Abstract
The dopamine and endocannabinoid neurotransmitter systems have been implicated in delay discounting, a measure of impulsive choice, and obesity. The current study was designed to determine the extent to which haloperidol and rimonabant affected delay discounting in rats fed standard-chow and high-fat diets. Sprague-Dawley rats were allowed to free-feed under a high-fat diet (4.73 kcal/g) or a standard-chow diet (3.0 kcal/g) for three months. Then, operant sessions began in which rats (n = 9 standard chow; n = 10 high-fat) chose between one sucrose pellet delivered immediately vs. three sucrose pellets after a series of delays. In another condition, carrot-flavored pellets replaced sucrose pellets. After behavior stabilized, acute injections of rimonabant (0.3-10 mg/kg) and haloperidol (0.003-0.1 mg/kg) were administered i.p. before some choice sessions in both pellet conditions. Haloperidol and rimonabant increased discounting in both groups of rats by decreasing percent choice for the larger reinforcer and area-under-the-curve (AUC) values. Rats in the high-fat diet condition demonstrated increased sensitivity to haloperidol compared to chow-fed controls: haloperidol increased discounting in both dietary groups in the sucrose condition,, but only in the high-fat-fed rats in the carrot-pellet condition. These findings indicate that blocking D2 and CB1 receptors results in increased delay discounting, and that a high-fat diet may alter sensitivity to dopaminergic compounds using the delay-discounting task.
Keywords: cannabinoids, dopamine, delay discounting, haloperidol, high-fat diet, obesity, rimonabant, rat
Introduction
Delay discounting, which describes a decrease in preference for a reinforcer as the delay to its receipt increases, has been implicated as a behavioral mechanism of obesity. Obese humans discount delayed reinforcers, such as money (Appelhans et al., 2011, 2012; Best et al., 2012; Fields et al., 2011; Weller et al., 2008) and food-related stimuli (Hendrickson & Rasmussen, 2013; Rasmussen et al., 2010), to a greater extent than healthy-weight controls. These findings have also been demonstrated in nonhumans, where obese rats choose smaller-sooner food reinforcers more often than lean controls (Boomhower et al., 2013).
One explanation for these differences in discounting patterns for food may be alterations (either separately or concomitantly) in the dopamine and endocannabinoid neurotransmitter systems, such as those that result from exposure to high-fat diets. For example, rodents fed a high-fat diet initially have elevated levels of dopamine-2 (D2) receptors in the nucleus accumbens compared to controls (South & Huang, 2008), after which D2-receptor levels decrease as diet exposure lengthens (i.e., after 10 to 20 weeks) (Huang et al., 2006; Johnson & Kenny, 2010). Dopamine signaling is closely involved with endocannabinoid activity (see reviews by Fitzgerald et al. 2012 and El Khoury et al. 2012) and extended exposure to high-fat diets also reduces the number of cannabinoid-1 (CB1) receptors in the rat nucleus accumbens compared to chow-fed controls (Harrold et al., 2002). Indeed, high-fat diet exposure results in increased sensitivity to both D2 and CB1 agonists (see Baladi et al., 2012) and may alter the reinforcing properties of food when exposure occurs early in life (la Fleur et al., 2007; Teegarden et al., 2010). However, no research to date has examined the contribution of D2- and CB1-specific blockade on delay discounting in rats exposed to a high-fat diet.
The dopamine neurotransmitter system is heavily involved in a variety of behavioral mechanisms related to food procurement (see Salamone et al., 2009), including delay discounting. Dopamine agonists, for example, typically decrease delay discounting by increasing preference for larger, delayed reinforcers (de Wit et al. 2002; Huskinson et al. 2012; Krebs and Anderson 2012; Wade et al. 2000) and non-selective D2/D3 antagonists (i.e., flupenthixol and raclopride) increase discounting (Wade et al., 2000). Though haloperidol (a non-selective D2 antagonist) increased choice for smaller, immediate food reinforcers in a study using a T-maze (Denk et al., 2005), other studies have found haloperidol does not affect delay discounting (e.g., Evenden & Ryan, 1996). These discrepant findings may result from procedural variables, such as floor effects in responding, that could mask drug effects. For example, in some delay conditions employed by Evenden and Ryan (1996), choice for a larger-later reinforcer under vehicle was 20% on average, which may have prevented haloperidol from reducing larger-later reinforcer choice (i.e., increasing discounting) further.
Recently, researchers have begun examining the role of cannabinoids in delay discounting (Boomhower et al., 2013; McDonald et al., 2003; Navarrete et al., 2012; Pattij et al., 2007; Wiskerke et al., 2011), though the results are complex. For example, Boomhower et al. (2013) found that rimonabant (a selective CB1 antagonist and inverse agonist) increased delay discounting in lean Zucker rats and decreased discounting in obese Zuckers, strains that differ in densities of CB1 receptors (Thanos, et al., 2008). However, other studies reported that CB1 antagonists (i.e., rimonabant or O-2050) increased discounting for food only when they were co-administered with amphetamine (Wiskerke et al., 2011). The mechanism through which rimonabant and other CB1 antagonists may alter discounting is unclear, as the potential for dopaminergic compounds to alter the behavioral effects of endocannabinoids has not been fully established. Thus, more research is needed to clarify the role of cannabinoids in delay discounting.
While D2 and CB1 activity is involved in delay discounting for food, the degree to which a high-fat diet may alter D2- and CB1-related changes in discounting has not been explored. The current study examined the extent to which haloperidol and rimonabant affected delay discounting in rats fed standard-chow and high-fat diets. Because high-fat diet exposure alters sensitivity to dopamine and cannabinoid agonists (Baladi et al. 2012; Wiley et al., 2011), we expected haloperidol and rimonabant to increase discounting in rats with a history of high-fat diet exposure to a greater extent than chow-fed controls. We used a procedure that systematically presented choices between a small, immediate food reinforcer and a larger food reinforcer delivered after a series of delays. The delays were individualized for each rat, limiting floor effects that may have been present in other studies. In addition, the effects of pellet type were examined. Some reports have indicated that the effectiveness of D2- and CB1-receptor blockers in decreasing food consumption may be increased when food is palatable (i.e., high in sugar or fat content) (Droste et al., 2010; Pritchett and Hajnal, 2011), though no research has examined if this increased effectiveness translates to a delay-discounting paradigm. Therefore, the effects of haloperidol and rimonabant on delay discounting were examined using both sucrose and carrot-flavored pellets, which differ in their sucrose concentration and reinforcing efficacy (e.g., Buckley and Rasmussen, 2012).
