Conservation physiology has great potential to help us understand how migratory animals interact with current and future anthropogenic threats. Migration is inherently challenging such that additional stressors derived from altered environments or interaction with human infrastructure or activities could lead to long-term changes to migratory phenotypes.
Keywords: Behaviour, energetics, human impacts, mechanism, movement
Abstract
Migration is a widespread phenomenon among many taxa. This complex behaviour enables animals to exploit many temporally productive and spatially discrete habitats to accrue various fitness benefits (e.g. growth, reproduction, predator avoidance). Human activities and global environmental change represent potential threats to migrating animals (from individuals to species), and research is underway to understand mechanisms that control migration and how migration responds to modern challenges. Focusing on behavioural and physiological aspects of migration can help to provide better understanding, management and conservation of migratory populations. Here, we highlight different physiological, behavioural and biomechanical aspects of animal migration that will help us to understand how migratory animals interact with current and future anthropogenic threats. We are in the early stages of a changing planet, and our understanding of how physiology is linked to the persistence of migratory animals is still developing; therefore, we regard the following questions as being central to the conservation physiology of animal migrations. Will climate change influence the energetic costs of migration? Will shifting temperatures change the annual clocks of migrating animals? Will anthropogenic influences have an effect on orientation during migration? Will increased anthropogenic alteration of migration stopover sites/migration corridors affect the stress physiology of migrating animals? Can physiological knowledge be used to identify strategies for facilitating the movement of animals? Our synthesis reveals that given the inherent challenges of migration, additional stressors derived from altered environments (e.g. climate change, physical habitat alteration, light pollution) or interaction with human infrastructure (e.g. wind or hydrokinetic turbines, dams) or activities (e.g. fisheries) could lead to long-term changes to migratory phenotypes. However, uncertainty remains because of the complexity of biological systems, the inherently dynamic nature of the environment and the scale at which many migrations occur and associated threats operate, necessitating improved integration of physiological approaches to the conservation of migratory animals.
Introduction
Migration is one of nature's most captivating phenomena. Migratory movements can be as vast as the transcontinental treks of African wildebeest or as minute as diel vertical migration of zooplankton within metres of the water surface. Movement is an inextricable component of animal behaviour, but migration is distinct from dispersal or station-keeping behaviours because it is predictable, directional and persistent (Dingle and Drake, 2007), owing to physiological changes that underlie the migratory life stage. Migration is exhibited by every major animal taxon and, ultimately, maximizes survival and reproductive success through the utilization of key habitats, food sources and breeding grounds and/or the avoidance of adverse environmental conditions (Dingle and Drake, 2007). The movement of large numbers of animals from one region to another can benefit ecosystems by linking nutrient sources and increasing local diversity; these factors may increase ecosystem resilience in times of disturbance (Bauer and Hoye, 2014). Conversely, the unique physiological demands of migration may leave migratory species more susceptible to disturbance (Wilcove and Wikelski, 2008).
Life-history phenotypes such as migration are expressed through behaviours exhibited by individuals in response to internal changes to their physiology (Ricklefs and Wikelski, 2002). Physiology therefore plays an important and foundational role in animal migration, and migratory physiology is a mechanistic sub-discipline of migration and movement biology (Bowlin et al., 2010; Dingle, 2014; Jachowski and Singh, 2015). Changes to the central nervous system characterize migration, because migrating animals are undistracted by cues that would otherwise elicit vegetative responses (Dingle, 1996). Moreover, physiological mechanisms control the timing, locomotion and synchronicity of migration, which dictate migratory behaviour and ultimate success (Wingfield et al., 1990). Migration is composed of complex and dynamic interactions among individual genetics, behaviour, physiology, biomechanics and the environment (Dingle, 2006). In addition, migrations are inherently challenging; large-scale movement across complex landscapes requires vast amounts of energy (Wikelski et al., 2003; Bowlin et al., 2005; Bishop et al., 2015). Furthermore, some species conduct migrations without interruption for refuelling, working off fixed energy reserves (Stephens et al., 2009). Given that a failed migration directly affects lifetime fitness of individuals (Dingle, 1980), natural selection has the potential to alter populations and migratory phenotypes rapidly. In some cases, this can lead to changes in population structure, evolutionary bottlenecks, inbreeding depression and extirpation or extinction (Wilcove and Wikelski, 2008), which have broader impacts on animal communities and entire ecosystems.
Over the last several decades, global changes and biodiversity losses have created a challenging landscape for conservation science. Climate change, habitat alteration, species invasions and pollution are altering landscapes and creating new challenges for animals. Migratory species represent a unique challenge because of their high mobility and their reliance on multiple habitats to complete their life history, meaning that they may be subject to multiple and varied threats in different habitats such that predicting and understanding their ability to adapt is difficult (Robinson et al., 2009; Sih et al., 2011; Gienapp, 2010). Conservation is a varied and dynamic science, the goals of which extend beyond simply avoiding extinction risk to understanding and conserving the traits and attributes of species that make them successful (Redford et al., 2011). Novel methodologies and solutions are constantly developing in an effort to achieve conservation objectives, including an increasing synergy between conservation and physiology (Wikelski and Cooke, 2006; Coristine et al., 2014; Lennox and Cooke, 2014). Conservation physiology focuses on understanding and predicting the responses of animals to environmental change and the potential for solving diverse conservation problems using physiological knowledge, approaches and tools (Cooke et al., 2013a,b). Given the importance of physiological mechanisms to animal migration, there are opportunities to implement physiology to enhance our understanding of migratory species and populations as well as develop novel conservation approaches that are informed by animal physiology. Here, we review the physiology of animal migration and demonstrate conservation physiology approaches for future research on human-induced environmental changes focused on key conservation questions where conservation physiology has the potential to play an important role. Although we consider all animal taxa in our review, the conservation physiology of migration literature is disproportionately rich in studies focused on fish and birds, which is reflected to some extent in the coverage below.
Review
Orientation and navigation
The success of migration depends on an animal's ability to orient and navigate along migratory paths and requires physiological mechanisms for taking the best migratory route (Åkesson and Hedenström, 2007; Bauer et al., 2011; Fig. 1). Birds (Mouritsen and Hore, 2012), sea turtles (Lohmann 1991; Luschi et al., 2007), bats (Holland et al., 2006; Tian et al., 2010), salamanders (Phillips and Borland, 1992) and salmon (Putman et al., 2014) are among species that use magnetic signals for orientation (Wiltschko and Wiltschko, 2005; Lohmann et al., 2007, 2008). Cellular mechanisms supporting magnetoreception have not been unequivocally demonstrated to date (Tian et al., 2010; Edelman et al., 2015), but magnetite integrated into sensory tissue has been located in bird beaks (Fleissner et al., 2003), salamander thymoids and turtle heads. Magnetic orientation is usually used to orient at long distances, such as in the open ocean (Putman et al., 2013, 2014). Long-distance navigation can be disturbed by a variety of human developments that create or modify magnetic signatures used by animals. The specific factors that affect animal navigation or homing depend on the navigation techniques used by the animal. There is some evidence that geomagnetic detection by birds can be disrupted by magnetic fields created around cities (Ritz et al., 2004, 2009; Engels et al., 2014). However, evidence that this is occurring in the wild is lacking.
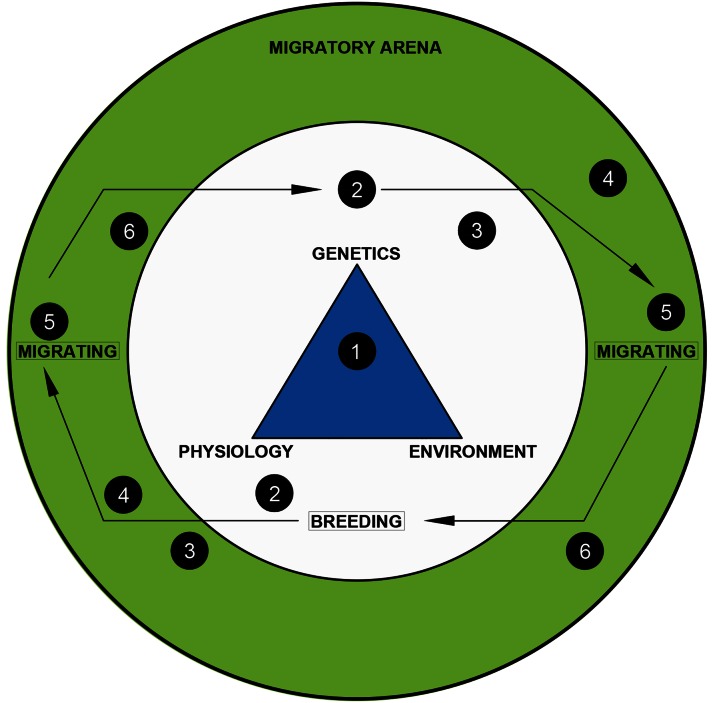
Figure 1:
Migration is a suite of physiological changes that manifest as predictable, persistent, oriented movement of animals between environments in order to exploit seasonal productivity and maximize fitness. Genetics, physiology (including metabolism and condition) and environmental conditions can influence transition to the migratory life stage (1). Prior to entering the migratory arena, animals accumulate fuel, and their bodies often undergo physiological remodelling to reduce the cost of transport during migration, including atrophy of some organs and hypertrophy of exercise muscles or organs (2). The timing of migration is synchronized with distant environmental conditions via hormonal regulation, with an important role being played by melatonin produced by the photosensitive pineal gland in response to changing day lengths (3). Upon departure, animals use a variety of behavioural strategies to maximize the energetic efficiency, including soaring or gliding (4), as well as periodic stopovers to replenish energy stores (5). To find their target habitat, migrating animals have a variety of strategies for orienting, wayfinding and interpreting visual, olfactory and other sensory information from the environment that indicates their proximity to high-quality habitat (6). Once migrating animals reach their target habitat, they exit the migratory arena and resume vegetative behaviour.
