Abstract
In a previously described peptidomimetic series, we reported the development of bifunctional µ-opioid receptor (MOR) agonist and δ-opioid receptor (DOR) antagonist ligands with a lead compound that produced antinociception for 1 h after intraperitoneal administration in mice. In this paper, we expand on our original series by presenting two modifications, both of which were designed with the following objectives: 1) probing bioavailability and improving metabolic stability, 2) balancing affinities between MOR and DOR while reducing affinity and efficacy at the Κ-opioid receptor (KOR), and 3) improving in vivo efficacy. Here we establish that through N-acetylation of our original peptidomimetic series, we are able to improve DOR affinity and increase selectivity relative to KOR while maintaining the desired MOR agonist/DOR antagonist profile. From initial in vivo studies, one compound (14a) was found to produce dose-dependent antinociception after peripheral administration with an improved duration of action of longer than 3 h.
INTRODUCTION
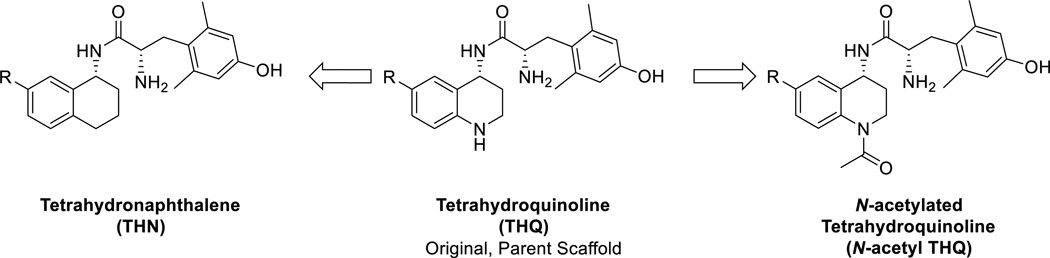
Several studies have reported the utility and application of compounds with µ-opioid receptor (MOR) activation and reduced δ-opioid receptor (DOR) signaling.1–5 Co-administration of a MOR agonist with a δ-opioid receptor DOR antagonist6–8 produces antinociception with reduced risk of tolerance and dependence, implicating the importance of reduced DOR signaling in mitigating these undesired properties. However, multi-drug regimens may produce pharmacokinetic complications and lead to low patient compliance.9–11 Thus, we1–2, 12–14 and others3–5,15,16 have pursued the development of bifunctional, mixed-efficacy MOR agonist/DOR antagonist ligands. We first reported the synthesis of a high-affinity (nanomolar binding) opioid receptor peptidomimetic with selectivity for MOR in 1998. In this initial report, a tetrahydroquinoline (THQ) scaffold was implemented to mimic the binding conformations of the high-affinity tetrapeptide JOM-13 (Tyr-c[d-Cys-Phe-d-Pen]OH) and related peptides, while eliminating the disulfide-containing moiety of the peptide.17 Since then, we have developed several additional mixed-efficacy peptides that have helped elucidate which functionalities yield high affinity binding across the receptor subtypes.13,14,18,19 For example, we have found that in our peptides, bulky aromatics (1-naphthyl, 2-naphthyl, and 2-indanyl groups) are especially useful for obtaining a more balanced MOR agonist/DOR antagonist profile.13,14 Given the promising results from the peptide series, we sought to transfer the key binding elements from a peptide scaffold to a more bioavailable peptidomimetic scaffold that incorporated the bulky aromatic moieties that enhanced the MOR agonist/DOR antagonist profile we desired. We reported a series of mixed-efficacy opioid peptidomimetics employing a THQ scaffold that displayed a MOR agonist/DOR antagonist profile and whose lead compound produced antinociception for a duration of about 1 h in the mouse warm water tail withdrawal (WWTW) assay after peripheral (intraperitoneal, ip) administration.1 Although our original opioid peptidomimetic series displayed the desired profile and validated our THQ scaffold as being a suitable and bioavailable template, there remained opportunity to improve in vitro and in vivo properties. For example, compounds in the original series displayed 10- to 130-fold binding affinity preference for MOR over DOR, and many also exhibited considerable affinity and efficacy at the κ-opioid receptor (KOR). In the subsequent generation of compounds, we have explored two different modifications to our original series in an attempt to 1) probe and improve bioavailability and metabolic stability, 2) balance the affinity at MOR and DOR while reducing KOR affinity and efficacy, and 3) increase in vivo efficacy and duration of action. Here we display our findings from two parallel series of analogues, which include replacement of our THQ scaffold with a tetrahydronaphthalene (THN) scaffold or N-acetylation of the nitrogen in the THQ core of our original series (Figure 1).
Figure 1.
Modifications to THQ Scaffold
Through both of these modifications, our goal was to reduce the metabolic lability associated with the nitrogen heteroatom in the THQ ring, as two common metabolic concerns associated with this moiety include N-oxidation and oxidation α to the heteroatom.20–24 Furthermore, the amine in the THQ core is also part of an aniline system, which is susceptible to aromatization, not only in vivo,25 but potentially in ambient atmosphere with trace acid present.26 As described below, we observed that N-acetylation of our parent compound 14i to form 14e improved DOR affinity, without altering MOR affinity, resulting in a better balance of MOR and DOR binding. Consequently we decided to explore parallel series, with variable substituent R (Figure 1) with and without N-acetylation of the core THQ nitrogen to examine effects on bioavailability and relative MOR and DOR affinities. We also explored the effect of eliminating the aniline nitrogen entirely by replacing the THQ scaffold with THN. As described below, the N-acetylation of the THQ scaffold improved DOR affinity across our parallel series without significantly altering efficacy profiles or binding at MOR, thereby creating bi-functional ligands with a more balanced MOR agonist/DOR antagonist profile. In addition, one of the new analogues, 14a, displays full antinociception in the mouse WWTW assay for a duration of longer than 3 h after peripheral administration, a significant improvement on our original in vivo results for the parent 14i.
RESULTS
Diversification of THQ-containing Analogues via Suzuki Coupling
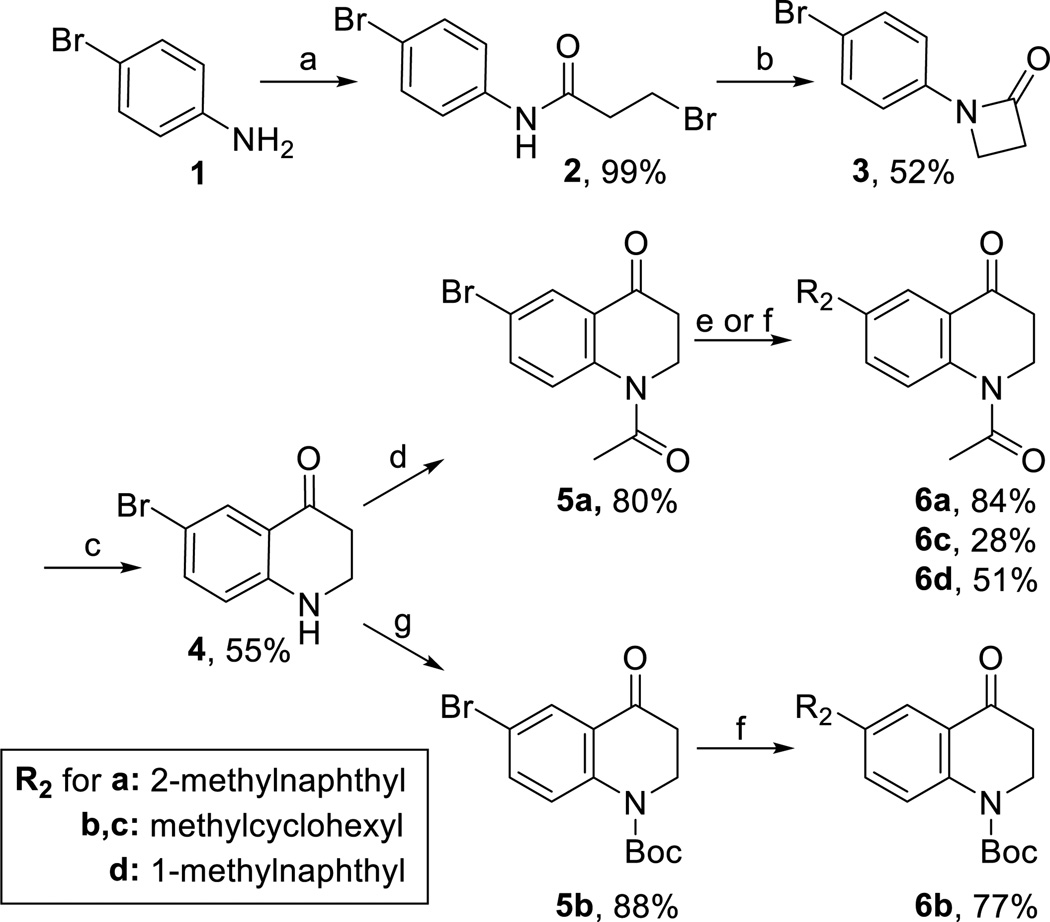
The core THQ intermediate 4 (Scheme 1) was synthesized using the methodology developed by Schmidt et al27 and described in our previous paper1, with the single modification of using p-bromoaniline as the starting material. Briefly, p-bromoaniline 1 was acylated using 3-bromopropionyl chloride to yield 2, cyclized under basic conditions to form lactam 3, and then treated with trifluoromethanesulfonic acid to form intermediate 4. The nitrogen of 4 was protected with a tert-butyloxycarbonyl (Boc) group or an acetyl group (later referred to as “R1”) to form intermediates 5a and 5b, which were then subjected to Suzuki cross-coupling28,29 to yield compounds 6a-d with diverse R2 substituents.
Scheme 1.
Synthesis of THQ-Containing Analogues Diversified via Suzuki Coupling
(a) 3-bromopropionyl chloride (1.02 eq), K2CO3 (2.05 eq), DCM, RT, (b) NaOtBu (1.05 eq), DMF, RT (c) TfOH (3 eq), DCE, RT (d) neat Ac2O (excees), refulx, (e) For synthesis of 6a and 6d: R2Bpin (2 eq), Pd(dppf)Cl2(0.1 eq), K2CO3 (3 eq), 3:1 acetone:H2O, MW 100°C, 300 W, (f) For synthesis of 6c and 6d: R2-B(OH)2 (2 eq), Ag2O (2.5 eq), Pd(dppf)Cl2 (0.1 eq), K2CO3 (3 eq), THF, MW 100°C, 300 W (g) Bco2O (1.2-2 eq), DMAP (0.1 eq), DIPEA (1.2-2 eq), DCM, 60°C
Diversification of THQ-containing Analogues via Condensation Chemistry
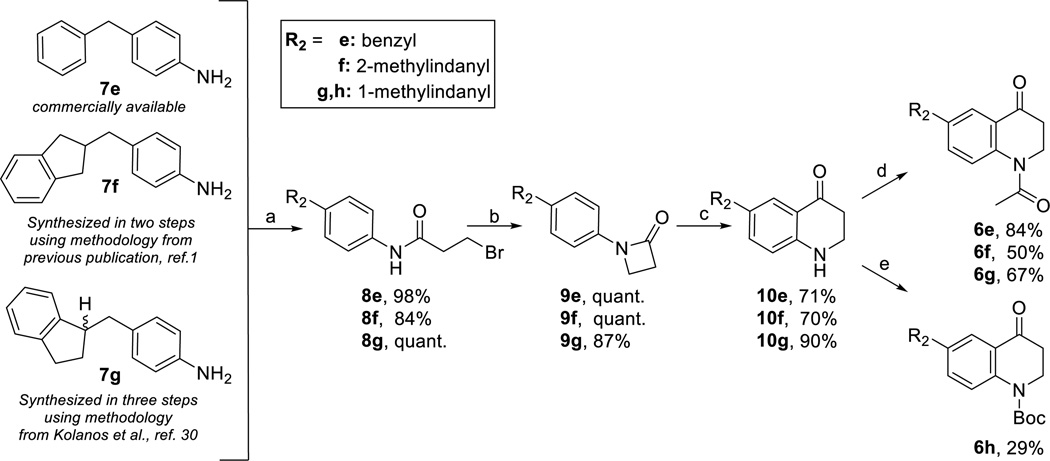
While starting material for 7e, which bears a benzyl R2-substiuent, was commercially available, compounds containing a THQ core with a 1- or 2-methylindanyl substituent were synthesized by incorporating the R2 substituent during the first synthetic step via a condensation reaction. Specifically, the 2-methylindanyl analogue was synthesized via an aldol condensation followed by a hydrogenation as previously described1 to form aniline intermediate 7f. The 1-methylindanyl analogues were synthesized as a racemic mixture using a slight modification of methodology originally developed by Kolanos et al30 that begins with a condensation reaction between indene and 4-acetamidobenzaldehyde, the product of which was hydrogenated and deprotected to yield an aniline intermediate 7g. The aniline intermediates 7e-g were then acylated, cyclized to form lactams 9e-g, and then treated with trifluoromethanesulfonic acid to form 10e-g, as shown previously1,27 (Scheme 2). Intermediates 10e-g were then protected with Boc or an acetyl group (later referred to as “R1”) to form intermediates 6e-h with diverse substituents.
Scheme 2.
Synthesis of THQ-Containing Analogues via Condensation Reactions
(a) 3-bromopropionyl chloride (1.02 eq), K2CO3 (2.05 eq), DCM, RT (b) NaOtBu (1.05 eq), DMF, RT (c) TfOH (3 eq), DCE, RT (d) For synthesis of 6e, 6g, 6g neat Ac2O (excess), reflux (e) For synthesis of 6h: Boc2O (1.2–2 eq), DMAP (0.1 eq), DIPEA (1.2–2 eq), DCM, 60°C
Completion of Synthesis of THQ-containing Analogues from Common Intermediate
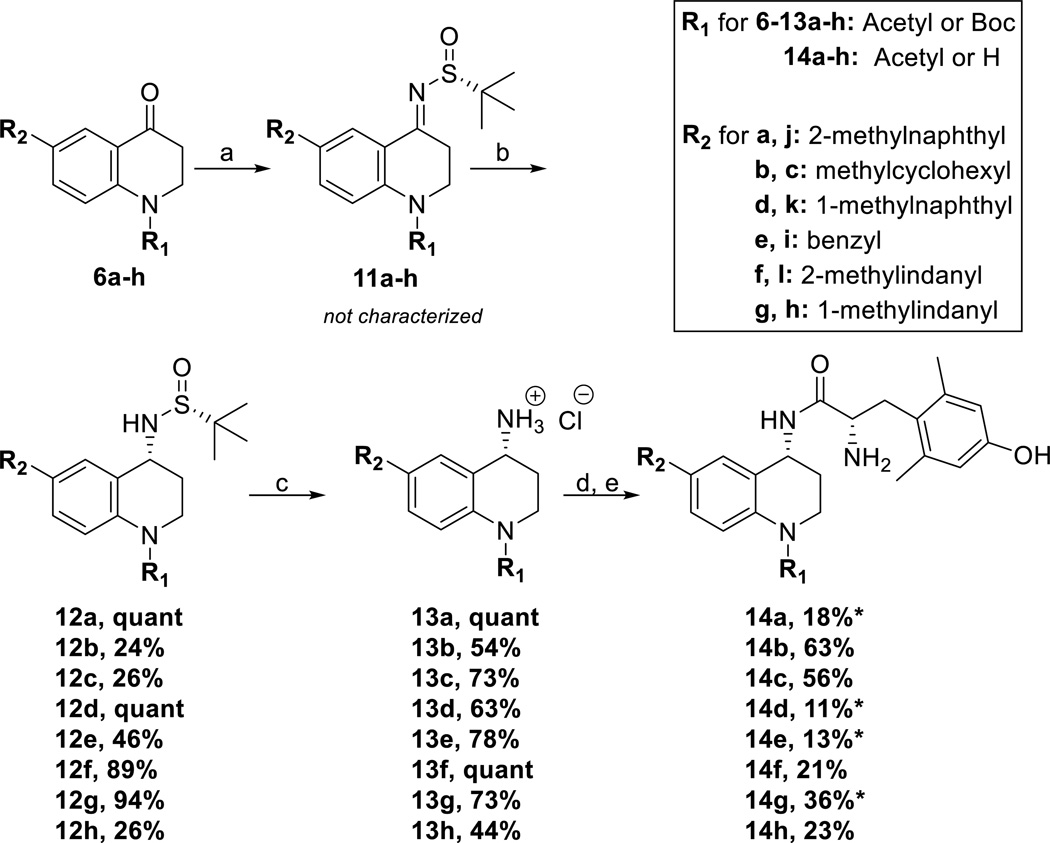
Once the R2 substituent was implemented into the THQ scaffold, the synthesis of final compounds 14a-h converged beginning with 6a-h (Scheme 3). Intermediates 6a-h were treated with (R)-t-butanesulfinamide and Ti(OEt)4 to yield imines 11a-h, which were reduced in situ with NaBH4 to form the desired R-stereochemistry of intermediates 12a-h.31–33 The Ellman auxiliary was cleaved using concentrated hydrochloric acid forming primary amines 13a-h, 31–33 which were then coupled to di-Boc protected 2,6-dimethyl-L-tyrosine (diBoc-DMT) and deprotected to yield compounds 14a-h. The syntheses for analogues 14i-l, which also contain a THQ core, were previously published.1
Scheme 3.
Completion of Synthesis of THQ-Containing Analogues
(a) Dihydroquinolinone intermediate, 6a-h (1 eq), (R)-t-Butanesulfinamide (2–3 eq), THF, Ti(OEt)4 (4–6 eq), 0°C, then reflux at 75°C (b) NaBH4 (6 eq), THF, −50°C to RT, then MeOH, RT (c) HCl (6 eq), dioxane, RT (d) diBoc-DMT (1.05 eq), PyBOP (1 eq), 6Cl-HOBt (1 eq), DIPEA (10 eq), DMF, RT (e) 1:1 TFA:DCM (excess). Compounds 14i-14l previously published, see ref. 1. * indicates starting material was recovered, but not included in yield calculation.