Methods
Subjects and diets
Forty male Sprague-Dawley rats were obtained (Simonsen Labortories, Gilroy, CA, USA) at three weeks of age and housed in individually clear plexiglass cages with free access to water. Subjects were housed in a temperature- and humidity-controlled room and maintained on a 12 h light:dark schedule (lights on at 07.00 h). Upon arrival, rats were given free access to either of two diets (TestDiet®, Richmond, IN) for three months: a high-fat diet (n = 20; 4.73 kcal/g, 45% from fat) or a standard-chow diet (n = 20; 3.0 kcal/g, 17% from fat). These diets and exposure duration were chosen because similar methods have resulted in significant alterations in D2- and CB1-receptor expression in rodents (see Harrold et al., 2002; Huang et al., 2006). After three months of feeding on their respective diets, the ten rats with the highest body mass (g) from the high-fat diet group and the ten rats with the lowest body mass (g) from the standard-chow diet group were selected for experimental sessions. Selection by body mass was determined based on the last two days’ weights. One rat in the standard-chow group died for reasons unrelated to the experiment before sessions began. The nineteen remaining rats were then maintained at 85% of their free-feeding weights to establish food as a reinforcer for the experiment. During this time, rats continued to receive their assigned diet directly after experimental sessions, but only enough to maintain their weights at 85% of their free-feeding weight. Therefore the rats were continuously exposed to their assigned diets throughout the experiment. All procedures were approved by the Idaho State University Institutional Animal Care and Use Committee.
Apparatus
Seven Coulbourn® Habitest (Coulbourn Instruments, Whitehall, PA, USA) rat operant chambers were used for data collection. Chambers were equipped with two levers on the right sidewall panel and were 5 cm above a grid floor with a food alcove centered between the levers. When response requirements were met, and depending on the experimental condition, one or more 45-mg sucrose pellets (95% sucrose, 3.4 kcal/g; TestDiet®, Richmond, IN, USA) or carrot-flavored pellets (3% sucrose, 3.3 kcal/g; TestDiet®, Richmond, IN, USA) were delivered to the alcove. These pellet types were chosen because these same carrot-flavored pellets were less preferred than sucrose pellets in Sprague-Dawley rats and Zucker rats in a previous study (see Buckley & Rasmussen, 2012). Two 28-V stimulus lights were situated above each lever as well as a 28-V houselight that was 28 cm above the alcove. A 5 cm X 5 cm fan circulated air in the upper left corner of the left sidewall panel and white noise was generated from a speaker in the upper right corner of the left sidewall panel. Each chamber was placed in a sound-attenuating cubicle and Graphic State® software (Coulbourn Instruments, Whitehall, PA, USA) on a Windows-based computer (located in an adjacent room) controlled all data collection within 0.01-s resolution.
Procedure
Delay-discounting procedure
Rats were trained to press two levers in a manner similar to that described in Boomhower et al. (2013). After training, a two-lever choice procedure was implemented based on Huskinson et al. (2012), who report a modified version of the procedure used by Evenden and Ryan (1996). In this procedure, a response on one lever resulted in the immediate delivery of a single pellet and a response on the opposite lever resulted in the delivery of three pellets after a series of delays that was individualized for each rat (see below). The lever that resulted in three pellets remained constant for a rat throughout the study, and whether the left or right lever resulted in three pellets was counterbalanced across subjects. After the delivery of a reinforcer, an intertrial interval (ITI) was initiated such that each trial lasted 60 s, regardless of which lever was chosen.
An experimental session consisted of five blocks of trials, where each block comprised two forced-choice trials followed by ten free-choice trials. At the start of a forced-choice trial, the stimulus light above a randomly selected lever was illuminated. For example, if the three-pellet lever was selected by the program, a single response on this lever resulted in three pellets delivered after a delay. The stimulus light flashed (0.5 s on, 0.5 s off) above the lever until the delivery of the three pellets, which then was coupled with three 0.5-s flashes of the houselight. All lights then were extinguished for the duration of the ITI. The second forced-choice trial followed in a manner similar to the first, except that the stimulus light above the opposite lever was illuminated and a single response on this lever resulted in the delivery of one pellet immediately accompanied with a single flash of the houselight. Free-choice trials were identical to forced-choice trials in every manner except that both stimulus lights above the levers were illuminated at the start of a trial. A response on either lever extinguished the stimulus light above the opposite lever (which became inactive for the remainder of the trial) and resulted in the programmed delivery of (a) pellet(s) immediately or after a delay. If a response was not emitted before 30 s had elapsed then the trial was considered omitted, all lights were extinguished, and a 60-s ITI began, after which the next free-choice trial began. Omissions were not programmed in forced-choice trials. A session ended after 2 h or the completion of 5 blocks (10 forced-choice and 50 free-choice trials), whichever came first. Sessions were conducted between 09.00 and 17.00 h Monday through Friday at the same time every day (±15 min), and the number of high-fat diet and standard-chow rats in each session was counterbalanced across group and time of day at which they were run.
