Abstract
Low birth weight serves as a crude proxy for impaired growth during fetal life and indicates a failure for the fetus to achieve its full growth potential. Low birth weight can occur in response to numerous etiologies that include complications during pregnancy, poor prenatal care, parental smoking, maternal alcohol consumption or stress. Numerous epidemiological and experimental studies demonstrate that birth weight is inversely associated with blood pressure and coronary heart disease. Sex and age impact the developmental programming of hypertension. In addition, impaired growth during fetal life also programs enhanced vulnerability to a secondary insult. Macrosomia, which occurs in response to maternal obesity, diabetes and excessive weight gain during gestation, is also associated with increased cardiovascular risk. Yet, the exact mechanisms that permanently change the structure, physiology and endocrine health of an individual across their lifespan following altered growth during fetal life are not entirely clear. Transmission of increased risk from one generation to the next in the absence of an additional prenatal insult indicates an important role for epigenetic processes. Experimental studies also indicate that the sympathetic nervous system, the renin angiotensin system, increased production of oxidative stress and increased endothelin play an important role in the developmental programming of blood pressure in later life. Thus, this review will highlight how adverse influences during fetal life and early development program an increased risk for cardiovascular disease including high blood pressure and provide an overview of the underlying mechanisms that contribute to the fetal origins of cardiovascular pathology.
INTRODUCTION
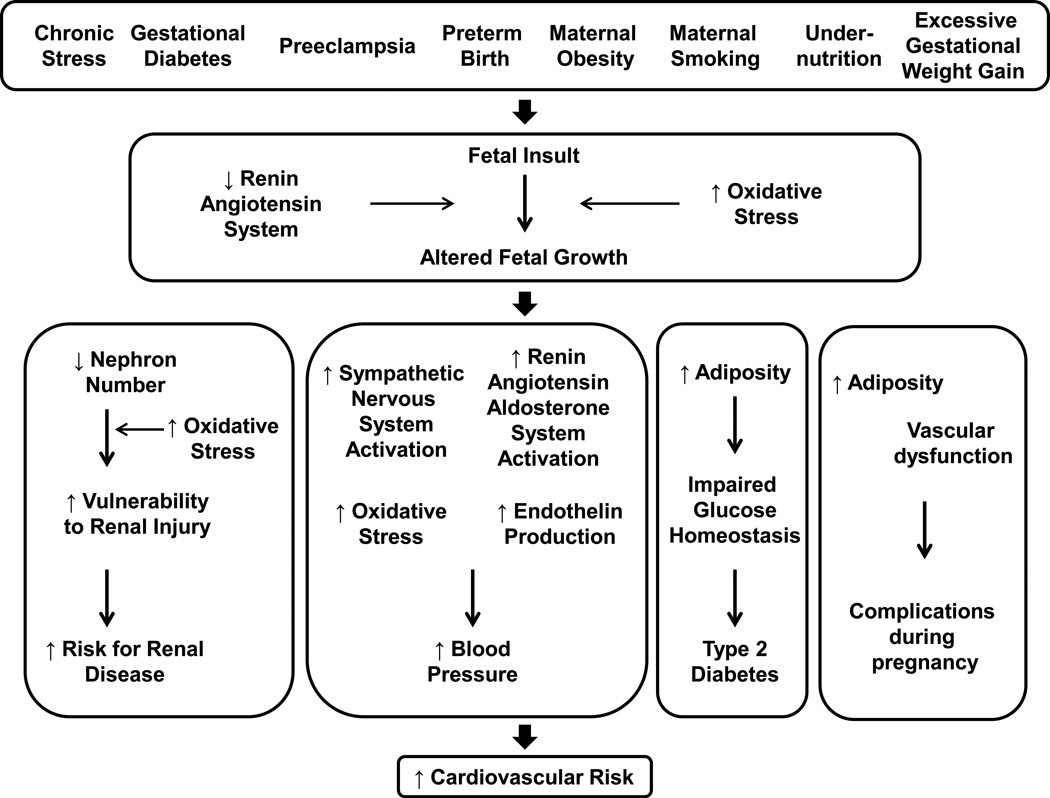
This review will provide an overview of the fetal or developmental origins of cardiovascular (CV) disease and pathology. Numerous studies implicate an association between influences during fetal life that slow or accelerate fetal growth with an increased risk in later life for hypertension, death from coronary heart disease, metabolic disease and chronic kidney disease (9, 10, 20, 87, 104). Experimental models investigating the underlying mechanisms that link insults during fetal life with increased risk for chronic disease mimic the many causes that impair fetal growth in the human population including maternal complications during pregnancy such as hypertension and diabetes, maternal obesity and excessive weight gain during pregnancy, or parental smoking, maternal alcohol consumption and maternal stress (3, 32, 103, 114, 116, 123, 141, 241). Sex and age impact programmed CV risk (3, 21, 32, 84, 132, 145, 249) and events that alter growth during fetal life increase susceptibility to a second insult in later life (143, 252). Risk for a pregnancy complicated by preeclampsia, diabetes or preterm delivery is increased in low birth weight women (80, 81, 82). Thus, influences during early life that alter growth and development exert long-term consequences on the CV health of an individual across their lifespan. Impaired growth during fetal life also impacts the CV health of the next generation (6) implicating epigenetic processes (222) in the etiology of disease that has its origins during fetal life and early development. Experimental studies not only provide proof of principle that adverse influences during fetal life program long-term CV consequences, but they also indicate that the sympathetic nervous system, the renin angiotensin system, increased oxidative stress and increased production of endothelin contribute to the etiology of CV disease that has its origins in fetal life.
HISTORY & ETIOLOGY OF THE FETAL ORIGINS OF CARDIOVASCULAR DISEASE
Historical perspective
From the 1940’s through the 1970’s Widdowson and McCance examined the impact of early undernutrition on later growth and development (227). In the 1970’s Forsdahl noted that poverty in early life followed by prosperity in later life increased the risk for CV disease (56). However, following Barker’s hypothesis in the 1980’s (9) the foundation for the fetal programming of chronic disease, now referred to as the Developmental Origins of Health and Disease (DOHaD) was formulated. Barker postulated that CV disease might have its origins in fetal life based on the observation that the geographical distribution of infant mortality in the early 1900’s closely resembled that of death from ischemic heart disease approximately 60 years later (9). He noted that areas with the greatest mortality were the regions that were the most deprived (9). Using birth weight as a crude marker for poor fetal growth, Barker noted an inverse relationship between birth weight and blood pressure (10) further strengthening the hypothesis that adverse influences during fetal life that slow growth and increase the risk for infant mortality in early life also program an increased risk for CV disease in those that survive (8). He postulated that when fetal nutritional demand was less that nutritional supply, the fetus would adapt for survival during gestation resulting in a redistribution of blood flow to spare the brain at the expense of other organs which in turn would alter the structure, metabolism, physiology, and endocrinology of the impacted areas in a manner that would “program” an increased risk for higher blood pressure and CV disease after birth (8). Since Barker’s first observation, numerous studies have examined the inverse relationship between birth weight and blood pressure (104). Hypertension serves as a risk factor for CV disease and birth weight is also inversely associated with other CV risk factors including chronic kidney disease (233), endothelial dysfunction (109), and cholesterol (34). Numerous maternal factors such as a mother’s body composition, her health and nutrition during pregnancy in addition to parental smoking, maternal alcohol consumption or maternal age, and prenatal care can impact fetal growth and ultimately birth weight. Accelerated weight gain in early postnatal life enhances the adverse impact following slow fetal growth (193) and accelerated growth during the early postnatal period is sufficient to impact systolic blood pressure (SBP) as well as diastolic blood pressure (DBP) (12). Accelerated weight gain not only impacts CV and metabolic parameters following low birth weight, but cardiometabolic risk is also aggravated in children born large for gestational age followed by accelerated post-natal growth (119). Thus, these studies indicate that the period of susceptibility to long-term programming of CV risk extends beyond birth. Hence, Barker’s original hypothesis involved a focus on fetal life. However, expansion of the period of vulnerability now highlights the post-natal period expanding the theory to include the developmental origins of adverse events in early life in importance on later chronic and CV health.
Low birth weight and the impact of fetal undernutrition was the initiating factor in the development of the DOHaD theory. Low birth weight, or 5.5 pounds full term is used as a crude marker indicative of failure to meet one’s full growth potential during fetal life (8). Low birth weight and asymmetric growth restriction reflect one fetal condition related increased CV risk in later life (8). However, recent studies indicate that intrauterine growth restriction (IUGR) indicative of poor fetal growth impacts later chronic health may also include preterm delivery (38). Preterm birth, <37 weeks, is linked to higher blood pressure in later life (38, 108). Total fat mass (22) and insulin resistance (54) are increased in individuals born preterm, but total cholesterol and triglycerides are not altered in young adulthood in individuals born preterm (33). Nephrogenesis continues following birth prior to 34–36 weeks gestation. Total kidney volume is reduced in preterm infants relative to term infants (92) suggesting that nephron number may be reduced although studies have yet to confirm. Preterm individuals exhibit abnormal glomerular morphology (15) although all glomeruli may not be affected (15). Therefore, these studies indicate that the mechanisms that program increased CV risk and high blood pressure following preterm birth differ from those that contribute to increased risk in those born low birth weight but at full term.
Obesity is a growing epidemic worldwide and maternal adiposity and excessive maternal weight gain during gestation greatly impacts the CV health of the offspring in later life in addition to increasing the risk for Type 2 Diabetes (T2D) (50, 126). Fetal exposure to maternal obesity also has an adverse impact on later chronic health of the offspring including increased body mass index (BMI), total body and abdominal fat mass, SBP, insulin levels and lower high-density lipoprotein cholesterol levels (61). Experimental models utilizing diet-induced obesity in the mouse (178) or rat (180) as a developmental insult report that fetal exposure to maternal obesity programs an increase in blood pressure in the offspring providing proof of principle that obesity in one generation programs impaired chronic health in the next generation. Fetal exposure to a maternal diet rich in fat also programs a marked increase in blood pressure in the offspring (95) indicating that fetal exposure to overnutrition is just as detrimental as fetal exposure to undernutrition in regards to long-term CV health. Whether nephron number is compromised following fetal exposure to overnutrition or maternal obesity is not yet known. However, these studies indicate that the developing fetus is sensitive to nutritional supply regardless of whether it involves excessive calories or is restricted in relation to access to proper nutritional needs.
To summarize, based on the similar geographical distribution of infant mortality to death from ischemic heart disease (9), Barker proposed that poor nutrition during early life increases susceptibility to later CV risk (8). Langley-Evans et al. provided proof of concept that undernutrition during fetal life programs an increase in blood pressure in later life utilizing an animal model of maternal protein restriction during gestation in the rat (103). Since this first direct test of Barker’s hypothesis, numerous other experimental models using the rat and different species have been developed to study the mechanisms that link insults during prenatal life with increased risk for CV, renal and metabolic disease in later life. Many of these mimic the maternal causes of low birth weight such as maternal undernutrition, maternal stress or alcohol consumption, and maternal smoking (103, 114, 141, 241, 249) whereas others mimic complications during pregnancy that are linked to increased CV risk in the offspring such as preeclampsia and gestational diabetes (3, 37, 238). These studies demonstrate that numerous factors contribute to the etiology of impaired growth (53, 97) and impact the physiology of an individual during development leading to increased CV risk and chronic disease (83). In addition these studies demonstrate that despite differences in the type of maternal or nutritional insult, an increase in SBP indicative of increased CV risk is observed in later life.
Etiology of impaired growth during fetal life
Complications during pregnancy that include preeclampsia or diabetes, maternal obesity or excessive weight gain during pregnancy, parental smoking, maternal stress or alcohol consumption in addition to maternal age and poor prenatal care impact fetal growth and contribute to the development of increased risk for CV disease in the offspring (50, 52, 60, 69, 126, 142, 201). Some of these factors are preventable; all of these adverse early life exposures impact long-term CV health in manner that may be sex and age dependent.
Maternal undernutrition
The theory of fetal programming originated from studies noting an inverse relationship between birth weight and blood pressure (10, 105). Low birth weight indicative of poor fetal nutrition can occur due to numerous etiologies including maternal undernutrition induced under conditions of famine. The Dutch Famine birth cohort includes men and women born as singleton births following in utero exposure to famine during the Dutch Hunger Winter of 1944–1945. Birth records from the Dutch Famine Birth cohort indicate that late gestational exposure to famine is associated with a reduction in birth weight (197). Early gestational exposure to famine is associated with increased prevalence of coronary heart disease (173). Exposure to famine during fetal life is also associated with an increased risk for hypertension (218) and elevated triacylglycerol concentrations in later life (39). In men HDL-cholesterol concentrations are lower (39) and the effect of famine on lipid profiles is independent of current BMI (172). Exposure to Holocaust conditions during early life is also linked to greater prevalence of CV risk including increased BMI, hypertension, diabetes, congestive heart failure and dyslipidemia (13). Nutritional restriction during fetal life in the human is usually associated with extreme economic conditions or a military crisis. However, these studies provide direct support for Barker’s hypothesis and indicate that the human fetus is sensitive to insults that restrict nutritional needs during development and indicate that undernutrition during fetal life is associated with an increased risk for CV and metabolic disease in later life.
Numerous experimental models use maternal undernutrition as a means to examine the mechanisms that link birth weight and blood pressure. Global undernutrition (70% food restriction) during gestation in the rat programs a marked increase in blood pressure associated with vascular dysfunction in the offspring in later life (152). Global undernutrition also programs an increase in blood pressure in the mouse that is associated with cardiac enlargement and an increase in coronary perivascular fibrosis (93). Maternal protein restriction (8% versus 18%) during gestation programs an increase in blood pressure in rat offspring that occurs in the absence of hypertension in the mother (100). Maternal protein restriction during fetal life also programs hypertension in the mouse (64), sheep (62) or cow (135) indicating that the long-term impact of nutritional insults during fetal life on later CV health is not species-specific. A reduction in nephron number is another common outcome observed in models induced by nutrient restriction in early life (62, 241). Sex influences the severity of programmed insult with female offspring exposed to a moderate reduction in maternal protein restriction protected from the development of hypertension and a reduction in nephron number in early life (242).
Preeclampsia
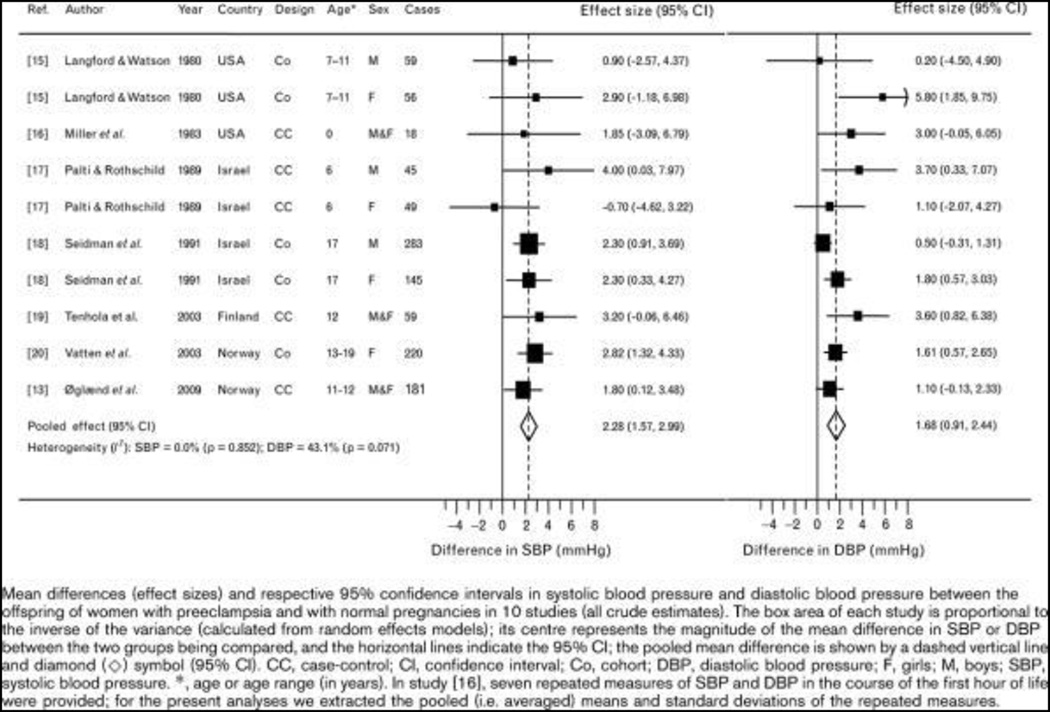
Preeclampsia is major health issue that impacts maternal and fetal health during gestation (5). Chronic hypertension, preeclampsia in a previous pregnancy, age of 35 years or older, diabetes and renal disease in addition to obesity and autoimmune disease increases the risk for preeclampsia (5). The diagnosis for preeclampsia was recently reevaluated and the revised criteria no longer includes proteinuria but can involve any of the following including a SBP of 160 mmHg or higher, thrombocytopenia, impaired liver function, progressive renal insufficiency, pulmonary edema, or new-onset cerebral or visual disturbances (5). Recent studies indicate that preeclampsia not only increases the risk for CV disease in the later life of the mother (82), but it also implicates a greater risk for CV disease in the offspring (35, 82, 83). Adolescent offspring of preeclamptic pregnancies exhibit an increase in adiposity (227) and SBP (35, 52, 142) that persists into adulthood (125) (Figure 1) (52). In female offspring the association between fetal exposure to preeclampsia and SBP in adolescence is confounded by higher weight gain and BMI (221). Thus, fetal exposure to pregnancy complicated by preeclampsia programs increased CV risk in the offspring.
Figure 1.
Relationship between offspring of women with preeclampsia and systolic blood pressure. (Used with permission, Figure 1, 35).
The placenta plays a central role in the etiology of preeclampsia and placental insufficiency is the initiating event that leads to the development of preeclampsia and increased CV risk in the offspring (153). Different models of placental insufficiency are used to investigate the link between fetal exposure to reduced uteroplacental perfusion and later programmed risk (3, 157, 238). Ligation of the uterine arteries, the bilateral uterine ligation model, when initiated at day 18 of gestation in the rat programs a reduction in nephron number (238) associated with vascular dysfunction (208), and hypertension in male offspring (238). Initiation of bilateral uterine ligation at day 19 of gestation in the rat also programs a reduction in nephron number (157). A mechanical reduction in flow to the uteroplacental unit initiated at day 14 of gestation in the rat (100), the reduced uterine perfusion model, induces hypertension (3) and impaired vascular function (155) in the offspring in a manner that is sex-specific (Figure 2) (3) and age-dependent (84). Placental insufficiency in the sheep results in greater extracellular matrix deposition and arterial stiffness (45) indicating that reduced perfusion to the fetus programs increased CV risk despite the species used for study. Although all of these models of placental insufficiency do not result in maternal hypertension during pregnancy, they do program increased CV risk and many exhibit similar mechanistic pathways in the etiology of programmed hypertension indicating a reduction in placental perfusion as a contributory factor.
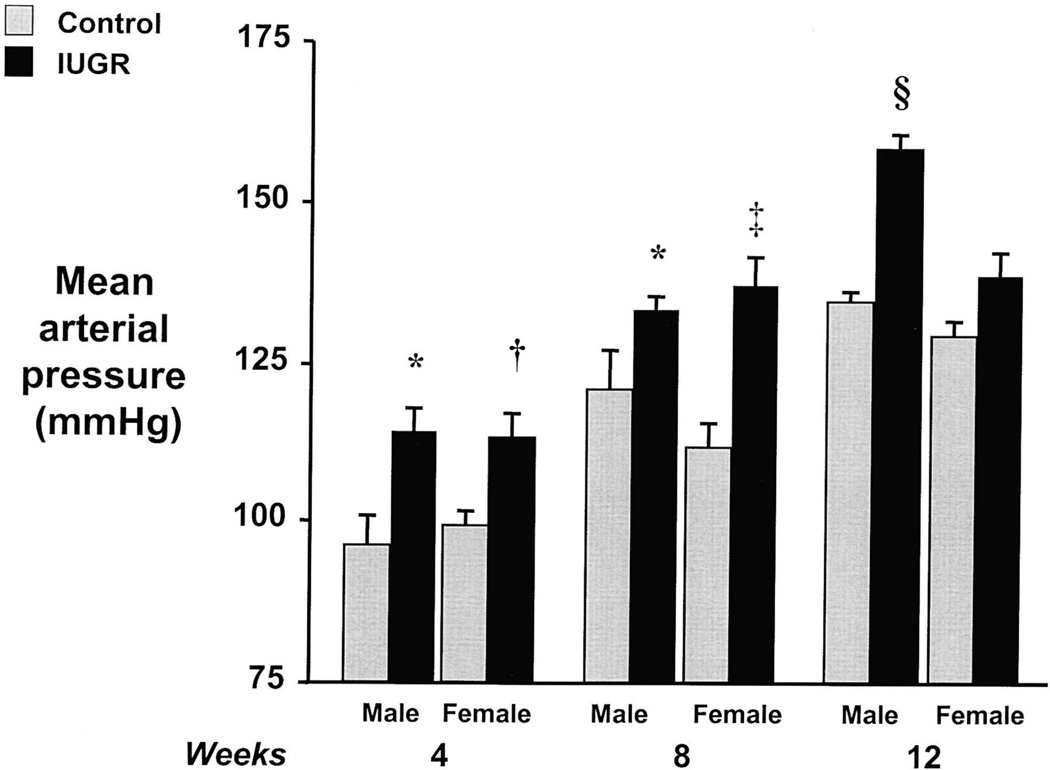
Figure 2.
