Abstract
Context
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide, and have been linked to acute interstitial nephritis. Less is known about the relationship between PPI use and chronic kidney disease (CKD).
Objective
To quantify the association between PPI use and incident CKD in a population-based cohort.
Design, Setting and Participants
10,482 participants in the Atherosclerosis Risk in Communities (ARIC) study with an estimated glomerular filtration rate (eGFR) of ≥60mL/min/1.73m2 were followed from a baseline visit (1996–1999) to December 31, 2011. Findings were replicated in an administrative cohort of 248,751 patients with eGFR ≥60mL/min/1.73m2 from Geisinger Health System.
Exposure
Self-reported PPI use in ARIC, or an outpatient PPI prescription in the replication cohort. Histamine-2 receptor (H2) antagonist use was considered a negative control and active comparator.
Main Outcome Measure
Incident CKD, using diagnostic codes indicating CKD at hospital discharge or death. In the replication cohort, incident CKD was defined by outpatient eGFR <60 mL/min/1.73 m2.
Results
Compared to non-users, PPI-users were more often white, obese, and taking antihypertensive medication. In ARIC, PPI use was associated with incident CKD in unadjusted analysis (hazard ratio [HR], 1.45; 95% confidence interval [CI], 1.11–1.90), analysis adjusted for demographic, socioeconomic, and clinical parameters (HR, 1.50; 95% CI, 1.14–1.96), and in analysis with PPI ever-use modeled as a time-varying variable (adjusted HR, 1.35; 95% CI, 1.17–1.55). The association persisted when baseline PPI users were compared directly to H2-antagonist users (adjusted HR, 1.39; 95% CI, 1.01–1.91), and to propensity-score matched non-users (HR, 1.76; 95% CI, 1.13–2.74). In the replication cohort, PPI use was associated with CKD in all analyses, including a time-varying new user design (adjusted HR 1.24; 95% CI, 1.20–1.28). Twice-daily PPI dosing was associated with a higher risk (adjusted HR, 1.46; 95% CI, 1.28–1.67) than once-daily dosing (adjusted HR, 1.15; 95% CI, 1.09–1.21).
Conclusions
PPI use is associated with a 20%–50% higher risk of incident CKD. Future research should evaluate whether limiting PPI use reduces the incidence of CKD.
INTRODUCTION
Chronic kidney disease (CKD) affects approximately 13.6% of adults in the US,1 is associated with a substantially increased risk of death and cardiovascular events,2 and accounts for a disproportionately large burden on Medicare’s financial resources.1 The increasing prevalence of CKD in the community cannot be fully explained by trends in known risk factors such as diabetes mellitus and hypertension, suggesting that other factors may contribute to the disease process.3, 4 Medications may be a potential factor, particularly given trends towards polypharmacy.5 Identifying iatrogenic risk factors for CKD may help to promote the rational use of medications and reduce the burden of CKD worldwide.
Proton pump inhibitors (PPI) are one of the most commonly prescribed medications in the US, and it has been estimated that between 25% and 70% of prescriptions have no appropriate indication.6 The duration of use frequently extends beyond recommended guidelines.7, 8 There is also a trend towards PPI use in infants and children.9, 10 Since the introduction of PPIs to the US market in 1990, several observational studies have linked PPI use to uncommon but serious adverse health outcomes, including hip fractures,11 community acquired pneumonia,12 Clostridium difficile infections,13 acute interstitial nephritis (AIN),14, 15 and acute kidney injury (AKI).16–18 It is plausible that PPI use may also be a risk factor for CKD, potentially mediated by recurrent AKI19, 20 or hypomagnesemia, which has been associated with both PPI use21 and incident CKD. 22 To the best of our knowledge, no population-based studies have evaluated the relationship between PPI use and the risk of CKD.
The objective of this study was to quantify the association between PPI use and incident kidney disease in the general population. We hypothesized that PPI use is an independent risk factor for CKD, and that the use of histamine-2 receptor (H2) antagonists, another common class of medications used to treat gastroesophageal reflux disease, is not. As a secondary outcome, we also evaluated the relationship between PPI use and AKI. Analyses were performed in the Atherosclerosis Risk in Communities (ARIC) study, a long-running population-based cohort, and replicated in patients receiving care in Geisinger Health System, an integrated health system in rural Pennsylvania.