The delay to the three pellets was 0 s in the first block of trials and increased in each subsequent block, while the delay to the one pellet remained constant (0 s). Subjects were first exposed to 0-s probe sessions, where both reinforcers were delivered immediately for each block. This continued for three sessions and until larger reinforcer choice was at least 90% (9 of 10 free-choice trials). After this criterion was met, the first delay series was implemented: 0-, 1-, 2-, 4-, and 8-s delays. In this series, the delay to the larger reinforcer was 0 s in the first block, 1 s in the second, 2 s in the third, 4 s in the fourth, and 8 s in the last block. The delay was increased in this manner for each subject until larger reinforcer choice decreased to less than 50% in at least one block after behavior stabilized (criteria described below). If larger reinforcer choice was 50% or above in all blocks after behavior stabilized under this delay series, then a 0-, 2-, 4-, 8-, 16-s delay series was implemented. If larger reinforcer choice still remained above 50% in all blocks, a 0-, 5-, 10-, 20-, 40-s delay series was implemented. Whether the delay series was ascending (0-, 1-, 2-, 4-, and 8-s) or descending (8-, 4-, 2-, 1-, and 0-s) across the session was counterbalanced across subjects and held constant for a rat throughout the study. All rats were exposed to two pellet conditions in which both the immediate and delayed reinforcers were either sucrose pellets or carrot-flavored pellets. The order of these pellet conditions was counterbalanced across subjects and determination of a terminal delay series for a subject was evaluated separately for both the sucrose- and carrot-pellet conditions.
Stability criteria and acute drug administration
When a delay series was implemented, baseline (non-injection) sessions were conducted for a minimum of five sessions. To ensure sensitivity to the variations in the amount and delay of reinforcement, 0-s probe sessions, in which delays to both outcomes were 0 s, were conducted every Wednesday. If larger reinforcer choice was <90% in any block, subsequent 0-s probe sessions were conducted until larger reinforcer choice was at least 90% in each block. We considered behavior as stable when four criteria used by Huskinson et al. (2012) and Krebs & Anderson (2012) were met. First, the total number of larger reinforcer choices across a session could not vary by more than 20% of the grand mean of the last five sessions. Second, choice in the 0-s delay block had to be at least 90% for the last five sessions. Third, the previous 0-s probe session had to be passed within one session. Fourth, there were no three consecutive sessions during the last five sessions in which total larger reinforcer choice increased or decreased.
After behavior under baseline met the four stability criteria (above), acute drug administrations commenced. Haloperidol (0.003-0.1 mg/kg i.p.) or vehicle were administered 30 min before some experimental sessions, and rimonabant (0.3-10 mg/kg i.p.) or vehicle were administered 1 h before some experimental sessions. However, injections were given only when the previous 0-s probe session was passed within one session and when larger reinforcer choice in the 0-s delay block in the previous session was at least 90%. For rimonabant, doses were injected on Tuesdays and Fridays, and for haloperidol, injections were administered on Fridays. Vehicle injections were administered on Thursdays, and all other days served as non-injection control days. Whether rimonabant or haloperidol was injected first was counterbalanced across subjects, and at least a seven-day washout period separated dose-response determinations of rimonabant and haloperidol. The injection order of the low and moderate doses of rimonabant (1-3 mg/kg) and haloperidol (0.01-0.03 mg/kg) was randomized across subjects and the highest dose of drug was injected last to minimize the chance of overdose. If fewer than five trials were completed in any block following an injection of the highest dose of haloperidol (0.1 mg/kg) or rimonabant (10 mg/kg), a smaller drug dose was administered (0.003 mg/kg haloperidol or 0.3 mg/kg rimonabant). In this way, all rats received at least three injections of drug and a vehicle injection.
Drugs
Haloperidol (Sigma-Aldrich, USA) was dissolved in a 1:1:18 solution of 3% lactic acid, a buffering agent, and saline (1 mL/kg volume). Rimonabant (National Institute of Mental Health Chemical Synthesis and Drug Supply Program) was dissolved in a 1:1:18 solution of ethanol (Sigma), Cremaphor (Sigma), and saline (1 mL/kg). These dose ranges of haloperidol and rimonabant were selected because they are behaviorally effective and lie outside the toxic range.
Data Analysis
Percent choice for the larger reinforcer was analyzed using a repeated-measures ANOVA (PASW Statistics 18.0) with diet condition (high-fat vs. standard-chow) as a between-subjects variable, delay block as a within-subjects variable, and dose of either rimonabant (1-10 mg/kg) or haloperidol (.01-0.1 mg/kg) as a within-subjects variable. A repeated-measures ANOVA was conducted separately to determine effects of pellet condition (sucrose vs. carrot) as a within-subjects variable. Area-under-the-curve values (AUC; Myerson et al. 2001) were calculated by plotting percent choice for the larger reinforcer as a function of delay series (0-8, 0-16, or 0-40 s) for each subject, summing the area of the trapezoids that were formed from this curve, and dividing by the total area possible to normalize data. Thus, AUC values ranged from 0 to 1, where lower AUC values indicated steeper discounting functions. A repeated-measures ANOVA with diet condition as a between-subjects variable and drug dose as a within-subjects variable was conducted on AUC data, which were transformed into percent of vehicle values to determine drug effects.
A point about analyses of drug effects should be noted. Table 1 shows the delay series and dose range of haloperidol and rimonabant for each rat. Across the study, there were nine rats in which increased sensitivity—defined as completing fewer than five trials in a delay block—was demonstrated to the highest doses of haloperidol or rimonabant. These rats were administered lower doses (0.003 mg/kg haloperidol or 0.3 mg/kg rimonabant). Across the carrot-pellet condition, one standard-chow and one high-fat diet rat met this criterion under haloperidol. However, across the sucrose-pellet condition, two and four high-fat diet rats (6 total) met this criterion for rimonabant and haloperidol, respectively, compared to one standard-chow rat, which was given a smaller dose of haloperidol. We report this for two reasons. First, a greater number of rats in the high-fat diet condition requiring smaller doses may indicate differential sensitivity to the drugs as a function of diet exposure. Second, we conducted repeated-measures ANOVAs that excluded these rats because they did not complete sufficient trials to warrant meaningful analysis. However, to ensure adequate power, we also conducted separate repeated-measures ANOVAs with vehicle, “low,” “moderate,” and “high” as levels of drug dose. Because the results from these analyses were similar, we report the results from the original repeated-measures ANOVAs, which excluded subjects given lower doses of haloperidol and rimonabant. We included these subjects’ data in the means for 0.01-0.03 mg/kg haloperidol and 1-3 mg/kg rimonabant in the figures, however, since the means did not change substantially with their removal.
