Abstract
Background:
Postsynaptically generated 2-arachidonoylglycerol activates the presynaptic cannabinoid type-1 receptor, which is involved in synaptic plasticity at both glutamatergic and GABAergic synapses. However, the differential function of 2-arachidonoylglycerol signaling at glutamatergic vs GABAergic synapses in the context of animal behavior has not been investigated yet.
Methods:
Here, we analyzed the role of 2-arachidonoylglycerol signaling selectively in hippocampal glutamatergic neurons. Monoacylglycerol lipase, the primary degrading enzyme of 2-arachidonoylglycerol, is expressed at presynaptic sites of excitatory and inhibitory neurons. By adeno-associated virus-mediated overexpression of monoacylglycerol lipase in glutamatergic neurons of the mouse hippocampus, we selectively interfered with 2-arachidonoylglycerol signaling at glutamatergic synapses of these neurons.
Results:
Genetic modification of monoacylglycerol lipase resulted in a 50% decrease in 2-arachidonoylglycerol tissue levels without affecting the content of the second major endocannabinoid anandamide. A typical electrophysiological read-out for 2-arachidonoylglycerol signaling is the depolarization-induced suppression of excitation and of inhibition. Elevated monoacylglycerol lipase levels at glutamatergic terminals selectively impaired depolarization-induced suppression of excitation, while depolarization-induced suppression of inhibition was not significantly changed. At the behavioral level, mice with impaired hippocampal glutamatergic 2-arachidonoylglycerol signaling exhibited increased anxiety-like behavior but showed no alterations in aversive memory formation and seizure susceptibility.
Conclusion:
Our data indicate that 2-arachidonoylglycerol signaling selectively in hippocampal glutamatergic neurons is essential for the animal’s adaptation to aversive situations.
Keywords: Monoacylglycerol lipase, endocannabinoids, hippocampus, anxiety, epilepsy
Introduction
The endocannabinoid system involves 2 major signaling molecules, 2-arachidonoyl glycerol (2-AG) and anandamide (AEA), which activate the cannabinoid type-1 receptor (CB1R). Unlike classical neurotransmitters such as amino acids and peptides, which are stored in synaptic vesicles, endocannabinoids are thought to be produced and released on demand (Alger, 2012; Castillo et al., 2012). Hence, their endogenous levels determine the magnitude and duration of CB1R stimulation and are generally regulated by endocannabinoid synthesizing and degrading enzymes. In the adult brain, 2-AG is primarily produced by diacylglycerol lipase-α (DAGLα) at the postsynaptic site and hydrolyzed to glycerol and arachidonic acid by monoacylgycerol lipase (MAGL) at the presynaptic site (Gao et al., 2010; Schlosburg et al., 2010; Tanimura et al., 2010; Pan et al., 2011; Uchigashima et al., 2011). MAGL is heterogeneously distributed with high expression levels in brain regions where CB1R is also abundant, such as hippocampus, cerebral cortex, and cerebellum (Dinh et al., 2002; Gulyas et al., 2004; Uchigashima et al., 2011).
Postsynaptically generated endocannabinoids mediate retrograde signaling mechanisms called depolarization-induced suppression of excitation (DSE) and inhibition (DSI) at excitatory and inhibitory synapses, respectively (Alger, 2012). These forms of short-term depression were shown to involve 2-AG–induced activation of CB1R, because DSE and DSI are abolished in DAGLα knockout mice (Gao et al., 2010; Tanimura et al., 2010) and prolonged in MAGL-deficient mice (Pan et al., 2011; Zhong et al., 2011). However, DAGLα and MAGL knockout animal models have not allowed discrimination between 2-AG signaling effects at glutamatergic vs GABAergic synapses. In this regard, it is also relevant to mention that cell type-selective CB1R deficiencies revealed different roles of CB1R in these 2 neuronal populations in the forebrain, eg, regarding anxiety-like behavior (Rey et al., 2012; Ruehle et al., 2013), fear memory (Ruehle et al., 2013), feeding behavior (Soria-Gómez et al., 2014), social interaction (Häring et al., 2011), and susceptibility to chemically-induced seizures (Monory et al., 2006).
