Abstract
Background:
Findings of substantial remaining morbidity in treated major depressive disorder (MDD) led us to review controlled trials of treatments aimed at preventing early relapses or later recurrences in adults diagnosed with MDD to summarize available data and to guide further research.
Methods:
Reports (n = 97) were identified through systematic, computerized literature searching up to February 2015. Treatment versus control outcomes were summarized by random-effects meta-analyses.
Results:
In 45 reports of 72 trials (n = 14 450 subjects) lasting 33.4 weeks, antidepressants were more effective than placebos in preventing relapses (response rates [RR] = 1.90, confidence interval [CI]: 1.73–2.08; NNT = 4.4; p < 0.0001). In 35 reports of 37 trials (n = 7253) lasting 27.0 months, antidepressants were effective in preventing recurrences (RR = 2.03, CI 1.80–2.28; NNT = 3.8; p < 0.0001), with minor differences among drug types. In 17 reports of 22 trials (n = 1 969) lasting 23.7 months, psychosocial interventions yielded inconsistent or inconclusive results.
Conclusions:
Despite evidence of the efficacy of drug treatment compared to placebos or other controls, the findings further underscore the substantial, unresolved morbidity in treated MDD patients and strongly encourage further evaluations of specific, improved individual and combination therapies (pharmacological and psychological) conducted over longer times, as well as identifying clinical predictors of positive or unfavorable responses and of intolerability of long-term treatments in MDD.
Keywords: Antidepressants, depression, major depression, psychotherapy, recurrence, relapse
Introduction
Major depressive disorder (MDD) and other forms of clinical depression are characterized by persistent low mood with associated changes in behavior, cognition, sleep and appetite, impaired social and occupational functioning, increased risk of self-harm or suicide, and increased mortality due to co-occurring general medical disorders. Based on World Health Organization data (2012), there is an approximately 10% lifetime international prevalence of depression, with perhaps 350 million persons currently suffering from this leading worldwide illness burden. In 2000, adult depression cost the UK approximately £9 billion, with an estimated 110 million working days lost (Thomas and Morris, 2003). In the USA in the same year, depression accounted for costs totalling over $80 billion for treatment and disability (Greenburg et al., 2003). These considerations indicate that depression has a major, international, clinical, public health, and economic impact.
Currently, pharmacological and non-pharmacological treatments are relatively well established as having at least moderate efficacy for the short-term treatment of acute major depression (Undurraga and Baldessarini, 2012; Baldessarini, 2013; Bauer et al., 2015). Effective antidepressants include selective serotonin (SSRIs) and serotonin-norepinephrine re-uptake inhibitors (SNRIs), a growing number of other types of modern antidepressants (such as bupropion and mirtazapine), older tricyclic (TCA) and monoamine oxidase inhibitor (MAOI) antidepressants, electroconvulsive therapy (ECT; Baldessarini, 2013; Bauer et al., 2015), and a variety of psychotherapies, including prevalent cognitive-behavioral therapy (Bondolfi et al., 2010; Sheets et al., 2013).
Despite generally effective short-term treatments for acute episodes of major depression, many patients experience relapses (early return of symptoms within the expected duration of a current episode, of perhaps 3–12 months) or later recurrences (new episodes) following initial short-term improvement or remission (Georgotas 1985; Ferreri et al., 1997; Forte et al., 2015). Recurrence rates are over 85% within a decade of an index depressive episode, and average approximately 50% or more within six months of apparent clinical remission if the initially-effective treatment was not continued (Baldessarini, 2013). The median time to a new episode with antidepressant treatment continued has averaged approximately 40 months, compared to just over 1 year if treatment was discontinued (Baldessarini, 2013; Rosenthal et al., 2013). With such high rates of relapse and recurrence, it has become usual practice to continue initially-effective antidepressant treatments for at least 6–12 months following apparent clinical remission of acute depression, and often longer for patients who have experienced multiple recurrences (Baldessarini, 2013). Nevertheless, clinically-treated patients diagnosed with MDD in long-term observational studies are depressed 40–50% of the time at follow-up (Forte et al., 2015).
Earlier reviews and meta-analyses and recent treatment guidelines for long-term treatment of MDD are generally supportive of treatments continued for several months as well as more than a year, particularly pharmacotherapies (Geddes et al., 2003; Lam et al., 2009; Pilling et al., 2009; Williams et al., 2009; Glue et al., 2010; Bauer et al., 2015). In view of the clinical importance of evaluating the effectiveness of continued and long-term treatment to prevent relapses and recurrences of major depression, we updated and extended previous reviews of portions of available research findings by separately examining reports of research trials involving continuation (up to 12 months following initial treatment) or long-term treatments (more than a year), as well as long-term trials of psychotherapy, all aimed at prophylaxis or prevention of relapses and recurrences of depressive illness.
Methods
Search Strategy and Selection Criteria
References for this review were identified through searches of the National Centre of Biotechnology Information, Pubmed/Medline, Scieverse, SciDirect, and Web-of-Science databases for reports published up to February 2015. Search terms were combinations of: adult; depress; major depress; long-term; prevent; relapse; recurrence; treatment; and generic names of specific antidepressant drugs. We reviewed reports identified in these searches and in relevant references cited in identified articles.
Inclusion/Exclusion Criteria
A report was selected for inclusion if: (a) it examined relapse or recurrence of major depressive disorder in adults treated with pharmacological or non-pharmacological means; and (b) it was reported in English. Reports were excluded if they concerned: (a) bipolar depression; (b) seasonal depression; (c) juvenile depression; or (d) studied only adverse events or costs.
Data Extraction
For each included report two investigators (Drs J Sim and Lau) independently extracted relevant data, including numbers and types of subjects, study design, treatments and controls, definitions of relapse and recurrence, nominal duration of treatment, dropout rates, and outcomes as rates of relapse or recurrence. Discrepancies were resolved by discussions and consensus amongst all team members.
Data Analysis
Extracted data were organized in digitalized spreadsheets and then summarized in tables to guide assessments. We subjected comparisons of active and control treatments to random-effects meta-analysis to obtain relative response rates (RRs) for active treatments versus controls, with their 95% confidence intervals (CIs), as well as rate differences (RDs) and their reciprocals (1/RD = number-needed-to-treat [NNT]), both with CIs. Averaged data are presented as means ± standard deviation (SD) or with CI. Statistical analyses employed data spreadsheets based on Statview.5 (SAS Institute) and Stata.13 statistical software (StataCorp.).
Results
Retrieved Studies
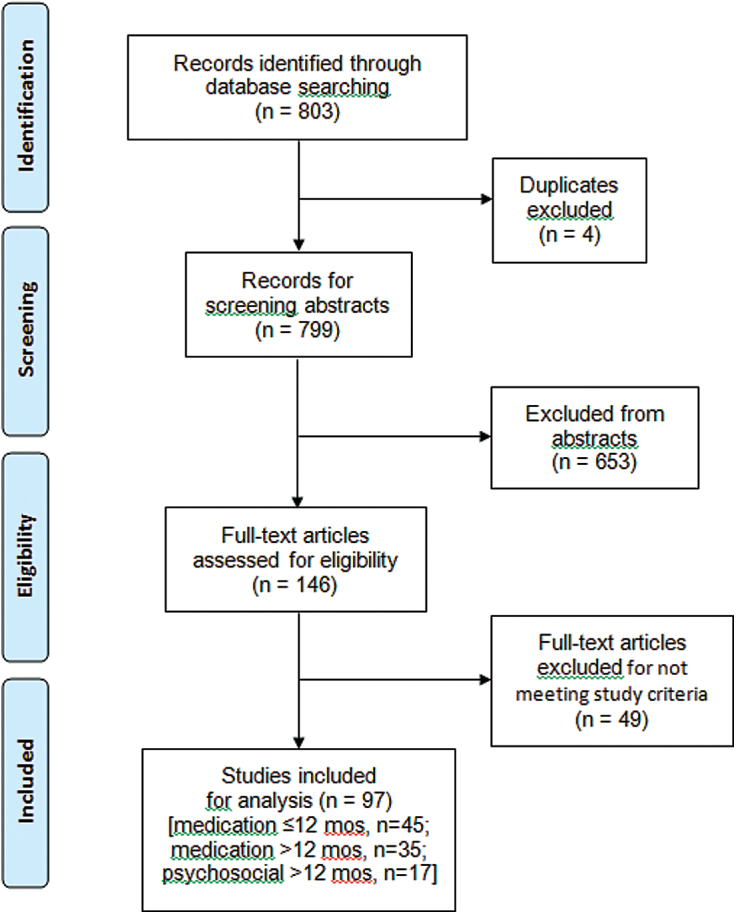
Initially, we identified 803 candidate reports, of which four represented second reports of the same study; of the 799 remaining citations, we excluded 653 as not meeting study inclusion/exclusion criteria. The remaining 146 full reports were reviewed in detail; 49 were excluded for having participants with diagnoses other than MDD, involving participants below 18 years old, or not specifying subject numbers and trial durations. This process left 97 reports for inclusion; two provided data for more than one of the following categories (Hollon et al., 2005; Segal et al., 2010). Reported study categories were: (a) intermediate-term (1–12 months) continuation trials of antidepressant treatment (n = 45); (b) long-term (≥12 months) trials of antidepressants (n = 35); and (c) long-term trials of psychosocial interventions (n = 17; supplementary Tables S1–S3). The screening and selection process is summarized in a Prisma flow chart (Figure 1).
Figure 1.
Flow chart of study selection process: 803 reports screened, 146 reviewed in detail, and 97 included for analysis, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations (http://www.prisma-statement.org/statement.htm).
Continuation Trials of Antidepressants
We identified 45 reports involving 72 placebo-controlled trials of different antidepressants, representing a total of 14 450 participants (Tables 1 and S1). Of these, four studies included two active treatments or two doses of one antidepressant versus a single placebo-treated group (Lauritzen et al., 1996; Montgomery et al., 1998; Schimdt et al., 2000; Sackeim et al., 2001). Observed relapse rates in each randomized treatment arm of the trials, and other salient characteristics of each study, are shown in Table S1.
Table 1.
