Abstract
The purpose of this study was to evaluate the approach of using diclofenac acid (DA) prodrugs for enhancing transdermal delivery. Methanol diclofenac ester (MD), ethylene glycol diclofenac ester (ED), glycerol diclofenac ester (GD), and 1,3-propylene glycol diclofenac ester (PD) were synthesized and evaluated for their physicochemical properties such as solubilities, octanol/water partition coefficients, stratum corneum/water partition coefficients, hydrolysis rates, and bioconversion rates. In vitro fluxes across human epidermal membrane (HEM) in Franz diffusion cell were determined on DA, MD, ED, GD, and PD saturated aqueous solutions. The formation of GD and ED led to the prodrugs with higher aqueous solubilities and lower partition coefficients than those of the parent drug. Prodrugs with improved aqueous solubility showed better fluxes across HEM in aqueous solution than that of the parent drug, with GD showing the highest aqueous solubility and also the highest flux. There is a linear relationship between the aqueous solubility and flux for DA, ED and PD, but GD and MD deviated from the linear line. Overall, diclofenac prodrugs with improved hydrophilicity than the parent drug could be utilized for enhancing transdermal diclofenac delivery.
Keywords: Diclofenac, Prodrug, Transdermal, Flux, Permeability, Solubility
Introduction
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID) with strong analgesic and anti-inflammatory effect. It has been used for pain relief in conditions such as osteoarthritis, usually in oral dosage forms. However, long-term use of oral NSAIDs like diclofenac can cause stomach problems including ulcers and bleeding [1], and they may also increase the risk of heart attacks and strokes [2]. On the other hand, topical NSAIDs may show less side effects commonly observed in the oral products due to less systemic exposure [3]. FDA recently approved several diclofenac products for topical pain relief: Voltaren (diclofenac sodium) gel, Pennsaid (diclofenac sodium) solution, and Flector (diclofenac epolamine) patch. Clinical studies have demonstrated that topical diclofenac products show better pain relief than the placebo counterparts [4, 5].
It has been postulated that the efficacy of topical diclofenac is due to direct penetration of the drug to affected joint tissue [3]. One study showed that application of diclofenac gel on one of the knees in patients with osteoarthritis in both knees, the drug synovial fluid concentrations in both knees were not significantly different [6]. This result indicates that diclofenac present in synovial fluid was not from direct penetration but from redistribution by systemic blood circulation. On the other hand, oral diclofenac showed significantly higher plasma concentration, synovial membrane tissue concentration and synovial fluid concentration, and also demonstrated better therapeutic efficacy than those by topical applications [7]. Additionally, topical diclofenac gel was only modestly effective against osteoarthritis pain in comparison to the placebo, since the placebo group also had substantial improvements in pain scores and function scores, possibly from the manual massage on the affected joint during application [8]. Those results suggest that current topical diclofenac products might need improvement in diclofenac delivery to the local tissues for better efficacy. One possible approach for enhancing topical delivery to the local tissues would be to increase the diclofenac drug permeation rate across the skin.
Prodrug approach has been widely investigated for enhancing transdermal delivery [9, 10]. By modifying the chemical structure of the parent drug, the formed prodrug may show different physicochemical properties including solubility, lipophilicity, and partition coefficient, which may render the prodrug more favorable for skin penetration. After entering into the body, the prodrug will convert back to its parent drug to exhibit the pharmacological effect. Diclofenac is a weak acid with an octanol/water partition coefficient of 4.9 from the Drug Bank website report, which is above the optimal partition coefficient (usually between 1 and 3) for transdermal drug delivery [11]. Presumably, converting diclofenac to some appropriate prodrugs within the optimal partition coefficient range could achieve higher transdermal penetration. Diclofenac prodrugs have been widely explored, but mostly for the purposes of reducing gastric irritation after oral administration or improving anti-inflammatory efficacy[12-14], with the exception of two studies on diclofenac prodrugs for enhancing transdermal delivery [15, 16]. In those two studies, diclofenac prodrugs were formed by converting diclofenac to diclofenac esters (formed ester with ethylene glycol in one study, and formed esters with different chain length of polyoxyethylene glycols in the other study), and better transdermal permeation was observed. In the present study, we were to investigate a wider range of diclofenac prodrugs (forming diclofenac ester prodrugs with methanol, ethylene glycol, glycerol, and 1,3-propylen glycol), with the purpose of better understanding diclofenac prodrugs for enhancing transdermal diclofenac delivery.
Methods
2.1 Material
Diclofenac sodium salt (USP grade) was obtained from Gallipot (St Paul, Minnesota). Methanol, propylene glycol, 1,3-propylene glycol, ethylene glycol, glycerol, N,N-Dicyclohexylcarbodiimide (DCC) and 4-pyrrolidonopyridine were obtained from Sigma (St. Louis, MO). Ethyl acetate and hexane were obtained from BDH chemicals. Silica gel, ultra pure, 40 to 60 μm was obtained from ACROS organics (New Jersey, USA). Dimethyl Sulfoxide – d6 was obtained from Cambridge Isotopes Laboratories (Andover, MA). Isopropyl Myristate (IPM) and 1-Octanol was obtained from Spectrum Chemicals (New Brunswick, NJ). 0.5% trypsin solution was obtained from Atlanta Biologicals (Lawrenceville, GA). Human cadaver skin was obtained from New York Fire Fighter Skin Bank. Human epidermal membrane (HEM), the epidermis layer of the skin, was prepared by heat stripping method as reported before [17].
2.2 Converting Diclofenac Sodium to Diclofenac Acid (DA)
Diclofenac sodium (10 grams) was dispersed into 200 ml purified water, and then 6.8 ml of hydrochloride acid (6N) was gradually added to the mixture under continuous stirring. After all the acid was added, the mixture was sonicated in a sonication bath for 0.5 hour. After sonication, 50 ml of ethyl acetate was added to the beaker to dissolve the solid. The ethyl acetate layer was separated in a separation funnel, and washed with purified water twice. Then the ethyl acetate solution was mixed with anhydrous zinc sulfate to eliminate the water content, and filtered through No.1 filter paper. Then ethyl acetate was evaporated and diclofenac acid was obtained.
