Abstract
Background
The ventromedial prefrontal cortex (VMPFC) is a key center of affect regulation and processing, fundamental aspects of emotional competence which are disrupted in mood disorders. Structural alterations of VMPFC have consistently been observed in adult major depression and are associated with depression severity, yet it is unknown whether young children with depression demonstrate similar abnormalities. We investigated cortical thickness differences in the VMPFC of children with a history of preschool-onset depression (PO-MDD).
Methods
Participants in a longitudinal study of PO-MDD underwent structural brain imaging between the ages of 7 to 12 years. Using local cortical distance metrics, cortical thickness of the VMPFC was compared in children with and without a history of PO-MDD.
Results
Children previously diagnosed with PO-MDD (n=34) had significantly thinner right VMPFC versus children without a history of PO-MDD [(n=95); F(1,126)=5.97, p=0.016)]. This effect was specific to children with a history of PO-MDD vs. other psychiatric conditions and was independent of comorbid anxiety or externalizing disorders. Decreases in right VMPFC thickness were predicted by preschool depressive symptoms independent of depressive symptoms in school age.
Limitations
Results are cross-sectional and cannot distinguish whether thinner right VMPFC represents a vulnerability marker of MDD, consequence of MDD, or marker of remitted MDD. Longitudinal imaging is needed to contextualize how this difference relates to normative VMPFC structural development.
Conclusions
Onset of depression at preschool age was associated with decreased cortical thickness of right VMPFC. This finding implicates the VMPFC in depression from very early stages of brain development.
Keywords: depression, brain development, magnetic resonance imaging, preschool, ventromedial prefrontal cortex
Introduction
Major Depressive Disorder (MDD) is associated with structural and functional alterations of limbic prefrontal-subcortical regions, and neuroimaging studies of adults with depression have made significant contributions towards identifying this neurocircuitry. However, research investigating the neurobiology of very early-onset depression is also needed to elucidate the role of specific neurocircuitry in the pathogenesis of affective disorders. Preschool-onset depression (PO-MDD), the earliest validated form of depression, demonstrates symptom specificity, familial transmission, and shared biological correlates with adolescent and adult depression (Luby et al., 2003; Luby et al., 2002). Additionally, PO-MDD shows homotypic continuity with school-age depression (Luby et al., 2009), a finding that has been replicated using current DSM-5 criteria (Luby et al., 2014). PO-MDD is therefore a compelling model to clarify the pathophysiology of depression throughout the lifespan, particularly during early life, when the brain undergoes rapid development.
A major component of the limbic-prefrontal neurocircuitry implicated in MDD is the ventromedial prefrontal cortex (VMPFC), a region comprised of portions of anterior cingulate cortex (ACC), including subgenual prefrontal cortex (SGPFC), and medial orbitofrontal cortex (mOFC) (Price and Drevets, 2012). Neuroanatomical studies of macaques and rodents have observed a high degree of interconnections specific to these subregions vs. other areas of prefrontal cortex (Ongur and Price, 2000). These studies have shown that output from VMPFC projects to visceral control centers including the hypothalamus, while human studies have demonstrated that activity in VMPFC correlates with visceral responses to emotive experiences (Price and Drevets, 2012). Lesions of VMPFC in humans result in loss of normative visceral responses to emotional stimuli (Bechara et al., 2005), and the occurrence of such lesions in childhood has been linked to disrupted socioemotional development (Taber-Thomas et al., 2014). Convergent evidence thus supports a conserved function of the VMPFC within a “visceromotor network,” whereby it mediates emotional behavior and promotes emotional development.
Early findings from PET imaging in individuals with depression demonstrated that metabolism in the VMPFC (after controlling for total VMPFC volume) is increased in MDD (Drevets et al., 1997) and correlates with greater symptom severity (Drevets et al., 2008). Functional MRI imaging studies have repeatedly shown differences in the SGPFC and adjacent VMPFC in resting and activation paradigms testing response to affective stimuli in individuals with mood disorders (reviewed by Price and Drevets, 2012; Todd and Botteron, 2002). Disturbances of VMPFC function in depression have been demonstrated to normalize under treatment with antidepressants (Mayberg et al., 2000) and deep brain stimulation (Mayberg et al., 2005), suggesting that neuroplasticity of the VMPFC is relevant to resolution of MDD. A growing body of work implicates the VMPFC in processes linked to the pathophysiology of depression, including negative affect processing (Elliott et al., 2000), emotional regulation (Rive et al., 2013), stress responsivity (Kern et al., 2008), and self-referential functions of the default mode network (Gusnard et al., 2001).
Structural differences in the VMPFC have also been associated with MDD. Volumetric neuroimaging studies have shown decreased volumes in the SGPFC in adults with familial MDD (Drevets et al., 1997) and recurrent MDD (Coryell et al., 2005; Hastings et al., 2004), where decreases are greatest in those with recurrent illness (Salvadore et al., 2011). Decreased VMPFC volumes in MDD have most frequently been localized to the left, suggesting that lower left VMPFC volumes may serve as a biomarker for depression. Postmortem neuropathology studies have demonstrated that although neuronal numbers in the SGPFC are often the same for individuals with and without depression, reduced dendritic branching and fewer glial cells are observed (Ongur et al., 1998; Rajkowska, 2000). Thus, mechanisms including disrupted cellular development, neuronal atrophy, or degenerative glial cell loss could account for decreases in VMPFC volume.