Results
Body mass
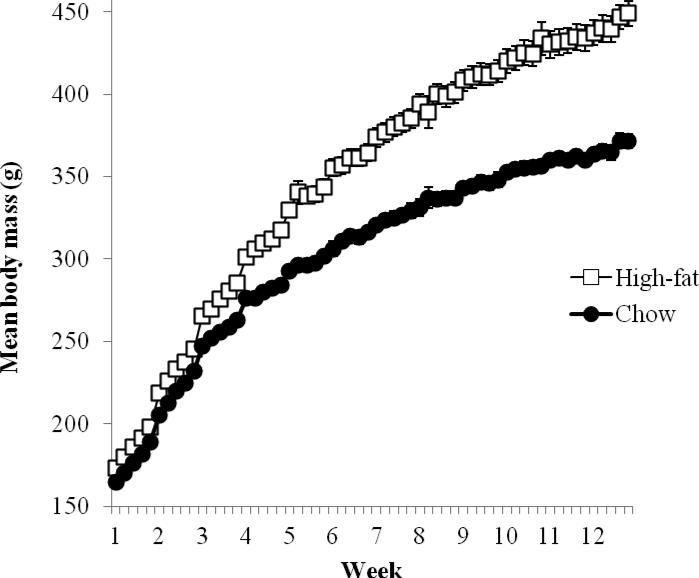
Fig. 1 shows body weight (g) of the rats in the standard-chow (n = 9) and high-fat (n = 10) diet conditions during the three-month diet exposure. For the rats selected for experimental sessions, the ten high-fat diet rats had significantly higher body weights (M = 449.02 g, SEM = 7.64) compared to the nine standard-chow rats [M = 371.61 g, SEM = 4.01; t(17) = 8.68, p < 0.001] by the end of the diet-exposure period.
Fig. 1.
Mean (±SEM) body weight of rats given free access to chow (n = 9) and high-fat (n = 10) diets for three months
Baseline delay discounting
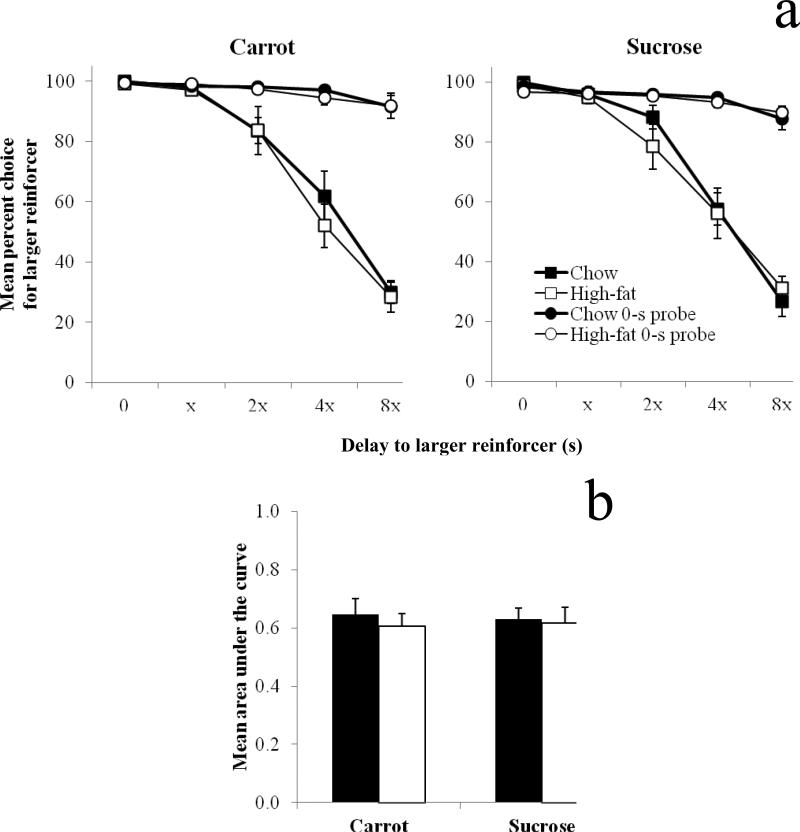
All data are presented as if the delay series were ascending for all subjects, given that there were no effects of delay order on larger reinforcer choice. Fig. 2 shows mean percent choice for the larger reinforcer (a) as a function of delay in both the delay and 0-s probe sessions for standard-chow and high-fat diet rats. Data from the carrot- and sucrose-pellet conditions are shown, and delays are presented as a function of the lowest non-zero delay in a delay series (see Table 1 for which delay series each rat received and whether it was ascending, e.g., 0-8, or descending, e.g., 8-0). In both the carrot and sucrose conditions, there was a significant main effect of delay block [carrot: F(4,68) = 124.82, p < 0.001, ηp2 = 0.88; sucrose: F(4, 68) = 136.33, p < 0.001, ηp2 = 0.89], indicating that as the delay to the larger reinforcer increased larger reinforcer choice decreased. There were no main effects or interactions involving diet or pellet condition.
Fig. 2.
Mean (±SEM) percent choice for the larger reinforcer as a function of delay in both the delay (squares) and 0-s probe (circles) sessions (a) and mean (±SEM) area under the curve (b) for standard-chow (black bars) and high-fat (white bars) diet rats in the carrot- and sucrose-pellet conditions. Because delay series were individualized for each rat, delays to the larger reinforcer are presented as a function of the lowest non-zero delay (x) in a delay series
Fig. 2 also shows mean area under the curve (b) for standard-chow and high-fat diet rats in the carrot- and sucrose-pellet conditions. There were no significant main effects or interaction involving pellet or diet condition, indicating that the delay series implemented for each rat were functionally equivalent in reducing larger reinforcer choice.