Complete Synthesis of THN Peptidomimetic Analogues
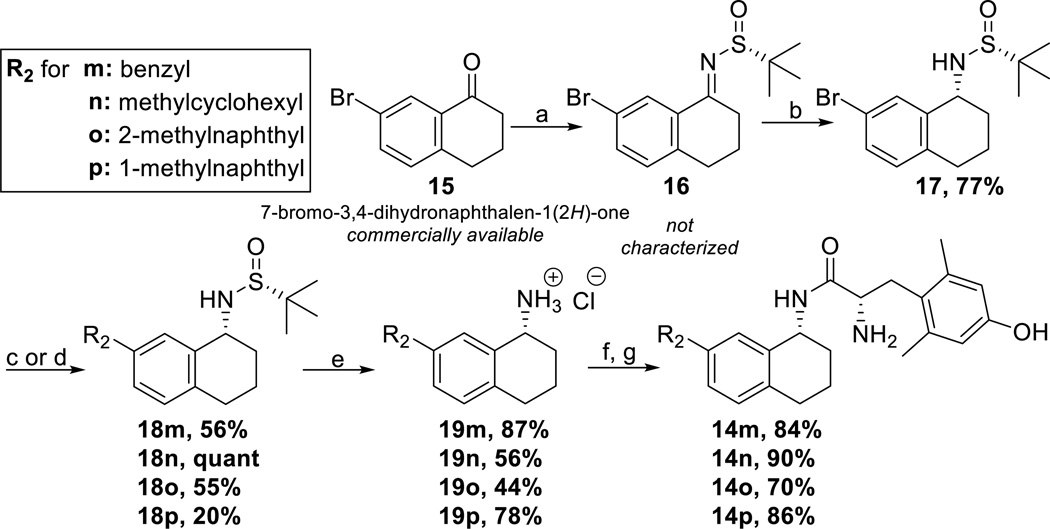
Synthesis of THN compounds 14m-p began by converting the ketone of commercially available 7-bromo-dihydronaphthalenone, 15, to the chiral imine 16 using (R)-t-butanesulfinamide and Ti(OEt)4 followed by in situ reduction to form 17 using NaBH4 to afford the desired R-stereochemistry of the sulfonamide31–33 (Scheme 4). Next, various R2 substituents were incorporated via Suzuki coupling to form intermediates 18m-p.28,29 Intermediates 18m-p were then treated with concentrated hydrochloric acid to cleave the Ellman auxiliary affording primary amines 19m-p.31–33 In the final step, the amines 19m-p were coupled to diBoc-DMT and deprotected to yield compounds 14m-p.
Scheme 4.
Synthesis of THN-Containing Analogues
(a) 15 (1 eq), (R)-t-Butanesulfinamide (2-3 eq), THF, Ti(OEt)4 (4-6 eq) 0°C, then refulx at 75°C (b) NaBH4 (4-6 eq), THF, −50°C to RT, then MeOH, RT (c) for synthesis of 18m, 18o, 18p: R2-Bpin (1 eq), pd(dppf)Cl2 (0.1 eq), K2CO3 (3 eq), 3:1 acetone:H2O,MW 110°C, 300 W (d) For synthesis of 18n: R2-B(OH)2 (2 eq), Ag2O (2.5 eq), pd(dppf)Cl2(0.1 eq), K2CO3 (3 eq), THF, MW 80°C, 300 W (e) HCl (6 eq), dioxane, RT(f) diBoc-DMT (1.05 eq), PyBOP (1 eq), 6Cl-HOBt (1 eq), DIPEA (10 eq), DMF, RT (g) 1:1 TFA:DCM (excess)
Opioid Receptor Binding and Efficacy
Binding affinities (Ki) for the final compounds were determined from competitive displacement of radiolabeled [3H]diprenorphine in membrane preparations from C6 cells stably expressing MOR (C6-MOR) or DOR (C6-DOR) or CHO cells stably expressing KOR (CHO-KOR), as previously described34,35(Table 1). Efficacy of the compounds was assessed by agonist-stimulated [35S]GTPγS binding to G protein36 in cell membrane preparations of C6-MOR, C6-DOR, and CHO-KOR (Table 1).
Table 1.
Opioid Receptor Binding Affinities and Efficacies of Peptidomimetics.
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binding, Ki(nM)a,c | DOR Ki/ MOR Ki Ratio |
EC50 (nM)b,c | % stimulationb,c | ||||||||||
| Cpd | X | R1 | R2 | MOR | DOR | KOR | MOR | DOR | KOR | MOR | DOR | KOR | |
| 14ie | N | H |  |
0.22 (0.02) |
9.4 (0.8) |
68 (2) |
43 | 1.6 (0.3) |
110 (6) |
540 (70) |
81 (2) |
16 (2) |
22 (2) |
| 14e | N | 0.13 0.02) |
1.8 (0.1) |
87 (10) |
14 | 6.0 (1) |
68 (2) |
>1300 (90) |
76 (4) |
26 (3) |
29 (5) |
||
| 14m | CH2 | -- | 0.045 (0.03) |
4.0 (1) |
19 (7) |
89 | 2.9 (0.6) |
dns | dns | 64 (9) |
dns | dns | |
| 14b | N | H | 0.043 (0.005) |
3.4 (1) |
6.2 (1) |
79 | 2.6 (1) |
dns | 97 (30) |
57 (5) |
dns | 36 (4) |
|
| 14c | N |  |
0.03 (0.01) |
0.32 (0.1) |
8.4 (2) |
11 | 0.61 (0.2) |
14 (8) |
240 (70) |
70 (6) |
26 (5) |
33 (6) |
|
| 14n | CH2 | -- | 0.12 (0.02) |
14 (6) |
20 (5) |
117 | dns | dns | 350 (80) |
dns | dns | 25 (6) |
|
| 14je | N | H | 0.078 (0.007) |
10 (2) |
54 (7) |
128 | 0.53 (0.08) |
dns | dns | 96 (3) |
dns | dns | |
| 14a | N |  |
0.04 (0.01) |
0.23 (0.02) |
48 (20) |
6 | 0.93 (0.2) |
dns | dnsd | 87 (3) |
dns | dnsd | |
| 14o | CH2 | -- | 0.06 (0.01) |
12 (4) |
92 (20) |
140 | 4.4 (2) |
dns | dns | 72 (4) |
dns | dns | |
| 14ke | N | H | 0.76 (0.1) |
6.0 (0.7) |
17 (1) |
8 | 0.84 (0.4) |
69 (40) |
dns | 93 (5) |
15 (1) |
dns | |
| 14d | N |  |
0.06 (0.02) |
1.3 (0.4) |
4.3 (2) |
22 | 0.48 (0.2) |
dns | dnsd | 70 (4) |
dns | dnsd | |
| 14p | CH2 | -- | 0.39 (0.08) |
5.0 (0.3) |
63 (20) |
13 | 14 (7) |
dns | dnsd | 58 (2) |
dns | dnsd | |
| 14le | N | H | 0.16 (0.04) |
4.1 (2) |
1.2 (0.4) |
26 | 0.24 (0.03) |
dns | dns | 86 (1) |
dns | 38 (2) |
|
| 14f | N |  |
0.05 (0.00) |
0.44 (0.07) |
12 (4) |
9 | 0.56 (0.1) |
dns | 610 (250) |
84 (2) |
dns | 60 (10) |
|
| 14h | N | H | 0.05 (0.02) |
6.8 (2) |
19 (10) |
136 | 9.0 (6) |
dns | 180 (80) |
16 (8) |
dns | 52 (7) |
|
| 14g | N |  |
0.03 (0.00) |
0.84 (0.13) |
15 (0.2) |
28 | 15 (10) |
dns | >1000 | 39 (10) |
dns | 26 (8) |
|
Binding affinities (Ki (nM)) were obtained by competitive displacement of radiolabeled [3H]diprenorphine in membrane preparations.
Efficacy data were obtained using agonist induced stimulation of [35S]GTPγS binding. Efficacy is represented as percent maximal stimulation relative to standard agonist DAMGO (MOR), DPDPE (DOR), or U69,593 (KOR) at 10 µM.
All values are expressed as the mean with S.E.M. in parentheses for n=3 independent assays in duplicate, unless otherwise noted.
n=2 independent assays in duplicate. dns: does not stimulate.
Syntheses and data for these compounds were previously published (see ref. 1)
While a total of six compound sets were synthesized, (each set defined by the R2 substituent), only four of these sets incorporated all three of the different scaffolds; these sets include analogues in which the R2 substituent is a benzyl, a 1-methylnaphthyl, a 2- methylnaphthyl, or a methylcyclohexyl. The effects of both scaffold modifications on the binding affinities are shown in Table 1. The most noticeable trend across these four sets of compounds is seen with the N-acetylated THQ series at DOR. This N-acetylated series (regardless of R2) shows a significantly higher affinity at DOR than the unsubstituted THQ counterpart. Additionally, N-acetylation maintains, and in some cases slightly improves affinity at MOR while slightly decreasing affinity at KOR. The effect of the N-acetylated THQ scaffold on efficacy (Table 1) across all receptors is minimal, with most compounds retaining similar efficacy as seen with the parent THQ series. When considering the effects of the N-acetylation on the THQ scaffold as a whole, this modification appears to balance the MOR/DOR binding profile while maintaining the desired MOR agonist/DOR antagonist efficacy profile.
In contrast, the effect of the THN scaffold on binding affinities at all three receptors (Table 1), when compared with the parent THQ scaffold, showed no consistent or advantageous trends. For example, when comparing 14m (THN scaffold) with 14i (THQ scaffold), 14m shows increased affinity across all three receptors. Additionally, when comparing 14n (THN scaffold) with 14b (THQ scaffold), 14n has decreased affinity across all three receptors. Furthermore, a decrease in efficacy is seen at MOR regardless of R2 substituent when compared to the THQ scaffold. Because the THN scaffold presented no apparent advantage, the 1- and 2-indanyl THN analogues were not pursued.
In vivo WWTW Assay
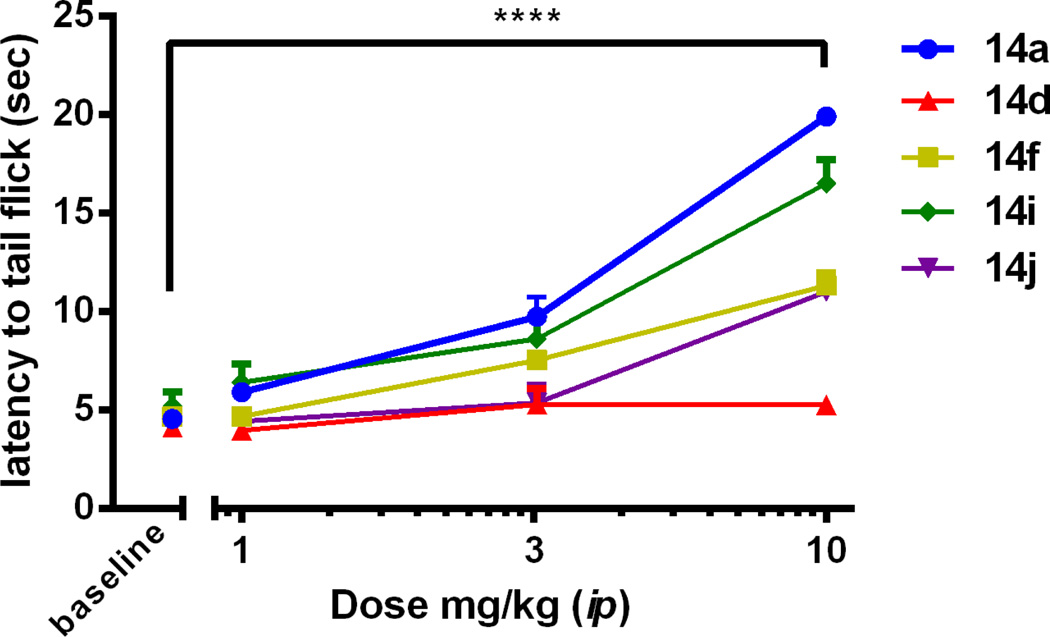
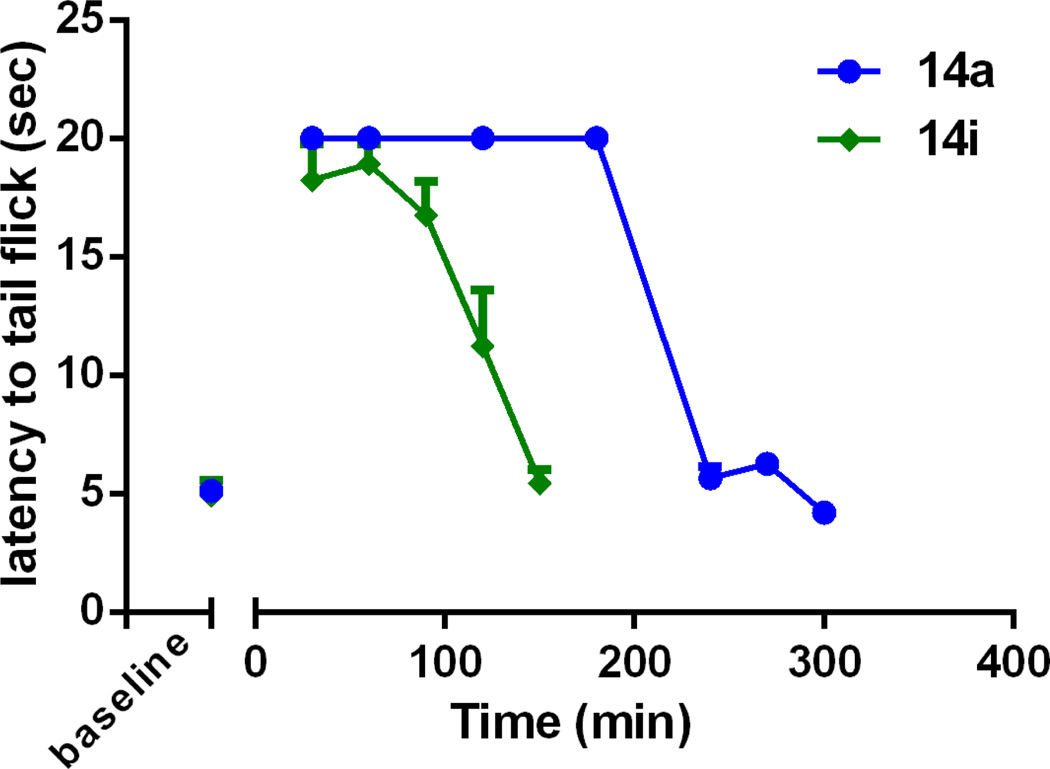
Several compounds were chosen based on in vitro data to be assayed for antinociception in the mouse WWTW assay following intraperitoneal (ip) injection.37 Of the compounds assayed (Table 2, Figure 2), only three compounds displayed dose-dependent antinociceptive activity. Compounds 14f and 14j displayed partial antinociception at the maximum dose tested, whereas 14a displayed full antinociception with ED50 = 4.72 +/− 0.01 mg/kg. By comparison, the ED50 of morphine, under the same conditions, was 4.73 +/− 0.001 mg/kg. Because compound 14a displayed full antinociception at 10 mg/kg in the initial WWTW assay, a time course study was completed to determine duration of action (Figure 3). As can be seen in Figure 3, 14a produces maximal antinociception with a rapid on-set which is maintained for approximately 200 min. This 200 min duration of action is greater than 3 times longer compared to the duration of action of our original lead peptidomimetic 14i1 (and similar to that observed for the same dose of morphine,1) suggesting better bioavailability or metabolic stability. Interestingly, despite being structural isomers with a similar in vitro profile, 14a and 14d displayed drastically different in vivo results when administered via ip injection (Figure 2). While 14a displayed full antinociception after ip administration, 14d displayed no antinociception at the same dose after ip administration, suggesting that subtle differences in chemical structure create substantial differences in pharmacokinetics.
Table 2.
In vivo Activity of Select Peptidomimetics.
| Cmpd | Scaffold | R2 substituent | In vivo Activity following ip administrationb |
|---|---|---|---|
| 14ia | THQ |  |
Full antinociception at 10 mg/kg with duration of action >1h |
| 14e | N-acetyl THQ | No activity up to 10 mg/kg | |
| 14m | THN | Partial antinociception with latency of 14 sec at 10 mg/kg | |
| 14b | THQ |  |
No activity up to 10 mg/kg |
| 14c | N-acetyl THQ | No activity up to 10 mg/kg | |
| 14j | THQ |  |
Partial antinociception with latency of 10 sec at 10 mg/kg |
| 14a | N-acetyl THQ | Full antinociception at 10 mg/kg with duration of action of >3 h | |
| 14o | THN | No activity up to 10 mg/kg | |
| 14d | N-acetyl THQ |  |
No activity up to 10 mg/kg |
| 14p | THN | No activity up to 10 mg/kg | |
| 14l | THQ |  |
No activity up to 10 mg/kg |
| 14f | N-acetyl THQ | Partial antinociception with latency of 11 sec at 10 mg/kg |
Summary of antinociceptive effects of select analogues (n=3 for all analogues, except for 14a n=6) in mouse WWTW assay following intraperitoneal (ip) administration, with a cut-off time of 20 sec.
see ref. 1.
Full antinociception indicates that the cut-off time was reached.
Figure 2.
Cumulative antinociceptive dose response curves of select analogues (n=3 for all analogues, except for 14a n=6) in the mouse WWTW assay following ip administration. Plotted as average +/− S.E.M. ****, p < 0.0001 for 14a, 14f, 14i, 14j for the 10 mg/kg dose when compared to baseline. Data for 14i from ref 1.
Figure 3.
Time course of antinociception of 14a and 14i (n=6) in the mouse WWTW assay following ip administration of 10 mg/kg. Plotted as average +/− S.E.M. Data for 14i from ref 1.