Although work in pediatric depression is not as extensive as in adults, emerging findings support involvement of the VMPFC. Several functional imaging studies, including both task-based and resting state, have shown altered activation of the VMPFC during emotional processing tasks and increased limbic connectivity with the VMPFC (Kerestes et al., 2013 for review). Structural neuroimaging studies in adolescent depression have also demonstrated involvement of the VMPFC. Botteron et al. found decreased left SGPFC volume in depressed vs. healthy adolescent females (Botteron et al., 2002), similar in lateralization to adult MDD. A study of adolescents with MDD and borderline personality disorder (BPD) demonstrated that the group with MDD and BPD had smaller gray matter ACC volumes within the VMPFC (Goodman et al., 2011). These studies show that in adolescents, as in adults, the structure and function of the VMPFC are disrupted in depression, yet it remains unclear when this effect occurs, whether it is lateralized, or whether these differences represent early risk factors or a consequence of very early-onset affective dysregulation.
Much of the research described above examined VMPFC volume. Recently, advances in neuromorphometry have substantiated the use of higher-resolution metrics for quantifying differences in cortical thickness related to psychopathology, which may enhance identification of structural biomarkers of MDD. Studies of cortical thickness in adult MDD have demonstrated cortical thinning in several brain regions (Truong et al., 2013; Wagner et al., 2012), including regions within the VMPFC (Jarnum et al., 2011). To date, the one study examining whole brain cortical thickness in pediatric MDD did not find differences in the VMPFC (Fallucca et al., 2011). A study in children and adults at risk for familial depression observed thinner cortex throughout the right hemisphere in at-risk individuals and cortical thickening in several regions of the VMPFC (Peterson et al., 2009). In healthy children, an investigation of cortical thickness and subclinical anxious/depressive symptoms found a negative relationship between right VMPFC cortical thickness and anxious/depressive symptoms pre-pubertally, but a positive relationship post-pubertally (Ducharme et al., 2013). Based on these studies, evidence that specific regional differences in cortical thickness represent a biomarker of depression is mixed and variable depending on developmental stage. However, most of these global thickness studies have relied on whole brain approaches, which have less power to detect regional differences due to the increased burden of multiple comparisons.
Here we report on neuroimaging data of VMPFC thickness from a longitudinal study of depressed preschoolers originally identified between 3 and 6 years of age (Luby, et al., 2009). We followed the trajectory of depressive symptoms in these children and comparison groups of children with no psychiatric diagnosis as well as those with other psychiatric conditions. An initial wave of neuroimaging was completed when subjects were between the ages of 7–12, allowing a unique investigation of school-age neural correlates of depressive symptoms from preschool age. We used a region-of-interest approach, which preserves statistical power, to examine whether thickness of the VMPFC was affected in children with a history PO-MDD. Given the importance of the VMPFC to emotional behavior and the frequent association between adult depression and decreased left VMPFC volume, we hypothesized that children with PO-MDD, who demonstrate deficiencies in emotional processing and regulation, would show decreased left VMPFC cortical thickness compared to healthy children and those with other psychiatric diagnoses.
Methods
Participants
Neuroimaging data were analyzed from 129 children within a larger sample enrolled in the 10-year longitudinal Preschool Depression Study (Luby, et al., 2009). The original sample (N=305) was recruited from metropolitan St. Louis daycares and preschools using a screener to include healthy children while oversampling preschoolers with depressive symptoms. Children and caregivers participated in 3–6 comprehensive annual diagnostic and developmental assessments prior to the first neuroimaging session. Three diagnostic groups underwent initial brain imaging at 7–12 years of age, including a group with a history of preschool onset depression (PO-MDD), a group with no current or prior history of psychiatric diagnosis (Healthy), and a comparison group with other psychiatric disorders (OPD). Exclusion criteria included pervasive developmental disorder; contraindications for MRI scanning; head injury with loss of consciousness>5 min; stroke, seizure disorder, or other neurological or medical illness with known neural impact. Data from children with gestational age<34 weeks, maternal alcohol consumption during all trimesters of pregnancy, and IQ<80 were also excluded. One hundred ninety-nine participants met these inclusion/exclusion criteria. Of these, 129 participants had images of both right and left VMPFC that passed quality control (QC) for overall image quality, segmentation of the VMPFC, and segmentation of the total cortical gray matter with Freesurfer. Informed consent from the child’s guardian and assent from the child were obtained prior to study participation and all study procedures were reviewed and approved by the Institutional Review Board at Washington University School of Medicine.
Measures
Diagnostic Status
Subjects were assessed annually using the Preschool Age Psychiatric Assessment (PAPA, parent interview, used from ages 3–8) (Egger et al., 2006) and the Child and Adolescent Psychiatric Assessment (CAPA, parent/child interview, used for age≥ 9) (Angold and Costello, 1995). The PAPA and CAPA are age-adjusted versions of the same interview and probe the same psychiatric constructs according to symptoms defined by standardized diagnostic categories of the DSM-IV-TR and ICD-10. Children identified as having PO-MDD met developmentally adjusted criteria for MDD on the PAPA prior to age 6. These criteria, which required 4 out of 9 (rather than 5 out of 9) core depressive symptoms and removed the 2-week duration criterion, have demonstrated homotypic continuity with later depression meeting full DSM criteria (Gaffrey et al., 2011; Luby et al., 2014). A PO-MDD severity score, previously shown to be a sensitive measure of illness severity (Luby et al., 2004), was derived from the sum of core depressive symptoms averaged from annual assessments on the PAPA before age 6. A severity score based on the average of yearly depressive symptom scores after age 6, the school-age MDD severity score (SA-MDD severity score), was similarly calculated based on either the PAPA for ages≤8 or the CAPA for age≥ 9.
In analyses accounting for comorbidity, children were identified as having preschool-onset anxiety (PO-Anxiety) if they met criteria for Generalized Anxiety Disorder, Separation Anxiety Disorder, or Post-traumatic Stress Disorder before age 6. Preschool-onset externalizing (PO-externalizing) was identified if a child met criteria for Attention Deficit Hyperactivity Disorder, Oppositional Defiant Disorder, and/or Conduct Disorder before age 6.
Depressive Symptoms at Time of Scan
Children and parents both completed a Children’s Depression Inventory, a measure of depressive symptom severity, on the day of scan (Kovacs, 1985).
