Abstract
The conventional method for measuring brain ATP synthesis is 31P saturation transfer (ST), a technique typically dependent on prolonged pre-saturation at γ-ATP. In this study, ATP synthesis rate in resting human brain is evaluated using EBIT (Exchange Kinetics by Band Inversion Transfer), a technique based on slow recovery of γ-ATP magnetization in the absence of B1 field following co-inversion of PCr and ATP resonances with a short adiabatic pulse. The unidirectional rate constant for the Pi → γ-ATP reaction is 0.21 ± 0.04 sec−1 and the ATP synthesis rate is 9.9 ± 2.1 mmol/min/kg in human brain (n = 12 subjects), consistent with the results by ST. Therefore EBIT could be a useful alternative to ST in studying brain energy metabolism in normal physiology and under pathological conditions. In addition to ATP synthesis, all detectable 31P signals are analyzed to determine the brain concentration of phosphorus metabolites including UDPG at ~ 10 ppm, a previously reported resonance in liver tissues and now confirmed in human brain. Inversion recovery measurements indicate that UDPG, like its diphosphate analogue NAD, has a shorter apparent T1 than monophosphates (Pi, PMEs and PDEs) but longer than triphosphate ATP, highlighting the significance of 31P-31P dipolar mechanism in T1 relaxation of polyphosphates. Another interesting finding is the observation of ~40% shorter T1 at intracellular Pi relative to extracellular Pi, attributed to the modulation by the intracellular phosphoryl exchange reaction Pi ↔ γ-ATP. The sufficiently separated intra- and extra-cellualr Pi signals also permit the distinction of pH between intra- and extra-cellular environments (pH 7.0 vs pH 7.4). In summary, the quantitative 31P MRS in combination with ATP synthesis, pH and T1 relaxation measurements may offer a promising tool to detect biochemical alternations at early stages of brain dysfunctions and diseases.
Keywords: brain metabolism, magnetization transfer, ATP, chemical exchange, T1 relaxation time, inversion transfer, pH, MRS
Graphical abstract
INTRODUCTION
The rate of ATP synthesis may be measured in functioning tissue by 31P NMR magnetization transfer (MT) methods (1–3). However, due to presence of multiple competing magnetization exchange pathways and nature of MT pulsing technique, in vivo applications of 31P MT for ATP synthesis is generally challenging (3,4), especially in the brain where one has to consider additional limiting factors such as tight SAR restriction, and the presence of compartmentalized phosphates (2) and broad signal from less mobile tissue phosphates (5). So far, the only 31P MRS technique that has proven useful for measuring human brain ATP synthesis is saturation transfer (ST), a subset of MT method based on the 31P chemical exchange saturation transfer between Pi and γ-ATP (1). Technically, ST relies on selective irradiation of γ-ATP with a long RF pulse or pulse train, resulting in a reduced Pi signal through ATPase mediated reaction (Pi → γ-ATP). While ST is commonly used for probing ATP energy metabolism, it typically requires a prolonged irradiation at γ-ATP (5 – 9 sec) for two major reasons: 1) to build up a steady-state MT effect at Pi, so that the rate constant of ATP synthesis kPi → γATP, can be easily evaluated using a simplified formula (3); and 2) to overcome the rapid drainage of γ-ATP magnetization to other “unwanted” pathways, especially the exchange reaction PCr ↔ γ-ATP, which occurs at a much faster rate due to the larger pool size of PCr and the higher enzymatic activity of creatine kinase (CK) (2,4). Unfortunately, the prolonged RF pulsing is prone to undesirable side effects such as increasing SAR exposure, and off-resonance saturation, the so-called “spillover” effect (6,7). This latter artifact is expected to worsen at an increased magnetic field due to shortened T1 and T2 at γ-ATP (which demands a higher B1 power for saturation) (4,8,9).
Selective inversion of the γ-ATP is an alternative approach to ST (15), but the magnitude of its magnetization transfer to Pi is small (4,10). However, when γ-ATP is co-inverted with PCr using a band inversion approach termed EBIT (Exchange Kinetics by Band Inversion Transfer), a multi-fold enhancement in MT is achieved, resulting in a Pi reduction comparable to that generated by ST but with much improved time efficiency (11). A key concept in EBIT is the role of PCr as a “magnetization buffer”. Upon co-inversion with γ-ATP by EBIT, PCr continuously replenishes a diminishing γ-ATP pool via the rapid exchange reaction PCr ↔ γ-ATP (11,12). This leads to an increased exchange time for the MT effect to build up between Pi and γ-ATP, equivalent to several seconds of ST saturation pulsing. The primary aim of this study is to evaluate the feasibility of this EBIT technique for measuring ATP synthesis rate in the human brain. Since band inversion transfer impacts much of the 31P NMR spectrum (all of the ATP signals plus phosphocreatine and UDP glucose), we also measured the apparent T1 relaxation times of all observable phosphates and their concentrations.
METHODS
Human Subjects
The protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Prior to the MRS study, informed written consent was obtained from all participants. Twelve subjects (7 male and 5 female), aged 43 ± 15 yr, BMI 24 ± 4, resting heart rate 70 ± 13, and peripheral capillary oxygen saturation (SpO2) 96 ± 2% participated the study. All subjects were in good general health with no history of peripheral vascular, systemic, myopathic, cancer, psychiatric or neurodegenerative diseases. Heart rate and blood oxygen saturation levels were monitored during the scan. The study was well-tolerated by all subjects.
MRS Protocol
All subjects were positioned head-first and supine in the MRI scanner (7T Achieva, Philips Healthcare, Cleveland, OH), with the back of the head positioned in the center of detection RF coil (Philips Healthcare, Cleveland, OH). The coil was a partial volume, double-tuned 1H/31P quadrature TR coil consisting of two tilted, partially overlapping 10 cm loops. Axial, coronal, and sagittal T2-weighted turbo spin echo images were acquired for shimming voxel. Typical imaging parameters included field-of-view 180 × 180 mm (FOV), repetition time (tR) 2.5 s, echo time (TE) 80 ms, turbo factor 15, in-plane spatial resolution 0.6 × 0.7 mm2, slice thickness 6 mm, gap 1 mm, bandwidth 517 Hz, number of acquisitions (NA) one, and acquisition time 2.1 min. Second order 1H-based automatic volume shimming was applied prior to 31P spectral acquisitions.