DISCUSSION AND CONCLUSIONS
Our previously described results1 were promising in that we were able to transfer pharmacophore elements from a MOR agonist/DOR antagonist peptide to a peptidomimetic scaffold, as seen in our parent peptidomimetic, 14i, which was shown to have antinociceptive activity after peripheral administration in mice. While our original series of compounds displayed the desired MOR agonist/DOR antagonist profile, there remained opportunity to improve the balance of binding affinities at MOR and DOR, and reduce any remaining KOR binding and efficacy. Although the optimal balance of “MOR agonism” with “DOR antagonism” is yet to be determined, we reasoned that a low nanomolar, balanced affinity profile (~1:1 MOR Ki: DOR Ki) was a logical place to start, since this would ensure that both MOR agonist and DOR antagonist character would be represented in the in vivo outcome and would provide useful information for future studies. Our initial series displayed relatively unbalanced MOR and DOR affinities, where our compounds were 10- to 130- fold selective for MOR over DOR (Table 1). In the present study, we set out to improve upon our initial compounds in two ways. First, we wished to explore modification of the THQ scaffold in order to avoid metabolic susceptibility due to the THQ ring nitrogen, while also examining the effect of these modifications on binding affinity and efficacy. Secondly, we wished to explore the additional effect of modifying the R2 substituent, in the search for a MOR agonist/DOR antagonist with a better MOR/DOR affinity balance.
The initial in vitro results for the parallel series of scaffold modifications yield interesting insight into both the electronic and steric requirements for optimal binding. The data in Tables 1 and 2 indicate that the series incorporating the THN scaffold, when compared to the unsubstituted THQ scaffold, leads to greater disparity in the binding affinities at MOR and DOR. These disparities are caused by either increasing affinity at MOR more so than at DOR or by decreasing affinity at DOR more than at MOR, relative to the parallel analogue with a THQ core. Both of these effects result in a less desirable affinity profile, and sometimes result in a profile with reduced affinity at both MOR and DOR. Furthermore, the THN scaffold does not improve selectivity over KOR across all sets of compounds. In addition to the effects that the THN scaffold has on the binding affinities at MOR, DOR, and KOR, the THN core also results in reduced efficacy at MOR, when compared to our parent THQ series. In contrast, the N-acetylated THQ series appears superior to both the THN series and the original THQ series. When compared to the original THQ scaffold, the corresponding N-acetylated analogues not only maintain MOR agonist activity and DOR antagonism, but also show increased DOR binding affinity (regardless of R2 substituent) while MOR affinity remains relatively unchanged, thereby creating a series of ligands with more balanced MOR and DOR affinities (Table 1). The only exception to this trend is in the case of compound 14d, where the MOR and DOR affinity both increase, with the MOR affinity increasing to a greater extent, resulting in a higher preference for MOR over DOR.
In order to explain the results of the N-acetylated THQ series, we looked at computational docking of one of the THQ/N-acetylated ligand pairs docked in the binding pocket of the inactive state of DOR (DORi), as previously described.13, 38 Modeling 14j and 14a in the DORi binding pocket (Figure 5) as a representative example, it can be seen that the N-acetyl of 14a extends further into the DORi binding pocket to create a polar contact with Tyr129 in transmembrane helix 3 (TM3) of the receptor (Figure 5, Panel B), an interaction that appears to be unavailable with the unsubstituted THQ core (Figure 5, Panel A). It seems that the N-acetyl can increase DORi binding through three modes: 1) The carbonyl of the N-acetyl forms a hydrogen bond with the hydroxyl in Tyr129, and once the hydrogen bond is formed, this could orient the ligand in the receptor to form a tighter hydrogen bonding network between 2) the primary amine of the 2,6-dimethyl tyrosine (Dmt) moiety of the ligand and Asp128 of the receptor, and 3) the Dmt hydroxyl moiety of the ligand and His278. It has been previously reported39–44 that ligand interaction with His278 and Asp128 are important for opioid ligand binding in all three classical receptors. While the DOR Tyr129 residue is conserved in MOR (Tyr148), the N-acetylation does not appear to have as profound of an effect in the MOR binding pocket, with the measured distance between 14j and 14a to the Tyr residue 4.7 Å to 4.3 Å, respectively (not pictured). When both 14j and 14a are overlaid with the crystal structure of the recently reported44 MOR agonist/DOR antagonist, DIPP-NH2, (Figure 5, Panels C and D, respectively) in DORi, it can be seen that 14a aligns better with the Dmt and free amine moiety of DIPP-NH2 than 14j. This suggests that the N-acetyl moiety has a subtle, yet impactful effect on the orientation of the ligand resulting in increased binding affinity at DORi. We hypothesize that the interaction between Tyr129 in the binding pocket of DORi and the N-acetylated ligand 14a (and other N-acetylated analogues) may bring 14a into closer proximity to His279 and Asp128, facilitating a better binding network within the DORi binding pocket. While the increase in DOR affinity across the N-acetylated analogues is the most apparent trend when considering the in vitro data in their entirety, it is clear that some substituents display superior profiles over others. For example, the methylcyclohexyl (14b, 14c, 14n) and 1- and 2-methylindanyl (14h, 14g, and 14l, 14f) analogues pick up considerable undesired KOR affinity and agonism, while the 1- and 2-methylnaphthyl analogues (14k, 14d, 14p, and 14j, 14a, 14o) display agonist activity at MOR, little or no agonist activity at DOR, and significantly reduced binding and efficacy at KOR, suggesting that these compounds behave as functional antagonists at DOR. To confirm antagonist activity at DOR, 14a and 14d were tested against DPDPE, and as expected from the high binding affinity and lack of stimulation of [35S]GTPγS binding at DOR, 14a and 14d both antagonized the DOR agonist DPDPE, with antagonist affinity constants (Ke) of 1.98 nM and 27.5 nM, respectively (data not shown).
Figure 5.
(A) 14j (purple) in DORinactive (DORi) with key residues shown. (B) 14a (teal) in DORi with key residues shown. Dashed lines represent calculated distances between ligand and receptor residues, indicating a possible interaction between N-acetyl moiety and Tyr129 that may increase binding affinity at DORi. (C) overlay of 14j (purple) and DIPP-NH2 (orange) in DORi. (D) Overlay of 14a (teal) and DIPP-NH2 (orange) in DORi.
Based on results from our initial in vitro screening, we chose twelve candidates for in vivo screening evaluation in the mouse WWTW assay. As seen in Figure 6, the in vitro results and cLogP calculations were both poor predictors of in vivo activity. Surprisingly, 14d displayed no in vivo activity at 10 mg/kg ip while 14a, a structural isomer of 14d, displayed full antinociception at 10 mg/kg ip. In order to explore this unexpected result, we submitted both 14a and 14d for plasma stability testing performed by Quintara Discovery (San Francisco, CA, U.S.). These results revealed that both analogues tested were fully stable in plasma after 30 min, suggesting compound degradation in the plasma does not account for the differing activities in vivo. Planned pharmacokinetic studies on 14a and 14d will be helpful for understanding the basis of the disparate in vivo results.
Figure 6.
Summary of in vivo activity following ip administration, cLogP, and molecular weight (MW, g/mol) for select compounds. Compounds 14e, 14o, 14p, 14l, 14m do not display antinociception following ip administration and structures are not shown. 1see ref. 1 for in vivo activity.
In summary, we have expanded upon our original THQ scaffold by incorporating a THN and an N-acetylated THQ scaffold with the intention of 1) probing and improving bioavailability and metabolic stability, 2) balancing further the affinity at MOR and DOR while reducing KOR affinity and efficacy, and 3) increasing in vivo activity and duration of action. Through N-acetylation of the THQ nitrogen not only were we able to better balance the affinity at MOR and DOR while maintaining the desired MOR agonist/DOR antagonist profile in vitro, but we were also able to decrease binding affinity and efficacy at KOR, thereby creating a more desirable compound profile. Furthermore, three of the compounds presented in this paper show in vivo activity when administered peripherally, one of which (14a) displays full antinociception in the mouse WWTW assay for >3 h, a promising improvement upon our original lead compound.1
EXPERIMENTAL SECTION
Chemistry
All reagents and solvents were obtained from commercial sources and used without additional purification. Suzuki couplings were performed on a Discover S-class (CEM) microwave in a closed vessel with maximum power input of 300 W and temperature set at 110 °C for 10–60 min under the standard method from their Synergy software. Hydrogenations were performed on a Parr hydrogenator apparatus from Parr Instrument Company, model 3916EA, at the pressures specified using 10% Pd/C as the catalyst. Flash column chromatography was carried out using P60 silica gel (230–400 mesh). Purification of final compounds was performed using a Waters semipreparative HPLC with a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 15% solvent B (0.1% TFA in acetonitrile) in solvent A (0.1% TFA in water) to 50% solvent B in solvent A at a rate of either 0.5% or 1% per minute and monitoring UV absorbance at 230 nm. Purity of synthesized compounds was determined on a Waters Alliance 2690 analytical HPLC instrument and a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 0% solvent B in solvent A to 45%, 70%, or 90% solvent B in solvent A in 45, 70, or 90 min, respectively, and UV absorbance at 230 nm (gradient A). Purities of the final compounds used for testing were ≥95% as determined by HPLC. 1H NMR and 13C NMR data were obtained on either a 400 or 500 MHz Varian spectrometer using CDCl3 or CD3OD solvents. The identity of each compound was verified by mass spectrometry using an Agilent 6130 LC-MS mass spectrometer in positive mode.
General procedure A for the synthesis of the 3-Bromo-N-propanamides.1,27 To a flame-dried round bottom flask under Ar was added the aniline compound (1.0 eq) and K2CO3 (2.05 eq). The reaction vessel was placed back under vacuum and anhyd. DCE was added via syringe. The reaction solution stirred under vacuum for 5 min. After 5 min, the reaction vessel was then flooded with Ar and 3-bromopropionyl chloride (1.02 eq) was added via syringe. The reaction stirred under Ar at RT for 1 h and was monitored by TLC using a ninhydrin stain for disappearance of aniline compound. Once the reaction was complete, it was quenched with dI H2O and the layers separated. The organic layer was washed with dI H2O (1 × 50 mL) followed by brine (1 × 30 mL), then dried over MgSO4, filtered, and concentrated under reduced pressure to yield the pure product.
General procedure B for the synthesis of phenylazetidin-2-ones.1,27 To a round bottom flask already containing the dried, desiccated 3-bromo-N-propanamide (1.0 eq) was added NaOtBu (1.05 eq). The reaction vessel was placed under vacuum and anhyd. DMF was added via syringe. The solution stirred under vacuum for 5 min, and then was flooded with Ar. The reaction stirred under Ar at RT for up to 3 h and was monitored by TLC. Once complete, the solvent was removed under reduced pressure and the resulting crude residue was re-suspended in DCM and dI H2O, and the layers separated. The organic layer was washed once with dI H2O (1 × 30 mL), then brine (1 × 30 mL), then dried over MgSO4, filtered, and concentrated under reduced pressure to yield the crude product, which was then purified using silica gel chromatography to yield the pure product.
General procedure C for the synthesis of 2,3-dihydroquinolin-4(1H)-ones.1,27 To the round bottom flask already containing the dried, desiccated phenylazetidin-2-ones (1.0 eq) was added anhyd. DCE under vacuum. The reaction vessel stirred under vacuum for 5 min then was flooded with Ar. Next, triflic acid (TfOH) (3.0 eq) was added via syringe. The reaction stirred under Ar at RT for up to 3 h and was monitored by TLC. Once complete, the reaction was quenched with dI H2O (20 mL) and solid K2CO3 (one spatula full) and the layers separated. The organic layer was washed once with dI H2O (1 × 30 mL), then sat. NaHCO3 (1 × 30 mL), then brine (1 × 30 mL), then dried over MgSO4, filtered, and concentrated under reduced pressure to yield the crude product, which was then purified using silica gel chromatography to yield the pure product.
General Procedure D for the synthesis of 1-acetyl-2,3-dihydroquinolin-4(1H)-ones. To a flame-dried round bottom flask under Ar was added the 2,3-dihydroquinolin-4(1H)-one. The reaction vessel was placed back under vacuum for 5 min, then excess Ac2O was added via syringe and the solution stirred for 5 min. The round bottom flask was flooded with Ar, equipped with a condenser, and placed in oil bath at 100°C. The reaction stirred at reflux for 12–20 h under Ar and was monitored by TLC. Once the reaction was complete, the solvent was removed under reduced pressure yielding a crude yellow oil which was purified using silica gel chromatograph to obtain the pure product.
General procedure E for the synthesis of tert-butyl 4-oxo-3,4-dihydroquinoline-1(2H)-carboxylates. To a flame-dried round bottom flask under Ar was added the 2,3-dihydroquinolin-4(1H)-one (1.0 eq), Boc2O (1.2–2.0 eq), and DMAP (0.1 eq). The reaction vessel was placed back under vacuum for 5 min, then anhyd. DCM was added via syringe and the solution stirred for 5 min under vacuum. The round bottom flask was flooded with Ar, and DIPEA (1.2–2.0 eq) was added via syringe. The reaction vessel was equipped with a condenser and placed in oil bath at 60°C. The reaction stirred at reflux for 12–16 h under Ar and was monitored by TLC. Once significant conversion to product was seen, the reaction was quenched using dI H2O (20 mL) and the layers were separated. The organic layer was washed with sat. NaHCO3 solution (1 × 20 mL) and brine (1 × 20 mL), then dried over MgSO4, filtered, and concentrated under reduced pressure to yield a crude yellow oil which was purified using silica gel chromatography to obtain the pure product.
General procedure F for Suzuki couplings using an aromatic boronic ester. A solution of 3:1 acetone:dI H2O was degassed for 1 h, then Ar was bubbled through solution for 1 h to ensure removal and displacement of ambient oxygen. When all the reagents are solid: aromatic bromide (1.0 eq), boronic ester (2.0 eq), K2CO3 (3.0 eq), and Pd(dppf)Cl2 (0.1 eq), were added to a microwave tube and the tube was placed under vacuum for 15 min, then flooded with Ar. Roughly 1–2 mL of the 3:1 acetone:dI H2O solution was added to tube via syringe, then tube was placed in microwave for 30–60 min with a maximum power of 300 W and a maximum temperature of 100°C with the “PowerMax” option enabled. When the boronic ester is liquid: aromatic bromide (1.0 eq) , K2CO3 (3.0 eq), and Pd(dppf)Cl2 (0.1 eq), were added to a microwave tube and the tube was placed under vacuum for 15 min, then flooded with Ar. Roughly 1–2 mL of the 3:1 acetone:dI H2O solution was added to tube via syringe, followed by addition of the boronic ester (2.0 eq) via syringe. The tube was placed in microwave for 30–60 min with a maximum power of 300 W and a maximum temperature of 100°C with the “PowerMax” option enabled. Once the microwave reaction was complete, reaction mixture was filtered through a pad of Celite to remove palladium, and solvents were removed under reduced pressure to yield a crude brown residue which was purified using silica gel chromatography to obtain the pure product. Procedure adapted from ref. 28.
General procedure G for Suzuki couplings using an aliphatic boronic acid. The aromatic bromide (1.0 eq), boronic acid (1.1–2.0 eq), K2CO3 (3.0 eq), Ag2O (2.5 eq), and Pd(dppf)Cl2 (0.1 eq), were added to a microwave tube and the tube was placed under vacuum for 15 min, then flooded with Ar. Roughly 1–2 mL of anhyd THF was added to tube via syringe, then tube was placed in microwave for 1 h with a maximum power of 300 W and a maximum temperature of 80°C with the “PowerMax” option enabled. Once the microwave reaction was complete, reaction mixture was filtered through a pad of Celite to remove palladium, and solvents were removed under reduced pressure to yield a crude brown residue which was purified using silica gel chromatography to obtain the pure product. Procedure adapted from ref. 29.
General procedure H for the synthesis of (R, R) THQ and THN sulfinamides.31–33 To a round bottom flask already containing dried, desiccated 6-substituted dihydroquinolinone intermediate (1.0 eq) was added (R)-2-methylpropane-2-sulfinamide (2.0–3.0 eq), then the round bottom flask was placed under vacuum for 10 min. Meanwhile, a reflux condenser was flame-dried under vacuum, and then flooded with Ar. Next, anhyd. THF (~20 mL) was added to the reaction vessel containing starting reagents via syringe. The reaction solution allowed to stir under vacuum for ~5 min and then was flooded with Ar. The round bottom flask was placed in ice bath and allowed to equilibrate. Next, Ti(OEt)4 (4.0–6.0 eq) was added slowly via syringe. Once addition was complete, the reaction vessel was taken out of ice bath and placed in oil bath at 70°C-75°C, equipped with condenser, and stirred for 16–40 h under Ar. The reaction was monitored by TLC for loss of ketone. Once sufficient conversion to the tert-butanesulfinyl imine was observed, reaction vessel was taken out of oil bath and cooled to ambient temperature. Meanwhile, an additional round bottom flask containing a stir bar was flame-dried under vacuum, then flooded with Ar, then NaBH4 was added quickly, and then reaction vessel was placed back under vacuum for 5 min. Minimal anhyd. THF was added (~5 mL) and vessel allowed to stir under vacuum for ~ 5 min, then was flooded with Ar. The round bottom flask was placed in dry ice/xylenes bath and allowed to equilibrate. Contents from the round bottom flask containing the imine intermediate were transferred to round bottom flask containing NaBH4 via cannula. Once contents completely added, the reaction was taken out of dry ice/xylenes bath and allowed to warm to room temperature. The reaction stirred at ambient temperature for 2–3 h. Once the reaction was complete, MeOH was added to quench. The solvent was removed under reduced pressure yielding a solid residue. The residue was re-suspended in DCM, solid remained the remaining solid was removed by filtration through a cotton plug and the mother liquor was concentrated and purified using silica gel chromatography to yield pure sulfinamide.
General procedure I for the synthesis of (R)-1,2,3,4-tetrahydroquinolin-4-amines and (R)-1,2,3,4-tetrahydronaphthalen-1-amines. To a round bottom flask already containing sulfinamide intermediate was added 15–20 mL dioxane followed by conc. HCl (6.0 eq). The reaction stirred at RT for up to 3 h. Solvent was removed under reduced pressure to yield slightly yellow, clear residue. The residue was re-suspended in Et2O. If a white solid precipitated (the HCl salt of the amine): solid was removed via filtration as product without any further purification necessary. If a white solid did not precipitate, but residue remains as film on flask: residue washed with fresh Et2O (3 × 5 mL) and dried without any further purification necessary.