2.3 Synthesis of Diclofenac Ester Prodrugs
Diclofenac ester prodrugs were synthesized following the method for Ketoraolac ester prodrug synthesis method [18] with some modifications. Specifically, diclofenac acid (0.00304 mol) was dissolved in 19 ml of acetone, then 24 mg of 4-pyrrolidinopyridine was added and dissolved. After that, 0.00916 mole of alcohol (methanol, ethylene glycol, glycerol, or 1,3-propylene glycol, depending on the prodrug to be synthesized) was added to the solution and stirred. To the solution, 777 mg of DCC was added. This reaction mixture was kept on continuous stirring for 18 hours at room temperature. Then the reaction mixture was centrifuged at 1000 rpm for 10 minutes. Supernatant was collected, and the remaining solid was washed with additional 20 ml acetone under vortexing for 5 mins, and then centrifuged again at 1000 rpm for 10 minutes to collect the supernatant. Both supernatants were combined and evaporated in a vacuum oven at room temperature to obtain the crude ester product. The crude product was then dissolved in 1 ml of ethyl acetate and purified through flash chromatography. 1H NMR spectra were obtained in DMSO-d6 at 300 MHz, JEOL ECX300 instrument. The yield was determined based on the amount (moles) of prodrug obtained after purification compared to the amount (moles) of diclofenac acid added.
-
1)
Diclofenac Acid (DA) 1H NMR (300 MHz DMSO-d6): δ = 7.50 (2H, d, 1, 3 ), δ = 7.22 – 7.13 (3H, m, -NH, 5, 7), δ = 7.03 (1H, t, 2), δ = 6.82 (1H, t, 6), δ = 6.25 (1H, d, 4), δ = 3.67 (2H, s, 8).
-
2)
Methanol Diclofenac Ester (MD): MD was purified by flash silica gel column chromatography eluting with 90% Hexane and 10% ethyl acetate and obtained with about 90% yield. The final product is a white crystal solid. 1H NMR (300 MHz DMSO-d6): δ = 7.51 (2H, d, 1, 3), δ = 7.21-7.14 (2H, m, 5, 7), δ = 7.07-7.00 (2H, m, -NH, 6), δ = 6.81 (1H, t, 2), δ = 6.21 (1H, d, 4), δ = 3.78 (2H, s, 8), δ = 3.62 (3H, s, a).
-
3)
Ethylene Glycol Diclofenac Ester (ED): ED was purified by flash silica gel column chromatography eluting with 60% Hexane and 40% ethyl acetate and obtained with about 80% yield. The final product is a white waxy semisolid. 1H NMR (300Mz, DMSO-d6): δ = 7.51 (2H, d, 1, 3), δ = 7.20-7.15 (2H, m, 5, 7), δ = 7.06-7.00 (2H, m, -NH, 6), δ = 6.82 (1H, t, 2), δ = 6.22 (1H, d, 4), δ = 4.82 (1H, t, -OH), δ = 4.07 (2H, t, b), δ = 3.78 (2H, s, 8), δ = 3.59-3.54 (2H, m, c).
-
4)
Glycerol Diclofenac Ester (GD): GD was purified by flash silica gel column chromatography eluting with 20% Hexane and 80% ethyl acetate and obtained with about 70% yield. The final product is a transparent viscous liquid. 1H NMR (300 MHz, DMSO-D6): δ = 7.54 (2H, d, 1, 3), δ = 7.24-7.18 (2H, m, 5, 7), δ = 7.08-7.06 (2H, m, -NH, 6), δ = 6.85 (1H, t, 2), δ = 6.25 (1H, d, 4), δ = 4.94 (1H, d, -OH), δ = 4.66 (1H, t, -OH), δ = 4.12 (1H, dd, d), δ = 3.99 (1H, dd, d), δ = 3.82(2H, s, 8), δ = 3.72(1H, m, e), δ = 3.37-3.34(2H, m, f).
-
5)
1,3 Propylene Glycol Diclofenac Ester (PD): PD was purified by flash silica gel column chromatography eluting with 80% Hexane and 20% ethyl acetate and obtained with about 80% yield. The final product is a pale yellow solid. 1H NMR (300 MHz, DMSO-D6): δ = 7.50 (2H, d, 1, 3), δ = 7.20-7.15 (2H, m, 5, 7), δ = 7.05-7.00 (2H, m, -NH, 6), δ = 6.81 (1H, t, 2), δ = 6.22 (1H, d, 4), δ = 4.50 (1H, t, -OH), δ = 4.10 (2H, t, g), δ = 3.76 (2H, s, 8), δ = 3.42-3.38 (2H, m, i), δ = 1.70 (2H, p, h).
2.4 Solubility Determination
The solubilities of the diclofenac prodrugs in deionized water or diclofenac acid in 0.02 M phosphate buffer at pH 2.5 (0.02 M NaH2PO4 solution adjusted to pH 2.5 with 6N HCl) were determined by adding excessive amount of drug or prodrugs into 10 ml of the solvent and equilibrating at room temperature under stirring condition. At 3, 6, 12, and 24 hours, the solution was centrifuged at 3000 rpm for 10 min, 0.5 ml of supernatant was taken and diluted with equal volume of water immediately and then analyzed with HPLC.
The solubilities of the diclofenac prodrugs and diclofenac acid in 5% (v/v) propylene glycol in water or the phosphate buffer solution at pH 2.5 at room temperature were also determined with the same method above. In addition, the solubilities of the prodrugs and diclofenac acid in hexane and in isopropylmyristate (IPM) was also determined in similar way, except the samples in IPM was diluted with DMSO, and samples in hexane were first evaporated to dry and then re-dissolved with DMSO. All solubility experiments were conducted in triplicate.