Drug effects on delay discounting
Percent choice for the larger reinforcer
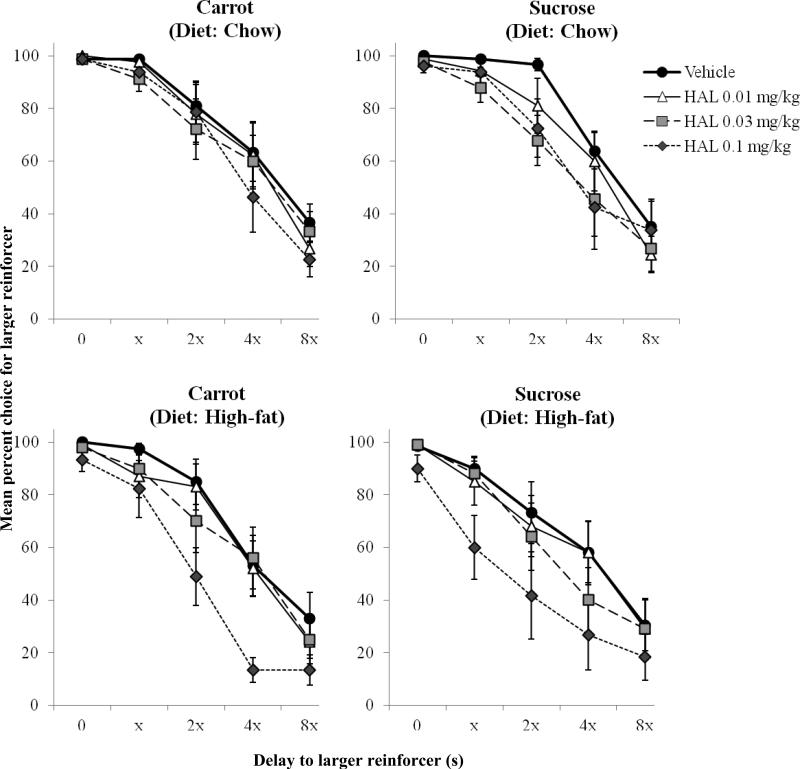
Because of increased trial omissions following haloperidol and rimonabant, some rats were excluded from drug analyses (shown in Table 1). Fig. 3 shows mean percent choice for the larger reinforcer as a function of its delay for rats given standard-chow (top panels) and a high-fat diet (bottom panels) and varying doses of haloperidol. Data from the carrot- (left panels) and sucrose- (right panels) pellet conditions are shown. The vehicle curves (bolded) represent data from sessions in which a vehicle injection was given beforehand. Vehicle injections occurred once for each pellet condition and each drug, resulting in four different vehicle curves for one rat. In the carrot condition, there was a significant main effect of delay block [F(4,60) = 57.69, p < 0.001, ηp2 = 0.79], as percent choice for the larger reinforcer decreased as delay increased. Haloperidol caused a dose-dependent leftward shift in the discounting function [F(3,45) = 5.59, p < 0.01, ηp2 = 0.27] by reducing percent choice for the larger reinforcer. There was also a significant delay block X dose interaction [F(12,180) = 3.61, p < 0.001, ηp2 = 0.19], as haloperidol decreased percent choice for the larger reinforcer when it was delayed, but did not affect larger reinforcer percent choice in the 0-s delay block. In the sucrose condition, there were similarly a significant main effects of delay block [F(4,48) = 47.78, p < 0.001, ηp2 = 0.80], and haloperidol [F(3,36) = 9.35, p < 0.001, ηp2 = 0.44], and a significant delay block X dose interaction [F(12,144) = 2.32, p = 0.01, ηp2 = 0.16]. There were no significant main effects or interactions involving diet condition or pellet type.
Fig. 3.
Effects of haloperidol on mean (±SEM) percent choice for the larger reinforcer as a function of its delay for rats exposed to standard-chow (upper panels) and high-fat diets (lower panels). Data from the carrot- and sucrose-pellet conditions are on the left and right, respectively
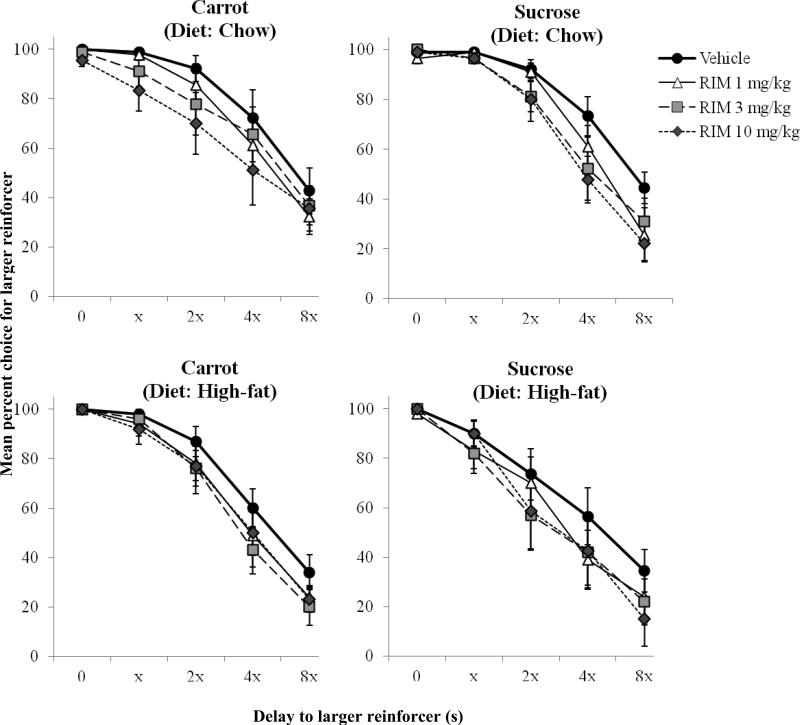
Fig. 4 shows mean percent choice for the larger reinforcer as a function of dose of rimonabant. In the carrot condition, there was a significant main effect of delay block [F(4,68) = 77.86, p < 0.001, ηp2 = 0.82], in that percent choice for the larger reinforcer was reduced as the delay increased. Rimonabant also dose-dependently shifted the discounting function to the left [F(3,51) = 6.94, p = 0.001, ηp2 = 0.29]. There were no significant interactions. In the sucrose condition, there were similarly significant main effects of delay block [F(4,60) = 59.86, p < 0.001, ηp2 = 0.80] and dose [F(3,45) = 4.86, p < 0.01, ηp2 = 0.25]. There was also a significant interaction [F(12,180) = 3.10, p = 0.001, ηp2 = 0.17], as rimonabant decreased percent choice for the larger reinforcer when it was delayed, but did not affect larger reinforcer percent choice in the 0-s delay block. There were no main effects or interactions involving diet condition or pellet type.