Light from the sun and other celestial bodies can be entrained by migrating animals for orientation (Able, 1982). Specifically, animals can detect polarized light from the sun to correct their movement path (Helbig, 1991; Reppert et al., 2004). Artificial lights can distract animals from their movement path (e.g. Dacke et al., 2003; Mazor et al., 2013); for example, beach, street and pathway lighting can entrain hatchling sea turtles, resulting in fewer successful migrations to the sea and reduced recruitment (Tuxbury and Salmon, 2005). Furthermore, seabirds can be disoriented at night when exposed to artificial light sources such as ships, lighthouses and oil and gas platforms, often ending in collision between the bird and the structure (Montevecchi, 2006). Light pollution from buildings, ships, aeroplanes and other structures has the potential to distract and disorient migrating animals and can increase the risk of death via collision or exhaustion when animals follow lights indefinitely (Jones and Francis, 2003; Merkel, 2010).
At close range, migrating animals generally rely on olfactory or visual cues to locate fine-scale areas in a habitat by searching for landmarks or appraising habitat qualities (Lohmann et al., 2008; Ueda, 2012). Experiments disrupting visual and olfactory pathways in migrating Oncorhynchus masou in coastal regions demonstrated a reduced ability to locate and enter natal streams (Ueda et al., 2000). Likewise, sea turtles use water- and airborne olfactory cues, which are believed to provide a source of navigational information throughout migrations (Koch et al., 1969; Lohmann et al., 2008). Turtle species that demonstrate high nesting site fidelity are thought to imprint on chemical gradients from natal grounds to guide reproductive migrations (Hasler and Scholz, 1983; Endres and Lohmann, 2013). Olfactory systems can also detect conspecific pheromones; indeed, red-sided garter snakes (Thamnophis radix parietalis) use these chemical signatures to follow movements of conspecifics to feeding areas and hibernacula and to locate partners during vernal breeding migrations (Lemaster et al., 2001). Alteration of chemical signatures in target habitats can mask or dilute chemical cues, causing animals to lose track of scents and become lost on migration. Acidification of inland waters from acid rain or pollution completely stopped further upstream migration of sockeye salmon (Oncorhynchus nerka; Ikuta et al., 2001). Stormwater runoff from roadways flushes chemicals into rivers, some of which (e.g. copper from brake pads) can impair olfactory sensitivity in coho salmon (Oncorhynchus kisutch) after even temporary exposure (McIntyre et al., 2008), perhaps interfering with navigation. Changes to water flow from redirection of water associated with irrigation, hydroelectric power generation and/or infilling of headwaters for development can redistribute or dilute chemical signatures, reducing the ability of aquatic animals to navigate, make successful migrations and recruit (Sato et al., 2000; Burnett et al., 2014a).
Energetics
Migratory species travelling long distances between habitats require adaptations to optimize energetic output. Endurance during migration is a function of energy availability; therefore, accumulation of fuel is an essential mechanism supporting migration (Fig. 1). Fatty acids are the most efficient fuel source per unit weight and are important for reducing the cost of transport for migrating animals (Butler and Woakes 1990; Jenni and Jenni-Eiermann, 1998; McWilliams et al., 2004; Del Raye et al., 2013). To accumulate fuel, animals can pre-empt migration with hyperphagia, dietary changes and/or increased food assimilation efficiency (Bairlein, 2002; Santra et al., 2008). However, animals preparing to migrate must have access to food of sufficient quality and quantity in order to execute migration; therefore, identification and conservation of key food sources and habitat types is essential (e.g. Alonso-Mejía et al., 1997). When key habitats and food sources are degraded or lost, actions such as supplemental feeding or enrichment may mitigate impacts on migratory populations. To reduce maintenance costs during the migration, atrophy of non-essential organs (e.g. alimentary; Piersma and Lindström, 1997; Piersma et al., 1999) and hypertrophy of locomotory and cardiac muscle (Piersma et al., 1999; King et al., 2015) take place. To compensate for low metabolism, reptiles may circulate thyroxine (Southwood and Avens, 2010) to increase oxygen consumption, heart mass and metabolic enzyme activity, suggesting an endocrine role in facilitating migratory activity (see also Bishop et al., 1995). Summer growing seasons are extending and winters are shortening, which can prolong residence at summering grounds, increase risk of pathogen incubation (e.g. Bartel et al., 2011) and reduce the time available for the essential preparatory process of feeding and establishing fuel stores, resulting in decreased energy available for migration (e.g. beluga whale, Delphinapterus leucas; Bailleul et al., 2012). Ensuring protection of or supplementing existing habitats necessary for animals preparing to migrate might be beneficial for conserving migrants; for example, Masero (2003) found that anthropogenic salinas in Spain provided valuable replacement habitat for shorebirds initiating hyperphagia prior to migration. Increased temperatures result in higher costs of activity during migration; activity in warm temperatures increases cardiac stress and limits distribution of oxygen to tissues (e.g. Pörtner and Farrell, 2008; Eliason et al., 2013). Correspondingly, there is a need to understand better how plasticity and evolution in animals under thermal stress contribute to resilience (Anttila et al., 2014). Rapid dehydration can occur when birds experience warm temperatures, increasing the need for stopover during migration and delaying arrival (McKechnie and Wolf, 2009), potentially necessitating the protection of larger tracts of land that are important for stopover. In fact, some species may become incapable of or lose their will to move when temperatures are high, which can delay migration and result in mismatched timing of arrival relative to peak environmental conditions (e.g. fish: Baisez et al., 2011; Eliason et al., 2013; mammals: Post and Forchhammer, 2008).
Behavioural strategies are combined with physiological mechanisms to limit the cost of transport and maximize distance that it is possible to travel per unit of fuel. During migration, animals exploit wind and ocean currents for conveyance along the migration path (Wikelski et al., 2006). In addition, animals may maintain activity near to but not exceeding their upper aerobic limit to sustain endurance; such findings can contribute to improved design and management of fish passage structures at dams for fish migrating to and from reproductive sites (Burnett et al., 2014b; Silva et al., 2015). Given that it is costly to transport large amounts of fuel along migration, some species interrupt migration to refuel (Alerstam et al., 2003; Dingle and Drake, 2007; Sawyer and Kauffman, 2011). The need to feed during migration makes movement paths somewhat predictable and can allow for protected areas or strategic shipping/aircraft routes. Indeed, patterns of foraging behaviour and corresponding dive physiology of sea turtles moving to and from nesting habitat can allow for better management of shipping operations (e.g. Eckert et al., 1989; Plot et al., 2015). Other animals, such as birds and whales, make stopovers in highly productive feeding areas to refuel. Stopover time is influenced by food availability, fuel load and the rate of fuel deposition (Hedenström and Alerstam, 1997; Eikenaar and Bairlein, 2014). At stopover sites, birds will restore their alimentary organs, feed quickly and then re-atrophy the organs prior to departure. However, other animals spend much of their migration at stopover sites, allowing them to maximize energy intake and migrate synchronously with plant phenology (Mate et al., 2011; Jones et al., 2014; Sawyer et al., 2009). Urbanization and habitat degradation are affecting the availability of the key stopover sites where animals replenish energy stores, and the fragmented habitats can exacerbate stress (Ellis et al., 2012). A lack of suitable stopover habitat can exhaust the energy available for migration (Faaborg et al., 2010; Braithwaite et al., 2015) or might concentrate many individuals at some rare, productive islands in a landscape. Stopover habitats are disappearing, and establishment of alternative stopover sites (Garaita and Arizaga 2015) or the use of supplemental feeding (Jones et al., 2014) may be necessary to conserve migratory animals. Although natural areas offer many benefits to animals, Liu and Swanson (2014) found that birds using modified habitat did not have higher stress than those using natural habitat, indicating the potential for adaptation to the loss of natural stopover habitat. Nonetheless, continued reduction and replacement of natural stopover habitat for migrants could encourage mass aggregations, which can rapidly deplete the available resources and increase disease transmission among individuals (Altizer et al., 2011). For this reason, immunology is an increasingly important aspect of the conservation physiology of migratory animals (e.g. Mallory et al., 2015). Ultimately, understanding the importance of stopover habitats and their role in replenishing fuel provides necessary information for conservation. Bonter et al. (2007) inferred the importance, and thereby the conservation priority, of migratory bird corridor habitats by measuring the body condition of birds and identifying the most important sites. Whitlock et al. (2015) further suggested seasonal protection of key foraging habitats for Pacific bluefin tuna (Thunnus orientalis) based on observations of high energy intake in certain hotspots of the Pacific Ocean.