Measure of MAP in a rat model of IUGR induced by reduced uterine perfusion. Data shown is for both male and female IUGR versus control offspring, at 4, 8, and 12 weeks of age. *P<0.05 vs. male control; †P<0.05 vs. female control; ‡P<0.01 vs. control; and §P<0.01 vs. control. All data are mean±SEM. (Used with permission, Figure 1, 3).
Placental insufficiency results in a reduction in the supply of nutrients and oxygen to the fetus. Whereas some investigators utilize nutrient restriction as an insult during fetal life or placental insufficiency, fetal exposure to maternal hypoxia is also used to mimic the maternal conditions that restrict fetal growth and program increased CV risk in the offspring (113, 236). Vascular function is impaired in rat offspring at 4 months of age following maternal hypoxia (11.5% O2 vs. 21% from days 15 to 21 of gestation) (134) whereas left ventricular hypertrophy, left ventricular diastolic dysfunction and pulmonary hypertension are present by 12 months of age (176). Prenatal exposure to hypoxia programs hypertension in male but not female offspring by 14 months of age (21) demonstrating a sex-specific response to a fetal insult that is apparent in models induced via nutrient restriction (241, 242) and placental insufficiency (3, 238). In addition, these studies that utilize prenatal hypoxia or placental insufficiency indicate that age may augment programmed CV risk (84, 207)
Maternal diabetes and diabetes during pregnancy
Pregnancies complicated by diabetes have an increased risk for high birth weight (225) and development of the Metabolic Syndrome (18). High birth weight is an increased risk factor for obesity in offspring (88). Yet, increased adiposity in offspring exposed to maternal diabetes is not always associated with increased adiposity in the mother (154). Insulin resistance is higher in offspring exposed to gestational diabetes (18, 94). Vascular markers for endothelial dysfunction including E-selectin, vascular adhesion molecule 1 are increased in children exposed to maternal diabetes during fetal life (231). Leptin, waist circumference (231), BMI (217, 231), and SBP (217, 231) are also increased in childhood and adolescence following fetal exposure to maternal diabetes implicating the long-term adverse impact of maternal diabetes on later CV and metabolic health in the offspring. Yet, increased adiposity in offspring of pregnancies complicated by diabetes may contribute to the increased SBP (248). Diabetes during pregnancy is also associated with a greater risk for a low birth weight delivery (75). A systematic review and meta-analysis reveals a strong correlation between diabetes in the mother and increased SBP during childhood in the offspring (2) with a stronger association noted for male offspring, but not female offspring (2). Thus, further studies are needed to determine if sex impacts the programming of later CV risk following a pregnancy complicated by diabetes.
Based on findings from epidemiological studies, fetal exposure to diabetes during pregnancy is another experimental model utilized to examine mechanisms that program chronic disease following a fetal insult. Diabetes induced by injection of streptozotocin in late gestation in the rat programs hypertension in the male (91, 253) but not the female offspring (91). Renal function is impaired is association with hypertension in response to fetal exposure to maternal diabetes (140, 171). Offspring of a pregnancy complicated by diabetes also exhibit salt sensitivity (140). Exposure to maternal diabetes throughout gestation also programs vascular dysfunction in association with hypertension and reduced renal function (170) implicating this as a viable model for study of mechanisms linking fetal insults with later chronic disease.
Maternal obesity and excessive weight gain during pregnancy
Women that are overweight prior to pregnancy increase the risk for abdominal obesity in their offspring regardless of other maternal health factors (159). Increased weight gain during gestation also increases offspring BMI in early adulthood (60, 126). Thus, these studies suggest that weight gain prior to and during pregnancy adversely impacts body composition in the offspring. However, the impact of maternal weight and weight gain during pregnancy is not limited to just adverse influences on body composition in the offspring. Gestational weight gain is associated with an increase in SBP in the offspring in early adulthood (60, 126). Maternal obesity also programs a greater risk for coronary heart disease and T2D in adulthood; observations noted in a large birth cohort independent of socioeconomic measures (50). However, the prevalence for T2D is greater in women than men and the association between maternal obesity and stroke is present only in women (50). Thus, this study indicates that sex impacts the adverse impact of exposure to maternal obesity during gestational life with women exhibiting greater sensitivity to programmed risk relative to men following fetal exposure to maternal obesity. Race impacts perinatal outcome with risk for low birth weight increased in African American women regardless of maternal BMI (127).
The increasing prevalence of obesity in the US and its impact on gestational health and fetal and maternal outcomes after delivery (130) warrant further studies to elucidate the mechanisms that programmed increased CV risk. In the mouse diet-induced obesity in the dam programs an increase in adiposity associated with an increase in nighttime (active phase) blood pressure in male and female offspring with a greater effect observed in female offspring of obese dams (178). Fasting insulin and glucose levels are also elevated in later life in mice exposed to maternal obesity during gestation (178). Diet-induced obesity in the rat also programs an increase in blood pressure in male and female offspring that is associated with a marked increase in leptin, insulin and triglycerides (180).
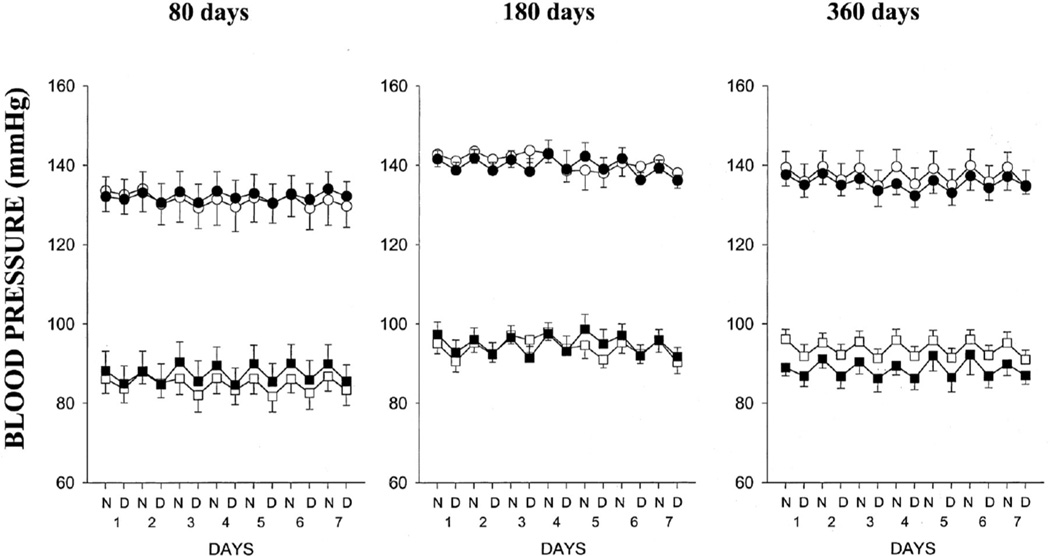
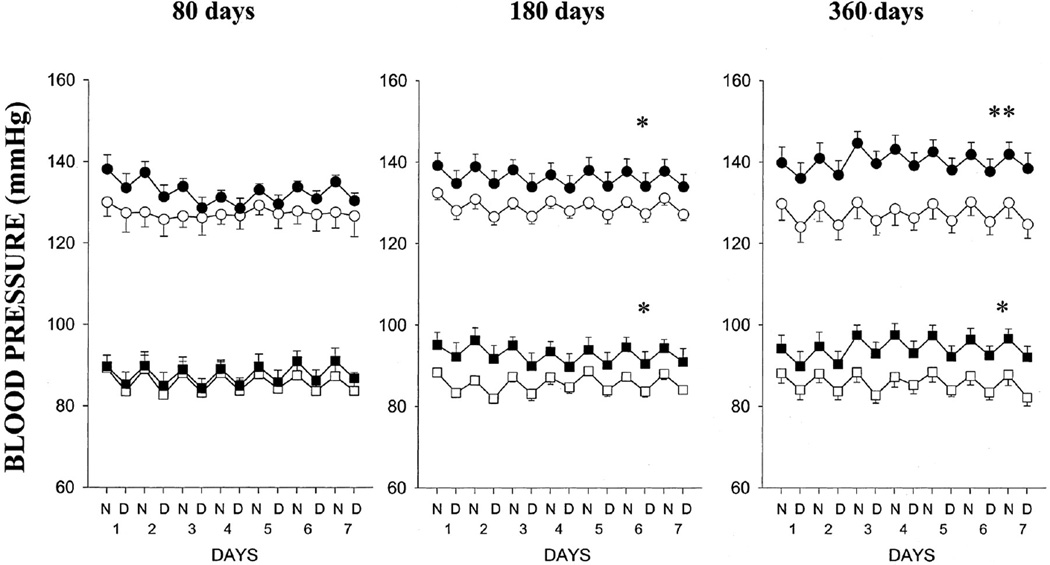
Overnutrition during gestation also impacts later chronic health of the offspring. Male and female offspring of lard-fed dams exhibit impaired vascular function (95) suggesting that fetal exposure to overnutrition is a potential mediator of CV risk that originates during development. However, male offspring of lard-fed rats are normotensive (Figure 3) (95) whereas female offspring are hypertensive in young adulthood (Figure 4) (95) indicating that sex impacts the effect of this nutritional insult on later blood pressure regulation. Whether age will impact these findings is not yet known. Both male and female offspring develop hypertension following fetal exposure to dams fed a high sucrose diet (179). Yet, only female offspring of sucrose fed dams develop obesity and impaired glucose tolerance, (179). Thus, these studies indicate that female offspring exhibit greater sensitivity to over-nutritional insults during fetal life and provide further evidence that sex impacts CV risk following a developmental insult.
Figure 3.
Systolic and diastolic blood pressures in male offspring of control (○, □) or lard-fed dams (•, ▪) at 80, 180, and 360 days old (n=6 for all groups at all-time points). Data are expressed as night (N) and day (D) 12-hour averages over 7 days (1–7). Values are mean±SEM. (Used with permission, Figure 2, 95).
Figure 4.
Systolic and diastolic blood pressures in female offspring of control dams (○, □) at 80 days (n=6), 180 days (n=5), and 360 days (n=6) or lard-fed dams (•, ▪) at 80 days (n=6), 180 days (n=5), and 360 days (n=6). Data are expressed as night (N) and day (D) 12-hour averages over 7 days (1–7). Values are mean±SEM. *P<0.05, **P<0.01. (Used with permission, Figure 3, 95).
Maternal smoking and alcohol consumption
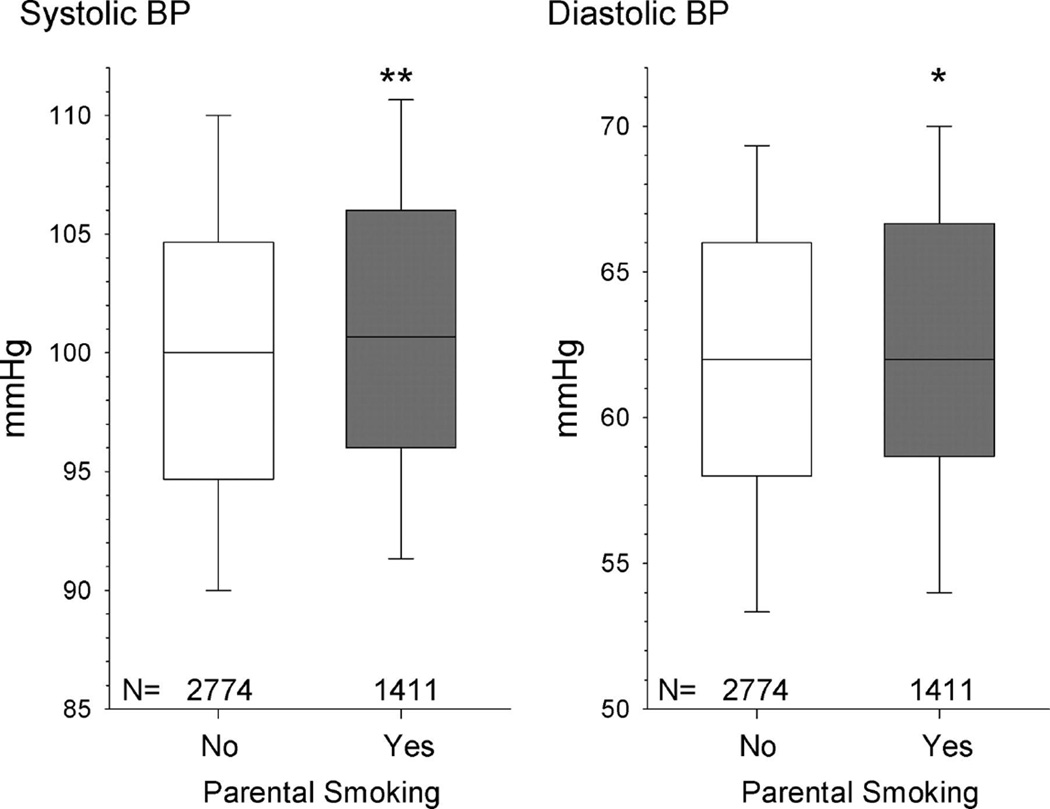
Adverse maternal lifestyle factors are associated with increased disease risk in their offspring. Parental cigarette use during pregnancy increases the risk for IUGR and low birth weight (96, 196). Additionally, parental smoking during pregnancy is associated with increased CV risk factors in the offspring in later life including increased BMI (44) and SBP (Figure 5) (191). Alcohol consumption during pregnancy is also associated with low birth weight and preterm birth (141). Yet, parental use of cigarette products and alcohol are modifiable risk factors. Thus, these studies highlight the importance of public awareness regarding the adverse impact of maternal smoking or alcohol consumption on the CV health of the unborn baby and indicate a need for experimental studies to provide mechanistic investigation into the etiology of programmed CV risk.
Figure 5.
Influence of current parental smoking on BP in preschool children (**P=0.0001, *P<0.05). The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. (Used with permission, Figure 1, 191.
Prenatal exposure to nicotine in the rat does not program an increase in baseline blood pressure in male offspring at 5 months of age (249). However, the blood pressure response to an acute infusion of Angiotensin II (Ang II) is significantly greater in the male offspring exposed to acute Ang II relative to control counterparts in young adulthood (Figure 6) (249) implicating increased CV risk originates from fetal exposure to nicotine. Female offspring in this model of fetal programming do not exhibit an enhanced blood pressure response to acute Ang II in early adulthood (Figure 6) (249). Yet, the blood pressure response to acute Ang II is increased n female offspring exposed to prenatal nicotine versus their female control counterparts by 22 months of age (207) suggesting that age abolishes this protective effect in the female. Age also leads to the development of hypertension at 22 months of age in male offspring exposed to prenatal nicotine (207) implicating that age as a second hit may be necessary to induce the development of hypertension in this model. Prenatal exposure to alcohol reduces nephron number in rat (65) and sheep offspring (66) and it also programs hypertension in rat offspring (65). Thus, these studies indicate that modifiable maternal risk factors can adversely impact chronic health in later life indicating that further mechanistic investigation is needed to fully understand the long-term impact of these insults on the CV health of the offspring across the lifespan.
Figure 6.
Effect of nicotine on Ang II–induced BP response in adult male and female offspring. SBP, DBP, and MAP responses to Ang II (10 µg/kg) were measured in adult male and female offspring that had been exposed in utero to saline control or nicotine. Data are means±SEMs and were analyzed by 2-way ANOVA with a posthoc test. *P<0.05, nicotine vs control. (Used with permission, Figure 1, 249).
Chronic stress in early life
The prevalence of low birth weight is increased two-fold in the African American population relative to the Caucasian population (139). Health prior to conception does not explain the racial/ethnic disparity in low birth weight (202). However, chronic stress prior to conception increases the risk of low birth weight (201). In addition, psychosocial stress in early life can impact later CV and metabolic health (For review, see 115). The mechanisms that contribute to the racial disparities in the prevalence of low birth weight are not clear. However, societal stress resulting in race-specific inequalities in educational, health care, housing and employment opportunities may serve as a determinant that contributes to the African American reproductive disadvantage (46). The Amsterdam Born Children and their Development Study (219) and the Adverse Childhood Experiences Study (47) are being used to examine the link between stress in early life and later chronic disease. Although early life stress may require a second stressor in later life to impact chronic disease, early life stress in addition to racial/ethnic disparities in fetal health may contribute to the developmental origins of increased CV risk.
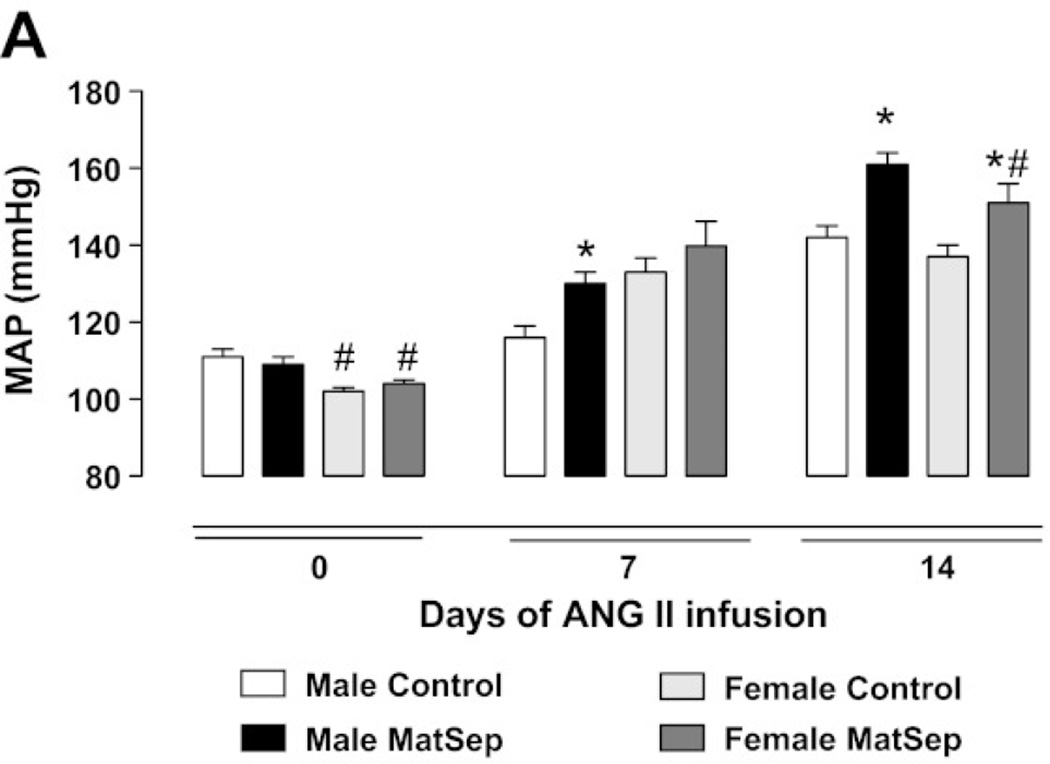
Based on studies demonstrating that stress during pregnancy is associated with a significant reduction in birth weight (189) and that adverse childhood experiences are associated with an increase in ischemic heart disease (47), experimental models have been developed to investigate the mechanisms by which psychological factors in early life contribute to impaired CV health in later life. Maternal separation during lactation is a model of early life stress (ELS) that does not program overt hypertension in offspring (116). However, blood pressure in response to chronic Ang II is significantly elevated in offspring exposed to ELS relative to hypertension that develops in control offspring in response to chronic Ang II (116). Thus, sensitivity to vasoactive factor Ang II is enhanced following this developmental insult. Female ELS offspring exhibit a delay in the development of Ang II-induced hypertension relative to male ELS (Figure 7) (115) Furthermore, the degree of Ang II-induced hypertension is attenuated in female ELS offspring compared to male ELS offspring (Figure 7) (115) indicating that sex impacts the programming of CV risk in this model of ELS. Therefore, females in this model, like that observed in models induced by nutritional insults, are protected to some degree relative to their male littermates. In addition, this model involves a developmental insult that is induced during postnatal period emphasizing that adverse programming of CV risk is not just limited to fetal life; hence the transition of terminology from fetal to developmental programming of chronic health and disease.
Figure 7.
The effect of chronic Ang II on blood pressure in male and female offspring exposed to early life stress (ELS) relative to control conterpart. ANG II-induced hypertension is delayed and attenuated in female MatSep rats compared with control rats. B*P < 0.05 vs. corresponding sex control group, #P < 0.05 vs. corresponding male group. (Used with permission, Figure 1A, 115).
Summary
Events that influence growth and development during early life exert a long-term impact on chronic health that persists across the lifespan. Stressors during early life that impact later health include nutritional insults, maternal disorders, maternal smoking and alcohol consumption, and psychological stress. Although numerous investigators note a relationship between the environment during early life and later chronic health, the development of a formal theory outlining what is now referred to as the DOHaD was first postulated by Barker and now encompasses a broad and greatly expanding field of investigative research. The etiology of impaired fetal growth and adverse influences that program an increase in later CV risk and chronic disease are varied. Despite the timing, pre- or post-natal, or the insult, stress, maternal weight gain, parental smoking or maternal alcohol consumption, undernutrition or overnutrition, these numerous adverse exposures during early life program increased risk for CV disease. An extensive number of experimental models have been developed to mimic the numerous factors that contribute to impaired fetal growth within the human population. These animal studies indicate that in addition to the gestational period, the period of plasticity that can program later chronic health extends beyond birth. Importantly, experimental models using early pre- and postnatal influence are contributing to our understanding of the etiology of the developmental origins of health and disease.
