Abstract
Multiple recruitment strategies are often needed to recruit an adequate number of participants, especially hard to reach groups. Technology-based recruitment methods hold promise as a more robust form of reaching and enrolling historically hard to reach young adults. The TARGIT study is a randomized two-arm clinical trial in young adults using interactive technology testing an efficacious proactive telephone Quitline versus the Quitline plus a behavioral weight management intervention focusing on smoking cessation and weight change. All randomized participants in the TARGIT study were required to be a young adult smoker (18–35 years), who reported smoking at least 10 cigarettes per day, had a BMI < 40 kg/m2, and were willing to stop smoking and not gain weight. Traditional recruitment methods were compared to technology-based strategies using standard descriptive statistics based on counts and proportions to describe the recruitment process from initial pre-screening (PS) to randomization into TARGIT. Participants at PS were majority Black (59.80%), female (52.66%), normal or over weight (combined 62.42%), 29.5 years old, and smoked 18.4 cigarettes per day. There were differences in men and women with respect to reasons for ineligibility during PS (p < 0.001; ignoring gender specific pregnancy-related ineligibility). TARGIT experienced a disproportionate loss of minorities during recruitment as well as a prolonged recruitment period due to either study ineligibility or not completing screening activities. Recruitment into longer term behavioral change intervention trials can be challenging and multiple methods are often required to recruit hard to reach groups.
ClinicalTrials.gov Identifier NCT01199185
The NHLBI funded TARGIT as part of a U01 Cooperative Agreement and as such the study design was approved. They did not have input into the data collection, analysis, or the interpretation of the data or in the writing of this report.
Keywords: Recruitment methods, Barriers to recruitment, Minority participation, Young adult smokers, Preventing post cessation weight gain, Interactive technology
1. Introduction
Recruitment into behavioral intervention studies can be expensive, time consuming, and complex depending on the population of interest. Multiple recruitment strategies are often needed to recruit an adequate number of participants, especially hard to reach groups. Study design issues such as eligibility criteria along with participant behaviors can challenge investigators to identify effective and timely recruitment strategies during screening [1]. Also adequate participation and representativeness of the study sample can be compromised during recruitment due to ineligibility, disinterest in participating, or inability maintaining contact with those interested. These dynamic behavioral factors limiting inclusion and diversity during recruitment of the study sample can influence external validity [2]. Population subgroups of interest that have historically been difficult to enroll in primary prevention trials include younger persons, smokers, and African Americans [3]. In smoking cessation trials, successful enrollment of minorities [4], men and younger adults [4], [5], [6] oftentimes has presented challenges. In prevention trials specific for overweight and obese persons, similar recruitment patterns, as have been seen with smokers, have shown noticeably lower eligibility rates among African Americans [7] with markedly lower participation rates among men and young adults (18–35 years) [8], [9]. This lack of adequate representation of young adults and African Americans in behavioral intervention trials has raised concerns regarding the generalizability of primary prevention outcomes to younger persons [9] and minorities [10] along with adequate translation of prevention science evidence.
Technology-based recruitment methods hold promise as a more robust form of reaching and enrolling historically hard to reach young adults [11], as do technology delivered interventions for greater dissemination of behavioral treatments to diverse populations [12]. However, information on technology recruitment methods is lacking, especially among the historically harder to reach groups previously identified. This paper will describe the recruitment process employed during the screening and enrollment phases of the National Heart, Lung and Blood Institute (NHLBI) funded study “Treating Adults at Risk for Weight Gain with Interactive Technology” (TARGIT) designed to recruit young adult smokers at risk for weight gain to stop smoking without gaining weight. Additionally, this study elucidates how the TARGIT experience helps to ascertain which recruitment methods (i.e., traditional versus technology-based strategies) were effective for randomizing the final study cohort.
2. Material and methods
2.1. Design
The TARGIT study is a randomized two-arm clinical trial in young adults using interactive technology testing an efficacious proactive telephone Quitline versus the Quitline plus a behavioral weight management intervention focusing on smoking cessation and weight change. The University of Tennessee Health Science Center (UTHSC), Department of Preventive Medicine, clinical trials research center in Memphis, TN was the study site. The project was approved by the Institutional Review Board (IRB) at UTHSC. TARGIT was a participating study in the cooperative group funded by the NHLBI U01 “Early Adult Reduction of weight through LifestYle intervention (EARLY) Trials” [13].
2.2. Population
This paper includes analyses on all smoking individuals who contacted the TARGIT study for possible enrollment. All randomized participants in the TARGIT study were required to be a young adult smoker (18–35 years), who reported smoking at least 10 cigarettes per day, had a BMI < 40 kg/m2, and were willing to stop smoking and not gain weight. The lower bound eligibility criteria for BMI was initially ≥22, but was decreased to ≥20 during the recruitment phase in an effort to increase enrollment (IRB approved change became effective 08/08/11). Before that change, 66 individuals were excluded during phone screen (PS) and one during screening visit (SV) because their BMI was <22, but would have passed the later applicable criterion of BMI ≥ 20, therefore, these individuals were invited to re-screen for the study. This approach ultimately allowed TARGIT to enroll additional participants whereby among the final 330 randomized, 21 (6.4%) were enrolled with a BMI in the affected range (e.g., they would not have been enrolled without the lowered BMI criteria). Additionally, TARGIT participants were required to have the ability to understand consent procedures (in English), have access to a telephone and the internet, demonstrate ability to access a specific web site, and demonstrate the ability to receive and respond to email. Further, interested persons had to be willing to accept random assignment to one of two intervention arms and be able to participate in a 24 month behavioral lifestyle change intervention to stop smoking and not gain weight. A full list of inclusion and exclusion criteria is found in Appendix 1.