Therefore, we aimed at selectively interfering with the availability of 2-AG at the synaptic terminal of a defined neuronal population within a brain region with established endocannabinoid system functions. Therefore, we generated a mouse model in which MAGL is selectively overexpressed at glutamatergic synapses in the hippocampus. Using an established electrophysiological read-out, DSE was found to be significantly reduced with no concomitant alterations on DSI. At the behavioral level, mice overexpressing MAGL showed an increased anxiety-like behavior but no changes in aversive memory formation or seizure susceptibility to kainic acid (KA).
Materials and Methods
Animals
Adult male mice were housed in groups in a temperature- and humidity-controlled room (22°C±1; 50%±1) with a 12-hour light-dark cycle and access to food and water ad libitum. All experiments were approved by the local animal care committee (Muenster: LANUV-NRW 8.87-51.05.20.10.218; Mainz: 23-177-07/G13-1021 and 23-177-07/G10-1037). NEX-Cre mice (C57BL/6N background) drive Cre expression in dorsal telencephalic glutamatergic neurons, with the exception of granule cells of the dentate gyrus (Goebbels et al., 2006; Monory et al., 2006). Note that in adult NEX-Cre mice, Cre recombinase is expressed in hippocampal pyramidal neurons (Guggenhuber et al., 2010).
Adeno-Associated Virus Vector Generation and Administration
The N-terminally hemagglutinin (HA)-tagged mouse MAGL cDNA was cloned into an adeno-associated virus (AAV) plasmid containing the cytomegalovirus enhancer/chicken β-actin promoter, the woodchuck hepatitis virus posttranscriptional regulatory element, and the bovine growth hormone polyadenylation sequence flanked by AAV2 inverted terminal repeats. A transcriptional terminator element (“Stop”), flanked by loxP sites, was used to enable cell type-selective transgene expression (Guggenhuber et al., 2010; Häring et al., 2012). Production of pseudotyped rAAV1/2 mosaic vectors and determination of genomic titers were performed as described (Guggenhuber et al., 2010). Adult male mice (6–9 weeks of age) were anesthetized by intraperitoneal injection of fentanyl (0.05mg/kg), midazolam (5mg/kg), and medetomidin (0.5mg/kg) and positioned in a small animal stereotaxic frame (Kopf instruments, Tujunga, CA). One microliter of AAV-Stop-MAGL vector (1.5×1011 vector copies/mL) was injected bilaterally into the hippocampus (-2.0mm AP, ±2.0mm ML, -2.0mm DV of bregma) at a rate of 150 nL/min using a microprocessor controlled mini-pump with 34G beveled needles (World Precision Instruments, Sarasota, FA). AAV-Stop-MAGL vector injection into hippocampi of NEX-Cre mice led to the excision of the transcriptional Stop cassette in all Cre recombinase-positive cells, resulting in transcriptional activation and overexpression of virally encoded HA-MAGL cDNA in hippocampal glutamatergic neurons of the CA1-3 region but not in dentate gyrus granule cells. Transgenic HA-MAGL expression was analyzed by immunohistochemistry against the HA tag in hippocampal sections of every mouse examined in behavioral tests. AAV-Glu-MAGL mice were excluded from the study, if HA immunostaining did not substantially cover CA1-CA3 areas of the hippocampus.
Immunohistochemistry
The rostral-caudal extent of transgene expression was assessed by immunohistochemistry. Brain sections were immunostained as previously described (Guggenhuber et al., 2010) using a rabbit anti-HA primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000). For colocalization studies, rabbit anti-MAGL (generous gift from Ken Mackie, 1:200), guinea pig anti-VGluT1 (Millipore, 1:500), and rabbit anti-CB1R (Frontier Institute, Hokkaido, Japan, 1:500) primary antibodies were used.