Fig. 4.
Effects of rimonabant on mean (±SEM) percent choice for the larger reinforcer as a function of its delay for rats exposed to standard-chow (upper panels) and high-fat (lower panels) diets. Data from the carrot- and sucrose-pellet conditions are on the left and right, respectively
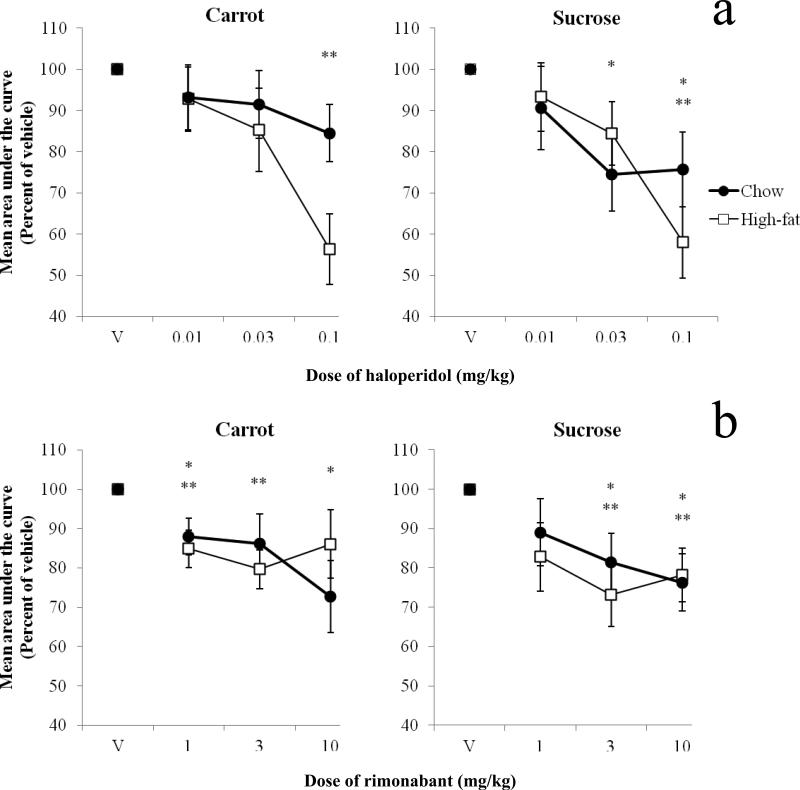
Area under the curve
Fig. 5 shows mean area-under-the-curve values expressed as percent of vehicle values as a function of dose of haloperidol (a) and rimonabant (b) in standard-chow and high-fat diet rats. Data from the carrot and sucrose condition are on the left and right, respectively. In the carrot-pellet condition, there were significant main effects of dose of haloperidol [F(3,45) = 6.90, p = 0.001, ηp2 = 0.32] and rimonabant [F(3,51) = 6.45, p = 0.001, ηp2 = 0.28] in that both haloperidol and rimonabant reduced AUC. At the 0.1 mg/kg dose of haloperidol, high-fat diet rats demonstrated significantly lower AUC values than the standard-chow rats [t(15) = 2.30 p < 0.05]. In the sucrose-pellet condition, there were also significant main effects of dose of haloperidol [F(3,36) = 8.51, p < 0.001, ηp2 = 0.42] and rimonabant [F(3,45) = 5.09, p < 0.01, ηp2 = 0.25]. There were no significant main effects or interactions involving diet condition or pellet type.
Fig. 5.
Mean (±SEM) area under the curve expressed as a percent of vehicle scores as a function of dose of haloperidol (a) and rimonabant (b) for standard-chow and high-fat diet rats in the carrot- (left panels) and sucrose-pellet (right panels) conditions. Note that the symbols for standard-chow and high-fat overlap under vehicle.
*p < 0.05 vs. vehicle for standard chow
**p < 0.05 vs. vehicle for high fat
Discussion
The procedure used in the current study demonstrated that haloperidol and rimonabant increased delay discounting in rats fed high-fat and standard-chow diets. Specifically, both haloperidol and rimonabant decreased the percentage of choices allocated to larger-later reinforcers and decreased AUC values in both groups of rats. These effects also were replicated across two foods that differed in sucrose concentration and reinforcing efficacy (Buckley & Rasmussen, 2012)—what some may describe as palatability. In regard to haloperidol, this study replicates and extends previous research showing that non-selective D2 antagonists decrease choice for larger, delayed reinforcers in rats (Cardinal et al., 2000; Denk et al., 2005; Wade et al., 2000). As for rimonabant, previous studies have reported that rimonabant increases discounting in lean Zucker rats, but decreases discounting in obese Zuckers (Boomhower et al., 2013). However, it should be noted that Boomhower et al. (2013) observed these effects in Zucker rats—a strain that differs from Sprague Dawley rats in leptin, endocannabinoid, and dopamine signalling (DiMarzo et al., 2001; Thanos et al, 2008a,b)—using an adjusting-delay procedure, factors that may explain why the current study found that rimonabant increased discounting in both dietary groups, rather than just the rats fed standard chow. Further, the current study also extends the literature on dopamine and endocannabinoid involvement in delay discounting by using rats with a history of consuming high-fat food. To our knowledge, this study is the first to show that haloperidol and rimonabant increase discounting in rats fed high-fat and standard-chow diets.