Appendix.
Inclusion and exclusion criteria for TARGIT.
| Inclusion criteria |
|
|
|
|
|
|
|
|
|
| Exclusion Criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3. Recruitment strategies
Recruitment for the TARGIT study took place over a two-year period from December 2010 to August 2012 and included both traditional- and technology-based methods. Traditional recruitment approaches included media (i.e., television, radio), print (i.e., mass mailings of postcards to age appropriate persons identified by driver's license registries, newspaper ads, rack cards, flyers), and community (i.e., events at college campuses, health fairs, word of mouth). Technology recruitment approaches included website (TARGIT study), internet (i.e., Google ads, Craig's List), social media (TARGIT Facebook page) and email list serves (i.e., academic, healthcare, corporate, professional).
2.4. Screening and enrollment
Enrollment into TARGIT was as follows: During PS the TARGIT study was explained to individuals and eligibility criteria were assessed. Persons who were eligible and interested were scheduled for an onsite SV. At SV, written informed consent was obtained and participants were evaluated for additional study eligibility requirements. Eligible and willing participants were asked to record dietary intake online at the study website and were scheduled for a randomization visit (RV). Only those eligible who demonstrated a successful dietary recording were randomized into an intervention assignment for treatment of smoking cessation (Quitline only versus Quitline plus technology-delivered weight management).
2.5. Screening and enrollment variables
A completed PS contact determined an individual's age, height/weight, gender, ethnicity/race, number of cigarettes currently smoked per day, history of current substance abuse, indications of an unstable psychiatric condition, current medications, and whether the individual was pregnant or planning to become pregnant in the next 2 years. In addition, the individual was asked how he/she learned about the TARGIT study. PS contacts were terminated whenever a reason for ineligibility was first determined in order to decrease participant/staff burden. The purpose of this study was to recruit eligible participants and not to determine the reasons for ineligibility, thus resulting in missing information for the variables not yet queried. During the subsequent on-site SV additional demographic information was obtained such as education, income, or marital status. The main focus of this manuscript is to identify the most effective recruitment strategies for eliciting interest in screening that resulted in successful study randomization. Further, evaluation of whether demographic variables were predictive of how far an individual progressed in the recruitment process and what patterns in recruitment flow emerged are discussed.
2.6. Statistical methods
We used standard descriptive statistics based primarily on counts and proportions to describe the recruitment process from initial PS to randomization into TARGIT. Statistical significance was based on t-tests, Wilcoxon and Krushkal–Wallis tests, as well as chi-square and Fisher's exact tests. Logistic regression with randomization coded as “yes/no” was used to verify the significance of the identified traits of importance in a multivariable setting. In addition, this approach was used to address the relative predictive power of the identified model (Area under the Receiver Operating Curve – AUC). When cell-counts became small and the standard assumptions underlying the chi-square test were not satisfied, we based conclusions on Fisher's exact test. In those cases, we also re-estimated significance with the low-count subgroups removed in order to verify that statistically significant findings were not driven by the possibly erratic distribution of cases in these small-frequency subgroups (e.g., in Table 2 the p-value was computed when the recruitment source “Other” was removed (only 2 out of 29 phone screened individuals were actually randomized).
Table 2.
Self-reported source of information about the TARGIT study that triggered interest and contact to the study.
| Source | Phone screen (N = 3093) | Eligible after phone screen (N = 1588) | Screening visit (N = 534) | Randomized (N = 330) | p-valuea |
|---|---|---|---|---|---|
| Technology methods | 152 (5.47) | 98 (6.27) | 32 (6.12) | 20 (6.23) | 0.006 |
| Traditional methods | |||||
| Media | 1316 (47.34) | 691 (44.24) | 206 (39.39) | 123 (38.32) | |
| 852 (30.65) | 497 (31.82) | 180 (34.42) | 122 (38.01) | ||
| Community | 431 (15.50) | 262 (16.77) | 102 (19.50) | 54 (16.82) | |
| Other | 29 (1.04) | 14 (0.90) | 3 (0.57) | 2 (0.62) | |
Note: Missing or “don't know” answers not shown in table. Cells show N (%).
Fisher's exact test whether the relative composition between Technology Methods, Media, Print, Community, and Other differs between the actually randomized and all other phone screened that were not randomized.