Meta-Analyses of Placebo-Controlled Trials of Continuation Treatments (≤12 months) for Major Depression
| Trial | Treatment | Subjects | RR (95% CI) | NNT (95% CI) | Weight (%) |
|---|---|---|---|---|---|
| Mindham et al., 1973 | Amitriptyline | 61 | 2.83 (1.46–5.49) | 2.3 (1.5–4.9) | 1.15 |
| Mindham et al., 1973 | Imipramine | 31 | 1.07 (0.25–4.49) | 80 (3.4 to >100) | 0.36 |
| Klerman et al., 1974 | Amitriptyline | 50 | 2.33 (0.68–8.01) | 6.2 (2.6 to >100) | 0.46 |
| Coppen et al., 1978 | Amitriptyline | 32 | 1.56 (0.45–5.43) | 8.9 (2.4 to >100) | 0.45 |
| Stein et al., 1980 | Amitriptyline | 55 | 2.23 (1.22–4.06) | 2.6 (1.6–7.3) | 1.28 |
| Van Praag and De Haan, 1980 | Clomipramine | 20 | 2.66 (0.98–7.22) | 2.0 (1.1–8.1) | 0.65 |
| Bialos et al., 1982 | Amitriptyline | 17 | 12.4 (0.83–184) | 1.2 (0.9–2.0) | 0.11 |
| Kane et al., 1982 | Imipramine+Lithium | 14 | 8.00 (1.28–50.0) | 1.1 (0.8–1.8) | 0.23 |
| Kane et al., 1982 | Lithium | 13 | 3.50 (1.08–11.3) | 1.4 (0.9–2.9) | 0.50 |
| Kane et al., 1982 | Imipramine | 12 | 1.50 (0.85–2.64) | 3.0 (1.4 to >100) | 1.37 |
| Davidson and Raft, 1984 | Phenelzine | 15 | 6.12 (0.98–38.3) | 1.4 (0.9–2.6) | 0.23 |
| Lendresse et al., 1985 | Nomifensine | 143 | 2.03 (1.17–3.52) | 5.0 (2.9–18) | 1.40 |
| Cook et al., 1986 | Tricyclics | 15 | 4.90 (0.30–80.7) | 3.0 (1.5 to >100) | 0.10 |
| Harrison et al., 1986 | Phenelzine | 12 | 5.00 (0.87–28.9) | 1.2 (0.8–2.4) | 0.25 |
| Montgomery et al., 1988 | Fluoxetine | 182 | 2.20 (1.49–3.25) | 3.2 (2.2–5.6) | 1.88 |
| Georgotas et al., 1989 | Nortriptyline | 36 | 1.21 (0.67–2.17) | 8.8 (2.2 to >100) | 1.32 |
| Georgotas et al., 1989 | Phenelzine | 38 | 4.89 (1.30–18.4) | 1.9 (1.3–3.9) | 0.41 |
| Quitkin et al., 1989 | Imipramine | 54 | 1.69 (1.10–2.60) | 3.0 (1.7–11) | 1.75 |
| Quitkin et al., 1989 | Phenelzine | 53 | 1.93 (1.19–3.12) | 2.6 (1.6–6.6) | 1.59 |
| Rouillon et al., 1989 | Maprotiline | 1 141 | 1.44 (1.13–1.84) | 14 (8.2–45) | 2.38 |
| Doogan and Caillard, 1992 | Sertraline | 295 | 3.50 (2.29–5.36) | 3.1 (2.3–4.5) | 1.77 |
| Montgomery et al., 1993b | Citalopram | 155 | 3.05 (1.53–6.09) | 4.6 (2.8–14) | 1.09 |
| Montgomery et al., 1993a | Paroxetine | 135 | 2.68 (1.46–4.91) | 3.7 (2.4–8.1) | 1.27 |
| Mynors-Wallis et al., 1995 | Amitriptyline | 61 | 3.10 (1.43–6.73) | 2.5 (1.6–5.5) | 0.94 |
| Robert and Montgomery, 1995 | Citalopram | 226 | 1.76 (1.00–3.10) | 9.5 (4.6 to >100) | 1.37 |
| Lauritzen et al., 1996 | Paroxetine | 58 | 6.33 (2.11–19.1) | 1.8 (1.3–2.9) | 0.56 |
| Lauritzen et al., 1996 | Imipramine | 58 | 2.11 (1.16–3.86) | 2.9 (1.7–9.7) | 1.28 |
| Cunningham, 1997 | Venlafaxine | 278 | 1.37 (0.99–1.90) | 8.9 (4.3 to >100) | 2.10 |
| Stewart et al., 1997 | Phenelzine | 28 | 3.76 (1.36–10.3) | 1.6 (1.1–2.9) | 0.64 |
| Stewart et al., 1997 | Imipramine | 32 | 1.13 (0.52–2.48) | 18 (2.5 to >100) | 0.92 |
| M Fava et al., 1998 | Paroxetine | 73 | 1.00 (0.46–2.17) | >100 | 0.95 |
| M Fava et al., 1998 | Fluoxetine | 74 | 0.72 (0.28–1.90) | >100 | 0.69 |
| Montgomery et al., 1998 | Mirtazapine | 387 | 6.78 (3.32–13.9) | 4.2 (5.9–6.8) | 1.04 |
| Montgomery et al., 1998 | Amitriptyline | 386 | 2.45 (1.56–3.86) | 6.0 (4.1–11) | 1.68 |
| Feiger et al., 1999 | Nefazodone | 467 | 1.96 (1.39–2.75) | 6.3 (4.2–12) | 2.05 |
| Silverstone and Ravindran, 1999 | Fluoxetine | 237 | 1.53 (1.05–2.23) | 7.3 (3.9–52) | 1.93 |
| Silverstone and Ravindran, 1999 | Venlafaxine-XR | 240 | 1.39 (0.97–1.98) | 9.0 (4.3 to >100) | 2.00 |
| Versiani et al., 1999 | Reboxetine | 283 | 2.57 (1.79–3.69) | 3.1 (2.4–4.7) | 1.98 |
| Bauer et al., 2000 | Lithium | 28 | 1.50 (0.94–2.40) | 2.0 (1.3–4.3) | 0.11 |
| Schmidt et al., 2000 | Fluoxetine 90 mg | 312 | 1.36 (1.05–1.76) | 7.6 (4.1–51) | 2.34 |
| Schmidt et al., 2000 | Fluoxetine 20 mg | 311 | 1.93 (1.43–2.60) | 4.2 (2.9–7.6) | 2.19 |
| Sackeim et al., 2001 | Nortriptyline+Lithium | 48 | 2.15 (1.25–3.68) | 2.2 (1.4–4.9) | 1.44 |
| Sackeim et al., 2001 | Nortriptyline | 50 | 1.40 (0.97–2.01) | 4.2 (2.1 to >100) | 1.98 |
| Thase et al., 2001 | Mirtazapine | 156 | 2.41 (1.45–4.00) | 3.6 (2.4–7.3) | 1.52 |
| Bschor et al., 2002 | Lithium | 22 | 0.70 (0.17–2.81) | >100 | 0.38 |
| Golden et al., 2002 | Paroxetine-CR | 423 | 0.62 (0.32–1.21) | >100 | 1.14 |
| Golden et al., 2002 | Paroxetine-IR | 428 | 0.38 (0.21–0.70) | >100 | 1.27 |
| Weihs et al., 2002 | Bupropion | 828 | 1.41 (1.20–1.64) | 6.7 (4.6–12) | 2.64 |
| Fava et al., 2005 | Hypericum | 88 | 1.28 (0.81–2.03) | 9.0 (3.1 to >100) | 1.66 |
| Fava et al., 2005 | Fluoxetine | 90 | 1.05 (0.69–1.58) | 45 (4.4 to >100) | 1.81 |
| Amsterdam and Bodkin 2006 | Selegiline-transdermal | 312 | 1.95 (1.34–2.83) | 5.2 (3.4–11) | 1.94 |
| Perahia et al., 2006 | Duloxetine | 269 | 1.63 (1.04–2.58) | 9.1 (4.8–91) | 1.67 |
| van den Broek et al., 2006 | Imipramine | 26 | 4.40 (1.22–15.8) | 1.6 (1.1–3.2) | 0.43 |
| Goodwin et al., 2009 | Agomelatine | 492 | 1.96 (1.48–2.59) | 5.1 (3.5–8.9) | 2.26 |
| Rickels et al., 2010 | Desvenlafaxine | 374 | 1.77 (1.30–2.40) | 5.4 (3.6–11) | 2.17 |
| Yildiz et al., 2010 | Antidepressants | 46 | 2.14 (1.08–4.26) | 2.9 (1.6–13) | 1.10 |
| Rosenthal et al., 2013 | Desvenlafaxine | 548 | 2.08 (1.46–2.96) | 6.8 (4.7–13) | 2.01 |
| Borges et al., 2014 | Not stated | 224 | 1.96 (1.29–2.97) | 5.0 (3.1–14) | 1.80 |
| Borges et al., 2014 | Not stated | 258 | 1.78 (1.24–2.56) | 5.4 (3.3–14) | 1.97 |
| Borges et al., 2014 | Not stated | 226 | 2.14 (1.60–2.88) | 2.9 (2.1–4.7) | 2.21 |
| Borges et al., 2014 | Not stated | 147 | 2.14 (1.08–4.24) | 6.6 (3.3 to >100) | 1.10 |
| Borges et al., 2014 | Not stated | 298 | 4.87 (2.85–8.30) | 3.3 (2.4–5.0) | 1.45 |
| Borges et al., 2014 | Not stated | 125 | 2.72 (1.24–5.98) | 5.1 (3.0–17) | 0.92 |
| Borges et al., 2014 | Not stated | 213 | 1.72 (0.97–3.05) | 9.9 (4.8 to >100) | 1.34 |
| Borges et al., 2014 | Not stated | 292 | 3.20 (1.86–5.50) | 4.7 (3.3–8.1) | 1.43 |
| Borges et al., 2014 | Not stated | 156 | 1.49 (1.07–2.08) | 5.2 (2.9–26) | 2.08 |
| Borges et al., 2014 | Not stated | 417 | 7.15 (2.56–20.0) | 8.4 (5.9–15) | 0.62 |
| Borges et al., 2014 | Not stated | 312 | 1.93 (1.40–2.66) | 4.3 (3.0–7.9) | 2.12 |
| Borges et al., 2014 | Not stated | 273 | 1.69 (1.22–2.34) | 5.4 (3.3–15) | 2.10 |
| Borges et al., 2014 | Not stated | 269 | 2.22 (1.50–3.29) | 4.1 (2.9–7.5) | 1.87 |
| Borges et al., 2014 | Not stated | 374 | 1.42 (1.11–1.82) | 7.0 (4.1–23) | 2.36 |
| Borges et al., 2014 | Not stated | 548 | 1.93 (1.40 -2.66) | 6.7 (4.5–13) | 2.11 |
| Pooled (72 comparisons) | ––– | 14 450 | 1.90 (1.73–2.08) | 4.4 (3.8–5.2) | 100 |
By random-effects meta-analysis, pooled relative risk (RR 1.90; z = 13.3) and pooled number needed to treat (NNT 4.4; z = 12.7) were both highly significant, even in a sensitivity meta-analysis, omitting eight trials with RR ≥ 5.0 (RR 1.81, CI 1.66–1.97; z = 12.7; all p<0.0001); 23/72 trials (31.9%) individually yielded non-significant drug-placebo differences.