Quantitative 31P spectra were acquired using a non-localized block-shaped excitation pulse with B1 59 μT, pulse width 0.22 ms, flip angle 55°, and pulse dead-time DT (the delay prior to FID sampling for suppression of the broad 31P signals from less mobile tissue phosphates) 0.6 ms, with the transmitter frequency centered at 700 Hz upfield from the resonance of PCr. Precautions were taken to avoid signal contribution from the neck muscles by positioning the head chin-up, aligning the occipital lobe at the coil iso-center, and placing a supporting cushion pad underneath the neck. Other 31P NMR parameters include TR 30 sec, sampling points 4 K, zero-filled to 8 K prior to FT, 6 acquisition averages and scan time 3 min. For quantitative comparison of different 31P peaks, the transmitter excitation profile was obtained by monitoring 31P signal intensity against transmitter carrier frequency which was varied from downfield PC to upfield β-ATP in 600 Hz step to cover the whole chemical shift range of all metabolites. The chemical shifts of all 31P metabolites were referenced to PCr at 0 ppm. Gaussian apodization (6 Hz) was applied to each FID prior to Fourier transformation using the scanner software (SpectroView, Philips Healthcare).
The EBIT sequence consisted of an adiabatic inversion pulse, followed by a variable post-inversion delay time t, a hard 90° readout pulse, and a recovery period with TR = 30 sec. A total of 12 delay times (tD = 3, 147, 341, 647, 1088, 1559, 2275, 3254, 4414, 5673, 7541 and 10000 msec) were used. The band-inversion pulse was a short trapezoid-shaped adiabatic pulse (pulse width pw = 42 msec, including 7 msec of pre- and post-ramp time), maximal B1 11.5 μT, inversion bandwidth of 2500 Hz, and centered at 150 Hz upfield from α-ATP at −7.5 ppm. The total data acquisition time for EBIT was 36 min for 6 acquisition average.
To measure the T1 relaxation time by the inversion-recovery method, a 5-ms adiabatic inversion pulse with a 7000 Hz bandwidth was used to invert all 31P peaks. A total of 12 post-inversion delay times (t = 5, 385, 801, 1259, 1768, 2343, 3002, 3775, 4708, 5887, 7489 and 10000 msec) were used at a constant TR of 30 sec. A preparatory dummy scan was applied prior to data acquisition. This experiment was conducted on 6 subjects.
31P Spectral Analysis
The frequency-domain 31P spectra were baseline corrected, and each of the 31P peaks was fitted by a Voigt lineshape (a combination of Gaussian and Lorentzian lineshape) using ACD software (Advanced Chemistry Development, Inc., Toronto, Canada). The area of the fitted 31P peaks (Sm) was converted to 31P metabolite concentration (Cm), in reference to γ-ATP as an internal standard (CγATP = 3 mM in occipital lobe of brain (1,2)), based on the following formula:
| [1] |
where ρm(Δf) represents the transmitter excitation profile for a given metabolite peak at offset frequency Δf (the difference between the resonance frequency of the 31P peak and the center frequency of the RF excitation pulse).
Evaluation of Rate Constant for ATP Synthesis
For the kinetic exchange reaction Pi↔ γ − ATP, the evolution of Pi Z-magnetization in the inversion delay time t, can be described by the Bloch-McConnell equation:
| [2] |
where is the rate of Pi Z-magnetization change at any given delay time t, and k denotes pseudo first-order kinetic rate constant, with the forward and reverse rate constants related by
| [3] |
in which and denote the Z-magnetization at Boltzmann thermal equilibrium for Pi and γ-ATP, respectively. Substituting kγATP→Pi in Eq [2] by Eq [3] gives:
| [4] |
where m is the normalized Z-magnetization defined by:
| [5] |
Since at mPi(t) = 1 at t = 0, the kinetic rate constant for ATP synthesis kPi→ATP can be evaluated by the following formula (derived from Equation [4]):
| [6] |
where mγATP(0) is γ-ATP Z-magnetization (normalized) immediately after inversion, which can be obtained experimentally; is the initial rate of Pi Z-magnetization change, which can be derived by fitting Pi Z-magnetization according to the following bi-exponential equation (with satisfaction of the boundary conditions mPi|t = 0 = 1 and mPi|t→∞ = 1):
| [7] |
where a, λ1 and λ2 are three fitting constants. This leads to the following first-order derivative:
| [8] |
from which can be obtained at t = 0. Thus the rate constant kPi→γATP can be evaluated either by Eq [6] using the initial magnetization rate as derived from bi-exponential data fitting to , or by Eq [4] using data fitting to the measured (1 − mPi(t)) and (mPi(t) – mγATP(t)).
Evaluation of pH in Brain
The intra- and extra-cellular pH values were evaluated from the chemical shift of the corresponding Pi peaks (δPi, in ppm) in reference to PCr (δ = 0 ppm) using the following formula:
| [9] |
where the H2PO4− ↔ H+ + HPO42− deprotonation constant pKa = 6.73, and the 31P limiting shifts δa = 3.275 ppm (for acidic protonated species H2PO4−) and δb = 5.685 ppm (for basic deprotonated species HPO42−) were used in the data analysis.
Evaluation of Mg2+ and MgATP Concentration in Brain
The free Mg2+ concentration in brain was evaluated from the chemical shift difference between α- and β-ATP (δα−β, in ppm) using the following formula (13):
| [10] |
where the MgATP effective disassociation constant kd = 0.05 mM and the limiting shift constants δATP = 10.82 ppm and δMgATP = 8.32 ppm were used in the data analysis.
The total concentration of all species of MgATP complexes was calculated by:
| [11] |
where [ATP]t represents the total concentration ATP in the brain (= 3 mM]).
Statistical Analysis
All data are reported as mean ± standard deviation, calculated using Matlab.
RESULTS
In Vivo Brain 31P Spectrum
A group-averaged (n = 12 subjects) in vivo 31P spectrum collected from resting human brain at 7T is shown in Figure 1a. After baseline correction (Figure 1b), the 31P signal intensities are evaluated by lineshape fitting (Figure 1c) and then converted to metabolite concentrations in reference to γ-ATP peak at −2.52 ppm assuming [ATP] = 3 mM (Table 1). The conversion takes into account the number of contributing phosphate groups and the non-uniformity of 31P excitation profile.
FIG. 1.