METHODS
Study Design and Setting: The ARIC Study
The ARIC study is a prospective cohort study of 15,792 adults aged between 45 and 64 years who were recruited as a population-based sample from four US communities (Forsyth, NC; Jackson, MS; suburban Minneapolis, MN; Washington County, MD). Participants attended the first visit between 1987–1990, and attended subsequent visits at 3-year intervals until their fourth visit between 1996–1999; visit 5 occurred between 2011–2013. All participants were followed through an annual telephone survey and review of community hospital discharge lists until December 31, 2011. Deaths were determined by telephone survey of alternative contacts and surveillance of local newspaper obituaries, state death lists and death certificates from the Department of Vital Statistics. Further details about the ARIC cohort have been published previously.23
Participants: The ARIC Study
For the present study, we included the 11,656 participants who attended visit 4. Urinary albumin to creatinine ratio (ACR), an important risk factor for CKD, was first collected at this visit, and only small numbers of participants reported PPI use prior to 1996. Participants missing data for either estimated glomerular filtration rate (eGFR) or ACR (N=215), or who had an eGFR <60 mL/min/1.73 m2 (N=725), were excluded. Participants with missing data for years of education, health insurance status, smoking status, body mass index (BMI), mean resting systolic blood pressure, prevalent hypertension, diabetes mellitus, cardiovascular disease, or use of antihypertensive or anticoagulant medications (N=234), were also excluded, resulting in a study population of 10,482 participants. Use of the full dataset with multiple imputation for missing variables did not change inference; thus, we used complete case analysis. The study population for the secondary outcome of AKI excluded persons with known ESRD or an eGFR <15 mL/min/1.73 m2 (N=50), and therefore included some participants with eGFR <60mL/min/1.73 m2, but was otherwise similarly constructed (N=11,145).
Measurement of Incident Kidney Disease: The ARIC Study
Incident CKD was defined by diagnostic codes that indicated CKD at hospital discharge (International Classification of Disease (ICD), Ninth Revision, Clinical Modification, ICD-9-CM) or death (Tenth Revision, ICD-10-CM), or incident ESRD, as determined through linkage with the United States Renal Data System (USRDS) registry.24, 25 In an earlier validation study that used a ≥25% decline in eGFR to <60mL/min/1.73 m2 at a follow up outpatient visit as a reference standard for CKD, the sensitivity of diagnostic codes for defining CKD was 35.5%, and specificity was 95.7%.24 Incident acute kidney injury (AKI) was defined by hospitalization or death with ICD-9-CM or ICD-10-CM diagnostic codes of 584.x or N17.x, respectively.26 Participants who died before developing CKD, were lost to follow up, or had disease-free survival to the end of December 31, 2011 were censored.
Measurement of Proton Pump Inhibitor Use and Other Covariates: The ARIC Study
Use of PPI and H2-antagonists was measured at the baseline study visit through direct visual inspection of pill bottles for all medications used during the preceding 2 weeks. Exposure to antihypertensive, anticoagulant, aspirin, statin, diuretic, and non-steroidal anti-inflammatory medications was measured in the same way. Subsequent exposure to PPI and H2-antagonists was obtained as part of annual telephone follow-up, which included questions about medication use starting in September 2006. At each telephone follow-up from 2006, participants were asked to assemble all medications they were taking and to “read the names of all the medications prescribed by a doctor”.
Baseline plasma and urinary creatinine were measured by the modified kinetic Jaffé method.24 The equation developed by the CKD Epidemiology Collaboration was used to calculate eGFR.27 Urinary albumin was measured using either the Dade Behring BN100 or Beckman IMMAGE Nephelometer.24 Three domains of socioeconomic status were measured: self-reported highest level of education, health insurance status, and household income in the previous 12 months. Cigarette smoking status was defined categorically as current, former or never smoker at baseline, and body mass index (BMI) was derived from the weight in kilograms divided by the height in meters squared. Prevalent hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or self-reported use of antihypertensive medication within the past 2 weeks. Prevalent diabetes mellitus was defined by a fasting blood glucose concentration ≥126 mg/dL, random glucose level of ≥200 mg/dL, self-report of a physician diagnosis of diabetes, or reported use of medication for diabetes in the last 2 weeks. Prevalent cardiovascular disease was defined as a composite outcome of prevalent coronary heart disease or stroke at visit 4.
Replication Cohort: Geisinger Health System
The replication cohort consisted of 248,751 patients with an outpatient eGFR ≥60 mL/min/1.73 m2 receiving care between February 13, 1997 and October 9, 2014 in Geisinger Health System, a large rural healthcare system in central and northeastern Pennsylvania. Participants were selected at the earliest time-point when they had both creatinine and systolic blood pressure available. Incident CKD was defined as the first outpatient eGFR <60 mL/min/1.73 m2 that was sustained at all subsequent assessments of eGFR, or the development of ESRD, which was ascertained through linkage to the US Renal Data System registry. Incident AKI was defined as an ICD-9-CM code of 584.x, and death was ascertained through linkage to the National Death Index. Individuals who did not develop the outcome of interest were censored at their last follow-up or death. Medication use was determined by prescriber prescription within 90 days prior to baseline. The frequency of PPI use was categorized as once daily or twice daily according to the prescription, and assumed to be once daily if not specified. Comorbidities were captured by inpatient and outpatient billing codes.