THE UNDERLYING MECHANISMS THAT PROGRAM AN INCREASE IN CARDIOVASCULAR RISK FOLLOWING A FETAL INSULT
The experimental models used to investigate the underlying mechanisms that link adverse events in early life with later increased CV risk vary, but despite the method of developmental insult, common outcomes are observed with involvement of similar mechanistic pathways reported in the developmental programming of increased CV risk. In addition, adverse events that alter growth and development during fetal life also program an enhanced vulnerability to an additional insult in later life which can impact programmed CV risk. This section will highlight the potential mechanisms that contribute to the etiology of the developmental origins of CV disease and also discuss the impact of additional factors such as sex, age and enhanced susceptibility to a second hit that contribute to increased CV risk that has its origins in early life.
Prenatal glucocorticoids
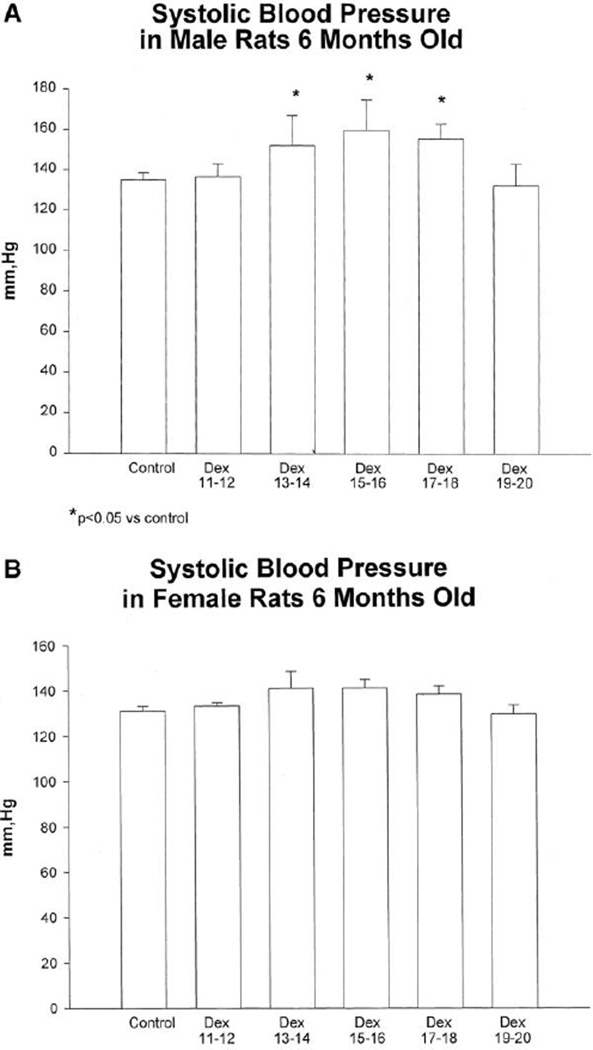
A reduction in placental expression of 11 beta-hydroxysteroid dehydrogenase type 2 (11B-HSD2) is observed in pregnancies complicated by preeclampsia (185) and IUGR (19). Placental 11B-HSD2 activity is reduced in late gestation in the experimental model induced via maternal protein restriction (102) implicating a commonality between human and experimental studies. 11B-HSD2 inactivates cortisol protecting the fetus from exposure to maternal glucocorticoids. Thus, these studies suggest that disruption of placental 11B-HSD2 may be a contributory factor in the fetal programming of increased CV risk. Fetal exposure to glucocorticoids induced via direct administration of exogenous glucocorticoids (245) or through pharmacological inhibition of maternal 11B-HSD2 in the rat (111) restricts growth during fetal life (111, 165) and programs an increase in blood pressure (111, 245), a reduction in nephron number (43, 150), and impaired vascular function in the offspring (72). Adverse programming of CV risk induced by fetal exposure to maternal undernutrition is prevented by pharmacological blockade of maternal glucocorticoid synthesis (101) suggesting a direct role for glucocorticoids as a critical mediator in the etiology of programmed risk. However, direct administration of glucocorticoids during gestation does reduce food intake in the pregnant rat (240) suggesting that the mechanisms involved in the programming of CV risk in this model may also be mediated by nutrient restriction. Prenatal dexamethasone also programs a sex difference with administration on days 13–18 of gestation in the rat associated with a marked increase in blood pressure in male offspring but not female counterparts (Figure 8) (151). In addition, the impact of prenatal exposure to glucocorticoids on birth weight in one generation is transmitted to the next in the absence of an additional gestational insult suggesting epigenetic mechanisms may contribute to inherited risk (48)
Figure 8.
Systolic blood pressures in 6-month-old rats that received prenatal dexamethasone or vehicle. Blood pressure was taken in trained rats using a tail cuff. Male rats (A) that received prenatal dexamethasone on days 13 and 14, 15 and 16, and 17 and 18 had elevated blood pressure compared with control rats. Female rats (B) were not hypertensive. There are at least 10 male rats in each group. There were 8 female rats in each group except days 11 and 12 and days 19 and 20, in which there were 5 rats. (Used with permission, Figure 5, 151).
The hypothalamo-pituitary-adrenal (HPA) axis plays a critical role during development and is very sensitive to exposure to glucocorticoids during fetal life. Alterations in the HPA axis can result from fetal exposure to stress (121), alcohol (229), undernutrtion (110) or overnutrition (195) and lead to long-term hyper- or hyposensitivity to stimuli (239). Hyperresponsiveness of the HPA may involve activation of the fetal sympathetic nervous system (SNS) (254) resulting in the developmental programming of greater stress reactivity contributing to increased CV risk in the offspring in later life (for review see 74).
Sex steroids
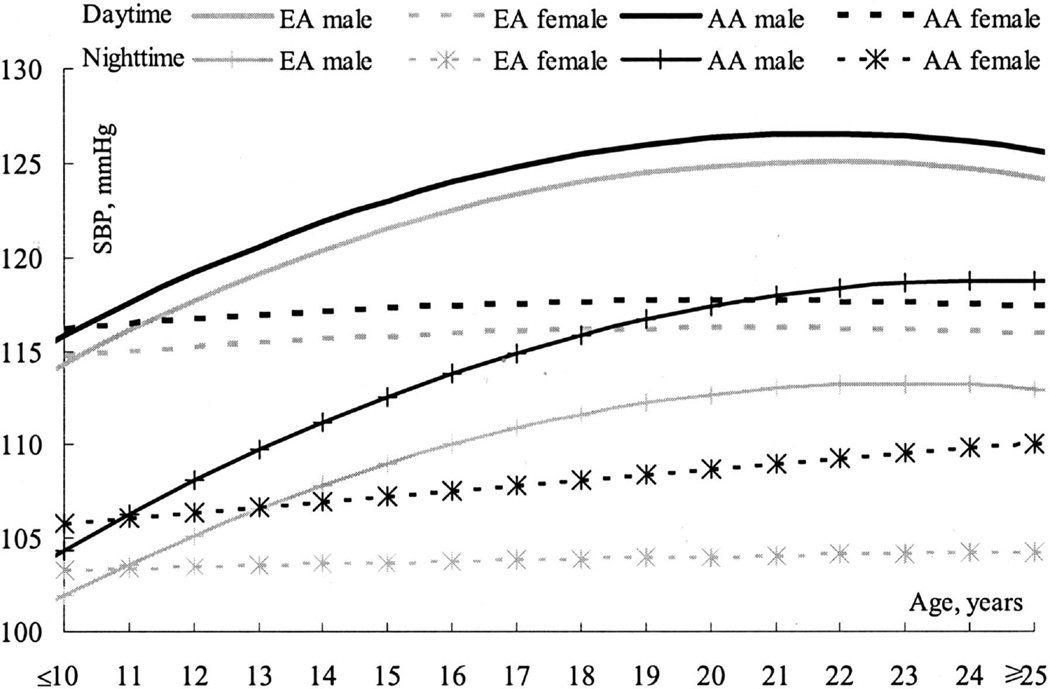
It is well-established that within the general population men exhibit a higher blood pressure relative to age-matched females prior to menopause (Figure 9) (226). Whether sex impacts programmed CV risk following an adverse insult during early life is not as clear. An inverse association between birth weight and blood pressure is noted in both men and women (7, 30, 31). Lawlor et a. investigated whether sex impacts this association using a meta-analysis of all observational studies and noted no sex difference during childhood or adulthood (107). However, absolute risk for coronary heart disease is increased in low birth weight men in young adulthood relative to age-matched low birth weight women suggesting that sex may impact the increased risk for CV disease that originates in early life (223). Many models of fetal and developmental programming exhibit a sex difference in blood pressure with female offspring protected to a greater degree relative to male counterparts (3, 21, 37, 115, 132, 151, 242; for an extensive review see 83). Males are more susceptible to the development of hypertension in models of fetal undernutrition, an observation noted in the rat (241), and the sheep (62). Yet, females are more susceptible to the developmental programming of hypertension following prenatal exposure to a maternal diet rich in lard (95) suggesting that the sex-specific programming of CV risk is insult specific. Prenatal exposure to hypoxia programs left ventricular hypertrophy in male but not female offspring at 12 months of age; yet, both males and females exposed to prenatal hypoxia exhibit echocardiographic signs of left ventricular dysfunction (1174).
Figure 9.
The increase of daytime and nighttime SBP with age by ethnicity and gender. (Figure 1, 226).
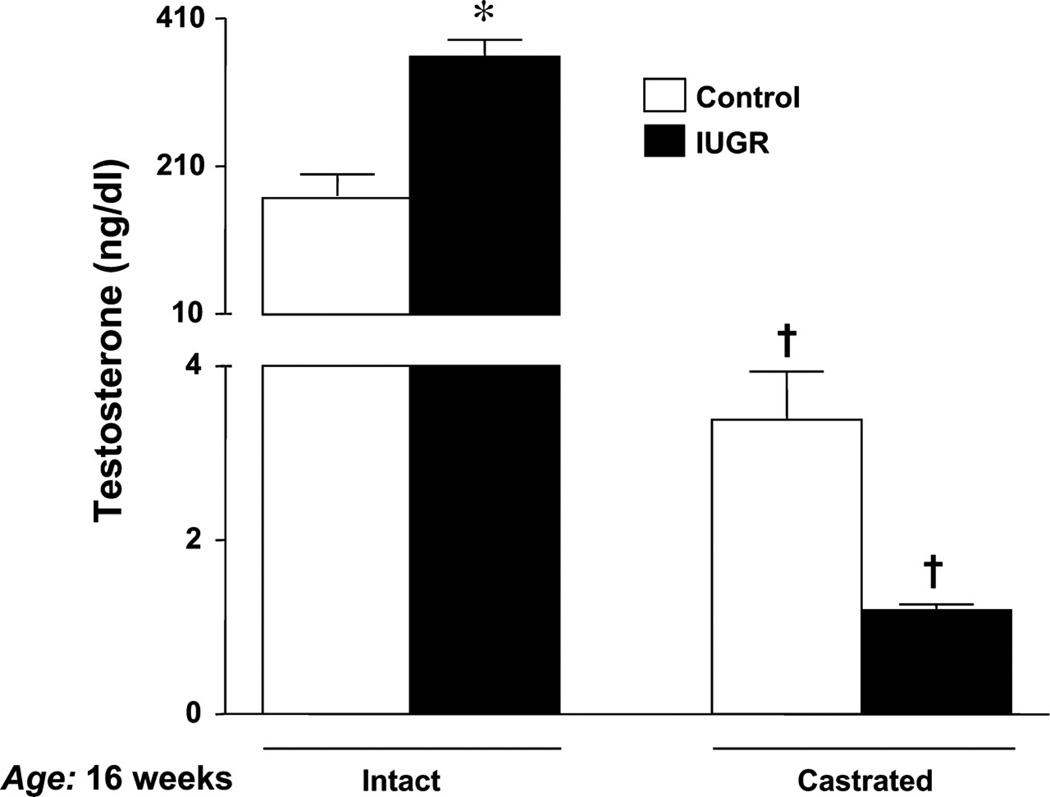
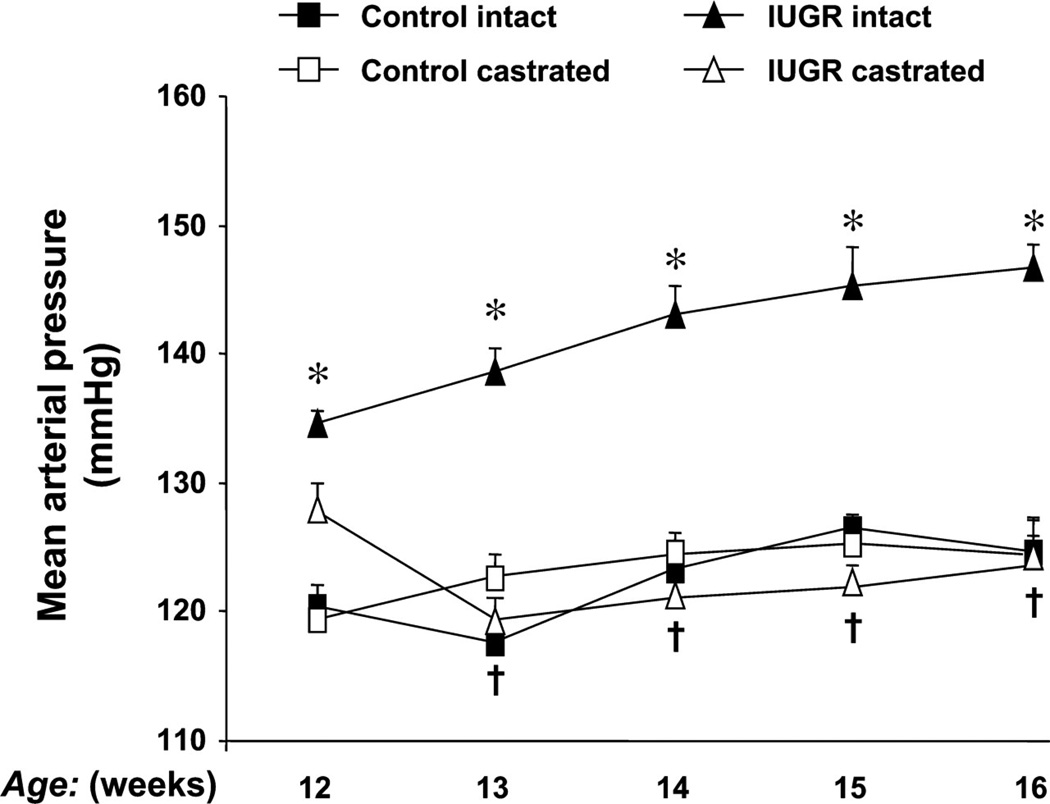
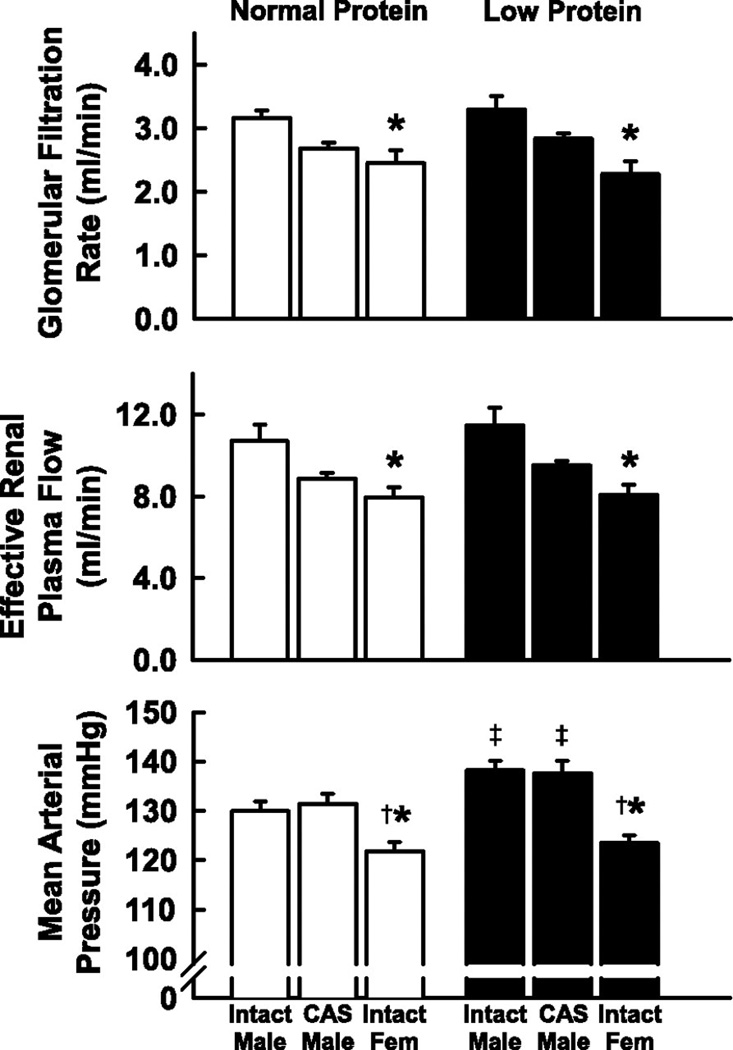
In the model of programming induced via exposure to placental insufficiency, male but not female IUGR rats exhibit hypertension in young adulthood (3) suggesting a role for sex steroids in mediating the sex difference in adult blood pressure. Plasma levels of testosterone are elevated two-fold in male IUGR rats relative to age-matched male control in young adulthood in this model (Figure 10) (145). Hypertension is abolished by castration in the male IUGR (Figure 11) (145) implicating that hypertension programmed by fetal exposure to placental insufficiency during late gestation in the rat is testosterone-dependent indicative of a correlative role for testosterone in the etiology of hypertension in male IUGR offspring in this model of developmental insult. Castration; however, does not abolish hypertension in male rats programmed by prenatal exposure to maternal protein restriction, 9% vs. 18% (Figure 12) (243). Circulating testosterone levels are not elevated in this model where male but not female offspring are hypertensive (243). Therefore, other causative factors contribute to the sex difference in adult blood pressure that is programmed by fetal exposure to moderate maternal protein restriction. Although testosterone does not differ in male rats exposed to early life stress (ELS) under baseline conditions, chronic Ang II induces a marked increase in circulating testosterone levels associated with enhanced Ang II hypertension in the ELS male rats relative to control counterparts; observations abolished by castration (115). Castration also abolishes the enhanced pressor response to acute Ang II in male IUGR rats programmed in response to placental insufficiency (149). Thus, these studies suggest that testosterone may serve as a contributory modulator to the sexual dimorphism of developmental programming of blood pressure.
Figure 10.
Serum testosterone levels measured in control and IUGR adult offspring. Blood collection followed decapitation at 16 wk of age. Control intact (n = 17), IUGR intact (n = 16), control CTX (n = 16), and IUGR CTX (n = 15) *P < 0.05 vs. control; †P < 0.05 vs. intact counterpart. All data are expressed as means ± SE. (Used with permission, Figure 5, 145).
Figure 11.
The effect of castration on blood pressure in a rat model of intrauterine growth restriction (IUGR) induced by placental insufficiency. Mean arterial pressure (MAP) was measured by radio telemetry from 12 to 16 wk of age in conscious, free-moving animals that underwent either sham (intact) or castration (CTX) at 10 wk of age. Control intact (n = 9), control CTX (n = 8), IUGR intact (n = 8), and IUGR CTX (n = 7). *P < 0.05 vs. control intact; †P < 0.05 vs. IUGR intact. All data are expressed as means ± SE. (Used with permission, Figure 1, 145).
Figure 12.
Arterial pressure and renal hemodynamics in adult intact male, castrated (CAS) male and female offspring of rats fed either a normal or protein-restricted diet during pregnancy. Values are means ± SE. *P < 0.05 compared with value for intact males of same diet group. †P < 0.05 compared with value for CAS males. ‡P < 0.05 compared with normal protein animals of same sex group. (Used with permission, Figure 1, 243).
