait. There is increasing evidence of a relationship between cognition and gait that may naturally be emphasized more in a group setting.67,68 A class involves more interaction, which could result in a greater emphasis on divided attention and cognitive function when compared to exercising alone. Gait variability improved only in the group class, which may relate to cognition. In cognitively impaired adults and in persons with PD, gait variability increases under dual task conditions.67,69 Furthermore, PD with FOG results in even more gait variability under dual task conditions.70 Gait variability is reportedly associated with falls and is increased in people with FOG.67,70-72
Although it is commonly believed that exercise improves quality of life, findings for the PD population have been mixed.9,73 In our study, quality of life improved across all groups, although the largest improvement was in the class. Reportedly, quality of life is correlated with depression and apathy, both which improved in the individual therapy group as well.74 Self-efficacy is a major determinant in successful continuation of exercise participation.75,76 Again, we found that the individual group was the only one group to significantly improve in self-efficacy, after oneon-one sessions with a physical therapist.
Results from this study suggest that exercise led by a physical therapist, either individually or in a group setting, may be critical to overcoming obstacles associated with comorbidities such as mild cognitive impairment, disease severity, BMI, number of medications and depression. People who had higher levels of comorbidities did not improve with home exercises like they did in the physical therapist-led programs. These comorbidities should be factored in to determine if a home exercise program is appropriate.
There are several limitations to this study. The lack of a non-exercising control group does not allow direct comparison of exercise versus no exercise. Furthermore, 4 weeks of exercise may not be long enough to see significant improvements in all groups and outcomes. Since we were unable to progress the intensity and complexity of the home exercise group as we did the other groups, we do not know if a progressive home exercise program would have shown differences. Finally, we did not have a follow-up period to determine whether the effects of exercise lasted over time. It should be noted that all of our interventions were led entirely by highly experienced physical therapists. Further research should consider if similar results would be obtained using less experienced physical therapists, physical therapist assistants or exercise trainers.
Our results suggest that an unsupervised home exercise program is the least effective way to deliver therapeutic exercise to people with PD. In addition, individual and group exercise has differing benefits. Group class may be most effective for improving gait, particularly those associated with cognitive challenges. In contrast, individual physical therapy may be the best method to improve function and balance. A combination of both group and one-on-one administered physical therapy may be the most effective way to treat mobility disability for people with PD. Furthermore, people with PD who have depression, high number of comorbidities, mild cognitive impairment, high BMI and advance disease severity should be seen in a physical therapist-supervised program. Taken together, the findings from this study call into question the usefulness of an unsupervised home exercise program to improve mobility in people with PD and other accompanying comorbidities.
Supplementary Material
Acknowledgments
Funding Sources: This work was funded by the Foundation for Physical Therapy; Clagett Family Research Grant and partially supported by the Oregon Clinical and Translational Research Institute (OCTRI) (KL2TR000152) from the National Center for Advancing Translational Sciences at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not represent the official views of the NIH. OCTRI provided facilities and staff, grant support 1 UL1 RR024140 01. The funding agency did not have a role in the design, conduct, or reporting of the study or in the decision to submit the article for publication.
Footnotes
Financial Disclosure: OHSU and Dr. Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
This work has previously been presented in poster format at the APTA Combined Sections Meeting 2014.
List of supplemental digital content
Supplemental Digital Content 1 (Video Abstract): JNR_abstract.mp4
Supplemental Digital Content 2 (Online only):
References
- 1.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: A meta-analysis of the effect of exercise and motor training. Movement Disorders. 2011;26(9):1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 3.Brienesse LA, Emerson MN. Effects of resistance training for people with Parkinson's disease: a systematic review. Journal of the American Medical Directors Association. 2013;14(4):236–241. doi: 10.1016/j.jamda.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematic review across the disability spectrum. Journal of Neurologic Physical Therapy. 2009;33(1):14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- 5.Earhart GM. Dance as therapy for individuals with Parkinson disease. European journal of physical and rehabilitation medicine. 2009;45(2):231. [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: A systematic review and meta-analysis. Movement Disorders. 2008;23(5):631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 7.Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Movement Disorders. 2007;22(4):451–460. doi: 10.1002/mds.21244. [DOI] [PubMed] [Google Scholar]