In these continuation trials, drug treatments continued for an average of 8.35 (CI 7.55–9.15) months. Risk of relapse within several months of initial remission averaged 23.3% with antidepressant continued versus 49.4% after discontinuing treatment, a 2.1-fold difference (Table S1). The meta-analytically computed, pooled relative, placebo/drug relapse rate was 1.90 (CI: 1.73–2.08), a highly significant decrease (z-score = 13.3, p < 0.0001; Table 1). Even when eight trials with unusually high drug-placebo differences (>5-fold; Table S1) were removed in a sensitivity re-analysis, the difference between continued antidepressant treatment and placebo remained highly significant. The overall computed number-needed-to-treat (NNT), or number of cases to be treated to obtain one responding better with drug than with placebo was low (4.4, CI 3.8–5.2).
Long-Term Antidepressant Treatment
We identified a total of 35 reports involving treatment with antidepressants continued for more than one year (2.27, CI 1.88–2.62 years) following an index episode of acute major depression, with a total of 7253 participants in 37 drug-control comparisons (Tables 2 and S2). All but one of these trials compared an antidepressant to a placebo; the single exception involved a small, 5.4-year comparison of antidepressant treatment alone versus with continued ECT, in which the addition of ECT yielded a significantly lower recurrence rate (27.6% vs. 82.8%; Gagne et al., 2000).
Table 2.
Meta-analysis of long-term (>12 months) trials of antidepressants versus placebo in major depressive disorder
| Trial | Treatment | Subjects | RR [95%CI] | NNT [95%CI] | % Weight | |
|---|---|---|---|---|---|---|
| Bjork 1983 | Zimelidine | 38 | 2.67 [1.34–5.32] | 1.9 [1.3–3.8] | 1.83 | |
| Glen et al. 1984 | Amitriptyline | 67 | 1.41 [1.04–1.84] | 3.9 [2.0–59] | 3.67 | |
| Glen et al. 1984 | Lithium | 78 | 1.37 [1.03–1.92] | 4.1 [2.1–139] | 3.78 | |
| Prien et al. 1984 | Amitriptyline±Lithium | 150 | 1.91 [1.32–2.78] | 3.5 [2.3–7.4] | 3.28 | |
| Frank et al. 1990 | Imipramine+IPT | 51 | 2.72 [1.28–5.78] | 2.4 [1.5–6.0] | 1.64 | |
| Robinson et al. 1991 | Phenelzine | 47 | 2.80 [1.54–5.09] | 1.9 [1.3–3.7] | 2.17 | |
| Kupfer et al. 1992 | Imipramine+IPT | 20 | 7.33 [1.07–50.3] | 1.7 [1.1–4.5] | 0.35 | |
| Maj et al. 1992 | Tricyclics±Lithium | 72 | 1.22 [0.97–1.52] | 6.3 [2.9 to >100] | 4.17 | |
| OADIG 1993 | Dothiepin | 69 | 1.83 [1.01–3.32] | 4.0 [2.1–37] | 2.19 | |
| Kishimoto et al. 1994 | Mianserin | 22 | 2.25 [1.08–4.67] | 1.8 [1.1–4.3] | 1.71 | |
| Entsuah et al. 1996 | Venlafaxine | 448 | 1.69 [1.23–2.32] | 7.2 [4.6–18] | 3.61 | |
| Kocsis et al. 1996 | Desipramine | 129 | 4.79 [2.29–10.0] | 2.4 [1.8–3.8] | 1.68 | |
| Keller et al. 1998 | Sertraline | 161 | 3.48 [1.37–8.88] | 6.2 [3.8–18] | 1.20 | |
| Terra and Montgomery 1998 | Fluvoxamine | 436 | 2.75 [1.86–4.06] | 4.4 [3.3–6.8] | 3.20 | |
| Reynolds et al. 1999 | Nortriptyline | 107 | 2.42 [1.60–3.68] | 2.2 [1.6–3.5] | 3.04 | |
| Alexopoulos et al. 2000 | Nortriptyline | 43 | 2.88 [1.09–7.64] | 2.9 [1.6–13] | 1.13 | |
| Gagne et al. 2000 | ECT+Antidepressants | 58 | 3.00 [1.63–5.54] | 1.8 [1.3–3.0] | 2.11 | |
| Rouillon et al. 2000 | Milnacipran | 214 | 1.45 [0.83–2.50] | 14 [5.6 to >100] | 2.38 | |
| Gilaberte et al. 2001 | Fluoxetine | 253 | 2.02 [1.34–3.04] | 5.0 [3.2–11] | 3.07 | |
| Hochstrasser et al. 2001 | Citalopram | 264 | 3.45 [2.47–4.82] | 1.9 [1.6–2.3] | 3.51 | |
| Klysner et al. 2002 | Citalopram | 121 | 2.12 [1.41–3.20] | 2.8 [1.9–5.3] | 3.08 | |
| Gelenberg et al. 2003 | Nefazodone | 160 | 1.57 [1.05–2.37] | 5.8 [3.1–40] | 3.09 | |
| Wilson et al. 2003 | Sertraline | 113 | 1.18 [0.80–1.73] | 13 [3.8 to >100] | 3.24 | |
| Lepine et al. 2004 | Sertraline | 288 | 1.97 [1.29–3.00] | 6.1 [3.7–18] | 3.02 | |
| Montgomery et al. 2004 | Venlafaxine | 225 | 2.51 [1.70–3.70] | 3.0 [2.2–4.7] | 3.20 | |
| Hollon et al. 2005 | Antidepressants | 69 | 1.64 [1.10–2.44] | 3.3 [1.9–12] | 3.14 | |
| Keller et al. 2005 | Gepirone | 420 | 1.52 [1.12–2.07] | 8.4 [4.9–30] | 3.66 | |
| Kornstein et al. 2006 | S-Citalopram | 139 | 2.38 [1.57–3.59] | 2.6 [1.9–4.5] | 3.06 | |
| Reynolds et al. 2006 | Paroxetine+CM | 53 | 1.92 [1.11–3.31] | 3.0 [1.8–11] | 2.40 | |
| Reynolds et al. 2006 | Paroxetine+PT | 63 | 1.50 [0.82–2.72] | 5.4 [1.8 to >100] | 2.18 | |
| Keller et al. 2007a | Venlafaxine | 131 | 5.89 [2.43–14.3] | 2.7 [2.2–4.3] | 1.31 | |
| Kocsis et al. 2007 | Venlafaxine-ER | 336 | 1.82 [1.31–2.52] | 5.5 [3.5–11] | 3.56 | |
| Kasper et al. 2008 | Hypericum | 426 | 1.42 [0.98–2.06] | 13 [6.3 to >100] | 3.29 | |
| Kelin et al. 2010 | Duloxetine | 514 | 2.53 [1.75–3.67] | 5.3 [3.8–8.3] | 3.30 | |
| Liebowitz et al. 2010 | Quetiapine-ER | 771 | 2.42 [1.83–3.20] | 5.0 [3.8–7.0] | 3.84 | |
| Segal et al. 2010 | Antidepressants | 58 | 2.60 [1.32–5.11] | 2.3 [1.5–5.2] | 1.88 | |
| Boulenger et al. 2012 | Vortioxetine | 639 | 1.97 [1.29–3.01] | 7.8 [4.9–20] | 3.00 | |
| Pooled (37 comparisons) | ––– | 7253 | 2.03 [1.80–2.28] | 3.8 [3.3–4.6] | 100 | |
Based on 35 placebo-controlled trials (except for Gagne et al., with antidepressants-only as controls), with 37 drug/control comparisons, lasting >12 (14–60) months shown in eTable S2, with random-effects meta-analytic modeling. Total N includes 9 controls used twice (for Glen et al. 1984). The pooled RR (recurrence rate with placebo/drug) indicates highly significant overall superiority versus placebo (z=11.7, p<0.0001); 5/37 trials (13.5%) individually yielded nonsignificant drug-control differences. Abbreviations: CM, clinical management; ECT, electroconvulsive treatment; ER, extended release; IPT, interpersonal psychotherapy; NNT = number-needed-to-treat to yield a selective response to drug > placebo; PT, psychotherapy.