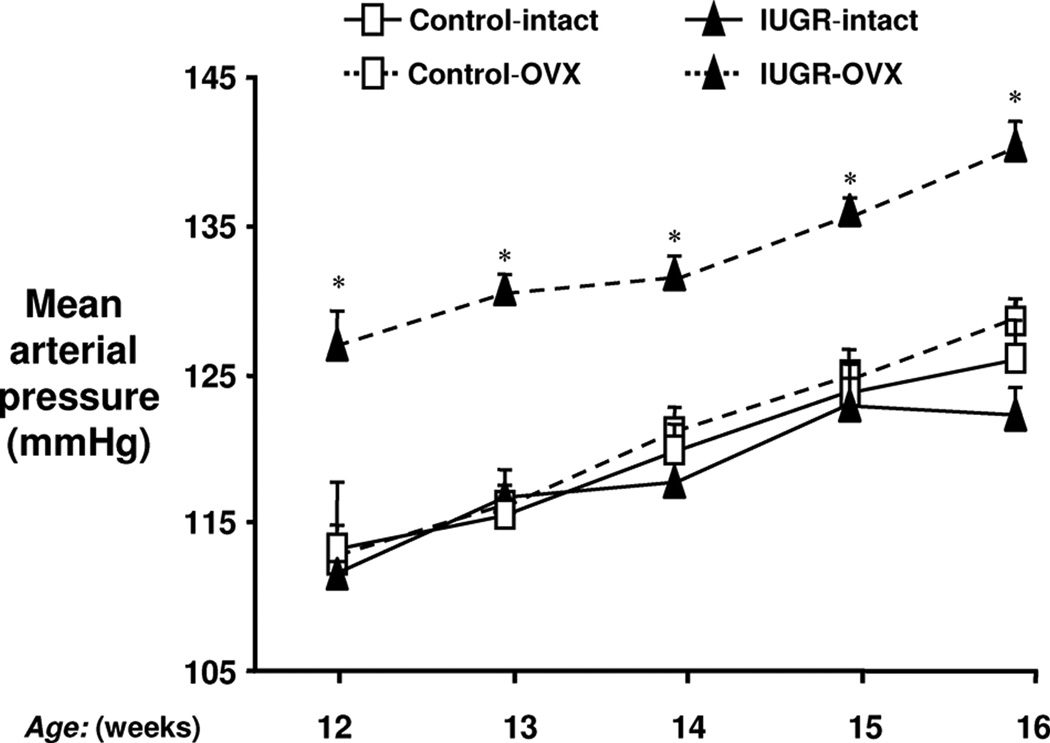
Although a permissive role is indicated for testosterone in mediating the sex difference in blood pressure following specific developmental insults, estrogen is also reported to play a protective role against programmed CV risk. Female IUGR rats exposed to placental insufficiency induced by a mechanical reduction in uteroplacental perfusion are normotensive after puberty (3). Estrogen levels do not differ in the female IUGR rats relative to age-matched female control after puberty; yet, ovariectomy in young adulthood induces hypertension in the female IUGR relative to the intact female IUGR and the intact and ovariectomized female control (Figure 13) (144). Estrogen replacement restores blood pressure back to normotensive levels (144) and also attenuates enhanced responsiveness to acute Ang II in the female IUGR rat (147) suggesting estradiol serves as protective factor against the programming of hypertension in the female IUGR in this model of developmental insult. Female offspring exposed to placental insufficiency induced by bilateral uterine ligation are also normotensive in young adulthood relative to male counterparts and female control (132). Whether estrogen mediates the sex difference in blood pressure in this model is not yet known. Prenatal exposure to nicotine also programs a sex difference in CV risk (249). Male offspring exposed to prenatal nicotine exhibit an enhanced blood pressure response to acute Ang II that is not observed in the female counterparts in young adulthood (Figure 6) (249). Ovariectomy induces a heightened acute pressor response to acute Ang II in the female rat exposed to nicotine during fetal life, an effect that is abolished in ovariectomized animals by estrogen replacement (Figure 14) (251). Thus, these studies provide compelling data indicating that estrogen is a protective modulator against the adverse programming of hypertension and increased CV risk in female offspring in early adulthood. However, age may abolish protection against programmed risk in female offspring exposed to a developmental insult (84).
Figure 13.
Ovariectomy and blood pressure in IUGR offspring. MAP was measured by radiotelemetry from 12 to 16 weeks of age in animals that underwent either sham (intact) or OVX at 10 weeks of age. Control intact (n=7), control OVX (n=7), IUGR intact (n=7), and IUGR OVX (n=8). *P<0.01 vs IUGR intact. All data are expressed as mean±SEM. (Used with permission, Figure 1, 144).
Figure 14.
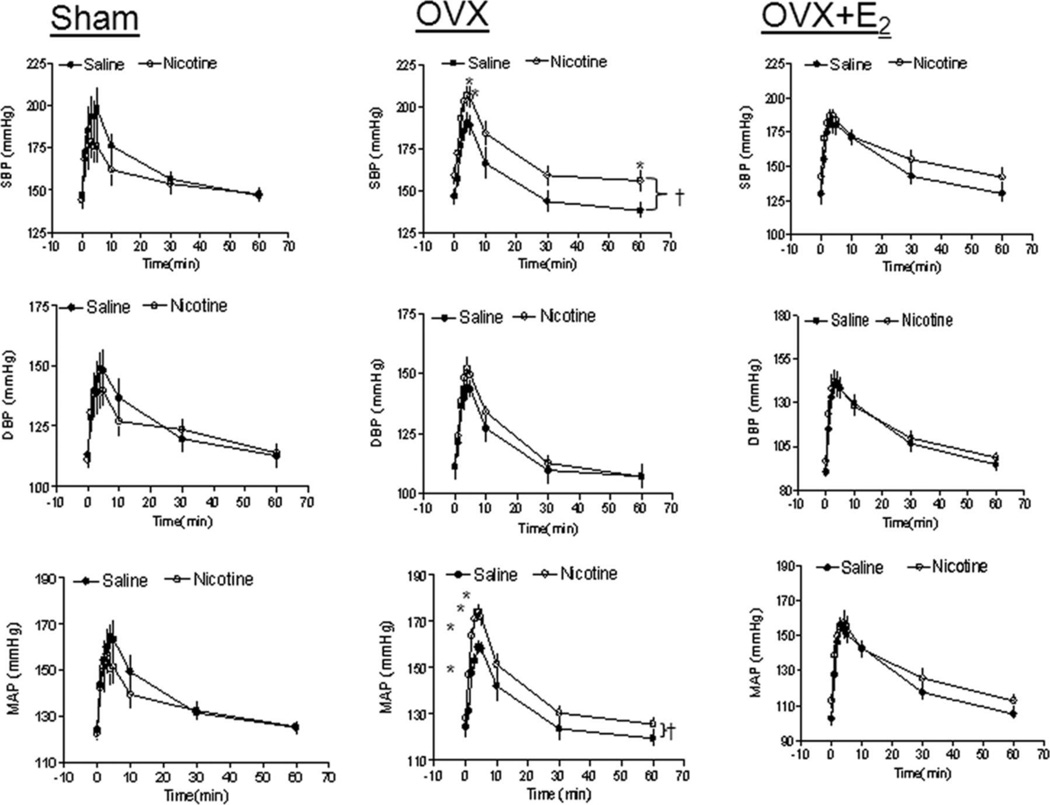
Effect of nicotine on angiotensin II (Ang II)–induced blood pressure (BP) response in sham, ovariectomy (OVX), and OVX+E2 offspring. Diastolic BP (DBP), systolic BP (SBP), and mean arterial BP (MAP) responses to Ang II (10 µg/kg) were measured in sham, OVX, and OVX+E2 groups of female offspring that had been exposed in utero to saline control or nicotine. All of the data are expressed as means±SEM from 6 animals in each group. †P<0.05 vs saline group; *P<0.05 vs data points in saline group. (Used with permission, Figure 1, 251).
Female IUGR offspring exposed to placental insufficiency that are normotensive in young adulthood (3) develop hypertension by 12 months of age (84). Enhanced sensitivity to acute Ang II also develops with age in female rats exposed to prenatal nicotine (207). Age impacts the association between birth weight and blood pressure in men and women (106). Birth weight predicts blood pressure in women at 60 but not 50 years of age (7). Birth weight is also strongly associated with an increased risk for coronary heart disease in women after menopause (106). Whether age evokes a differential impact on the effect of low birth weight on later blood pressure in men versus women has not yet been specifically addressed. In addition, whether menopause impacts the developmental origins of CV disease in women is not yet clear.
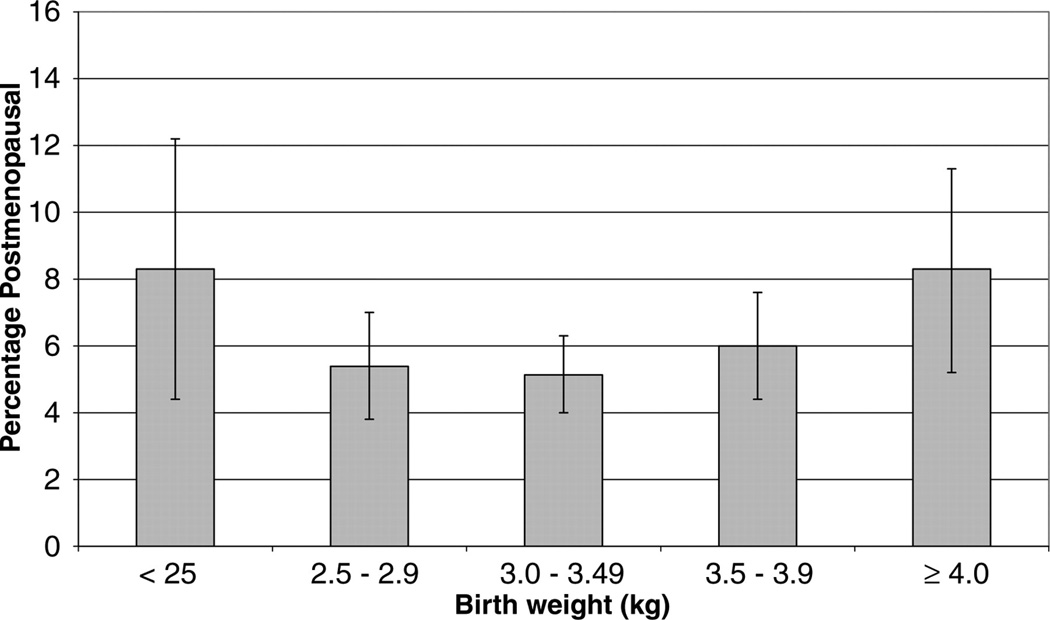
Many factors can impact the normal age at menopause (182). Menopause normally occurs around 50 to 55 years of age (23, 182). Smoking and severe weight loss or vigorous exercise accelerates early age at menopause (182). Undernutrition during fetal life is also associated with early age at menopause (49) suggesting that events during fetal life impact reproductive aging. Age at menopause increases the risk for CV disease (182) and birth weight impacts the timing of menopause with low (198, 215) and high (215) birth weight (Figure 15) (215) in addition to fetal exposure to maternal smoking associated with earlier age at menopause (215). Undernutrition in the rat programs accelerated ovarian failure (14) and early estrous acyclicity (28, 71) indicative of early reproductive senescence. Thus, early reproductive aging also has its origins in fetal life. Whether early reproductive aging is directly related to an increase in CV risk in low birth weight women is not yet known and further studies are needed to clearly address this disparity in sex-specific risk.
Figure 15.
Percentage (SE) post-menopausal by age 44–45 years by birthweight. (Used with permission, Figure 2, 215).
To summarize, the developmental programming of chronic disease by experimental models that slow fetal growth including placental insufficiency, nutrient restriction, prenatal nicotine and early life stress program a sex difference in blood pressure under basal conditions and in the enhanced blood pressure response to vasoactive factors such as Ang II. Sex steroids may contribute to this sexual dimorphism with testosterone acting as a permissive modulator whereas estrogen may serve as a protective modulator against the developmental programming of CV risk in young adulthood. Whether sex impacts CV health in individuals born low birth weight is not clear and the exact impact of age and reproductive health on CV risk that originates during fetal life is not yet known. Further studies are needed to elucidate the importance of sex steroids, the exact mechanisms by which sex steroids contribute to the development of hypertension, and how sex and reproductive health contribute to the complexity of chronic disease that is programmed by exposure to adverse influences during early life.
Renin-angiotensin-aldosterone system
The renin angiotensin aldosterone system (RAAS) is a critical mediator of salt and water homeostasis. Angiotensin (Ang II) is the most biologically active peptide in the RAS and it exerts its effects on sodium reabsorption and vasoconstriction via its angiotensin type 1 receptor (AT1R) (131). Angiotensin converting enzyme (ACE) converts Ang I to Ang II; ACE2 contributes to the production of Ang (1–9) and Ang (1–7), peptides that counteract the vasoconstrictor actions of Ang II in conjunction with the angiotensin type 2 receptor (AT2R) (131). The contribution of the RAAS to the developmental programming of hypertension in later life originates during fetal life and involves changes in expression of the RAS that are age- and sex-specific.
Children born to preeclamptic pregnancies have alterations in the RAAS that includes increased circulating levels of aldosterone (129, 227). Boys born small for gestational age exhibit a significant increase in ACE activity and circulating Ang II associated with a marked increase in SBP (58). Whether increases in components of the RAAS directly contribute to the increase in blood pressure in boys born small has not been directly tested. However, the marked increase in SBP in girls born small for gestational age is not associated with an increase in circulating levels of ACE and Ang II or other components of the RAAS (58). Thus, other factors may contribute to the developmental programming of increased blood pressure in female children exposed to slow growth during fetal life. Whether expression of the RAAS alters with age in the human population exposed to a developmental insult is not yet known.
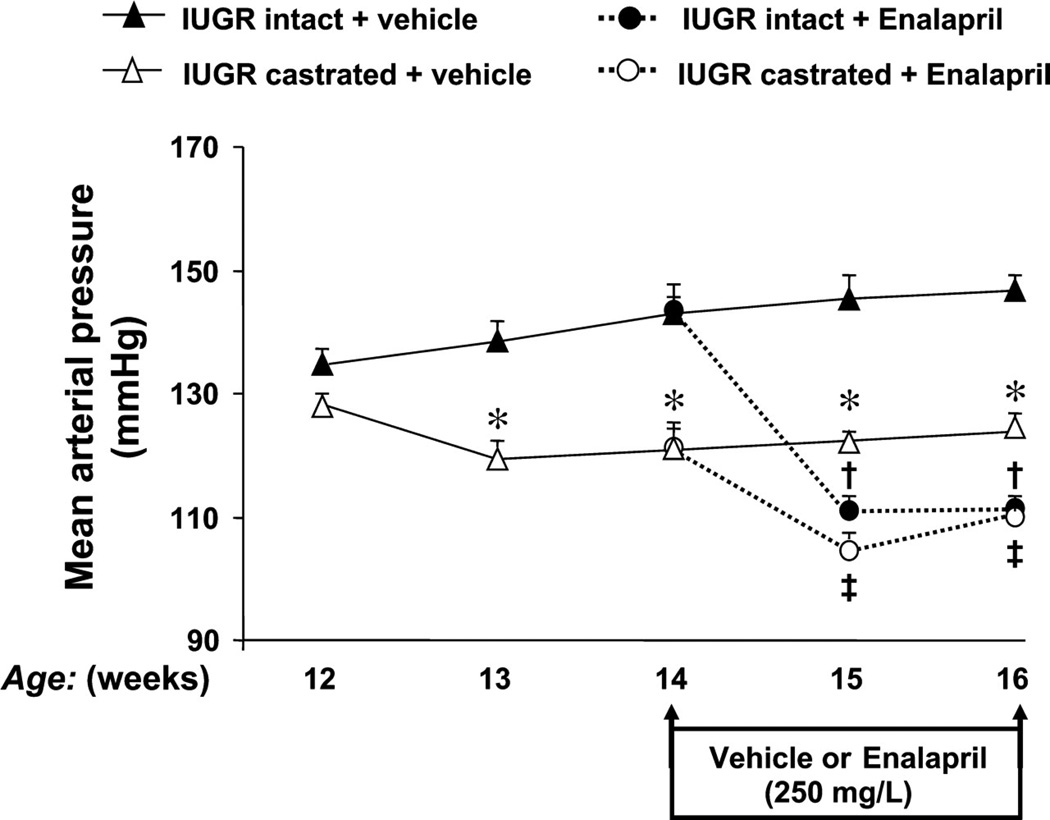
During nephrogenesis renal expression of renin is decreased in rats exposed to placental insufficiency (64) and maternal protein restriction (241) (for an extensive review see Table 2, Moritz et al, 131). The RAAS is important for proper nephrogenesis (70) suggesting that nutritional insults during fetal life that reduce renal expression of the RAAS contribute to impaired renal development resulting in a reduction in nephron number in the adult offspring (247). The RAAS also exerts a long-term impact on the developmental programming of hypertension that initiates in postnatal life prior to puberty. Blockade of the RAAS from 2–4 weeks of age with the ACE inhibitor, captopril, prevents the development of hypertension up to 12 weeks of age in offspring exposed to maternal protein restriction (190). This study suggests that the period sensitive to the long-term programming of increased CV risk by a developmental insult extends up to 4 weeks of age in the rat, well beyond the end of nephrogenic period. Yet, blockade of the RAAS via ACE inhibition with enalapril from 2 to 4 weeks of age only impacts blood pressure in male IUGR rats during the treatment period in a model of hypertension programmed by placental insufficiency (67). Therefore these studies indicate that the extended period of susceptibility may be insult specific or that early blockade may be unable to reverse the adverse programming of hypertension following specific developmental insults.
Expression of the RAAS in adult animals varies by developmental insult and is sex-specific (for an extensive review, see Table 2 in Moritz et al, 131). Although expression of renal renin and angiotensinogen are reduced at birth in IUGR rats exposed to placental insufficiency, renal renin and renal angiotensinogen expression are increased in conjunction with hypertension in young adulthood (67). Renal ACE activity is also elevated in male IUGR rats exposed to placental ischemia during fetal life (67). Yet, renal Ang II peptide expression, renal AT1R density (67) and plasma renin activity (145) do not differ upon comparison of hypertensive male IUGR relative to their age-matched normotensive male controls. Thus, inadequate suppression of the RAS occurs in response to increased blood pressure in this model of developmental insult. Deregulating of the RAAS is also observed in other models of developmental insult. Rats exposed to protein restriction during fetal life exhibit a marked increase in plasma renin activity (116). Prenatal exposure to glucocorticoids up-regulates expression of the renal RAS (192). Thus, inappropriate activation of the RAS may occur in response to a developmental insult. The importance of the RAS is indicated in studies whereby blockade of the RAS abolishes hypertension programmed by a developmental insult (123, 145, 160). Hypertension programmed in response to placental insufficiency in the male IUGR rat is abolished by ACE inhibition with enalapril (Figure 16) (145). Hypertension in offspring exposed to maternal protein restriction is also abolished by the ACE inhibitor enalapril (123, 160). Thus, these studies indicate that the RAAS contributes to the etiology of hypertension that originates during fetal life and that the developmental programming of hypertension involves common mechanistic pathways despite the method of developmental insult.
Figure 16.
The effect of renin-angiotensin system (RAS) blockade with angiotensin converting enzyme (ACE) inhibition in adult male IUGR offspring. The ACE inhibitor enalapril was administrated at a dose of 250 mg/l via drinking water for 2 wk starting at 14 wk of age in IUGR offspring. MAP was measured by radio telemetry from 12 to 16 wk of age in conscious and free-moving animals that underwent either sham (intact) or castration (CTX) at 10 wk of age. IUGR intact untreated (n = 8), IUGR CTX untreated (n = 7), IUGR intact treated (n = 8), IUGR CTX treated (n = 8). *P < 0.05 vs. IUGR intact, †P < 0.05 vs. IUGR intact; and ‡P < 0.05 vs. IUGR CTX. All data are expressed as means ± SE. (Used with permission, Figure 2, 145).
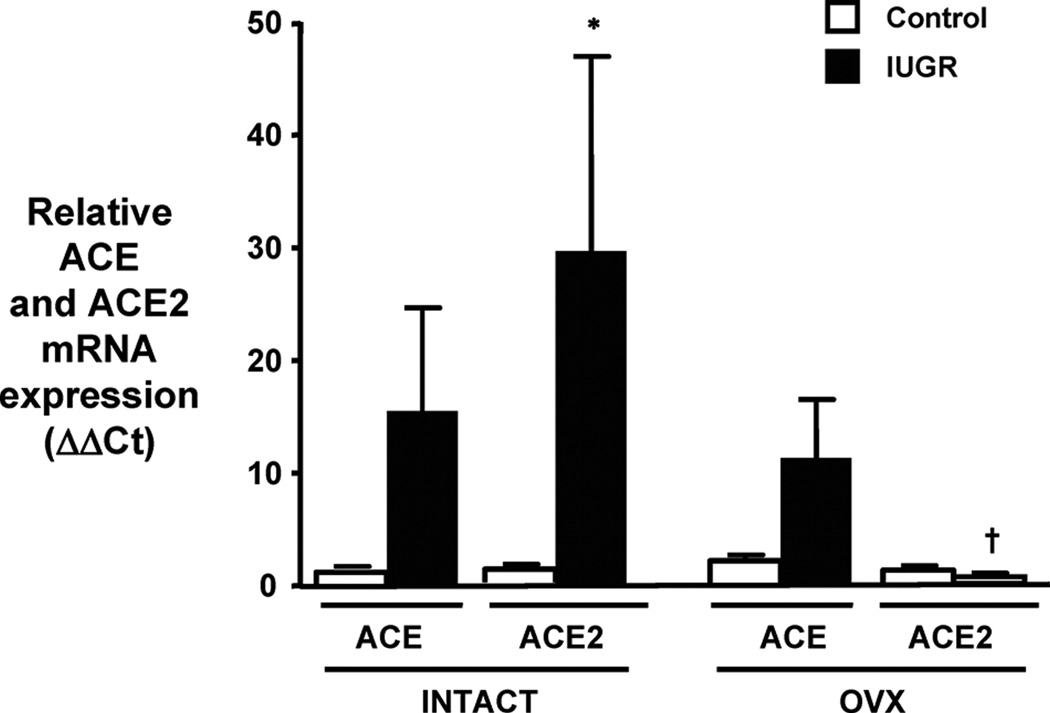
Sex differentially impacts expression of the RAAS in experimental models of developmental insult (For an extensive review see Mortiz et al, 131). Female IUGR offspring exposed to placental insufficiency in the rat are normotensive in young adulthood but develop a marked increase in blood pressure following ovariectomy (144). ACE inhibition induced via a 2 week treatment with enalapril abolishes hypertension induced by ovariectomy in the female IUGR rat (144) demonstrating an important role for the RAAS in hypertension that develops following loss of the ovarian hormones. Hypertension induced via ovariectomy in the female IUGR rat is associated with a marked reduction in renal ACE2 mRNA expression (Figure 17) (144). Renal ACE2 mRNA and protein expression and activity are up-regulated in the intact female IUGR rat (144) that is normotensive in young adulthood (3) suggesting that modulation of the RAAS by estrogen may contribute to the sex difference in blood pressure programmed by this developmental insult and serve as a protective mechanism against the development of hypertension in the female IUGR rat. Yet, sex-specific modulation of the RAAS may vary by prenatal insult. Down-regulation of renal AT2R mRNA and protein expression are associated with an increase in renal AT1R mRNA and protein expression in female offspring exposed to IL-6 during fetal life (177). Renal ACE activity is also increased in the female but not male offspring exposed to IL-6 during prenatal life (177). The programming of increased blood pressure following prenatal exposure to IL-6 is significantly greater in female rats relative to their male littermates (177). In addition, circulating aldosterone levels are significantly elevated in the female but not the male rats exposed to prenatal IL-6 (177) suggesting that sex-specific alterations in the RAAS may also predispose the female to greater risk in a manner that is insult specific.