3. Results
3.1. Recruitment flow and study eligibility
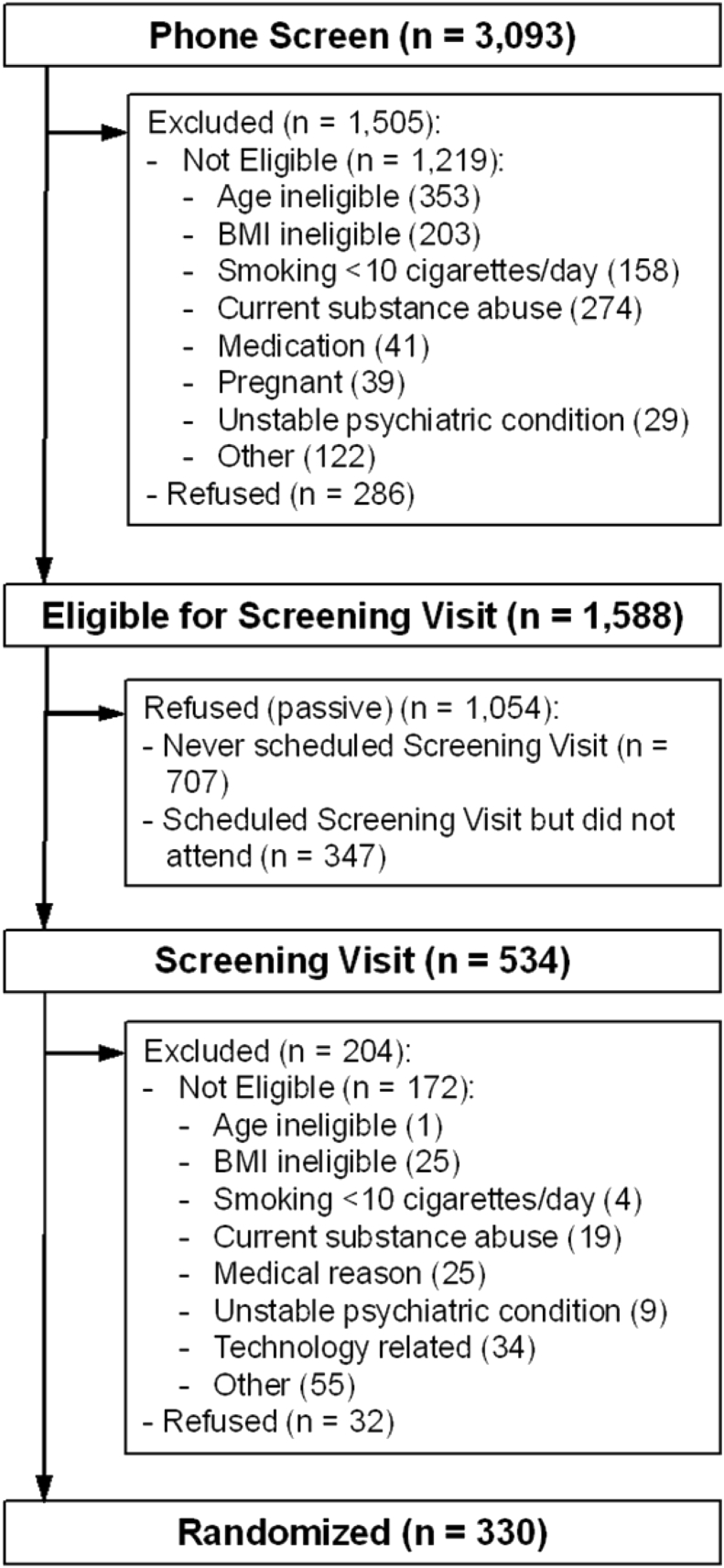
Recruitment flow for the TARGIT study is shown in Fig. 1. Of the 3093 participants who took part in PS, 9.25% (n = 286) declined further participation during the call. Another 39.41% (n = 1219) were not eligible with age, current substance abuse, and BMI being the most common reasons. More than half of callers were found to be eligible for continued screening (51.34%; n = 1588). Of those eligible following PS, 44.52% (n = 707) declined scheduling SV despite several attempts to make a convenient on-site appointment. There were 21.85% (n = 347) who scheduled an SV, but never attended despite multiple appointment reschedules. Of the 534 participants who completed an SV, 32.21% (n = 172) were not eligible due to technology-related, BMI, and medical conditions being the most common reasons along with an additional 5.99% (n = 32) refusing further participation. By the end of screening, there were 330 participants eligible (i.e., 10.67% of all initial PS contacts) and who were randomly assigned to either proactive Quitline or proactive Quitline plus Weight Management Intervention delivered via interactive technology.
Fig. 1.
Recruitment flow from first phone contact to randomization. Note: “Technology related” includes “Did not demonstrate ability to respond to email” [3] and “Did not demonstrate ability to access specific web site” (31).
Participants at PS were majority Black (59.80%), female (52.66%), normal or over weight (combined 62.42%), 29.5 years old, and smoked 18.4 cigarettes per day, see Table 1.
Table 1.