2.5 Partition Coefficient Determination
To determine the partition coefficient in octanol/water system, the diclofenac prodrugs or diclofenac acid were first dissolved in octanol, and then 10 times the volume of purified water (or 0.02 M phosphate buffer at pH 2.5 for DA) was added and equilibrated on a shaking platform at room temperature for 24 hours. The octanol phase and water phase were separated by centrifugation, and the drug concentrations in the aqueous phase and the octanol phase after equilibrium were measured by HPLC method. The partition coefficient was calculated by equation: LogPO/W = Log (Coctanol / Cwater).
To determine the partition coefficient of diclofenac acid and its prodrugs in stratum corneum / water system, the stratum corneum was first isolated with a previous method [19] with some modifications. HEM was placed with the viable epidermis side down on a filter paper in a petridish. The filter paper was wetted with phosphate buffer saline (PBS) solution at pH7.4 and then 0.5% trypsin was added to get a final concentration of 0.25% with the liquid level match the top of the HEM. The petridish was incubated at 37°C for 24 hrs. After the treatment, the HEM was removed carefully and put into deionized water. After the epidermis layer fell off, the stratum corneum was taken and rinsed three times in deionized water, and then air dried. Diclofenac acid or the prodrugs were dissolved in 5% propylene glycol solution in water (or 0.02 M phosphate buffer at pH 2.5 for DA), and the dried stratum corneum was weighed and put into the drug solutions. The solutions were equilibrated under shaking condition with an orbital shaker for 24 hrs. Then the stratum corneum was removed from the solution and the any excess solution was blotted away carefully, and weighed immediately. The stratum corneum was then extracted by soaking in 5 ml methanol under shaking condition for 24 hrs and then the methanol solution was analyzed with HPLC for the amount of drug taken by the stratum corneum. In addition, the drug concentration of aqueous solution after equilibrium was also measured. The drug concentration in stratum corneum is determined based on the stratum corneum dry weight and also the amount of drug taken by it. The partition coefficient was calculated by equation: LogKSC/W = Log (CStratum Corneum/CWater). All partition coefficient experiments were conducted in triplicate.
2.6 Degradation Rate Determination
Diclofenac prodrugs of appropriate concentration in PBS solution (pH 7.4) with addition of 30% DMSO were prepared. 30% of DMSO in PBS at pH 7.4 was used for the purpose of increasing the solubility of prodrugs in the solution. The initial concentrations of the prodrugs in the solutions were similar. The solutions were placed in a water bath shaker at 37 °C. Samples were taken from the solution at 0 min, 10 mins, 20 mins, 30 mins, 1hour, 2hours, 4hours, 8hours, 24hours and 48 hours, and analyzed with HPLC method. The samples were analyzed for the decreasing in concentration of the prodrugs over time. The pseudo first order hydrolysis rate constants for all the prodrugs were determined. All degradation experiments were conducted in triplicate.
2.7 Bioconversion Rate Determination
Diclofenac prodrugs solution with appropriate concentrations in DMSO is prepared, and the solution (about 5% of the plasma volume) was spiked into freshly prepared rat plasma, and then placed in 37°C water bath shaker. Aliquots were taken from the plasma periodically into a methanol solution to stop the bioconversion and precipitate out the plasma protein. Then the samples were centrifuged and supernatants were analyzed by HPLC method. The bioconversion rates for all the prodrugs were determined. All bioconversion experiments were conducted in triplicate.
2.8 In Vitro Flux Study of Diclofenac Acid and Its Prodrugs in Aqueous Solution
Individual saturated solutions of each of the prodrugs and diclofenac acid in 5% propylene glycol in water was made on the day of the experiment, by adding excess drug to the solution (approximately 1% of the drug in the suspension) and sonicated in a water bath for 20 min and set aside for 1 hour before use. The HEM was mounted on the vertical Franz diffusion cell with the stratum corneum facing the donor cell. The diffusion area is 0.64 cm2. The receptor cell was filled with 5 ml of 0.15 M phosphate buffer saline (PBS) solution at pH 7.4, and the donor cell was filled with 0.5 ml of the fresh made saturated drug solution. The donor cell was covered with a parafilm to prevent the evaporation of the donor solution. The experiment was conducted at 37 °C. The whole 5 ml of receptor solution was taken, and fresh PBS solution was added at time 2, 4, 6, 8, 12, 24, 30, 36, and 48 hour. Samples were analyzed with HPLC method. The flux of each of the drug or prodrug was determined by the Equation 1 [20]: J = ΔQ / (AQΔt) (1). Where J is the flux, A is the diffusion surface area, Q is the cumulative amount of the drug transported into the receiver chamber and t is the time. Under steady state, the ΔQ/Δt was the slope of the linear regression of the cumulative amount of the drug verses the later experiment time points. All in vitro flux experiments were conducted in triplicate.
2.9 HPLC analysis method
The DA and its prodrugs were analyzed with a HP 1050 HPLC with quaternary pump and DAD detector. The column used was a Zorbax XDB-C18 4.6 × 150 mm 5.0 micron HPLC column. The mobile phases were acetonitrile and 0.02 M citrate buffer at pH 6.0 with a gradient method. The flow rate was 1 ml/min, and the detection UV wavelength was 280 nm.