Timing
The timing of migration exerts a considerable influence on fitness because it affects resource availability. Properly timed migration is important for avoiding unfavourable conditions and arriving at stopover sites and the ultimate destination at an appropriate time when environmental conditions are suitable. Mismatched timing may result in migrations coinciding with depleted food sources at stopover sites or reduced breeding opportunities at the destination (Meltofte, 1985). The timing of migration is somewhat determined by genetics (Berthold, 1996) and circadian/circannual biorhythms; however, the environment exerts a secondary influence on migration (Richardson, 1990; Fig. 1). Together, biotic and abiotic cues combine to control the endocrine system of migratory animals, which regulates the physiological and morphological changes necessary to prepare for departure, locomotion and arrival (Fig. 1).
Preparation for migration begins prior to departure and ensures that energetic reserves are sufficient for the journey (Ramenofsky and Wingfield, 2007). Consequently, departure is influenced in part by fuel reserves and body condition (Brodersen et al., 2008), which cue the release of behaviour-mediating hormones. In insects, juvenile hormone is the principal endocrine cue for initiating migration behaviour (Chapman et al., 2015). In vertebrates, fluxes in melatonin influence preparation for migration. The photosensitive pineal gland entrains information about photoperiod and controls melatonin secretion, which tracks circadian and circannual changes (Bradshaw and Holzapfel, 2007; Tosches et al., 2014; Winkler et al., 2014). In turn, melatonin stimulates androgen production (Crossin et al., 2010), a primary cue for the breeding migration of fish and birds (Wingfield et al., 1990). Among birds, melatonin concentrations regulate migratory restlessness (i.e. Zugunruhe) and a transition to nocturnal activity prior to departure (Gwinner, 1996). In addition to melatonin, fluxes of glucocorticoids, catecholamines, thyroxine, prolactin and leptin contribute to the timing of migration (Cornelius et al., 2013).
An important consequence of relying on fixed signals, such as photoperiod, is that changes to the climate result in the temporal mismatch of key life-history events (e.g. migration, breeding) of migratory animals from suitable environmental conditions (e.g. plant flowering, insect emergence). However, there is evidence of plasticity in the timing of migration because birds can adjust their migration timing, for example to compensate for poor weather (Richardson, 1990; Cochran and Wikelski 2005; Ramenofsky, 2011). Nonetheless, there are limits to such plasticity (DeWitt et al., 1998), and climate change may advance too rapidly for plasticity to compensate (e.g. Gauthier et al., 2013). Reed et al. (2011) predicted that natural advances in the timing of migration are likely to facilitate persistence of salmon, and efforts to manipulate migration timing might prove beneficial, such as by artificially cuing freshwater migration (e.g. by manipulating temperature or flow in rivers). In addition, it remains uncertain how endogenous clocks that are sensitive to photoperiod will respond to extreme and accelerated environmental change rather than to gradual changes (Kumar et al., 2010).
The rate of migration and timing of arrival are controlled by multiple factors, including intraspecific differences, particularly when early arrival confers fitness benefits. The rate of movement is likely to be related to optimization of energy use (e.g. Braithwaite et al., 2015), and stopover timing is probably controlled by circadian rhythms (Bartell and Gwinner, 2005; Sauman et al., 2005). However, birds that arrive in unfavourable conditions can depart and return later (Hahn et al., 2004). Glucocorticoids influence the arrival of migrating birds, with high corticosterone corresponding to earlier arrival (Lobato et al., 2010). The influence of glucocorticoids (e.g. corticosterone, cortisol) is important from a conservation perspective because they fluctuate throughout life to stimulate life-history transitions but can be manipulated by stress, which can potentially interfere with the expression of key life-history events and/or have measurable fitness consequences (Bush and Hayward, 2009). The exact location where individuals terminate migration depends on the species, with some exhibiting strict philopatry (e.g. Lea et al., 2015) and others simply seeking suitable habitat. Understanding the difference is non-trivial because philopatric species have less flexible migrations and can therefore be more susceptible to environmental change. Competition can also influence patterns of settlement that drive selection for the timing of migration (Møller, 1994; Drent et al., 2003). Arrival at breeding grounds coincides with reproductive maturation for most migratory species associated with territoriality, meaning that social groups or flocks of migrants (Ramenofsky and Wingfield, 2007) break, and individuals that were cooperative during migration become antagonistic.
Human interference can also affect the timing of animal migration, an important example of which is because of interactions with fisheries (Raby et al., 2015b). The mobility of many migratory species exposes them to fisheries and, indeed, many of the most important fisheries resources are migratory species, including salmonids, tunas, billfishes and cods. However, many non-teleost migrants are affected by fisheries as bycatch, particularly elasmobranchs, cetaceans, sea turtles and seabirds (Hall et al., 2000; Raby et al., 2011). For bycatch (which includes target species protected by harvest restrictions), interactions with fisheries are stressful and can have lethal and sublethal effects on fitness. Encounters with fisheries can cause physical damage to tissues (e.g. bleeding, barotrauma), reflex impairment from muscular exhaustion or insufficient oxygen delivery to the brain (Raby et al., 2015b), physiological disturbance in the muscle and blood (Cooke et al., 2013a) or external infection. After fisheries interactions, animals may require hours or days to restore homeostasis, during which time migration is delayed and predation risk is enhanced (Raby et al., 2014). In Atlantic salmon (Salmo salar) fisheries, release from recreational fisheries is associated with anomalous downriver movement, migratory delays and shorter migration (Lennox et al., 2015). Such delays or alterations to the migratory schedule can impair fitness of migrating animals, and research is ongoing in fisheries sectors to understand how migrations are affected by these human interactions and interferences. Efforts to reduce the impact of fisheries on aquatic resources rely on physiological knowledge and tools and include strategies for developing assessment protocols (e.g. Raby et al., 2012) and recovery strategies and tools (Farrell et al., 2001; Donaldson et al., 2013; Raby et al., 2015a; Robinson et al., 2015) for animals destined to be released by fishers. However, further efforts are needed to explore revival of non-teleost species that are often affected by fisheries.
Synthesis
Although migration is ‘behaviour’, it is the manifestation of integrated physiological processes in animals (Berthold, 1996; Dingle, 2006). Migration incorporates considerable physiological adaptation as well as genetic, ontogenetic and morphological traits underlying a migratory syndrome (Dingle, 2006). Our overview of the physiological mechanisms controlling migration provides a mechanistic model of migration (see Fig. 1), explaining how and why it occurs, how it is regulated by animals, and some documented and potential changes to migration faced by animals in a changing world. Our physiological model of migration generalizes complex processes that sometimes have considerable variation among taxa but performs reasonably well in summarizing the important physiological variables that regulate migratory behaviour and its fitness end points. However, as a discipline focused on the cellular, biomechanical and biochemical processes of organisms, physiology has the capacity to provide more than information about individuals and can also generate knowledge that informs conservation (Tracy et al. 2006; Wikelski and Cooke, 2006; Cooke et al. 2013b; Madliger et al., 2016; Fig. 2).
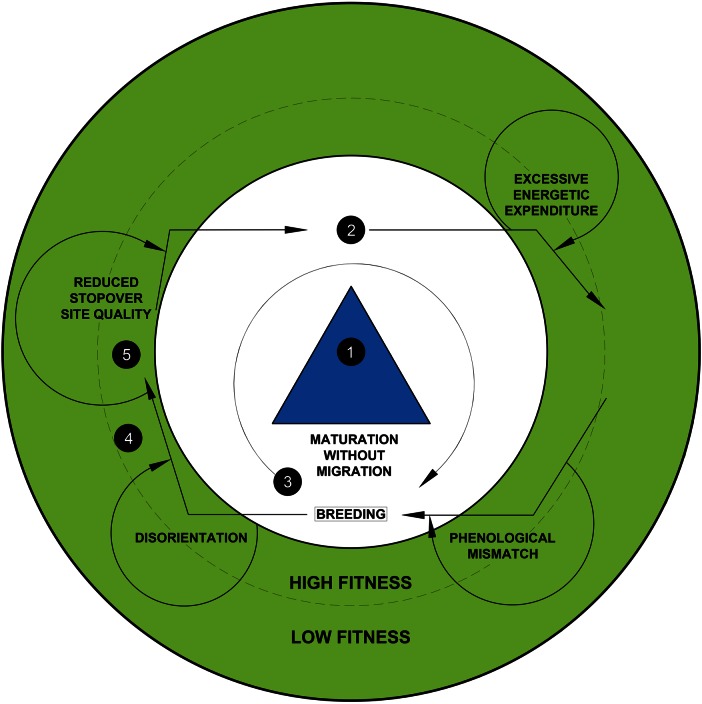
Figure 2:
Migration is a physiologically challenging life-history stage, and there are many adaptations that animals have evolved for optimizing fitness (Fig. 1). Migratory species should optimally move through areas of high fitness in the migratory arena; however, anthropogenic change is altering the path through the migratory arena, which has consequences for lifetime fitness (represented by curved arrows). Some important conservation challenges are highlighted in this figure to demonstrate how they interface with fitness impairment. Conservation agendas must focus on mitigating such challenges to maintain high fitness of individual migrants and conserve migratory phenotypes, populations and species.