Demographics and characteristics of initial contacts (PS) and subsequent subgroups of those eligible after the PS for participation in the SV, actually participated in SV, and ultimately recruited (randomized).
| Variable | Phone screen (N = 3,093) | Eligible after phone screen (N = 1,588) | Screening visit (N = 534) | Randomized (N = 330) | p-value |
|---|---|---|---|---|---|
| Age mean (std) | 29.5 (6.9) | 28.4 (4.6) | 29.46 (4.32) | 29.70 (4.18) | 0.623a |
| Gender N (%) | |||||
| Male | 1212 (47.34) | 776 (49.40) | 277 (51.87) | 169 (51.21) | 0.132a |
| Female | 1348 (52.66) | 795 (50.60) | 257 (48.13) | 161 (48.79) | |
| Ethnicity N (%) | |||||
| Hispanic or Latino | 64 (2.51) | 45 (2.87) | 15 (2.84) | 11 (3.33) | 0.308a |
| Not Hispanic or Latino | 2482 (97.49) | 1525 (97.13) | 514 (97.16) | 319 (96.67) | |
| Race (grouped) N (%) | |||||
| Black or African American (only selection) | 1525 (59.80) | 868 (55.39) | 229 (42.88) | 123 (37.27) | <0.001a |
| White (only selection) | 946 (37.10) | 642 (40.97) | 275 (51.5) | 189 (57.27) | |
| Other (incl. multiple) | 79 (3.10) | 57 (3.64) | 30 (5.62) | 18 (5.45) | |
| Education N (%) | |||||
| At most high school graduate of GED | 172 (36.83) | 108 (32.73) | 0.004b | ||
| At least some vocational of training school after high school | 295 (63.17) | 222 (67.27) | |||
| Personal income (annual) | |||||
| < $16,000 | 217 (46.47) | 144 (43.64) | 0.001b | ||
| $16,000 – $49,999 | 185 (39.61) | 142 (43.03) | |||
| >= $50,000 | 37 (7.92) | 31 (9.39) | |||
| Don't know | 28 (6.00) | 13 (3.94) | |||
| Marital Status N (%) | |||||
| Single or casually dating | 194 (41.45) | 124 (37.69) | 0.021b | ||
| Married or other committed relationship | 248 (52.99) | 188 (57.14) | |||
| Separated, divorced, or widowed | 26 (5.56) | 17 (5.17) | |||
| Smoking data mean (std) | |||||
| Cigarettes smoked per day | 18.4 (12.0) | 19.0 (8.5) | 18.93 (8.15) | 18.23 (6.79) | 0.808a |
| BMI N (%) | |||||
| Underweight (<18.5) | 25 (0.96) | 6 (0.38)c | 2 (0.37) | 0 (0.00) | <0.001a,e |
| Normal (18.5–24.9) | 817 (31.27) | 487 (30.67) | 131 (24.53) | 76 (23.03) | |
| Overweight (25.0–29.9) | 814 (31.15) | 532 (33.50) | 188 (35.21) | 124 (37.58) | |
| Obese (>30) | 957 (36.62)d | 563 (35.45) | 213 (39.89) | 130 (39.39) | |
Note: Education, income, and marital status was not collected at PS but first during SV. Because screening was terminated as soon as the first ineligibility reason was encountered, most variables have missing data (n missing not shown in table).
Comparing randomized group vs. all others in PS group.
Comparing randomized group vs. all others attending SV.
Self-reported underweight did not automatically preclude individuals from attending the onsite SV: of these 6 individuals 4 never scheduled a SV and the remaining 2 attended the SV but were not enrolled/randomized.
Of these n = 174 (18%) were Obese > 40.
When restricting comparison to those with eligible BMI (20–40) in PS group the p-value is <0.001.
Almost all were non-Hispanic (97.49%), with no difference in the proportion of males versus females between races (p = 0.317, data not shown). Age and number of daily cigarettes did not predict final study enrollment (p = 0.623 and 0.808, respectively), however, several demographic variables were associated with recruitment flow, most notably was race. The final study cohort randomized was comprised of mostly Whites (57.27%) despite representing only 37.10% of PS-eligible participants (initial contacts; p < 0.001). During PS a larger proportion of Blacks (43.08%) compared to Whites (32.14%) did not qualify to proceed on to SV with the reasons for ineligibility being different (p < 0.001 for association test of race vs. type of ineligibility reason; data not shown): Blacks were more likely to be excluded due to current substance abuse (34.46% vs. 18.99% of Whites), but less likely due to prescription medications (2.18% vs. 13.97% of Whites) or an unstable psychiatric condition (2.57% vs. 7.26% Whites). Other major exclusions affecting both races equally included age (13.66% and 12.85%, respectively) and BMI (21.19% vs. 17.88%). There were no differences between Blacks and Whites with respect to refusing to participate given equal eligibility (14.90% Blacks vs. 16.30% Whites, p = 0.420), but of those who could have attended SV, only 26.38% of Blacks while 42.83% of Whites attended the in-person screening (p < 0.001).