Pubertal Staging
The Tanner Staging Questionnaire was used to estimate children’s pubertal status at the time of scan based on parent or child report (dependent on age and developmental stage of child).
MRI Acquisition and Processing
Two 3D T1-weighted magnetization prepared rapid gradient echo scans (MPRAGEs) were acquired on a Siemens 3.0-T Tim Trio scanner without sedation (sagittal acquisition, repetition time 2,300ms, echo time 3.16ms, inversion time 1,200ms, flip angle 8°, 160 slices, 256 × 256 matrix, field of view 256mm, 1.0-mm3 voxels, total time 12:36min). The images were co-registered and summed to increase the signal-to-noise ratio (Holmes et al., 1998). A gain field correction using a 3D parabolic model of the gain field with ten free parameters (Styner et al., 2000) was applied to minimize the effects of image inhomogeneity, and volume intensity was converted to 8-bits. Because the funding for neuroimaging began at a different time than established annual behavioral assessments, scans were conducted within a 6 month window of a diagnostic assessment.
VMPFC boundaries were manually defined in an anterior commissure-posterior commissure orientation (see Figure 1 for boundary definitions). Interrater reliability for manual identification of VMPFC boundaries was 0.96, based on intraclass correlation coefficients comparing regional VMPFC volume differences in five subjects across six raters.
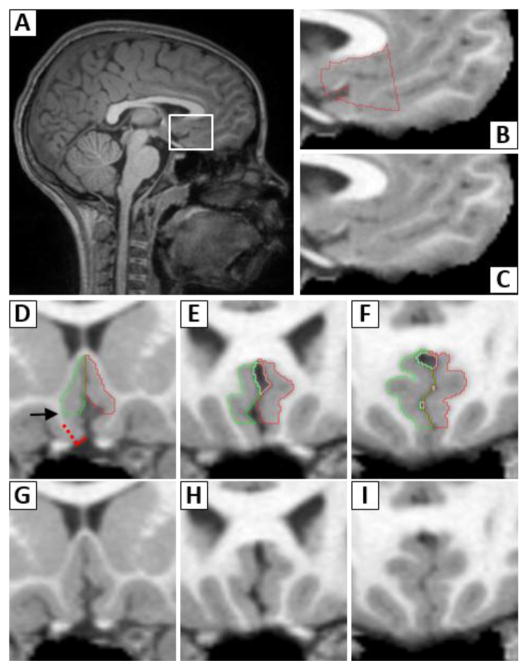
Figure 1. Boundary Definitions for VMPFC Region of Interest (ROI).
A sagittal brain view (A) shows the ventromedial prefrontal cortex (VMPFC) enclosed by the white box, with magnified views in B and C. The VMPFC ROI, shown in red in B, is framed superiorly by the corpus callosum. The anterior boundary is perpendicular to the genu of the corpus callosum and the posterior boundary falls where olfactory nerve lies inside the olfactory sulcus. Panels D–I show anterior to posterior coronal views of the ROI. The right and left VMPFC ROIs are in green and red, respectively. Details for obtaining the inferior boundary are illustrated on the right VMPFC in D. The inferior boundary is first defined by a line bisecting the cortex (red hatched line) and is perpendicular to the maximum inflection point of the gyrus rectus (solid red line). To eliminate frequent saturation artifacts in this region, the bottom 27% of the VMPFC is removed, and the adjusted inferior boundary falls at the level of the arrow.
A Bayesian tissue segmentation of the VMPFC, which was fully automated using in-house software (Lee et al., 2008; see supplement for further details), was limited to an area including the VMPFC and adjacent structures. Use of this subcube reduced the overall intensity inhomogeneity (in comparison to inhomogeneity across in the entire brain), thereby facilitating accurate tissue segmentation. Based on this tissue segmentation, the cortical thickness was calculated using Labeled Cortical Distance Mapping (LCDM). LCDM is a well-validated, high resolution approach that has successfully been used to characterize difference in cortical thickness in psychopathology including Alzheimer’s and schizophrenia (Miller et al., 2003; Ratnanather et al., 2013, 2014; Takayanagi et al., 2013; Wang et al., 2007; Karnik-Henry et al., 2012). The LCDM technique characterizes cortical morphometry in terms of the distribution of labeled distances of GM voxels from the grey matter/white matter (GM/WM) surface (Ceyhan et al., 2011, 2013; Miller et al., 2000; Ratnanather et al., 2001). Because the LCDM is derived from the distribution of distances of GM voxels from the GM/WM surface over the entire ROI, no averaging of individual surface-to-surface distances is required. Rather, cortical thickness is calculated as the 95th percentile of the GM voxel distribution (Harms et al., 2010; Ratnanather et al., 2013). This percentile cutoff excludes excludes the most distant GM voxels which have the highest probability of falling beyond the cortex and resulting in a misclassification of the tissue (see Supplemental figure 1).
Measures of whole brain cortical thickness and volume, both of which were used as covariates for VMPFC thickness in statistical analyses, were generated separately using Freesurfer 5.1. See supplement for detailed QC criteria.
All VMPFCs analyzed were from participants with two MPRAGES passing image QC. In scans with two available MPRAGES (180 of 199 eligible participants), 83% of VMPFC ROIs passed QC of the VMPFC tissue segmentation by a trained rater (see supplement for details on QC of the VMPFC tissue segmentation). Participants were excluded if either (or both) right or left VMPFC failed (n=41), leaving n=139 participants. Ten additional participants were excluded for failing QC of Freesurfer whole brain measures, leaving a final total of n=129 participants for analysis. See supplement for information regarding exclusions by diagnostic groups.