Results and Discussion
3.1 Diclofenac and Its Prodrugs Physicochemical Properties
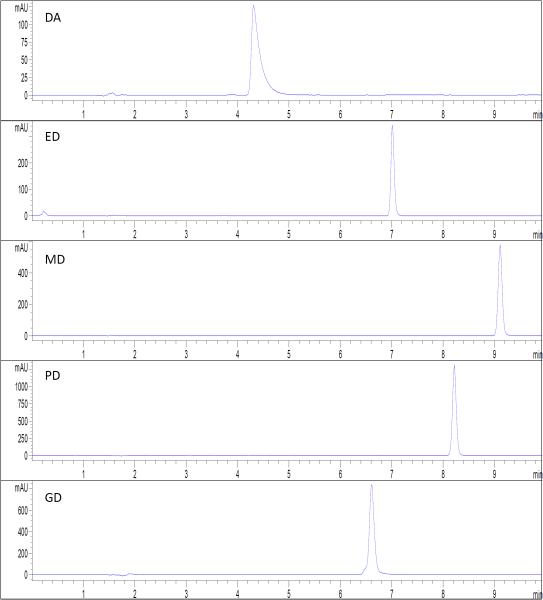
We obtained the diclofenac prodrugs with purity estimated to be above 95%, since no obvious impurity peak was observed from the HPLC chromatography (see Figure 2). The HPLC retention time was 4.4 min for DA, 6.6 min for GD, 7.1 min for ED, 8.2 min for PD, and 9.1 min for MD. The purity of each prodrug was also confirmed from 1H NMR spectra with no significant impurity observed (data not showed).
Figure 2.
The HPLC chromatograph of the standard solutions of diclofenac acid and the four prodrugs.
The solubilities of diclofenac acid and its prodrugs in a series of solvents are listed in Table 1. In the determination of the aqueous solubility of diclofenac acid (DA), 0.02 M phosphate buffer at pH 2.5 was used instead of purified water for other prodrugs. In this way, the solubility determined is the intrinsic solubility of the unionized DA. Methanol diclofenac ester (MD) showed lower solubility than that of the unionized DA in water (more than 2 folds decrease). Propylene glycol diclofenac ester (PD) showed similar solubility to that of DA. On the other hand, ethylene glycol ester (EG) and glycerol diclofenac ester (GD) showed much higher solubility in purified water than that of the unionized DA (more than 10 and 100 fold increase, respectively). In addition, the addition of 5% propylene glycol (PG) into water showed a slight increase (20-30%) in the aqueous solubility of DA, ED, PD and GD; but basically no effect on the MD solubility. The addition of 5% PG in water was for the purpose of better wetting the chemicals and increasing the dissolution rate of the chemicals.
Table 1.
Summary of the physicochemical properties of the diclofenac acid and its prodrugs.
| Drug | DA | MD | ED | GD | PD |
|---|---|---|---|---|---|
| Solubility in water (μmol/ml) | 0.0034a | 0.0019 | 0.0747 | 0.551 | 0.0033 |
| Solubility in 5% PG water (μmol/ml) | 0.0041a | 0.0019 | 0.0918 | 0.662 | 0.0048 |
| Solubility in Hexane (μmol/ml) | 0.134 | 21.0 | 1.76 | 0.386 | 2.22 |
| Solubility in IPM (μmol/ml) | 12.6 | 43.0 | 49.3 | 69.7 | 13.6 |
| Partition coefficient (LogP octanol/water) | 4.57a | 5.00 | 3.83 | 3.30 | 4.08 |
| Hydrolysis in PBS (half life in hours) | -- | 34.6 | 50.2 | 25.9 | 63.9 |
| Bioconversion in plasma (half life in min) | -- | 1.62 | 1.66 | 11.3 | 2.41 |
Water was substituted with 0.02 M phosphate buffer at pH 2.5.
The solubilities of the five chemicals in a non-polar organic solvent hexane are also listed in Table 1. The formation of the ester prodrugs showed higher solubilities in hexane than that of DA, with MD being the highest. The solubilities of those five chemicals in isopropylmyristate (IPM), an organic solvent with similar lipophilicity to the lipid structure in stratum corneum are in the order of: GD > ED > MD > PD > DA. All five chemicals showed good solubilities in IPM, but the formation of ester prodrugs showed higher solubility than the parent drug in IPM.
The partition coefficients in octanol/water for the five chemicals follow the order of: MD > DA > PD > ED > GD. MD showed highest partition coefficient or highest lipophilicity, which is consistent with its lowest solubility in purified water. On the other hand, the formation of GD showed lowest partition coefficient or highest hydrophilicity, which is also consistent with its highest solubility in purified water. Our experimental DA partition coefficient value is 4.57, which is close to the Drug Bank website value of 4.9.
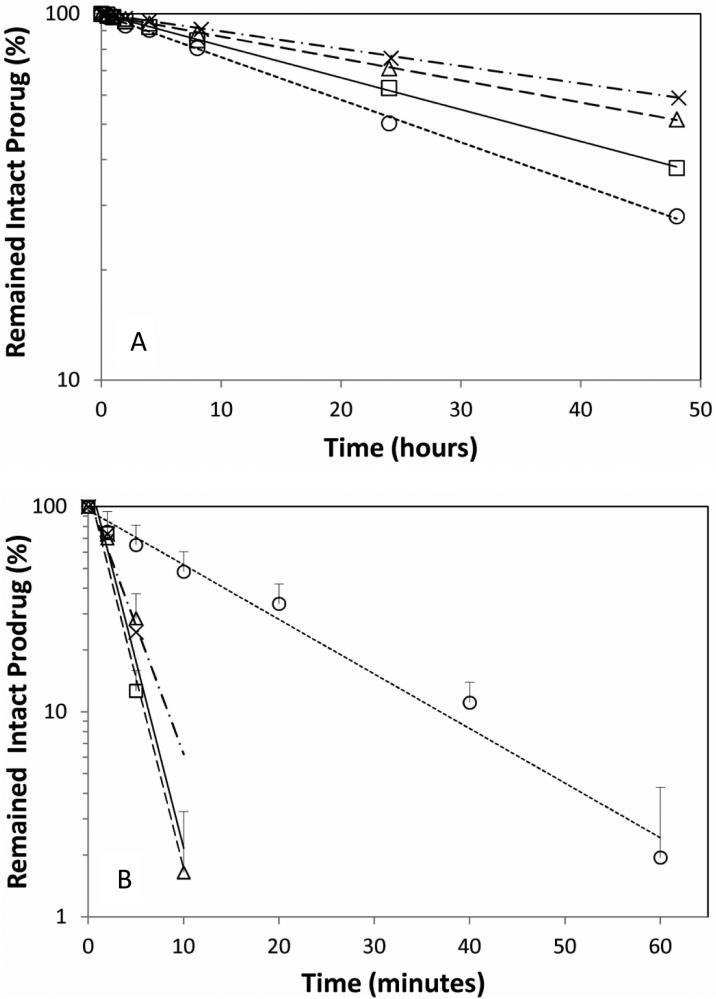
The hydrolysis rates of the diclofenac prodrugs in PBS at pH 7.4 are shown in Figure 3A, in which the intact prodrug concentrations in the PBS solution at different time points have been normalized by the initial starting concentration (in terms of percentage), and plotted against the experiment time. A linear relationship between the intact prodrug concentrations (in logarithm scale) vs. time was observed for each prodrug. This indicates that the hydrolysis was following a pseudo first order reaction mechanism. The half lives of the hydrolysis of the four prodrugs are listed in Table 1, based on the slopes determined from the linear regression. PD showed the longest half life in PBS solution, followed by ED and MD, and GD showed the shortest half life. The shortest half life of GD could be due to the catalytic effect of its hydroxyl group to the hydrolysis of the ester. Overall, the half lives are in the range of one to three days at 37 °C, which indicates that these diclofenac prodrugs are not very stable in aqueous solution.