General Procedure J for diBoc-DMT coupling to form final product. The (R)-amine intermediate and diBoc-DMT (1.05 eq) and the coupling reagents PyBOP (1.0 eq), HOBt-Cl (1.0 eq), were dissolved in DMF (10–15 mL) followed by the addition of the and DIPEA (10.0 eq). The reaction mixture stirred for 18 h at room temperature. After concentration under reduced pressure, the crude residue was dissolved in a 1:1 mixture of DCM and TFA (10 mL) and stirred for 1 h. The mixture was concentrated and purified by semipreparative HPLC to yield the final compound. Note that while other coupling reagents could be used, the coupling reagents used here were chosen to minimize racemization. Additionally, diBoc-DMT was used instead of monoBoc-DMT to prevent any possible ester formation at the phenol of tyrosine that might occur under these coupling conditions, especially at longer reaction times.
3-Bromo-N-(4-bromophenyl)propanamide (2) was synthesized following general procedure A starting from commercially available 1 (2.0 g, 11.6 mmol, 1.0 eq), K2CO3 (3.29 g, 23.8 mmol, 2.05 eq), and 3-bromopropionyl chloride (1.20 mL, 11.9 mmol, 1.02 eq) to yield title compound as an off-white solid (3.53 g, 98.9%) with no additional purification necessary after quench and work-up. 1H NMR (500 MHz, CDCl3) δ 7.47 – 7.39 (m, 4H), 3.70 (t, J = 6.5 Hz, 2H), 2.94 (t, J = 6.5 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 167.9, 136.4, 132.0, 128.3, 121.6, 40.7, 26.8.
1-(4-Bromophenyl)azetidin-2-one (3) was synthesized following general procedure B starting with 2 (3.53 g, 11.5 mmol, 1.0 eq) and NaOtBu (1.16 g, 12.1 mmol, 1.05 eq). Following solvent removal and work-up, the crude product was chromatographed on silica gel (equil in 100% hex, run in 1:4 EA:hex) to yield title compound as a pure white, flaky solid (1.36 g, 52.1%). 1H NMR (500 MHz, CDCl3) δ 7.46 – 7.42 (m, 2H), 7.27 – 7.22 (m, 2H), 3.62 (td, J = 4.6, 2.0 Hz, 2H), 3.13 (td, J = 4.6, 1.9 Hz, 2H). No 13C spectrum acquired.
6-Bromo-2,3-dihydroquinolin-4(1H)-one (4) was synthesized following general procedure C starting with 3 (1.36 g, 6 mmol, 1.0 eq) and TfOH (1.60 mL, 18.0 mmol, 3.0 eq) to yield a crude yellow oil. Following work-up, the crude material was chromatographed on silica gel (equil in 100% hex, run in 2:3 EA:hex) to yield title compound as a pure yellow powder (739 mg, 54.5%). 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 2.4 Hz, 1H), 7.35 (dd, J = 8.7, 2.4 Hz, 1H), 6.58 (d, J = 8.7 Hz, 1H), 4.43 (s, 1H), 3.58 (t, 2H), 2.69 (t, 2H). 13C NMR (126 MHz, CDCl3) δ 192.3, 150.6, 137.6, 130.0, 120.5, 117.7, 110.2, 42.0, 37.6.
1-Acetyl-6-bromo-2,3-dihydroquinolin-4(1H)-one (5a) was synthesized following general procedure D starting with 4 (739 mg, 3.27 mmol, 1.0 eq) fo 16 h. Following removal of solvent, the resulting crude yellow oil was purified using silica gel chromatography (equil in 100%, run in 1:3 EA:hex) to yield title compound as a white, waxy solid (704 mg, 80.4%). 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 2.4 Hz, 1H), 7.64 (dd, J = 8.7, 2.5 Hz, 1H), 7.47 (s, 1H), 4.21 (t, J = 6.3 Hz, 2H), 2.81 (t, J = 6.3 Hz, 2H), 2.35 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 192.4, 169.0, 142.6, 136.6, 130.3, 127.0, 125.8, 118.7, 44.2, 39.1, 23.1.
Tert-Butyl 6-bromo-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (5b) was synthesized following general procedure E using 4 (300 mg, 1.33 mmol, 1.0 eq), Boc2O (348 mg, 1.59 mmol, 1.2 eq), DMAP (16.2 mg, 0.13 mmol, 0.1 eq) and DIPEA (0.277 mL, 1.59 mmol, 1.2 eq). Following the quench and work-up, the crude product was chromatographed on silica gel (equil in 100% hex, run in 2:3 EA:hex) to yield pure product as a white solid (380 mg, 87.8%). 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 2.4 Hz, 1H), 7.70 (d, J = 8.9 Hz, 1H), 7.57 (dd, J = 9.0, 2.4 Hz, 1H), 4.15 (t, J = 6.3 Hz, 2H), 2.77 (t, J = 6.3 Hz, 2H), 1.55 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 192.8, 152.4, 143.1, 136.6, 129.9, 126.1, 125.5, 117.1, 82.6, 44.2, 38.7, 28.3.
1-Acetyl-6-(naphthalen-2-ylmethyl)-2,3-dihydroquinolin-4(1H)-one (6a) was synthesized following general procedure F using 5a (50 mg, 0.19 mmol, 1.0 eq), 4,4,5,5-tetramethyl-2-(naphthalen-2-ylmethyl)-1,3,2-dioxaborolane(100 mg, 0.37 mmol, 2.0 eq), K2CO3 (78 mg, 0.56 mmol, 3.0 eq), and Pd(dppf)Cl2 (14 mg, 0.019 mmol, 0.1 eq). The contents were placed microwave tube and reacted in microwave with max temp of 110°C, max power of 250 W for 30 min, with the “Powermax” option enabled. Once crude mixture was filtered through Celite, the solvent was removed and the residue was purified via silica gel chromatography (equil in 100% hex, run in 1:1 EA:hex) to yield title compound (51 mg, 83.6%) as clear, colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.91 (s, 1H), 7.80 – 7.74 (m, , 3H), 7.63 (s, 1H), 7.48 – 7.36 (m, 3H), 7.31 – 7.26 (m, 1H), 4.24 – 4.15 (m, 2H), 4.13 (s, 2H), 2.80 – 2.69 (m, 2H), 2.29 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 194.0, 169.2, 142.1, 138.6, 137.4, 134.6, 133.5, 132.1, 128.3, 127.60, 127.57, 127.5, 127.3, 127.2, 127.1, 126.1, 125.9, 125.5, 124.2, 43.9, 41.3, 39.4, 23.0.
Tert-Butyl 6-(cyclohexylmethyl)-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (6b) was synthesized following general procedure G using 5b (100 mg, 0.31 mmol, 1.0 eq), (cyclohexylmethyl)boronic acid (87 mg, 0.61 mmol, 2.0 eq), K2CO3 (127 mg, 0.92 mmol, 3.0 eq), Ag2O (178 mg, 0.77 mmol, 2.5 eq), and Pd(dppf)Cl2 (22 mg, 0.031 mmol, 0.1 eq). The contents were placed microwave tube and reacted in microwave with max temp of 80°C, max power of 300 W for 60 min, with the “Powermax” option disabled. Once filtered through Celite, the solvent was removed and the crude residue purified via silica gel chromatography (equil in 100% pet. ether, run in 5:1 pet ether:Et2O) to yield title compound as clear, colorless oil (81 mg, 77.1%). 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 2.2 Hz, 1H), 7.66 (d, J = 8.5 Hz, 1H), 7.28 (d, J = 2.3 Hz, 1H), 4.19 – 4.08 (m, 2H), 2.82 – 2.71 (m, 2H), 2.47 (d, J = 7.1 Hz, 2H), 1.77 – 1.59 (m, 5H), 1.55 (s, 9H), 1.51 (s, 1H), 1.18 – 1.12 (m, 2H), 0.97 – 0.90 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 194.5, 152.8, 137.1, 135.0, 133.9, 125.5, 124.6, 123.3, 82.0, 44.3, 43.2, 39.6, 39.1, 36.0, 33.0, 28.3, 26.2.
1-Acetyl-6-(cyclohexylmethyl)-2,3-dihydroquinolin-4(1H)-one (6c) was synthesized following general procedure G using 5a (100 mg, 0.37 mmol, 1.0 eq), (cyclohexylmethyl)boronic acid (58 mg, 0.41 mmol, 1.1 eq), K2CO3 (155 mg, 1.12 mmol, 3.0 eq), Ag2O (216 mg, 0.93 mmol, 2.5 eq), and Pd(dppf)Cl2 (27 mg, 0.37 mmol, 0.1 eq). The contents were placed microwave tube and reacted in microwave with max temp of 80°C, max power of 300 W for 60 min, with the “Powermax” option disabled. Once filtered through Celite, crude residue purified via silica gel chromatography (equil in 100% hex, run in 1:3 EA:hex) to yield title compound as clear, colorless oil (30 mg, 28.3%). Additionally, 51 mg of 5a was recovered; this was not considered when calculating percent yield. 1H NMR (500 MHz, CDCl3) δ 7.78 (s, 1H), 7.33 (d, J = 6.8 Hz, 1H), 4.29 – 4.19 (m, 2H), 2.79 (t, J = 6.2 Hz, 2H), 2.50 (d, J = 7.1 Hz, 2H), 2.33 (s, 3H), 1.73 – 1.59 (m, 5H), 1.52 (m, 1H), 1.19 – 1.14 (m, 3H), 0.99 – 0.90 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 194.4, 169.3, 141.7, 139.1, 135.0, 127.8, 125.7, 123.8, 43.2, 39.6, 35.3, 34.1, 33.0, 26.4, 26.2, 23.1.
1-Acetyl-6-(naphthalen-1-ylmethyl)-2,3-dihydroquinolin-4(1H)-one (6d) was synthesized following general procedure F using 5a (50 mg, 0.19 mmol, 1.0 eq), 4,4,5,5-tetramethyl-2-(naphthalen-1-ylmethyl)-1,3,2-dioxaborolane (100 mg, 0.37 mmol, 2.0 eq), K2CO3 (78 mg, 0.56 mmol, 3.0 eq), and Pd(dppf)Cl2 (14 mg, 0.019 mmol, 0.1 eq). Contents placed microwave tube and reacted in microwave with max temp of 110°C, max power of 250 W for 30 min with “Powermax” enabled. Once crude mixture was filtered through Celite, the residue was purified via silica gel chromatography (equil in 100% hex, run in 9:1 EA:hex) to yield title compound as clear, colorless oil (31 mg, 50.8%). 1H NMR (500 MHz, CDCl3) δ 7.96 – 7.90 (m, 2H), 7.89 – 7.83 (m, 1H), 7.80 – 7.75 (m, 1H), 7.49 – 7.40 (m, 3H), 7.32 (d, J = 7.1 Hz, 2H), 4.44 (s, 2H), 4.23 – 4.13 (m, 2H), 2.75 (t, J = 6.2 Hz, 2H), 2.28 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 194.0, 169.2, 142.1, 138.3, 135.4, 134.2, 134.0, 133.6, 131.8, 128.8, 127.6, 127.5, 127.4, 126.1, 125.7, 125.5, 124.2, 124.0, 43.9, 39.5, 38.3, 23.0.
1-Acetyl-6-benzyl-2,3-dihydroquinolin-4(1H)-one (6e) was synthesized following general procedure D using 10e (170 mg, 0.72 mmol, 1.0 eq) and excess Ac2O. The reaction stirred at 100°C for 16 h. Once complete solvent was removed under reduced pressure and the crude product was chromatographed on silica gel (equil in 100% hex, run in 3:2 EA:hex) to yield the title compound as a clear, colorless oil (168 mg, 84.0%)1H NMR (400 MHzCDCl3) δ 7.87 – 7.84 (bs, 1H), 7.37 – 7.33 (m, 2H), 7.31 – 7.24 (m, 2H), 7.23 – 7.15 (m, 3H), 4.20 (t, J = 6.2 Hz, 2H), 3.98 (s, 2H), 2.76 (t, J = 6.2 Hz, 2H), 2.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 194.0, 169.3, 142.1, 140.0, 138.9, 134.6, 128.8, 128.7, 127.6, 126.4, 126.0, 124.2, 43.9, 41.2, 39.5, 23.1.
1-Acetyl-6-((2,3-dihydro-1H–inden-2-yl)methyl)-2,3-dihydroquinolin-4(1H)-one (6f) was synthesized following general procedure D using 10f (55 mg, 0.24 mmol, 1.0 eq). The reaction stirred at reflux for 20 h. Once the reaction was complete, solvent was removed and the crude residue was purified using silica gel chromatography (equil in 100% hex, run in 2:3 EA:hex) to yield title compound as a clear oil (39 mg, 50.4%). 1H NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 7.41 (d, J = 6.7 Hz, 2H), 7.20 – 7.07 (m, 4H), 4.24 (t, J = 6.3 Hz, 2H), 3.00 (dd, J = 15.3, 6.3 Hz, 2H), 2.87 – 2.76 (m, 5H), 2.67 (dd, J = 15.4, 6.2 Hz, 2H), 2.35 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 194.2, 169.4, 142.7, 141.9, 139.0, 134.7, 127.5, 126.2, 125.9, 124.4, 124.0, 43.9, 41.1, 40.7, 39.4, 38.7, 23.1.
1-Acetyl-6-((2,3-dihydro-1H–inden-1-yl)methyl)-2,3-dihydroquinolin-4(1H)-one (6g) was synthesized following general procedure D using 10g (245 mg, 0.88 mmol, 1.0 eq). The reaction stirred at reflux for 16 h. Once the reaction was complete, the solvent was removed and the crude residue was purified using silica gel chromatography (equil in 100% hex, run in 2:3 EA:hex) to yield the title compound as a clear, colorless oil (190 mg, 67.4%). 1H NMR (500 MHz, CDCl3) δ 7.88 (s, 1H), 7.36 (d, J = 6.2 Hz, 1H), 7.21 (d, J = 5.7 Hz, 1H), 7.18 – 7.09 (m, 4H), 4.23 (s, 2H), 3.44 (p, J = 7.0 Hz, 1H), 3.15 (dd, J = 13.7, 5.5 Hz, 1H), 2.88 (ddd, J = 14.1, 8.2, 5.5 Hz, 2H), 2.84 – 2.75 (m, 4H), 2.69 (dd, J = 13.7, 9.5 Hz, 1H), 2.34 (s, 3H), 2.13 (ddd, J = 13.0, 10.4, 6.7 Hz, 1H), 1.81 – 1.67 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 194.0, 169.1, 146.1, 143.8, 141.9, 138.3, 134.7, 127.5, 126.5, 125.9, 125.7, 124.5, 123.9, 123.5, 46.0, 43.8, 40.4, 39.4, 31.5, 30.9, 23.0.
tert-Butyl 6-((2,3-dihydro-1H–inden-1-yl)methyl)-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (6h) was synthesized following general procedure E using 10g (214 mg, 0.77 mmol, 1.0 eq), Boc2O (337 mg, 1.54 mmol, 2.0 eq), DMAP (9 mg, 0.077 mmol, 0.1 eq), DIPEA (0.268 mL, 1.54 mmol, 2.0 eq). The reaction stirred at reflux for 16 h. Once enough starting material was converted to product, the crude yellow oil was purified using silica gel chromatography (equil in 100% hex, run in 2:3 EA:hex) to yield the title compound as a yellow oil (83 mg, 28.5%). Additionally, 122 mg of starting material 10g was recovered, this was not considered when calculating percent yield. 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 8.4 Hz, 1H), 7.21 (d, J = 5.9 Hz, 2H), 7.15 (d, J = 8.3 Hz, 4H), 4.16 (t, J = 6.3 Hz, 2H), 3.43 (p, J = 6.9 Hz, 1H), 3.13 (dd, J = 13.7, 5.4 Hz, 1H), 2.88 – 2.83 (m, 1H), 2.83 – 2.78 (m, 1H), 2.78 – 2.74 (t, J = 6.3 Hz, 2H), 2.67 (dd, J = 13.6, 9.5 Hz, 1H), 2.13 (dq, J = 13.1, 7.8 Hz, 1H), 1.74 (dq, J = 13.9, 7.3 Hz, 1H), 1.56 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 194.3, 152.1, 146.4, 144.0, 142.2, 136.5, 134.8, 127.2, 126.5, 126.0, 124.6, 124.5, 123.6, 123.5, 82.0, 77.3, 77.0, 76.8, 46.2, 44.3, 40.5, 39.0, 31.6, 31.0, 28.3.
4-((2,3-Dihydro-1H–inden-2-yl)methyl)aniline (7f) was synthesized according to published procedure1 to yield title compound. NMR data matched previously reported literature values.1
4-((2,3-Dihydro-1H–inden-1-yl)methyl)aniline (7g) was synthesized according to published procedure26 to yield title compound. NMR data matched previously reported literature values.26
N-(4-Benzylphenyl)-3-bromopropanamide (8e) was synthesized according to general procedure A using commercially available 7e (2.15 g, 11.7 mmol, 1.0 eq) to yield title compound as tan solid (2.63 g, 98.0%) with no additional purification necessary. 1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 7.4 Hz, 2H), 7.22 – 7.12 (5H, m), 3.95 (s, 2H), 3.71 (t, J = 6.6 Hz, 2H), 2.92 (t, J = 6.6 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 167.7, 141.9, 137.7, 135.4, 129.5, 128.9, 128.5, 126.1, 120.3, 41.3, 40.7, 27.1.