Statistical Analyses
To identify significant covariates of VMPFC thickness, Pearson’s correlations for continuous variables or t-tests or univariate analysis of variance (ANOVA) for categorical variables were conducted separately for right and left VMPFC thickness. Covariates were included in subsequent analyses if significant for either right or left VMPFC thickness. Tested variables included gender, age at scan, pubertal status (prepubertal/pubertal), handedness (right/non-right), and use of psychotropic medication (yes/no) within 48 hours of scan. Average whole brain cortical thickness (WBT) and whole brain volume (WBV) were included as covariates in all subsequent analyses to control for differences related to overall cortical thickness or scaling effects from WBV.
Repeated measures univariate analysis of covariance (ANCOVA) was used to test whether school-age children’s right or left VMPFC thickness differed as a function of PO-MDD diagnosis. Cortical thickness served as the dependent variable, with PO-MDD diagnosis (yes/no) and hemisphere (right/left) serving as independent variables. In these analyses, hemisphere was inputted as the repeated measure, since variability in right and left VMPFC thickness are expected to covary within a subject and would therefore not be independent measures. Effects on right or left VMPFC were examined as a function of the interaction between diagnosis and hemisphere. Additional repeated measures ANCOVAs examined specificity of the hypothesized effect of PO-MDD on left and right VMPFC relative to other psychiatric diagnoses. Separate analyses used 2-level independent variables to test differences in VMPFC thickness predicted by diagnoses of 1) PO-MDD vs. Healthy and 2) PO-MDD vs OPD. In another set of analyses, PO-Anxiety (yes/no) and/or PO-Externalizing (yes/no) were included as covariates to test their effect on the interaction between independent variables of diagnosis (PO-MDD yes/no) and hemisphere and on VMPFC thickness (dependent variable).
Hierarchical regression analyses were used to investigate the relationship between the independent variables of PO-MDD symptom severity and school-age depressive symptom severity, with the dependent variables of either right or left VMPFC thickness. School-age depressive symptom severity was entered first into the model, prior to adding PO-MDD severity in the next step.
Results
Participant Characteristics
Participants with PO-MDD showed no significant differences in age at time of scan, gender, pubertal status, income, caregiver education, or ethnicity vs. children in the Healthy and OPD groups (Table 1). Children with PO-MDD displayed greater use of psychotropic medications (χ2=5.290, p=.021) and lower IQ (t=−2.041, p=.047) than the Healthy group only. Children with PO-MDD showed a trend toward a greater proportion of right-handedness compared to both groups (PO-MDD vs. Healthy: χ2=3.499, p=.085; PO-MDD vs. OPD: χ2=4.524, p=.057). Because handedness and IQ differed across samples, these variables were examined in subsequent covariate analyses.
Table 1.
Participant Characteristics
| Healthy n=58 | Psychiatric Comparison n=37 | PO-MDD n=34 | |
|---|---|---|---|
| Age in Years at Scan; Mean (SD) | 9.78 (1.43) | 9.81 (1.33) | 9.71 (1.12) |
| Age range (years) | 7–12 | 7–12 | 7–11 |
| Gender; %Female (n) | 52% (30) | 46% (17) | 44% (15) |
| Pubertal Status; %Pre-pubertal (n) | 52% (30) | 54% (20) | 56% (19) |
| IQ; Mean (SD) | 110 (13.69)* | 108 (13.21) | 103 (15.48) |
| Income; %<60K (n) | 57% (33) | 43% (16) | 65% (22) |
| Caregiver Education; %< 4yr Degree (n) | 47% (27) | 35% (13) | 53% (18) |
| Ethnicity; %Non-White (n) | 43% (25) | 38% (14) | 44% (15) |
| Handedness; %Right Handed (n) | 85% (49) | 81% (30) | 97% (33) |
| Psychotropic within 48 hours of Scan; % (n) | 0% (0)* | 5.4% (2) | 8.8% (3) |
| PO-MDD Severity; Mean (SD) | .05 (.05)** | .10 (.05)** | .23 (.11) |
| SA-MDD Severity; Mean (SD) | .04 (.03)** | .10 (.06)** | .17 (.12) |
| Preschool-Onset Internalizing (n) | 0 | 12 | 17 |
| GAD | 0 | 5 | 15 |
| SAD | 0 | 7 | 13 |
| PTSD | 0 | 2 | 7 |
| Preschool-Onset Externalizing (n) | 0 | 18 | 17 |
| ADHD | 0 | 9 | 10 |
| ODD | 0 | 10 | 14 |
| CD | 0 | 8 | 10 |
| CDI-Child Total T-Score Day of Scan | 41 (5.33) | 42(6.69) | 44 (8.27) |
| CDI-Parent Total T-Score Day of Scan | 42 (6.64)** | 46 (6.80)* | 50 (9.34) |
indicates p<.001 vs. PO-MDD group.
indicates p<.05 vs. PO-MDD group.
Covariate Analyses
Results indicated that neither right nor left VMPFC cortical thickness differed significantly in relation to children’s gender, age at time of scan, pubertal status, handedness, IQ, and/or recent use of psychotropic medication (within 48 hours of scan). Thus, only average whole brain cortical thickness (WBT) and whole brain volume (WBV) were included in all analyses.
Effects of PO-MDD
A repeated measures ANCOVA tested whether school-age children’s left and/or right VMPFC thickness differed relative to their prior diagnosis of PO-MDD. Results indicated a significant interaction of prior diagnosis of PO-MDD and hemisphere on VMPFC cortical thickness, Wilks’ Lambda=.943, F(1, 125)=7.531, p=.007, with a small effect size (d=.24). Univariate follow-up analyses indicated that school children previously diagnosed with PO-MDD had significantly thinner right VMPFC, F(1, 125)=5.97, p=.016, compared to those without a prior diagnosis of PO-MDD (Figure 2). There was no significant difference in left VMPFC thickness relative to PO-MDD, F(1, 125)=0.042, p=.838. The result for right VMPFC remained significant when controlling for handedness and IQ (see supplement).