Western Blot
Immunoblot was performed as previously described (Ruehle et al., 2013) using the primary antibodies rabbit anti-HA (Santa Cruz Biotechnology; 1:1000), rabbit anti-MAGL (gift from Ken Mackie; 1:2000), rabbit anti-DAGLα (gift from Ken Mackie, 1:500), rabbit anti-CB1R (Frontier Institute, Hokkaido, Japan, 1:500), and mouse anti-tubulin (Sigma-Aldrich, St. Louis, MO; 1:100000). Quantification analyses were done on 4 independent samples for HA-MAGL and MAGL and on 8 independent samples for CB1R and DAGLα. Signal detection was achieved by ECL Prime Western blotting detection reagent (GE healthcare) and the Peqlab FUSION-SL Advance 4.2 MP analyzer. Densitrometric data were related to tubulin and normalized to the wild-type condition.
MAGL Activity Assay
The MAGL activity assay is based on the hydrolysis of 4-nitrophenyl acetate (4-NPA) by MAGL, resulting in the product 4-nitrophenol, a chromogenic molecule with absorbance at 405 to 412nm. Isolated dorsal mouse hippocampi were homogenized in 400 μL ice-cold assay buffer (100mM Tris-HCl, pH 7.4) and sonicated (Brandelin, Berlin, Germany). Protein content of the samples was determined using the BCATM protein assay kit (Pierce, Rockford, IL) according to the manufacturer’s protocol. Lysates were stored in aliquots at -80°C until use. For the standard curve, 4-nitrophenol (Sigma Aldrich, St. Louis, MO) was dissolved in 70% ethanol and diluted with assay buffer to obtain the following final concentrations: 15.625 μM, 31.25 μM, 62.5 μM, 125 μM, 250 μM, and 500 μM 4-NPA (Sigma Aldrich, St. Louis, MO). All standard and sample measurements were performed in duplicates in a 96-well plate in a total volume of 200 μL. Four μg of sample protein were incubated with the appropriate substrate concentration at 37°C, and the absorbance at 405nm was measured in the FLUOstar apparatus (BMG Labtech, Ortenberg, Germany) at time zero (baseline values) and after 20 minutes of incubation. As 4-NPA underlies chemical hydrolysis, measurements from wells containing buffer and 4-NPA only (no tissue) were used as controls, and their values were systematically subtracted from the sample values. The amount of 4-nitrophenol produced was calculated according to the standard curve, and baseline values were subtracted to obtain exclusively the metabolized 4-nitrophenol produced within the 20-minute incubation step.
Lipid Extraction and Liquid Chromatography/Multiple Reaction Monitoring
The extraction method used to obtain the results shown in Figure 3C-E was previously reported (Schulte et al., 2012). For results shown in Figure 6D-F, the extraction method was optimized as previously described (Lomazzo et al., 2015). Quantification of endocannabinoids was performed as reported before (Lomazzo et al., 2015).