3-Bromo-N-(4-((2,3-dihydro-1H–inden-2-yl)methyl)phenyl)propanamide (8f) was synthesized following general procedure A using 7f (2.1 g, 9.5 mmol, 1.0 eq) to yield title compound as brown solid (2.87 g, 84.4%) with no additional purification necessary to yield title compound. NMR data matched previously reported values.1
3-Bromo-N-(4-((2,3-dihydro-1H–inden-1-yl)methyl)phenyl)propanamide (8g) was synthesized following general procedure A using 7g (473 mg, 2.1 mmol, 1.0 eq), K2CO3 (600 mg, 4.3 mmol, 2.05 eq), and 3-bromopropionyl chloride (220 mL, 2.2 mmol, 1.02 eq) to yield title compound as an off-white solid (759 mg, quant.) with no additional purification necessary. 1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 7.0 Hz, 2H), 7.34 (s, 1H), 7.26 (d, J = 1.5 Hz, 0H), 7.22 (d, J = 6.8 Hz, 1H), 7.14 (dd, J = 19.7, 9.8 Hz, 6H), 5.29 (d, J = 1.5 Hz, 2H), 3.71 (t, J = 6.5 Hz, 2H), 3.41 (p, J = 7.1 Hz, 1H), 3.09 (dd, J = 13.7, 5.6 Hz, 1H), 2.94 (d, J = 6.6 Hz, 1H), 2.90 – 2.84 (m, 1H), 2.79 (dt, J = 15.7, 7.7 Hz, 1H), 2.66 (dd, J = 13.6, 9.2 Hz, 1H), 2.17 – 2.08 (m, 1H), 1.74 (dq, J = 15.1, 8.0, 7.6 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 167.8, 146.7, 144.1, 137.4, 135.3, 129.6, 126.5, 126.0, 124.5, 123.7, 120.0, 53.4, 46.4, 40.8, 31.8, 31.1, 27.1.
1-(4-Benzylphenyl)azetidin-2-one (9e) was synthesized following general procedure B using 8e (3.63 g, 1.1 mmol, 1.0 eq) and NaOtBu (1.15 g, 1.2 mmol, 1.05 eq) in anhyd. DMF. Following aqueous washes, the title compound was isolated as a tan solid (2.70 g, quant.) and was taken ahead to next step (formation of 10e) without purification, isolation, or characterization.
1-(4-((2,3-Dihydro-1H–inden-2-yl)methyl)phenyl)azetidin-2-one (9f) was synthesized following general procedure B using 8f (2.87 g, 8.0 mmol, 1.0 eq), NaOtBu (808 mg, 8.41 mmol, 1.05 eq) to yield the title compound as a brown solid (2.22 g, quant.) with no additional purification necessary. NMR data matched previously reported values.1
1-(4-((2,3-Dihydro-1H–inden-1-yl)methyl)phenyl)azetidin-2-one (9g) was synthesized following general procedure B using 8g (759 mg, 2.12 mmol, 1.0 eq), NaOtBu (214 mg, 2.22 mmol, 1.05 eq) to yield the title compound as a light tan solid (512 mg, 87.4%) with no additional purification necessary. 1H NMR (500 MHz, CDCl3) δ 7.29 (d, J = 8.3 Hz, 2H), 7.25 – 7.05 (m, 8H), 3.60 (t, J = 4.4 Hz, 2H), 3.40 (p, J = 7.1 Hz, 1H), 3.09 (t, J = 4.5 Hz, 3H), 3.06 (d, J = 6.0 Hz, 1H), 2.90 – 2.73 (m, 3H), 2.66 (dd, J = 13.6, 9.0 Hz, 1H), 2.11 (ddd, J = 15.9, 7.9, 5.4 Hz, 1H), 1.73 (dq, J = 15.1, 7.2 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 164.2, 146.6, 144.0, 136.6, 136.4, 129.6, 126.4, 125.9, 124.5, 123.7, 116.0, 46.4, 40.8, 38.0, 36.0, 31.7, 31.1.
6-Benzyl-2,3-dihydroquinolin-4(1H)-one (10e) was synthesized following general procedure C using 9e (2.70 g, 1.1 mmol, 1.0 eq) and TfOH (3.0 mL, 3.4 mmol, 3.0 eq). Following column purification (equil in 100% hex, run in 2:3 EA:hex) title compound was isolated as a yellow solid (1.91 g, 70.7%). NMR data matched previously reported values.1
6-((2,3-Dihydro-1H–inden-2-yl)methyl)-2,3-dihydroquinolin-4(1H)-one (10f) was synthesized following general procedure C using 9f (2.22 g, 8.00 mmol, 1.0 eq) and TfOH (2.12 mL, 24.0 mmol, 3.0 eq) to yield a crude dark red oil. Crude residue was purified using silica gel chromatography (equil in 100%, run in 1:3 EA:hex) to yield the title compound as a yellow powder (1.55 g, 69.7%). NMR data matched previously reported values.1
6-((2,3-Dihydro-1H–inden-1-yl)methyl)-2,3-dihydroquinolin-4(1H)-one (10g) was synthesized following general procedure C using 9g (512 mg, 1.85 mmol, 1.0 eq) and TfOH (0.490 mL, 5.54 mmol, 3.0 eq) to yield title compound as a slightly impure yellow solid (459 mg, 89.6%) with no additional purification necessary. 1H NMR (500 MHz, CDCl3) δ 7.70 (s, 1H), 7.19 (s, 1H), 7.11 (m, 4H), 6.60 (d, J = 8.3 Hz, 1H), 3.52 (t, J = 6.5 Hz, 2H), 3.37 – 3.32 (m, 1H), 3.01 (dd, J = 13.7, 5.0 Hz, 1H), 2.85 (m, 1H), 2.77 (m, 1H), 2.67 (m, 2H), 2.56 (m, 1H), 2.11 (dt, J = 12.3, 6.5 Hz, 1H), 1.72 (dq, J = 13.8, 7.4, 6.9 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 193.9, 150.5, 146.6, 144.0, 136.2, 130.2, 127.1, 126.3, 125.9, 124.4, 123.6, 119.0, 115.8, 46.3, 42.3, 40.2, 38.1, 31.5, 31.0.
(R)-N-((R)-1-Acetyl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12a) was synthesized following general procedure H from 6a (51 mg, 0.155 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (56 mg, 0.464 mmol, 3.0 eq), and Ti(OEt)4 (0.195 mL, 0.929 mmol, 6.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11a) in situ. Once sufficient ketone was converted into imine intermediate (after 40 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (35 mg, 0.929 mmol, 6.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in 100% hex, run in 100% EA) to yield the title compound as a clear, colorless oil (67 mg, quant. from 6a) which was taken ahead to the next step (formation of 13a) without further characterization.
tert-Butyl(R)-4-(((R)-tert-butylsulfinyl)amino)-6-(cyclohexylmethyl)-3,4-dihydroquinoline-1(2H)-carboxylate (12b) tert-Butyl(R)-4-(((R)-tert-butylsulfinyl)amino)-6-(cyclohexylmethyl)-3,4-dihydroquinoline-1(2H)-carboxylate (12b) was synthesized following general procedure H using 6b (78 mg, 0.227 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (55 mg, 0.454 mmol, 2.0 eq), and Ti(OEt)4 (0.191 mL, 0.909 mmol, 4.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11b) in situ. Once sufficient ketone was converted into imine intermediate (after 40 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (34 mg, 0.909 mmol, 4.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in 100% hex, run in 1:3 EA:hex ) to yield the title compound as a dark, yellow oil (102 mg, 23.5% from 6b). 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 8.6 Hz, 1H), 7.10 (s, 1H), 7.02 (d, J = 8.5 Hz, 1H), 4.55 (s, 1H), 3.99 (d, J = 12.8 Hz, 1H), 3.55 (t, J = 12.1 Hz, 1H), 3.27 (s, 1H), 2.46 – 2.37 (m, 2H), 2.17 (d, J = 13.3 Hz, 1H), 1.97 (t, J = 12.8 Hz, 1H), 1.71 – 1.59 (m, 5H), 1.52 (s, 9H), 1.46 (d, J = 7.5 Hz, 1H), 1.22 (s, 9H), 1.17 – 1.12 (m, 2H), 0.99 – 0.87 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 153.6, 136.9, 136.1, 129.3, 128.9, 128.0, 123.5, 81.0, 55.6, 50.3, 43.2, 40.0, 39.6, 33.1, 33.0, 29.6, 28.3, 26.5, 26.2, 22.6.
(R)-N-((R)-1-Acetyl-6-(cyclohexylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12c) was synthesized following general procedure H using 6c (56 mg, 0.196 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (48 mg, 0.392 mmol, 2.0 eq), and Ti(OEt)4 (0.165 mL, 0.785 mmol, 4.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11c) in situ. Once sufficient ketone was converted into imine intermediate (after 40 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (30 mg, 0.785 mmol, 4.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in 100% hex, run in 4:1 EA:hex ) to yield the title compound as a clear, yellow oil (20 mg, 26.1% from 6c) that was taken ahead to the next step (formation of 13c) without further characterization.
(R)-N-((R)-1-Acetyl-6-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12d) was synthesized following general procedure H using 6d (31 mg, 0.0941 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (34 mg, 0.282 mmol, 3.0 eq), and Ti(OEt)4 (0.118 mL, 0.565 mmol, 6.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11d) in situ. Once sufficient ketone was converted into imine intermediate (after 40 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (21 mg, 0.565 mmol, 6.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. The resultant solid was removed, to yield a clear, colorless residue (41 mg, quant), that was taken ahead to the next step (formation of 13d) without further purification, isolation, or characterization.
(R)-N-((R)-1-Acetyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12e) was synthesized following general procedure H using 6e (168 mg, 0.601 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (219 mg, 1.80 mmol, 3.0 eq), and Ti(OEt)4 (0.757 mL, 3.61 mmol, 6.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11e) in situ. Once sufficient ketone was converted into imine intermediate (after 40 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (137 mg, 3.61 mmol, 6.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (4:1 EA:hex ) to yield the title compound as a clear, colorless oil (105 mg, 45.5% from 6e), that was taken ahead to the next step (formation of 13e) without further characterization.
(R)-N-((R)-1-Acetyl-6-((2,3-dihydro-1H–inden-2-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12f) was synthesized following general procedure H using 6f (39 mg, 0.122 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (30 mg, 0.244 mmol, 2.0 eq), and Ti(OEt)4 (0.102 mL, 0.488 mmol, 4.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11f) in situ. Once sufficient ketone was converted into imine intermediate (after 24 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (19 mg, 0.488 mmol, 4.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in 100% hex, run in 9:1 EA:hex) to yield the title compound as a clear, colorless oil (46 mg, 88.9% from 6f). 1H NMR (500 MHz, CDCl3) δ 7.40 (s, 1H), 7.29 (s, 1H), 7.21 – 7.15 (m, 2H), 7.15 – 7.09 (m, 3H), 4.77 – 4.66 (m, 1H), 4.57 (bs, 1H), 3.01 (dd, J = 15.3, 6.0 Hz, 2H), 2.83 – 2.72 (m, 3H), 2.67 (dd, J = 15.2, 5.7 Hz, 2H), 2.43 – 2.34 (m, 1H), 2.28 – 2.20 (m, 4H), 2.13 – 2.02 (m, 1H), 1.22 (s, 9H). No 13C spectrum acquired.
(R)-N-((4R)-1-Acetyl-6-((2,3-dihydro-1H–inden-1-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (12g) was synthesized as a mixture of diastereomers following general procedure H using 6g (176 mg, 0.551 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (200 mg, 1.65 mmol, 3.0 eq), and Ti(OEt)4 (0.693 mL, 3.31 mmol, 6.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11g) in situ. Once sufficient ketone was converted into imine intermediate (after 16 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (125 mg, 3.31 mmol, 6.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in and run in 100% EA) to yield the title compound as a clear, colorless oil of a mixture of diastereomers (219 mg, 93.6% from 6g). 1H NMR (500 MHz, CDCl3) δ 7.29 – 7.25 (m, 1H), 7.24 – 7.19 (m, 1H), 7.17 – 7.09 (m, 4H), 7.09 – 7.05 (m, 1H), 4.54 (bs, 1H), 3.99 – 3.87 (m, 2H), 3.84 – 3.72 (m, 1H), 3.44 (bs, 1H), 3.19 – 3.04 (m, 1H), 2.96 – 2.75 (m, 2H), 2.68 (q, J = 12.4 Hz, 1H), 2.25 (s, 3H), 2.23 – 2.19 (m, 1H), 2.19 – 2.12 (m, 1H) 2.12 – 2.05 (m, 1H), 1.84 – 1.71 (m, 1H), 1.24 – 1.14 (s, 9H). 13C NMR (126 MHz, CDCl3 δ 169.7, 146.3, 143.8, 138.1, 136.4, 128.7, 128.6, 126.4, 125.9, 125.8, 124.4, 124.3, 123.6, 123.5, 55.6, 55.0, 50.9, 46.1, 46.0, 40.6, 40.5, 31.7, 31.6, 30.9, 30.9, 30.5, 23.2, 22.4, 22.0, 20.9, 14.0.
Tert-Butyl(4R)-4-(((R)-tert-butylsulfinyl)amino)-6-((2,3-dihydro-1H–inden-1-yl)methyl)-3,4-dihydroquinoline-1(2H)-carboxylate (12h) was synthesized as a mixture of diastereomers following general procedure H using 6h (83 mg, 0.220 mmol, 1.0 eq), (R)-2-methylpropane-2-sulfinamide (80 mg, 0.660 mmol, 3.0 eq), and Ti(OEt)4 (0.277 mL, 0.132 mmol, 6.0 eq) to form the (R)-tert-butanesulfinyl imine intermediate (11h) in situ. Once sufficient ketone was converted into imine intermediate (after 16 h), the reaction mixture was transferred to a round bottom flask containing NaBH4 (50 mg, 0.132 mmol, 6.0 eq) and stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (equil in 100%, run in 2:3 EA:hex) to yield the title compound as a clear, colorless oil of a mixture of diastereomers (27 mg, 25.7% from 6h). 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 1H), 7.31 – 7.03 (m, 7H), 4.55 (s, 1H), 4.00 (d, J = 12.8 Hz, 1H), 3.58 (t, J = 12.0 Hz, 1H), 3.44 – 3.35 (m, 1H), 3.28 (s, 1H), 3.07 (ddd, J = 29.4, 13.6, 5.2 Hz, 1H), 2.94 – 2.84 (m, 1H), 2.83 – 2.74 (m, 1H), 2.64 (ddd, J = 19.0, 13.7, 9.6 Hz, 1H), 2.23 – 2.10 (m, 2H), 1.99 (dd, J = 23.8, 10.6 Hz, 1H), 1.76 (tt, J = 14.4, 7.6 Hz, 2H), 1.53 (s, 9H), 1.44 (bs, 3H), 1.22 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 153.6, 146.7, 144.1, 136.41, 136.37, 136.3, 129.22, 129.16, 128.9, 128.8, 128.31, 128.28, 126.5, 126.0, 125.9, 124.49, 124.46, 123.8, 123.72, 123.69, 81.1, 55.6, 50.3, 46.4, 46.3, 40.6, 40.5, 40.03, 39.99, 31.9, 31.7, 31.06, 31.05, 29.5, 28.4, 24.2, 22.6.
(R)-1-Acetyl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13a) was synthesized following general procedure I using 12a (56 mg, 0.135 mmol, 1.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid did not crash out. Residue was washed 3 x with fresh Et2O and dried to yield product as a clear, yellow oil (56 mg, quant.). 1H NMR (500 MHz, CD3OD) δ 7.83 – 7.67 (m, 4H), 7.54 – 7.24 (m, 6H), 4.56 (dd, J = 7.7, 4.0 Hz, 1H), 4.16 (s, 2H), 3.96 – 3.81 (m, 2H), 2.46 – 2.35 (m, 1H), 2.26 (s, 3H), 2.13 – 2.09 (m, 1H). 13C NMR (126 MHz, CD3OD) δ 172.5, 140.8, 139.6, 138.1, 135.1, 133.6, 130.8, 129.2, 128.6, 128.5, 128.4, 128.1, 127.1, 126.5, 48.2, 42.3, 29.8, 23.3, 21.5.
(R)-1-(Tert-Butoxycarbonyl)-6-(cyclohexylmethyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13b) was synthesized following general procedure I using 12b (22 mg, 0.0490 mmol, 1.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. After washing the solid 3 x with fresh Et2O, the remaining Et2O was decanted off, yielding the title compound as an off-white solid (10 mg, 53.5%). 1H NMR (500 MHz, CDCl3) δ 8.79 (s, 3H), 7.70 (d, J = 8.4 Hz, 1H), 7.32 (s, 1H), 7.06 (d, J = 8.4 Hz, 1H), 4.39 (s, 1H), 4.08 (d, J = 13.5 Hz, 1H), 3.64 – 3.54 (m, 1H), 2.40 (d, J = 7.0 Hz, 2H), 2.19 (s, 2H), 1.70 – 1.56 (m , 5H), 1.51 (s, 10H), 1.19 – 1.08 (m, 3H), 0.98 – 0.85 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 153.1, 137.1, 136.3, 130.0, 129.4, 123.9, 122.1, 81.4, 47.6, 43.2, 39.9, 39.5, 33.1, 33.0, 28.3, 27.8, 26.5, 26.3, 26.3.
(R)-1-Acetyl-6-(cyclohexylmethyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13c) was synthesized following general procedure I using 12c (20 mg, 0.0512 mmol, 1.0 eq) and conc. HCl (7.5 µL, 0.31 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. After washing the solid 3 x with fresh Et2O, the remaining Et2O was decanted off, yielding an off-white solid (12 mg, 72.7%). 1H NMR (500 MHz, CD3OD) δ 7.27 (s, 1H), 7.21 (d, J = 8.2 Hz, 1H), 4.58 (t, J = 6.7 Hz, 1H), 3.91 (h, J = 8.3, 7.9 Hz, 2H), 2.53 (d, J = 7.1 Hz, 2H), 2.48 – 2.39 (m, 1H), 2.28 (s, 3H), 2.10 – 1.99 (m, 1H), 1.75–1.65 (m, 5H), 1.61–1.53 (m, 1H), 1.26 – 1.19 (m, 3H), 1.02–0.94 (m, 2H). No 13C spectrum acquired.
(R)-1-Acetyl-6-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13d) was synthesized following general procedure I using 12d (41 mg (from theoretical yield of 12d), 0.0943 mmol, 1.0 eq) and conc. HCl (14 µL, 0.566 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. Solid was filtered off and washed 3 x with fresh Et2O and dried to yield product as a white solid (22 mg, 62.9%). 1H NMR (500 MHz, CD3OD) δ 8.02 (d, J = 7.1 Hz, 1H), 7.87 (d, J = 8.1, 1H), 7.78 (d, J = 7.1 Hz, 1H), 7.50 – 7.43 (m, 4H), 7.43 – 7.39 (m, 2H), 7.30 – 7.23 (m, 1H), 4.53 (t, J = 6.7 Hz, 1H), 4.49 (s, 2H), 3.87 (dtd, J = 8.8, 6.7, 6.2, 3.4 Hz, 2H), 2.39 (dq, J = 12.5, 5.7 Hz, 1H), 2.25 (s, 3H), 2.04 (dd, J = 14.2, 7.1 Hz, 1H). 13C NMR (126 MHz, CD3OD) δ 172.5, 137.3, 135.6, 134.6, 133.3, 130.5, 129.8, 128.8, 128.7, 128.5, 127.08, 127.07, 126.71, 126.70, 126.6, 126.5, 125.3, 48.2, 39.4, 29.9, 23.3.