Figure 2. Children with PO-MDD Display Decreased Right VMPFC thickness.
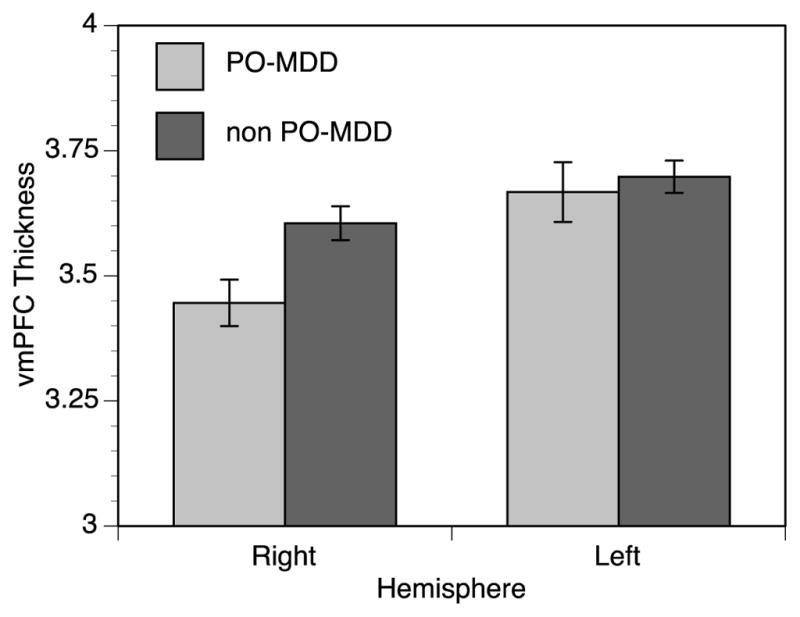
Children with PO-MDD (preschool-onset depression) showed 4.4% thinner cortex in the right VMPFC than children without PO-MDD. The left VMPFC was .85% thinner in children with PO-MDD, a difference that was not statistically significant (t=−.421, p=.638).
Specificity to PO-MDD
The analyses above compared children with PO-MDD to children without PO-MDD, a group including both healthy children (healthy) and those with other psychiatric disorders (OPD). To examine the specificity of the effect of prior PO-MDD relative to each of these groups, we conducted two additional repeated measures ANCOVAs, one comparing PO-MDD and Healthy groups and another comparing PO-MDD and OPD groups. The analysis of PO-MDD vs. Healthy again revealed a significant interaction of diagnosis and hemisphere on children’s VMPFC thickness, Wilks’ Lambda=.939, F(1, 88)=5.745, p=.019, d=.25, with the PO-MDD group showing significantly thinner right VMPFC, F(1, 88)=5.211, p=.025 (Figure 3) but not left VMPFC. Similarly, the ANCOVA comparing PO-MDD and OPD groups revealed a significant interaction of diagnosis by hemisphere on VMPFC thickness, Wilks’ Lambda =.902, F(1, 67)=7.319, p=.009, d=.31, with the PO-MDD group showing significantly thinner right VMPFC, F(1, 67)=5.028, p=.028 but not left VMPFC (Figure 3). In both analyses, results for right VMPFC remained significant when controlling for IQ and handedness (see Supplement).
Figure 3. Decreased Right VMPFC Thickness Is Specific to Children with PO-MDD.
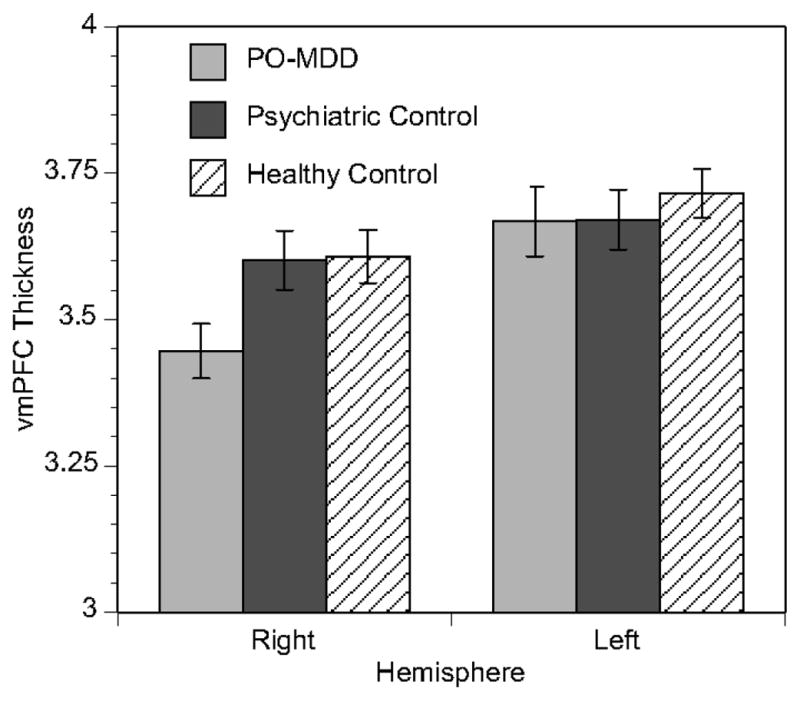
Children with preschool-onset depression (PO-MDD) showed 4.5% lower cortical thickness than healthy children and 4.3% thinner right VMPFC than children with other psychiatric disorders. Left VMPFC thickness was also lowest in the PO-MDD group, but the difference was not significant (PO-MDD vs. other: .11%, t=−.039, p=.969; PO-MDD vs. healthy: 1.3%, t=−.675, p=.501).