Statistical Analysis
Baseline characteristics of PPI-users and non-PPI users were compared using t-tests for continuous variables and chi-squared tests for categorical variables. The Wilcoxon rank-sum test was used for continuous variables that were not normally distributed. Cox proportional hazards regression was used to estimate the hazard ratio and 95% confidence intervals of incident CKD associated with PPI use. The proportional hazards assumption was tested using Schoenfeld residuals. Exposure to PPI was modeled as a binary variable at baseline and, in secondary analyses, as a time-varying ever-use variable, in which a participant was considered an ever-user at the first instance of PPI use and at all time points thereafter. In ARIC, time-varying PPI use represented baseline use with updates in 2006 and yearly thereafter; in the replication cohort, it was evaluated by assessing all provider prescriptions throughout the study period. In ARIC, adjustment was performed for demographic variables (age, sex, race and study center), socioeconomic status (health insurance status and highest level of education), clinical measurements (baseline eGFR, logarithm of ACR, cigarette smoking, mean systolic blood pressure, BMI), prevalent comorbidities (diabetes, cardiovascular disease) and concomitant use of medications (antihypertensive medications and anticoagulant medications). Household income and concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, diuretic, or statin medications were considered to be possible confounders a priori; however, they did not affect the results of adjusted analyses and thus were not included in the final model. In the replication cohort, fewer comorbidities were available; thus, analyses were adjusted for age, sex, race, eGFR, smoking status, BMI, systolic blood pressure, diabetes, history of cardiovascular disease, antihypertensive medication use, anticoagulant medication use, and statin, aspirin, and NSAID use. Subgroup analyses were performed, stratified by median age, sex, race (in ARIC only), diabetes, and medication status. In Geisinger, the risk of CKD was also evaluated in “once-daily” and “twice-daily” PPI-users. Similar analyses were performed for the secondary outcome of AKI. Absolute risk differences were estimated as the difference between the expected 10-year risk among PPI users and the expected 10-year risk had they not used PPIs.
Five sensitivity analyses were performed. First, the study population was limited to participants using H2-antagonists or PPIs, and the risk of kidney disease associated with PPI use was assessed using H2-antagonists as the active comparator. Second, the association between PPI use and incident kidney disease was examined in a propensity-score matched cohort, where logistic regression was used to estimate the probability of PPI use based on observable predictors of PPI use, and non-PPI-using controls were selected using 1:1 nearest neighbor matching. Third, a new-user design was used, whereby the risk associated with time-varying ever PPI use was assessed only among persons not using PPI at baseline.28 Given that new use was not available until 2006 in ARIC, this analysis was performed only in the replication cohort. Fourth, the associations between H2-antagonist use and incident kidney disease were assessed as a negative control. Fifth, persons with baseline ACR >30 mg/g (or, in Geisinger, 1+ protein on dipstick) were excluded from the study population. All analyses were performed using Stata/IC, version 13.1 (StataCorp, LP).
RESULTS
Study Population