(R)-1-Acetyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13e) was synthesized following general procedure I using 12e (105 mg 0.273 mmol, 1.0 eq) and conc. HCl (40 µL, 1.64 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. Solid was filtered off and washed 3 x with fresh Et2O and dried to yield product as a white solid (68 mg, 78.2%). 1H NMR (500 MHz, CD3OD) δ 7.43 – 7.37 (m, 1H), 7.32 – 7.21 (m, 4H), 7.20 – 7.15 (m, 1H), 4.62 – 4.53 (m, 1H), 4.01 (s, 2H), 3.95 – 3.86 (m, 2H), 2.49 – 2.38 (m, 1H), 2.28 (s, 3H), 2.07 (s, 1H). 13C NMR (126 MHz, CD3OD) δ 172.4, 142.1, 140.9, 138.1, 130.7, 129.9, 129.6, 129.0, 127.3, 126.5, 48.3, 42.2, 29.9, 23.3.
(R)-1-Acetyl-6-((2,3-dihydro-1H–inden-2-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13f) was synthesized following general procedure I using 12f (46 mg 0.108 mmol, 1.0 eq) and conc. HCl (16 µL, 0.650 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and residue crashed out. Residue was gummy and sticky so it was washed 3 x with fresh Et2O and dried to yield product as a tan solid (39 mg, quantitative). Solid was taken ahead to next reaction (formation of 14f) without further isolation, purification, or characterization.
(4R)-1-Acetyl-6-((2,3-dihydro-1H–inden-1-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13g) was synthesized as a mixture of diastereomers following general procedure I using 12g (219 mg 0.516 mmol, 1.0 eq) and conc. HCl (76 µL, 3.10 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. Solid was filtered off and washed 3 x with fresh Et2O and dried to yield title compound as a white solid (135 mg, 73.4%). 1H NMR (500 MHz, CD3OD) δ 7.46 – 7.38 (1H), 7.27 (bs, 1 H), 7.22 – 7.17 (m, 1H), 7.16 – 7.08 (m, 3H), 4.60 (s, 1H), 3.93 (qt, J = 8.3, 4.4 Hz, 2H), 3.53–3.44 (m, 1H), 3.17 (dt, J = 13.4, 6.6 Hz, 1H), 2.94 – 2.84 (m, 1H), 2.84 – 2.74 (m, 1H), 2.74 – 2.67 (m, 1H), 2.46 (dq, J = 11.5, 4.7 Hz, 1H), 2.30 (s, 3H), 2.18–2.05 (m, 2H), 1.78 (dp, J = 13.2, 6.6 Hz, 1H). 13C NMR (126 MHz, CD3OD) δ 172.43, 172.41, 147.7, 147.6, 145.11, 145.08, 138.1, 131.0, 129.44, 129.37, 127.7, 127.10, 127.06, 126.3, 125.48, 125.47, 124.73, 124.70, 48.3, 47.6, 41.8, 41.8, 32.9, 32.8, 31.9, 30.0, 23.4, 23.3.
(4R)-1-(tert-Butoxycarbonyl)-6-((2,3-dihydro-1H–inden-1-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride (13h) was synthesized as a mixture of diastereomers following general procedure I using 12h (27 mg 0.0559 mmol, 1.0 eq) and conc. HCl (8 µL, 0.336 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. Solid was filtered off and washed 3 x with fresh Et2O and dried to yield title compound as a white solid (10 mg, 43.5%). 1H NMR (500 MHz, CD3OD) δ 7.71 (d, J = 8.5 Hz, 1H), 7.28 (s, 1H), 7.24 – 7.17 (m, 2H), 7.10 (m, 3H), 4.56 (d, J = 4.7 Hz, 1H), 3.95 – 3.78 (m, 2H), 3.51 – 3.40 (m, 1H), 3.13 (dt, J = 12.8, 6.4 Hz, 1H), 2.95 – 2.74 (m, 2H), 2.68 (dd, J = 13.5, 9.2 Hz, 1H), 2.39 – 2.30 (d, J = 4.1 Hz, 1H), 2.18 – 2.03 (m, 2H), 1.78 (dp, J = 13.2, 6.5 Hz, 1H), 1.54 (d, J = 1.4 Hz, 9H). 13C NMR (126 MHz, CD3OD) δ 154.8, 147.7, 145.1, 138.41, 138.37, 138.0, 131.0, 130.9, 129.5, 129.4, 127.7, 127.1, 127.0, 125.7, 125.5, 124.7, 83.0, 47.6, 41.9, 41.7, 32.8, 31.9, 29.2, 28.5.
(S)-N-((R)-1-Acetyl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14a) was synthesized following general procedure J starting from the (R) amine intermediate 13a (56 mg, 0.153 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (17 mg, 30.4%). Starting material 13a was recovered, but not considered when calculating percent yield. 1H NMR (500 MHz, CD3OD) δ 7.80 – 7.71 (m, 4H), 7.60 (s, 1H), 7.47 – 7.36 (m, 2H), 7.28 (dd, J = 8.6, 1.8 Hz, 1H), 7.19 (s, 1H), 7.12 (dd, J = 8.6, 2.1 Hz, 1H), 6.51 (s, 2H), 4.94 (q, J = 6.8 Hz, 1H), 4.07 (s, 2H), 3.87 (dd, J = 11.5, 5.0 Hz, 1H), 3.24 (dd, J = 13.7, 11.5 Hz, 1H), 3.04 (dd, J = 13.7, 5.0 Hz, 1H), 2.26 (s, 6H), 2.17 (s, 3H), 1.89 – 1.79 (m, 1H), 1.51–1.41 (m, 1H). 13C NMR (126 MHz, CD3OD) δ 172.5, 169.2, 157.4, 151.9, 140.1, 139.8, 135.1, 133.6, 129.5, 129.1, 128.6, 128.5, 128.4, 127.9, 127.1, 126.5, 125.8, 123.3, 116.5, 53.5, 47.2, 47.1, 42.3, 31.9, 31.2, 23.4, 20.4. HPLC (gradient A): retention time = 38.8. ESI-MS 522.3 [M+H]+.
(S)-2-Amino-N-((R)-6-(cyclohexylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14b) was synthesized following general procedure J starting from the (R) amine intermediate 13b (10 mg, 0.0262 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (9 mg, 63.2%). 1H NMR (500 MHz, CD3OD) δ 8.09 (d, J = 8.0 Hz, 1H), 6.85 (d, J = 2.0 Hz, 1H), 6.83 (s, 1H), 6.61 (d, J = 8.1 Hz, 1H), 6.39 (s, 2H), 4.89 (q, J = 5.0 Hz, 1H), 3.78 (dd, J = 11.6, 5.1 Hz, 1H), 3.16 (dd, J = 13.6, 11.6 Hz, 1H), 2.99 (dt, J = 12.5, 4.3 Hz, 1H), 2.93 (dd, J = 13.7, 5.1 Hz, 1H), 2.52 (td, J = 11.7, 2.6 Hz, 1H), 2.31 – 2.22 (m, 2H), 2.18 (s, 6H), 1.77 – 1.67 (m, 1H), 1.61 – 1.48 (m, 5H), 1.47 – 1.40 (m, 1H), 1.37 – 1.27 (m, 1H), 1.12–1.02 (m, 3H), 0.87 – 0.74 (m, 2H). 13C NMR (126 MHz, CD3OD) δ 168.6, 157.4, 140.0, 136.3, 131.8, 131.0, 123.3, 118.9, 116.4, 53.4, 45.8, 44.3, 41.2, 39.0, 34.3, 34.1, 31.9, 28.7, 27.6, 27.38, 27.36, 20.5. HPLC (gradient A): retention time = 31.0. ESI-MS 458.2 [M+Na]+.
(S)-N-((R)-1-Acetyl-6-(cyclohexylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14c) was synthesized following general procedure J starting from the (R) amine intermediate 13c (12 mg, 0.0372 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (12 mg, 55.5%). 1H NMR (500 MHz, CD3OD) δ 8.15 (d, J = 8.1 Hz, 1H), 7.06 – 7.00 (m, 2H), 6.51 (s, 2H), 4.99 – 4.93 (m, 1H), 3.88 (dd, J = 11.5, 5.0 Hz, 1H), 3.79 (s, 1H), 3.26 (dd, J = 13.6, 11.5 Hz, 1H), 3.22 – 3.10 (m, 1H), 3.05 (dd, J = 13.7, 5.0 Hz, 1H), 2.48 – 2.38 (m, 2H), 2.28 (s, 6H), 2.19 (s, 3H), 1.90 – 1.82 (m, 1H), 1.74 – 1.59 (m, 5H), 1.53 – 1.37 (m, 2H), 1.26 – 1.12 (m, 3H), 0.99 – 0.87 (m, 2H). 13C NMR (126 MHz, CD3OD) δ 172.5, 169.2, 157.5, 140.1, 129.5, 125.4, 123.3, 116.5, 53.5, 47.0, 44.4, 41.0, 34.3, 34.2, 32.0, 31.3, 27.6, 27.36, 27.35, 23.33, 20.44. HPLC (gradient A): retention time = 39.5. ESI-MS 478.3 [M+Na]+.
(S)-N-((R)-1-Acetyl-6-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14d) was synthesized following general procedure J starting from the (R) amine intermediate 13d (22 mg, 0.060 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (4 mg, 12.9%). Starting material 13d was recovered, but not considered when calculating percent yield. 1H NMR (500 MHz, CD3OD) δ 7.99 – 7.95 (m, 1H), 7.85 (d, J = 7.8 Hz, 1H), 7.78 – 7.74 (m, 1H), 7.48 – 7.37 (m, 4H), 7.27 (d, J = 7.0 Hz, 1H), 7.22 – 7.17 (m, 1H), 7.02 (d, J = 8.4 Hz, 1H), 6.51 (s, 2H), 4.93 (bs, 1H) 4.40 (s, 2H), 3.84 (dd, J = 11.4, 5.0 Hz, 1H), 3.24 (t, J = 12.6 Hz, 1H), 3.05 – 2.98 (m, 1H), 2.27 (d, J = 1.4 Hz, 6H), 2.17 (s, 3H), 1.86 (dt, J = 11.6, 5.7 Hz, 1H), 1.49 (bs, 1H). No 13C NMR Spectrum acquired. HPLC (gradient A): retention time = 38.3. ESI-MS 544.3 [M+Na]+.
(S)-N-((R)-1-Acetyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14e) was synthesized following general procedure J starting from the (R) amine intermediate 13e (68 mg, 0.215 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (16 mg, 12.7%). Starting material 13e was recovered, but not considered when calculating percent yield. 1H NMR (500 MHz, CD3OD) δ 7.26 – 7.20 (m, 2H), 7.18 – 7.12 (m, 4H), 7.06 (d, J = 8.3 Hz, 1H), 6.51 (s, 2H), 4.94 (t, J = 6.0 Hz, 1H), 3.94 – 3.83 (m, 3H), 3.27 – 3.22 (m, 2H), 3.16 (m, 1H), 3.05 (dd, J = 13.8, 5.0 Hz, 1H), 2.27 (s, 6H), 2.18 (s, 3H). No 13C NMR spectrum acquired. HPLC (gradient A): retention time = 32.7. ESI-MS 494.3 [M+Na]+.
(S)-N-((R)-1-Acetyl-6-((2,3-dihydro-1H–inden-2-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14f) was synthesized following general procedure J starting from the (R) amine intermediate 13f (39 mg, 0.108 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as a TFA salt (14 mg, 20.6%).1H NMR (500 MHz, CD3OD) δ 7.12 (s, 4H), 7.09 – 7.04 (m, 2H), 6.51 (s, 2H), 4.97 (s, 1H), 3.85 (dd, J = 11.2, 4.3 Hz, 1H), 3.29 – 3.19 (m, 2H), 3.04 (dd, J = 13.7, 4.1 Hz, 2H), 2.98 – 2.87 (m, 2H), 2.73 (d, J = 10.3 Hz, 3H), 2.62 (d, J = 15.6 Hz, 2H), 2.28 (s, 6H), 2.21 (s, 3H). No 13C NMR spectrum acquired. HPLC (gradient A): retention time = 38.4. ESI-MS 534.3 [M+Na]+.
(S)-N-((R)-1-Acetyl-6-(((R/S)-2,3-dihydro-1H–inden-1-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14g) was synthesized following general procedure J starting from the (R) amine intermediate 13g (20 mg, 0.056 mmol, 1.0 eq) to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as the TFA salt of a mixture of diastereomers (8 mg, 22.9%). Starting material 13g was recovered, but not considered when calculating percent yield. 1H NMR (500 MHz, CD3OD) δ 7.21 – 6.98 (m, 7H), 6.52 (s, 2H), 5.03 – 4.93 (1, 2H), 3.87 (dt, J = 10.5, 4.9 Hz, 1H), 3.81 (s, 1H), 3.40 (q, J = 7.3 Hz, 1H), 3.30–3.21 (m, 1H), 3.08 – 3.00 (m, 2H), 2.90–2.81 (m, 1H), 2.80 – 2.70 (m, 1H), 2.67 – 2.55 (m, 1H), 2.28 (s, 6H), 2.21 (s, 3H), 2.15 – 2.03 (m, 1H), 1.94 – 1.82 (m, 1H), 1.71 (dq, J = 14.3, 7.4 Hz, 1H), 1.45 (s, 1H). No 13C NMR spectrum acquired. HPLC (gradient A): retention time = 38.4. ESI-MS 534.3 [M+Na]+.
(S)-2-Amino-N-((R)-6-(((R/S)-2,3-dihydro-1H–inden-1-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14h) was synthesized following general procedure J starting from the (R) amine intermediate 13h (10 mg, 0.0241 mmol, 1.0 eq) as a mixture of diastereomers to yield crude product which was purified by semipreparative HPLC then lyophilized to yield the title compound as the TFA salt of a mixture of diastereomers (5 mg, 35.7%). 1H NMR (500 MHz, CD3OD) δ 7.18 – 7.14 (m, 1H), 7.12 – 7.06 (m, 2H), 7.05 – 7.00 (m, 1H), 6.97 – 6.91 (m, 2H), 6.62 (dd, J = 8.4, 3.4 Hz, 1H), 6.49 (s, 2H), 4.97 (t, J = 4.7 Hz, 1H), 3.86 (dt, J = 11.7, 4.8 Hz, 1H), 3.38–3.31 (m, 1H) 3.29 – 3.22 (m, 1H), 3.09 – 2.97 (m, 3H), 2.88 – 2.79 (m, 1H), 2.78 – 2.69 (m, 1H), 2.64 – 2.43 (m, 3H), 2.28 (s, 6H), 2.13 – 1.98 (m, 1H), 1.79 (t, J = 12.6 Hz, 1H), 1.69 (ddd, J = 15.1, 13.0, 7.0 Hz, 1H), 1.58 – 1.49 (m, 1H). No 13C NMR spectrum acquired . HPLC (gradient A): retention time 30.7. ESI-MS 492.2 [M+Na]+.
(S)-2-Amino-N-((R)-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14i) was previously synthesized. See ref. 1 for characterization details.
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)propanamide (14j) was previously synthesized. See ref. 1 for characterization details.
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-6-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)propanamide (14k) was previously synthesized. See ref. 1 for characterization details.
(S)-2-Amino-N-((R)-6-((2,3-dihydro-1H–inden-2-yl)methyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14l) was previously synthesized. See ref. 1 for characterization details.
(S)-2-Amino-N-((R)-7-benzyl-1,2,3,4-tetrahydronaphthalen-1-yl)-3-(4-hydroxy-2,6-dimethylphenyl) propanamide (14m) was synthesized following general procedure J starting from the (R) amine intermediate 19m (44 mg, 0.161 mmol) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound as a TFA salt (58 mg, 84%). 1H NMR (400 MHz, CD3OD) δ 8.09 (d, J = 8.4 Hz, 1H), 7.19 (dd, J = 8.8, 6.8 Hz, 2H), 7.16 – 7.07 (m, 3H), 7.04 (s, 1H), 6.91 (d, J = 1.2 Hz, 2H), 6.47 (s, 2H), 4.92 (s, 1H), 3.87 (d, J = 4.9 Hz, 1H), 3.83 (s, 2H), 3.24 (dd, J = 13.6, 11.5 Hz, 1H), 3.01 (dd, J = 13.7, 5.0 Hz, 1H), 2.62 – 2.51 (m, 2H), 2.26 (s, 6H), 1.69 – 1.45 (m, 2H), 1.45 – 1.34 (m, 1H), 1.32 – 1.19 (m, 1H). 13C NMR (101 MHz, CD3OD) δ 167.4, 155.9, 141.3, 138.8, 138.5, 135.14, 135.10, 135.0, 129.2, 128.8, 128.3, 127.9, 127.8, 125.5, 121.8, 115.0, 52.1, 48.2, 48.0, 47.8, 47.6, 47.4, 47.3, 47.2, 47.0, 40.9, 30.6, 29.2, 28.1, 19.1, 18.9. HPLC (gradient A): retention time = 40.6 ESI-MS 451.2 [M+Na]+.