Among those who were eligible but did not attend a SV, approximately two-thirds never scheduled the in-person visit, and the remaining one-third scheduled but did not attend despite several attempts to reschedule a convenient appointment. No differences were observed between Blacks and Whites with respect to these two “modes” of not continuing with the in-person visit (p = 0.298; data not shown). Differences between races were noted again at SV (p < 0.001), whereby, 68.73% of Whites and 53.71% of Blacks proceeded to RV. The reasons for exclusion at SV were markedly different between the racial groups (p = 0.009). Similar to recruitment flow at PS, Blacks were relatively more likely to be excluded due to current substance abuse (13.98% vs. 8.82% of Whites), while relatively less likely to be excluded due to medical reasons (10.75% vs. 19.12%, respectively) or unstable psychiatric conditions (1.08% vs. 10.29%, respectively). Blacks were also more likely to exceed the upper BMI criteria at SV (13.98% vs. 4.41% of Whites with similar proportions for low BMI exclusion of 5.38% and 4.41%, respectively) and were more often excluded due to technology-related reasons (22.58% vs. 16.18%, respectively).
3.2. Other baseline characteristics affecting recruitment for TARGIT
There were differences in men and women with respect to reasons for ineligibility during PS (p < 0.001; ignoring gender specific pregnancy-related ineligibility). Males were relatively more likely to be excluded for current substance abuse (41.14% vs. 24.39% females) and were less likely BMI-ineligible (13.71% vs. 28.46%, respectively). There were no systematic differences in eligibility between males and females at SV. About one out of five (34/172) persons excluded at SV was due to a technology-related reason which was an eligibility requirement not addressed during PS. Other demographic variables that influenced recruitment flow included education (p = 0.004; higher educated participants were more likely to be randomized), personal income (p = 0.001; higher income groups were more likely to be randomized), and marital status (p = 0.021; married individuals or those living in a committed relationship were more likely to be randomized). These variables were first collected at SV, thus, the comparisons are between those randomized versus those who attended SV but were not randomized. BMI (obtained at PS) is clearly associated with recruitment flow with persons in the normal BMI category being less likely to randomize (p < 0.001). This finding remains consistent even when limiting the comparison to only those that have BMI at PS comparison to only those within the eligible range for the TARGIT study (p < 0.007).
Differences in recruitment source are also of note during screening and randomization for TARGIT (Table 2). Traditional sources of recruitment (e.g., media, print, and community) yielded larger numbers of participants attending all visit types than any technology source. At PS, television ads (36.40%; subgroup of Media; not shown in Table 2), direct mail (24.12%; subgroup of Print), and word of mouth (13.35%; subgroup of Community) were the most common recruitment sources self-reported by participants whereas all types of technology combined (e.g., email, internet ad, social media ad, study website) were only self-reported by 5.47% of callers. However, at RV direct mail (31.21%) was the most commonly reported recruitment source above that of television ads (29.39%), with technology sources accounting for only 6.23% of those randomized. Of note, radio was seldom mentioned (only 6.14% of callers and 7.88% of randomized participants). Among those randomized, a significant association was found between race and the type of recruitment strategies reported (p-value = 0.030). Blacks compared to Whites were more likely to respond to media advertisement (48.78% and 31.67%, respectively), while Whites were more responsive to print materials (41.67% in Whites and 32.52% in Blacks). Fewer participants were reached by technology methods overall; 4.07% (n = 5) and 7.78% (n = 14) among either Blacks or Whites, respectively (Table 3).
Table 3.
Self-reported source of information about the TARGIT study that triggered interest and contact to the study for Blacks and Whites.
| Source | Phone screen (N = 3093) |
Randomized (N = 330) |
||||
|---|---|---|---|---|---|---|
| Black/AA (n = 1525) | White (n = 946) | p-valuea | Black/AA (n = 123) | White (n = 189) | p-valuea | |
| Technology methods | 59 (3.91) | 82 (8.85) | <0.001 | 5 (4.07) | 14 (7.78) | 0.030 |
| Traditional methods | ||||||
| Media | 753 (49.93) | 388 (41.86) | 60 (48.78) | 57 (31.67) | ||
| 458 (30.37) | 298 (32.15) | 40 (32.52) | 75 (41.67) | |||
| Community | 224 (14.85) | 152 (16.40) | 18 (14.63) | 32 (17.78) | ||
| Other | 14 (0.93) | 7 (0.76) | 0 (0.00) | 2 (1.11) | ||
Note: Missing or “don't know” answers not shown in table. Cells show N (%).
Fisher's exact test whether the relative composition between Technology Methods, Media, Print, Community, and Other differs between the two races.
Interestingly, significant differences in recruitment source existed between male and female callers (p = 0.001) but not in the subgroup of randomized participants (p = 0.815) where the vast majority of males and females were recruited by media or print, (Table 4).
Table 4.
Self-reported source of information about the TARGIT study that triggered interest and contact to the study by gender.
| Source | Phone screen (N = 3093) |
Randomized (N = 330) |
||||
|---|---|---|---|---|---|---|
| Females (n = 1332) | Males (n = 1191) | p-valuea | Females (n = 158) | Males (n = 163) | p-valuea | |
| Technology methods | 66 (4.95) | 81 (6.80) | 0.001 | 10 (6.33) | 10 (6.13) | 0.815 |
| Traditional methods | ||||||
| Media | 667 (50.08) | 504 (42.32) | 62 (39.24) | 61 (37.42) | ||
| 391 (29.35) | 396 (33.25) | 61 (38.61) | 61 (37.42) | |||
| Community | 194 (14.56) | 201 (16.88) | 25 (15.82) | 29 (17.79) | ||
| Other | 14 (1.05) | 9 (0.76) | 0 (0.00) | 2 (1.23) | ||
Note: Missing or “don't know” answers not shown in table. Cells show N (%).