Figure 3.
(A) The hydrolysis of the diclofenac prodrugs in pH 7.4 PBS solutions at 37 °C. (B) The bioconversion of the diclofenac prodrugs in rat fresh plasma at 37 °C. Symbols: (○) GD; (□) MD; (Δ) ED; (x) PD.
The diclofenac prodrugs have to convert to the parent drug in the body to exhibit pharmacology effect. Thus the bioconversion rates of the prodrugs in fresh rat plasma were determined. The intact prodrug concentrations (in terms of percentage normalized with the initial prodrug concentration) at different time points in rat plasma were plotted against the reaction time under the logarithm scale (see Figure 3B). A linear relationship was also observed for each prodrug in the bioconversion reaction, and the half lives of the bioconversion are listed in Table 1. The bioconversion in rat plasma for the four prodrugs are much faster (half lives in minutes) than their hydrolysis in PBS, due to the presence of esterases in the plasma. MD and ED showed comparable bioconversion rates with half life only about 1.6 min, PD showed a half life of 2.4 min, and GD showed relatively slower bioconversion rate with half life of 11 min, possibly due to the bulky glycerol group in the GD partially hindering esterase enzyme access for its bioconversion.
3.2 In Vitro Flux Study of Diclofenac Acid and Its Prodrugs
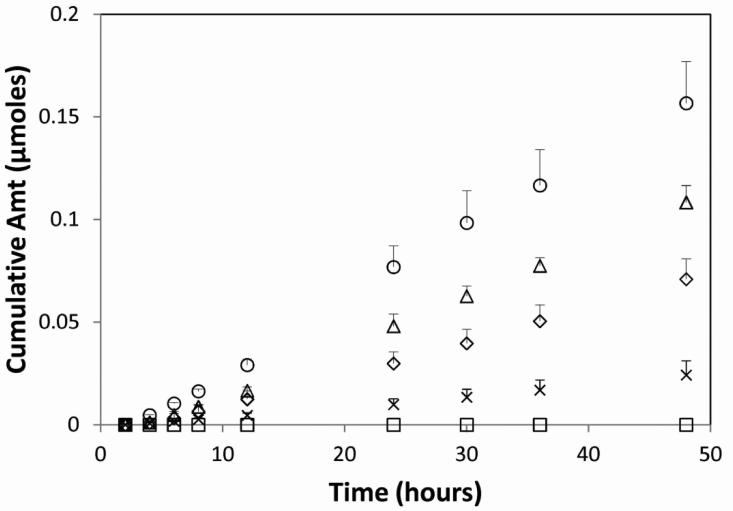
The cumulative amount of DA, MD, ED, GD, and PD permeated through human epidermal membrane over time in the in vitro flux study with their saturated aqueous solutions are shown in Figure 4. GD showed the highest cumulative amount permeated across the HEM, followed by ED, DA and PD, but MD did not show any detectable amount permeated across HEM. The steady state fluxes of the drug or prodrugs are summarized in Table 2. In comparing the steady state fluxes with the octanol/water partition coefficients of the drugs, we can discover that the addition of polar group to the diclofenac molecule to form more hydrophilic ester prodrugs (ED and GD) with increased aqueous solubilities enhanced the transdermal permeation, and on the other hand, the addition of methyl group to form more hydrophobic ester prodrug (MD) with decreased aqueous solubility inhibited the transdermal permeation. This observation is in agreement with previous findings that there is a parabolic relationship between the transdermal flux and the octanol/water partition coefficient with maximum flux value observed at partition coefficient (LogP) values between 1 and 3 [11, 18].
Figure 4.
The in vitro flux across HEM of the diclofenac or its prodrugs in the 5% propylene glycol aqueous saturated solutions (y-axis is the cumulative amount of the drug detected in the receiver chamber). Symbols: (○) GD; (Δ) ED; (◇) DA; (□) MD; (x) PD.
Table 2.
Summary the relationship among the partition coefficient in stratum corneum, drug solubility, and the flux across the human epidermal membrane in aqueous solution (the value in the bracket is the standard deviation of the flux).
| Drug | DA | MD | ED | GD | PD |
|---|---|---|---|---|---|
| Partition coefficient (LogK SC/ 5% PG water) | 2.97a | 3.56 | 1.74 | 1.56 | 2.58 |
| Solubility in 5% PG water (C0 in μmol/ml) | 0.0041a | 0.0019 | 0.0918 | 0.662 | 0.0048 |
| K·C0 (μmol/g) | 3.78 | 7.03 | 5.04 | 24.0 | 1.82 |
| Aqueous solution flux (nmol·cm−2·hr−1) | 2.57(0.29) | 0 | 3.99(0.80) | 5.54(0.78) | 0.84(0.25) |
Water was substituted with 0.02 M phosphate buffer at pH 2.5.