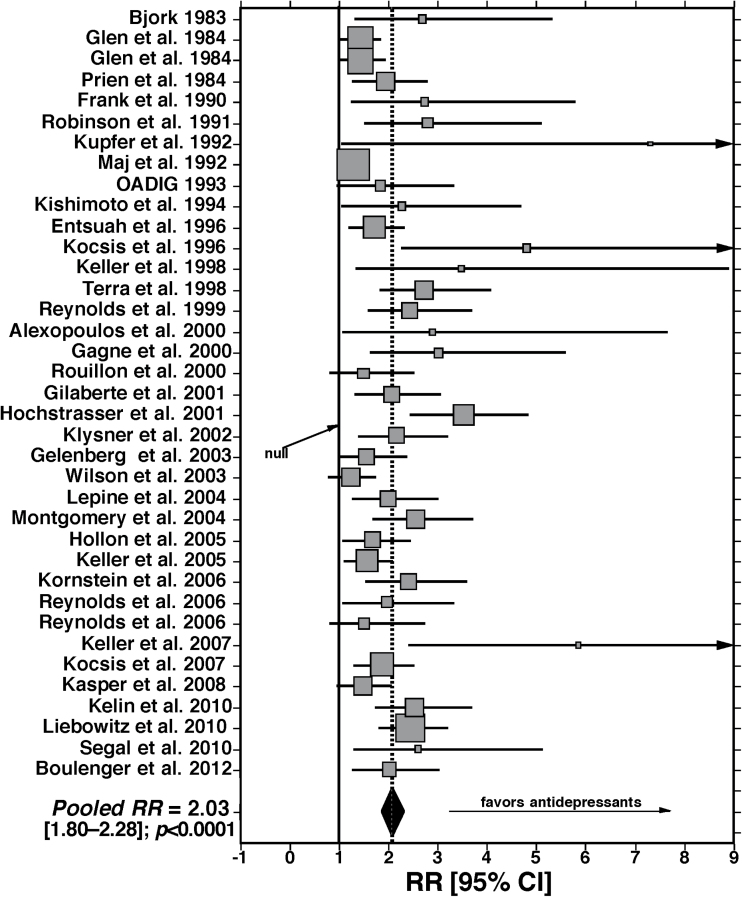
Based on random-effects meta-analysis (Table 2, Figure 2), all but five of the 37 (86.4%) drug-placebo comparisons trials yielded statistically superior benefits of active agents over placebo treatment in reducing recurrence risk. All but five trials (Maj et al., 1992; Rouillon et al., 2000; Wilson et al., 2003; Reynolds et al., 2006; Kasper et al., 2008) yielded statistically superior benefits of active agents over placebo treatments in reducing recurrence risk. These failed trials involved antidepressants such as tricyclic antidepressants (Maj et al., 1992), SSRIs (Wilson et al., 2003; Reynolds et al., 2006), the anxiolytic SNRI milnacipran (Rouillon et al., 2000), and a preparation of the plant Hypericum perforatum (St. John’s Wort; Kasper et al., 2008). Overall, the long-term, controlled trials yielded significantly greater protection from recurrences with antidepressants versus placebo treatments (RR 2.03, CI 1.80–2.28; z = 11.7, p < 0.0001), with a low number-needed-to-treat (NNT 3.8, CI 3.3–4.6). That is, recurrence risk was reduced by 2.03-fold or 50.7% with long-term antidepressant treatment (Figure 2).
Figure 2.
Findings from random-effects meta-analysis of 37 controlled, long-term trials (>12 months) of antidepressants vs. placebos in major depression. The pooled ratio (RR) of recurrence risk with placebo vs. antidepressants, of 2.03 [CI: 1.80-2.28]) is highly significant (z-score = 11.7, p < 0.0001).
Psychosocial Treatments
A variety of psychosocial methods were tested in 17 trials with 22 tested treatments, averaging 1.98 (CI 1.49–2.46) years and involving 1 969 participants (Tables 3 and S3). Treatment techniques included various forms of cognitive or cognitive-behavioral psychotherapy (CT or CBT), sometimes with a mindfulness orientation (MCT), and patient- or family-based psychoeducation, with or without antidepressant treatment. Controls typically involved treatment as usual (often involving continued use of antidepressants, or placebo controls for comparisons in which antidepressants were involved). This heterogeneous group of studies yielded varied findings, as might be expected. Statistically nonsignificant results were reported in at least one component of 11 of the trials (50.0%; Tables 3 and S3). A notable finding in four of the trials (all involving CT or MCT) is that presumably more severely ill patients, with at least three or five pre-trial recurrences of depressive episodes, showed greater responses to treatment than subjects with fewer recurrences (Ma and Teasdale, 2004; Bockting et al., 2005, 2009; Teasdale et al., 2000).
Table 3.
Meta-Analysis of Controlled, Long-Term Trials of Psychosocial Treatments for Major Depression
| Trials | Subjects | Treatment | Controls | RR (95% CI) | NNT (95% CI) | Weight (%) |
|---|---|---|---|---|---|---|
| Shea et al., 1992 | 37 | CBT | Pbo | 0.92 (0.37–2.26) | >100 | 3.06 |
| Shea et al., 1992 | 36 | IPT | Pbo | 1.00 (0.39–2.55) | >100 | 2.93 |
| GA Fava et al., 1998 | 40 | CBT+AD | AD | 3.20 (1.45–7.05) | 1.8 (1.2–3.4) | 3.58 |
| Gortner et al., 1998 | 151 | CBT | CM | 0.73 (0.50–1.08) | >100 | 6.06 |
| Teasdale et al., 2000 a | 73 | MCT (high) | TAU | 1.64 (1.04–2.59) | 3.8 (2.1–25) | 5.63 |
| Teasdale et al., 2000 b | 72 | MCT (low) | TAU | 0.55 (0.31–0.98) | >100 | 4.82 |
| Jarrett et al., 2001 | 156 | CBT | TAU | 3.00 (1.44–6.26) | 4.9 (3.1–12) | 3.86 |
| Katon et al., 2001 | 386 | PsychoEd | TAU | 0.98 (0.75–1.29) | >100 | 6.85 |
| Klein et al., 2004 | 82 | CBT | TAU | 3.25 (1.15–9.14) | 4.6 (2.6–20) | 2.58 |
| Ma & Teasdale, 2004 a | 38 | MCT (high) | TAU | 0.98 (0.68–1.26) | 1.4 (1.1–1.9) | 0.98 |
| Ma & Teasdale, 2004 b | 38 | MCT (low) | TAU | 0.40 (0.15–1.06) | >100 | 2.81 |
| Bockting et al., 2005 a | 71 | CT (high) | TAU | 1.54 (1.02–2.32) | 4.1 (2.1–41) | 5.92 |
| Bockting et al., 2005 b | 101 | CT (low) | TAU | 0.93 (0.68–1.26) | >100 | 6.66 |
| Hollon et al., 2005 | 70 | CT | Pbo | 2.45 (1.46–4.13) | 2.2 (1.5–4.0) | 5.16 |
| Dobson et al., 2008 | 79 | CT | AD | 2.19 (1.08–4.42) | 3.6 (2.1–14) | 4.03 |
| Dobson et al., 2008 | 76 | BA | AD | 2.00 (1.00–4.02) | 3.8 (2.1–23) | 4.09 |
| Kuyken et al., 2008 | 123 | MCT | AD | 1.26 (0.90–1.75) | 8.2 (3.4 to >100) | 6.46 |
| Bockting et al., 2009 a | 86 | CT (high) | TAU | 1.28 (1.06–1.53) | 11 (2.8–16) | 7.33 |
| Bockting et al., 2009 b | 86 | CT (low) | TAU | 0.97 (0.79–1.20) | >100 | 7.22 |
| Bondolfi et al., 2010 | 60 | MCT | TAU | 1.19 (0.56–2.50) | 18 (3.4 to >100) | 3.81 |
| Segal et al., 2010 | 54 | MCT | Pbo | 2.45 (1.30–4.60) | 2.4 (1.5–5.6) | 4.46 |
| Shimazu et al., 2011 | 54 | FamPsychoEd | TAU | 6.00 (1.52–23.7) | 2.4 (1.6–4.8) | 1.71 |
|
Pooled
(22 comparisons) |
1 969 | ––– | ––– | 1.39 (1.13–1.70) | 6.0 (3.8–14) | 100 |
Based on random-effects meta-analysis of data in Supplementary Table S3, omitting drug arms and trials lacking separate control arms. The pooled RR of 1.39 is statistically significant (z = 3.15, p = 0.002), although 11 of 22 (50.0%) comparisons involved non-significant differences between experimental psychosocial treatments and controls.
In four studies, differences were found among subgroups with relatively ahigh (≥3 or ≥5) vs. blower numbers of previous depressive recurrences. With high recurrences, pooled RR = 1.63 (1.04–2.57; z = 2.14, p = 0.03); with low recurrences, RR = 0.80 (0.58–1.09; z = 1.41, p = 0.16).
AD, antidepressants; BA, behavioral activation therapy; CBT, cognitive behavioral therapy; CI, confidence interval; CT, cognitive psychotherapy; FamPsychoEd, family psychoeducation; IPT, interpersonal psychotherapy; MCT, mindfulness-oriented cognitive therapy; Pbo, placebo; PsychoEd, psychoeducation; RR, recurrence rate; TAU, treatment as usual.
Meta-Regression Analyses
Following random-effects meta-analyses, we used meta-regression modeling to test for associations of outcomes of trials reported in Tables 1–3 and S1–S3 with available potential covariates. These factors included: (a) year study was reported; (b) trial size (total number of subjects); (c) mean age of subjects; (d) proportion of women participants; (e) estimated counts of previous depressive episodes; (f) initial weeks of treatment prior to randomization; (g) month duration of continuation or long-term trials; (h) type of antidepressant (older agents [TCAs, the atypical agents dothiepin and mianserin, or MAO inhibitors] vs. newer agents [SSRIs or SNRIs, mirtazapine, or bupropion]).
Regarding meta-analysis of intermediate-term continuation trials (Tables 1 and S1), meta-regression found only one of the eight stated factors to be significantly associated with efficacy of active treatments over placebo. It was the duration (weeks) of initial treatment and clinical stabilization of subjects presenting in acute depressive episodes prior to randomization into a continuation phase of each trial. That is, longer initial treatment was associated significantly with a larger drug-placebo contrast (greater effect size, whether based on placebo/drug RR or the difference in relapse rates between placebo and antidepressant, RD) during continuation treatment (β slope function = +0.048; CI 0.024–0.073; z = 3.90, p < 0.001).
Meta-regression analysis for long-term medication maintenance trials (Tables 2 and S2) also found only one factor to be significantly associated with outcomes in meta-analysis. That is, longer duration (months) had a positive effect on drug-placebo contrasts (β slope function = +0.104; CI 0.044–0.163; z = 3.44, p < 0.001). In addition, with linear regression modeling, recurrence rates with both drug and placebo increased with longer exposure time, as expected, although recurrence risk with placebo grew much more than with drug (r = +0.57 vs. +0.30, respectively). It follows that both their RR and RD grew larger, with longer treatment indicating greater effect size (r = +0.47 and +0.41, respectively, both t-scores ≥2.56, with p ≤ 0.01), consistent with the meta-regression analysis.