In ARIC, 10,482 participants were followed for a median of 13.9 years. In the validation cohort, 248,751 participants were followed for a median of 6.2 years. At baseline in both cohorts, PPI-users were more likely to have a higher BMI and take antihypertensive, aspirin or statin medications than non-users (Table 1). H2-antagonist users showed similar characteristics to PPI-users. The prevalence of ever-use of PPIs increased substantially during the follow up period (Figure 1).
Table 1.
Baseline Characteristics of Study Populations: The Atherosclerosis Risk in Communities Cohort and the Replication Cohort
| Atherosclerosis Risk in Communities Cohort |
Geisinger Health System Replication Cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PPI- Users |
H2- Antagonist Users* |
Non- Users |
P- value |
PPI- Users |
H2- Antagonist Users* |
Non- Users |
P- value |
||
| N | 322 | 956 | 9,204 | 16,900 | 6,640 | 225,211 | |||
| Age, mean (SD), y | 62.8 (5.5) |
63.1 (5.5) | 62.5 (5.6) |
0.008 | 50.0 (15.9) |
50.3 (16.3) | 49.5 (16.3) |
<0.001 | |
| Male, % | 42.5 | 39.3 | 44.4 | 0.01 | 43.2 | 42.6 | 43.5 | 0.32 | |
| White, % | 86.0 | 84.2 | 77.9 | <0.001 | 94.6 | 96.4 | 95.5 | <0.001 | |
| Education ≥ 12 years, % |
81.7 | 79.4 | 81.8 | 0.18 | NA | NA | NA | ||
| Health insurance, % | 92.2 | 88.9 | 85.6 | <0.001 | NA | NA | NA | ||
| Annual household income, % | 0.22 | ||||||||
| ≥$25,000 | 72.0 | 66.4 | 66.2 | NA | NA | NA | |||
| <$25,000 | 23.6 | 29.7 | 29.7 | NA | NA | NA | |||
| No response | 4.3 | 3.9 | 4.2 | NA | NA | NA | |||
| eGFR, mean (SD), mL/min/1.73m2 |
87.8 (13.4) |
86.5 (13.5) | 88.9 (13.1) |
<0.001 | 94.9 (17.7) |
95.2 (18.2) | 96.0 (18.0) |
<0.001 | |
| ACR, median (IQR), mg/g |
4.0 (5.6) |
3.6 (5.3) | 3.7 (5.8) |
0.71 | NA | NA | NA | ||
| Cigarette smoking, % |
0.23 | <0.001 | |||||||
| Current | 11.5 | 15.5 | 15.2 | 25.7 | 26.1 | 23.9 | |||
| Former | 48.4 | 44.2 | 43.2 | 26.4 | 25.4 | 23.9 | |||
| Never | 40.1 | 40.3 | 41.6 | 47.9 | 48.5 | 52.2 | |||
| Body Mass Index, median (IQR), kg/m2 |
28.8 (5.6) |
28.7 (6.7) | 27.8 (6.7) |
<0.001 | 30.8 (7.3) |
30.8 (7.4) | 30.2 (7.1) |
<0.001 | |
| Mean systolic blood pressure, mean (SD), mmHg |
126.5 (18.3) |
128.2 (18.6) |
127.0 (18.8) |
0.16 | 126.4 (15.8) |
128.2 (16.7) |
128.0 (17.7) |
<0.001 | |
| Prevalent medical condition, % |
|||||||||
| Hypertension | 54.3 | 50 | 44.8 | <0.001 | 33.3 | 34.0 | 30.2 | <0.001 | |
| Diabetes mellitus | 14.9 | 18 | 15.6 | 0.14 | 10.8 | 9.7 | 10.4 | 0.06 | |
| Cardiovascular disease |
13.7 | 14.1 | 10.8 | 0.003 | 11.3 | 11.8 | 8.7 | <0.001 | |
| Medication use, % | |||||||||
| Antihypertensive | 55.3 | 48.5 | 39.9 | <0.001 | 32.0 | 31.3 | 20.6 | <0.001 | |
| ACE-I or ARB | 16.8 | 13.4 | 12.9 | 0.12 | 15.5 | 13.4 | 9.6 | <0.001 | |
| Diuretics | 16.1 | 12.1 | 9.6 | <0.001 | 13.8 | 12.6 | 8.3 | <0.001 | |
| Aspirin | 64.9 | 67.6 | 54.9 | <0.001 | 7.8 | 5.9 | 3.9 | <0.001 | |
| NSAID | 27.6 | 32.8 | 33.2 | 0.11 | 13.9 | 14.4 | 9.5 | <0.001 | |
| Statin | 20.2 | 13.6 | 10.3 | <0.001 | 13.9 | 11.7 | 6.1 | <0.001 | |
| Anticoagulant | 1.9 | 2.8 | 1.7 | 0.04 | 2.5 | 2.9 | 1.1 | <0.001 | |
Abbreviations: PPI, proton pump inhibitor; H2, histamine 2 receptor; SD, standard deviation; NC, North Carolina; MD, Maryland; MN, Minnesota; MS, Mississippi; NA, data not available; eGFR, estimated glomerular filtration rate; ACR, urinary albumin creatinine ratio; IQR, inter-quartile range; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSAID, non-steroidal anti-inflammatory drug