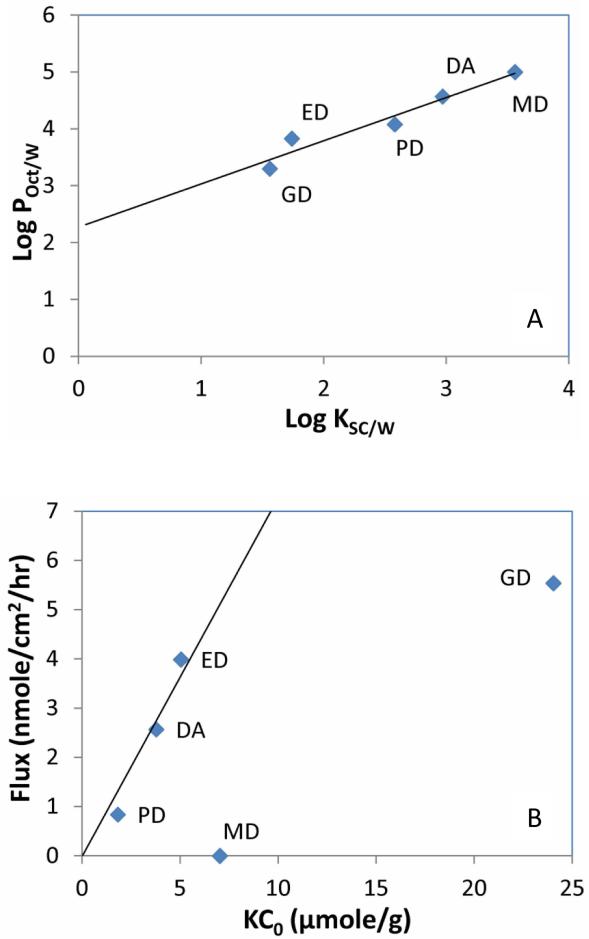
It is generally believed that permeating through stratum corneum is the rate limiting step for transdermal delivery and the steady state flux across the human epidermis can be explained by the Equation 2 [11]: J = K·D·C0 / h (2). Where J is the flux, K is the stratum corneum and formulation partition coefficient of the drug, D is the diffusion coefficient of the drug in stratum corneum, h is the path length in the stratum corneum, and Co is the drug concentration in the formulation. The partition coefficients between the stratum corneum and the 5% PG water solutions for DA, MD, ED, GD and PD are reported in Table 2. There is a linear relationship between the octanol/ water partition coefficients (LogPO/W) and stratum corneum/ PG water partition coefficient (LogKSC/W) for the diclofenac drug and its prodrugs as demonstrated by Figure 5A. Based on Equation 2, if we assume the path length (h) in stratum corneum is the same for all five chemicals and they have similar diffusion coefficient (D) because of their relatively close molecular weights and chemical structures, then there would be a linear relationship between the flux J and the value of KCo, where Co is the concentration of the drug or prodrug in the 5% PG solution. As we used saturated drug solution for the in vitro transdermal flux study, the concentration C0 would be the drug or prodrug solubility in the 5% PG water solution as showed in Table 2. As we plotted the J verses KCo in Figure 5B, the DA, ED, and PD fell into the linear relationship very well (as evidenced by the linear regression line forced to pass the origin point), but MD and GD deviated from the linear line. It was reported that molecule with an extra hydrogen bonding group could lower its diffusion coefficient in stratum corneum by an order of magnitude [11]. GD is significantly more hydrophilic and has an extra hydroxyl group comparing to DA, ED and PD. The deviation from the linear relationship for GD could be due to its diffusion coefficient in the stratum corneum being significantly lower than that of DA, ED, or PD. We observed that there is some increase in the flux with significant increase in the aqueous solubility from ED to GD, but further increase of the aqueous solubility of diclofenac prodrug with more additional hydroxyl groups may not contribute to much significant increase in the flux across the skin due to the offset from the decrease of the diffusion coefficient of the prodrugs.
Figure 5.
(A) The linear relationship between the stratum corneum water partition coefficients (Log KSC/W) and the octanol water partition coefficients (Log POct/W). Linear line is the linear regression of the data points with regression equation of y = 0.7598x+2.2701 and R2= 0.9388. (B) The relationship between the in vitro fluxes across HEM in 5% PG solutions and the values of KC0 (the stratum corneum water partition coefficient times the solubility of the drug or prodrug in 5% PG solution). The linear line is the linear regression of the data points of DA, ED and PD with forcing the linear line through the origin point, with a linear regression equation of y = 0.7284x and R2 = 0.9248.
MD has even one less hydroxyl group than DA, ED or PD, thus it could have a higher diffusion coefficient value in the stratum corneum. Based on its KC0, MD would have a flux value even higher than DA. But experimentally, it showed zero flux. As it is known, the permeability of a drug across the whole epidermis (PEP) is dependent on the permeability across stratum corneum (PSC) and the permeability across viable epidermis (PVE) as described by the Equation 3 [11] : 1/PEP = 1/PSC + 1/PVE (3). Usually, the permeability across the epidermis is controlled by the permeability across the stratum corneum (Psc << PVE). However, for water insoluble drug like MD with very high octanol/water partition coefficient, its permeability across the epidermis could be controlled by the viable epidermis (because of its extreme low aqueous solubility, its permeability across viable epidermis could be significant smaller than its permeability across stratum corneum). DA also has a very low aqueous solubility in unionized form, but its solubility under physiological pH condition is several orders of magnitude higher, thus would have much larger viable epidermis permeability.