PO-MDD and Co-Morbidity
Structural differences in VMPFC have been associated with both anxiety (Ducharme, et al., 2013; van Tol et al., 2010) and impulsivity (Boes et al., 2009), the latter a feature of externalizing disorders. An additional repeated measures ANCOVA therefore tested whether children’s left and/or right VMPFC cortical thickness differed in relation to prior PO-MDD after covarying for a prior diagnosis of preschool-onset anxiety (PO-Anxiety) and/or externalizing disorders (PO-Externalizing). As in the previous analyses, results indicated a significant diagnosis by hemisphere effect on VMPFC cortical thickness, Wilks’ Lambda =.943, F(1, 123)=7.503, p=.007, with an effect size similar to the results above (d=.24). VMPFC cortical thickness did not differ significantly in relation to PO-Anxiety, (Wilks’ Lambda=1.000., F(1, 123)=0.543, p=.463, d=.062), and/or PO-Externalizing, (Wilks’ Lambda=.025, F(1, 123)=0.025, p=.875, d=.00), when the main effect of PO-MDD was included.
PO-MDD Severity in Relation to VMPFC Thickness
Volumetric changes in depression have been observed to be greater in individuals with more severe depression (Chen et al., 2007) and with recurrent vs. remitted depression (Salvadore, et al., 2011). Thus, we tested whether the severity of depressive symptoms during the preschool period and after the preschool period were associated with decreased thickness in VMPFC. Hierarchical multiple regressions tested whether depressive symptom scores before age 6 (PO-MDD severity) or after age 6 prior to time of scan (school-age MDD severity: SA-MDD severity) had main effects or an interaction in predicting right or left VMPFC thickness. Children’s WBT, WBV, and CDI scores at time of scan were entered into step 1 of the model, allowing these effects to be covaried at all later steps of the model. SA-MDD severity was entered into step 2, PO-MDD severity was entered into step 3, and an interaction term for PO-MDD and SA-MDD severity was entered at step 4.
At the second step of the model, SA-MDD severity, when entered without PO-MDD severity, demonstrated a negative relationship with right VMPFC thickness (R2adjusted=.123, F(5, 95)=6.31, p<.005; SA-MDD predictor: t=−2.773, p<.007).
When PO-MDD severity and SA-MDD severity were entered simultaneously at step 3, the overall model improved, R2adjusted=.18, F(6, 95)=4.486, p<.001, and PO-MDD severity had a significant main effect on children’s right VMPFC thickness, t=−2.685, p<.008. In contrast to the second step of the model, SA-MDD severity did not have a significant effect on right VMPFC thickness (t=−.649; p<.518), and a significant correlation was observed between SA-MDD severity and PO-MDD severity (r=.645, p<.001). Similarly, CDI scores at time of scan, which at the first step of the model were associated with right VMPFC thickness (child CDI: t=2.081, p=.040; parent CDI=−2.104, p=.038), showed no significant association when entered with PO-MDD severity and SA-MDD severity simultaneously (child: t=1.989, p=.050; parent: t=−.286, p=.776).
There was no significant interaction of PO-MDD and SA-MDD severity in predicting right VMPFC thickness (t=1.271, p<.207). Analyses for left VMPFC thickness found no main or interaction effect of PO-MDD and/or SA-MDD severity scores, (R2adjusted=.027, F(7, 95)=1.380, p=.224).
Discussion
This is the first report of an investigation of VMPFC cortical thickness in a population with a history of PO-MDD, a pediatric, early-onset form of depression which exhibits homotypic continuity with later-onset depression (Luby et al., 2014). In school-age children with prior PO-MDD, right VMPFC cortical thickness was decreased relative to children without PO-MDD, and this decrease also correlated with increased PO-MDD severity scores. This finding extends previous observations of cortical thinning and volumetric decreases of VMPFC in depression at older ages. By demonstrating that altered VMPFC structure, a well-replicated finding in adult depression, occurs in children with a history of PO-MDD, these results provide further neurobiological support for PO-MDD as an early form of MDD and suggest that the VMPFC mediates affective regulation from an early stage of development.
Lateralization of VMPFC Effect in PO-MDD
Although volumetric decreases in VMPFC in MDD have been most commonly observed on the left, meta-analyses have discerned a smaller, similar effect in the right VMPFC (Hajek, et al., 2008). Work in rodents has implicated the right VMPFC in the regulation of the HPA axis (Sullivan and Gratton, 1999), suggesting a mechanism whereby right VMPFC dysfunction could promote depression. Literature on pediatric MDD also provides some evidence for a role of the right VMPFC. One study examining the effects of age of onset of MDD on brain volumetric differences found that right ACC volumes were lower in participants with onset prior to age 18 (van Tol, et al., 2010). Pediatric studies of structural and resting state functional connectivity of VMPFC with limbic structures have demonstrated a predominance of effects on the right. One study of adolescents with MDD demonstrated lower fractional anisotropy in the WM tract connecting subgenual ACC to amygdala in the right hemisphere (Cullen et al., 2009). Resting state functional connectivity studies have shown that connectivity of right ACC with midline structures is disrupted in adolescent MDD (Cullen, et al., 2009) and in children with prior PO-MDD (Gaffrey, et al., 2012). Hence, this finding contributes to a growing body of work suggesting that disruption of right VMPFC, in addition to left VMPFC, is a potential biomarker of depression, particularly in early-onset forms of the disorder.
VMPFC Effect Is Unrelated to PO-Anxiety or PO-Externalizing
Children in this sample, both with and without PO-MDD, demonstrated internalizing and externalizing symptoms. The VMPFC has been implicated not only in emotional regulation, but also impulsivity (Boes, et al., 2009) and anxiety (Ducharme, et al., 2013; van Tol, et al., 2010), and altered VMPFC structure has been associated with these behavioral characteristics. However, the lack of an effect of internalizing or externalizing disorders supports a primary relationship between decreased right VMPFC thickness and depression, while minimizing the potential confound of comorbidity. Nonetheless, future research in children with primary disorders other than PO-MDD is needed to confirm the specificity of this finding to depression.