Figure 17.
Renal ACE and ACE2 mRNA expression in intact and ovariectomized control and IUGR offspring. Renal ACE and ACE2 mRNA expressions were assessed using real-time PCR. Results were calculated using the 2−ΔΔCT method and expressed in folds increase/decrease of the gene of interest; control-intact (n=7), IUGR-intact (n=7), control-OVX (n=7), and IUGR-OVX (n=8). *P<0.05 vs control-intact ACE2. †P<0.05 vs IUGR-intact ACE2. All of the data are expressed as mean±SEM. (Used with permission, Figure 5, 144).
Sex also impacts blood pressure sensitivity to Ang II following a developmental insult. Male IUGR offspring in the model of programming induced by reduced uterine perfusion exhibit a greater blood pressure response to acute Ang II relative to control counterparts (149). The enhanced blood pressor response is specific to the vasoactive factor Ang II and does not involve alterations in vessel morphology (149). Castration abolishes hypersensitivity to acute Ang II in the male IUGR implicating that testosterone as a modulator of this interaction (149). Female IUGR in this model also exhibit a hyperresponsiveness that is specific to acute Ang II and is exacerbated by ovariectomy (147). Thus, estrogen is also a modulator of this response. Sex differences in Ang II sensitivity are not limited to just this model of developmental insult. Prenatal nicotine programs a sex-specific response with male offspring more sensitive in young adulthood relative to female counterparts (249); however, this sex difference is abolished with age (207). Hypertension induced in response to chronic Ang II is greater in male rats exposed to ELS relative to their female or control counterparts (118). In addition, the development of hypertension in response to chronic Ang II is delayed in female ELS rats relative to male rats exposed to ELS (118) indicating that females are less sensitive. Testosterone may also modulate enhanced Ang II hypertension in the male ELS rats (118). However, in this model estrogen does not appear to play a modulatory role (118). Although the exact mechanisms mediating hypersensitivity to Ang II are not yet known, enhanced sensitivity to Ang II following a developmental insult may involve programming of the HPA axis (74).
Adverse programming of the RAAS is not limited to just the kidney. Fetal exposure to a maternal high fat diet programs a marked increase in body weight and total fat mass associated with a marked increase in SBP at 6 months of age in the female offspring (68). Angiotensinogen expression in adipose tissue is up-regulated at birth and remains increased at 6 months of age in offspring exposed to a maternal high fat diet (68). ACE and AT2R expression are also elevated in the adipose tissue at 6 months of age (68). Whether circulating or intrarenal levels of the RAAS are altered is not reported but increased adipose angiotensinogen expression is observed in other animal models of obesity (89) and the RAAS impacts adipose cell mass in the presence of a high fat diet (255). Central expression of the AT1R is increased in offspring exposed to maternal protein restriction (160) and nicotine exposure (124). Blood pressure is not increased in young adulthood in offspring exposed to perinatal nicotine (249). Yet, the acute blood pressure response to Ang II is significantly enhanced (249) indicative of increased sensitivity to this vasoactive factor. The mechanisms involved may be multifactorial and include a decrease in baroreflex sensitivity (249) in conjunction with the generation of oxidative stress within the vasculature (250). Thus, multiple mechanisms contribute to increased CV risk following a developmental insult and include the RAAS, the sympathetic nervous system (SNS) and oxidative stress.
Sympathetic nervous system
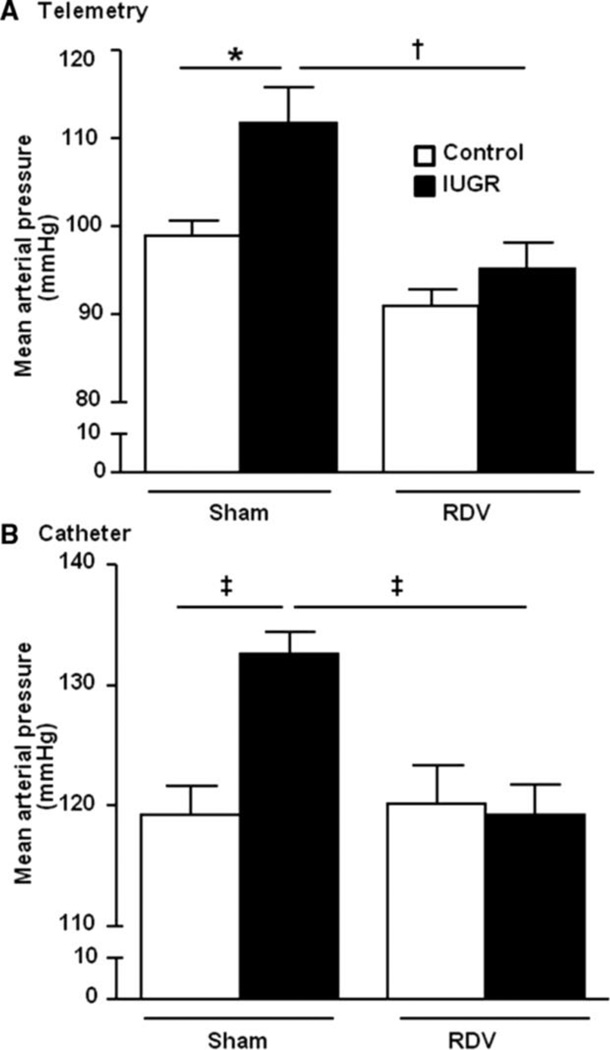
The SNS is thought to play an important role in the etiology of hypertension (51). Several studies indicate that SNS activation is increased in low birth weight individuals (17, 85, 181); yet not all studies agree. Muscle SNS activity is decreased in healthy low birth weight individuals in young adulthood (230). Children of women with greater pre-pregnancy weight do not exhibit an increase in heart rate, or sympathetic drive despite the increase in BMI and SBP at 5–6 years of age (60). However, increases in circulating catecholamines indicative of enhanced SNS activation are observed in different experimental models of low birth weight including models that use placental insufficiency in the rat (90) or sheep (76), or a model induced via protein restriction in the rat (156). Basal renal sympathetic nerve activity is elevated in rat offspring of diabetic dams that are hypertensive in adulthood (37) and in rabbits exposed to a high fat diet during development (162). Thus, these studies indicate that despite the species or method of fetal insult, activation of the SNS may contribute to the fetal programming of increased CV risk. Maternal obesity programs hypertension in rat offspring that is abolished by alpha-1/beta-adrenergic blockade (180) indicating a critical role for SNS activation in the fetal programming of hypertension in offspring of obese rats. Activation of the renal nerves is indicated to contribute to essential hypertension (51). Renal denervation abolishes hypertension in male offspring in adulthood in models of fetal programming induced by placental insufficiency (4), prenatal exposure to glucocorticoids (32) or maternal diabetes (37). Therefore, these studies provide proof that activation of the renal sympathetic nerves contributes to hypertension in adult offspring following a developmental insult. Renal denervation also prevents the development of hypertension in male IUGR offspring programmed by fetal exposure to placental insufficiency (148) implicating an inherent role for renal SNS activation in the etiology of fetal programming of CV risk. However, the renal nerves may also contribute to the development of hypertension that develops with age in female IUGR rats that are normotensive in young adulthood (3).
Female IUGR rats in the model of placental insufficiency induced via a mechanical reduction in uteroplacental perfusion develop an age-dependent increase in blood pressure that is associated with a concurrent increase in total fat mass and visceral fat by 12 months of age relative to age-matched, same-sex control counterparts (84). Circulating levels of leptin are also increased in the female IUGR rats by 12 months of age (84). Leptin, released from adipocyctes, activates the SNS (205) and blood pressure is increased in the mouse by infusion of leptin indicating a direct role for leptin in the etiology of obesity-related hypertension (205). Bilateral renal denervation normalizes blood pressure in the female IUGR rat at 12 months of age relative to its denervated control counterpart (Figure 18) (84) abolishing the hypertension that develops with age in female IUGR rats. Thus, in the model induced via placental insufficiency, the renal nerves contribute to the development of hypertension that is inherent and present prior to puberty in male IUGR rats (148) and the renal nerves also contribute to the development of hypertension that occurs in an age- and adiposity-dependent manner in female offspring (84). The exact source for renal sympathetic nerve activation in this model is not yet known, but involves a mechanism that is sex and age-dependent. SNS activity is also increased in obese women (58) and activation of the SNS contributes to obesity-induced hypertension (73). Thus, while the renal nerves have an innate role in the fetal programming of hypertension in the male IUGR rat, programming of hypertension in the female IUGR fat is age-dependent in a manner that may involve the development of age-dependent increases in adiposity.
Figure 18.
Mean arterial pressure in female control and IUGR rats measured by (a) telemetry or (b) chronically instrumented catheters in the conscious state 2 weeks post bilateral renal denervation. *P<0.05, †P<0.01, ‡P<0.001. Data values represent mean±SE. (Used with permission, Figure 5, 84).
The stimulus for innate activation of the renal sympathetic nervous system in male IUGR rats programmed in response to reduced uterine perfusion may have its origins in activation of the central RAS (41). Expression of the AT1R is significantly increased in regions of the brain central to CV control in rats programmed during fetal life by exposure to maternal protein restriction (160). Importantly, ICV administration of either an ACE inhibitor or an AT1R antagonist abolishes hypertension programmed in response to this fetal insult (160) implicating an important role for the central RAS in the etiology of hypertension programmed in response to this fetal insult. There is substantial evidence that Ang II within the central nervous system regulates the renal sympathetic nerves (For an extensive review, see 41). Whether suppression of the central RAS will abolish hypertension in models programmed via placental insufficiency or prenatal exposure to dexamethasone is not yet tested. However, these findings indicate that activation of the central RAS modulate sympathetic activity of the peripheral nerves contributing to the neural control of renal function and blood pressure.
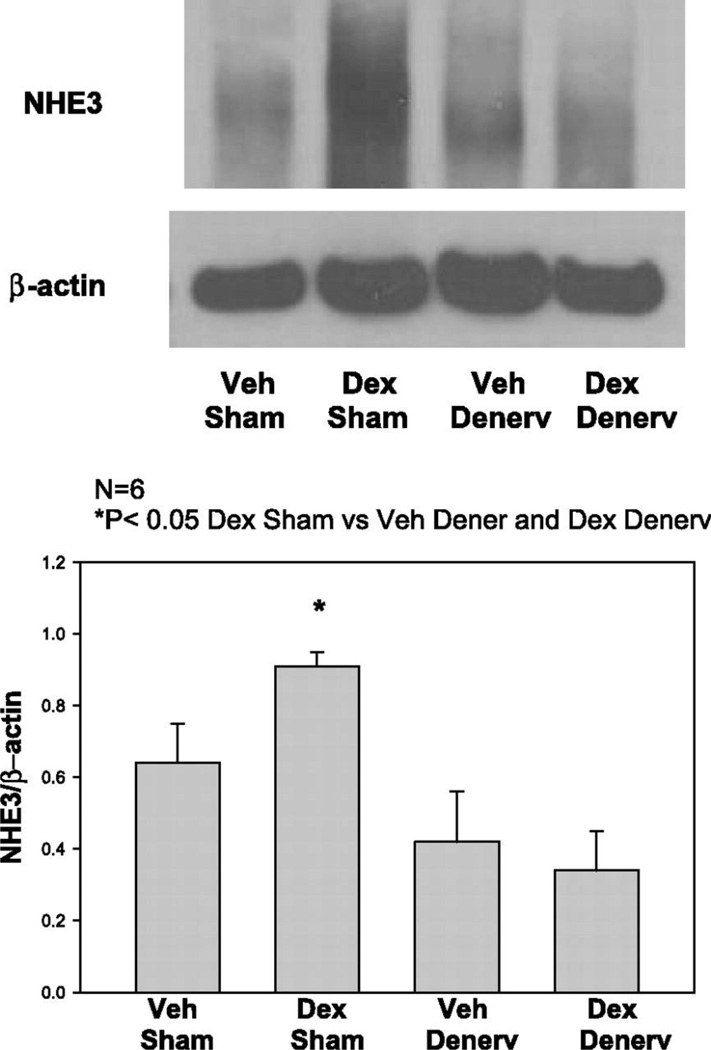
The underlying mechanism by which renal sympathetic nerve activation mediates the development of hypertension following a developmental insult is not clear but may involve modulation of sodium reabsorption along the tubule. All segments of the renal tubule are innervated (41) and an increase in renal sympathetic nerve activation increases sodium reabsorption within the renal tubule (163). Prenatal exposure to glucocorticoids programs a significant increase in renal Na+-K+-2Cl− cotransporter (NKCC2), thiazide-sensitive cotransport (NCC), and type 3 Na+/H+ exchanger (NHE3) protein abundance in male offspring that is abolished by bilateral renal denervation (Figure 19) (32). Thus, increased renal sympathetic nerve activation in male offspring exposed to a prenatal insult may involve an increase in sodium transport in the renal tubule and contribute to the programming of hypertension following a fetal insult.
Figure 19.
Effect of prenatal dexamethasone and renal denervation on type 3 Na+/H+ exchanger (NHE3) protein abundance. (Used with permission, Figure 3, 32).
Oxidative stress
Oxidative stress is indicated in the etiology of organ damage and chronic disease (235). Whether the production of reactive oxygen species is a cause or a consequence of disease pathology is not clear (235). Experimental studies indicate that oxidative stress contributes to pathophysiological changes in the vasculature in the heart and kidneys, and also contributes to the normal regulation of physiological function (235). Markers of oxidative stress including urinary F2-isoprostanes and thiobarbituric-acid-reactive-substances (TBARS) are elevated in children born small for gestational age (59). The level of TBARS is positively associated with SBP in these children (59) suggesting that increases in oxidative stress may contribute to elevations in SBP observed in children following a developmental insult. However, superoxide dismutase activity (SOD) activity is also elevated implicating a compensatory mechanism that may also be programmed in response to the impaired growth during fetal life (59).
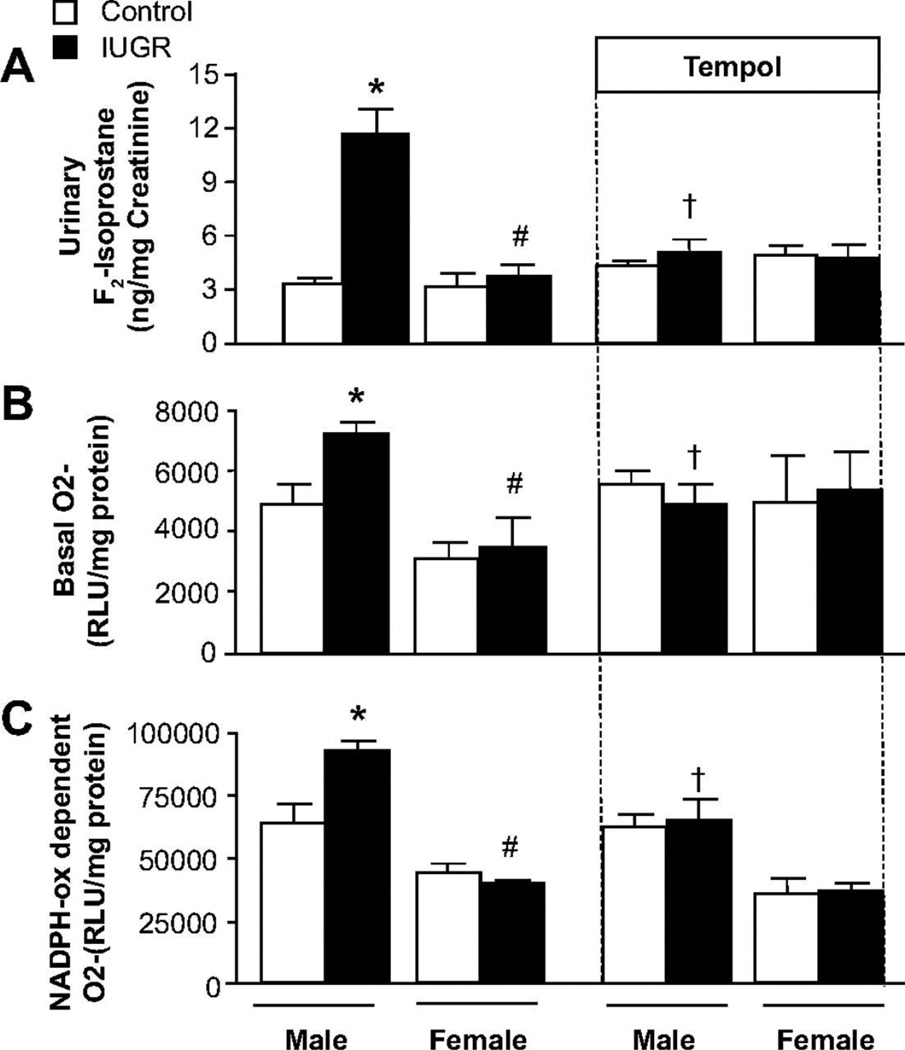
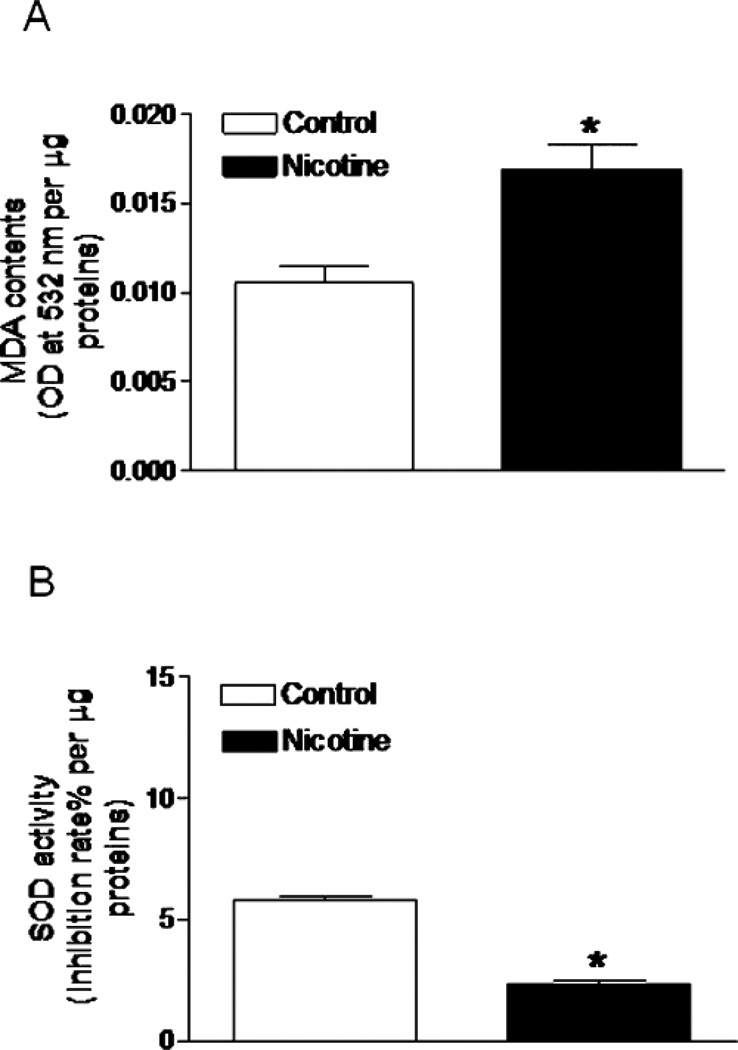
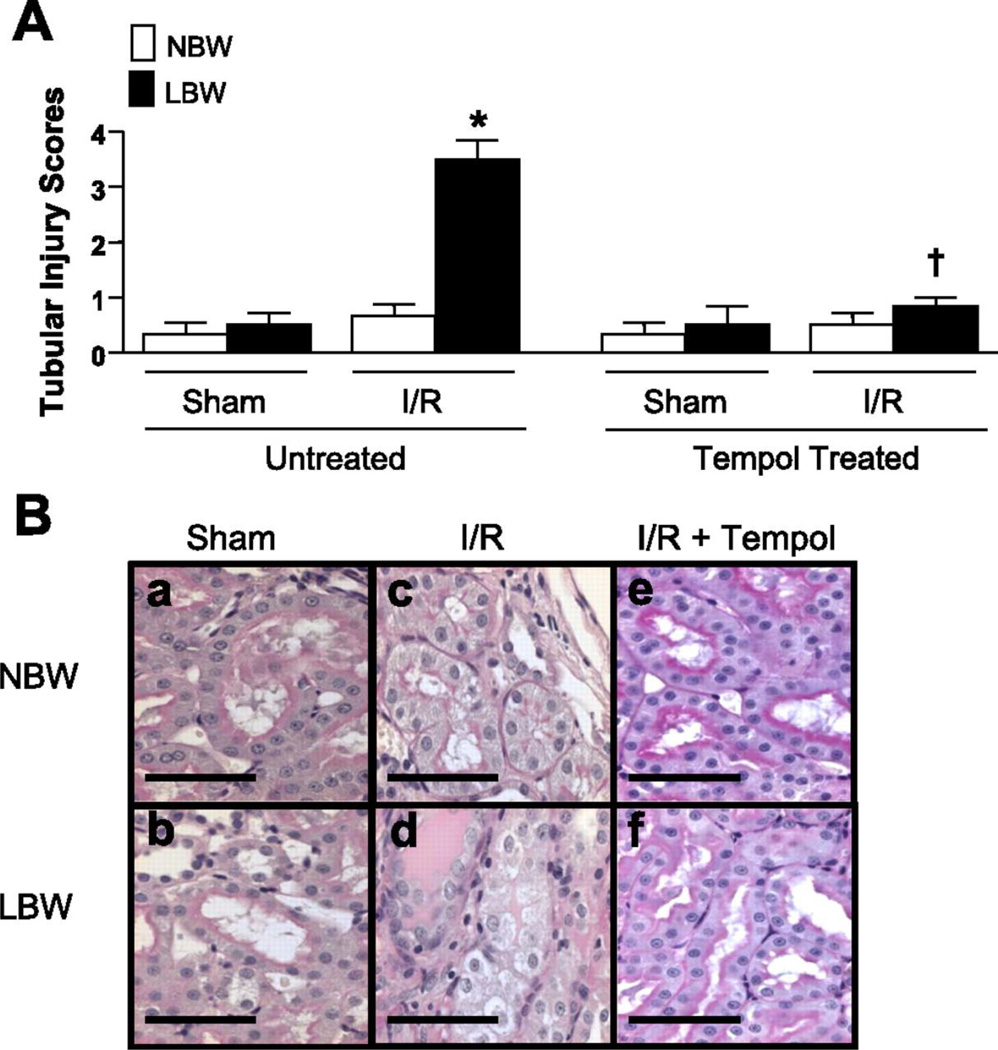
Experimental models also demonstrate an important role for oxidative stress in the developmental programming of increased CV risk. Renal markers of oxidative stress are elevated in male IUGR rats in association with hypertension in young adulthood (Figure 20) (146). Treatment with the antioxidant, tempol, abolishes hypertension in male IUGR offspring in this model of programming induced via placental insufficiency in the rat (146). Tempol also abolishes hypertension in male offspring exposed to maternal protein restriction that also exhibit an increase in renal markers of oxidative stress (123). Thus, these studies suggest that common mechanistic pathways contribute to the fetal programming of hypertension despite the model of fetal insult. However, fetal programming of oxidative stress may vary in a manner that is tissue specific. Expression and activity of anti-oxidants are not altered in the kidney of male IUGR programmed by placental insufficiency relative to male control (146). Yet, male offspring exposed to nicotine during fetal life exhibit a marked reduction in SOD activity in conjunction with an increase in vascular malondialdehyde, a marker of oxidative damage within the aorta (Figure 21) (233). Heightened vascular responsiveness to acute Ang II in male offspring exposed to prenatal nicotine is abolished by tempol and apocynin, a NADPH oxidase inhibitor (250) suggesting that oxidative stress also contributes to the fetal programming of increased CV risk in this model in a manner that involves an imbalance in both anti- and pro-oxidant production.