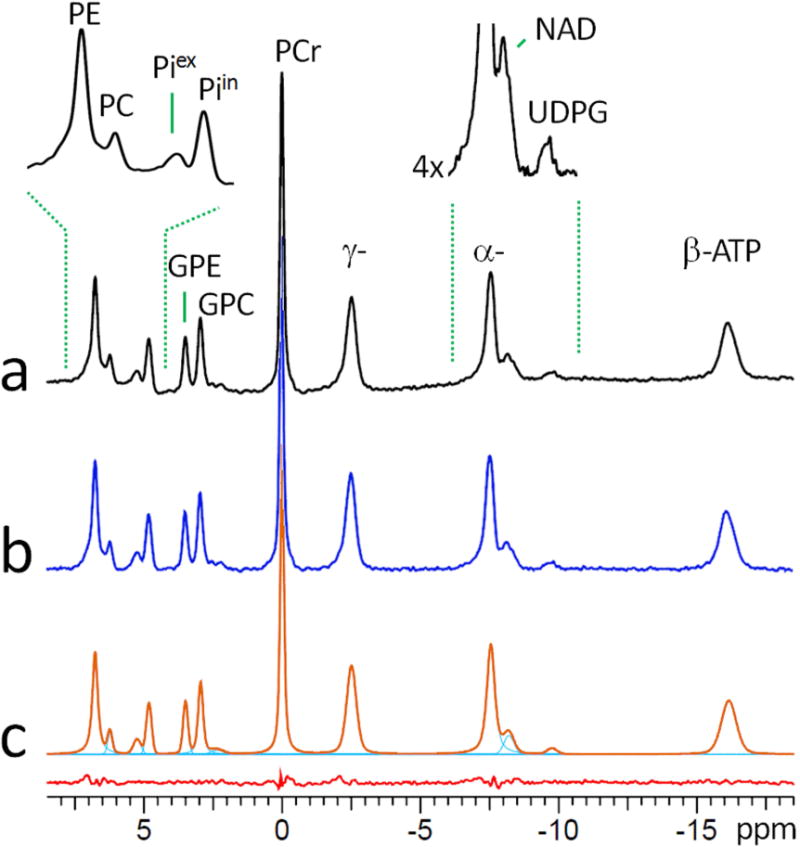
A 7T 31P MR spectrum, group-averaged for 12 subjects, acquired from resting human brain using a spatially nonselective single-pulse sequence with long TR of 30 sec, before (a) and after (b) baseline correction, and the result of 31P peak fitting (c). The fitting residual (bottom red trace) in (c) represents the spectral subtraction (b – c). Abbreviation: PE, phosphoethenolamine; PC, phosphocholine, GPE, glycerophosphoethanolamine; GPC, glycerophosphocholine; Piin and Piex, intra- and extracellular inorganic phosphate; PCr, phosphocreatine; ATP, adenosine triphosphate; NAD, nicotinamide adenine dinucleotide; UDPG, uridine diphosphate glucose.
Table 1.
31P Chemical shift (δ), line-width (LW1/2), apparent relaxation time (T1,app), and concentration (Conc.) of 31P metabolites in resting human brain at 7T (N = 12 subjects)
| # of PO4 groups | δ (ppm) | LW1/2 (Hz) | T1,app (s) | Conc. (mM) | |
|---|---|---|---|---|---|
| Monophosphates | |||||
| PE | 1 | 6.76 ± 0.05 | 71.1 ± 6.2 | 6.33 ± 1.10 | 2.27 ± 0.16 |
| PC | 1 | 6.24 ± 0.02 | 62.7 ± 13.2 | 4.31 ± 1.04 | 0.30 ± 0.12 |
| Piex | 1 | 5.24 ± 0.05 | 97.1 ± 27.7 | 5.80 ± 1.07 | 0.30 ± 0.09 |
| Piin | 1 | 4.82 ± 0.01 | 66.1 ± 5.3 | 3.70 ± 0.46 | 0.85 ± 0.11 |
| GPE | 1 | 3.50 ± 0.01 | 60.0 ± 5.5 | 6.79 ± 0.95 | 0.80 ± 0.18 |
| GPC | 1 | 2.95 ± 0.01 | 62.7 ± 4.4 | 5.82 ± 0.88 | 1.32 ± 0.18 |
| PCr | 1 | [0] | 48.4 ± 6.9 | 3.39 ± 0.17 | 4.37 ± 0.39 |
| diphosphates | |||||
| NAD | 2 | −8.21 ± 0.04 | 127.2 ± 17.8 | 2.07 ± 0.13 | 0.28 ± 0.13 |
| UDPG | 2 | −9.72 ± 0.04 | 101.7 ± 10.2 | 2.95 ± 0.36 | 0.08 ± 0.04 |
| triphosphates | |||||
| ATP | 3 | ||||
| γ- | −2.52 ± 0.01 | 122.6 ± 6.7 | 1.70 ± 0.15 | [3.0] | |
| α- | −7.56 ± 0.02 | 101.4 ± 3.3 | 1.35 ± 0.14 | 3.09 ± 0.23 | |
| β- | −16.15 ± 0.05 | 182.6 ± 11.9 | 1.13 ± 0.09 | 2.82 ± 0.25 | |
The 31P spectrum at 7T is able to clearly detect a small peak upfield at −9.7 ppm (Figure 1 inset), previously observed but not assigned in the human brain (8), although a similar signal has been observed in other spectra from human subjects (14). Based on its chemical shift, it is assigned to a di-phosphate metabolite UDPG (0.08 mM), a glucose precursor for synthesis of glycogen (13). Between PME and PDE peaks, two distinct pools of Pi with an intensity ratio of ~ 1 : 3 can also be observed, assigned to intracellular (δ(Piin) = 4.82 ppm) and extracellular (δ (Piex) = 5.24 ppm) inorganic phosphate (19), respectively. They correspond to a concentration of 0.85 mM (Piin) and 0.30 (Piex), and a pH value of 6.99 (Piin) and 7.38 (Piex).
ATP contributes three separate 31P signals at −2.5, −7.6 and −16.2 for γ-, α- and β-ATP, respectively. In principle, any one of them can be used as an internal reference to quantify other metabolites. However, given the fact that α-ATP signal is partially overlapped with neighboring NAD signal and that β-ATP signal is significantly broadened and away from other metabolite signals (Figure 1), we chose to use γ-ATP signal as the reference. By integral, the intensity ratio of γ- : α- : β-ATP signals is 1.00 : 1.03 ± 0.07 : 0.94 ± 0.09 after correction for excitation profile (Figure 4b). The slightly lower intensity of β-ATP relative to those of γ- and α-ATP is most likely due to T2 effect (4).
FIG. 4.