A pattern of choosing smaller-sooner reinforcers can result from (a) an increased sensitivity to (or aversion toward) the delay to the larger reinforcer and/or (b) a decreased sensitivity to the amount of the larger reinforcer. Indeed, an advantage of the procedure used in the current study is that it parsed a measure of delay sensitivity from amount sensitivity by testing larger reinforcer choice when both reinforcers were delivered immediately (i.e., in the 0-s delay block). Neither haloperidol nor rimonabant significantly affected larger reinforcer choice in the 0-s delay block, since larger reinforcer choice in the 0-s delay block remained at or above 90% on average. Therefore, haloperidol- and rimonabant-induced increases in discounting were not the result of changes in amount sensitivity, but rather they were the result of an increased sensitivity to the delays to reinforcement. Some research has demonstrated that the effects on delay discounting of dopaminergic drugs, such as amphetamine, depend on conditioned stimuli during the delay (e.g., a flashing light) (Cardinal et al. 2000; Slezak and Anderson 2009). Though we did not explicitly test whether the effects of haloperidol (or rimonabant) on discounting changed across differing stimulus conditions, it is possible that haloperidol and rimonabant were affecting sensitivity to conditioned reinforcers. Future research should consider manipulating the stimuli present during the delays to the larger reinforcer to answer this question.
Based on our statistical analyses, rats exposed to a high-fat diet showed no differences in sensitivity to rimonabant and slightly increased sensitivity to haloperidol compared to controls. Examination of Figure 3 reveals that the highest dose of haloperidol decreased choices for larger, delayed food for both pellet types to a greater extent in rats fed a high-fat diet compared to standard chow. In the upper left of Figure 5, this difference between groups is also reflected in the AUC values in the carrot-pellet condition, though not with sucrose pellets. Table 1, however, shows that in the sucrose condition, four (40%) rats in the high-fat diet condition were so sensitive to the 0.1 mg/kg dose of haloperidol, that they omitted trials to the point where their data were excluded from analysis; only one standard chow rat (11%) met this same criterion. To make an argument for increased sensitivity to haloperidol, we would expect to observe group differences at this highest dose across all measures and pellet types. However, these data cannot be disregarded, and may be interpreted at least as partial support for dopaminergic alterations induced by a high-fat diet. This would be consistent with other studies that have shown that rodents fed a high-fat diet for an extended duration demonstrate decreased D2 binding (Huang et al., 2006; Johnson & Kenny, 2010) in the brain and increased sensitivity to D2 agonists (Baladi et al., 2012).
Differences in baseline delay discounting were not observed between diet conditions. Baseline differences might be expected since high-fat diets affected D2- and CB1-receptor densities in rodents’ brains in other studies (Harrold et al., 2002; Huang et al., 2006; Johnson & Kenny, 2010; South & Huang, 2008). While it is unclear why differences in behavior were not observed, it should be pointed out that though rats received their assigned diet throughout the study, the free-feeding condition was in place only until operant sessions commenced. At that point, rats were only given enough of their assigned diets to maintain 85% of their free-feeding body mass. This food restriction may have reduced the potential for differences in brain neurochemistry (i.e., those specific to dopamine and cannabinoid regulation) between the dietary groups, though some research indicates mild food restriction from standard chow and diets high in fat may increase sensitivity to D2 agonists (see Baladi et al., 2012). Moreover, there is some evidence that mild food restriction may affect discounting in rats, but this is more so with females (e.g., Koot et al. 2009). Future research should consider examining the potential influence of food restriction on behavioral differences between animals with exposure to high fat and standard chow.
The current study also examined the extent to which haloperidol and rimonabant affected delay discounting when qualitatively different reinforcers (i.e., carrot vs. sucrose) were used. Previous studies have shown haloperidol (e.g., Pritchett and Hajnal, 2011) and rimonabant (Carai et al., 2006; Droste et al., 2010; but also see Buckley & Rasmussen, 2014) are more effective in reducing consumption of palatable food compared to less palatable food. In the current study, haloperidol increased discounting in both high-fat- and standard-chow-fed rats when sucrose was delivered, but only increased discounting in high-fat-fed rats when carrot pellets were delivered (see Figure 5). In regard to trial completions, more rats demonstrated increased sensitivity to haloperidol (four high-fat diet and one standard chow) when sucrose pellets were delivered compared to carrot pellets (one high-fat diet and one standard chow). It could be the case that the behavioral effects of haloperidol are increased when sucrose is delivered, but a history of consuming a high-fat diet increases sensitivity to D2 antagonists regardless of reinforcer palatability.
There could be some additional interpretations of the present results. First, the increases in delay discounting observed after injections of haloperidol and rimonabant may have been the result of aging or maturation throughout the experiment. For example, some research has shown that older rats choose smaller-sooner food reinforcers more often than younger rats (e.g., Simon et al., 2010), and these changes may be related to downregulation of D2 receptors and upregulation of CB1 receptors that occur in the striatum of aging rats (Thanos et al., 2008a,b). However, we find this unlikely for two reasons: (a) we did not observe any time-dependent increases in delay discounting across the study, and (b) the order of haloperidol and rimonabant was counterbalanced across subjects and therefore would distribute any drug effects related to age across groups equally. Second, the weights of the two groups of rats used in the current study differed on average by about 100 g, which may have influenced the pharmacokinetics of haloperidol and rimonabant. However, past research has demonstrated that increased sensitivity to dopaminergic compounds after high-fat diet exposure is most likely due to changes in dopamine functioning rather than alterations in pharmacokinetics attributable to weight differences (Baladi et al., 2011, 2012; Bowman et al., 1999). Finally, the effects of haloperidol and rimonabant on delay discounting observed in the current study may be operating through another behavioral process, such as timing or time estimation, in addition to affecting delay sensitivity. For example, both haloperidol (Buhusi & Meck, 2002) and rimonabant (Han & Robinson, 2001) have been shown to affect timing in rodents by increasing overestimates of time. Although the current study was not designed to measure timing, the finding that both haloperidol and rimonabant increased discounting is consistent with the effects of these drugs on timing. That is, if delays to reinforcement were “overestimated” after injections of haloperidol or rimonabant, one would expect decreases in behavior directed toward larger-later reinforcers.