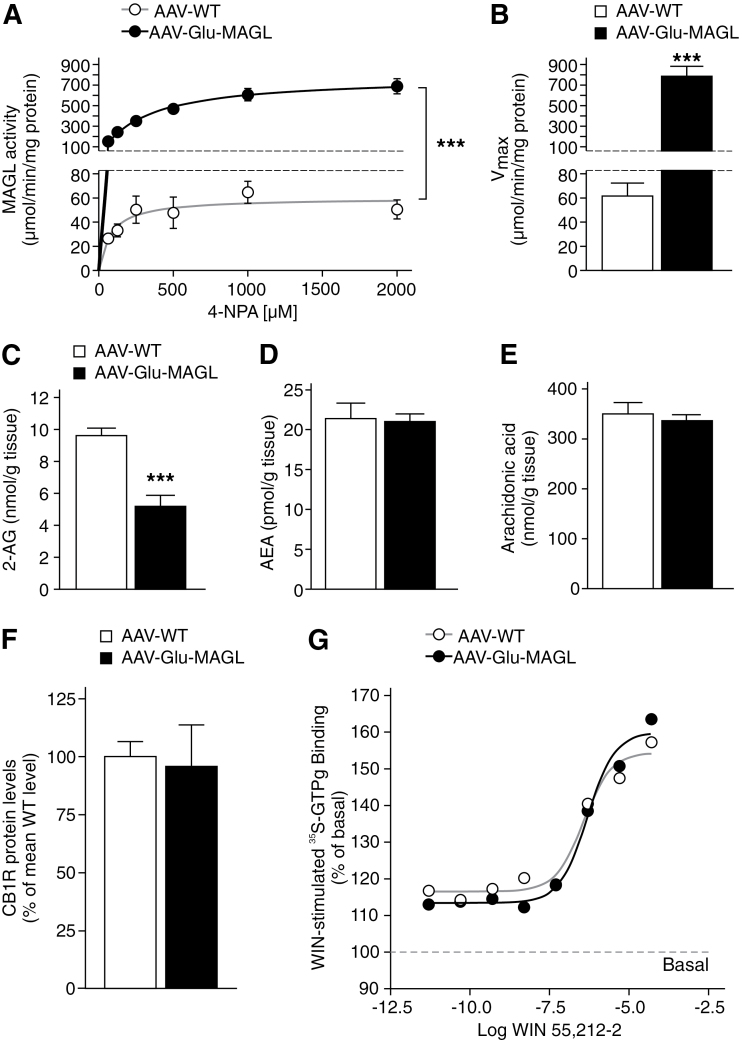
Figure 3.
Monoacylgycerol lipase (MAGL) overexpression in hippocampal pyramidal neurons leads to alteration in 2-arachidonoyl glycerol (2-AG) degradation and 2-AG levels. (A) Michaelis-Menten enzyme kinetics revealed highly elevated MAGL activity in adeno-associated virus (AAV)-Glu-MAGL mice compared with AAV-WT controls (n=4). (B) AAV-Glu-MAGL mice showed an increased maximum turnover rate of the substrate. (C-E) 2-AG levels were significantly lower in AAV-Glu-MAGL mice than in AAV-WT controls (n=6), while the levels of AEA and arachidonic acid were unchanged. ***P<.001. (F) Cannabinoid type-1 receptor (CB1R) protein levels were not altered in AAV-Glu-MAGL mice compared with AAV-WT (n=8). (G) Hippocampal homogenates were stimulated with increasing concentrations of WIN 55,212-2 (from 5 pM to 50 µM) to generate dose-response curves. EC50 values were similar in the 2 animal groups analyzed (0.36 µM and 0.48 µM in AAV-WT and AAV-Glu-MAGL, respectively). WIN 55,212-2-stimulated [35S]-GTPγS binding is expressed as percent of basal binding (n=4).
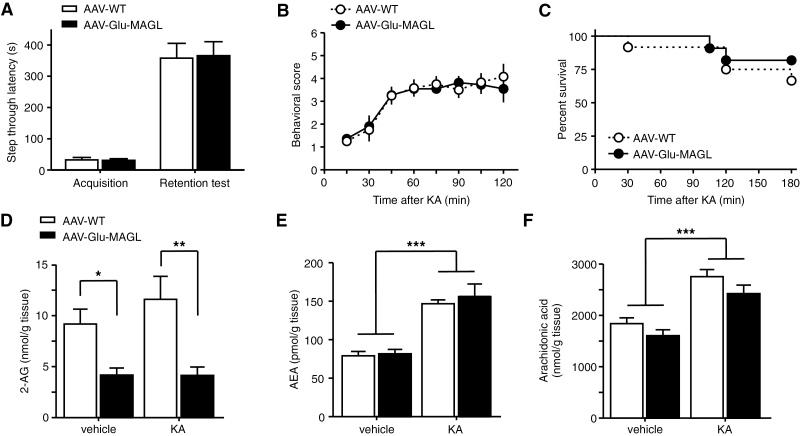
Figure 6.