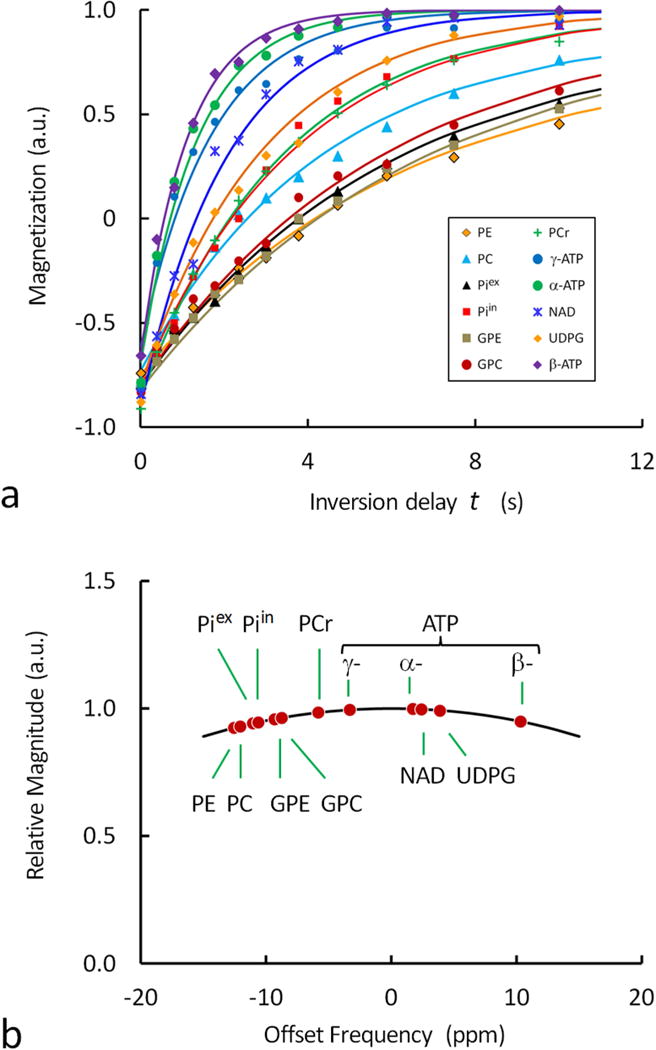
(a) Plots of normalized 31P magnetization against the inversion delay time t for the evaluation of apparent T1 relaxation time of brain metabolites. The data were from the signal intensity measurements in the inversion-recovery 31P spectra shown in Figure 3, and the solid curves represent the fitting based a mono-exponential process. Note the rapid relaxation of ATP, NAD and UDPG (P-metabolites with two or more coupling phosphate groups) as compared to PME, PDE, Pi and PCr (P-metabolites with a single phosphate group). (b) Excitation profile of the 31P spectrum showing the dependence of signal intensity on transmitter offset frequency. The excitation profile was used for correction of metabolite concentration based on signal intensity.
T1 Relaxation Time of 31P Metabolites
The T1 values of all 31P resonances are measured using the inversion recovery method (Figure 3). The dependence of 31P signal intensity on inversion delay time revealed a wide range of recovery rates for these different metabolites. Though the T1 relaxation (by dipolar interaction, plus CSA) may not be the only mechanism governing the inversion recovery of every 31P signal, all follow reasonably well a mono-exponential trend (Figure 4a). For the 12 31P signals observed, the recovery time constant is in the following order: β-ATP < α-ATP < γ-ATP < NAD < UDPG < PCr < Piin < PC < Piex ~ GPC < PE ~ GPE. The observed trend is that the 31P signals of diphosphates (including NAD, UDPG) and triphospahte (α-, β- and γ-ATP) recover more rapidly than those of monophosphates (including PE, PC, Pi, GPE, GPC and PCr). The recovery rates reflect the T1 relaxation times; however, for those 31P spins involving chemical exchange and/or cross-relaxation (such as Pi, PCr, α-, β- and γ-ATP), the observed recovery time constants represent the apparent T1 relaxation times.
FIG. 3.
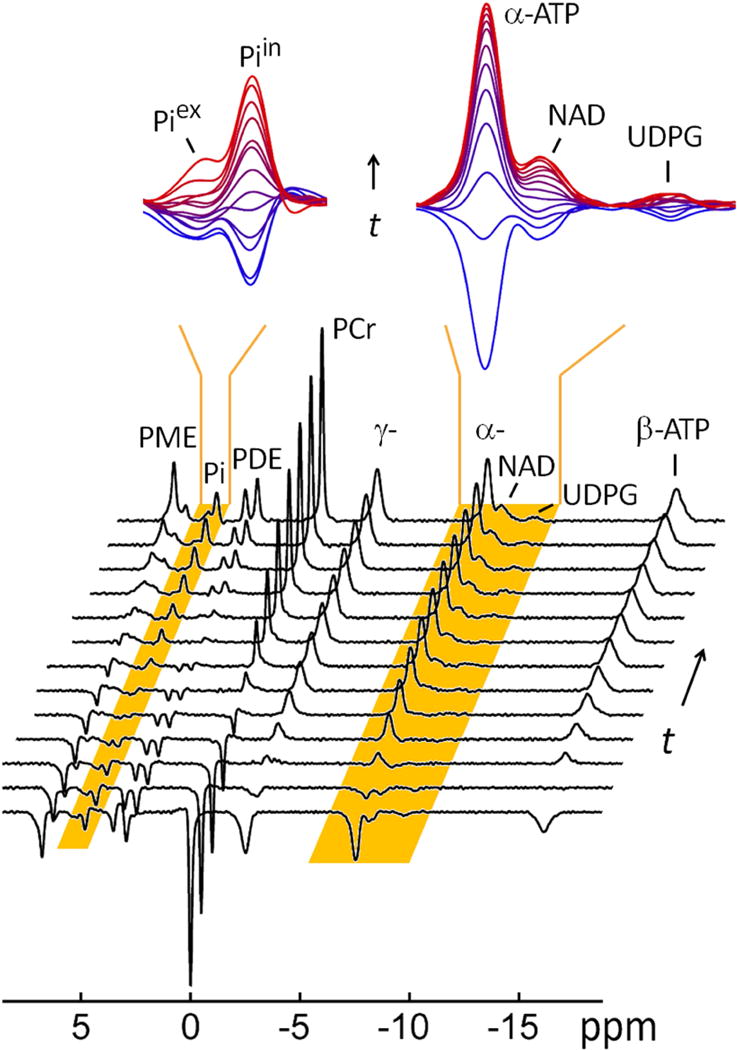
A typical 31P MR spectral series acquired from resting human brain at 7 T using a non-selective inversion-recovery sequence at TR 30 sec, NA = 6 and varying inversion delay time t. The insets showed the regional spectra enlarged for clear view of 31P signals of low-intensity in the chemical shift regions of 4.5 – 5.5 ppm (left) and −6.5 – −10.5 ppm (right); the spectra were colored coded from blue to red to indicate the gradual increase of the inversion delay time t. For comparison, the last trace of the spectral series represents the fully relaxed brain 31P MR spectrum acquired at TR 30 sec without applying the inversion pulse.