Fisher's exact test whether the relative composition between Technology Methods, Media, Print, Community, and Other differs between the two genders.
Neither ethnicity (p = 0.309), BMI (p = 0.324) nor the number of daily cigarettes (p = 0.446) were associated with the recruitment source among screened callers. Likewise, marital status (p = 0.968), education (p = 0.396), and income (p = 0.075) lacked association with recruitment source among SV-participants. Screened callers' age was significantly associated with recruitment source (p < 0.001), whereby technology-recruited callers were on average about 3 years younger than the traditional-media recruited participants (data not shown). However, those proceeding to RV are of comparable age and the association of age with recruitment source was only marginally present among the subgroup of randomized individuals (p = 0.054). The shift in statistically significant association of age with recruitment source is largely the result of our eligibility criteria that reduced the age range from 15 to 72 years of age (at phone screen; third quartile is 34 years old) to 18 to 35 (required by eligibility criteria for randomization).
A multivariable logistic regression analysis with randomization “yes/no” as dependent variable confirmed that age (p < 0.001; OR = 1.076, 95% CI. 1.048–1.105), race (p < 0.001; Black vs White OR = 0.360, 95% CI 0.280–0.463) and BMI (p = 0.002; Overweight vs Normal weight OR = 1.596, 95% CI 1.161–2.193; Obese vs. Normal weight OR = 1.710, 95% CI 1.248–2.341) obtained at PS are in combination “predictive” of successful enrollment whereas ethnicity, gender, and number of daily cigarettes do not predict subsequent enrollment (i.e., stepwise backward selection starting with named variables and with a cutoff of marginal p-value of 0.05). In this multivariable analysis, recruitment source is not included with a (marginal) p-value of 0.052. Despite the above statistically significant ORs, the predictive ability of the model is very limited with a Receiver Operating Characteristic (ROC) Area Under the Curve (AUC) of 0.678 (95% CI. 0.648–0.708). Based on the AUCs in univariate-versions of the model race only (AUC = 0.622) is the relatively best predictor followed by age only (AUC = 0.597) and BMI only (0.553).
4. Discussion
Failure to recruit adequate numbers along with insufficient diversity in the study population can compromise the results of clinical trials both in terms of not having adequate power to test the study hypotheses as well as not being able to generalize the treatment results to broader groups [14]. TARGIT successfully recruited the targeted sample size (N = 330). To accomplish this goal, focused recruitment strategies to increase study participation among historically hard to reach minority groups [15] representative of our community included multiple mass mailings, traditional ads on minority media outlets, community outreach activities, and social media advertising. Although initial recruitment efforts yielded a racial composition interested in participating that was similar to the Mid-south community at large (3093 of whom approx. 60% were African American and approx. 40% were White [16], [17]); TARGIT experienced a disproportionate loss of minorities during recruitment as well as a prolonged recruitment period due to either study ineligibility or not completing screening activities. Black participants were found to be ineligible at PS and SV to a greater extent than Whites and less likely to attend the in-person screening visit if found eligible by telephone. Specifically, the proportion of Blacks enrolled in one of two TARGIT interventions to stop smoking and not gain weight dropped from 59.80% to 37.27% among all interested callers who were ultimately randomized to treatment (Table 1). Final randomized participants into TARGIT were approximately 60% White and 40% Black; the inverse of call responders.
Although we are unable to make specific conclusions from these data regarding the disproportionate loss of minorities during recruitment, there is some previous work that helps shed light on the findings. For example, numerous barriers have been cited previously as reasons for inadequate study participation for recruitment of hard to reach study populations. Common challenges for participating in research studies may include lack of community involvement, misperceptions or distrust of the research purpose or of the informed consent process, language or literacy issues, study participation and/or treatment burden, and lack of interest and/or awareness of the health risk [18]. Further, personal circumstances such as lack of transportation, safety concerns, child care or family demands, job schedule conflicts, health issues, and being unwilling or unable to sustain regular contact with study staff and/or scheduled study visits may also effect recruitment success [19]. During TARGIT recruitment, more current substance abuse beyond that of smoking, medical reasons, and not completing web-based food diaries that adversely impacted study eligibility was observed among our minority participants. Exploring and potentially addressing these barriers to participation in future studies is warranted.
Therefore, given the interest seen by the positive responses to TARGIT advertising, coupled with the loss of follow through in attendance/adherence to recruitment processes suggests that general community perceptions of the value of the research was favorable; however, personal circumstances may have presented individual challenges to further recruitment participation. The proportion lost following phone eligibility was clearly more affected by inability to contact (two-thirds) and failure to attend (one-third) than eligibility which could indicate a lack of general interest in the program after the phone screen. Another interesting observation in this study was how gender composition remained almost a 50–50 split from call in to randomization (p = 0.132) with women having a slight majority during PS (52.66%) and remaining similarly proportioned in the final randomized sample (48.79%). Previously, studies have shown that men, especially minorities, have been less likely to be enrolled into lifestyle change trials for weight loss or weight gain prevention [20]. However, TARGIT demonstrates that men were equally as willing to stop smoking and not gain weight as women were. There has been limited information on recruiting younger persons into behavioral prevention trials [9], with recent data suggesting this group can be challenging [21]. TARGIT showed appeal to young adults as we successfully randomized age eligible participants (18–35 years) seeking to prevent cessation-related weight gain. One additional point of interest was how age seemed to matter in regards to responsivity to technology-based recruitment methods. Specifically, those who responded to technology methods were on average three years younger.