Physiology is positioned to directly inform conservation efforts for managing migratory species (e.g. Cooke et al., 2012), including those that are obligatorily migrants as well as partial or facultative migrants (e.g. Chapman et al., 2012). Human activities and global change are altering the migratory arena and changing the balance of costs and benefits associated with migration such that there is the potential for various severe fitness impairments for migrating animals (Fig. 2). In some cases, ecosystem connectivity is threatened by development and sprawl, construction of dams, roads and tall buildings as well as the deterioration of the acoustic environment associated with anthropogenic noise (Tennessen et al., 2014). At temperate latitudes, springtime is advancing, deciduous plants are blooming earlier (Menzel, 2002), rain is replacing snow (Knowles et al., 2006), and resident species are advancing activity and reproduction (Gibbs and Breisch, 2001). In polar zones, sea surface temperatures are rising, ice cover is receding (Parmesan, 2006), primary production is changing (Smol et al., 2005), and atmospheric circulation patterns are altering winter conditions in the Northern hemisphere (e.g. polar vortexes in Eastern North America; Kim et al., 2015). These changes are challenges for animals and particularly for migratory species, which rely on many different habitats and geographical areas to complete their life history. Challenges that arise during migration can manifest as reduced fitness of migratory species (Fig. 2); therefore, the fundamental challenge posed to conservation is to understand and mitigate fitness impairments of migratory species in a changing world. Addressing conservation challenges will increasingly rely on understanding the physiological mechanisms that define migration (e.g. Cooke et al., 2012). There are increasing examples of physiology informing conservation initiatives (Lennox and Cooke, 2014), although the nascence of conservation physiology means that the success stories are limited, but growing (Madliger et al., 2016). Therefore, we foresee considerable potential for migratory physiology to be applied for conservation, and offer some relevant examples.
Some animals navigate using temporally unstable navigational cues, meaning that it is necessary to predict where the animals will be in order to monitor them and conserve critical habitat. Putman et al. (2013) suggested that the direction that sockeye salmon migrate through Queen Charlotte Straight (i.e. entering from the north or south direction) is predictable based on sea surface temperature and geomagnetic field drift. The ability to forecast the timing or direction of migration by understanding the physiological mechanisms used by fish to orient has conservation implications, particularly for fisheries and water resource management. Furthermore, anthropogenic noise can create perturbations in otherwise stable navigational cues, such as the electromagnetic field. Electromagnetic noise is emitted everywhere humans use electronic devices. Inability to orient during migration will decrease the likelihood of survival, increase the energetic cost of migration, delay arrival and, ultimately, impair fitness. Indeed, nocturnally migrating songbird populations are currently in dramatic decline, and the effect of anthropogenic electromagnetic noise on migratory physiology may be an underappreciated factor in their conservation. In a more general sense, the ability to predict migrations can be used to influence the management of humans (e.g. by managing vehicle operations, fisher behaviour, or dam operations or by reducing electromagnetic noise) or, alternatively, to influence the animals themselves (i.e. alter the route taken by migrants).
Using knowledge of the physiological basis for migratory path selection to prevent interactions with barriers is an active area of research for terrestrial and aquatic organisms. Improved understanding of sensory mechanisms (Blumstein and Berger-Tal, 2015) can be used in the development of management strategies, such as deflecting animals away from turbines or barriers using visual (lights), auditory (blasts) or somatosensory (bubble curtains) cues (Noatch and Suski, 2012). Switching to green coloured lights, which exclude short-wavelength red light that may affect cellular mechanisms associated with orientation, has reduced collisions between birds and ships and oilrigs by allowing birds to maintain migratory trajectory (Wiltschko et al., 1993; Poot et al., 2008). Moreover, ultraviolet lights might increase perceptibility of aircraft for the Canada goose (Branta canadensis) and reduce bird strike (Blackwell et al., 2012). In the future, there are further opportunities for lighting to be adapted for guiding migrants away from dangerous areas (e.g. turbines, blasting).
Disappearance of stopover habitat results in energetic depletion, competition for limited resources and disease transmission among migrants concentrated at a few high-quality sites. The exposure of an animal to an earlier, often spatially distinct site may influence reproductive success months later (e.g. Ceriani et al., 2015), thousands of kilometres away, even if the breeding grounds are in pristine condition (known as a carry-over effect; Norris and Taylor, 2006; O'Connor et al., 2014). Unravelling how different stressors influence migratory animals is inherently challenging given the potential for these carry-over effects and the vast distances traversed by many animals (O'Connor and Cooke, 2015), making it necessary to conserve habitat along an entire migratory corridor. Strategically placed artificial stopover sites or areas where supplemental feed is deposited for migrants that are matched with energetically demanding portions of the migration could help to buffer the effects of habitat loss. Amano et al. (2007) simulated the use of supplemental feeding areas to reduce conflicts between migratory birds and agricultural crops but suggested that they would be unsuccessful unless carried out on a large scale. Supplemental feeding of birds and ungulates is common in some regions, and engineering feed to suit the nutritional requirements of migrants could provide a short-term solution for loss of stopover habitat, although in the long-term the preservation, remediation or replacement of stopover habitat is the only viable solution (Smith et al., 2015). Likewise, manipulating water levels in hydroelectric damn drawback areas may influence the extent to which migratory birds can refuel for their migration, although empirical studies of this have not been successful (Wagner et al., 2014).
Mistimed migrations have the potential to reduce the fitness of migratory animals by desynchronizing life-history events (including migration) and phenological processes that support those events, including favourable temperatures, vegetation bloom and insect emergence. It is difficult to use physiological knowledge or tools to counteract the likely effects of mismatched timing, particularly for animals that rely on fixed cues, such as photoperiod (Feder et al., 2010). The ability for animals to adapt their life history to climate change depends on evolutionary responses and phenotypic plasticity (Bradshaw and Holzapfel, 2008). However, one solution has been suggested as a response to climate warming, which is the use of artificial freshets for manipulating the timing of river entry for anadromous fishes (Huntsman, 1942). Once in the river, fish may find microhabitat to buffer high water temperatures; by entering earlier in the spring, they can avoid active migration during high summer temperatures when cardiorespiratory systems become strained to deliver sufficient oxygen (Eliason et al., 2013). Migrating fish may be less prone to significant mortality en route if their migrations are completed early in the season when water temperatures are lower. When migration is sufficiently disturbed that animals can no longer move, facilitated migration has shown promise for maintaining a population of migratory lobster (Green et al., 2010). Although manual transport is not a viable option for most species, it may be possible to facilitate migration of some species, particularly around physical barriers (e.g. around dams using specialized transport trucks; Sigourney et al., 2015; hydraulic pumps, such as the ‘salmon cannon’; Mesa et al., 2013). Moreover, Hartup et al. (2004) suggested that captive-rearing of greater sandhill cranes (Grus canadensis) and then training them to migrate from Wisconsin to Florida using ultralight aircraft was viable on the basis of their normal faecal corticosterone profiles during the assisted migration. Selective breeding of individuals that are adequately adapted to specific changing environmental conditions is a drastic measure that could improve stocks of migratory species via enhancement programmes. For example, there is evidence that different populations or stock complexes of salmon are better equipped for climate change as a result of aerobic acclimation to high water temperatures (Eliason et al., 2011). Stock enhancement programmes have the unique platform to proliferate these physiological phenotypes if extant populations do not sufficiently track climate change naturally. Of course, the broader ecological consequences of such intervention would have to be critically addressed before the implementation of such measures could be considered (e.g. Ford et al., 2015).
Conclusions
In an era of substantial human-induced rapid environmental change, science is increasingly focused on generating solutions to conservation problems (Soulé, 1985). There is imminent concern that climate change will affect migratory species (Robinson et al., 2009), but it is important to recognize that migration is a behaviour that has evolved to cope with extreme environmental variability and has persisted and continued to evolve over millions of years of global change. Indeed, it should be anticipated that an era of continued change would be met with further evolution and adaptation by these migratory species (Visser 2008). As a result, it is somewhat tempting to predict that many mobile species will be able to compensate for changes in environmental conditions by adjusting their migratory strategies via plasticity or microevolution. Already, there are examples of animals adapting their migratory phenotypes to account for climatic changes (Berthold et al., 1992; Able and Belthoff, 1998; Juanes et al., 2004), and models predict further adaptations, including smaller size (Clark et al., 2012) and advanced maturation (i.e. without migration; Morita et al., 2014), to cope with changing fitness landscapes (Fig. 2). In addition, improved conditions for feeding during prolonged temperate summers have the potential to decrease the need for migration of some species (Brodersen et al., 2008).