ATP Turnover Rate in Brain
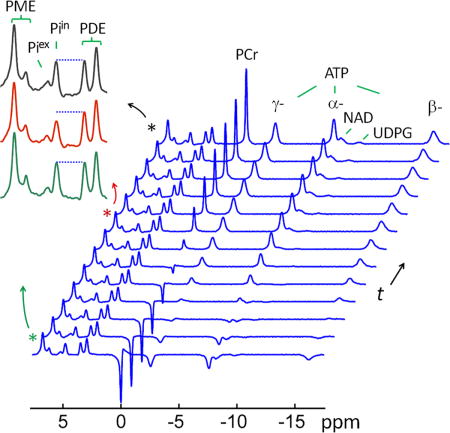
In order to evaluate ATP synthesis, the magnetization exchange effect between γ-ATP and Pi is monitored using EBIT sequence at varying inversion delay times (Figure 5). The band inversion pulse is tailored to invert PCr, α-, β- and γ-ATP but not any of the downfield spins (Pi, PMEs and PDEs). For both Piin and Piex, there is no measurable difference in signal intensity before and immediately after the band inversion. With an increase in inversion delay time, the Piin signal at 4.8 ppm clearly shows a trend of decreasing until it reaches an intensity minimum, occurred at t ~ 2.8 sec. After that time point, Piin starts to recover gradually toward its equilibrium state. The maximum Piin signal reduction is measured ~25% relative to its M° (Figure 5). In contrast, the Pex signal at 5.2 ppm remains largely invariant, i.e., independent of the change in inversion delay time, indicating that the extracellular phosphate is not involved in magnetization exchange with γ-ATP.
FIG. 5.
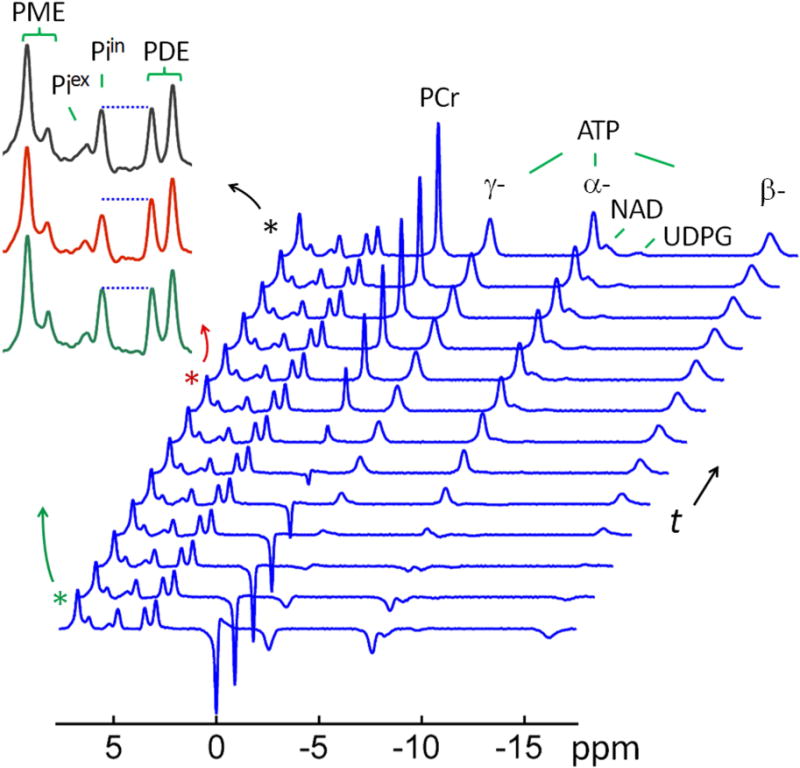
A typical 31P MR spectral series acquired with EBIT sequence using a band inversion pulse selectively inverting PCr and ATP spins, and followed by a varying delay time t All data are shown with identical y-scale. The insets show the regional spectra in the chemical shift region of 2 – 8 ppm, enlarged for a clear view of magnetization transfer effect at intracellular inorganic phosphate (Piin) from γ-ATP due to inversion delay (green trace: inversion delay time t = 3 ms; red trace: t = 3.2 sec; and black trace: fully relaxed spectrum). For comparison, the last trace of the spectral series represents the fully relaxed brain 31P MR spectrum acquired at TR 30 sec without applying the inversion pulse.
For γ-ATP, the inversion fraction is 55% (mγATP(0) = −0.55). After inversion, the recovery rate of γ-ATP signal lags behind α- and β-ATP, but it is faster than that of PCr (Figure 4). When Piin reaches its minimum intensity, the recovery of γ-ATP is at ~ 60% of its equilibrium magnetization (M°). According to Equation [4], at this time point, the net magnetization transfer between γ-ATP and Pi (= k (mPi − mγATP)) is balanced by the Pi relaxation (= (1 – mPi)/T1,Pi).
To evaluate the rate constant, kPi→γ-ATP, the mPi ~ t dataset collected for Piin is fitted to a bi-exponential equation (Equation [8]), yielding an initial rate constant (n = 12 subjects). Together with the measured γ-ATP inversion fraction (mγATP(0) = −0.55), this leads to a kPi→γ-ATP value of 0.203 ± 0.043 sec−1 ( ,Equation [6]), as comparable to kPi→γ-ATP = 0.214 ± 0.045 sec−1 obtained by fitting data to Equation [4], which also yields an intrinsic T1 value of 6.71 ± 1.82 sec for Piin (n = 12 subjects). With an intracellular phosphate concentration of 0.85 mM (Table 1), the unidirectional flux from Piin to γ- ATP in the brain is measured 10.9 ± 2.3 mM/min, or 9.9 ± 2.1 mmol/min/kg wet weight converted with a brain tissue density of 1.1 g/ml.
DISCUSSION
31P Metabolite Measurements
In this 31P MRS study, quantitative spectral data are acquired from resting human brain at 7T using a FID-based, long-TR, single pulse-acquire sequence. One important finding in this brain 31P MR study is the observation of UDPG (Figures 1, 3 and 5) at −9.72 ppm. Due to its low abundance, the presence of UDPG in vivo and in vitro has been rarely recognized or commented upon (8,15–17). Our UDPG assignment is based on an early in vivo 31P study by Malloy et al (13) in rat liver in which a high UDPG 31P signal was detected 2.15 ppm upfield from α-ATP, matching exactly the chemical shift observed here in the brain at 7T. UDPG 31P signal has also been identified in human liver at 7T (14), and as well as in cancer cells ex vivo using high-resolution 31P NMR (18). In retrospect, UDPG signal, though very weak, has appeared in some of the early 31P NMR spectra acquired from the human brain (1,8).