For the purposes of this table, participants using both a PPI and an H2-antagonist were classified as PPI users. In ARIC, this represented 24 of the 322 PPI users; in Geisinger, this represented 815 of the 6,640 PPI users.
Figure 1.
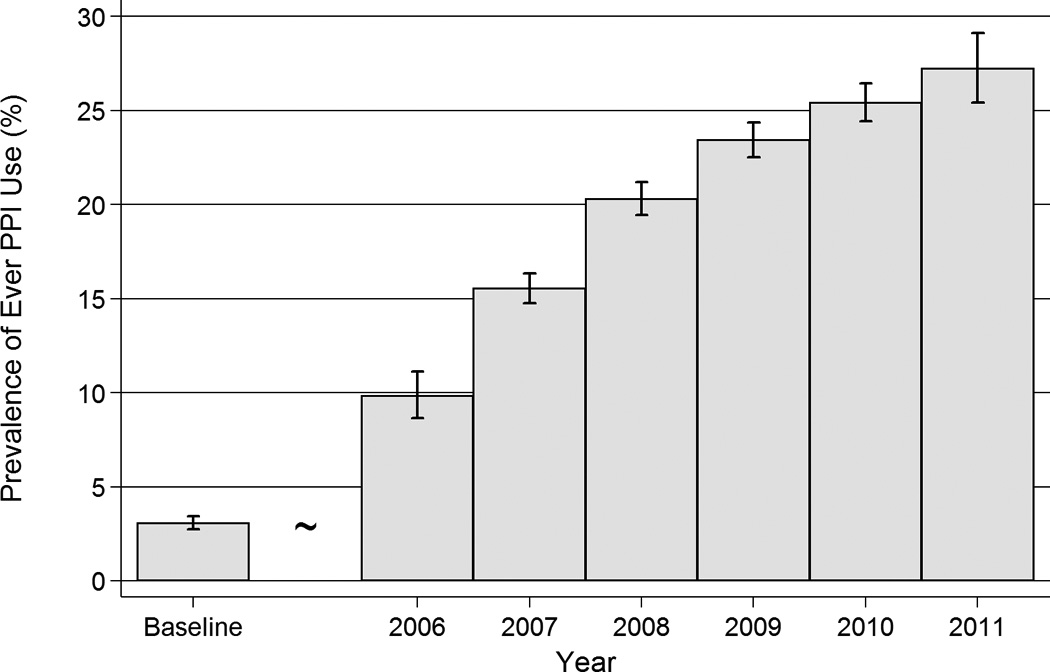
Prevalence of Ever Proton Pump Inhibitor Use Over Time in the Atherosclerosis Risk in Communities Cohort
Abbreviations: PPI, proton pump inhibitor
Association of PPI Use With Kidney Disease in ARIC
In ARIC, there were 56 incident CKD events among the 322 baseline PPI-users (14.2 per 1,000 person-years), and 1,382 events among 10,160 baseline non-users (10.7 per 1,000 person-years). In unadjusted analysis, participants who used PPIs at baseline had 1.45-times (95% confidence interval [CI], 1.11–1.90; P=.006) the risk of incident CKD relative to non-users (Table 2). The risk after adjustment for potential confounders, including demographics, socioeconomic status, clinical measurements, prevalent comorbidities and concomitant use of medications, was similar (HR, 1.50; 95% CI, 1.14–1.96; P=.003), as was the association when PPI use was modeled as a time-varying ever-use variable (HR, 1.35; 95% CI, 1.17–1.55; P<.001). Subgroup analyses were consistent with the primary results (Figure 2). The 10-year estimated absolute risk of CKD among the 322 baseline PPI users was 11.8%; the expected risk had they not used PPIs was 8.5% (absolute risk difference, 3.3%).
Table 2.
Proton Pump Inhibitor Use and Risk of Incident Chronic Kidney Disease
| Atherosclerosis Risk in Communities Cohort (N=10,482) |
Geisinger Health System Replication Cohort (N=248,751) |
|||
|---|---|---|---|---|
| Events | Total N | Events | Total N | |
| PPI-users | 56 | 322 | 1,921 | 16,900 |
| H2-antagonist users | 158 | 956 | 1,022 | 6,640 |
| Non PPI-, Non-H2-antagonist users | 1,224 | 9,204 | 27,204 | 225,221 |
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Associations between PPI use and Incident CKD | ||||
| Unadjusted Baseline PPI-use (Ref: No PPI-use) |
1.45 (1.11, 1.90) | 0.006 | 1.20 (1.15, 1.26) | <.001 |
| Adjusted Baseline PPI-use (Ref: No PPI-use) |
1.50 (1.14, 1.96) | 0.003 | 1.17 (1.12, 1.23) | <.001 |
| Time-varying Ever PPI use (Ref: No PPI-use) |
1.35 (1.17, 1.55) | <.001 | 1.22 (1.19–1.25) | <.001 |
| Baseline PPI-use (Ref: Baseline H2-antagonist use) |
1.39 (1.01, 1.91) | 0.05 | 1.29 (1.19–1.40) | <.001 |
| Propensity-score matched Baseline PPI use (Ref: No PPI-use) |
1.76 (1.13, 2.74) | 0.01 | 1.16 (1.09–1.24) | <.001 |
| Time-varying New PPI-use (Ref: No PPI-use) |
NA | NA | 1.24 (1.20, 1.28) | <.001 |
| Negative control | ||||
| Baseline H2-antagonist use (Ref: No H2-antagonist use) |
1.15 (0.98,1.36) | 0.10 | 0.93 (0.88–0.99) | 0.03 |
Abbreviations: CI, confidence interval; PPI, proton pump inhibitor; H2, histamine 2 receptor; Ref, reference group.
All analyses were adjusted unless otherwise specified. Adjustment variables for ARIC: age, sex, race, study center, education, health insurance status, baseline estimated glomerular filtration rate, urinary albumin creatinine ratio, cigarette smoking status, body mass index, systolic blood pressure, diabetes, cardiovascular disease, antihypertensive medication use, and anticoagulant medication use. Adjustment variables for the replication cohort: age, sex, race, eGFR, smoking status, BMI, systolic blood pressure, diabetes, history of cardiovascular disease, antihypertensive medication use, anticoagulant medication use, and statin, aspirin, and NSAID use. Propensity score analyses were adjusted for propensity score only, which were estimated using the same variables.
Figure 2.