- 8.Mehrholz J, Friis R, Kugler J, Twork S, Storch A, Pohl M. Treadmill training for patients with Parkinson's disease. Cochrane Database Syst Rev. 2010:1. doi: 10.1002/14651858.CD007830.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database Syst Rev. 2012:7. doi: 10.1002/14651858.CD002817.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson's disease? Clinical Journal of Sport Medicine. 2006;16(5):422–425. doi: 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- 11.de Goede CJ, Samyra P, Keus H, Gert Kwakkel P, Wagenaar RC. The effects of physical therapy in Parkinson's disease: a research synthesis. Arch Phys Med Rehabil. 2001;82(4):509–515. doi: 10.1053/apmr.2001.22352. [DOI] [PubMed] [Google Scholar]
- 12.Deane K, Jones D, Ellis-Hill C, Clarke C, Playford E, Ben-Shlomo Y. A comparison of physiotherapy techniques for patients with Parkinson's disease. The Cochrane database of systematic reviews. 2000;(1):CD002815–CD002815. doi: 10.1002/14651858.CD002815. [DOI] [PubMed] [Google Scholar]
- 13.Kwakkel G, De Goede C, Van Wegen E. Impact of physical therapy for Parkinson's disease: a critical review of the literature. Parkinsonism & related disorders. 2007;13:S478–S487. doi: 10.1016/S1353-8020(08)70053-1. [DOI] [PubMed] [Google Scholar]
- 14.van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson's disease. Movement Disorders. 2013;28(11):1587–1596. doi: 10.1002/mds.25658. [DOI] [PubMed] [Google Scholar]
- 15.Oldridge NB. Compliance and exercise in primary and secondary prevention of coronary heart disease: a review. Preventive Medicine. 1982;11(1):56–70. doi: 10.1016/0091-7435(82)90005-6. [DOI] [PubMed] [Google Scholar]
- 16.Suttanon P, Hill KD, Said CM, et al. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer's disease: a pilot randomized controlled trial. Clinical rehabilitation. 2013;27(5):427–438. doi: 10.1177/0269215512460877. [DOI] [PubMed] [Google Scholar]
- 17.Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early-or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012 doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home-based treadmill training for individuals with Parkinson's disease: a randomized controlled pilot trial. Clinical rehabilitation. 2012;26(9):817–826. doi: 10.1177/0269215511432652. [DOI] [PubMed] [Google Scholar]
- 19.Quinn L, Busse M, Khalil H, Richardson S, Rosser A, Morris H. Client and therapist views on exercise programmes for early-mid stage Parkinson's disease and Huntington's disease. Disability & Rehabilitation. 2010;32(11):917–928. doi: 10.3109/09638280903362712. [DOI] [PubMed] [Google Scholar]
- 20.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:183–189. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 21.Pluck G, Brown R. Apathy in Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(6):636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skorvanek M, Rosenberger J, Gdovinova Z, et al. Apathy in Elderly Nondemented Patients With Parkinson's Disease Clinical Determinants and Relationship to Quality of Life. Journal of Geriatric Psychiatry and Neurology. 2013;26(4):237–243. doi: 10.1177/0891988713500587. [DOI] [PubMed] [Google Scholar]
- 23.Kim YE, Jeon BS. Musculoskeletal problems in Parkinson's disease. Journal of Neural Transmission. 2013:1–6. doi: 10.1007/s00702-012-0960-2. [DOI] [PubMed] [Google Scholar]
- 24.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Movement Disorders. 2012;27(9):1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD-MCI. Movement disorders. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly M, McMinn D, Allan JL. A bidirectional relationship between physical activity and executive function in older adults. Frontiers in Human Neuroscience. 2015;8:1044. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King L, Salarian A, Mancini M, et al. Exploring Outcome Measures for Exercise Intervention in People with Parkinson's Disease. Parkinson's disease. 2013:2013. doi: 10.1155/2013/572134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009;89(4):384–393. doi: 10.2522/ptj.20080214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser M, Marinus J, van Hilten J, Schipper R, Stiggelbout A. Assessing Comorbidity in Patients With Parkinson's Disease. Movement Disorders. 2004;19:824–828. doi: 10.1002/mds.20060. [DOI] [PubMed] [Google Scholar]
- 30.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 31.Paschal KA, Oswald AR, Siegmund RW, Siegmund SE, Threlkeld JA. Test-retest reliability of the physical performance test for persons with Parkinson disease. Journal of Geriatric Physical Therapy. 2006;29(3):82–86. doi: 10.1519/00139143-200612000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation System's Test: the mini-BESTest. Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2010;42:323. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King LA, Mancini M, Priest K, Salarian A, Rodrigues-de-Paula F, Horak F. Do clinical scales of balance reflect turning abnormalities in people with Parkinson's disease? Journal of Neurologic Physical Therapy. 2012;36(1):25. doi: 10.1097/NPT.0b013e31824620d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of Reliability, Validity, and Responsiveness of the Mini-BESTest and Berg Balance Scale in Patients With Balance Disorders. Phys Ther. 2013;93:158–167. doi: 10.2522/ptj.20120171. [DOI] [PubMed] [Google Scholar]
- 35.Podsiadlo D, Richardson S. The timed“ Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American geriatrics Society. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 36.Shumway-Cook A, Woollacott M. Motor Control Theory and Applications. 1995:323–324. [Google Scholar]
- 37.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in communitydwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 38.Horak FB, Wrisley DM, Frank J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damiano AM, Snyder C, Strausser B, Willian MK. A review of health-related quality-of-life concepts and measures for Parkinson's disease. Qual Life Res. 1999;8:235–243. doi: 10.1023/a:1008823222574. [DOI] [PubMed] [Google Scholar]
- 40.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 41.Norman GJ, Vaughn AA, Roesch SC, Sallis JF, Calfas KJ, Patrick K. Development of decisional balance and self-efficacy measures for adolescent sedentary behaviors. Psychology & Health. 2004;19(5):561–575. [Google Scholar]