As showed in Figure 3A, the diclofenac prodrugs are prone to hydrolysis in aqueous solution. In the in vitro flux study, we removed the entire receiver solution for every sample point and analyzed for both the diclofenac acid and the prodrug concentration in the samples. For PD flux study, no diclofenac acid was detected in all the receiver samples; and for ED and GD flux studies, diclofenac acid was detected in the receiver samples only at the 24 hour and the 48 hour time points, with the amount less than 20% of the ester prodrug forms in the samples. Only at those two time points, the sampling time interval was 12 hours, and some of the prodrugs in the receiver chamber could be hydrolyzed to diclofenac acid in sufficient amount. There could be some prodrug hydrolyzed to diclofenac acid in the donor chamber, but the contribution of the diclofenac acid from the hydrolysis in the donor solution to the flux across HEM would be small to negligible since we did not detect significant amount of diclofenac acid in the receiver samples in other time points during the flux study. We combined both the prodrug and the diclofenac acid in the receiver samples for cumulative amount permeated across the HEM in Figure 4. In addition, we think that the HEM used in the in vitro flux study would have little enzymes to convert the diclofenac prodrugs to the parent drug. This could be due to the skin used was not fresh (more than 1 year from the date obtained from its donor) and also the heat strapping separation procedure could deactivate the enzymes. Unpublished results of flux study with fresh full thickness rat skin in our lab did show most of the diclofenac prodrugs being converted to the parent drug in the receiver samples in the first 8 hours. Similar results was also reported in Naproxen prodrugs in vitro skin permeation study with rat skin [9]. Since the permeation across stratum corneum is the rate limited step for most drugs and the prodrug bioconversion usually occurs in the viable epidermis and dermis layers, it would be adequate for using HEM instead of fresh full thickness skin in the in vitro flux evaluation in this study.
There is a linear relationship between LogP and the stratum corneum water partition coefficient as observed in this study (Figure 5A), thus drugs with higher LogP would have higher tendency to partition into the stratum corneum. However, the transdermal flux is also dependent on the solubility in the vehicle (5% PG water in this study) as showed in Equation 2. Conventionally, it is observed that drugs with octanol/water partition coefficient (LogP) in the range between 1 and 3 showed better flux across skin [11]. In this range, the drugs usually showed a balanced hydrophilicity and lipophilicity: adequate aqueous solubility and sufficient participation into the stratum corneum. For hydrophilic drug such as ketorolac (LogPO/W around 1), its prodrugs with increased lipophilicity improved the transdermal flux [18]. On the other hand, for lipophilic drug such as diclofenac acid (LogPO/W of 4.57), a prodrug with lower LogPO/W would improve the transdermal flux. Indeed, we observed that converting DA to prodrug with even higher LogPO/W (MD) showed significant decrease in the flux across skin; converting DA to prodrug with a little lower LogPO/W but not much improvement in aqueous solubility (PD) also showed lower flux than DA; only converting DA to prodrugs with lower LogPO/W and significant improvement in aqueous solubility (ED and GD) showed higher flux than DA.
A previous study also reported that ED showed better in vitro flux than the parent drug in its sodium salt form, but they used 5% PG in methanol as the vehicle and full thickness rats skin in their study [16], where methanol could provide significant disruption to the stratum corneum and enhancing the molecules transport across skin. In another study [15], diclofenac prodrugs formed with polyoxyethylene glycols with the polymer number from 2 to 6 was investigated and highest in vitro cumulative amount was observed for the prodrug with longest chain length (molecular weight around 560) which showed the lowest LogP and highest aqueous solubility. For comparison, the GD (molecular weight around 370) in our study showed similar LogP and solubility to the diclofenac polyoxyethylene glycol prodrug with the polymer number of 5 (molecular weight around 516) in that study. In our study, we noticed that GD deviated from the flux vs. KC0 linear line as demonstrated in Figure 5B, possible from the much lower diffusion coefficient of GD in the stratum corneum. The formation of diclofenac prodrug with long polyoxyethylene glycol would decrease the diffusion coefficient even more as they demonstrate to have much higher molecular weight and more hydrogen bonds. In that study [15], they used ethanol as the vehicle and 50% ethanol water as receiver media, which may cause significant skin disruption and therefore better flux for the diclofenac prodrug with longest polyoxyethylene glycol. We used 5% PG water, a more skin friendly vehicle for the in vitro flux study of diclofenac prodrugs. Through investigating the correlation between the flux and stratum corneum water partition coefficient, we provided better understanding in designing diclofenac prodrugs for enhancing transdermal delivery of diclofenac. However, since the diclofenac prodrugs in this study are not very stable in aqueous media (see Figure 3A), further exploring the diclofenac prodrugs in non-aqueous media for transdermal delivery may be needed.
Conclusions
Diclofenac prodrugs were synthesized and evaluated for the physicochemical properties and in vitro fluxes across human skin. The formation of less hydrophilic diclofenac acid prodrugs with alcohols like methanol and 1,3-propylene glycol rendered the prodrugs with lower aqueous solubility, higher partition coefficients, and lower fluxes across the skin in aqueous solutions. On the other hand, the formation of more hydrophilic diclofenac acid prodrugs with alcohols like ethylene glycol and glycerol rendered the prodrugs with higher aqueous solubilities, lower partition coefficients, and higher fluxes across the skin in aqueous solutions. In addition, the prodrugs demonstrated the potential to be converted to the parent drug in vivo. In summary, diclofenac prodrugs with improved aqueous solubilities showed the potential in enhancing transdermal diclofenac delivery.