In the midst of unprecedented change, a comprehensive understanding of how oncoming disturbances will affect ecosystems remains elusive and requires better baseline information about animal physiology. Migratory species are an important point of conservation emphasis given their ecological and economic importance. Madliger et al. (2016) demonstrated that conservation physiology is transitioning from a theoretical discipline to one that is materializing in conservation action, and although success stories for migratory species in the published literature are not yet common (but see Cooke et al., 2012), we anticipate a growing role for this synergy in the conservation of migratory species. However, our review has demonstrated that there are still key knowledge gaps related to conservation physiology of migrating animals and that there is a disproportionate focus on migratory birds and teleost fishes in the conservation physiology literature. These challenges are likely to be due in large part to difficulties in studying highly mobile animals across scales. Tracking, sampling or holding small-bodied insects or large-bodied and cryptic whales to gain insight on mechanisms of enormous, population-scale movements are challenges that must be overcome through the development and implementation of new techniques for gaining physiological insight (see Jachowski and Singh, 2015). Indeed, the complexity of biological systems, the inherent dynamic nature of the environment and the scale at which many migrations occur and associated multiple threats operate complicate links between physiological stressors, stress responses and fitness consequences (Bush and Hayward, 2009). Nonetheless, we submit that further integration of basic and applied physiological research, tools, knowledge and concepts (Blumstein and Berger-Tal 2015; Jachowski and Singh, 2015; Sopinka et al., 2015) with behavioural ecology and conservation science (see Cooke et al., 2014) will be important and necessary for developing and refining strategies to meet conservation and management objectives related to migratory species in a changing world.
Funding
R.J.L. and J.M.C. were supported by the Natural Science and Engineering Research Council of Canada (NSERC) Graduate Fellowships. S.J.C. was supported by NSERC (via a Discovery Grant, Strategic Grants and both NSERC HydroNet and Ocean Tracking Network Canada) and the Canada Research Chairs programme.
References
- Able KP. (1982) Skylight polarization patterns at dusk influence migratory orientation in birds. Nature 299: 550–551. [Google Scholar]
- Able KP, Belthoff JR (1998) Rapid ‘evolution’ of migratory behaviour in the introduced house finch of eastern North America. Proc Biol Sci 265: 2063–2071. [Google Scholar]
- Åkesson S, Hedenström A (2007) How migrants get there: migratory performance and orientation. BioScience 57: 123–133. [Google Scholar]
- Alerstam A, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103: 247–260. [Google Scholar]
- Alonso-Mejía A, Rendon-Salinas E, Montesinos-Patiño E, Brower LP (1997) Use of lipid reserves by monarch butterflies overwintering in Mexico: implications for conservation. Ecol Appl 7: 934–947. [Google Scholar]
- Altizer S, Bartel R, Han BA (2011) Animal migration and infectious disease risk. Science 331: 296–302. [DOI] [PubMed] [Google Scholar]
- Amano T, Ushiyama K, Fujita GO, Higuchi H (2007) Predicting grazing damage by white-fronted geese under different regimes of agricultural management and the physiological consequences for the geese. J Appl Ecol 44: 506–515. [Google Scholar]
- Anttila K, Couturier CS, Øverli Ø, Johnsen A, Marthinsen G, Nilsson GE, Farrell AP (2014) Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat Commun 5: 4252. [DOI] [PubMed] [Google Scholar]
- Bailleul F, Lesage V, Power M, Doidge D, Hammill M (2012) Migration phenology of beluga whales in a changing Arctic. Climate Res 53: 169–178. [Google Scholar]
- Bairlein F. (2002) How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 89: 1–10. [DOI] [PubMed] [Google Scholar]
- Baisez A, Bach JM, Leon C, Parouty T, Terrade R, Hoffmann M, Laffaille P (2011) Migration delays and mortality of adult Atlantic salmon Salmo salar en route to spawning grounds on the River Allier, France. Endanger Species Res 15: 265–270. [Google Scholar]
- Bartel RA, Oberhauser KS, De Roode JC, Altizer SM (2011) Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell PA, Gwinner E (2005) A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J Biol Rhythms 20: 538–549. [DOI] [PubMed] [Google Scholar]
- Bauer S, Hoye BJ (2014) Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344: doi:10.1126/science.1242552. [DOI] [PubMed] [Google Scholar]
- Bauer S, Nolet BA, Giske J, Chapman JW, Åkesson S, Hedenström A, Fryxell JM (2011) Cues and decision rules in animal migration. In Milner-Gulland EJ, Fryxell JM, Sinclair ARE, eds, Animal Migration: a Synthesis. Oxford University Press, Oxford, pp 68–87. [Google Scholar]
- Berthold P. (1996) Control of Bird Migration. Chapman and Hall, London. [Google Scholar]
- Berthold P, Helbig AJ, Mohr G, Querner U (1992) Rapid microevolution of migratory behaviour in a wild bird species. Nature 360: 668–670. [Google Scholar]
- Bishop CM, Butler PJ, Atkinson NM (1995) The effect of elevated levels of thyroxine on the aerobic capacity of locomotor muscles of the tufted duck, Aythya fuligula. J Comp Physiol B 164: 618–621. [Google Scholar]
- Bishop CM, Spivey RJ, Hawkes LA, Batbayar N, Chua B, Frappell PB, Milsom WK, Natsagdorj T, Newman SH, Scott GR et al. (2015) The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347: 250–254. [DOI] [PubMed] [Google Scholar]
- Blackwell BF, DeVault TL, Seamans TW, Lima SL, Baumhardt P, Fernández-Juricic E (2012) Exploiting avian vision with aircraft lighting to reduce bird strikes. J Appl Ecol 49: 758–766. [Google Scholar]
- Blumstein DT, Berger-Tal O (2015) Understanding sensory mechanisms to develop effective conservation and management tools. Curr Opin Behav Sci 6: 13–18. [Google Scholar]
- Bonter DN, Donovan TM, Brooks EW (2007) Daily mass changes in landbirds during migration stopover on the south shore of Lake Ontario. Auk 124: 122–133. [Google Scholar]
- Bowlin MS, Cochran WW, Wikelski MC (2005) Biotelemetry of New World thrushes during migration: physiology, energetics and orientation in the wild. Integr Comp Biol 45: 295–304. [DOI] [PubMed] [Google Scholar]
- Bowlin SM, Bisson IA, Shamoun-Baranes J, Reichard JD, Sapir N, Marra PP, Kunz TH, Wilcove DS, Hedenström A, Guglielmo CG et al. (2010) Grand challenges in migration biology. Integr Comp Biol 50: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38: 1–25. [Google Scholar]
- Bradshaw WE, Holzapfel CM (2008) Genetic response to rapid climate change: it's seasonal timing that matters. Mol Ecol 17: 157–166. [DOI] [PubMed] [Google Scholar]
- Braithwaite JE, Meeuwig JJ, Hipsey MR (2015) Optimal migration energetics of humpback whales and the implications of disturbance. Conserv Physiol 3: doi:10.1093/conphys/cov001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen J, Nilsson PA, Hansson LA, Skov C, Brönmark C (2008) Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 89: 1195–1200. [DOI] [PubMed] [Google Scholar]
- Burnett NJ, Hinch SG, Braun DC, Casselman MT, Middleton CT, Wilson SM, Cooke SJ (2014a) Burst swimming in areas of high flow: delayed consequences of anaerobiosis in wild adult sockeye salmon. Physiol Biochem Zool 87: 587–598. [DOI] [PubMed] [Google Scholar]
- Burnett NJ, Hinch SG, Donaldson MR, Furey NB, Patterson DA, Roscoe DW, Cooke SJ (2014b) Alterations to dam-spill discharge influence sex-specific activity, behaviour and passage success of migrating adult sockeye salmon. Ecohydrology 7: 1094–1104. [Google Scholar]
- Bush DS, Hayward LS (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. [Google Scholar]
- Butler BJ, Woakes AJ (1990) The physiology of bird flight. In Gwinner E, ed., Bird Migration. Springer, Berlin, Germany, pp 300–318. [Google Scholar]
- Ceriani SA, Roth JD, Tucker AD, Evans DR, Addison DS, Sasso CR, Ernhart LM, Weishampel JF (2015) Carry-over effects and foraging ground dynamics of a major loggerhead breeding aggregation. Mar Biol 162: 1955–1968. [Google Scholar]
- Chapman BB, Hulthén K, Brodersen J, Nilsson PA, Skov C, Hansson LA, Brönmark C (2012) Partial migration in fishes: causes and consequences. J Fish Biol 81: 456–478. [DOI] [PubMed] [Google Scholar]
- Chapman JW, Reynolds DR, Wilson K (2015) Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol Lett 18: 287–302. [DOI] [PubMed] [Google Scholar]
- Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ, Hinch SG, Patterson DA, Farrell AP (2012) Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS ONE 7: e39079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WW, Wikelski M (2005) Individual migratory tactics of New World Catharus thrushes. In Greenberg R, eds, Birds of Two Worlds: the Ecology and Evolution of Migration. Johns Hopkins University Press, Baltimore, MD, USA, pp 274–289. [Google Scholar]