(S)-2-Amino-N-((R)-7-(cyclohexylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (14n) was synthesized following general procedure J starting from the (R) amine intermediate 19n (20 mg, 0.0715 mmol) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound as a TFA salt (35 mg, 89.7%). 1H NMR (500 MHz, CD3OD) δ 8.08 – 8.02 (m, 1H), 6.95 – 6.89 (m, 3H), 6.49 (s, 2H), 4.98 – 4.93 (m, 1H), 3.92 – 3.81 (m, 1H), 3.25 (d, J = 12.2 Hz, 1H), 3.01 (d, J = 14.1 Hz, 1H), 2.60 – 2.54 (bs, 2H), 2.40 – 4.33 (bs, 2H), 2.28 (s, 6H), 1.73 – 1.59 (m, 6H), 1.58–1.52 (m, 1H), 1.47 – 1.37 (m, 2H), 1.33 – 1.24 (m, 1H), 1.22- 1.12 (m, 3H), 0.92 (m, 2H). 13C NMR (126 MHz, CD3OD) δ 168.7, 157.3, 140.1, 139.9, 136.2, 136.0, 130.7, 129.8, 129.5, 123.2, 116.5, 53.53, 53.49, 48.8, 44.7, 41.1, 34.4, 34.2, 32.0, 30.7, 29.6, 27.7, 27.39, 27.38, 20.5, 20.3. HPLC (gradient A): retention time = 47.3. ESI-MS 457.3 [M+H]+.
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-7-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)propanamide (14o) was synthesized following general procedure J starting from the (R) amine intermediate 19o (11 mg, 0.034 mmol) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound as a TFA salt (14 mg, 69.7%). 1H NMR (500 MHz, CD3OD) δ 8.09 (d, J = 8.2 Hz, 1H), 7.74 (dd, J = 25.4, 7.7 Hz, 4H), 7.56 (s, 1H), 7.40 (p, J = 7.0 Hz, 3H), 7.26 (d, J = 8.3 Hz, 1H), 7.11 (s, 1H), 7.03 – 6.94 (m, 3H), 6.49 (s, 2H), 5.01 – 4.93 (m, 1H), 4.03 (s, 2H), 3.85 (dd, J = 11.2, 4.7 Hz, 1H), 3.24 (t, J = 12.5 Hz, 1H), 3.00 (dd, J = 13.5, 4.7 Hz, 1H), 2.65 – 2.55 (m, 2H), 2.27 (s, 6H), 1.69 – 1.60 (m, 1H), 1.59 – 1.52 (m, 1H), 1.47 – 1.37 (m, 1H), 1.33 – 1.24 (bs, 1H). 13C NMR (126 MHz, CD3OD) δ 168.8, 157.3, 140.3, 140.0, 139.9, 136.7, 136.6, 135.1, 133.5, 130.7, 130.4, 129.5, 128.9, 128.6, 128.4, 127.7, 127.0, 126.4, 123.2, 116.5, 111.4, 53.5, 48.7 , 42.5, 32.0, 30.6, 29.6, 20.5, 20.3. HPLC (gradient A): retention time = 45.6. ESI-MS 479.3 [M+H]+.
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-7-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)propanamide (14p) was synthesized following general procedure J starting from the (R) amine intermediate 19p (7 mg, 0.0216 mmol) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound as a TFA salt (11 mg, 85.9%). 1H NMR (500 MHz, CD3OD) δ 7.97 (d, J = 8.1 Hz, 1H), 7.87 – 7.82 (m, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.49 – 7.34 (m, 3H), 7.22 (d, J = 7.0 Hz, 1H), 7.13 (s, 1H), 6.95 – 6.84 (m, 2H), 6.49 (d, J = 1.8 Hz, 2H), 4.99 – 4.91 (m, 1H), 4.35 (s, 2H), 3.84 (ddd, J = 11.5, 5.1, 1.8 Hz, 1H), 3.25 (ddd, J = 13.5, 11.4, 1.9 Hz, 1H), 3.00 (ddd, J = 13.9, 5.1, 1.7 Hz, 1H), 2.64 – 2.56 (m, 2H), 2.27 (s, 6H), 1.68–1.59 (m, 1H), 1.59 – 1.51 (m, 1H), 1.46 – 1.38 (m, 1H), 1.33 – 1.24 (m, 1H). 13C NMR (126 MHz, cd3od) δ 167.2, 155.9, 138.5, 138.3, 136.6, 135.2, 135.1, 134.1, 131.9, 129.0, 128.8, 128.2, 127.6, 126.71, 126.69, 125.4, 125.1, 125.0, 123.8, 121.8, 115.0, 52.1, 47.4, 38.1, 30.6, 29.2, 28.1, 19.1, 18.9. No HPLC retention time data. ESI-MS 501.1 [M+Na]+.
(R)-N-((R)-7-Bromo-1,2,3,4-tetrahydronaphthalen-1-yl)-2-methylpropane-2-sulfinamide (17) was synthesized following general procedure H using commercially available 15 (1.0 g, 4.44 mmol, 1 eq), (R)-2-methylpropane-2-sulfinamide (1076 mg, 8.88 mmol, 2.0 eq), and Ti(OEt)4 (3.73 mL, 17.771 mmol, 4 eq) to form the (R)-tert-butanesulfinyl imine intermediate (16) in situ. Once sufficient ketone was converted into imine intermediate (after 48 h), the reaction mixture was transferred via cannula to a round bottom flask containing NaBH4 (671.7 mg, 17.76 mmol, 4 eq) and 13 mL of THF in a xylenes dry ice bath, after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography (1:9 EA:hex) to yield the title compound as a clear, colorless oil (1126 mg, 77%) (from 15). 1H NMR (500 MHz, CDCl3) δ 7.56 (d, J = 2.1 Hz, 1H), 7.24 (dd, J = 8.2, 2.1 Hz, 1H), 6.93 (d, J = 8.2 Hz, 1H), 4.46 (q, J = 4.4 Hz, 1H), 3.31 (d, J = 4.0 Hz, 1H), 2.72 (dt, J = 17.0, 5.2 Hz, 1H), 2.61 (ddd, J = 17.0, 8.9, 5.7 Hz, 1H), 2.00 – 1.92 (m, 1H), 1.92 – 1.79 (m, 2H), 1.76 – 1.67 (m, 1H), 1.18 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 138.9, 138.0, 132.07, 132.06, 130.8, 130.5, 119.6, 77.3, 77.0, 76.7, 55.4, 52.5, 30.2, 28.4, 22.5, 18.0.
(R)-N-((R)-7-Benzyl-1,2,3,4-tetrahydronaphthalen-1-yl)-2-methylpropane-2-sulfinamide (18m) was synthesized following general procedure F using 17 (110 mg, 0.33 mmol, 1.0 eq), 2-benzyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (148.2 uL, 0.66 mmol, 2.0 eq), K2CO3 (138 mg, 0.99 mmol, 3.0 eq), and Pd(dppf)Cl2 (24.4 mg, 0.033 mmol, 0.1 eq). The reagents were placed in a microwave tube followed by 1–2 mL of the previously degassed 3:1 acetone/water solvent system and reacted in microwave with max temp of 110°C, max power of 300 W for 60 min with “Powermax” enabled. Once crude mixture was filtered through Celite, the residue was purified via silica gel chromatography (1:1 EA:hex) to yield title compound (64 mg, 56%) as a clear, colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.35 – 7.31 (m, 1H), 7.31 – 7.24 (m, 2H), 7.23 – 7.15 (m, 3H), 7.02 (t, J = 1.4 Hz, 2H), 4.56 (d, J = 3.9 Hz, 1H), 3.95 (s, 2H), 3.23 (d, J = 3.2 Hz, 1H), 2.78 (dt, J = 16.9, 4.9 Hz, 1H), 2.69 (ddd, J = 16.5, 9.5, 5.6 Hz, 1H), 2.08 – 1.99 (m, 1H), 1.99 – 1.82 (m, 2H), 1.80 – 1.70 (m, 1H), 1.22 (s, 9H).13C NMR (126 MHz, CDCl3) δ 141.2, 139.5, 136.8, 135.5, 129.9, 129.5, 128.83, 128.82, 128.5, 128.2, 126.0, 77.3, 77.1, 76.8, 55.4, 52.5, 41.6, 30.3, 28.7, 22.6, 18.1.
(R)-N-((R)-7-(Cyclohexylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)-2-methylpropane-2-sulfinamide (18n) was synthesized following general procedure G using 17 (100 mg, 0.30 mmol, 1.0 eq), (cyclohexylmethyl)boronic acid (86 mg, 0.61 mmol, 2.0 eq), K2CO3 (126 mg, 0.91 mmol, 3.0 eq), Ag2O (175 mg, 0.76 mmol, 2.5 eq), and Pd(dppf)Cl2 (22 mg, 0.031 mmol, 0.1 eq). The reagents were placed in a microwave tube followed by 1.5 mL anhyd. THF and reacted in microwave with max temp of 80°C, max power of 300 W for 60 min, “Powermax” option disabled. Once the crude mixture was filtered through Celite, the residue purified via silica gel chromatography (equil in 100% hex, run in 2:3 EA:hex) to yield the title compound as clear, colorless oil (105 mg, quant), which was taken to the next step (formation of 19n) without further characterization.
(R)-2-Methyl-N-((R)-7-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)propane-2-sulfinamide (18o) was synthesized following general procedure F using 17 (49 mg, 0.15 mmol, 1.0 eq), 4,4,5,5-tetramethyl-2-(naphthalen-2-ylmethyl)-1,3,2-dioxaborolane (80 mg, 3.0 mmol, 2.0 eq), K2CO3 (58 mg, 0.42 mmol, 3.0 eq), and Pd(dppf)Cl2 (11 mg, 0.015 mmol, 0.1 eq). The reagents were placed in a microwave tube followed by 1–2 mL of the previously degassed 3:1 acetone/water solvent system and reacted in microwave with max temp of 110°C, max power of 300 W for 60 min with “Powermax” enabled. Once crude mixture was filtered through Celite, the residue was purified via silica gel chromatography (equil in 100% hex, run in 1:1 EA:hex) to yield title compound (32 mg, 55.2%) as a clear, colorless oil which was taken ahead.
(R)-2-Methyl-N-((R)-7-(naphthalen-1-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-yl)propane-2-sulfinamide (18p) was synthesized following general procedure F using 17 (46 mg, 0.14 mmol, 1 eq), 4,4,5,5-tetramethyl-2-(naphthalen-1-ylmethyl)-1,3,2-dioxaborolane (75 mg, 0.28 mmol, 2 eq), K2CO3 (58 mg, 0.42 mmol, 3 eq), and Pd(dppf)Cl2 (10 mg, 0.014 mmol, 0.1 eq). The reagents were placed in a microwave tube followed by the previously degassed 3:1 acetone/water solvent system and reacted in microwave with max temp of 110°C, max power of 250 W for 30 min with “Powermax” enabled. Once crude mixture was filtered through Celite, the residue was purified via silica gel chromatography (equil in 100% hex, run in 1:4 EA:hex) to yield title compound (11 mg, 20.2%) as clear, colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.03 – 7.97 (m, 1H), 7.87 – 7.81 (m, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.48 – 7.38 (m, 3H), 7.37 (s, 1H), 7.31 (d, J = 6.9 Hz, 1H), 6.96 (s, 2H), 4.53 (q, J = 3.4 Hz, 1H), 4.40 (s, 2H), 3.20 (s, 1H), 2.79–2.71 (m, 1H), 2.70 – 2.60 (m, 1H), 2.05 – 1.97 (m, 1H), 1.94 – 1.79 (m, 2H), 1.77 – 1.65 (m, 1H), 1.17 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 138.9, 136.8, 136.6, 135.5, 133.9, 132.0, 129.7, 129.4, 128.6, 127.9, 127.2, 127.1, 125.9, 125.54, 125.47, 124.2, 55.4, 52.4, 38.6, 30.3, 28.7, 22.6, 18.1.
(R)-7-Benzyl-1,2,3,4-tetrahydronaphthalen-1-aminium chloride (19m) was synthesized following general procedure I using 18m (112 mg, 0.33 mmol, 1.0 eq) and 5 mL of 2M HCl in dioxane. The hydrochloride salt crashed out after the addition of the acid. The Et2O was filtered off leaving a white, flaky precipitate which was washed with cold Et2O, and dried under vacuum without any further purification to yield the title compound (78 mg, 87%). 1H NMR (400 MHz, CDCl3) δ 8.74 (s, 3H), 7.56 (s, 1H), 7.30 – 7.05 (m, 5H), 7.01 (s, 2H), 4.39 (dd, J = 6.7 Hz, 1H), 3.86 (s, 2H), 2.87 – 2.74 (m, 1H), 2.73 – 2.57 (m, 1H), 2.19 – 1.89 (m, 3H), 1.72 (dd, J = 14.7, 6.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 141.0, 139.5, 135.3, 131.1, 129.8, 129.5, 129.3, 128.9, 128.4, 126.0, 77.3, 77.0, 76.7, 49.6, 41.5, 28.3, 27.9, 18.7.
(R)-7-(Cyclohexylmethyl)-1,2,3,4-tetrahydronaphthalen-1-aminium chloride (19n) was synthesized following general procedure I using 18n (100 mg, 0.29 mmol, 1.0 eq) and conc. HCl (42 µL, 1.7 mmol, 6.0 eq). After removing solvent, the residue was re-suspended in Et2O and a white solid crashed out. The Et2O was filtered off leaving a white, flaky precipitate which was washed with cold Et2O, and dried under vacuum without any further purification to yield the title compound (45 mg, 55.9%). 1H NMR (500 MHz, CDCl3) δ 8.72 (s, 3H), 7.38 (s, 1H), 7.00 (s, 2H), 4.40 (q, J = 5.4 Hz, 1H), 2.81 (dt, J = 16.7, 5.4 Hz, 1H), 2.71 – 2.61 (m, 1H), 2.40 (d, J = 7.1 Hz, 2H), 2.18 – 2.07 (m, 2H), 2.05 – 1.94(m, 1H), 1.79 – 1.69 (m, 1H), 1.68 – 1.55 (m, 5H), 1.54 – 1.46 m, 1H), 1.22 – 1.08 (m, 3H), 1.01 – 0.84 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 139.7, 134.7, 130.6, 129.7, 129.6, 129.3, 49.7, 43.5, 39.5, 33.11, 33.09, 28.3, 27.93, 27.91, 26.6, 26.3, 26.3.
(R)-7-(Naphthalen-2-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-aminium chloride (19o) was synthesized following general procedure I using 18o (30 mg, 0.077 mmol, 1.0 eq) and conc. HCl (11 µL, 0.46 mmol, 6.0 eq). After removing solvent, the residue was re-suspended in Et2O, and solid did not crash out. Residue was washed 3 x with fresh Et2O then dried to yield the title compound as off white, sticky solid (11 mg, 44.3%). 1H NMR (500 MHz, CD3OD) δ 7.83 – 7.74 (m, 3H), 7.66 (s, 1H), 7.43 (p, J = 7.0 Hz, 2H), 7.35 – 7.29 (m, 2H), 7.23 (d, J = 8.0 Hz, 1H), 7.16 (d, J = 7.9 Hz, 1H), 4.45 (t, J = 5.6 Hz, 1H), 4.14 (s, 2H), 2.92 – 2.74 (m, 2H), 2.22 – 2.11 (m, 1H), 2.04 – 1.82 (m, 3H). 13C NMR (126 MHz, CD3OD) δ 141.2, 140.0, 136.9, 135.1, 133.6, 133.0, 131.2, 130.9, 129.8, 129.1, 128.6, 128.5, 128.4, 128.0, 127.1, 126.5, 50.3, 42.5, 29.4, 29.1, 19.6.
(R)-7-(Naphthalen-1-ylmethyl)-1,2,3,4-tetrahydronaphthalen-1-aminium chloride (19p) was synthesized following general procedure I using 18p (11 mg, 0.028 mmol, 1.0 eq) and conc. HCl (4 µL, 0.17 mmol, 6.0 eq). After removing solvent, residue was re-suspended in Et2O, and solid crashed out. After washing the solid 3 x with fresh Et2O, the remaining Et2O was decanted off, yielding the title compound as a white solid (7 mg, 77.8%). 1H NMR (400 MHz, CD3OD) δ 8.05 – 7.96 (m, 1H), 7.86 (dd, J = 7.0, 2.3 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.47 – 7.39 (m, 3H), 7.36 (d, J = 7.0 Hz, 1H), 7.30 (s, 1H), 7.17 – 7.07 (m, 2H), 4.47–3.38 (m, 3H), 2.90 – 2.69 (m, 2H), 2.14 (ddt, J = 14.5, 9.6, 4.6 Hz, 1H), 1.92 (ddt, J = 43.3, 20.4, 8.1 Hz, 3H). No 13C NMR spectrum acquired.
In Vitro Pharmacology. Cell Lines and Membrane Preparations. All tissue culture reagents were purchased from Gibco Life Sciences (Grand Island, NY, U.S.). C6-rat glioma cells stably transfected with a rat µ (C6-MOR) or rat δ (C6-DOR) opioid receptor30 and Chinese hamster ovary (CHO) cells stably expressing a human κ (CHO-KOR) opioid receptor31 were used for all in vitro assays. Cells were grown to confluence at 37 °C in 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum and 5% penicillin/streptomycin. Membranes were prepared by washing confluent cells three times with ice cold phosphate buffered saline (0.9% NaCl, 0.61 mM Na2HPO4, 0.38 mM KH2PO4, pH 7.4). Cells were detached from the plates by incubation in warm harvesting buffer (20 mM HEPES, 150 mM NaCl, 0.68 mM EDTA, pH 7.4) and pelleted by centrifugation at 1600 rpm for 3 min. The cell pellet was suspended in ice-cold 50 mM Tris-HCl buffer, pH 7.4, and homogenized with a Tissue Tearor (Biospec Products, Inc., Bartlesville, OK, U.S.) for 20 s. The homogenate was centrifuged at 15,000 rpm for 20 min at 4 °C. The pellet was rehomogenized in 50 mM Tris-HCl with a Tissue Tearor for 10 s, followed by recentrifugation. The final pellet was resuspended in 50 mM Tris-HCl and frozen in aliquots at 80 °C. Protein concentration was determined via a BCA protein assay (Thermo Scientific Pierce, Waltham, MA, U.S.) using bovine serum albumin as the standard.