Despite these alternative interpretations, the procedure used in the current study demonstrated that haloperidol and rimonabant increased delay discounting in rats fed high-fat and standard-chow diets. To our knowledge, this is the first study to find that haloperidol and rimonabant affect discounting in rats with different dietary histories. These findings provide support for the involvement of both the D2 and CB1 receptors in controlling delay discounting and emphasize the importance of understanding the effects of dietary history on behavioral responses to drugs that target the D2 and CB1 receptor.
Supplementary Material
Acknowledgements
We thank Dr. Sally Huskinson for help with data analysis and Starlie Belnap, Sarah Bushnell, Tiffany Doherty, Dave Judy, Jessica Kramer, Nic Maier, Aaron Miller, and Rebecca Miller for assisting with data collection. The first author is now at Auburn University, Auburn, AL, USA.
Sources of Funding
This research was supported by the Idaho INBRE program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences), the Idaho State University Humanities and Social Sciences Research committee, as well as undergraduate research grants from Psi Chi and Sigma Xi.
Footnotes
Conflicts of Interest
None declared.
References
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19(11):2175–82. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Waring ME, Schneider KL, Pagoto SL, Debiasse MA, Whited MC, Lynch EB. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 2012;59(2):576–84. doi: 10.1016/j.appet.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. The Journal of Pharmacology and Experimental Therapeutics. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Daws LC, France CP. You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012;63(1):76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, et al. Behavioral economic predictors of overweight children's weight loss. Journal of Consulting and Clinical Psychology. 2012;80(6):1086–96. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower SR, Rasmussen EB, Doherty TS. Impulsive-choice patterns for food in genetically lean and obese Zucker rats. Behavioural Brain Research. 2013;241:214–21. doi: 10.1016/j.bbr.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. The Journal of Pharmacology and Experimental Therapeutics. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Buckley JL, Rasmussen EB. Obese and lean Zucker rats demonstrate differential sensitivity to rates of food reinforcement in a choice procedure. Physiology & Behavior. 2012;108:19–27. doi: 10.1016/j.physbeh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JL, Rasmussen EB. Rimonabant's reductive effects on high densities of food reinforcement, but not palatability, in lean and obese Zucker rats. Psychopharmacology. 2014 doi: 10.1007/s00213-013-3366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116(2):291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Carai MAM, Colombo G, Maccioni P, Gessa GL. Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Reviews. 2006;12(2):91–9. doi: 10.1111/j.1527-3458.2006.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152(4):362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- De Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–25. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings K, Sharp T, Rushworth MFS, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179(3):587–96. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Droste SM, Saland SK, Schlitter EK, Rodefer JS. AM 251 differentially effects food-maintained responding depending on food palatability. Pharmacology, Biochemistry, and Behavior. 2010;95(4):443–8. doi: 10.1016/j.pbb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- El Khoury M-A, Gorgievski V, Moutsimilli L, Giros B, Tzavara ET. Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;38(1):36–50. doi: 10.1016/j.pnpbp.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128(2):161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fields SA, Sabet M, Peal A, Reynolds B. Relationship between weight status and delay discounting in a sample of adolescent cigarette smokers. Behavioural Pharmacology. 2011;22(3):266–8. doi: 10.1097/FBP.0b013e328345c855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Shobin E, Pickel VM. Cannabinoid modulation of the dopaminergic circuitry: implications for limbic and striatal output. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;38(1):21–9. doi: 10.1016/j.pnpbp.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behavioral Neuroscience. 2001;115(1):243–246. doi: 10.1037/0735-7044.115.1.243. [DOI] [PubMed] [Google Scholar]
- Harrold J, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Research. 2002;952(2):232–8. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Hendrickson K, Rasmussen E. Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behaviour Research and Therapy. 2013;51(7):399–409. doi: 10.1016/j.brat.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Huang XF, Zavitsanou K, Huang X, Yu Y, Wang HQ, Chen F, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behavioural Brain Research. 2006;175(2):415–9. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacology, Biochemistry, and Behavior. 2012;101(3):403–16. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G. Gender differences in delay-discounting under mild food restriction. Behavioural Brain Research. 2009;200(1):134–143. doi: 10.1016/j.bbr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Krebs CA, Anderson KG. Preference reversals and effects of D-amphetamine on delay discounting in rats. Behavioural Pharmacology. 2012;23(3):228–40. doi: 10.1097/FBP.0b013e32835342ed. [DOI] [PubMed] [Google Scholar]
- La Fleur SE, Vanderschuren LJMJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan R. a H. A reciprocal interaction between food-motivated behavior and diet-induced obesity. International Journal of Obesity. 2007;31(8):1286–94. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–43. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete F, Pérez-Ortiz JM, Manzanares J. Cannabinoid CB2 receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. British Journal of Pharmacology. 2012;165(1):260–73. doi: 10.1111/j.1476-5381.2011.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MCW, Schepers I, González-Cuevas G, de Vries TJ, Schoffelmeer ANM. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology. 2007;193(1):85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett CE, Hajnal A. Obesogenic diets may differentially alter dopamine control of sucrose and fructose intake in rats. Physiology & Behavior. 2011;104(1):111–6. doi: 10.1016/j.physbeh.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Lawyer SR, Reilly W. Percent body fat is related to delay and probability discounting for food in humans. Behavioural Processes. 2010;83(1):23–30. doi: 10.1016/j.beproc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiology of Aging. 2010;31(5):853–62. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behavioural Pharmacology. 2009;20(5-6):424–36. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- South T, Huang X-F. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochemical Research. 2008;33(3):598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-terms changes in dietary preferences and central reward signaling. Neuroscience. 2010;162(4):924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008a;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008b;62(9):637–42. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150(1):90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–9. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Jones AR, Wright MJ. Exposure to a high-fat diet decreases sensitivity to delta-9-tetrahydrocannabinol-induced motor effects in female rats. Neuropharmacology. 2011;60:274–283. doi: 10.1016/j.neuropharm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer ANM, Pattij T. Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PloS One. 2011;6(10):e25856. doi: 10.1371/journal.pone.0025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.