- Cooke SJ, Hinch SG, Donaldson MR, Clark TD, Eliason EJ, Crossin GT, Raby GD, Jeffries GD, Jeffries KM, Lapointe M et al. (2012) Conservation physiology in practice: how physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Phil Trans R Soc B Biol Sci 367: 1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Donaldson MR, O'Connor CM, Raby GD, Arlinghaus R, Danylchuk AJ, Hanson KC, Hinch SG, Clark TD, Patterson DA et al. (2013a) The physiological consequences of catch-and-release angling: perspectives on experimental design, interpretation, extrapolation and relevance to stakeholders. Fish Manage Ecol 20: 268–287. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013b) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 7: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Blumstein DT, Buchholz R, Caro T, Fernández-Juricic E, Franklin CE, Metcalfe J, O'Connor CM, St Clair CC, Sutherland WJ et al. (2014) Physiology, behaviour and conservation. Physiol Biochem Zool 87: 1–14. [DOI] [PubMed] [Google Scholar]
- Coristine LE, Robillard CM, Kerr JT, O'Connor CM, Lapointe D, Cooke SJ (2014) A conceptual framework for the emerging discipline of conservation physiology. Conserv Physiol 2: doi:10.1093/conphys/cou033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JM, Boswell T, Jenni-Eiermann S, Breuner CW, Ramenofsky M (2013) Contributions of endocrinology to the migration life history of birds. Gen Comp Endocrinol 190: 47–60. [DOI] [PubMed] [Google Scholar]
- Crossin GT, Hinch SG, Cooke SJ, Patterson DA, Lotto AG, Van Der Kraak G, Zohar Y, Klenke U, Farrell AP (2010) Testing the synergistic effects of GnRH and testosterone on the reproductive physiology of pre-adult pink salmon Oncorhynchus gorbuscha. J Fish Biol 76: 112–128. [DOI] [PubMed] [Google Scholar]
- Dacke M, Nilsson DE, Scholtz CH, Byrne M, Warrant EJ (2003) Animal behaviour: insect orientation to polarized moonlight. Nature 424: 33. [DOI] [PubMed] [Google Scholar]
- Del Raye G, Jorgensen SJ, Krumhansl K, Ezcurra JM, Block BA (2013) Travelling light: white sharks (Carcharodon carcharias) rely on body lipid stores to power ocean-basin scale migration. Proc Biol Sci 280: 20130836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13: 77–81. [DOI] [PubMed] [Google Scholar]
- Dingle H. (1980) Ecology and evolution of migration. In Cauthreaux A, eds, Animal Migration, Orientation, and Navigation. Academic Press, New York, pp 1–101. [Google Scholar]
- Dingle H. (1996) Migration: the Biology of Life on the Move , Ed 1. Oxford University Press, New York. [Google Scholar]
- Dingle H. (2006) Animal migration: is there a common migratory syndrome? J Ornithol 147: 212–220. [Google Scholar]
- Dingle H. (2014) Migration: the Biology of Life on the Move, Ed 2. Oxford University Press, New York. [Google Scholar]
- Dingle H, Drake VA (2007) What is migration? Bioscience 57: 113–121. [Google Scholar]
- Donaldson MR, Raby GD, Nguyen VN, Hinch SG, Patterson DA, Farrell AP, Rudd MA, Thompson LA, O'Connor CM, McConnachie SH et al. (2013) Evaluation of a simple technique for recovering fish from capture stress: integrating physiology, biotelemetry, and social science to solve a conservation problem. Can J Fish Aquat Sci 70: 90–100. [Google Scholar]
- Drent R, Both C, Green M, Madsen J, Piersma T (2003) Pay-offs and penalties of competing migratory schedules. Oikos 103: 274–292. [Google Scholar]
- Eckert SA, Eckert KL, Ponganis P, Kooyman GL (1989) Diving and foraging behavior of leatherback sea turtles (Dermochelys coriacea). Can J Zool 67: 2834–2840. [Google Scholar]
- Edelman NB, Fritz T, Nimpf S, Pichler P, Lauwers M, Hickman RW, Papadaki-Anastasopoulou A, Ushakova L, Heuser T, Resch GP et al. (2015) No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. Proc Natl Acad Sci USA 112: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenaar C, Bairlein F (2014) Food availability and fuel loss predict Zugunruhe. J Ornithol 155: 65–70. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hinch SG, Farrell AP (2013) Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv Physiol 1: doi:10.1093/conphys/cot008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RD, McWhorter TJ, Moron M (2012) Integrating landscape ecology and conservation physiology. Landscape Ecol 27: 1–12. [Google Scholar]
- Endres CS, Lohmann KJ (2013) Detection of coastal mud odors by loggerhead sea turtles: a possible mechanism for sensing nearby land. Mar Biol 160: 2951–2956. [Google Scholar]
- Engels S, Schneider NL, Lefeldt N, Hein CM, Zapka M, Michalik A, Elbers D, Kittel A, Hore PJ, Mouritsen H (2014) Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509: 353–356. [DOI] [PubMed] [Google Scholar]
- Faaborg J, Homes RT, Anders AD, Bildstein KL, Dugger KM, Gauthreaux SA, Heglund P, Hobson KA, Jahn AE, Johnson DH et al. (2010) Recent advances in understanding migration systems of New World land birds. Ecol Monogr 80: 3–48. [Google Scholar]
- Farrell AP, Gallaugher PE, Fraser J, Pike D, Bowering P, Hadwin AK, Parkhouse W, Routledge R (2001) Successful recovery of the physiological status of coho salmon on board a commercial gillnet vessel by means of a newly designed revival box. Can J Fish Aquat Sci 58: 1932–1946. [Google Scholar]
- Feder ME, Garland T, Marden JH, Zera AJ (2010) Locomotion in response to shifting climate zones: not so fast. Annu Rev Physiol 72: 167–190. [DOI] [PubMed] [Google Scholar]
- Fleissner G, Holtkamp-Rötzler E, Hanzlik M, Winklhofer M, Fleissner G, Petersen N, Wiltschko W (2003) Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol 458: 350–360. [DOI] [PubMed] [Google Scholar]
- Ford MJ, Murdoch A, Hughes M (2015) Using parentage analysis to estimate rates of straying and homing in Chinook salmon (Oncorhynchus tshawytscha). Mol Ecol 24: 1109–1121. [DOI] [PubMed] [Google Scholar]
- Garaita R, Arizaga J (2015) The benefits of a constructed lagoon for the conservation of Eurasian Spoonbills (Platalea leucorodia) in a tidal marsh. J Nat Conserv 25: 35–41. [Google Scholar]
- Gauthier G, Bêty J, Cadieux MC, Legagneaux P, Doiron M, Chevallier C, Lai S, Tarroux A, Berteaux D (2013) Long-term monitoring at multiple trophic levels suggests heterogeneity in responses to climate change in the Canadian Arctic tundra. Phil Trans R Soc B Biol Sci 368: 20120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JP, Breisch AR (2001) Climate warming and calling phenology of frogs near Ithaca, New York, 1900–1999. Conserv Biol 15: 1175–1178. [Google Scholar]
- Gienapp P. (2010) Migration. In Candolin U, Wong BB, eds, Behavioural Responses to a Changing World: Mechanisms and Consequences. Oxford University Press, Oxford, UK, pp 80–92. [Google Scholar]
- Green BS, Gardner C, Linnane A, Hawthorne PJ (2010) The good, the bad and the recovery in an assisted migration. PLoS ONE 5: e14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner E. (1996) Circadian and circannual programmes in avian migration. J Exp Biol 199: 39–48. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Sockman KW, Breuner CW, Morton ML (2004) Facultative altitudinal movements by mountain white-crowned sparrows (Zonotrichia leucophrys oriantha) in the Sierra Nevada. Auk 121: 1269–1281. [Google Scholar]
- Hall MA, Alverson DL, Metuzals KI (2000) By-catch: problems and solutions. Mar Poll Bull 41: 204–219. [Google Scholar]
- Hartup BK, Olsen GH, Czekala NM, Paul-Murphy J, Langenberg JA (2004) Levels of fecal corticosterone in sandhill cranes during a human-led migration. J Wildl Dis 40: 267–272. [DOI] [PubMed] [Google Scholar]
- Hasler AD, Scholz AT (1983) Olfactory Imprinting and Homing in Salmon. Springer, Berlin, Germany. [PubMed] [Google Scholar]
- Hedenström A, Alerstam T (1997) Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J Theor Biol 189: 227–234. [DOI] [PubMed] [Google Scholar]
- Helbig AJ. (1991) Dusk orientation of migratory European robins, Erithacus rubecula: the role of sun-related directional information. Anim Behav 41: 313–322. [Google Scholar]
- Holland RA, Thorup K, Vonhof MJ, Cochran WW, Wikelski M (2006) Navigation: bat orientation using Earth's magnetic field. Nature 444: 702–702. [DOI] [PubMed] [Google Scholar]
- Huntsman AG. (1942) Death of salmon and trout with high temperature. J Fish Res Board Can 5: 485–501. [Google Scholar]
- Ikuta K, Munakata A, Aida K, Amano M, Kitamura S (2001) Effects of low pH on upstream migratory behavior in land-locked sockeye salmon Oncorhynchus nerka. Water Air Soil Poll 130: 99–106. [Google Scholar]
- Jachowski DS, Singh NJ (2015) Toward a mechanistic understanding of animal migration: incorporating physiological measurements in the study of moving animals. Conserv Physiol 3: doi:10.1093/conphys/cov035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni L, Jenni-Eiermann (1998) Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29: 521–528. [Google Scholar]
- Jones J, Francis CM (2003) The effects of light characteristics on avian mortality at lighthouses. J Avian Biol 34: 328–333. [Google Scholar]