Figure 20.
Renal superoxide production and urinary excretion of F2-isoprostanes in male and female control and intrauterine growth-restricted (IUGR) offspring treated with the superoxide dismutase (SOD) mimetic Tempol (1 mmol/L) or vehicle (tap water ad libitum) from 14 weeks to 16 weeks of age. A, 24-hour urinary excretion of F2-isoprostane; B, Renal basal superoxide anion production; and C, Renal NADPH oxidase–dependent superoxide anion production. *P<0.05 vs untreated male control, #P<0.05 vs untreated male IUGR, †P<0.05 vs untreated male IUGR. Data values represent mean±SE. □, control; ■, IUGR. (Used with permission, Figure 3, 146).
Figure 21.
Effect of antenatal nicotine on vascular malondialdehyde (MDA) and superoxide dismutase (SOD) activities. Pregnant rats were treated with saline (control) or nicotine, MDA (A) and SOD activity (B) were determined in aortas isolated from 5 months old male offspring. Data are means ± SEM of tissues from five animals. *P < 0.05 versus control. (Used with permission, Figure 4, 233).
Sex also impacts the developmental programming of increased oxidative stress. Unlike their male counterparts, renal markers of oxidative stress are not increased in normotensive female IUGR exposed to placental insufficiency (Figure 20) (146). In addition, unlike their male counterparts, expression and activity of renal antioxidants are up-regulated in female IUGR relative to female control (146) indicating a sex-specific increase in anti-oxidant enzymes that may be preventative against the programming of hypertension in the female IUGR rat. Superoxide production is increased within the microvessels of female offspring exposed to maternal protein restriction (57). Vascular anti-oxidant enzyme levels do not differ, but treatment with apocynin decreases superoxide production within the mesenteric vessels and improves vascular responses to acetylcholine and bradykinin (57). Thus, activation of pro-oxidant enzymes rather than a loss of anti-oxidant enzymes within the vasculature are the contributory factors in that contribute to impaired vascular function in females exposed to fetal undernutrition. Fetal exposure to a high fat diet also increases markers of oxidative stress in the brain of the offspring (233) Thus, tissue-, sex- and insult-specific programming of oxidative stress is observed in different experimental models of fetal insult. Clearly, additional studies are warranted to fully elucidate the importance of pro- versus anti-oxidants in the developmental programming of CV health and disease and to determine if differential programming of oxidant enzymes contributes to sex difference in programmed CV risk.
Oxidative stress plays a critical role in normal placental development and contributes to fetal growth restriction when oxygen resources become limited in pregnancies complicated by hypoxia (136). Perinatal antioxidant treatment reduces blood pressure in later life in the Spontaneously Hypertensive rat (SHR) indicating that increased exposure to reactive oxygen species during fetal life contributes to the later development of hypertension in the SHR (164). Treatment with antioxidants during fetal life also prevents the development of hypertension in offspring exposed to protein restriction during fetal life (26). Maternal obesity compromises the pro-and anti-oxidant status within the fetal/placenta unit resulting in an increase in markers of oxidative stress (122). Antioxidant treatment during pregnancy prevents the development of increase adiposity and impaired glucose tolerance in offspring of pregnant rats fed a Western style diet rich in fat and sugar (188). Thus, these studies indicate that fetal exposure to oxidative stress contributes to the developmental programming of CV and metabolic risk.
Endothelin system
Early life stress induced by maternal separation during lactation programs an exaggerated acute stress-mediated pressor response in the adult animal that is abolished by blockade of the endothelin type A receptor (ETAR) and the endothelin type B receptor (ETBR) (114), receptors that mediate the actions of endothelin, a potent vasoconstrictor. Enhanced vascular sensitivity to endothelin is observed in male offspring exposed to maternal undernutrition (216) or fetal hypoxia (21) suggesting that programming of the endothelin system may contribute to developmental origins of CV risk. Only male offspring of hypoxic dams exhibit the enhanced responsiveness to endothelin (21). In addition, SBP in male rats exposed to fetal hypoxia are more responsive to endothelin receptor blockade that their female littermates (21) suggesting that endothelin may contribute to sex differences in the fetal programming of CV disease.
Role of inflammatory cytokines
Fetal exposure to maternal protein restriction programs an increase in renal inflammation (199) implicating a role for inflammation in the etiology of increased programmed CV risk. Prenatal exposure to the inflammatory cytokine interleukin-6 (IL-6) programs hypertension associated with alterations in expression of the RAS and sodium excretion in offspring exposed to this prenatal insult (177). Although investigation into the importance of inflammation in the programming of hypertension is limited, these studies implicate an important role for inflammatory processes in the etiology of chronic disease that originates in early life.
Epigenetic processes
Epigenetic processes involve heritable changes in gene function and expression that occur in the absence of changes in the DNA sequence (25). These processes can include alterations in the methylation pattern of the DNA, modifications in the histones, or changes in small non-coding RNAs (For an extensive review, see 25). The developmental programming of chronic disease in one generation following a developmental insult (F1) can be transmitted to the next (F2) generation (6, 78, 158) suggesting that epigenetic processes may contribute to the transgenerational programming of chronic disease. Placental insufficiency in the rat programs endothelial dysfunction in the F1 generation that is transmitted to the F2 generation in the absence of an additional prenatal insult (6, 161). Transmission of hypertension and vascular dysfunction is also present in the F3 generation of male offspring following global nutrient restriction in the F1 (161) Elevated fasting insulin levels and an enhanced insulin response to a glucose challenge is observed in the F2 offspring of F1 offspring exposed to nicotine during their gestational life (F1)(78). Transgenerational transmission of insulin resistance is also noted following IUGR in the F1 generation (209). Maternal protein restriction programs insulin resistance in the F1 and F2 generations (158) implicating the ability of insults in one generation to impact the chronic health of the subsequent F2 generation. Thus, these studies indicate that the transmission of programmed risk is observed in different models of insult providing additional support that epigenetics may be a common mechanism in the programming of later chronic disease.
Specific genes targeted by epigenetic processes that may contribute to the developmental programming of chronic disease including CV and metabolic disturbances include 11B-HSD2 (11), components of the RAS such as the ACE (64) in addition to the AT1R (16) and AT2R (64), and the glucose transporter 4 (GLUT4) (256) which contributes to glucose homeostasis via uptake of glucose in the skeletal muscle. Alterations in genes important for proper hepatic and cardiac metabolism such as peroxisomal proliferator-activated receptor (PPAR)-α and the liver X (LXR) gene which is involved in fatty aid metabolism are also impacted by epigenetic processes (120, 194, 220). Changes in expression of these gene products are associated with marked alterations in the methylation patterns in CpG sites within the promoter region (11, 64, 194, 220), the acetylation pattern of histones involved in modulation of gene expression (1, 11, 120), and up-or down-regulation of miRNAs, specific regulators of mRNA translation (64). A role for epigenetic processes in the developmental programming of chronic disease is reported in offspring exposed to fetal glucocorticoid exposure (16), placental insufficiency (11), prenatal nicotine (120), maternal protein restriction (64, 194, 220, 256) and a maternal diet high in fat (1) with epigenetic modifications noted for genes located in the brain (64), liver (1, 120, 220), kidney (11), heart (194) and muscle (256). In addition, epigenetic modifications reported in one generation following fetal exposure to undernutrition (F1) are present in the next (F2) generation (128) implicating the transmission and maintenance of epigenetic modifications from one generation to the next. Thus, these studies indicate that insults during development program changes in the expression of gene products via epigenetic manipulations.
Enhanced susceptibility to an additional stress
Influences that alter fetal growth not only enhance the risk for CV disease in later life, but also enhance susceptibility to an additional insult and impact natural stressors such as pregnancy. The impact of fetal growth on later chronic health is diverse an can result an increase in blood pressure an increased risk for metabolic disturbances which may augment CV risk in individuals already compromised by adverse exposures to a maternal insult during gestational life.
Enhanced susceptibility to renal disease
A reduction in nephron number is a common observation noted for many experimental models regardless of the developmental insult (132, 150, 237, 241). Although long-term follow-up indicates that the prevalence for hypertension and microalbuminuria are increased in kidney donors (183), renal function and blood pressure remain well preserved years following a donor uninephrectomy (63, 138). However, uninephrectomy induced during the nephrogenic period in the rat programs overt hypertension associated with a greater risk for renal injury (133, 241) implicating that a reduction in nephron number during “development” per se is sufficient to induce hypertension in later life. Yet, prenatal exposure to glucocorticoids programs hypertension in the presence or absence of a reduction in nephron number (151) suggesting that nephron number is not the sole determinant of programmed hypertension in this model of developmental insult. Reductions in nephron number are also observed in the absence of hypertension following fetal exposure to placental insufficiency in late gestation (151). Furthermore, a reduction in nephron number that occurs during development by blockade of factors important for proper glomerular development including the RAS (117, 244) or cyclooxygenase-2 (COX2) (166) program hypertension, altered renal function and increased CV risk in the offspring in a manner that is sex and age-dependent (166, 167). Thus, the development of hypertension in many models of developmental insult is present in the absence of a reduced nephron number (150) indicating that a reduction in the number of functional glomeruli that occurs during nephrogenesis is not the primary cause of increased blood pressure following a developmental insult. However, a reduction in nephron number and functioning glomeruli may predispose an individual to increased susceptibility to an additional or secondary insult. Low birth weight in humans is associated with a reduction in nephron number (79). The risk for end stage renal disease is increased in low birth weight individuals relative to their normal birth weight counterparts that are hypertensive, diabetic or both (100) suggesting that impaired kidney development during development predisposes an individual to increased susceptibility to renal disease and injury in later life when superimposed with other chronic disease risk. Evidence supporting this concept of increased vulnerability to a second insult is provided in the model of IUGR induced by placental insufficiency in the rat (143). Renal superoxide production is increased in association with a significant increase in markers of renal injury in male IUGR rats following a mild ischemia/reperfusion event that does not result in an increase in oxidative stress or renal injury in control male rats exposed to the same insult (143). Treatment with an antioxidant attenuates renal injury in the male IUGR implicating a role for increased production of reactive oxygen species by the IUGR rat in response to the mild ischemic event (Figure 22) (143). Prenatal exposure to hypoxia programs enhanced susceptibility to a cardiac ischemia/reperfusion injury in the IUGR offspring relative to control (176) indicating that enhanced sensitivity to a secondary insult is not limited to just the kidney. Postnatal exposure to a diet high in fat following prenatal hypoxia exacerbates susceptibility to cardiac injury following acute ischemia (175). The additional exposure of a postnatal diet rich in fat and sugar also exacerbates IUGR-induced hypertension (137) suggesting that a second hit may work synergistically to augment the programming of chronic disease. Thus, fetal programming enhances vulnerability to a second insult during postnatal life augmenting the adverse impact of increased CV risk that originates during fetal life.
Figure 22.
A: renal tubular injury scores in response to a mild I/R in NBW and LBW rats untreated and treated with tempol. The degree of tubular injury was scored using an established method of semiquantitive evaluation of 10 fields randomly selected per each examined kidney (high-power fields). All data are expressed as means ± SE. *P < 0.005 vs. all other groups. †P < 0.05 vs. LBW untreated counterpart. B: microphotographs representing renal tubular injury in response to a mild I/R in NBW and LBW rats untreated and treated with tempol. a: NBW sham. b: LBW sham. c: NBW mild renal I/R. d: LBW mild renal I/R. e: NBW mild renal I/R + tempol. f: LBW mild renal I/R + tempol. (All pictures are hematoxylin and eosin at ×400 magnification. Scale bars = 50 µm.) (Used with permission, Figure 8, 143).
Pregnancy – a second strike in LBW women
Pregnancy may serve as a second hit in women born with compromised growth during fetal life (82). Pregnancy involves significant anatomical and physiological changes in order to meet the needs of the developing fetus (207). Pregnancy also involves significant alterations in the regulation of glucose homeostasis that when disrupted results in the development of gestational diabetes (42). Preexisting factors such as increased adiposity (213) and endothelial dysfunction (191) contribute to the etiology of the maternal syndrome of preeclampsia (170) and are risk factors programmed in response to altered fetal growth (83). Birth weight is associated with an increased risk for metabolic disease (24, 50). Thus, factors that increase the risk for chronic disease following a developmental insult may also increase the risk for a pregnancy complicated by hypertension and diabetes in women with compromised growth during fetal life. Several studies indicate that maternal birth weight predicts a woman’s risk for a pregnancy complicated by preeclampsia (81) or gestational diabetes (80, 186). Maternal weight also increases the risk for preterm delivery (36). Complications during pregnancy such as preeclampsia and gestational diabetes are associated with a greater prevalence for CV and metabolic disease after delivery (55, 184). Thus, altered growth during fetal life not only increases the risk for chronic disease in later life, but it also increases a woman’s risk for complications during pregnancy. Complications that arise during pregnancy often serve as an indicator for increased CV risk after pregnancy. Thus, pregnancy may serve as a second hit that provides an additional adverse impact a woman’s health across her lifespan following compromised growth and development during her fetal life.
Developmental programming of impaired metabolic health and increased CV risk
Fetal exposure to complications during pregnancy including IUGR and maternal factors such as obesity and smoking that program an increased risk of hypertension and CV disease in the offspring are also associated with an increased risk for impaired glucose homeostasis and T2D (24, 27, 50, 169). CV disease is the most common cause of death in individuals with T2D (77). Many other CV risk factors including hypertension and obesity are observed in conjunction with T2D contributing to the strong association between diabetes and death from CV disease (77). Thus, insults during fetal life can have a broad impact on the overall chronic health of an individual through the developmental programming of hypertension (105), coronary heart disease (9), and impaired metabolic health (27, 169) with the developmental programming of impaired metabolic health serving as an additional or second hit against increased CV risk
Experimental models indicate that undernutrition during fetal life programs impaired glucose homeostasis in a manner that is sex and insult specific. Fetal exposure to maternal protein restriction in the rat is associated with an increase in fasting glucose levels in male offspring in young adulthood (156); bilateral uterine ligation in the rat programs an increase in fasting glucose levels in the female (86). Male rat offspring exposed to maternal protein restriction exhibit glucose intolerance (156); however, female rat offspring develop hyperinsulinemia associated with a reduction in insulin signaling implicating sex- and insult-specific pathways contribute to impaired glucose homeostasis following a developmental insult. Bilateral uterine ligation in the rat programs a reduction in beta-cell mass in male and female offspring in young adulthood (203) whereas maternal protein restriction in the rat programs a reduction in beta cell mass at a similar age only in the male (212). Impaired beta-cell development following an early life insult involves epigenetic modification of genes that regulate beta-cell development including Pdx-1 (200). Expression of Pdx-1 is reduced during the neonatal period in IUGR offspring exposed to placental insufficiency (200). Administration of a pancreatic beta-cell trophic factor, exendin-4, prevents the development of impaired glucose homeostasis in the IUGR rat by restoring beta-cell differentiation and preventing impaired beta-cell formation (200) implicating an important role for impaired pancreatic function in the developmental programming of impaired glucose homeostasis following fetal exposure to a under-nutritional insult. Adiposity is increased in rat offspring exposed to maternal obesity during gestation in association with hyperglycemia (40) indicating that the developmental programming of increased metabolic risk occurs in pregnancies complicated by overnutrition and maternal obesity in addition to undernutrition and IUGR.
Impaired glucose homeostasis may also involve a shift in hepatic gluconeogenesis. Placental insufficiency in the rat programs an increase in hepatic glucose production in IUGR offspring that exhibit insulin resistance (224). Gluconeogenesis is regulated by the PEPCK and glucose-6-phosphatase (G6PC) genes. Expression of these genes is up-regulated in the IUGR offspring suggesting impaired signaling in the liver contributes to the increase in gluconeogenesis (224). Maternal protein restriction in the rat also programs an increase in hepatic PEPCK and G6PC genes (112) suggesting that gluconeogenic production of glucose is impaired regardless of the nutritional developmental insult. These alterations are apparent prior to birth in IUGR sheep exposed to placental insufficiency (214) implicating a compensatory mechanism that may contribute to survival of the fetus in times of nutritional stress but when persists beyond birth, contributes to impaired glucose regulation and homeostasis.
Insulin resistance in the adult IUGR rat is also associated with impaired skeletal muscle glucose uptake (187). Thus, nutritional insults during development also program a disabled insulin responsiveness of the skeletal muscle to glucose. Impaired translocation of GLUT4 to the cell surface contributes to the development of insulin resistance following a developmental insult. Protein expression of the GLUT4 is decreased in the skeletal muscle of the IUGR rat in association with impaired GLUT4 translocation following fetal undernutrition (211). Compromised GLUT4 translocation in the IUGR rat involves a defect in pyruvate oxidative phosphorylation in the skeletal mitochondria that reduces ATP production (187). Offspring of diabetic dams also exhibit impaired translocation of GLUT4 in the skeletal and adipose tissue associated with glucose intolerance (210). Thus, insulin resistance involves impaired skeletal muscle uptake of glucose which may contribute to the later development of T2D following a developmental insult.
To summarize, insults during fetal life program impaired glucose homeostasis that involves alterations in the pancreas, skeletal muscle and liver impacting overall insulin release and glucose uptake mediated via impaired signaling that may result from epigenetic processes. In addition, programming of insulin resistance is transmitted to the next generation (209), an observation noted in the fetal programming of high blood pressure (7) further implicating an important role for epigenetic processes in the etiology of chronic disease that has its origins in early life.
Perspectives
Despite the extensive progress made during the past couple of decades in elucidating the underlying mechanisms that link early life with later chronic health and disease, consideration of an individual’s exposure to maternal complications during gestational life or birth weight as a marker of fetal growth are not considered in the management of chronic disease or prevention of increased CV risk. Lackland et al. addressed the influence of birth weight on antihypertensive therapy and reported that birth weight is associated with a greater use of calcium channel blockers in black women and ACE inhibitors in white men (99). This study suggests that birth weight influences the efficacy of antihypertensive medication and also notes the importance of sex in the consideration of clinical treatment. Yet studies beyond this initial investigation are lacking and the impact of birth weight is not yet considered when outlining options for treatment and prevention in individuals with birth weights that exceed the lower and upper limits in regards to size. In addition, very few studies separate outcome by sex. Experimental studies demonstrate that sex impacts the chronic health of the offspring in response to a fetal insult. Experimental studies also demonstrate that programming of key systems involved in the long-term control of blood pressure regulation are sex-specific; yet, these observations are not considered in the management of chronic health for low birth weight individuals. Age also impacts the developmental programming of chronic health. Yet, few studies are investigating the importance of age a modulator of programmed health. Costs related to aging studies in experimental protocols may be a contributory factor but in light of the aging population, additional studies are clearly warranted. The etiology of programming insults also varies greatly within the general population. Experimental studies note that similar mechanistic pathways contribute to increased blood pressure and CV risk that is programmed in response to a developmental insult, but whether different programming events during the early life of an individual contributes to increased programmed CV risk in a manner that differentially alters management of chronic disease is not yet known. Clearly studies are needed to address how birth weight and differing developmental insults impact clinical management and healthcare across the lifespan.