T1 Difference among Mono-, Di- and Tri-phosphates
The inversion recovery experiments reveal an interesting, previously unrecognized observation, that is, the (apparent) T1 value of the brain metabolites decreases in the general order of triphosphate < diphosphate < monophosphate (Table 1). This is reflected in the faster recovery of all signals in the upfield region than those in the downfield regions (Figures 3 and 4), and the brain 31P T1 values are consistent with the results previously reported in the skeletal muscle by us (4) and also by Bogner et al (9) at 7T. A possible explanation is that there may be a strong 31P dipole-dipole interaction within the di- and tri-phosphate metabolites (including ATP, NAD and UDPG) through the chemical bond –P–O–P–, while such a relaxation mechanism is apparently absent in the monophosphate metabolites (including PE, PC, GPE, GPC, Pi and PCr). Indeed, not only the T1 of tri- and di-phosphates is ~2 – 4 fold shorter than that of monophosphates (Table 1), but even within the triphosphate ATP, the central β-31P spin is featured with a shorter T1 (1.1 sec) than the terminal α- and γ-31P spins (1.4 – 1.7 sec, Table 1) as one would expect from dipolar relaxation mechanism.
Furthermore, of the six major monophosphates, both PCr and Pi have a reduced T1 in comparison to those remaining metabolites including PE, PC, GPE and GPC. This phenomenon could be reasonably explained by the effect of the chemical exchange with γ-ATP, since γ-ATP has a short T1 and is actively involved in phosphoryl exchange with both Pi and PCr, but not with PME and PDE metabolites.
In addition to dipolar mechanism, chemical shift anisotropy (CSA) might also play a role in 31P T1 relaxation. Of all P-metabolites reported here, the most likely candidate with a relative large CSA contribution is PCr, since the P atom of PCr is bonded to one N plus three O atoms, rather than to four O atoms; the latter has a more symmetric structure with a higher degree of shielding isotropy as featured in all monophosphates other than PCr. This may explain a previous observation of a shorter intrinsic T1 at PCr (5.4 sec) than at Pi (7.3 sec, ref(4)). It might also be part of the reason for the short apparent T1 observed here for PCr (3.4 sec, Table 1), but discerning such a mechanism is complicated by the mixing of MT effect. As for tri- di-phosphates, it is likely that dipolar mechanism, rather than CSA, is more responsible for the observed short T1. This is because all phosphate groups in these poly-phoshates have a similar isotropic structure P(O)4, which is distincly different from the less isotropic structure NP(O)3 as in PCr. Furthermore, the P-to-P spatial distance is only ~ 2.2 Å in the di- and tri-phosphates, well within the expected radius of dipolar interaction (typically ~ 5 Å).
Two Pools of Pi
Several 31P studies have reported the observation of two Pi signals in various organs (12,19–21). It is generally understood that the phenomenon is due to presence of two pools of Pi different in pH (ΔpH ~0.4). In the brain, the major upfield peak is assigned to intracellular Pi, and the minor downfield peak assigned to extracellular Pi (2). However, in skeletal muscle and perfused hearts, the minor Pi signal is considered to be from mitochondrial Pi, characterized by a shorter T1 than the major Pi signal (19,21). In sharp contrast, for brain, the T1 of the major Pi signal is 40% shorter than that of the minor Pi signal, due to being involved in chemical exchange with γ-ATP of short T1 (see below). We also noticed that, for the major Pi signal, the T1 is quite similar between the brain and skeletal muscle (3.7 ± 0.5 sec versus 4.3 ± 0.4 sec (19)). The major difference is in the minor Pi signal; the T1 is about 4-fold longer in the brain (5.8 ± 1.1 sec, Table 1) than in the skeletal muscle (1.4 ± 0.5 sec, (19)).
EBIT Measurement of ATP Synthesis
The EBIT data in Figures 5 and 6 clearly illustrate the modulation of the intracellular Pi signal by γ-ATP inversion as anticipated by chemical exchange effect. In contrast, the extracellular Pi signals remain invariant (Figure 6 top), indicative of lack of such chemical exchange effect. In terms of MT effect, the maximal Piin reduction by EBIT is 25% in the brain, which is reached 2.8 sec after the band inversion (Figure 5), equivalent to the MT effect generated by a continuous saturation at γ-ATP for ~ 2 sec using a frequency-selective pulse train by conventional ST (1,2). In comparison, a steady-state Pi reduction of ~ 30 – 38% was reported in the brain using ST, achieved after a prolonged irradiation at γ-ATP for duration 6 – 9 sec (1,2). Since the Pi reduction by EBIT occurs during the inversion delay period in absence of any B1 pulsing, one therefore can expect consistency in instrumental performance without concerns of potential issues that may be present with using prolonged repetitive saturation pulses (3).
FIG. 6.
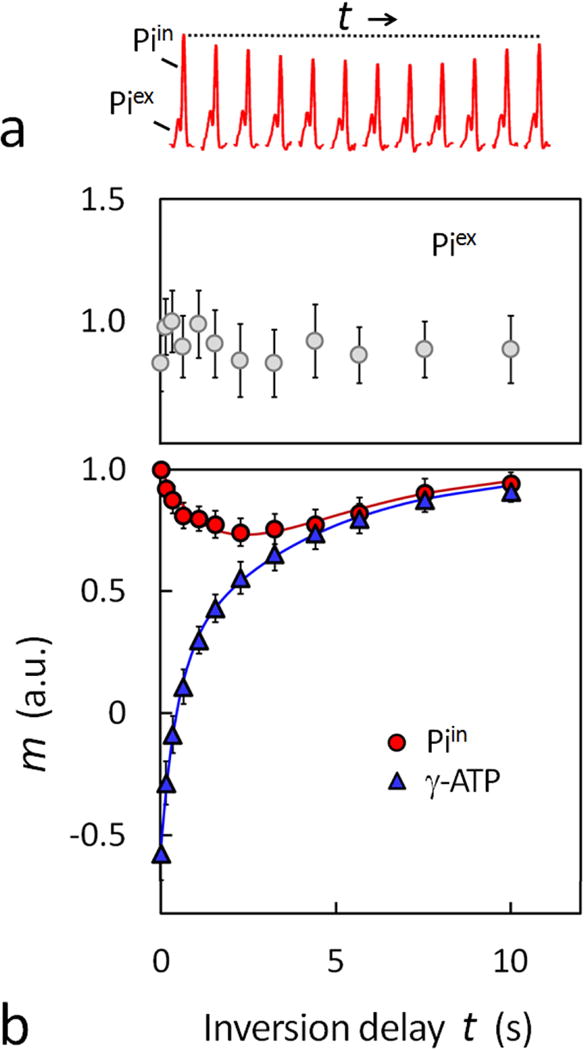
(a) Comparison of intra- and extracellular Pi signal changes in response to increase in the inversion recovery delay time t. All data are shown with identical y-scale, and taken from the EBIT spectra shown in Figure 5. (b) Normalized Z-magnetizations versus delay time t for extracellular Pi (top) and intracellular Pi and γ-ATP (bottom, averaged for 12 subjects). The solid curves in the plots represent the data fitting using a bi-exponential equation (Eq. [7]) for Pi and a mono-exponential equation for γ-ATP.