Specificity of PO-MDD Symptoms vs. Later Depressive Symptoms on VMPFC
We found that PO-MDD symptom severity, in addition to diagnosis, predicted decreases in right VMPFC thickness. In keeping with previous data showing a relationship between childhood subclinical depressive symptoms and right VMPFC thickness (Ducharme, et al., 2013), later school-age depressive symptoms were also negatively associated with right VMPFC thickness, although this was not significant after accounting for PO-MDD symptoms. Because this population was oversampled for PO-MDD, our finding does not address whether children with school-age onset of depressive symptoms also demonstrate decreases in right VMPFC thickness, an important question for future research.
There was also no interaction between PO-MDD severity and SA-MDD severity, which we had expected given the association between depression severity, symptom recurrence, and increased disturbances in brain structure (Chen, et al., 2007; Salvadore, et al., 2011). Although later timepoints are needed to determine whether structural changes related to SA-MDD symptoms arise later, our findings suggest an interpretation in which the right VMPFC appears more sensitive to cortical thinning relative to depressive symptoms during early life. This parallels a recent study showing that VMPFC lesions acquired in childhood, but not adulthood, disrupt aspects of socioemotional competency (Taber-Thomas, et al., 2014). It also dovetails with longitudinal studies in healthy children demonstrating that the relationship between VMPFC structure and depressive symptoms (Ducharme, et al., 2013) as well as VMPFC connectivity to limbic neurocircuitry (Gee et al., 2013), undergo substantial shifts throughout childhood. Hence, our findings may highlight the evolving function and relative sensitivity of VMPFC during early development. Future studies with larger sample sizes and designs to specifically test the relationship between age of onset and depression severity are now needed.
Limitations
While strengths of this study include longitudinal follow-up of depressive symptoms in a relatively large, unique sample, there are several limitations. Children with PO-MDD were imaged years after the onset of their symptoms, so we cannot establish whether decreased right VMPFC thickness is a vulnerability marker of MDD, a consequence of MDD, or a marker of remitted MDD. Nevertheless, given the young age of the sample, our findings are consistent with the notion that abnormalities of the VMPFC and related neurocircuitry occur early in development. Children were also imaged close to puberty, a stage in which relationships between VMPFC thickness and depressive symptoms may reverse (Ducharme, et al., 2013). Puberty was not significant in our covariate analysis, yet it is possible that development associated with puberty limited our ability to detect differences in VMPFC thickness. Another limitation regards the use of a parent-report diagnostic assessment at earlier waves of the study, in contrast to a combined parent/child-report version of the assessment at later waves. This approach, although standard for developmental studies in which children are too young to provide informative self-report of mood symptoms (Bird et al., 1992), is vulnerable to decreased reliability. Reassuringly, in this sample, the correlation between PO-MDD and SA-MDD symptoms was relatively strong (r=.645, p=.000), and previous work from our group has demonstrated stability of PO-MDD through school age using these reporter methods (Luby et al., 2014). Finally, the imaging results reported here, while incorporating longitudinal behavioral analysis, are cross-sectional. Longitudinal analyses that include children closer to the onset of PO-MDD and after puberty would be helpful to substantiate our interpretations.
Conclusion
These results support the role of the VMPFC in the pathophysiology of MDD earlier during development than has been previously shown. They are consistent with an altered developmental trajectory of the VMPFC in PO-MDD, which could relate to a prior, persistent neurodevelopmental deviation or an “atrophying effect” on VMPFC. Because the VMPFC is implicated in the regulation of emotions and stress, its early disruption could cumulatively compromise these functions throughout the lifespan. This concern, together with the inherent plasticity of the VMPFC, underscores the importance of early identification and intervention in mood disorders. Future longitudinal studies examining the neurodevelopment of this region during adolescence and young adulthood will be critical to guiding the implementation and assessment of such interventions.
Supplementary Material
Highlights.
Structure of ventromedial prefrontal cortex (VMPFC) is often altered in depression.
Children with prior preschool-onset depression (PO-MDD) show thinner right VMPFC.
Other preschool-onset behavioral disorders do not predict VMPFC thickness.
More preschool, but not school-age, depressive symptoms predict thinner right VMPFC.
PO-MDD is associated with disrupted VMPFC structure during development.
Acknowledgments
Role of the Funding Source: This work, including participant recruitment, data collection, and analysis, was supported by National Institute of Mental Health Awards MH064769 to J.L.; MH090786 to J.L., K.B., and D.B.; and P41 EB015909 to M.M. and T.R. N.M. received post-doctoral research support under 5T32MH100019.
We wish to thank the children and families for their participation in the study. This work was supported by National Institute of Mental Health Awards MH064769 to J.L.; MH090786 to J.L., K.B., and D.B.; and P41 EB015909 to M.M. and T.R. N.M. received support under 5T32MH100019.