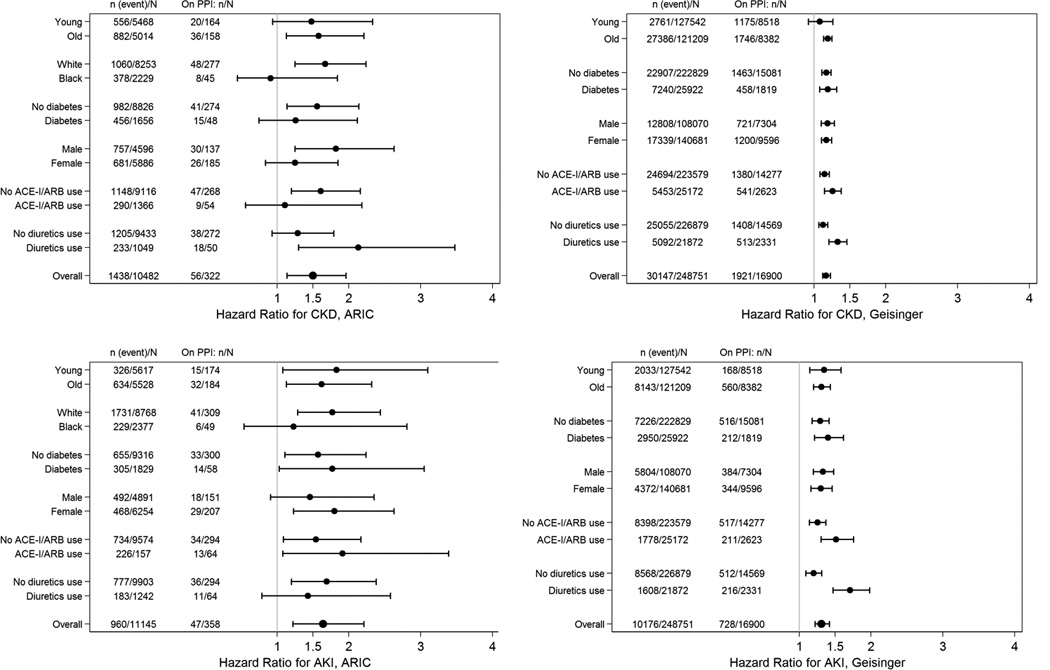
Association of Proton Pump Inhibitor Use with Incident Chronic Kidney Disease and Acute Kidney Injury, by Subgroups
Abbreviations: PPI, proton pump inhibitor; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; Young/Old refers to less than or equal or greater than the median age (62 years and 50 years in ARIC and Geisinger, respectively)
A slightly stronger association was seen between PPI use and AKI (Table 3). For example, in unadjusted analysis, participants who used PPIs at baseline had 1.72-times (95% CI, 1.28–2.30; P<.001) the risk of incident AKI relative to those who did not report use; corresponding risks were similar after adjustment for potential confounders (HR, 1.64; 95% CI, 1.22–2.21; P<.001) and when PPI use was analyzed as a time-varying ever-use variable (HR, 1.49; 95% CI, 1.25–1.77; P<.001).
Table 3.
Proton Pump Inhibitor Use and Risk of Incident Acute Kidney Injury
| Atherosclerosis Risk in Communities Cohort (N=11,145) |
Geisinger Health System Replication Cohort (N=248,751) |
|||
|---|---|---|---|---|
| Events | Total N | Events | Total N | |
| PPI-users | 47 | 358 | 728 | 16,900 |
| H2-antagonist users | 104 | 1,053 | 347 | 6,640 |
| Non PPI-, Non-H2-antagonist users | 809 | 9,734 | 9,101 | 225,211 |
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Associations between PPI use and Incident AKI | ||||
| Unadjusted Baseline PPI-use (Ref: No PPI-use) |
1.72 (1.28, 2.30) | <0.001 | 1.30 (1.21–1.40) | <.001 |
| Adjusted Baseline PPI-use (Ref: No PPI-use) |
1.64 (1.22, 2.21) | <0.001 | 1.31 (1.22–1.42) | <.001 |
| Time-varying Ever PPI use (Ref: No PPI-use) |
1.49 (1.25, 1.77) | <0.001 | 1.54 (1.47–1.60) | <.001 |
| Baseline PPI-use (Ref: Baseline H2-antagonist use) |
1.58 (1.05, 2.40) | 0.03 | 1.30 (1.13, 1.48) | <.001 |
| Propensity-score matched Baseline PPI use (Ref: No PPI-use) |
2.00 (1.24, 3.22) | 0.005 | 1.29 (1.16–1.43) | <.001 |
| Time-varying New PPI-use (Ref: No PPI-use) |
NA | NA | 1.66 (1.57–1.75) | <.001 |
| Negative control | ||||
| Baseline H2-antagonist use (Ref: No H2-antagonist use) |
1.03 (0.84, 1.26) | 0.78 | 0.98 (0.89–1.10) | 0.8 |
Abbreviations: CI, confidence interval; PPI, proton pump inhibitor; H2, histamine 2 receptor; Ref, reference group.
All analyses were adjusted unless otherwise specified. Adjustment variables for ARIC: age, sex, race, study center, education, health insurance status, baseline estimated glomerular filtration rate, urinary albumin creatinine ratio, cigarette smoking status, body mass index, systolic blood pressure, diabetes, cardiovascular disease, antihypertensive medication use, and anticoagulant medication use. Adjustment variables for the replication cohort: age, sex, race, eGFR, smoking status, BMI, systolic blood pressure, diabetes, history of cardiovascular disease, antihypertensive medication use, anticoagulant medication use, and statin, aspirin, and NSAID use. Propensity score analyses were adjusted for propensity score only, which were estimated using the same variables.