CONCLUSION
The etiology of impaired growth during fetal life varies and encompasses complications during pregnancy such as preeclampsia and gestational diabetes, maternal obesity, excessive weight gain or undernutrition during pregnancy, preterm delivery, paternal smoking, maternal alcohol consumption or stress (Figure 23). The mechanisms that link adverse exposures during early life with increased risk for CV disease in later life are many. Yet, despite the method of developmental insult or the species studied, experimental models report common mechanistic pathways that contribute to the underlying etiology of programmed CV risk. Human and experimental studies report inappropriate activation of the RAAS in association with an increase in blood pressure following a fetal insult. SNS activity is also elevated in low birth weight individuals. Animal studies that induce IUGR or expose offspring to obesity during fetal life provide proof of principle for the importance of the RAAS and the renal nerves in the fetal programming of hypertension (Figure 23). An imbalance in anti- and pro-oxidants resulting in increased oxidative stress is observed in low birth weight humans and experimental models of developmental insult and the importance for oxidative stress and endothelin as mediators of increased CV risk is also demonstrated in animal studies (Figure 23). A reduction in nephron number that originates during fetal life enhances vulnerability to a second insult and may be the contributory factor that facilitates the increased risk for end stage renal disease in low birth weight individuals that develop hypertension or T2D in later life (Figure 23). Preeclampsia is a risk factor for CV disease after delivery. Impaired growth during fetal life is associated with increased adiposity and vascular dysfunction, risk factors for preeclampsia and gestational diabetes. Birth weight predicts a woman’s risk for developing a pregnancy complicated by preeclampsia and diabetes (Figure 23) indicating the long-term impact that an adverse exposure during early life has on a woman’s health across her lifespan. Birth weight also predicts an individual’s risk for type 2 diabetes and experimental models demonstrated that adverse influences during fetal life program hyperglycemia and insulin resistance in the offspring contributing to the development of T2D and subsequent increased CV risk (Figure 23).
Figure 23.
Schematic diagram of the fetal programming of cardiovascular risk that originates from adverse exposure to maternal insults during gestational life and programs alterations in structure and physiology that contribute to the development of increased cardiovascular risk in later life.
To summarize, investigation into the developmental origins of health and disease is a rapidly expanding field. Adverse influences that slow or accelerate growth during early life have long-lasting effects on the health of an individual and can result in increased adiposity, vascular dysfunction, impaired glucose homeostasis, elevated blood pressure, and increased risk for renal and CV disease in later life. The gestational health of a woman has its origins in her own gestational life indicating that complications during pregnancy in one generation can significantly impact the gestational health of the next generation. Further studies are needed to fully elucidate the mechanisms that program increased CV risk in later life in order to improve the overall health of individuals that are at risk due to impaired growth during early life.
Acknowledgments
Dr Alexander is supported by National Institutes of Health grants HL074927, HL51971 and an American Heart Grant GRNT19900004, Dr Intapad is supported by an American Heart Association Post-Doctoral Fellowship Grant, 12POST1198002, and both receive support from the NIH P20GM104357.
Footnotes
CROSS-REFERENCES
Hypertension, Physiology and Pathophysiology
Hypertension, Renal Neural Mechanisms
Renin angiotensin aldosterone system and the renal regulation of sodium, potassium and blood pressure homeostasis
ROS and the CV system
REFERENCES
- 1.Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, Hyde MJ, Modi N. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55:3114–3127. doi: 10.1007/s00125-012-2689-8. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CM, Lopez F, Zimmer A, Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod. 2006;74:538–544. doi: 10.1095/biolreprod.105.045807. [DOI] [PubMed] [Google Scholar]
- 7.Andersen LG, Angquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, Baker JL, Sorensen TI. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5:e14126. doi: 10.1371/journal.pone.0014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker D. Mother, babies, and disease in later life. London: BMJ Publishing Group; 1994. pp. 1–12.pp. 132–134. [Google Scholar]
- 9.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baserga M, Kaur R, Hale MA, Bares A, Yu X, Callaway CW, McKnight RA, Lane RH. Fetal growth restriction alters transcription factor binding and epigenetic mechanisms of renal 11beta-hydroxysteroid dehydrogenase type 2 in a sex-specific manner. Am J Physiol Regul Integr Comp Physiol. 2010;299:R334–R342. doi: 10.1152/ajpregu.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension. 2008;52:638–644. doi: 10.1161/HYPERTENSIONAHA.108.114256. [DOI] [PubMed] [Google Scholar]
- 13.Bercovich E, Keinan-Boker L, Shasha SM. Long-term health effects in adults born during the Holocaust. Isr Med Assoc J. 2014;16:203–207. [PubMed] [Google Scholar]
- 14.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black MJ, Sutherland MR, Gubhaju L, Kent AL, Dahlstrom JE, Moore L. When birth comes early: effects on nephrogenesis. Nephrology (Carlton) 2013;18:180–182. doi: 10.1111/nep.12028. [DOI] [PubMed] [Google Scholar]
- 16.Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS One. 2010;5:e9237. doi: 10.1371/journal.pone.0009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boguszewski MC, Johannsson G, Fortes LC, Sverrisdóttir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. J Hypertens. 2004;22:1157–1163. doi: 10.1097/00004872-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 19.Börzsönyi B, Demendi C, Pajor A, Rigó J, Jr, Marosi K, Agota A, Nagy ZB, Joó JG. Gene expression patterns of the 11β-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. Eur J Obstet Gynecol Reprod Biol. 2012;161:12–17. doi: 10.1016/j.ejogrb.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Boubred F, Saint-Faust M, Buffat C, Ligi I, Grandvuillemin I, Simeoni U. Developmental Origins of Chronic Renal Disease: An Integrative Hypothesis. Int J Nephrol. 2013:346067. doi: 10.1155/2013/346067. Epub 2013. PMID: 24073334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62:753–758. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 22.Breukhoven PE, Kerkhof GF, Willemsen RH, Hokken-Koelega AC. Fat mass and lipid profile in young adults born preterm. J Clin Endocrinol Metab. 2012;97:1294–1302. doi: 10.1210/jc.2011-2621. [DOI] [PubMed] [Google Scholar]
- 23.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am. J. Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 24.Bruin JE, Kellenberger LD, Gerstein HC, Morrison KM, Holloway AC. Fetal and neonatal nicotine exposure and postnatal glucose homeostasis: identifying critical windows of exposure. J Endocrinol. 2007;194:171–178. doi: 10.1677/JOE-07-0050. [DOI] [PubMed] [Google Scholar]
- 25.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 26.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson S, Persson PG, Alvarsson M, Efendic S, Norman A, Svanström L, Ostenson CG, Grill V. Low birth weight, family history of diabetes, and glucose intolerance in Swedish middle-aged men. Diabetes Care. 1999;22:1043–1047. doi: 10.2337/diacare.22.7.1043. [DOI] [PubMed] [Google Scholar]
- 28.Chernoff N, Gage MI, Stoker TE, Cooper RL, Gilbert ME, Rogers EH. Reproductive effects of maternal and pre-weaning undernutrition in rat offspring: age at puberty, onset of female reproductive senescence and intergenerational pup growth and viability. Reprod Toxicol. 2009;28:489–494. doi: 10.1016/j.reprotox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham FG, Gant NF, Leveno KJ, et al. Diabetes. In: Cunningham FG, Gant NF, Leveno KJ, et al., editors. Williams Obstetrics. 21st. New York, NY: McGraw-Hill; 2001. pp. 1359–1381. [Google Scholar]
- 30.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 31.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 32.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalziel SR, Parag V, Rodgers A, Harding JE. Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol. 2007;36:907–915. doi: 10.1093/ije/dym067. [DOI] [PubMed] [Google Scholar]
- 34.Davies AA, Smith GD, Ben-Shlomo Y, Litchfield P. Low birth weight is associated with higher adult total cholesterol concentration in men: findings from an occupational cohort of 25,843 employees. Circulation. 2004;110:1258–1262. doi: 10.1161/01.CIR.0000140980.61294.4D. [DOI] [PubMed] [Google Scholar]
- 35.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 36.De B, Lin S, Lohsoonthorn V, Williams MA. Risk of preterm delivery in relation to maternal low birth weight. Acta Obstet Gynecol Scand. 2007;86:565–571. doi: 10.1080/00016340701223127. [DOI] [PubMed] [Google Scholar]
- 37.de Almeida Chaves Rodrigues AF, de Lima IL, Bergamaschi CT, Campos RR, Hirata AE, Schoorlemmer GH, Gomes GN. Increased renal sympathetic nerve activity leads to hypertension and renal dysfunction in offspring from diabetic mothers. Am J Physiol Renal Physiol. 2013;304:F189–F197. doi: 10.1152/ajprenal.00241.2012. [DOI] [PubMed] [Google Scholar]
- 38.deJong F, Monuteaux MC, vanElburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86:1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- 40.Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. Rat maternal obesity and high-fat program offspring metabolic syndrome. Am J Obstet Gunecol. 2014 doi: 10.1016/j.ajog.2014.03.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiBona GF. Peripheral and central interactions between the renin angiotensin system and the sympathetic nerves in the control of renal function. Ann N Y Sci. 2001;940:395–406. doi: 10.1111/j.1749-6632.2001.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Canni G, Miccoli R, Volpe L, Lencioni C, Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res Rev. 2003;19:259–270. doi: 10.1002/dmrr.390. [DOI] [PubMed] [Google Scholar]
- 43.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2006;292:R453–R461. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 44.Dior UP, Lawrence GM, Sitlani C, Enquobahrie D, Manor O, Siscovick DS, Friedlander Y, Hochner H. Parental smoking during pregnancy and offspring cardio-metabolic risk factors at ages 17 and 32. Atherosclerosis. 2014;235:430–437. doi: 10.1016/j.atherosclerosis.2014.05.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodson RB, Rozance PJ, Fleenor BS, Petrash CC, Shoemaker LG, Hunter KS, Ferguson VL. Increased arterial stiffness and extracellular matrix reorganization in intrauterine growth-restricted fetal sheep. Pediatr Res. 2013;73:147–154. doi: 10.1038/pr.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez TP. Adverse birth outcomes in African American women: the social context of persistent reproductive disadvantage. Soc Work Public Health. 2011;26:3–16. doi: 10.1080/10911350902986880. [DOI] [PubMed] [Google Scholar]
- 47.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 48.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 49.Elias SG, van Noord PA, Peeters PH, den Tonkelaar I, Grobbe DE. Caloric restriction reduces age at menopause: the effect of the 1944–1945 Dutch famine. Menopause. 2003;10:399–405. doi: 10.1097/01.GME.0000059862.93639.C1. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the Helsinki Birth Cohort Study. Ann Med. 2014;9:1–5. doi: 10.3109/07853890.2014.919728. [DOI] [PubMed] [Google Scholar]
- 51.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 52.Ferreira I, Peeters LL, Stehouwer CD. Preeclampsia and increased blood pressure in the offspring: meta-analysis and critical review of the evidence. J Hypertens. 2009;27:1955–1959. doi: 10.1097/HJH.0b013e328331b8c6. [DOI] [PubMed] [Google Scholar]
- 53.Filkaszova A, Chabada J, Stencl P, Drobny J, Sysak R, Urban H, Oravec J, Lamprechtova B, Oroszova V. Ultrasound diagnosis of macrosomia. Bratisl Lek Listy. 2014;115:30–33. doi: 10.4149/bll_2014_007. [DOI] [PubMed] [Google Scholar]
- 54.Finken MJ, Keijzer-Veen MG, Dekker FW, Frölich M, Hille ET, Romijn JA, Wit JM Dutch POPS-19 Collaborative Study Group. Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia. 2006;49:478–485. doi: 10.1007/s00125-005-0118-y. [DOI] [PubMed] [Google Scholar]
- 55.Forest JC, Girouard J, Masse J, Moutquin JM, Kharfi A, Ness RB, Roberts JM, Giquere Y. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol. 2005;105:1373–1380. doi: 10.1097/01.AOG.0000163252.02227.f8. [DOI] [PubMed] [Google Scholar]
- 56.Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br J PrevSoc Med. 1977;31:91–95. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco MC, Akamine EH, Rebouças N, Carvalho MH, Tostes RC, Nigro D, Fortes ZB. Long-term effects of intrauterine malnutrition on vascular function in female offspring: implications of oxidative stress. Life Sci. 2007;80:709–715. doi: 10.1016/j.lfs.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 58.Franco MC, Casarini DE, Carneiro-Ramos MS, Sawaya AL, Barreto-Chaves ML, Sesso R. Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin Sci (Lond) 2008;114:375–380. doi: 10.1042/CS20070284. [DOI] [PubMed] [Google Scholar]
- 59.Franco MC, Kawamoto EM, Gorjao R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatr Res. 2007;62:204–208. doi: 10.1203/PDR.0b013e3180986d04. [DOI] [PubMed] [Google Scholar]
- 60.Gademan MG, van Eijsden M, Roseboom TJ, van der Post JA, Stronks K, Vrijkotte TG. Maternal prepregnancy body mass index and their children's blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension. 2013;62:641–647. doi: 10.1161/HYPERTENSIONAHA.113.01511. [DOI] [PubMed] [Google Scholar]
- 61.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, Jaddoe VW. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63:683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: Hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldfarb DA, Martin SF, Braun WE, Schreiber MJ, Mastroianni B, Papajcik D, Rolin HA, Flecher S, Goormastic M, Novick AC. Renal outcomes 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 64.Goyal R, Longo LD. Maternal protein deprivation: sexually dimorphic programming of hypertension in the mouse. Hypertens Res. 2013;36:29–35. doi: 10.1038/hr.2012.129. [DOI] [PubMed] [Google Scholar]
- 65.Gray SP, Denton KM, Cullen-McEwen L, Bertram JF, Moritz KM. Prenatal alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Npehrol. 2010;21:1891–1902. doi: 10.1681/ASN.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray SP, Kenna K, Bertram JF, Hoy WE, Yan EB, Bocking AD, Brien JF, Walker DW, Harding R, Moritz KM. Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep . Am J Physiol Regul Integr Comp Physiol. 2008;295:R568–R574. doi: 10.1152/ajpregu.90316.2008. [DOI] [PubMed] [Google Scholar]
- 67.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R804–R811. doi: 10.1152/ajpregu.00725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guberman C, Jellyman JK, Han G, Ross MG, Desai M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol. 2013;209:262.e1–262.e8. doi: 10.1016/j.ajog.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guimarães AM, Bettiol H, Souza LD, Gurgel RQ, Almeida ML, Ribeiro ER, Goldaniv MZ, Barbieri MA. Is adolescent pregnancy a risk factor for low birth weight? Rev Saude Publica. 2013;47:11–19. doi: 10.1590/s0034-89102013000100003. [DOI] [PubMed] [Google Scholar]
- 70.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 71.Guzmán C, Cabrera R, Cárdenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188:435–442. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- 73.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Hayes DK, Feigal DW, Smith RA, Fuddy LJ. Maternal asthma, diabetes, and high blood pressure are associated with low birth weight and increased hospital birth and delivery charges; Hawai'i hospital discharge data 2003–2008. Hawaii J Med Public Health. 2014;73:49–57. [PMC free article] [PubMed] [Google Scholar]
- 76.Hiraoka T, Kudo T, Kishimoto Y. Catecholamines in experimentally growth-retarded rat fetus. Asia Oceania J Obstet Gynaecol. 1991;17:341–348. doi: 10.1111/j.1447-0756.1991.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 77.Hobbes FD. Reducing cardiovascular risk in diabetes: beyond glycemic and blood pressure control. Int J Cardio. 2006;110:137–145. doi: 10.1016/j.ijcard.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, Tarnopolsky MA. Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine. 2007;31:254–259. doi: 10.1007/s12020-007-0043-6. [DOI] [PubMed] [Google Scholar]
- 79.Hughson M, Farris AB, 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 80.Innes KE, Byers TE, Marshall JA, Barón A, Orleans M, Hamman RF. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534–2541. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 81.Innes KE, Marshall JA, Byers TE, Calonge N. A woman's own birth weight and gestational age predict her later risk of developing preeclampsia, a precursor of chronic disease. Epidemiology. 1999;10:153–160. [PubMed] [Google Scholar]
- 82.Intapad S, Alexander BT. Pregnancy Complications and Later Development of Hypertension. Curr Cardiovasc Risk Rep. 2013;7:183–189. doi: 10.1007/s12170-013-0303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology (Bethesda) 2014;29:122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.IJzerman RG, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation. 2003;108:566–571. doi: 10.1161/01.CIR.0000081778.35370.1B. [DOI] [PubMed] [Google Scholar]
- 86.Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- 87.Jiang X, Ma H, Wang Y, Liu Y. Early life factors and type 2 diabetes. J Diabetes Res. 2013;2013:485082. doi: 10.1155/2013/485082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2014 doi: 10.1111/ijpo.230. [Epub ahead of print] PMID: 24916852. [DOI] [PubMed] [Google Scholar]
- 89.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol. 1997;273:R236–R242. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- 90.Jones CT, Robinson JS. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. J Dev Physiol. 1983;5:77–87. [PubMed] [Google Scholar]
- 91.Katkhuda R, Peterson ES, Roghair RD, Norris AW, Scholz TD, Segar JL. Sex-specific programming of hypertension in offspring of late-gestation diabetic rats. Pediatr Res. 2012;72:352–361. doi: 10.1038/pr.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kandasamy Y, Smith R, Wright IM, Lumbers ER. Extra-uterine growth in preterm infants: oligonephropathy and prematurity. Pediatr Nephrol. 2013;28:1971–1796. doi: 10.1007/s00467-013-2462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawamura M, Itoh H, Yura S, Mogami H, Fujii T, Makino H, Miyamoto Y, Yoshimasa Y, Aoe S, Ogawa Y, Sagawa N, Kanayama N, Konishi I. Isocaloric high-protein diet ameliorates systolic blood pressure increase and cardiac remodeling caused by maternal caloric restriction in adult mouse offspring. Endocr J. 2009;56:679–689. doi: 10.1507/endocrj.k08e-286. [DOI] [PubMed] [Google Scholar]
- 94.Kelstrup L, Damm P, Mathiesen ER, Hansen T, Vaag AA, Pedersen O, Clausen TD. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab. 2013;98:3793–313801. doi: 10.1210/jc.2013-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 96.Ko TJ, Tsai LY, Chu LC, Yeh SJ, Leung C, Chen CY, Chou HC, Tsao PN, Chen PC, Hsieh WS. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55:20–27. doi: 10.1016/j.pedneo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Kramer MS. The epidemiology of low birthweight. Nestle Nutr Inst Workshop Ser. 2013;74:1–10. doi: 10.1159/000348382. [DOI] [PubMed] [Google Scholar]
- 98.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 99.Lackland DT, Egan BM, Syddall HE, Barker DJ. Associations between birth weight and antihypertensive medication in black and white medicaid recipients. Hypertension. 2002;39:179–183. doi: 10.1161/hy0102.100545. [DOI] [PubMed] [Google Scholar]
- 100.LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda) 2013;28:225–233. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 102.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 103.Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin Nutr. 1994;13:319–324. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 104.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 105.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 106.Lawlor DA, Davey Smith G, Ebrahim S. Birth weight is inversely associated with coronary heart disease in post-menopausal women: findings from the British women's heart and health study. J Epidemiol Community Health. 2004;58:120–125. doi: 10.1136/jech.58.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lawlor DA, Ebrahim S, Davey Smith G. Is there a sex difference in the association between birth weight and systolic blood pressure in later life? Findings from a meta-regression analysis. Am J Epidemiol. 2002;156:1100–1104. doi: 10.1093/aje/kwf154. [DOI] [PubMed] [Google Scholar]
- 108.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 109.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 110.Lesage J, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Breton C, Deloof S, Vieau D. Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress. 2006;9:183–981. doi: 10.1080/10253890601056192. [DOI] [PubMed] [Google Scholar]
- 111.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 112.Liu XM, Kong J, Song WW, Lu Y. Glucose metabolic and gluconeogenic pathways disturbance in the intrauterine growth restricted adult male rats. Chin Med Sci J. 2009;24:208–212. doi: 10.1016/s1001-9294(10)60003-x. [DOI] [PubMed] [Google Scholar]
- 113.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: A model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol. 2005;288:R16–R24. doi: 10.1152/ajpregu.00462.2004. [DOI] [PubMed] [Google Scholar]
- 114.Loria AS, D'Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R185–R191. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loria AS, Ho DH, Pollock JS. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiol (Oxf) 2013 doi: 10.1111/apha.12189. [Epub ahead of print] PMID: 2433084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension. 2010;55:494–499. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Changes in renal hemodynamics and excretory function induced by a reduction of ANG II effects during renal development. Am J Physiol Regul Integr Comp Physiol. 2007;293:R695–R700. doi: 10.1152/ajpregu.00191.2007. [DOI] [PubMed] [Google Scholar]
- 118.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R121–R129. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lurbe E, Garcia-Vicent C, Torro MI, Aguilar F, Redon J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension. 2014;63:1326–1332. doi: 10.1161/HYPERTENSIONAHA.114.03137. [DOI] [PubMed] [Google Scholar]