Tests of memory performance and seizure susceptibility. (A) Hippocampal memory performance was analyzed in the passive avoidance test. During memory acquisition and 24 hours later (retention test), latencies to enter the dark chamber of adeno-associated virus (AAV)-Glu-MAGL did not differ from AAV-WT controls (n=23). (B) Seizures were induced by intraperitoneal injection of kainic acid (KA; 35mg/kg). Seizure severity was not altered in AAV-Glu-MAGL compared with AAV-WT controls (n=11–12). (C) Kaplan-Meier survival curves during KA treatment did not differ between the groups (n=11–12). (D-F) At 30 minutes after KA injection, 2-arachidonoyl glycerol (2-AG) levels (D) did not change significantly in response to KA, but remained decreased in AAV-Glu-MAGL mice compared with AAV-WT controls. In contrast, KA administration increased the levels of anandamide (AEA) (E) and arachidonic acid (F) to a similar magnitude in both groups (n=8). *P<.05, **P<.01, ***P<.001.
[35S]-GTPγS Binding
Mouse hippocampi were dissected on ice and homogenized in 1.2mL of assay buffer containing 50mM Tris HCl, pH 7.4, 100mM NaCl, 3mM MgCl2, 0.2mM EGTA, 0.5% BSA, and protease inhibitors (Complete cocktail tablets, Roche) by mechanical disruption with a glass homogenizer. An aliquot from these homogenates was used to evaluate the expression of MAGL by Western blot to check the efficiency of MAGL overexpression in AAV-Glu-MAGL mice. Protein concentration was measured by the BCA method (Pierce). Whole homogenates (4 μg of protein) were added in assay tubes containing assay buffer, 30 µM GDP (Sigma-Aldrich), and 50 pM [35S]-labeled GTP (Perkin Elmer). Agonist-stimulated binding was measured by using increasing concentrations (from 5 pM to 50 µM) of the CB1R agonist WIN 55,212-2 (Biotrend). Basal GTP binding activity was measured in absence of agonist. Nonspecific binding was defined in the presence of 10 µM unlabeled GTP (Roche Diagnostics). Assays were carried out in 4 independent experiments (n=4). In each independent experiment, agonist-stimulated binding was carried out in triplicate for each concentration of WIN 55,212-2. Basal and nonspecific bindings were carried out in 10 and 5 replicates, respectively. Specific agonist-stimulated and specific basal bindings were defined by subtracting nonspecific binding values. Assay tubes were then incubated for 1 hour and a half at 30°C. Bound receptors were separated from the free ligand by vacuum filtration over Brandel GF/B glass-fiber filters. Filters were dried for 1 hour at room temperature and counted in a liquid scintillation counter.
Electrophysiological Recordings
Whole-cell patch clamp-recordings were obtained from CA1 pyramidal cells as previously described (Ruehle et al., 2013) in slices prepared 3 to 4 weeks after AAV-Stop-MAGL injection and performed at 30°C. The intracellular solution was as previously described (Ruehle et al., 2013). The EGTA concentration was kept low in view of the Ca2+-dependence of DSE and DSI (Lenz and Alger, 1999). Postsynaptic excitatory (eEPSCs) or inhibitory (eIPSCs) currents, evoked upon local electrical stimulation of the stratum radiatum, were pharmacologically isolated, and DSE or DSI was tested by application of a depolarization step (−70 to 0 mV, 3 seconds, and 10 seconds). At least 2 DSE or DSI tests (at each duration) were applied to each cell, and baseline was calculated from each evoked postsynaptic current (ePSC), divided by the average of all ePSCs before the depolarization step (multiplied by 100%). Magnitudes of DSE and DSI were calculated as follows: Δ of ePSCs=[(×2 – ×1)/×1] * 100, where ×1=mean of last 5 ePSCs amplitudes before the depolarization, and ×2=mean of first 3 ePSCs amplitudes immediately after the depolarization. A significant deviation from zero was considered as DSE or DSI.