Association of PPI Use With Kidney Disease in the Replication Cohort
In the replication cohort, there were 1,921 incident CKD events among 16,900 baseline PPI users (20.1 per 1,000 person-years) and 28,226 events among 231,851 baseline non-users (18.3 per 1,000 person-years). PPI use was significantly associated with incident CKD in unadjusted analyses (HR, 1.20; 95% CI, 1.15–1.26; P<.001), adjusted analyses (adjusted HR, 1.17; 95% CI, 1.12–1.23; P<0.001), and when estimated using a time-varying ever-use model (adjusted HR, 1.22; 95% CI, 1.19–1.25; P<.001). Twice-daily PPI dosing was associated with a higher risk of CKD (adjusted HR, 1.46; 95% CI, 1.28–1.67; P<.001) than once-daily dosing (adjusted HR, 1.15; 95% CI, 1.09–1.21; P<.001). The 10-year absolute risk of CKD among the 16,900 baseline PPI users was 15.6%; the expected risk had they not used PPIs was 13.9% (absolute risk difference, 1.7%).
Similar associations were seen with incident AKI: PPI use resulted in higher risk of incident AKI in unadjusted (HR, 1.30; 95% CI, 1.21–1.40; P<.001), adjusted (HR, 1.31; 95% CI, 1.22–1.42; P<.001), and time-varying ever-use analyses (adjusted HR, 1.54; 95% CI, 1.47–1.60; P<.001). Twice-daily PPI dosing was associated with a higher risk of AKI (adjusted HR, 1.62; 95% CI, 1.32–1.98; p<.001) than once daily dosing (adjusted HR, 1.28; 95% CI, 1.18–1.39; p<.001).
Sensitivity Analyses
When compared directly to H2-antagonist use, PPI use was associated with incident CKD in ARIC (adjusted HR, 1.39; 95% CI, 1.01–1.91; P=.05) and in the replication cohort (adjusted HR, 1.29; 95% CI, 1.19–1.40; P<.001). Baseline PPI use was also associated with incident CKD in propensity-matched analyses (ARIC: HR, 1.76; 95% CI, 1.13–2.74; P=.01; Geisinger: HR, 1.16; 95% CI, 1.09–1.24; P<.001), and in the new users analysis (adjusted HR, 1.24; 95% CI, 1.20–1.28; P<.001; Table 2). H2-antagonist use was not associated with increased risk of incident CKD in either cohort (ARIC: adjusted HR, 1.15; 95% CI, 0.98–1.36; P=.10; Geisinger: adjusted HR, 0.93; 95% CI, 0.88–0.99, P=.03). Similar results were obtained when persons with baseline albuminuria were excluded (ARIC: adjusted HR, 1.45; 95% CI, 1.09–1.96; P=.01; Geisinger, adjusted HR, 1.19; 95% CI, 1.13–1.25; P<.001). Sensitivity analyses using AKI as an outcome were also consistent (Table 3).
COMMENT
In this prospective community-based cohort of over 10,000 adults, we found that baseline use of PPIs was independently associated with a 20–50% higher risk of incident chronic kidney disease, after adjusting for several potential confounding variables, including demographics, socioeconomic status, clinical measurements, prevalent comorbidities and concomitant use of medications. The observed association persisted when PPI exposure was modeled as a time-varying ever-use variable, and was replicated in a separate administrative cohort of 248,751 people. The risk was specific to PPI medications, as the use of H2-antagonists, which is prescribed for the same indication as PPIs, was not independently associated with CKD. Similar findings were demonstrated for the outcome of AKI, and collectively suggest that PPI use is an independent risk factor for both CKD and AKI.
Previous studies have also identified an association between PPI use and AKI, most specifically in the form of AIN.14–18 Our study adds to the existing literature by describing an association between PPI use and incident CKD, suggesting a 20–50% higher risk among PPI users. We note that our study is observational, and thus does not provide evidence for causality. However, a causal relationship between PPI use and CKD could have considerable public health impact given the widespread extent of use. More than 15 million Americans used prescription PPIs in 2013, costing over $10 billion.29 Studies suggest that 70% of these prescriptions are without indication,6 and that 25% of long-term PPI-users could discontinue therapy without developing symptoms.30 Indeed, there are already calls for the reduction of unnecessary use of PPIs.31
Observational cohort studies are one of the best methods to study adverse effects of medications used in “real world” settings; however, several limitations inherent in observational design must be considered. First, unlike a randomized controlled trial, participants who are prescribed PPIs may be at higher risk of CKD for reasons unrelated to their PPI use. For example, PPI-users in both ARIC and the replication cohorts were more likely to be obese, have a diagnosis of hypertension, and have a greater burden of prescribed medications. In recognition of this potential bias, we performed adjustment for multiple confounders including BMI, hypertension, diabetes and other medications, compared PPI users directly to H2-antagonist users, and conducted propensity score matched analyses. Each of these sensitivity analyses showed a consistent relationship between PPI use and a higher risk of CKD.