Despite the difference in MT mechanism between EBIT and ST, both approaches yield comparable results for the ATP synthesis in the resting human brain. The unidirectional rate constant derived from EBIT data (kPi→γ-ATP = 0.21 ± 0.05 sec−1), is in reasonable agreement with the results reported by Lei et al (0.17 ± 0.04 sec−1, (1)) and Du et al (0.18 ± 0.05 sec−1, (2)) using ST. The forward flux for ATP synthesis, calculated by product of kPi→γ-ATP and Piin, is 9.9 ± 2.1 mmol/min/kg, comparable to the ST results of 12.1 ± 2.8 mmol/min/kg (calculated using a total Pi concentration of 1.30 mM, (1)) and 8.8 ± 1.9 mmol/min/kg (calculated using Piin of 0.90 mM, (2)).
From EBIT data, similar kPi→γ-ATP values are obtained by two different ways: 1) after fitting the bi-exponential Eq [7] to data and using the initial slope of the fit (Eq [6]); and 2) by fitting all of mPi(t) and mγATP(t) data to Eq [4]. The later approach is also able to evaluate T1,Pi in addition to kPi→γ-ATP. While the initial slope approach has long been applied to evaluate kPi→γ-ATP in IT data analysis for the reaction PCr → γ-ATP (22–25), measuring kPi→γ-ATP is technically much more challenging in vivo since the MT effect by Pi→γ-ATP pathway is one order of magnitude smaller than that by the competitive PCr→γ-ATP reaction (1,4,10). Using PCr as a “magnetization buffer”, EBIT approach amplifies the MT effect by Pi→γ-ATP pathway, allowing kPi→γ-ATP measured in the human brain as in the skeletal muscle, in spite of the quantitative difference in PCr/ATP ratio (1.5 for the brain vs 4.5 for the skeletal muscle).
There are potential technical limitations in this study that have to be considered. First, although our data acquisition scheme is able to clearly illustrate small change in MT effect at varying inversion delay times, the use of long TR and a large number of data points for evaluation of kPi→γ-ATP is time consuming. To be practical for clinical application, a time-saving data acquisition scheme is needed which could be achieved by use of short TR and less data points. While TR can be shortened to speed up data acquisition, but the trade-off is the complexity of data analysis, since the 31P magnetization at the end of TR can no longer be taken as a constant (Mzo) due to incomplete and delay-dependent recovery as previously described (4). Second, while the high SNR 31P data acquired in this study permit a more reliable determination signal intensity, the use of a surface coil is known to be susceptible to inhomogeneity in B1 and sensitive to regions only sufficiently close to the surface of the RF coil, mainly the occipital-parietal lobes. But it is possible that the energy metabolism may vary in different brain regions and depend on local cellular density of neuron and astrocytes, an issue worthy to address in future studies using volume coil in combination with suitable spatial encoding techniques. Third, the use of non-localized 31P acquisition strategy may be potentially prone to contamination from non-brain tissues, such as the muscle in the skull. However, it is very likely that the muscle contribution is negligible, judging from the fact that the observed brain 31P spectrum is distinctly different from the muscle 31P spectrum acquired under identical conditions. Specifically, in the brain the total PME signal is more than 2-fold larger than Pi, while in skeletal muscle the PME signal appeared to be negligibly small in comparison to Pi (11). Moreover, in the brain the GPE/GPC ratio is ~1 : 1.5, while in the skeletal muscle, this ratio is ~1 : 15 (11), a 10-fold difference. Finally, though the term ATP has been used in this work, strictly speaking, all forms of NTP, including ATP, CTP, GTP and UTP, which are indistinguishable by in vivo 31P MRS (1), contribute to the measured unidirectional exchange rate constant.
In summary, we have also demonstrated that the rate of ATP synthesis in the human brain can be measured at 7T using EBIT. This technique could become an alternative to the conventional ST approach in the study of brain energy metabolism in normal physiology and under pathological conditions. In addition to ATP synthesis, we also measured the brain concentration of all detectable phosphorous metabolites including UDPG, an essential precursor for glycogen synthesis. Furthermore, an analysis of the inversion recovery data indicates that the apparent T1 of 31P signals follows the order of monophosphate > diphosphate > triphospate, implying that dipolar mechanism may play a significant role in the T1 shortening of tri- and diphosphates in reference to monophosphates. With the enhanced spectral resolution, the 31P MRS at 7T is also able to distinguish extra- from intra-cellular Pi pools, yielding important information about intra- and extracellular pH. Early diagnosis and follow-up of neurodegenerative diseases are often hampered by the lack of reliable biomarkers; the quantitative 31P MRS in combination with ATP synthesis, pH and T1 relaxation measurements may offer a promising tool to detect biochemical alternations in such cases.
FIG. 2.

Brain concentration of P-metabolites (a) and Mg2+ (b), and intra- and extracellular pH (c), averaged for n = 12 subjects. Data quantification was based on the 31P peak fitting shown in Figure 1c, with P-metabolites concentrations evaluated by the integral of 31P peaks, [Mg2+] by chemical shift difference between α- and β-ATP, and pH by the chemical shift difference between Pi and PCr.
Acknowledgments
The authors are grateful for the technical support from Ivan Dimitrov (Philips Medical Systems), Salvador Pena for operational assistance. Jeannie Davis and Janet Jerrow recruited and managed the human subjects. This project was supported by the National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through P41EB015908, DK081186, R37-HL-034557, P01DK058398 and RO1AR050597, Department of Defense Grant W81XWH-06-2-0046.