Community partnerships and involvement, taking a bottom-up approach to clinical research and identifying community needs that could enhance participation and excitement for an intervention and health research may be important future directions for research into effective recruitment methods for clinical trials [22]. Another important implication of our findings is that use of technology appeared to be a very challenging method to recruit participants into the clinical trial even among this young adult group. Although we saturated the community with all possible recruitment sources, the ease of access to all technology based methods may have limited the responsivity particularly among minority participants. Future research could be aimed at identifying if a more effective use of technology as a recruitment strategy could be identified and what the effort/cost ratio per recruited participant would be.
Most randomized TARGIT participants responded to traditional recruitment methods (e.g., print, media) over that of technology-based approaches. It is also important to note that multiple methods of recruitment were necessary to enroll the final sample size in TARGIT and when a recruitment method appeared to be less effective alternate methods were employed. Further, when recruitment into the trial was slow a small modification of eligibility criteria allowed TARGIT to randomize additional persons with slightly lower BMI. Thus, it is important for research studies to monitor the recruitment process and continually re-evaluate the success of the recruitment methods used and whether eligibility criteria need to be modified. Also of note in TARGIT, several methods of recruitment were occurring simultaneously thus exposure to 2 or more methods for many of the participants is likely. For example, a participant may receive a recruitment postcard from the study (sent to every age eligible person in the area) and also hear the television advertisements running. Saturation of message delivery via multiple recruitment methods may be important to the ultimate response; however, TARGIT did not collect information about the potential exposure to more than one recruitment method and thus cannot contribute to its importance. The importance of printed study materials resulting in the majority of randomized participants suggests that it is beneficial to have these traditional methods available even if newer methods are being utilized. Future research into message saturation and exposure may be informative to improve recruitment into behavioral intervention trials.
5. Conclusions
Recruitment into longer term behavioral change intervention trials can be challenging and multiple methods are often required to recruit hard to reach groups. A limitation of this study was not being able to quantify the effort and cost of each recruitment method when comparing traditional versus technological approaches to study participation. Further, the differences that were observed between successful recruitment strategies for screenings versus randomization into the trial need additional investigation. Reaching a representative sample of the community and enrolling an adequate number of eligible study participants during the recruitment period limited TARGIT's ability to distinguish frequency and/or repetition of recruitment methods by participant self-report. Therefore, in addition to lower cost easily disseminated technology-based approaches to participation in research studies, traditional recruitment methods such as print and media remain important in the overall armamentarium for researchers to attain overall study goals. Examination of the importance of message saturation and tailored recruitment methods among hard to reach groups may be important for identifying optimal recruitment strategies. Moreover, determining the advantages of media versus technology driven approaches to study participation among minorities and younger persons could be helpful in informing future recruitment into behavioral intervention trials.
References
- 1.Leventhal H., Nerenz D.R., Leventhal E.A., Love R.R., Bendena L.M. The behavioral dynamics of clinical trials. Prev. Med. 1991;20(1):132–146. doi: 10.1016/0091-7435(91)90014-u. PubMed PMID: 2008422. [DOI] [PubMed] [Google Scholar]
- 2.Glasgow R.E., Strycker L.A., Kurz D., Faber A., Bell H., Dickman J.M. Recruitment for an internet-based diabetes self-management program: scientific and ethical implications. Ann. Behav. Med. 2010;40(1):40–48. doi: 10.1007/s12160-010-9189-1. a publication of the Society of Behavioral Medicine. PubMed PMID: 20411443. [DOI] [PubMed] [Google Scholar]
- 3.Hollis J.F., Satterfield S., Smith F., Fouad M., Allender P.S., Borhani N. Recruitment for phase II of the Trials of Hypertension Prevention. Effective strategies and predictors of randomization. Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann. Epidemiol. 1995;5(2):140–148. doi: 10.1016/1047-2797(94)00058-2. PubMed PMID: 7795832. [DOI] [PubMed] [Google Scholar]