Radioligand Binding Assays. Radiolabeled compounds were purchased from Perkin-Elmer (Waltham, MA, U.S.). Opioid ligand binding assays were performed by competitive displacement of 0.2 nM [3H]diprenorphine (250 µCi, 1.85 TBq/mmol) by the peptidomimetic from membrane preparations containing opioid receptors as described above. The assay mixture, containing membranes (20 µg protein/tube) in 50 mM Tris-HCl buffer (pH 7.4), [3H]diprenorphine, and various concentrations of test peptidomimetic, was incubated at room temperature for 1 h to allow binding to reach equilibrium. The samples were rapidly filtered through Whatman GF/C filters using a Brandel harvester (Brandel, Gaithersburg, MD, U.S.) and washed five times with 50 mM Tris-HCl buffer. Bound radioactivity on dried filters was determined by liquid scintillation counting, after saturation with EcoLume liquid scintillation cocktail, in a Wallac 1450 MicroBeta (Perkin-Elmer, Waltham, MA, U.S.). Nonspecific binding was determined using 10 µM naloxone. The results presented are the mean ± standard error (S.E.M.) from at least three separate assays performed in duplicate. Ki (nM) values were calculated using nonlinear regression analysis to fit a logistic equation to the competition data using GraphPad Prism, version 6.0c, for Mac OS X (GraphPad Software Inc., La Jolla, CA).
Stimulation of [35S]GTPγS Binding. Agonist stimulation of [35S]guanosine 5′-O-[γ-thio]triphosphate ([355S]GTPγS, 1250 Ci, 46.2 TBq/mmol) binding to G-protein was measured as described previously.32 Briefly, membranes (10–20 µg of protein/tube) were incubated 1 h at 25°C in GTPγS buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2, pH 7.4) containing 0.1 nM [35S]GTPγS, 30 µM guanosine diphosphate (GDP), and varying concentrations of test peptidomimetic. G-protein activation following receptor stimulation of [35S]GTPγS (% stimulation) with peptidomimetic was compared with 10 µM of the standard compounds [D-Ala2,N-MePhe4,Gly-ol]enkephalin (DAMGO) at MOR, D-Pen2,5-enkephalin (DPDPE) at DOR, or U69,593 at KOR. The reaction was terminated by vacuum filtration of GF/C filters that were washed 10 times with GTPγS buffer. Bound radioactivity was measured as described above. The results are presented as the mean ± standard error (S.E.M.) from at least three separate assays performed in duplicate; potency (EC50 (nM)) and % stimulation were determined using nonlinear regression analysis with GraphPad Prism, as above.
In Vivo Pharmacology
Animals. Adult male C57BL/6 mice weighing between 20 and 30 g at 8–16 weeks old purchased from Harlan (Indianapolis, IN). Mice were group-housed and had free access to food and water at all times. Experiments were conducted in the housing room, which was maintained on a 12 h light/dark cycle (with lights on at 0700). Each mouse was used only once and experiments were conducted between 10 a.m. and 5 p.m. Studies were performed in accordance with the University of Michigan Committee on the Use and Care of Animals and the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011 publication).
Antinociception. The antinociceptive effects of select peptidomimetics were evaluated in the WWTW assay using a cumulative dosing procedure.33 To determine tail withdrawal latencies, each mouse was placed briefly into a plastic, cylindrical restrainer and 2–3 cm of the tail tip was placed into a water bath maintained at 50 °C. The latency to withdraw the tail was recorded with a maximum cutoff time of 20 s. If the mouse did not remove its tail by the cutoff time, the experimenter removed its tail from the water to prevent tissue damage. Each animal received an injection of saline (intraperitoneal, ip) and then 30 min later, the baseline withdrawal latencies were recorded and ranged between 3 and 6 s. Following baseline determinations, three increasing doses (1, 2.2, and 6.8 mg/kg) of a final compound 14 analogue was given at 30 min intervals to provide final doses of 1, 3.2, and 10 mg/kg. Thirty minutes after each injection, the tail withdrawal latency was measured as described above. To determine the duration of antinociceptive action the tail-withdrawal test was performed at varying times following administration of a final compound 14 analogue (10 mg/kg, ip). To confirm that 14a produces antinociception via the opioid receptors, the cumulative dose response was repeated over the doses 3.2, 10, and 32 mg/kg following a 30 min pretreatment with 1 mg/kg naltrexone or saline (ip). The ED50 for mice receiving saline pretreatment is 4.73 +/− 0.08 mg/kg 14a, and the ED50 for the mice receiving 1 mg/kg naltrexone is 15.07 +/− 1.03 mg/kg 14a, suggesting that the antinociception 14a produces is MOR-mediated.
Computational Modeling. Three-dimensional models of opioid receptors in inactive conformation were produced as previously described39 using X-ray structures of the mouse MOR (PDB ID: 4dkl)41, the human DOR (PDB ID: 4n6h)45 and the human KOR (PDB ID: 4djh)43 as structural templates. The recently obtained crystal structure of mouse MOR in the active conformation (PDB ID: 5c1m)41 was used as a template for homology modeling of active conformations of DOR and KOR. Structures of receptor loops in the active state were kept similar to those in the crystal structures of corresponding receptors in the inactive state. N-termini of DOR (residues 33–45), KOR (residues 45–57) were modeled using the structure of MOR N-terminus in the active conformation with a few adjustment to satisfy formation of Zn-binding centers involving D216 (H54-D216) and H319 (H57-H319), which were previously suggested for MOR.47 Structures of peptidomimetic ligands were generated using 3D–Builder Application of QUANTA (Accelrys, Inc) followed by the Conformational Search included in the program package. Ligand conformations that demonstrated the best superposition of aromatic substituents of the THQ core with the pharmacophore elements (Tyr1 and Phe3) of receptor-bound conformations of cyclic tetrapeptides40,48 were selected and minimized with CHARMm implemented in QUANTA (Adopted-Basis Newton Raphson method, 100 steps, ε=10). Low energy conformations (within 2 kcal/mol) were manually positioned inside the receptor binding cavity to reproduce the binding modes of cyclic tetrapeptides. The docking pose of each ligand was subsequently refined using the solid docking module of QUANTA. Models of opioid ligand-receptor complexes are available upon request.
Acknowledgments
This study was supported by NIH grant DA003910 (H.I.M, E.M.J and J.R.T). JPA was supported by the Substance Abuse Interdisciplinary Training Program administered by NIDA: 5 T32 DA007267 and NWG by the Pharmacological Sciences Training Program: T32-GM007767. Tyler Trask, Evan Schramm, Aaron Chadderdon and Chao Gao are thanked for additional in vitro studies.
ABBREVIATIONS USED
- MOR
ε opioid receptor
- DOR
δ opioid receptor
- KOR
κ opioid receptor
- THQ
tetrahydroquinoline
- THN
tetrahydronaphthalene
- CHO
Chinese hamster ovary
- GTPγS
guanosine 5’-O-[γ-thio]triphosphate
- WWTW
warm water tail withdrawal
- TM
transmembrane
- Boc2O
di-tert -butyldicarbonate
- Boc
tert-butyldicarbonate
- DIPEA
N,N- diisopropylethylamine
- EA
ethyl acetate
- hex
hexanes
- TfOH
triflic acid
- PyBOP
benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate
- HOBt-Cl
6-chlorohydroxybenzotriazole
- diBoc-Dmt
tert-butyloxycarbonyl-protected 2,6-Dimethyltyrosine
REFERENCES
- 1.Mosberg HI, Yeomans L, Harland AA, Bender AM, Sobczyk-Kojiro K, Anand JP, Clark MJ, Jutkiewicz EM, Traynor JR. Opioid peptidomimetics: leads for the design of bioavailable mixed efficacy µ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands. J. Med. Chem. 2013;56:2139–2149. doi: 10.1021/jm400050y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM. Development of a bioavailable µ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J. Med. Chem. 2014;57:3148–3153. doi: 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J. Pharmacol. Exp. Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- 4.Fujita W, Gomes I, Dove LS, Prohaska D, McIntyre G, Devi LA. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem. Pharmacol. 2014;92:448–456. doi: 10.1016/j.bcp.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dove LS, Lembo A, Randall CW, Fogel R, Andrae D, Davenport JM, McIntyre G, Almenoff JS, Covington PS. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329–338. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of the delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 7.Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective delta opioid receptor antagonist TIPP(psi) Eur. J. Pharmacol. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- 8.Hepburn MJ, Little PJ, Gringas J, Khun CM. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J. Pharmacol. Exp. Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- 9.Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 11.Morphy R, Rankovic Z. Designed multiple ligands An emerging drug discovery paradigm. J. Med. Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- 12.Bender AM, Clark MJ, Agius MP, Traynor JR, Mosberg HI. Synthesis and evaluation of 4-substituted piperidines and piperazines as balanced affinity µ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands. Bioorg. Med. Chem. Lett. 2014;24:548–551. doi: 10.1016/j.bmcl.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purington LC, Pogozheva ID, Traynor JR, Mosberg HI. Pentapeptides displaying µ opioid receptor agonist and δ opioid receptor partial agonist/antagonist properties. J. Med. Chem. 2009;52:7724–7731. doi: 10.1021/jm9007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purington LC, Sobczyk-Kojiro K, Pogozheva ID, Traynor JR, Mosberg HI. Development and in vitro characterization of a novel bifunctional µ-agonist/δ-antagonist opioid tetrapeptide. ACS Chem. Bio. 2011;6:1375–1381. doi: 10.1021/cb200263q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, Bilsky EJ, Rothman RB. 14-Alkoxy- and 14-acyloxypyridomorphinans: µ agonist/δ antagonist opioid analgesics with diminished tolerance and dependence side effects. J. Med. Chem. 2012;55:8350–8363. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, Chung NN, Coderre TJ. The opioid mu agonist/delta antagonist DIPP-NH(2)[Psi] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J. Med. Chem. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, McFadyen IJ, Traynor JR, Mosberg HI. Design of a high affinity peptidomimetic opioid agonist from peptide pharmacophore models. Bioorg. Med. Chem. Lett. 1998;8:2685–2688. doi: 10.1016/s0960-894x(98)00472-7. [DOI] [PubMed] [Google Scholar]
- 18.Anand JP, Purington LC, Pogozheva ID, Traynor JR, Mosberg HI. Modulation of opioid receptor ligand affinity and efficacy using active and inactive state receptor models. Chem. Biol. Drug Des. 2012;80:763–770. doi: 10.1111/cbdd.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pryzdzial MJ, Pogozheva ID, Ho JC, Bosse KE, Sawyer E, Traynor JR, Mosberg HI. Design of high affinity cyclic pentapetide ligands for Κ-opioid receptors. J. Peptide Res. 2005;66:255–262. doi: 10.1111/j.1399-3011.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 20.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab. Rev. 2002;34:83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 21.de Groot MJ, Ackland MJ, Horne VA, Alex AA, Jones BC. Novel approach to predicting P450 mediated drug metabolism. The development of a combined protein and pharmacophore model for CYP2D6. J. Med. Chem. 1999;42:1515–1524. doi: 10.1021/jm981118h. [DOI] [PubMed] [Google Scholar]
- 22.de Groot MJ, Ackland MJ, Horne VA, Alex AA, Jones BC. A novel approach to predicting P450 mediated drug metabolism CYP2D6 catalyzed N-dealkylation reactions and qualitative metabolite predictions using a combined protein and pharmacophore model for CYP2D6. J. Med. Chem. 1999;42:4062–4070. doi: 10.1021/jm991058v. [DOI] [PubMed] [Google Scholar]
- 23.de Groot MJ, Alex AA, Jones BC. Development of a combined protein and pharmacophore model for cytochrome P450 2C9. J. Med. Chem. 2002;45:1983–1993. doi: 10.1021/jm0110791. [DOI] [PubMed] [Google Scholar]
- 24.Singh SB, Shen LQ, Shen LQ, Walker MJ, Sheridan RP. A model for predicting likely sites of CYP3A4-mediated metabolism on drug-like molecules. J. Med. Chem. 2003:46. doi: 10.1021/jm020400s. [DOI] [PubMed] [Google Scholar]
- 25.Gu C, Collins R, Holsworth DD, Walker GS, Voorman RL. Metabolic Aromatization of N-alkyl-1,2,3,4-tetrahydroquinoline substructures to quinolinium by human liver microsomes and horseradish peroxidase. Drug Metab. Dispos. 2006;34:2044–2055. doi: 10.1124/dmd.106.012286. [DOI] [PubMed] [Google Scholar]
- 26.Dobbelaar PH, Marzabadi CH. Povarov reactions of exo-glycals: preparation of C-linked, quinoline analogues. Tetrahedron. 2011;67:9273–9282. [Google Scholar]
- 27.Schmidt RG, Bayburt EK, Latshaw SP, Koenig JR, Daanen JF, McDonald HA, Bianchi BR, Zhong C, Joshi S, Honore P, Marsh KC, Lee CH, Faltynek CR, Gomtsyan AL. Chroman and tetrahydroquinoline ureas as potent TRPV1antagonists. Bioorg. Med. Chem. Lett. 2011;21:1338–1341. doi: 10.1016/j.bmcl.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 28.Bandgar BP, Bettigeri SV, Phopase JJ. Palladium catalyzed ligand-free Suzuki cross-coupling reactions of benzylic halides with aryl boronic acids under mild conditions. Tetrahedron Lett. 2004;45:6959–6962. [Google Scholar]
- 29.Zou G, Reddy YK, Falck JR. Ag(I)-promoted Suzuki-Miyaura cross-couplings of n-alkylboronic acids. Tetrahedron Lett. 2001;42:7213–7215. [Google Scholar]
- 30.Kolanos R, Siripurapu U, Pullagurla M, Riaz M, Setola V, Roth BL, Dukat M, Glennon RA. Binding of isotryptamines and indenes at h5-HT6 serotonin receptors. Bioorg. Med. Chem. Lett. 2005;15:1987–1991. doi: 10.1016/j.bmcl.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 31.Tanuwidjaja J, Peltier HM, Ellman JA. One-pot asymmetric synthesis of either diastereomer of tert-butanesulfinyl-protected amines from ketones. J. Org. Chem. 2007;72:626–629. doi: 10.1021/jo0616512. [DOI] [PubMed] [Google Scholar]
- 32.Borg G, Cogan DA, Ellman JA. One-pot asymmetric synthesis of tert-butanesulfinyl-protected amines from ketones by the in situ reduction of tert-butanesulfinyl ketimines. Tetrahedron Lett. 1999;40:6709–6712. [Google Scholar]
- 33.Colyer JT, Andersen NG, Tedrow JS, Soukup TS, Faul MM. Reversal of diastereofacial selectivity in hydride reductions of N, tert-butanesulfinyl imines. J. Org. Chem. 2006;71:6859–6862. doi: 10.1021/jo0609834. [DOI] [PubMed] [Google Scholar]
- 34.Lee KO, Akil H, Woods JH, Traynor JR. Differential binding properties of oripavines at cloned mu- and delta-opioid receptors. Eur. J. Pharmacol. 1999;378:323–330. doi: 10.1016/s0014-2999(99)00460-4. [DOI] [PubMed] [Google Scholar]
- 35.Husbands SM, Neilan CL, Broadbear J, Grundt P, Breeden S, Aceto MD, Woods JH, Lewis JW, Traynor JR. BU74, a complex oripavine derivative with potent kappa opioid receptor agonism and delayed opioid antagonism. Eur. J. Pharmacol. 2005;509:117–135. doi: 10.1016/j.ejphar.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SHY5Y Cells. Mol. Pharmacol. 1995;47:848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- 37.Lamberts JT, Jutkiewicz EM, Mortensen RM, Traynor JR. Mu-opioid receptor coupling to Gα(o) plays an important role in opioid antinociception. Neuropsychopharmacology. 2011;36:2041–2053. doi: 10.1038/npp.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anand JP, Porter-Barrus VR, Waldschmidt HV, Yeomans L, Pogozheva ID, Traynor JR, Mosberg HI. Translation of structure-activity relationships from cyclic mixed efficacy opioid peptides to linear analogues. Biopolymers. 2014;102:107–114. doi: 10.1002/bip.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansour A, Taylor LP, Fine JL, Thompson RC, Hoversten MT, Mosberg HI, Watson SJ, Akil H. Key residues defining the µ-opioid receptor binding pocket: a site-directed mutagensis study. J. Neurochem. 1997;68:344–353. doi: 10.1046/j.1471-4159.1997.68010344.x. [DOI] [PubMed] [Google Scholar]
- 40.Fowler CB, Pogozheva ID, Lomize AL, LeVine H, Mosberg HI. Complex of an active µ-opioid receptor with a cyclic peptide agonist modeled from experimental constraints. Biochemistry. 2004;43:15796–15810. doi: 10.1021/bi048413q. [DOI] [PubMed] [Google Scholar]
- 41.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the δ-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang X-P, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Structure of the human Κ-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenalti G, Giguere PM, Katritch V, Huang X-P, Thompson AA, Cherezov V, Roth BL, Stevens RC. Molecular control of d-opioid receptor signaling. Nature. 2014;506:191–196. doi: 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenalti G, Zatespin NA, Betti C, Giguere P, Han GW, Ishchenko A, Liu W, Guillemyn K, Zhang H, James D, Wang D, Weierstall U, Spence JC, Boutet S, Messerschmidt M, Williams GJ, Gati C, Yefanov OM, White TA, Oberthuer D, Metz M, Yoon CH, Barty A, Chapman HN, Basu S, Coe J, Conrad CE, Fromme R, Fromme P, Tourwe D, Schiller PW, Roth BL, Ballet S, Katritch V, Stevens RC, Cherezov V. Structural basis for bifunctional peptide recognition at human δ-opioid receptor. Nat. Struct. Mol. Biol. 2015;22:265–268. doi: 10.1038/nsmb.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, Granier S, Gmeiner P, Husbands SM, Traynor JR, Weis WI, Steyaert J, Dror RO, Kobilka BK. Structural insights into m-opioid receptor activation. Nature. 2015;524:315–321. doi: 10.1038/nature14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowler CB, Pogozheva ID, LeVine H, Mosberg HI. Refinement of a homology model of the mu-opioid receptor using distance constraints from intrinsic and engineered zinc-binding sites. Biochemistry. 2004;43:8700–8710. doi: 10.1021/bi036067r. [DOI] [PubMed] [Google Scholar]
- 48.Pogozheva ID, Przydzial MJ, Mosberg HI. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J. 2005;7:E434–E448. doi: 10.1208/aapsj070243. [DOI] [PMC free article] [PubMed] [Google Scholar]