Abbreviations used
- IT
inversion transfer
- ST
saturation transfer
- NOE
nuclear Overhauser effect
- MT
magnetization transfer
- SAR
specific absorption ratio
- CSA
chemical shift anisotropy
- EBIT
exchange kinetics by band inversion transfer
- Eq
Equation
- Pi
inorganic phosphate
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- PCr
phosphocreatine
- PME
phosphomonoesters
- PDE
phosphodiesters
- NAD
Nicotinamide ddenine dinucleotide
- UDPG
Uridine diphosphoglucose
References
- 1.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100(24):14409–14. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–14. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 3.Befroy DE, Rothman DL, Petersen KF, Shulman GI. 31P-magnetization transfer magnetic resonance spectroscopy measurements of in vivo metabolism. Diabetes. 2012 Nov;61(11):2669–78. doi: 10.2337/db12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren J, Yang B, Sherry AD, Malloy CR. Exchange kinetics by inversion transfer: Integrated analysis of the phosphorus metabolite kinetic exchanges in resting human skeletal muscle at 7 T. Magn Reson Med. 2015;73(4):1359–69. doi: 10.1002/mrm.25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresnen A, Duong TQ. Brain high-energy phosphates and creatine kinase synthesis rate under graded isoflurane anesthesia: An in vivo (31) P magnetization transfer study at 11.7 tesla. Magn Reson Med. 2015;73(2):726–30. doi: 10.1002/mrm.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingsley PB, Monahan WG. Corrections for off-resonance effects and incomplete saturation in conventional (two-site) saturation-transfer kinetic measurements. Magn Reson Med. 2000;43(6):810–9. doi: 10.1002/1522-2594(200006)43:6<810::aid-mrm6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Baguet E, Roby C. Off-Resonance Irradiation Effect in Steady-State NMR Saturation Transfer. J Magn Reson. 1997;128(2):149–60. doi: 10.1006/jmre.1997.1230. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Chen W, Zhu XH. Field dependence study of in vivo brain (31) P MRS up to 16.4 T. NMR Biomed. 2014;27(9):1135–41. doi: 10.1002/nbm.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogner W, Chmelik M, Schmid AI, Moser E, Trattnig S, Gruber S. Assessment of (31)P relaxation times in the human calf muscle: a comparison between 3 T and 7 T in vivo. Magn Reson Med. 2009;62(3):574–82. doi: 10.1002/mrm.22057. [DOI] [PubMed] [Google Scholar]
- 10.Balaban RS, Koretsky AP. Interpretation of 31P NMR saturation transfer experiments: what you can’t see might confuse you. Focus on “Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles”. Am J Physiol Cell Physiol. 2011;301(1):C12–5. doi: 10.1152/ajpcell.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren J, Sherry AD, Malloy CR. Amplification of the effects of magnetization exchange by 31 P band inversion for measuring adenosine triphosphate synthesis rates in human skeletal muscle. Magn Reson Med. 2014 doi: 10.1002/mrm.25514. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren J, Sherry AD, Malloy CR. A simple approach to evaluate the kinetic rate constant for ATP synthesis in resting human skeletal muscle at 7 T. NMR Biomed. 2015 doi: 10.1002/nbm.3310. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malloy CR, Cunningham CC, Radda GK. The metabolic state of the rat liver in vivo measured by 31P-NMR spectroscopy. Biochim Biophys Acta. 1986;885(1):1–11. doi: 10.1016/0167-4889(86)90032-7. [DOI] [PubMed] [Google Scholar]
- 14.Valkovič L, Bogner W, Gajdošík M, Považan M, Kukurová IJ, Krššák M, Gruber S, Frollo I, Trattnig S, Chmelík M. One-dimensional image-selected in vivo spectroscopy localized phosphorus saturation transfer at 7T. Magn Reson Med. 2014;72(6):1509–15. doi: 10.1002/mrm.25058. [DOI] [PubMed] [Google Scholar]
- 15.Potwarka JJ, Drost DJ, Williamson PC. Quantifying 1H decoupled in vivo 31P brain spectra. NMR Biomed. 1999;12(1):8–14. [PubMed] [Google Scholar]
- 16.Jensen JE, Drost DJ, Menon RS, Williamson PC. In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed. 2002;15(5):338–47. doi: 10.1002/nbm.776. [DOI] [PubMed] [Google Scholar]
- 17.Decking UK, Alves C, Wallimann T, Wyss M, Schrader J. Functional aspects of creatine kinase isoenzymes in endothelial cells. Am J Physiol Cell Physiol. 2001;281(1):C320–8. doi: 10.1152/ajpcell.2001.281.1.C320. [DOI] [PubMed] [Google Scholar]
- 18.Abramov Y, Carmi S, Anteby SO, Ringel I. Ex vivo 1H and 31P magnetic resonance spectroscopy as a means for tumor characterization in ovarian cancer patients. Oncol Rep. 2013;29(1):321–8. doi: 10.3892/or.2012.2071. [DOI] [PubMed] [Google Scholar]
- 19.Kan HE, Klomp DW, Wong CS, Boer VO, Webb AG, Luijten PR, Jeneson JA. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR Biomed. 2010;23(8):995–1000. doi: 10.1002/nbm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffrey FM, Storey CJ, Nunnally RL, Malloy CR. Effect of ischemia on NMR detection of phosphorylated metabolites in the intact rat heart. Biochemistry. 1989;28(13):5323–6. doi: 10.1021/bi00439a003. [DOI] [PubMed] [Google Scholar]
- 21.Garlick PB, Brown TR, Sullivan RH, Ugurbil K. Observation of a second phosphate pool in the perfused heart by 31P NMR; is this the mitochondrial phosphate? J Mol Cell Cardiol. 1983;15(12):855–8. doi: 10.1016/0022-2828(83)90347-4. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RA, Kushmerick MJ, Brown TR. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982;242:C1–C11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh PS, Balaban RS. Saturation and inversion transfer studies of creatine kinase kinetics in rabbit skeletal muscle in vivo. Magn Reson Med. 1988;7:56–64. doi: 10.1002/mrm.1910070107. [DOI] [PubMed] [Google Scholar]
- 24.Mora BN, Narasimhan PT, Ross BD. 31P magnetization transfer studies in the monkey brain. Magn Reson Med. 1992;26:100–115. doi: 10.1002/mrm.1910260111. [DOI] [PubMed] [Google Scholar]
- 25.McFarland EW, Kushmerick MJ, Moerland TS. Activity of creatine kinase in a contracting mammalian muscle of uniform fiber type. Biophys J. 1994;67:1912–1924. doi: 10.1016/S0006-3495(94)80674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