Behavior
Two weeks prior to behavioral phenotyping, animals were single-housed to avoid confounding influences of social status. All experiments were performed 4 weeks after AAV injection in the animal’s light phase in the following order: elevated plus maze (EPM), light/dark test, open field, passive avoidance, and KA-induced seizures. Tests for anxiety, locomotion, and avoidance learning were performed in 2 independent experiments, each group comprising 10 to 12 animals, to obtain robust and reproducible data.
EPM and light/dark test were performed as previously described (Ruehle et al., 2013). Light intensity in the open arms of EPM was set at 40 lux and for the light/dark test at 100 lux.
Open Field
Mice were placed in one corner of an illuminated (100 lux) white box (40 cm×40 cm×40cm) and allowed to freely explore the environment for 10 minutes. The center was defined as a square comprising 13cm × 13cm.
Passive Avoidance
The passive avoidance apparatus consisted of a white, highly illuminated compartment and a black, dark compartment, both connected by a door (Ugo Basile, Comerio, Italy). Mice were placed in the lit compartment, and after 30 seconds, the door was automatically opened. When mice entered the dark compartment, the door closed automatically and a mild foot shock (0.3 mA, 2 seconds) was delivered to the animal. Mice learn to associate the dark compartment with the foot shock. In the retention test 24 hours later, mice were subjected to the same procedure but without footshock, and the latency to enter the dark compartment was used as an indicator of learning and memory.
Chemically Induced Seizures
Acute epileptiform seizures were induced by intraperitoneal injection of KA (35mg/kg) and analyzed as previously described (Guggenhuber et al., 2010).
Data Analysis
Data are presented as means±SEM and were analyzed with SPSS 19 Statistics Software for Windows (IBM). Unpaired, 2-tailed t test was used to analyze normally distributed data. For the assessment of MAGL activity, data were analyzed by Michaelis-Menten kinetics with GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA) and nonlinear regression curve fitting revealing maximum turnover rates of the samples, followed by 2-way ANOVA. For [35S]-GTPγS binding assays, EC50 values were determined by fitting saturation binding data to one binding site by nonlinear regression analysis using the GraphPad Prism 4.0 software. WIN 55,212-2-stimulated binding was expressed as percent of basal binding. Data are presented as means±SEM from 4 independent experiments with each individual sample run in triplicate. Electrophysiological data were analyzed with GraphPad Prism and Statistica (Statsoft, Inc) using unpaired t test or 1-tailed Mann-Whitney test (for small sample size). Repeated-measures ANOVA was used to analyze seizure severity. The Kaplan-Meier method was used to evaluate survival, followed by the log rank test. Two-way ANOVA followed by simple effects analysis was used to analyze lipid levels after vehicle and KA injection. P<.05 was set as value to define statistical significance. Densitometric measurement of immunoblot signals was performed by using Bio1D software (Vilber Lourmat, Eberhardzell, Germany). Graphs were generated with GraphPad Prism software.
Results
Efficient Overexpression of MAGL in Hippocampal Pyramidal Neurons
We previously developed a viral approach to induce transgene expression in a selective cell population of a particular brain region induced by Cre recombinase-mediated excision of a transcriptional Stop element (Guggenhuber et al., 2010). In our construct, a human influenza HA epitope tag was fused to the N terminus of MAGL, and an AAV vector was produced (termed AAV-Stop-MAGL vector). To overexpress MAGL exclusively in hippocampal pyramidal neurons, the AAV-Stop-MAGL vector was injected bilaterally into the hippocampus of adult NEX-Cre mice (termed AAV-Glu-MAGL), which express Cre recombinase selectively in cortical and CA1-CA3 hippocampal glutamatergic neurons but not in granule cells of the dentate gyrus (Goebbels et al., 2006; Monory et al., 2006; Guggenhuber et al., 2010). In Cre recombinase-positive neurons, the transcriptional Stop cassette in the AAV-Stop-MAGL vector was excised, which enabled the transcription of the virally encoded HA-tagged MAGL gene, representing the AAV-Glu-MAGL mouse group. The control group was generated by injection of the AAV-Stop-MAGL vector into the hippocampus of wild-type littermates of the NEX-Cre line, which did not express Cre recombinase (termed AAV-WT).