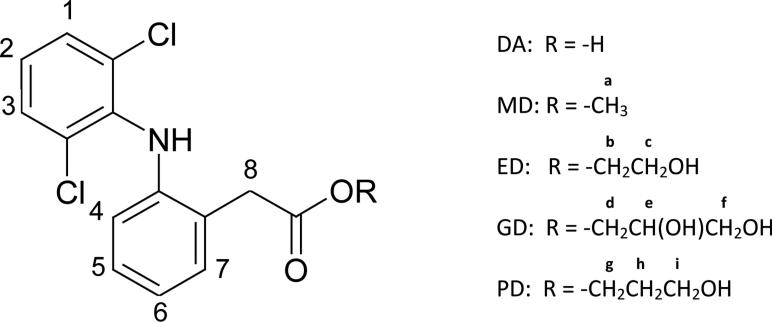
Figure 1.
The molecular structure of diclofenac acid and the prodrugs.
Acknowledgments
The project described was supported partly by the INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences).
Footnotes
Declaration of Interest
None of the authors has conflict of interest.
References
- 1.McCarthy D. Nonsteroidal anti-inflammatory drug-related gastrointestinal toxicity: definitions and epidemiology. Am J Med. 1998;105(5A):3S–9S. doi: 10.1016/s0002-9343(98)00274-5. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald VE, Jr., Bennett JS, Packer M, Roberts WC, Williams GW. The editor's roundtable: nonsteroidal anti-inflammatory drugs and cardiovascular risk. Am J Cardiol. 2008;102(8):1046–55. doi: 10.1016/j.amjcard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50(1):50–61. doi: 10.1177/0091270009336234. [DOI] [PubMed] [Google Scholar]
- 4.Barthel HR, Haselwood D, Longley S, 3rd, Gold MS, Altman RD. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum. 2009;39(3):203–12. doi: 10.1016/j.semarthrit.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Galer BS. A comparative subjective assessment study of PENNSAID(R) and Voltaren Gel(R), two topical formulations of diclofenac sodium. Pain Pract. 2011;11(3):252–60. doi: 10.1111/j.1533-2500.2010.00420.x. [DOI] [PubMed] [Google Scholar]
- 6.Radermacher J, Jentsch D, Scholl MA, Lustinetz T, Frolich JC. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol. 1991;31(5):537–41. doi: 10.1111/j.1365-2125.1991.tb05576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyatake S, Ichiyama H, Kondo E, Yasuda K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol. 2009;67(1):125–9. doi: 10.1111/j.1365-2125.2008.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Zhang W, Jones A, Doherty M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. Bmj. 2004;329(7461):324. doi: 10.1136/bmj.38159.639028.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautio J, Taipale H, Gynther J, Vepsalainen J, Nevalainen T, Jarvinen T. In vitro evaluation of acyloxyalkyl esters as dermal prodrugs of ketoprofen and naproxen. J Pharm Sci. 1998;87(12):1622–8. doi: 10.1021/js970465w. [DOI] [PubMed] [Google Scholar]
- 10.Valiveti S, Paudel KS, Hammell DC. In vitro/in vivo correlation of transdermal naltrexone prodrugs in hairless guinea pigs. Pharm Res. 2005;22(6):981–9. doi: 10.1007/s11095-005-4593-0. [DOI] [PubMed] [Google Scholar]
- 11.Guy R, Hadgraft J. Transdermal drug delivery. 2nd ed. Marcel Dekker Inc.; New York: 2003. [Google Scholar]
- 12.Abo-Ghalia MH, Shalaby AM, el-Eraqi WI, Awad HM. Synthesis and anti-phlogistic potency of some new non-proteinogenic amino acid conjugates of “Diclofenac”. Amino Acids. 1999;16(3-4):425–40. doi: 10.1007/BF01388181. [DOI] [PubMed] [Google Scholar]
- 13.Halen PK, Murumkar PR, Giridhar R, Yadav MR. Prodrug designing of NSAIDs. Mini Rev Med Chem. 2009;9(1):124–39. doi: 10.2174/138955709787001695. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro L, Silva N, Iley J. Aminocarbonyloxymethyl ester prodrugs of flufenamic acid and diclofenac: suppressing the rearrangement pathway in aqueous media. Arch Pharm (Weinheim) 2007;340(1):32–40. doi: 10.1002/ardp.200600145. [DOI] [PubMed] [Google Scholar]
- 15.Bonina FP, Puglia C, Barbuzzi T. In vitro and in vivo evaluation of polyoxyethylene esters as dermal prodrugs of ketoprofen, naproxen and diclofenac. Eur J Pharm Sci. 2001;14(2):123–34. doi: 10.1016/s0928-0987(01)00163-4. [DOI] [PubMed] [Google Scholar]
- 16.Jilani J, Shatnawi A, Idkaidek N. Evaluation of hydroxyethyldiclofenac as possible prodrug for topical application. Acta Pharmaceutical Turcica. 2003;45:227–233. [Google Scholar]
- 17.Peck KD, Ghanem AH, Higuchi WI. The effect of temperature upon the permeation of polar and ionic solutes through human epidermal membrane. J Pharm Sci. 1995;84(8):975–82. doi: 10.1002/jps.2600840813. [DOI] [PubMed] [Google Scholar]
- 18.Doh HJ, Cho WJ, Yong CS. Synthesis and evaluation of Ketorolac ester prodrugs for transdermal delivery. J Pharm Sci. 2003;92(5):1008–17. doi: 10.1002/jps.10353. [DOI] [PubMed] [Google Scholar]
- 19.He N, Li SK, Suhonen TM, Warner KS, Higuchi WI. Mechanistic study of alkyl azacycloheptanones as skin permeation enhancers by permeation and partition experiments with hairless mouse skin. J Pharm Sci. 2003;92(2):297–310. doi: 10.1002/jps.10269. [DOI] [PubMed] [Google Scholar]
- 20.Yan G, Xu Q, Anissimov YG, Hao J, Higuchi WI, Li SK. Alternating current (AC) iontophoretic transport across human epidermal membrane: effects of AC frequency and amplitude. Pharm Res. 2008;25(3):616–24. doi: 10.1007/s11095-007-9405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]