Meta-regression modeling for findings in long-term psychotherapy trials (Tables 3 and S3) indicated that none of the factors listed above was significantly associated with larger effect-sizes in random-effect meta-analysis. However, when trials involving high (≥3 or ≥5) versus low counts of previous depressive episodes were pooled for random-effects meta-analysis, there was a much greater effect of psychosocial interventions after higher recurrence counts.
Effect of Type of Treatment
We also summarized meta-analytic results for comparisons of specific types of antidepressants versus placebo controls in long-term trials summarized in Table 2. Only TCAs (n = 10) and the pool of SSRIs (n = 11) and SNRIs (n = 7) provided at least 10 trials for meta-analysis, and all yielded similar RR values (2.09, 2.10, and 2.17, respectively). Overall, computed RR values (relative recurrence with placebo/antidepressant: greater, better) ranked: ECT (RR = 3.00) > MAO inhibitor (2.80) > SSRIs (2.17) ≥ SNRIs (2.10) ≥ TCAs (2.09) > various standard antidepressants (1.91) > atypical agents (gepirone, hypericum, mianserin, nefazodone, or quetiapine-ER; 1.72) > lithium (1.39), but all active agent types were significantly superior to placebo.
Summary of Findings
Finally, salient characteristics of the information found regarding: (a) continuation of antidepressant treatment up to 12 months (72 trials with 14 450 subjects) after an acute depressive episode; (b) long-term maintenance treatment with antidepressants (37 trials with 7253 subjects); and (c) long-term use or addition of psychosocial treatments (22 trials with 1 969 subjects) are summarized in Table 4. By trial-type, meta-analytically computed values of effect-size (RR) ranked: b, long-term antidepressants (2.02) > a, continuation of antidepressants (1.90) > c, long-term psychosocial treatments (1.39). Similarly, computed NNT values ranked inversely in the same order (b, 3.8 < a, 4.4 ≤ c, 6.0), as did the rate of trials finding superior outcomes with test treatment versus controls (b, 86.5% > a, 68.1% > c, 50.0%).
Table 4.
Summary of Meta-Analytic Findings from Controlled Trials for Continuation or Maintenance Treatments for Recurrent Major Depression
| Measures | Drug Continuation | Drug Maintenance | Psychosocial Maintenance |
|---|---|---|---|
| Reports | 45 | 35 | 17 |
| Controlled trials | 72 | 37 | 22 |
| Years | 1 973–2 014 | 1 983–2 012 | 1 992–2 011 |
| Subjects | |||
| Total | 14 450 | 7253 | 1 969 |
| Per trial | 189 (138–239) | 188 (131–245) | 124 (92.1–156) |
| Mean age | 48.3 (46.0–50.6) | 47.5 (42.5–52.1) | 44.6 (44.0–48.2) |
| % Women | 60.4 (56.4–64.4) | 69.5 (37.3–71.7) | 69.6 (64.7–75.5) |
| Duration (mos) | 8.35 (7.55–9.15) | 26.8 (25.5–31.1) | 23.7 (18.4–29.0) |
| RR (95% CI) | 1.90 (1.73–2.08) | 2.03 (1.80-2.28) | 1.39 (1.13–1.70) |
| z-score (p-value) | 13.3 (<0.0001) | 11.7 (<0.0001) | 3.15 (0.002) |
| NNT (95% CI) | 4.4 (3.8–5.2) | 3.8 (3.3–4.6) | 6.0 (3.8–14) |
| Trials with significant superiority of test treatment (%)* | 49/72 (68.1%) | 32/37 (86.5%) | 11/22 (50.0%) |
Recurrence rate (RR) is meta-analytically pooled risk of new depression with placebo or control treatment vs. active experimental treatment. Data are derived from Tables 1–3 and Supplementary Tables S1–3. *Success-rate is significantly greater for drug vs. psychosocial maintenance treatments (χ2 = 6.29, p = 0.01) and for all drug vs. psychosocial treatments (χ2 = 4.72, p = 0.03), but not between continuation and maintenance drug treatment (χ2 = 2.80, p = 0.09).
CI, confidence interval; NNT, number needed to treat to yield a selective response to drug > placebo.
Discussion
Most pharmacotherapy trials for MDD patients found significant reductions of early relapse (up to 12 months) or of long-term recurrence rates with active drugs compared to placebo controls (Tables 1, 12, S1, and S2, Figure 2). Differences between specific types of treatments or specific agents were insufficiently studied except for TCA, SSRI, and SNRI antidepressants, all of which appeared to provide similar long-term benefits. Long-term trials of psychotherapy for depression were relatively fewer and have yielded variable findings (Tables 3 and S3). Family psychoeducation appeared to be useful long-term in two studies reported from Japan (Shimazu et al., 2011; Shimodera et al., 2012).
Continuation and Long-Term Pharmacological Treatments
In most continuation and long-term maintenance trials, antidepressant drugs proved to be more effective than placebo controls (Tables 1 and 2). Of the total of 109 such trials of efficacy in preventing relapses or recurrences of major depression, 81 (74.3%) yielded statistically significant differences favoring a variety of types of antidepressants (68.1% of continuation trials and 86.5% of maintenance trials). The most frequently studied antidepressants were SSRIs and SNRIs, which outperformed placebo controls in 11 of 20 (55.0%) continuation trials and 13 of 15 (86.7%) maintenance trials. Based on the results of meta-analysis, the relative risk of new episodes of depression with these modern drugs and TCAs was very similar, and the numbers of trials of other types of treatments was too limited to support secure conclusions about comparative efficacy. These findings appear to support the clinical value of extending antidepressant treatment for up to 12 months after initial improvement or remission of acute depression, as well as the value of long-term antidepressant treatment, at least when indications based on the demonstrated risk of recurrences, and of probable benefit, are present.
Non-Pharmacological Treatments
The effects of psychosocial treatments, with or without antidepressants, yielded inconsistent and largely inconclusive findings (Table 3). Among these, notably, family psychoeducation continued for up to 10 months was examined in two Japanese studies and found to be clinically effective and cost-effective in reducing recurrence risks in major depressive disorder patients (Shimazu et al., 2011; Shimodera et al., 2012). Mindfulness-based cognitive psychotherapy aims at teaching patients to disengage from cognitive processes that are proposed to increase vulnerability to depression, by improving awareness and acceptance of negative thoughts and feelings. Several trials of this treatment method did not provide superior protection against recurrences of depression than control conditions (Kuyken et al., 2008; Bondolfi et al., 2010; Mathew et al., 2010). However, one trial found it to be about as effective as long-term treatment with an antidepressant (Segal et al., 2010), and others found beneficial effects among patients with at least three previous depressive episodes (Teasdale et al., 2000; Ma and Teasdale, 2004). Widely clinically employed cognitive-behavioral therapy was associated with significantly lower long-term recurrence rates of depression than with a control condition involving treatment as usual or nonspecific clinical management, again, but the benefits appeared to be limited largely to patients with three or more previous episodes (GA Fava et al., 1998; Bockting et al., 2005, 2009). There is also some evidence that use of CBT during an index episode of acute depression may have long-lasting beneficial effects in limiting risks of relapse or recurrences for up to 2 years after initial remission (Hollon et al., 2005; Dobson et al., 2008).
In the present findings, notably, some trials found that psychosocial methods outperformed or significantly added to benefits of pharmacotherapies (e.g. GA Fava et al. 1998; Dobson et al. 2008; Kuykun et al. 2008; Table 3). Such findings encourage additional, well-designed, larger trials to test for long-term benefits of psychosocial methods, including their potential additional benefits when combined with antidepressants.
Finally, studies of effects of long-term ECT remain scarce. Two were identified that examined patients who had responded to ECT in an acute episode of major depression. Participants were then randomized to continue ECT with or without antidepressant medication, compared to other treatments that included an antidepressant plus lithium. Their findings favored ECT added or compared with medicinal treatments (Gagne et al., 2000; Kellner et al., 2006). A small “mirror-image” study found that maintenance ECT was associated with fewer and shorter psychiatric hospitalizations over 8 years compared to similar periods of illness before use of maintenance ECT (Gupta et al., 2008).
Effect of Multiple Depressive Recurrences
Several of the studies reviewed found that treatment effects appeared to be superior among patients with multiple (especially ≥3) previous episodes of depression (Montgomery et al., 1993; Teasdale et al., 2000; Lepine et al., 2004; Ma and Teasdale, 2004; Kelin et al., 2010). This relationship was more likely with psychotherapy (Teasdale et al., 2000; Ma and Teasdale, 2004; Bockting et al., 2005, 2009) than with pharmacotherapies (Montgomery and Dunbar, 1993; M Fava et al., 1998; Lepine et al., 2004; Keller et al., 2007a; 2007b; Kocsis et al., 2007; Kuyken et al., 2008; Bondolfi et al., 2010; Kelin et al., 2010; Mathew et al., 2010; Thase et al., 2011). Mechanisms involved are not clear, but more recurrent depressive disorders may be particularly responsive to efforts to apply psychological methods to modify demoralization and adverse patterns of thinking and behavior proposed to contribute to a risk of depression (Bockting et al., 2005). An alternative possibility is that multiple previous recurrences may select for cases with particularly high future risks of recurrence, and so provide a statistical advantage in controlled trials.
Effect of Duration of Initial and Later Treatment
In meta-regression analyses, the only factors that appeared to be associated with greater drug-placebo differences were a longer initial treatment of index episodes of acute depression and a longer duration of long-term treatment that followed. Presumably, longer initial treatment places antidepressant-treated patients at an advantage in terms of adequate recovery from an acute depressive episode, with greater stabilization and resistance to relapses within several initial months, and longer treatment is disadvantageous for placebos. We also found recently that shorter duration of initial treatment with an antidepressant, especially less than 4–6 months, is followed by higher relapse risks with a placebo (Baldessarini et al., 2015). The possibility that such effects influenced the outcome of continuation trials could not be tested since data on the duration of initial placebo treatment in index depressive episodes were not available.