- Jones JD, Kauffman MJ, Monteith KL, Scurlock BM, Albeke SE, Cross PC (2014) Supplemental feeding alters migration of a temperate ungulate. Ecol Appl 24: 1769–1779. [DOI] [PubMed] [Google Scholar]
- Juanes F, Gephard S, Beland KF (2004) Long-term changes in migration timing of adult Atlantic salmon (Salmo salar) at the southern edge of the species distribution. Can J Fish Aquat Sci 61: 2392–2400. [Google Scholar]
- Kim BM, Son SW, Min SK, Jeong JH, Kim SJ, Zhang X, Shim T, Yoon JH (2015) Weakening of the stratospheric polar vortex by Arctic sea-ice loss. Nat Commun 5: doi:10.1038/ncomms5646 [DOI] [PubMed] [Google Scholar]
- King MO, Zhang Y, Carter T, Johnson J, Harmon E, Swanson DL (2015) Phenotypic flexibility of skeletal muscle and heart masses and expression of myostatin and tolloid-like proteinases in migrating passerine birds. J Comp Physiol B 185: 333–342. [DOI] [PubMed] [Google Scholar]
- Knowles N, Dettinger MD, Cayan DR (2006) Trends in snowfall versus rainfall in the western United States. J Climate 19: 4545–4559. [Google Scholar]
- Koch AL, Carr A, Ehrenfeld DW (1969) The problem of open-sea navigation: the migration of the green turtle to Ascension Island. J Theor Biol 22: 163–179. [DOI] [PubMed] [Google Scholar]
- Kumar V, Wingfield JC, Dawson A, Ramenofsky M, Rani S, Bartell P (2010) Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol Biochem Zool 83: 827–835. [DOI] [PubMed] [Google Scholar]
- Lea JS, Wetherbee BM, Queiroz N, Burnie N, Aming C, Sousa LL, Mucientes GR, Humphries NE, Harvey GM, Harvey GM et al. (2015) Repeated, long-distance migrations by a philopatric predator targeting highly contrasting ecosystems. Sci Rep 5: doi:10.1038/srep11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaster MP, Moore IT, Mason RT (2001) Conspecific trailing behaviour of red-sided garter snakes, Thamnophis sirtalis parietalis, in the natural environment. Anim Behav 61: 827–833. [Google Scholar]
- Lennox R, Cooke SJ (2014) State of the interface between conservation and physiology: a bibliometric analysis. Conserv Physiol 2: doi:10.1093/conphys/cou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox RJ, Uglem I, Solem Ø, Thorstad EB, Havn T, Næsje T, Whoriskey FG, Cooke SJ (2015) Does catch-and-release angling alter the behaviour and fate of adult Atlantic salmon during upriver migration? Trans Am Fish Soc 144: 400–409. [Google Scholar]
- Liu M, Swanson DL (2014) Stress physiology of migrant birds during stopover in natural and anthropogenic woodland habitats of the Northern Prairie region. Conserv Physiol 2: doi:10.1093/conphys/cou046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato E, Moreno J, Merino S, Morales J, Tomás G, Martínez J, Vasquez RA, Kuchar A, Mostl E, Osorno JL (2010) Arrival date and territorial behavior are associated with corticosterone metabolite levels in a migratory bird. J Ornithol 151: 587–597. [Google Scholar]
- Lohmann KJ. (1991) Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J Exp Biol 155: 37–49. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Lohmann CM, Putman NF (2007) Magnetic maps in animals: nature's GPS. J Exp Biol 210: 3697–3705. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Lohmann CM, Endres CS (2008) The sensory ecology of ocean navigation. J Exp Biol 211: 1719–1728. [DOI] [PubMed] [Google Scholar]
- Luschi P, Benhamou S, Girard C, Ciccione S, Roos D, Sudre J, Benvenuti S (2007) Marine turtles use geomagnetic cues during open-sea homing. Curr Biol 17: 126–133. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Baldwin DH, Meador JP, Scholz NL (2008) Chemosensory deprivation in juvenile coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ Sci Technol 42: 1352–1358. [DOI] [PubMed] [Google Scholar]
- McKechnie AE, Wolf BO (2009) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 11: rsbl20090702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams SR, Guglielmo C, Pierce B, Klaassen M (2004) Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35: 377–393. [Google Scholar]
- Madliger CL, Cooke SJ, Crespi EJ, Funka JL, Hultines KR, Hunt KE, Rohr JR, Sinclair BJ, Suski CD, Willis CKR et al. (2016) Success stories and emerging themes in conservation physiology. Conserv Physiol 4: doi:10.1093/conphys/cov057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory ML, Little CM, Boyd ES, Ballard J, Elliott KH, Gilchrist HG, Hipfner JM, Petersen A, Shutler D (2015) Leucocyte profiles of Arctic marine birds: correlates of migration and breeding phenology. Conserv Physiol 3: doi:10.1093/conphys/cov028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masero JA. (2003) Assessing alternative anthropogenic habitats for conserving waterbirds: salinas as buffer areas against the impact of natural habitat loss for shorebirds. Biodivers Conserv 12: 1157–1173. [Google Scholar]
- Mate BR, Best PB, Lagerquist BA, Winsor MH (2011) Coastal, offshore, and migratory movements of South African right whales revealed by satellite telemetry. Mar Mamm Sci 27: 455–476. [Google Scholar]
- Mazor T, Levin N, Possingham JP, Levy Y, Rocchini D, Richardson AJ, Kark S (2013) Can satellite-based night lights be used for conservation? The case of nesting sea turtles in the Mediterranean. Biol Conserv 159: 63–72. [Google Scholar]
- Meltofte H. (1985) Populations and breeding schedules of waders, Charadrii, in high arctic Greenland. Meddelelser om Grønland (Monographs on Greenland, Bioscience Series, Vol. 16). pp. 1–44. 16: 1-43. [Google Scholar]
- Menzel A. (2002) Phenology: its importance to the global change community. Clim Chang 54: 379–385. [Google Scholar]
- Merkel FR. (2010) Light-induced bird strikes on vessels in Southwest Greenland. Pinngortitaleriffirk, Greenland: Greenland Institute of Natural Resources Technical Report No. 84; 26 pp. [Google Scholar]
- Mesa MG, Gee LP, Weiland LK, Christiansen HE (2013) Physiological responses of adult rainbow trout experimentally released through a unique fish conveyance device. N Am J Fish Manage 33: 1179–1183. [Google Scholar]
- Møller AP. (1994) Phenotype-dependent arrival time and its consequences in a migratory bird. Behav Ecol Sociobiol 35: 115–122. [Google Scholar]
- Montevecchi WA. (2006) Influences of artificial light on marine birds. In Rich C, Longcore T, eds, Ecological Consequences of Artificial Night Lighting. Springer, Berlin, Germany, pp 94–113. [Google Scholar]
- Morita K, Tamate T, Kuroki M, Nagasawa T (2014) Temperature-dependent variation in alternative migratory tactics and its implications for fitness and population dynamics in a salmonid fish. J Anim Ecol 83: 1268–1278. [DOI] [PubMed] [Google Scholar]
- Mouritsen H, Hore PJ (2012) The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr Opin Neurobiol 22: 343–352. [DOI] [PubMed] [Google Scholar]
- Noatch MR, Suski CD (2012) Non-physical barriers to deter fish movements. Environ Rev 20: 71–82. [Google Scholar]
- Norris DR, Taylor CM (2006) Predicting the consequences of carry-over effects for migratory populations. Biol Lett 2: 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Cooke SJ (2015) Ecological carryover effects complicate conservation. Ambio 44: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Norris DR, Crossin GT, Cooke SJ (2014) Biological carryover effects: linking common concepts and mechanisms in ecology and evolution. Ecosphere 5: art28 doi:10.1890/ES13-00388.1. [Google Scholar]
- Parmesan C. (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37: 637–669. [Google Scholar]
- Phillips JB, Borland SC (1992) Behavioural evidence for use of a light-dependent magnetoreception mechanism by a vertebrate. Nature 359: 142–144. [Google Scholar]
- Piersma T, Lindström Å (1997) Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol Evol 12: 134–138. [DOI] [PubMed] [Google Scholar]
- Piersma T, Gudmundsson GA, Lilliendahl K (1999) Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Phys Biochem Zool 72: 405–415. [DOI] [PubMed] [Google Scholar]
- Plot V, de Thoisy B, George J-Y (2015) Dispersal and dive patterns during the post-nesting migration of olive ridley turtles from French Guiana. Endanger Species Res 26: 221–234. [Google Scholar]
- Poot H, Ens BJ, de Vries H, Donners MA, Wernand MR, Marquenie JM (2008) Green light for nocturnally migrating birds. Ecol Soc 13: 47. [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Post E, Forchhammer MC (2008) Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil Trans R Soc B Biol Sci 363: 2367–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman NF, Lohmann KJ, Putman EM, Quinn TP, Klimley AP, Noakes DL (2013) Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr Biol 23: 312–316. [DOI] [PubMed] [Google Scholar]
- Putman NF, Jenkins ES, Michielsens CGJ, Noakes DLG (2014) Geomagnetic imprinting predicts spatio-temporal variation in homing migration of pink and sockeye salmon. J Roy Soc Interface 11: doi:10.1098/rsif.2014.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby GD, Colotelo AH, Blouin-Demers G, Cooke SJ (2011) Freshwater commercial bycatch: an understated conservation problem. BioScience 61: 271–280. [Google Scholar]