- 42.Sockeel P, Dujardin K, Devos D, Deneve C, Destée A, Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(5):579–584. doi: 10.1136/jnnp.2005.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahn S, Elton RL. Unified Parkinson's disease rating scale. Recent developments in Parkinson's disease. 1987;2:153–163. [Google Scholar]
- 44.Ramaker C, Marinus J, Stiggelbout AM, van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Movement Disorders. 2002;17(5):867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 45.APDM. APDM movement monitoring solutions. 2013 [Google Scholar]
- 46.Salarian A, Russmann H, Vingerhoets FJG, et al. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. Biomedical Engineering, IEEE Transactions on. 2004;51:1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 47.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a sensitive and reliable measure of mobility. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2010;18:303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giladi N, Shabtai H, Simon E, Biran S, Tal J, Korczyn A. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism & related disorders. 2000;6(3):165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 49.Miller MD, Parrdis CF, Houck PR, et al. Rating Chronic Medical Illness Burdgen in Geropsychiatric Practice and Research: Application of the Cumulative Illness Rating Scale. Psychiat Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 50.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 51.Gill DJ, Freshman A, Blender Ja, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 52.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 53.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. Journal of the American Geriatrics Society. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 55.Moe-Nilssen R, Nordin E, Lundin-Olsson L. Criteria for evaluation of measurement properties of clinical balance measures for use in fall prevention studies. Journal of evaluation in clinical practice. 2008;14(2):236–240. doi: 10.1111/j.1365-2753.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 56.Maria Manuela C-C, editor. Handbook of Research on Serious Games as Educational, Business and Research Tools (2 Volumes) IGI Global; Hershey, PA, USA: 2012. [Google Scholar]
- 57.Chow S-C, Liu J-P. Design and Analysis of Clinical Trials: Concepts and Methodologies. 3rd ed Vol. 507. John Wiley & Sons; 2008. [Google Scholar]
- 58.Iskander M. Innovations in E-learning, Instruction Technology, Assessment and Engineering Education. 1 ed Springer Science & Business Media; 2007. [Google Scholar]
- 59.Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Movement Disorders. 2009;24(5):655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 60.King MB, Judge JO, Whipple R, Wolfson L. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Phys Ther. 2000;80(1):8–16. [PubMed] [Google Scholar]
- 61.Lohnes CA, Earhart GM. External validation of abbreviated versions of the activities-specific balance confidence scale in Parkinson's disease. Movement Disorders. 2010;25(4):485–489. doi: 10.1002/mds.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cyarto EV, Brown WJ, Marshall AL, Trost SG. Comparative effects of home-and group-based exercise on balance confidence and balance ability in older adults: cluster randomized trial. Gerontology. 2008;54(5):272–280. doi: 10.1159/000155653. [DOI] [PubMed] [Google Scholar]
- 63.Harrison MB, Wylie SA, Frysinger RC, et al. UPDRS activity of daily living score as a marker of Parkinson's disease progression. Movement disorders. 2009;24(2):224–230. doi: 10.1002/mds.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the unified Parkinson's disease rating scale. Movement Disorders. 2006;21(8):1200–1207. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]
- 65.Hauser RA, Auinger P. Determination of minimal clinically important change in early and advanced Parkinson's disease. Movement Disorders. 2011;26(5):813–818. doi: 10.1002/mds.23638. [DOI] [PubMed] [Google Scholar]
- 66.Ellis T, de Goede CJ, Feldman RG, Wolters EC, Kwakkel G, Wagenaar RC. Efficacy of a physical therapy program in patients with Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. 2005;86(4):626–632. doi: 10.1016/j.apmr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson's disease. Journal of Geriatric Psychiatry and Neurology. 2003;16(1):53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 68.van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Rikkert MGO. Executive functions are associated with gait and balance in community-living elderly people. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(12):1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 69.Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. Journal of neuroengineering and rehabilitation. 2011;8(1):2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hausdorff J, Schaafsma J, Balash Y, Bartels A, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Experimental Brain Research. 2003;149(2):187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 71.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Movement Disorders. 2004;19(8):871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 73.Antunes HKM, Stella SG, Santos RF, Bueno OFA, Mello MTd. Depression, anxiety and quality of life scores in seniors after an endurance exercise program. Revista Brasileira de Psiquiatria. 2005;27(4):266–271. doi: 10.1590/s1516-44462005000400003. [DOI] [PubMed] [Google Scholar]
- 74.Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in Parkinson disease. Journal of geriatric psychiatry and neurology. 2010;23(1):35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- 75.Desharnais R, Bouillon J, Godin G. Self-efficacy and outcome expectations as determinants of exercise adherence. Psychological Reports. 1986;59(3):1155–1159. [Google Scholar]
- 76.Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91(12):1838–1848. doi: 10.2522/ptj.20100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.