Abbreviations
- VMPFC
ventromedial prefrontal cortex
- PO-MDD
preschool-onset depression
Footnotes
Contributors:
Natasha Marrus: analysis and interpretation of the data; preparation, review, and approval of the manuscript
Andrew Belden: analysis and interpretation of the data; preparation, review, and approval of the manuscript
Tomoyuki Nishino: collection and management of the data; preparation, review, and approval of the manuscript
Ted Handler: collection of the data; preparation, review, and approval of the manuscript
Tilak Ratnanather: analysis and interpretation of the data; preparation, review, and approval of the manuscript
Michael Miller: analysis and interpretation of the data; review and approval of the manuscript
Deanna Barch: design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, and approval of the manuscript
Joan Luby: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript
Kelly Botteron: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript
Conflicts of Interest: None. The authors (N.M., A.B., T.N., T.H., T.R., M.M., D.B., J.L., and K.B.) confirm that there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angold A, Costello EJ. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C) Psychological Medicine. 1995;25(4):755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cognitive Science. 2005;9(4):159–162. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(1):78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience. 2009;4(1):1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry. 2002;51(4):342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Ceyhan E, Hosakere M, Nishino T, Alexopoulos J, Todd R, Botteron KN, Millier MI, Ratnanather JT. Statistical Analysis of Cortical Morphometrics Using Pooled Distances Based on Labeled Cortical Distance Maps. Journal of Mathematical Imaging and Vision. 2011;40(1):20–35. doi: 10.1007/s10851-010-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, Bullmore E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological Psychiatry. 2007;62(5):407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. American Journal Psychiatry. 2005;162(9):1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, Evans AC, Karama S. Anxious/Depressed Symptoms are Linked to Right Ventromedial Prefrontal Cortical Thickness Maturation in Healthy Children and Young Adults. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11(8):1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Fallucca E, MacMaster FP, Haddad J, Easter P, Dick R, May G, Stanley JA, Rix C, Rosenberg DR. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Archives of General Psychiatry. 2011;68(5):527–533. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. Journal of Affective Disorders. 2011;133(3):537–545. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. Journal Psychiatric Research. 2011;45(6):803–807. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Kozeny J, Kopecek M, Alda M, Hoschl C. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. Journal of Psychiatry and Neuroscience. 2008;33(2):91–99. [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, Ratnanather JT, Miller MI, Barch DM, Csernansky JG. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. British Journal of Psychiatry. 2010;196(2):150–7. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29(5):952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal Computer Assisted Tomography. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jarnum H, Eskildsen SF, Steffensen EG, Lundbye-Christensen S, Simonsen CW, Thomsen IS, Frund ET, Theberge J, Larrson EM. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatrica Scandinavica. 2011;124(6):435–446. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: A systematic review. Neuroimage Clinical. 2013;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik-Henry MS, Wang L, Barch DM, Harms MP, Campanella C, Csernansky JG. Medial temporal lobe structure and cognition in individuals with schizophrenia and in their non-psychotic siblings. Schizophrenia Research. 2012;138:128–35. doi: 10.1016/j.schres.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lee NA, Priebe CE, Miller MI, Ratnanather JT. Validation of Alternating Kernel Mixture Method: Application to Tissue Segmentation of Cortical and Subcortical Structures. Journal of Biomedicine and Biotechnology. 2008:346129. doi: 10.1155/2008/346129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of Preschool Disorders to Full DSM Depression at School Age and Early Adolescence: Continuity of Preschool Depression. American Journal Psychiatry. 2014;171(7):768–776. doi: 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60(12):1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(8):928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. American Journal of Psychiatry. 2004;161(11):1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Archives of General Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48(8):830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller MI, Massie AB, Ratnanather JT, Botteron KN, Csernansky JG. Bayesian construction of geometrically based cortical thickness metrics. Neuroimage. 2000;12:676–87. doi: 10.1006/nimg.2000.0666. [DOI] [PubMed] [Google Scholar]
- Miller MI, Hosakere M, Barker AR, Priebe CE, Lee N, Ratnanather JT, Wang L, Gado M, Morris JC, Csernansky JG. Labeled cortical mantle distance maps of the cingulate quantify differences between dementia of the Alzheimer type and healthy aging. Proceedings National Academy Science USA. 2003;100:15172–7. doi: 10.1073/pnas.2136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings National Academy Science USA. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams RB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proceedings National Academy Sciences USA. 2009;106(15):6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cognitive Science. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Poynton CB, Pisano DV, Crocker B, Postell E, Cebron S, Ceyhan E, Honeycutt NA, Mahon PB, Barta PE. Morphometry of superior temporal gyrus and planum temporale in schizophrenia and psychotic bipolar disorder. Schizophrenia Research. 2013;150:476–483. doi: 10.1016/j.schres.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnanather JT, Cebron S, Ceyhan E, Postell E, Poynton CB, Pisano DV, Crocker B, Honeycutt NA, Mahon PB, Barta PE. Cortical thickness of superior temporal gyrus and planum temporale in schizophrenia and psychotic bipolar disorder. Frontiers in Psychiatry. 2014;5:94. doi: 10.1016/j.schres.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37(10 Part 2):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA, Jr, Drevets WC. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011;54(4):2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Brechbühle C, Székely G, Gerig G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE Transactions on Medical Imaging. 2000;19(3):153–165. doi: 10.1109/42.845174. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. Journal of Neuroscience. 1999;19(7):2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber-Thomas BC, Asp EW, Koenigs M, Sutterer M, Anderson SW, Tranel D. Arrested development: early prefrontal lesions impair the maturation of moral judgement. Brain. 2014;137(Pt 4):1254–1261. doi: 10.1093/brain/awt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi M, Wentz J, Takayanagi Y, Schretlen DJ, Ceyhan E, Wang L, Suzuki M, Sawa A, Barta PE, Ratnanather JT, Cascella NG. Reduced anterior cingulate gray matter volume and thickness in subjects with deficit schizophrenia. Schizophrenia research. 2013;150:484–490. doi: 10.1016/j.schres.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Botteron KN. Etiology and genetics of early-onset mood disorders. Child and Adolescent Psychiatric Clinics of North America. 2002;11(3):499–518. doi: 10.1016/s1056-4993(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, Hall GB. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Research: Neuroimaging. 2013;214(3):204–211. doi: 10.1016/j.pscychresns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, VanBuchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Archives General Psychiatry. 2010;67(10):1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. Journal of Psychiatric Research. 2012;46(11):1449–1455. doi: 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Hosakere M, Trein JC, Miller A, Ratnanather JT, Barch DM, Thompson PA, Qiu A, Gado MH, Miller MI, Csernansky JG. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophrenia Research. 2007;93:66–78. doi: 10.1016/j.schres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.