- Raby GD, Donaldson MR, Hinch SG, Patterson DA, Lotto AG, Robichaud D, English KK, Willmore WG, Farrell AP, Davis MW et al. (2012) Validation of reflex indicators for measuring vitality and predicting the delayed mortality of wild coho salmon bycatch released from fishing gears. J Appl Ecol 49: 90–98. [Google Scholar]
- Raby GD, Packer JR, Danylchuk AJ, Cooke SJ (2014) The understudied and underappreciated role of predation in the mortality of fish released from fishing gears. Fish Fish 15: 489–505. [Google Scholar]
- Raby GD, Wilson SM, Patterson DA, Hinch SG, Clark TD, Farrell AP, Cooke SJ (2015a) A physiological comparison of three techniques for reviving sockeye salmon exposed to a severe capture stressor during upriver migration. Conserv Physiol 3: doi:10.1093/conphys/cov015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby GD, Hinch SG, Patterson DA, Hills JA, Thompson LA, Cooke SJ (2015b) Mechanisms to explain purse seine bycatch mortality of coho salmon. Ecol Appl 25: 1757–1775. [DOI] [PubMed] [Google Scholar]
- Ramenofsky M. (2011) Reconsidering the role of photoperiod in relation to effects of precipitation and food availability on spring departure of a migratory bird. Proc Biol Sci 282: doi:10.1098/rspb.2011.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramenofsky M, Wingfield JC (2007) Regulation of migration. BioScience 57: 135–143. [Google Scholar]
- Redford KH, Amato G, Baillie J, Beldomenico P, Bennett EL, Clum N, Cook R, Fonseca G, Hedges S, Launay F et al. (2011) What does it mean to successfully conserve a (vertebrate) species? BioScience 61: 39–48. [Google Scholar]
- Reed TE, Schindler DE, Hague MJ, Patterson DA, Meir E, Waples RS, Hinch SG (2011) Time to evolve? Potential evolutionary responses of Fraser River sockeye salmon to climate change and effects on persistence. PLoS ONE 6: e20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Zhu H, White RH (2004) Polarized light helps monarch butterflies navigate. Curr Biol 14: 155–158. [DOI] [PubMed] [Google Scholar]
- Richardson WJ. (1990) Timing of bird migration in relation to weather: updated review. In Gwinner E, eds, Bird Migration. Springer, Berlin, Germany, pp 78–101. [Google Scholar]
- Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17: 462–468. [Google Scholar]
- Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltshko W (2004) Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429: 177–180. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, Thalau P, Timmel CR, Wiltschko W (2009) Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J 96: 3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KA, Hinch SG, Raby GD, Donaldson MR, Robichaud D, Patterson DA, Cooke SJ (2015) Influence of postcapture ventilation assistance on migration success of adult sockeye salmon following capture and release. Trans Am Fish Soc 144: 693–704. [Google Scholar]
- Robinson RA, Crick HQ, Learmonth JA, Maclean I, Thomas CD, Bairlein F, Forchhammer MC, Francis CM, Gill JA, Bodley BJ et al. (2009) Travelling through a warming world: climate change and migratory species. Endanger Species Res 7: 87–99. [Google Scholar]
- Santra AK, Pan S, Samanta AK, Das S, Halder S (2008) Nutritional status of forage plants and their use by wild elephants in South West Bengal, India. Tropic Ecol 49: 251–257. [Google Scholar]
- Sato K, Shoji T, Ueda H (2000) Olfactory discriminating ability of lacustrine sockeye and masu salmon in various freshwaters. Zool Sci 17: 313–317. [DOI] [PubMed] [Google Scholar]
- Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, Stalleicken J, Yuan Q, Casselman A, Reppert SM (2005) Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 46: 457–467. [DOI] [PubMed] [Google Scholar]
- Sawyer H, Kauffman MJ (2011) Stopover ecology of a migratory ungulate. J Anim Ecol 80: 1078–1087. [DOI] [PubMed] [Google Scholar]
- Sawyer H, Kauffman MJ, Nielson RM, Horne JS (2009) Identifying and prioritizing ungulate migration routes for landscape-level conservation. Ecol Appl 19: 2016–2025. [DOI] [PubMed] [Google Scholar]
- Sigourney DB, Zydlewski JD, Hughes E, Cox O (2015) Transport, dam passage, and size selection of adult Atlantic Salmon in the Penobscot River, Maine. N Am J Fish Manage 35: 1164–1176. [Google Scholar]
- Sih A, Ferrari MC, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4: 367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AT, Hatry C, Thiem JD, Gutowsky LF, Hatin D, Zhu D, Dawson JW, Katopodis C, Cooke SJ (2015) Behaviour and locomotor activity of a migratory catostomid during fishway passage. PLoS ONE 10: e0123051 doi:10.1371/journal.pone.0123051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Miller AC, Merchant CR, Sankoh AF (2015) Local site variation in stopover physiology of migrating songbirds near the south shore of Lake Ontario is linked to fruit availability and quality. Conserv Physiol 3: doi:10.1093/conphys/cov036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smol JP, Wolfe AP, Birks HJB, Douglas MS, Jones VJ, Korhola A, Pienitz R, Ruhland K, Sorvari S, Antoniades D et al. (2005) Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA 102: 4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopinka NM, Patterson LD, Redfern JC, Pleizier NK, Belanger CB, Midwood JD, Crossin GT, Cooke SJ (2015) Manipulating glucocorticoids in wild animals: basic and applied perspectives. Conserv Physiol 3: doi:10.1093/conphys/cov031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulé ME. (1985) What is conservation biology? A new synthetic discipline addresses the dynamics and problems of perturbed species, communities, and ecosystems. BioScience 35: 727–734. [Google Scholar]
- Southwood A, Avens L (2010) Physiological, behavioral, and ecological aspects of migration in reptiles. J Comp Physiol B 180: 1–23. [DOI] [PubMed] [Google Scholar]
- Stephens PA, Boyd IL, McNamara JM, Houston AI (2009) Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90: 2057–2067. [DOI] [PubMed] [Google Scholar]
- Tennessen JB, Parks SE, Langkilde T (2014) Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs. Conserv Physiol 2: doi:10.1093/conphys/cou032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Lin W, Zhang S, Pan Y (2010) Bat head contains soft magnetic particles: evidence from magnetism. Bioelectromagnetics 31: 499–503. [DOI] [PubMed] [Google Scholar]
- Tosches MA, Bucher D, Vopalensky P, Arendt D (2014) Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy CR, Nussear KE, Esque TC, Dean-Bradley K, Tracy CR, DeFalco LA, Castle KT, Zimmerman LC, Espinoza RE, Barber AM (2006) The importance of physiological ecology in conservation biology. Integr Comp Biol 46: 1191–1205. [DOI] [PubMed] [Google Scholar]
- Tuxbury SM, Salmon M (2005) Competitive interactions between artificial lighting and natural cues during seafinding by hatching marine turtles. Biol Conserv 121: 311–316. [Google Scholar]
- Ueda H. (2012) Physiological mechanisms of imprinting and homing migration in Pacific salmon Oncorhynchus spp. J Fish Biol 81: 543–558. [DOI] [PubMed] [Google Scholar]
- Ueda H, Leonard JBK, Naito Y (2000) Physiological biotelemetry research on the homing migration of salmonid fishes. In Moore A, Russell I, eds, Advances in Fish Telemetry. Crown Copyright, Lowestoft, UK, pp 89–97. [Google Scholar]
- Visser ME. (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc Biol Sci 275: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DN, Green DJ, Pavlik M, Cooper J, Williams TD (2014) Physiological assessment of the effects of changing water levels associated with reservoir management on fattening rates of neotropical migrants at a stopover site. Conserv Physiol 2: doi:10.1093/conphys/cou017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock RE, Hazen EL, Walli A, Farwell C, Bograd SJ, Foley DG, Castleton M, Block BA (2015) Direct quantification of energy intake in an apex marine predator suggests physiology is a key driver of migrations. Sci Adv 18: e1400270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser (2003) Avian metabolism: costs of migration in free-flying songbirds. Nature 423: 704. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML (2006) Simple rules guide dragonfly migration. Biol Lett 2: 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove DS, Wikelski M (2008) Going, going, gone: is animal migration disappearing. PLoS Biol 6: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R (2005) Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 675–693. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Munro U, Ford H, Wiltschko R (1993) Red light disrupts magnetic orientation of migratory birds. Nature 364: 525–527. [Google Scholar]
- Wingfield JC, Schwabl H, Mattocks PW Jr (1990) Endocrine mechanisms of migration. In Gwinner E, eds, Bird Migration. Springer, Berlin, Germany, pp 232–256. [Google Scholar]
- Winkler DW, Jørgensen C, Both C, Houston AI, McNamara JM, Levey DJ, Partecke J, Fudickar A, Kacelnik A, Roshier D et al. (2014) Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Movement Ecol 2: 10. [Google Scholar]