- 4.King A.C., Cao D.C., Southard C.C., Matthews A. Racial Differences in Eligibility and Enrollment in a Smoking Cessation Clinical Trial. Health Psychol. 2011;30(1):40–48. doi: 10.1037/a0021649. PubMed PMID: WOS:000287287800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuyemi K.S., Cox L.S., Nollen N.L., Snow T.M., Kaur H., Choi W. Baseline characteristics and recruitment strategies in a randomized clinical trial of African-American light smokers. Am. J. Health Promot. 2007;21(3):183–191. doi: 10.4278/0890-1171-21.3.183. http://dx.doi.org/10.4278/0890-1171-21.3.183 PubMed PMID: 17233236. [DOI] [PubMed] [Google Scholar]
- 6.Webb M.S., Seigers D., Wood E.A. Recruiting African American smokers into intervention research: relationships between recruitment strategies and participant characteristics. Res. Nurs. Health. 2009;32(1):86–95. doi: 10.1002/nur.20299. PubMed PMID: WOS:000262521500009. [DOI] [PubMed] [Google Scholar]
- 7.Mount D.L., Davis C., Kennedy B., Raatz S., Dotson K., Gary-Webb T.L. Factors influencing enrollment of African Americans in the look ahead trial. Clin. Trials. 2012;9(1):80–89. doi: 10.1177/1740774511427929. PubMed PMID: 22064686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin H.J., O'Connor H.T., Rooney K.B., Steinbeck K.S. Effectiveness of strategies for recruiting overweight and obese Generation Y women to a clinical weight management trial. Asia Pac. J. Clin. Nutr. 2013;22(2):235–240. doi: 10.6133/apjcn.2013.22.2.16. PubMed PMID: 23635367. [DOI] [PubMed] [Google Scholar]
- 9.Gokee-LaRose J., Gorin A.A., Raynor H.A., Laska M.N., Jeffery R.W., Levy R.L. Are standard behavioral weight loss programs effective for young adults? Int. J. Obes. 2009;33(12):1374–1380. doi: 10.1038/ijo.2009.185. PubMed PMID: 19786967; PubMed Central PMCID: PMC2996044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaya F.T., Gbarayor C.M., Yang H.W.K., Agyeman-Duah M., Saunders E. A perspective on African American participation in clinical trials. Contemp. Clin. Trials. 2007;28(2):213–217. doi: 10.1016/j.cct.2006.10.001. PubMed PMID: WOS:000243716100011. [DOI] [PubMed] [Google Scholar]
- 11.Heffner J.L., Wyszynski C.M., Comstock B., Mercer L.D., Bricker J. Overcoming recruitment challenges of web-based interventions for tobacco use: the case of web-based acceptance and commitment therapy for smoking cessation. Addict. Behav. 2013;38(10):2473–2476. doi: 10.1016/j.addbeh.2013.05.004. PubMed PMID: 23770645; PubMed Central PMCID: PMC3725211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Free C., Phillips G., Galli L., Watson L., Felix L., Edwards P. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. PubMed PMID: 23349621; PubMed Central PMCID: PMC3548655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lytle L.A., Svetkey L.P., Patrick K., Belle S.H., Fernandez I.D., Jakicic J.M. The early trials: a consortium of studies targeting weight control in young adults. Transl. Behav. Med. 2014;4(3):304–313. doi: 10.1007/s13142-014-0252-5. PubMed PMID: 25264469; PubMed Central PMCID: PMC4167899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey K.R. Generalizing the results of randomized clinical trials. Control Clin. Trials. 1994;15(1):15–23. doi: 10.1016/0197-2456(94)90024-8. PubMed PMID: 8149769. [DOI] [PubMed] [Google Scholar]
- 15.Yancey A.K., Ortega A.N., Kumanyika S.K. Effective recruitment and retention of minority research participants. Annu. Rev. Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. PubMed PMID: 16533107. [DOI] [PubMed] [Google Scholar]
- 16.Shelby County QuickFacts from the U.S. Census Bureau [Internet] 2014. U.S. Census Bureau: State and County QuickFacts; Population Estimates. [Google Scholar]
- 17.Memphis (city) QuickFacts from the US Census Bureau [Internet] 2014. U.S. Census Bureau: State and City QuickFacts; Population Estimates. [Google Scholar]
- 18.Lovato L.C., Hill K., Hertert S., Hunninghake D.B., Probstfield J.L. Recruitment for controlled clinical trials: Literature summary and annotated bibliography. Control Clin. Trials. 1997;18(4):328–352. doi: 10.1016/s0197-2456(96)00236-x. PubMed PMID: WOS: A1997XP11800008. [DOI] [PubMed] [Google Scholar]
- 19.Coday M., Boutin-Foster C., Goldman Sher T., Tennant J., Greaney M.L., Saunders S.D. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Ann. Behav. Med. 2005;29(Suppl):55–65. doi: 10.1207/s15324796abm2902s_9. a publication of the Society of Behavioral Medicine. PubMed PMID: 15921490. [DOI] [PubMed] [Google Scholar]
- 20.Pagoto S.L., Schneider K.L., Oleski J.L., Luciani J.M., Bodenlos J.S., Whited M.C. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity. 2012;20(6):1234–1239. doi: 10.1038/oby.2011.140. PubMed PMID: 21633403. [DOI] [PubMed] [Google Scholar]
- 21.Tate D.F., LaRose J.G., Griffin L.P., Erickson K.E., Robichaud E.F., Perdue L. Recruitment of young adults into a randomized controlled trial of weight gain prevention: message development, methods, and cost. Trials. 2014;15:326. doi: 10.1186/1745-6215-15-326. PubMed PMID: 25128185; PubMed Central PMCID: PMC4150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Las Neuces D.D.H.K., DiGirlamo A., Hicks L. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv. Res. 2012;3(47):1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]