Limitations
This review has several noteworthy limitations. First, there were relatively few well-designed studies involving non-pharmacological treatments, and very few involving specific combinations of treatment methods that are commonly employed clinically on an empirical basis. Second, follow-up periods ranged from several months to five years (average: up to 2 years), with a probable excess of new episodes within the early months, although the actual times to new episodes usually were not reported. That is, not all trials are likely to represent fair testing of protection against recurrences (not contaminated by relapses) of major depression, which probably requires several years given the natural history of the illness, in which episodes typically reoccur less than yearly (Baldessarini, 2013). Third, many studies involve “enriched” designs, in which patients were required to respond favorably to short-term treatment of an initial episode of acute major depression. Such case selection is likely to limit potential generalization of findings to broader clinical populations. Fourth, many intermediate- or long-term trials involve discontinuation of an active treatment in patients barely recovering from an acute episode of depression. Such treatment discontinuation itself may contribute to inferior outcomes in the placebo arms of trials, especially if treatment discontinuation is abrupt or rapid (Baldessarini et al., 2010, 2015; Baldessarini, 2013). Fifth, we did not include studies of juvenile depression or study mainly geriatric depression. However, recent reviews of maintenance trials in these groups indicate that the evidence available is encouraging, but much less abundant than in adult MDD (Kok et al., 2011; Cox et al., 2012; Wilkinson and Izmeth, 2012). Additional limitations include the need to re-use single placebo arms in some trials that compared more than one active treatment; however, we carried out sensitivity analyses based on including only one treatment/control comparison from each of such studies, and found very minor effects on the meta-analytically pooled outcomes (not shown). In addition, there is a general lack of relevant clinical details concerning previous depressive episodes in many studies, and little consideration of subjective wellness and improved functioning as clinically relevant components of outcomes, and we found very little evidence that specific clinical or biological characteristics were considered that might contribute to predicting the likelihood of accepting, tolerating, and benefitting from particular long-term treatments.
Conclusions
Most of the pharmacotherapy trials reviewed showed significantly lower rates of relapse or recurrence of depressive episodes among adult patients diagnosed with MDD compared to placebos. These treatments involved the use of SNRIs, SSRIs, and other agents with demonstrated short-term efficacy in acute major depressive episodes (Undurraga and Baldessarini, 2012; Baldessarini, 2013). ECT also had some benefit in recurrence prevention, but remains inadequately tested in the long term. Fewer long-term, monotherapy trials of psychotherapies have been reported; most involve cognitive-behavioral techniques, sometimes with antidepressants continued. Nevertheless, most psychotherapy trials have found some evidence of long-term benefit, especially in patients with three or more previous episodes of major depression. Despite the evidence of efficacy of antidepressants, and suggestive support for some forms of psychosocial interventions compared to placebos or other controls, the present findings underscore the substantial rates of long-term unresolved morbidity in treated MDD patients (Forte et al., 2015); with treatment, relapse rates averaged 23.3% (in 8.4 months), and recurrences averaged 24.6% to 36.8% (in 24–27 months; Tables S1–3). That conclusion strongly encourages further efforts to develop more effective and better-accepted treatments and evaluations of the effects of dosing and of specific combination therapies, conducted over periods longer than a year, and efforts to design trials so as to limit biases associated with case selection and artifacts associated with treatment discontinuation, especially soon after initial apparent clinical recovery, as well as to examine predictors of positive or unfavorable outcomes and of nonadherence to long-term treatments.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
No author or immediate family member has financial relationships with commercial organizations that might represent the appearance of potential conflicts of interest with the material presented here.
Supplementary Material
Acknowledgments
Supported in part by a grant from the Bruce J Anderson Foundation and by the McLean Private Donors Psychopharmacology Research Fund (to Dr Baldessarini).
References
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J. (2000) Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 57:285–290. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Bodkin JA. (2006) Selegiline transdermal system in the prevention of relapse of major depressive disorder: 52-week, double-blind, placebo-substitution, parallel-group clinical trial. J Clin Psychopharmacol 26:579–586. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. (2013) Chemotherapy in Psychiatry, 3rd edition. New York: Springer Press. [Google Scholar]
- Baldessarini RJ, Tondo L, Ghiani C, Lepri B. (2010) Illness risk following rapid versus gradual discontinuation of antidepressants. Am J Psych 167:934–941. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Lau WK, Sim J, Sum MY, Sim K. (2015) Duration of initial antidepressant treatment and subsequent relapse of major depression. J Clin Psychopharmacol 35:75–76. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bschor T, Kunz D, Berghofer A, Strohle A, Miller-Oerlinghausen B. (2000) Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psych 157:1429–1435. [DOI] [PubMed] [Google Scholar]
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ. (2015) World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Unipolar Depressive Disorders. Guidelines for biological treatment of unipolar depressive disorders. World J Biol Psychiatry 16:76–95. [DOI] [PubMed] [Google Scholar]
- Bialos D, Giller E, Jatlow P, Docherty J, Harkness L. (1982) Recurrence of depression after discontinuation of long-term amitriptyline treatment. Am J Psych 139:325–329. [DOI] [PubMed] [Google Scholar]
- Bjork K. (1983) The efficacy of zimeldine in preventing depressive episodes in recurrent major depressive disorders: double-blind placebo-controlled study. Acta Psychiatr Scand 308:182–189. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Schene AH, Spinhoven P, Koeter MW, Wouters LF, Huyser J, Kamphuis JH. (2005) Preventing relapse/recurrence in recurrent depression with cognitive therapy: randomized controlled trial. J Consult Clin Psychol 73:647–657. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Spinhoven P, Wouters LF, Koeter MW, Schene AH. (2009) Long-term effects of preventive cognitive therapy in recurrent depression: 5.5-year follow-up study. J Clin Psychiatry 70:1621–1628. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, der Linden MV, Gex-Fabry M, Bizzini L, Rouget BW, Myers-Arrazola L, Gonzalez C, Segal Z, Aubry JM, Bertschy G. (2010) Depression relapse prophylaxis with Mindfulness-Based Cognitive Therapy: replication and extension in the Swiss health care system. J Affect Disord 122:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S, Chen Y-F, Laughren TP, Temple R, Patel HD, David PA, Mathis M, Unger E, Yang P, Khin NA. (2014) Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry 75:205–214. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Loft H, Florea I. (2012) Randomized clinical study of Lu-AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol 26:1408–1416. [DOI] [PubMed] [Google Scholar]
- Bschor T, Berghöfer A, Ströhle A, Kunz D, Adli M, Müller-Oerlinghausen B, Bauer M. (2002) How long should the lithium augmentation strategy be maintained? One-year follow-up of a placebo-controlled study in unipolar refractory major depression. J Clin Psychopharmacol 22:427–430. [DOI] [PubMed] [Google Scholar]
- Cook BL, Helms PM, Smith RE, Tsai M. (1986) Unipolar depression in the elderly: reoccurrence on discontinuation of tricyclic antidepressants. J Affect Disord 10:91–94. [DOI] [PubMed] [Google Scholar]
- Coppen A, Ghose K, Montgomery S, Rama RV, Bailey J, Jorgensen A. (1978) Continuation therapy with amitriptyline in depression. Br J Psychiatry 133:28–33. [DOI] [PubMed] [Google Scholar]
- Cox GR, Fisher CA, De Silva S, Phelan M, Akinwale OP, Simmons MB, Hetrick SE. (2012) Interventions for preventing relapse and recurrence of a depressive disorder in children and adolescents. Cochrane Database Syst Rev 11:CD007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LA. (1997) Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Ann Clin Psychiatry 9:157–164. [DOI] [PubMed] [Google Scholar]
- Davidson J, Raft D. (1984) Use of phenelzine in continuation therapy. Neuropsychobiology 11:191–194. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB., Kohlenberg RJ, Gallop RJ, Rizvi SL, Gollan JK, Dunner DL, Jacobson NS. (2008) Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol 76:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doogan DP, Caillard V. (1992) Sertraline in the prevention of depression. Br J Psychiatry 160:217–222. [DOI] [PubMed] [Google Scholar]
- Entsuah AR, Rudolph RL, Hackett D, Miska S. (1996) Efficacy of venlafaxine and placebo during long-term treatment of depression: pooled analysis of relapse rates. Int Clin Psychopharmacol 11:137–145. [PubMed] [Google Scholar]
- Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. (1998) Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 55:816–820. [DOI] [PubMed] [Google Scholar]
- Fava M, Amsterdam JD, Deltito JA, Salzman C, Schwaller M, Dunner DL. (1998) Double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Ann Clin Psychiatry 10:145–150. [DOI] [PubMed] [Google Scholar]
- Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, Zajecka J, Murck H, Rosenbaum JF. (2005) Double-blind, randomized trial of St John’s wort, fluoxetine, and placebo in major depressive disorder. J Clin Psychopharmacol 25:441–447. [DOI] [PubMed] [Google Scholar]
- Feiger AD, Bielski RJ, Bremner J, Heiser JF, Trivedi M, Wilcox CS, Roberts DL, Kensler TT, McQuade RD, Kaplita SB, Archibald DG. (1999) Double-blind, placebo-substitution study of nefazodone in the prevention of relapse during continuation treatment of outpatients with major depression. Int Clin Psychopharmacol 14:19–28. [DOI] [PubMed] [Google Scholar]