A second limitation of our study is the potential for surveillance bias, whereby outcome assessment might have occurred more often in persons using PPIs. In the ARIC study, incident CKD was detected using hospitalization discharge codes; in Geisinger, outpatient creatinine values were used. However, the association between PPI use and new CKD persisted after accounting for predictors of more frequent contact with the medical system, such as insurance status and comorbid illness. A third limitation is the low sensitivity of hospital discharge codes for diagnosing CKD in ARIC. However, study results were replicated in the Geisinger cohort, where CKD was defined by direct laboratory measurements. Fourth, including baseline PPI users can invoke selection bias, whereby baseline users represent a special group of PPI-users who tolerate the medication without development of CKD. In our study, there were relatively few prevalent PPI users at baseline, which should lead to less bias.32 In addition, the results were replicated in a new-user design in Geisinger, where baseline PPI users were excluded. A fifth potential limitation is that neither PPI nor H2-antagonist use was captured as directly observed therapy. In recent years, both have become available over-the-counter in the US, and thus medication exposure in both ARIC and Geisinger may have been misclassified.
Notable strengths of the ARIC study include a large representative community-based sample, a baseline visit occurring soon after PPIs were introduced into the US, visual confirmation of medications, comprehensive data pertaining to potential confounders, and close monitoring for over 13 years of follow up. Sensitivity analyses, including a time-varying exposure model, propensity-score matching, and replication in a large second cohort, showed robust results. We also demonstrated specificity to PPI use, rather than H2-antagonist use.
In summary, we found that PPI use, but not H2-antagonist use, was an independent risk factor for CKD and AKI. Further research is required to investigate whether PPI use itself causes kidney damage and, if so, the underlying mechanisms of this relationship.
Acknowledgments
MG receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK092287).
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
MG had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Contributor Information
Benjamin Lazarus, Email: blazaru1@jhu.edu.
Yuan Chen, Email: ychen178@jhu.edu.
Francis P. Wilson, Email: francis.p.wilson@yale.edu.
Yingying Sang, Email: ysang1@jhu.edu.
Alex R. Chang, Email: chaalex@gmail.com.
Josef Coresh, Email: coresh@jhu.edu.
REFERENCES
- 1.United States Renal Data System. 2014 USRDS Annual Data Report: An overview of the epidemiology of kidney disease in the United States. 2014. [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Grams ME, Juraschek SP, Selvin E, et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis. 2013 Aug;62(2):253–260. doi: 10.1053/j.ajkd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014 Jan;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008 Jan 5;336(7634):2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant K, Al-Adhami N, Tordoff J, Livesey J, Barbezat G, Reith D. Continuation of proton pump inhibitors from hospital to community. Pharm World Sci. 2006 Aug;28(4):189–193. doi: 10.1007/s11096-006-9028-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013 Jul;6(4):443–451. doi: 10.1586/17512433.2013.811206. [DOI] [PubMed] [Google Scholar]
- 9.Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007 Oct;45(4):421–427. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne P, Christiaens T, Vander Stichele R, Van Winckel M. Changes in prescription patterns of acid-suppressant medications by Belgian pediatricians: analysis of the national database, [1997–2009] J Pediatr Gastroenterol Nutr. 2014 Feb;58(2):220–225. doi: 10.1097/MPG.0b013e3182a3b04e. [DOI] [PubMed] [Google Scholar]
- 11.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006 Dec 27;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 12.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE. 2015;10(6):e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. Cmaj. 2004 Jul 6;171(1):33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014 Oct;86(4):837–844. doi: 10.1038/ki.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra F, Suarez M, Rey M, Vela MF. Systematic review: Proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007 Aug 15;26(4):545–553. doi: 10.1111/j.1365-2036.2007.03407.x. [DOI] [PubMed] [Google Scholar]
- 16.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. Cmaj. 2015;3(2):E166–E171. doi: 10.9778/cmajo.20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard CE, Freeman CP, Newcomb CW, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf. 2012 Nov;21(11):1155–1172. doi: 10.1002/pds.3329. [DOI] [PubMed] [Google Scholar]
- 19.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012 Mar;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmark L, van der Wiel HE, de Groot MC, van Grootheest AC. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol. 2007 Dec;64(6):819–823. doi: 10.1111/j.1365-2125.2007.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS ONE. 2014;9(11):e112558. doi: 10.1371/journal.pone.0112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2014 Oct 1; doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 24.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014 Aug;64(2):214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebholz CM, Coresh J, Ballew SH, et al. Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis. 2015 Mar 12; doi: 10.1053/j.ajkd.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014 Apr;9(4):682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015 Jul;11(7):437–441. doi: 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IMS Institute for Healthcare Informatics. Medicine use and shifting costs of healthcare. A review of the use of medicines in the United States in 2013. 2014 [Google Scholar]
- 30.Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006 Sep 15;24(6):945–954. doi: 10.1111/j.1365-2036.2006.03084.x. [DOI] [PubMed] [Google Scholar]
- 31.Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007 Jan;83(975):66–68. doi: 10.1136/pgmj.2006.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012 Feb 15;175(4):250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]