- 120.Ma N, Nicholson CJ, Wong M, Holloway AC, Hardy DB. Fetal and neonatal exposure to nicotine leads to augmented hepatic and circulating triglycerides in adult male offspring due to increased expression of fatty acid synthase. Toxicol Appl Pharmacol. 2014;275:1–11. doi: 10.1016/j.taap.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 122.Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–416. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 123.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 124.Mao C, Zhang H, Xiao D, Zhu L, Ding Y, Zhang Y, Wu L, Xu Z, Zhang L. Perinatal nicotine exposure alters AT 1 and AT 2 receptor expression pattern in the brain of fetal and offspring rats. Brain Res. 2008;1243:47–52. doi: 10.1016/j.brainres.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mamun AA, Kinarivala MK, O'Callaghan M, Williams G, Najman J, Callaway L. Does hypertensive disorder of pregnancy predict offspring blood pressure at 21 years? Evidence from a birth cohort study. J Hum Hypertens. 2012;26:288–294. doi: 10.1038/jhh.2011.35. [DOI] [PubMed] [Google Scholar]
- 126.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 127.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Racial disparities in pregnancy outcomes in obese women. J Matern Fetal Neonatal Med. 2014;27:122–126. doi: 10.3109/14767058.2013.806478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martínez D, Pentinat T, Ribó S, Daviaud C, Bloks VW, Cebrià J, Villalmanzo N, Kalko SG, Ramón-Krauel M, Díaz R, Plösch T, Tost J, Jiménez-Chillarón JC. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered lxra DNA methylation. Cell Metab. 2014;3(19):941–951. doi: 10.1016/j.cmet.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 129.Martinez-Aguayo A, Aglony M, Bancalari R, Avalos C, Bolte L, Garcia H, Loureiro C, Carvajal C, Campino C, Inostroza A, Fardella C. Birth weight is inversely associated with blood pressure and serum aldosterone and cortisol levels in children. Clin Endocrinol (Oxf) 2012;76:713–718. doi: 10.1111/j.1365-2265.2011.04308.x. [DOI] [PubMed] [Google Scholar]
- 130.Mission JF, Marshall NE, Caughey AB. Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv. 2013;68:389–399. doi: 10.1097/OGX.0b013e31828738ce. [DOI] [PubMed] [Google Scholar]
- 131.Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta. 2010;31:S40–S46. doi: 10.1016/j.placenta.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 132.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587:2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moritz KM, Wintour EM, Dodic M. Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension. 2002;39:1071–1076. doi: 10.1161/01.hyp.0000019131.77075.54. [DOI] [PubMed] [Google Scholar]
- 134.Morton JS, Rueda-Clausen CF, Davidge ST. Flow-mediated vasodilation is impaired in adult rat offspring exposed to prenatal hypoxia. J Appl Physiol (1985) 2011;110:1073–1082. doi: 10.1152/japplphysiol.01174.2010. [DOI] [PubMed] [Google Scholar]
- 135.Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JL, Hildebrandt TB, Lonergan P, Ireland JJ, Evans AC. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol Reprod. 2013;88:92. doi: 10.1095/biolreprod.112.107235. [DOI] [PubMed] [Google Scholar]
- 136.Murray AJ. Oxygen delivery and fetal-placental growth: beyond a question of supply and demand? Placenta. 2012;33:e16–e22. doi: 10.1016/j.placenta.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 137.Myrie SB, MacKay DS, Van Vliet BN, Bertolo RF. Early programming of adult blood pressure in the low birth weight Yucatan miniature pig is exacerbated by a post-weaning high-salt-fat-sugar diet. Br J Nutr. 2012;108:1218–1225. doi: 10.1017/S0007114511006696. [DOI] [PubMed] [Google Scholar]
- 138.Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miller PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43:1110–1115. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- 139.National Center for Health Statistics, final natality data. [Retrieved February 4th, 2004]; from www.marchofdimes.com/peristats. [Google Scholar]
- 140.Nehiri T, Duong Van Huyen JP, Viltard M, Fassot C, Heudes D, Freund N, Deschênes G, Houillier P, Bruneval P, Lelièvre-Pégorier M. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes. 2008;57:2167–2175. doi: 10.2337/db07-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nykjaer C, Alwan NA, Greenwood DC, Simpson NA, Hay AW, White KL, Cade JE. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J Epidemiol Community Health. 2014;68:542–549. doi: 10.1136/jech-2013-202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Øglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. 2009;27:2051–2054. doi: 10.1097/HJH.0b013e328330052a. [DOI] [PubMed] [Google Scholar]
- 143.Ojeda NB. Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Renal Physiol. 2011;301:F420–F426. doi: 10.1152/ajprenal.00045.2011. [DOI] [PubMed] [Google Scholar]
- 144.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292:R758–R763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 146.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension. 2012;60:114–122. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ojeda NB, Intapad S, Royals TP, Black JT, Dasinger JH, Tull FL, Alexander BT. Hypersensitivity to acute ANG II in female growth-restricted offspring is exacerbated by ovariectomy. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1199–R1205. doi: 10.1152/ajpregu.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol. 2007;34:1212–1216. doi: 10.1111/j.1440-1681.2007.04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1421–R1427. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. ActaPhysiol (Oxf) 2013;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Page KA, Romero A, Buchanan TA, Xiang A. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. J Pediatr. 2014;164:807–810. doi: 10.1016/j.jpeds.2013.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension. 2003;42:768–774. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- 156.Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE. Catecholamine levels and receptor expression in low protein rat offspring. Diabet Med. 2000;17:848–853. doi: 10.1046/j.1464-5491.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 157.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285:R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 158.Pinheiro AR, Salvucci ID, Augila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (London) 2008;114:381–392. doi: 10.1042/CS20070302. [DOI] [PubMed] [Google Scholar]
- 159.Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Järvelin MR, Vääräsmäki M. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–1121. doi: 10.2337/dc09-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pladys P, Lahaie I, Cambonie G, Thibault G, Lê NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 161.Ponzio BF, Carvalho MH, Fortes ZB, do Carmo Franco M. Implications of maternal nutrient restriction in transgenerational programming of hypertension and endothelial dysfunction across F1–F3 offspring. Life Sci. 2012;90:571–577. doi: 10.1016/j.lfs.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 162.Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63:338–345. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 163.Quan A, Baum M. Renal nerve stimulation augments effect of intraluminal angiotensin II on proximal tubule transport. Am J Physiol Renal Physiol. 2002;282:F1043–F1048. doi: 10.1152/ajprenal.00279.2001. [DOI] [PubMed] [Google Scholar]
- 164.Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–88. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- 165.Reinisch JM, Simon NG, Karwo WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intra-uterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 166.Reverte V, Tapia A, Baile G, Gambini J, Gíménez I, Llinas MT, Salazar FJ. Role of angiotensin II in arterial pressure and renal hemodynamics in rats with altered renal development: age- and sex-dependent differences. Am J Physiol Renal Physiol. 2013;304:F33–F40. doi: 10.1152/ajprenal.00424.2012. [DOI] [PubMed] [Google Scholar]
- 167.Reverte V, Tapia A, Loria A, Salazar F, Llinas MT, Salazar FJ. COX2 inhibition during nephrogenic period induces ANG II hypertension and sex-dependent changes in renal function during aging. Am J Physiol Renal Physiol. 2014;306:F534–F541. doi: 10.1152/ajprenal.00535.2013. [DOI] [PubMed] [Google Scholar]
- 168.Ribeiro MM, Trombetta IC, Batalha LT, Rondon MU, Forjaz CL, Barretto AC, Villares SM, Negrão CE. Muscle sympathetic nerve activity and hemodynamic alterations in middle-aged obese women. Braz J Med Biol Res. 2001;34:475–478. doi: 10.1590/s0100-879x2001000400006. [DOI] [PubMed] [Google Scholar]
- 169.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 170.Roberts JM, Gammill HS. Preeclampsia. Hypertension. 2005;46:1246–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 171.Rocha SO, Gomes GN, Forti AL, do CarmoPinho Franco M, Fortes ZB, de FátimaCavanal M, Gil FZ. Long-term effects of maternal diabetes on vascular reactivity and renal function in rat male offspring. Pediatr Res. 2005;58:1274–1279. doi: 10.1203/01.pdr.0000188698.58021.ff. [DOI] [PubMed] [Google Scholar]
- 172.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–1106. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 173.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 175.Rueda-Clausen CF, Morton JS, Dolinsky VW, Dyck JR, Davidge ST. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R418–R426. doi: 10.1152/ajpregu.00148.2012. [DOI] [PubMed] [Google Scholar]
- 176.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011;90:285–294. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 177.Samuelsson AM, Alexanderson C, Mölne J, Haraldsson B, Hansell P, Holmäng A. Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol. 2006;575:855–867. doi: 10.1113/jphysiol.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 179.Samuelsson AM, Matthews PA, Jansen E, Taylor PD, Poston L. Sucrose feeding in mouse pregnancy leads to hypertension, and sex-linked obesity and insulin resistance in female offspring. Front Physiol. 2013;4:14. doi: 10.3389/fphys.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 181.Sandboge S, Moltchanova E, Blomstedt PA, Salonen MK, Kajantie E, Osmond C, Barker DJ, Eriksson JG. Birth-weight and resting metabolic rate in adulthood - sex-specific differences. Ann Med. 2012;44:296–303. doi: 10.3109/07853890.2010.549147. [DOI] [PubMed] [Google Scholar]
- 182.Sapre S, Thakur R. Lifestyle and dietary factors that determine age at menopause. J Midlife Health. 2014;5:3–5. doi: 10.4103/0976-7800.127779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saran R, Marshall S, Madsen R, Keavey P, Tapson JS. Long-term follow-up of kidney donors: a longitudinal study. Nephrol Dial Transplant. 1997;12:1615–1621. doi: 10.1093/ndt/12.8.1615. [DOI] [PubMed] [Google Scholar]
- 184.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Dörr HG, Rascher W, Dötsch J. Decreased gene expression of 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase in human placenta of patients with preeclampsia. J Clin Endocrinol Metab. 2001;86:1313–1317. doi: 10.1210/jcem.86.3.7311. [DOI] [PubMed] [Google Scholar]
- 186.Seghieri G, Anichini R, De Bellis A, Alviggi L, Franconi F, Breschi MC. Relationship between gestational diabetes mellitus and low maternal birth weight. Diabetes Care. 2002;25:1761–1765. doi: 10.2337/diacare.25.10.1761. [DOI] [PubMed] [Google Scholar]
- 187.Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E130–E137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- 188.Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010;59:3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Seng JS, Low LK, Sperlich M, Ronis DL, Liberzon I. Post-traumatic stress disorder, child abuse history, birthweight and gestational age: a prospective cohort study. BJOG. 2011;118:1329–1339. doi: 10.1111/j.1471-0528.2011.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 191.Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wühl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation. 2011;123:292–298. doi: 10.1161/CIRCULATIONAHA.110.958769. [DOI] [PubMed] [Google Scholar]
- 192.Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579:503–513. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Singhal A, Cole TJ, Fewtrell M, Kennedy K, Stephenson T, Elias-Jones A, Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115:213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]
- 194.Slater-Jefferies JL, Lillycrop KA, Townsend PA, Torrens C, Hoile SP, Hanson MA, Burdge GC. Feeding a protein-restricted diet during pregnancy induces altered epigenetic regulation of peroxisomal proliferator-activated receptor-α in the heart of the offspring. J Dev Orig Health Dis. 2011;2:250–255. doi: 10.1017/S2040174410000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Spencer SJ, Tilbrook A. Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology. 2009;34:1133–1143. doi: 10.1016/j.psyneuen.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Spracklen CN, Ryckman KK, Harland KK, Saftlas AF. Effects of smoking and preeclampsia on birthweight for gestational age. J Matern Fetal Neonatal Med. 2014;4:1–20. doi: 10.3109/14767058.2014.928853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–836. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 198.Steiner AZ, D'Aloisio AA, DeRoo LA, Sandler DP, Baird DD. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. 2010;172:140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 200.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth-retarded rat. Diabetes. 2003;52:734–740. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- 201.Strutz KL, Hogan VK, Siega-Riz AM, Suchindran CM, Halpern CT, Hussey JM. Preconception stress, birth weight, and birth weight disparities among US women. Am J Public Health. 2014;12:e1–e8. doi: 10.2105/AJPH.2014.301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Strutz KL, Richardson LJ, Hussey JM. Selected preconception health indicators and birth weight disparities in a national study. Womens Health Issues. 2014;24:e89–e97. doi: 10.1016/j.whi.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Styrud J, Eriksson UJ, Grill V, Swenne I. Experimental intrauterine growth retardation in the rat causes a reduction of pancreatic B-cell mass, which persists into adulthood. Biol Neonate. 2005;88:122–128. doi: 10.1159/000086136. [DOI] [PubMed] [Google Scholar]
- 204.Szpera-Gozdziewicz A, Breborowicz GH. Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front Biosci (Landmark Ed) 2014;19:734–746. doi: 10.2741/4240. [DOI] [PubMed] [Google Scholar]
- 205.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 206.Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 207.Tao H, Rui C, Zheng J, Tang J, Wu L, Shi A, Chen N, He R, Wu C, Li J, Yin X, Zhang P, Zhu Z, Tao J, Xiao J, Mao C, Xu Z. Angiotensin II-mediated vascular changes in aged offspring rats exposed to perinatal nicotine. Peptides. 2013;44:111–119. doi: 10.1016/j.peptides.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 208.Tare M, Parkington HC, Bubb KJ, Wlodek ME. Uteroplacental insufficiency and lactational environment separately influence arterial stiffness and vascular function in adult male rats. Hypertension. 2012;60:378–386. doi: 10.1161/HYPERTENSIONAHA.112.190876. [DOI] [PubMed] [Google Scholar]
- 209.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PW, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2007;292:E1270–E1279. doi: 10.1152/ajpendo.00462.2006. [DOI] [PubMed] [Google Scholar]
- 210.Thamotharan M, McKnight RA, Thamotharan S, Kao DJ, Devaskar SU. Aberrant insulin-induced GLUT4 translocation predicts glucose intolerance in the offspring of a diabetic mother. Am J Physiol Endocrinol Metab. 2003;284:E901–E914. doi: 10.1152/ajpendo.00516.2002. [DOI] [PubMed] [Google Scholar]
- 211.Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth restricted adult rat female offspring. Am J Physiol Endocrinol Metab. 2005;288:E935–E947. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- 212.Theys N, Bouckenooghe T, Ahn MT, Remacle C, Reusens B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1516–R1525. doi: 10.1152/ajpregu.00280.2009. [DOI] [PubMed] [Google Scholar]
- 213.Thompson ML, Ananth CV, Jaddoe VW, Miller RS, Williams MA. The association of maternal adult weight trajectory with preeclampsia and gestational diabetes mellitus. Paediatr Perinat Epidemiol. 2014;28:287–296. doi: 10.1111/ppe.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thorn SR, REgnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod. 2010;25:791–798. doi: 10.1093/humrep/dep451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Torrens C, Hanson MA, Gluckman PD, Vickers MH. Maternal undernutrition leads to endothelial dysfunction in adult male rat offspring independent of postnatal diet. Br J Nutr. 2009;101:27–33. doi: 10.1017/S0007114508988760. [DOI] [PubMed] [Google Scholar]
- 217.Tsadok MA, Friedlander Y, Paltiel O, Manor O, Meiner V, Hochner H, Sagy Y, Sharon N, Yazdgerdi S, Siscovick D, Elchalal U. Obesity and blood pressure in 17-year-old offspring of mothers with gestational diabetes: insights from the Jerusalem Perinatal Study. Exp Diabetes Res. 2011;2011:906154. doi: 10.1155/2011/906154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van Abeelen AF, de Rooij SR, Osmond C, Painter RC, Veenendaal MV, Bossuyt PM, Elias SG, Grobbee DE, van der Schouw YT, Barker DJ, Roseboom TJ. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694–698. doi: 10.1016/j.placenta.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 219.van Eijsden M, Vrijkotte TG, Gemke RJ, van der Wal MF. Cohort profile: the Amsterdam Born Children and their Development (ABCD) study. Int J Epidemiol. 2011;40:1176–1186. doi: 10.1093/ije/dyq128. [DOI] [PubMed] [Google Scholar]
- 220.van Straten EM, Bloks VW, Huijkman NC, Baller JF, van Meer H, Lütjohann D, Kuipers F, Plösch T. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol. 2010;298:R275–R282. doi: 10.1152/ajpregu.00413.2009. [DOI] [PubMed] [Google Scholar]
- 221.Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol. 2003;101:529–533. doi: 10.1016/s0029-7844(02)02718-7. [DOI] [PubMed] [Google Scholar]
- 222.Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6:2165–2178. doi: 10.3390/nu6062165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Birth size and coronary heart disease risk score in young adulthood. The Atherosclerosis Risk in Young Adults (ARYA) study. Eur J Epidemiol. 2006;21:33–38. doi: 10.1007/s10654-005-4658-8. [DOI] [PubMed] [Google Scholar]
- 224.Vuquin P, Raab E, Liu B, Barzilai N, Simmons R. Hepatic insulin reistance precedes the development of diabetes in a model of intrauterine growth restriction. Diabetes. 2004;53:2617–2622. doi: 10.2337/diabetes.53.10.2617. [DOI] [PubMed] [Google Scholar]
- 225.Wahabi HA, Fayed AA, Alzeidan RA, Mandil AA. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr Disord. 2014;14:47. doi: 10.1186/1472-6823-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 227.Washburn LK, Brosnihan KB, Chappell MC, Diz DI, Gwathmey TM, Nixon PA, Russell GB, Snively BM, O'Shea TM. The renin-angiotensin-aldosterone system in adolescent offspring born prematurely to mothers with preeclampsia. J Renin Angiotensin Aldosterone Syst. 2014 doi: 10.1177/1470320314526940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Washburn L, Nixon P, Russell G, Snively BM, O'Shea TM. Adiposity in adolescent offspring born prematurely to mothers with preeclampsia. J Pediatr. 2013;162:912–917. doi: 10.1016/j.jpeds.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weitz G, Wellhoener P, Heindl S, Fehm HL, Dodt C. Relationship between metabolic parameters, blood pressure, and sympathoendocrine function in healthy young adults with low birth weight. Exp Clin Endocrinol Diabetes. 2005;113:444–450. doi: 10.1055/s-2005-865709. [DOI] [PubMed] [Google Scholar]
- 231.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–507. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 233.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Widdowson EM, McCance RA. Physiological undernutrition in the newborn guinea-pig. Br J Nutr. 1955;9:316–321. doi: 10.1079/bjn19550043. [DOI] [PubMed] [Google Scholar]
- 235.Wilcox CS, Gutterman D. Focus on oxidative stress in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2005;288:H3–H6. doi: 10.1152/ajpheart.00854.2004. [DOI] [PubMed] [Google Scholar]
- 236.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. Am J Physiol Heart Circ Physiol. 2005;289:H674–H682. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am SocNephrol. 2007;18:1688–1696. doi: 10.1681/ASN.2007010015. [DOI] [PubMed] [Google Scholar]
- 238.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008;74:187–195. doi: 10.1038/ki.2008.153. [DOI] [PubMed] [Google Scholar]
- 239.Wood CE. Development and programming of the hypothalamus-pituitary-adrenal axis. Clin Obstet Gynecol. 2013;56:610–621. doi: 10.1097/GRF.0b013e31829e5b15. [DOI] [PubMed] [Google Scholar]
- 240.Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1069–R1075. doi: 10.1152/ajpregu.00753.2005. [DOI] [PubMed] [Google Scholar]
- 241.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 242.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 243.Woods LL, Morgan TK, Resko JA. Castration fails to prevent prenatally programmed hypertension in male rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1111–R1116. doi: 10.1152/ajpregu.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number and renal function in rats. Am J Physiol Regul IntegrComp Physiol. 1998;275:R1593–R1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]
- 245.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 246.Woods LL, Weeks DA, Rasch R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension. 2001;38:337–342. doi: 10.1161/01.hyp.38.3.337. [DOI] [PubMed] [Google Scholar]
- 247.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 248.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22:215–220. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xiao D, Huang X, Yang S, Zhang L. Estrogen normalizes perinatal nicotine-induced hypertensive responses in adult female rat offspring. Hypertension. 2013;61:1246–1254. doi: 10.1161/HYPERTENSIONAHA.113.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs post-ischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 253.Yan J, Li X, Su R, Zhang K, Yang H. Long-term effects of maternal diabetes on blood pressure and renal function in rat male offspring. PLoS One. 2014;9:e88269. doi: 10.1371/journal.pone.0088269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Young JB. Programming of sympathoadrenal function. Trends Endocrinol Metab. 2002;13:381–385. doi: 10.1016/s1043-2760(02)00661-6. [DOI] [PubMed] [Google Scholar]
- 255.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 256.Zheng S, Rollet M, Pan YX. Protein restriction during gestation alters histone modifications at the glucose transporter 4 (GLUT4) promoter region and induces GLUT4 expression in skeletal muscle of female rat offspring. J Nutr Biochem. 2012;23:1064–1071. doi: 10.1016/j.jnutbio.2011.05.013. [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Boubred F, Saint-Faust M, Buffat C, Ligi I, Grandvuillemin I, Simeoni U. Developmental origins of chronic renal disease: an integrative hypothesis. Int J Nephrol. 2013;2013:346067. doi: 10.1155/2013/346067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatford KL, Simmons RA. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin Obstet Gynecol. 2013;56:520–528. doi: 10.1097/GRF.0b013e31829e5b29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology (Bethesda) 2014;29:122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Burdge GC. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab. 2012;26:667–676. doi: 10.1016/j.beem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta. 2010;31:S40–S46. doi: 10.1016/j.placenta.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- Segovia SA, Vickers MH, Gray C Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975. doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]