- Ferreri M, Colonna L, Leger JM. (1997) Efficacy of amineptine in the prevention of relapse in unipolar depression. Int Clin Psychopharmacol 12:S39–45. [DOI] [PubMed] [Google Scholar]
- Forte A, Baldessarini RJ, Tondo L, Vázquez G, Pompili M, Girardi P. (2015) Long-term morbidity in bipolar-I, bipolar-II, and major depressive disorders. J Affect Disord 178:71–78. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ. (1990) Three-year outcomes of maintenance therapies in recurrent depression. Arch Gen Psychiatry 47:1093–1099. [DOI] [PubMed] [Google Scholar]
- Gagne GG, Jr, Furman MJ, Carpenter LL, Price LH. (2000) Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psych 157:1960–1965. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM. (2003) Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 361:653–661. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, et al. (2003) Randomized, placebo-controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biol Psychiatry 54:806–817. [DOI] [PubMed] [Google Scholar]
- Georgotas A. (1985) Affective disorders: pharmacotherapy. Compr Psychiatry 4:821–833. [Google Scholar]
- Georgotas A, McCue RE, Cooper TB. (1989) Placebo-controlled comparison of nortriptyline and phenelzine in maintenance therapy of elderly depressed patients. Arch Gen Psychiatry 46:783–786. [DOI] [PubMed] [Google Scholar]
- Gilaberte I, Montejo AL, de la Gandara J, Perez-Sola V, Bernardo M, Massana J, Martin-Santos R, Santiso A, Noquera R, Casais L, Perez-Camo V, Arias M, Judge R. (2001) Fluoxetine in the prevention of depressive recurrences: double-blind study. J Clin Psychopharmacol 21:417–424. [DOI] [PubMed] [Google Scholar]
- Glen AI, Johnson AL, Sheperd M. (1984) Continuation therapy with lithium and amitriptyline in unipolar depressive illness: randomized, double-blind, controlled trial. Psychol Med 14:37–50. [DOI] [PubMed] [Google Scholar]
- Glue P, Donovan MR, Kolluri S, Emir B. (2010) Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Aus New Zeal J Psychiatry 44:697–705. [DOI] [PubMed] [Google Scholar]
- Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dubé EM. (2002) Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. J Clin Psychiatry 63:577–584. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Emsley R, Rembry S, Rouillon F. (2009) Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 70:1128–1137. [DOI] [PubMed] [Google Scholar]
- Gortner ET, Gollan JK, Dobson KS, Jacobson NS. (1998) Cognitive-behavioral treatment for depression: relapse prevention. J Consult Clin Psychol 66:377–384. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Leong SA, Birnbaum HG, Robinson RL. (2003) Economic burden of depression with painful symptoms. J Clin Psychiatry 64:17–23. [PubMed] [Google Scholar]
- Gupta S, Tobiansky R, Bassett P, Warner J. (2008) Efficacy of maintenance electroconvulsive therapy in recurrent depression: a naturalistic study. J ECT 24:191–194 [DOI] [PubMed] [Google Scholar]
- Harrison W, Rabkin J, Stewart JW, McGrath PJ, Tricamo E, Quitkin F. (1986) Phenelzine for chronic depression: a study of continuation treatment. J Clin Psychiatry 47:346–349. [PubMed] [Google Scholar]
- Hochstrasser B, Isaksen PM, Koponen H, Lauritzen L, Mahnert FA, Rouillon F, Wade AG, Andersen M, Pedersen SF, Swart JC, Nil R. (2001) Prophylactic effect of citalopram in unipolar, recurrent depression: placebo-controlled study of maintenance therapy. Br J Psychiatry 178:304–310. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, Lovett ML, Young PR, Haman KL, Freeman BB, Gallop R. (2005) Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry 62:417–422. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. (2001) Preventing recurrent depression using cognitive therapy with and without a continuation phase: randomized clinical trial. Arch Gen Psychiatry 58:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Quitkin FM, Rifkin A, Ramos-Lorenzi JR, Nayak DD, Howard A. (1982) Lithium carbonate and imipramine in the prophylaxis of unipolar and bipolar I1 illness: prospective, placebo-controlled comparison. Arch Gen Psychiatry 39:1065–1069. [DOI] [PubMed] [Google Scholar]
- Kasper S, Volz HP, Moller HJ, Dienel A, Kieser M. (2008) Continuation and long-term maintenance treatment with Hypericum extract WS-5570 after recovery from an acute episode of moderate depression: double-blind, randomized, placebo controlled long-term trial. Eur Neuropsychopharmacol 18:803–813. [DOI] [PubMed] [Google Scholar]
- Katon W, Rutter C, Ludman EJ, Von Korff M, Lin E, Simon G, Bush T, Walker E, Unützer J. (2001) Randomized trial of relapse prevention of depression in primary care. Arch Gen Psychiatry 58:241–247. [DOI] [PubMed] [Google Scholar]
- Kelin K, Berk M, Spann M, Sagman D, Raskin J Walker D, Perahia D. (2010) Duloxetine 60mg/day for the prevention of depressive recurrences: post hoc analyses from a recurrence prevention study. Int J Clin Pract 64:719–726. [DOI] [PubMed] [Google Scholar]
- Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, Schatzberg A, Russell J, Hirschfeld R, Klein D, McCullough JP, Fawcett JA, Kornstein S, LaVange L, Harrison W. (1998) Maintenance phase efficacy of sertraline for chronic depression: randomized controlled trial. JAMA 280:1665–1672. [DOI] [PubMed] [Google Scholar]
- Keller MB, Ruwe FJ, Janssens CJ, Sitsen JM, Jokinen R, Janczewski J. (2005) Relapse prevention with gepirone-ER in outpatients with major depression. J Clin Psychopharmacol 25:79–84. [DOI] [PubMed] [Google Scholar]
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, Friedman ES, Gelenberg AJ, Kocsis JH, Dunner DL, Hirschfeld RM, Rothschild AJ, Ferguson JM, Schatzberg AF, Zajecka JM, Pedersen RD, Yan B, Ahmed S, Musgnung J, Ninan PT. (2007a) Prevention of recurrent episodes of depression with venlafaxine for two years (PREVENT) study: outcomes from the 2-year and combined maintenance phases. J Clin Psychiatry 68:1246–1256. [DOI] [PubMed] [Google Scholar]
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, Friedman ES, Gelenberg AJ, Kocsis JH, Dunner DL, Dunlop BW, Hirschfeld RM, Rothschild AJ, Ferguson JM, Schatzberg AF, Zajecka JM, Pedersen R, Yan B, Ahmed S, Schmidt M. (2007b) Prevention of recurrent episodes of depression with venlafaxine for two years (PREVENT) study: outcomes from the acute and continuation phases. Biol Psychiatry 62:1371–1379. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, Mueller M, Bernstein HJ, O’Connor K, Smith G, Biggs M, Bailine SH, Malur C, Yim E, McClintock S, Sampson S, Fink M. (2006) Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry 63:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A, Mizukawa R, Matsuzaki F, Hazama H, Kamase H, Tanaka K, Kunimoto N. (1994) Prophylactic effect of mianserin on recurrent depression. Acta Psychiatr Scand 89:46–51. [DOI] [PubMed] [Google Scholar]
- Klein DN, Santiago NJ, Vivian D, Blalock JA, Kocsis JH, Markowitz JC, McCullough JP, Jr, Rush AJ, Trivedi MH, Arnow BA, Dunner DL, Manber R, Rothbaum B, Thase ME, Keitner GI, Miller IW, Keller MB. (2004) Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. J Consult Clin Psychol 72:681–688. [DOI] [PubMed] [Google Scholar]
- Klerman GL, DiMascio A, Weissman M, Prusoff B, Paykel ES. (1974) Treatment of depression by drugs and psychotherapy. Am J Psych 131:186–191. [DOI] [PubMed] [Google Scholar]
- Klysner R, Bent-Hansen J, Hansen HL, Lunde M, Pleidrup E, Poulsen DL, Andersen M, Petersen HE. (2002) Efficacy of citalopram in the prevention of recurrent depression in elderly patients: placebocontrolled study of maintenance therapy. Br J Psychiatry 181:29–35. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, Parides M. (1996) Maintenance therapy for chronic depression. A controlled clinical trial of desipramine. Arch Gen Psychiatry 53:769–776. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Thase ME, Trivedi MH, Shelton RC, Kornstein SG, Nemeroff CB, Friedman ES, Gelenberg AJ, Dunner DL, Hirschfeld RM, Rothschild AJ, Ferguson JM, Schatzberg AF, Zajecka JM, Pederson RD, Yan B, Ahmed S, Musgnung J, Ninan PT, Keller MB. (2007) Prevention of recurrent episodes of depression with venlafaxine-ER in a 1-year maintenance phase from the PREVENT Study. J Clin Psychiatry 68:1014–1023. [DOI] [PubMed] [Google Scholar]
- Kok RM, Heeren TJ, Nolen WA. (2011) Continuing treatment of depression in the elderly: a systematic review and meta-analysis of double-blinded randomized controlled trials with antidepressants. Am J Geriat Psychiatry 19:249–255. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Bose A, Li D, Saikali KG, Gandhi C. (2006) Escitalopram maintenance treatment for prevention of recurrent depression: randomized, placebo-controlled trial. J Clin Psychiatry 67:1767–1775. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Perel JM, Cornes C, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ. (1992) Five-year outcome for maintenance therapies in recurrent depression. Arch Gen Psychiatry 49:769–773. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Barrett B, Byng R, Evans A, Mullan E, Teasdale JD. (2008) Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol 76:966–978. [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, Parikh SV, Patten SB, Ravindran AV. (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord 117(Supp 1):S26–43. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Odgaard K, Clemmesen L, Lunde M, Ohrstrom J, Black C, Bech P. (1996) Relapse prevention by means of paroxetine in ECT-treated patients with major depression: a comparison with imipramine and placebo in medium-term continuation therapy. Acta Psychiatr Scand 94:241–251. [DOI] [PubMed] [Google Scholar]
- Lendresse PH, Chen MC, LeMarie JC. (1985) Traitement prolongé par nomifensine 75mg dans les états dépressifs neurotiques et reactionnels. Psychiatrie Francaise 16:156–158. [Google Scholar]
- Lepine JP, Caillard V, Bisserbe JC, Troy S, Hotton JM, Boyer P. (2004) Randomized, placebo-controlled trial of sertraline for prophylactic treatment of highly recurrent major depressive disorder. Am J Psych 161:836–842. [DOI] [PubMed] [Google Scholar]
- Liebowitz M, Lam RW, Lepola U, Datto C, Sweitzer D, Eriksson H. (2010) Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: a randomized, placebo-controlled trial. Depress Anxiety 27:964–976. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. (2004) Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol 72:31–40. [DOI] [PubMed] [Google Scholar]
- Maj M, Veltro F, Pirozzi R, Lobrace S, Magliano L. (1992) Pattern of recurrence of illness after recovery from an episode of major depression: prospective study. Am J Psych 149:795–800. [DOI] [PubMed] [Google Scholar]
- Mathew KL, Whitford HS, Kenny MA, Denson LA. (2010) Long-term effects of mindfulness-based cognitive therapy as a relapse prevention treatment for major depressive disorder. Behav Cogn Psychother 38:561–576. [DOI] [PubMed] [Google Scholar]
- Mindham RH, Howland C, Shepherd M. (1973) Evaluation of continuation therapy with tricyclic antidepressants in depressive illness. Psychol Med 3:5–17. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Dunbar G. (1993a) Paroxetine is better than placebo in relapse prevention and the prophylaxis of recurrent depression. Int Clin Psychopharmacol 8:189–195. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Dufour H, Brion S, Gailledreau J, Laqueille X, Ferrey G, Moron P, Parant-Lucena N, Singer L, Danion JM, Beuzen JN, Pierredoin MA. (1988) Prophylactic efficacy of fluoxetine in unipolar depression. Br J Psychiatry 153:S69–76. [PubMed] [Google Scholar]
- Montgomery SA, Rasmussen JG, Tanghøj P. (1993b) A 24-week study of 20mg citalopram, 40mg citalopram, and placebo in the prevention of relapse of major depression. Int Clin Psychopharmacol 8:181–188. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Reimitz PE, Zivkov M. (1998) Mirtazapine vs. amitriptyline in the long-term treatment of depression: double-blind placebo-controlled study. Int Clin Psychopharmacol 13:63–73. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Entsuah R, Hackett D, Kunz NR, Rudolph RL. (2004) Venlafaxine versus placebo in the preventive treatment of recurrent major depression. J Clin Psychiatry 65:328–336. [DOI] [PubMed] [Google Scholar]
- Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D. (1995) Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. The BMJ 310:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old Age Depression Interest Group (OADIG) (1993) How long should the elderly take antidepressants? Double-blind placebo-controlled study of continuatiodprophylaxis therapy with dothiepin. Br J Psychiatry 162:175–182. [PubMed] [Google Scholar]
- Perahia DG, Gilaberte I, Wang F, Wiltse CG, Huckins SA, Clemens JW, Montgomery SA, Montejo AL, Detke MJ. (2006) Duloxetine in the prevention of relapse of major depressive disorder: double-blind placebo-controlled study. Br J Psychiatry 188:346–353. [DOI] [PubMed] [Google Scholar]
- Pilling S, Anderson I, Goldberg D, Meader N, Taylor C. (2009) Two guideline development groups: depression in adults, including those with a chronic physical health problem: summary of NICE guidance. The BMJ 339:b4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, Johnson WE. (1984) Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 41:1096–1104. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, McGrath PJ, Stewart JW, Harrison W, Wager SG, Nunes E, Rabkin JG, Tricamo E, Markowitz J, Klein DF. (1989) Phenelzine and imipramine in mood reactive depressives: further delineation of the syndrome of atypical depression. Arch Gen Psychiatry 46:787–793. [DOI] [PubMed] [Google Scholar]
- Reynolds CF III, Frank E, Perel JM, Imber SD, Cornes C, Miller MD, Mazumdar S, Houck PR, Dew MA, Stack JA, Pollock BG, Kupfer DJ. (1999) Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: randomized controlled trial in patients older than 59 years. JAMA 28:39–45. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, Houck PR, Mazumdar S, Butters MA, Stack JA, Schlernitzauer MA, Whyte EM, Gildengers A, Karp J, Lenze E, Szanto K, Bensasi S, Kupder DJ. (2006) Maintenance treatment of major depression in old age. N Engl J Med 354:1130–1138. [DOI] [PubMed] [Google Scholar]
- Rickels K, Montgomery SA, Tourian KA, Guelfi JD, Pitrosky B, Padmanabhan SK, Germain JM, Leurent C, Brisard C. (2010) Desvenlafaxine for the prevention of relapse in major depressive disorder: results of a randomized trial. J Clin Psychopharmacol 30:18–24. [DOI] [PubMed] [Google Scholar]
- Robert P, Montgomery SA. (1995) Citalopram in doses of 20–60mg is effective in depression relapse prevention: placebo-controlled 6 month study. Int Clin Psychopharmacol 10:S29–35. [PubMed] [Google Scholar]
- Robinson DS, Lerfald SC, Bennett B, Laux D, Devereaux E, Kayser A, Corcella J, Albright D. (1991) Continuation and maintenance treatment of major depression with the monoamine oxidase inhibitor phenelzine: a double-blind placebo-controlled discontinuation study. Psychopharmacol Bull 27:31–39. [PubMed] [Google Scholar]
- Rosenthal JZ, Boyer P, Vialet C, Hwang E, Tourian KA. (2013) Efficacy and safety of desvenlafaxine 50mg/day for prevention of relapse in major depressive disorder:a randomized controlled trial. J Clin Psychiatry 74:158–166. [DOI] [PubMed] [Google Scholar]
- Rouillon F, Phillips R, Serrurier D, Ansart E, Gérard MJ. (1989) Recurrence of unipolar depression and efficacy of maprotiline. Encephale 15:527–34. [PubMed] [Google Scholar]
- Rouillon F, Warner B, Pezous N, Bisserbe JC. (2000) Milnacipran efficacy in the prevention of recurrent depression: 12-month placebo-controlled study. Int Clin Psychopharmacol 15:133–140. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J. (2001) Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: randomized controlled trial. JAMA 285:1299–1307. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Fava M, Robinson JM, Judge R. (2000) Efficacy and safety of a new enteric-coated formulation of fluoxetine given once weekly during the continuation treatment of major depressive disorder. J Clin Psychiatry 61:851–857. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Bloch R, Levitan RD. (2010) Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry 67:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF, Pilkonis PA, Beckham E, Glass DR, Dolan RT, Parlodd MB. (1992) Course of depressive symptoms over follow-up: findings from the National Institute of Mental Health treatment of depression collaborative research program. Arch Gen Psychiatry 49:782–787. [DOI] [PubMed] [Google Scholar]
- Sheets ES, Wilcoxon-Craighead L, Brosse AL, Hauser M, Madsen JW, Edward-Craighead W. (2013) Prevention of recurrence of major depression among emerging adults by a group cognitive behavioral/interpersonal intervention. J Affect Disord 147:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu K, Shimodera S, Mino Y, Nishida A, Kamimura N, Sawada K, Fujita H, Furukawa TA, Inoue S. (2011) Family psychoeducation for major depression: randomised controlled trial. Br J Psychiatry 198:385–390. [DOI] [PubMed] [Google Scholar]
- Shimodera S, Furukawa TA, Mino Y, Shimazu K, Nishida A, Inoue S. (2012) Cost-effectiveness of family psychoeducation to prevent relapse in major depression: results from a randomized controlled trial. BMC Psychiatry 12:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone PH, Ravindran A. (1999) Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry 60:22–28. [DOI] [PubMed] [Google Scholar]
- Stein MK, Rickels K, Weise CC. (1980) Maintenance therapy with amitriptyline: controlled trial. Am J Psych 137:370–371. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Tricamo E, McGrath PJ, Quitkin FM. (1997) Prophylactic efficacy of phenelzine and imipramine in chronic atypical depression: likelihood of recurrence on discontinuation after 6 months’ remission. Am J Psych 154:31–36. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. (2000) Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 68:615–623. [DOI] [PubMed] [Google Scholar]
- Terra JL, Montgomery SA. (1998) Fluvoxamine prevents recurrence of depression: results of a long-term, double-blind, placebo-controlled study. Int Clin Psychopharmacol 13:55–62. [PubMed] [Google Scholar]
- Thase ME, Nierenberg AA, Keller MB, Panagides J. (2001) Efficacy of mirtazapine for prevention of depressive relapse: placebo-controlled double-blind trial of recently remitted high-risk patients. J Clin Psychiatry 62:782–788. [DOI] [PubMed] [Google Scholar]
- Thase ME, Gelenberg A, Kornstein SG, Kocsis JH, Trivedi MH, Ninan P, Li T, Pedersen R, Keller M. (2011) Comparing venlafaxine extended release and fluoxetine for preventing the recurrence of major depression: results from the PREVENT study. J Psychiatr Res 45:412–420. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Morris S. (2003) Cost of depression among adults in England in 2000. Br J Psychiatry 183:514–519. [DOI] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ. (2012) A 30-year meta-analytic review of antidepressant efficacy in acute major depression. Neuropsychopharmacology 37:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusekom BS, van den Broek WW, Birkenhäger TK. (2007) Long-term follow-up after successful electroconvulsive therapy for depression: 4- to 8-year naturalistic follow-up study. J ECT 23:17–20. [DOI] [PubMed] [Google Scholar]
- van den Broek WW, Birkenhager TK, Mulder PG, Bruijn JA, Moleman P. (2006) Imipramine is effective in preventing relapse in electroconvulsive therapy-responsive depressed inpatients with prior pharmacotherapy treatment failure: randomized, placebo-controlled trial. J Clin Psychiatry 67:263–268. [DOI] [PubMed] [Google Scholar]
- Van Praag H, De Haan S. (1980) Depression vulnerability and 5-hydroxytryptophan prophylaxis. Psychiatry Res 3:75–83. [DOI] [PubMed] [Google Scholar]
- Versiani M, Mehilane L, Gaszner P, Arnaud CR. (1999) Reboxetine, a unique selective NRI, prevents relapse and recurrence in long-term treatment of major depressive disorder. J Clin Psychiatry 60:400–406. [DOI] [PubMed] [Google Scholar]
- Weihs KL, Houser TL, Batey SR, Ascher JA, Bolden-Watson C, Donahue RM, Metz A. (2002) Continuation phase treatment with bupropion-SR effectively decreases the risk for relapse of depression. Biol Psychiatry 51:753–761. [DOI] [PubMed] [Google Scholar]
- Wilkinson P, Izmeth Z. (2012) Continuation and maintenance treatments for depression in older people. Cochrane Database Syst Rev 11:CD006727. [DOI] [PubMed] [Google Scholar]
- Williams N, Simpson AN, Simpson K, Nahas Z. (2009) Relapse rates with long-term antidepressant drug therapy: a meta-analysis. Hum Psychopharmacol 24:401–408. [DOI] [PubMed] [Google Scholar]
- Wilson KCM, Mottram PG, Ashworth L, Abou-saleh MT. (2003) Older community residents with depression: long-term treatment with sertraline. Br J Psychiatry 182:492–497. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2012) Global burden of disease: depression, 2012. http://www.who.int/mediacentre/factsheets/fs369/en/: Accessed 7 May 2015. [Google Scholar]
- Yildiz A, Mantar A, Simsek S, Onur E, Gokmen N, Fidaner H. (2010) Combination of pharmacotherapy with electroconvulsive therapy in prevention of depressive relapse: pilot controlled trial. J ECT 26:104–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.