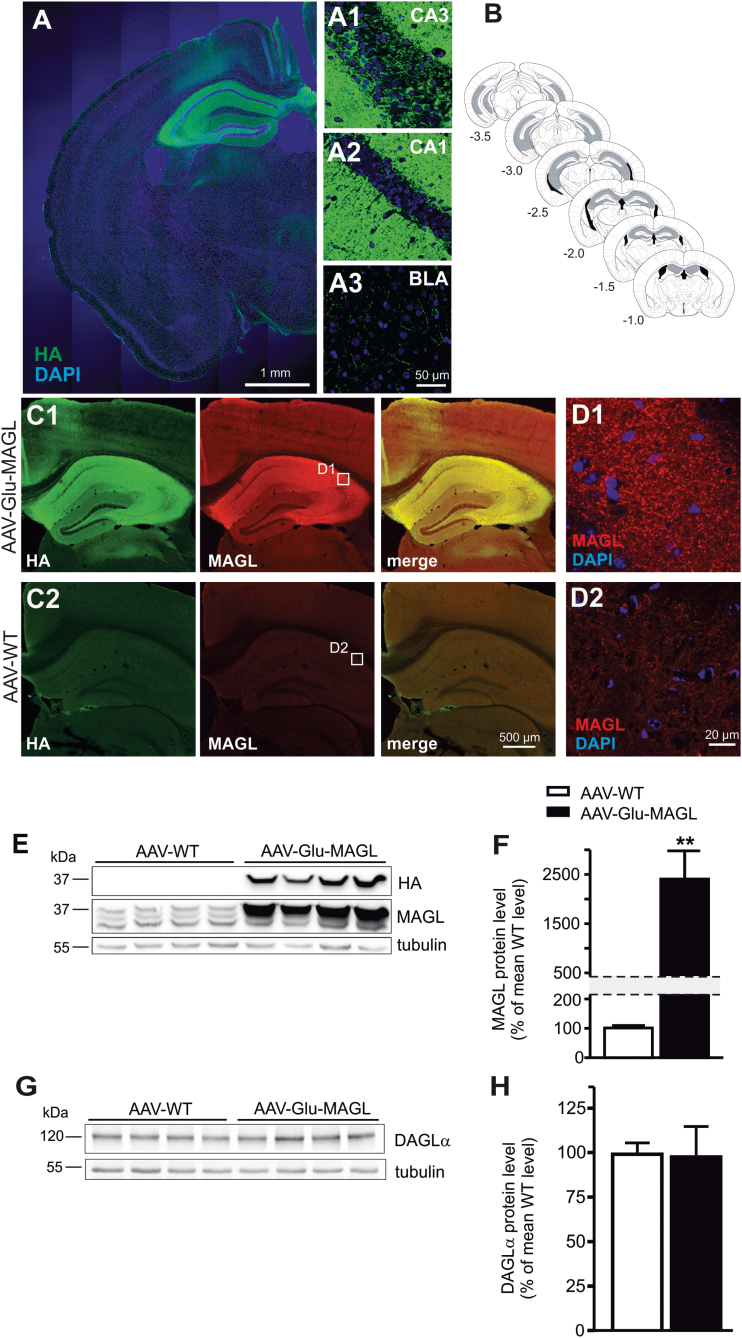
Immunostaining for the HA-tag revealed widespread transgene expression in the hippocampus of AAV-Glu-MAGL mice (Figure 1A) but also in extra-hippocampal areas, such as the basolateral amygdala (BLA), although at highly reduced levels (Figure 1A 3) compared with hippocampus (Figure 1A 1-2). The rostrocaudal AAV vector spread in the hippocampus was >2.5mm (Figure 1B). As detected by immunohistochemistry using the anti-MAGL antibody, strong hippocampal overexpression of HA-tagged MAGL in AAV-Glu-MAGL (Figure 1C 1) was observed compared with endogenous MAGL in AAV-WT (Figure 1C 2). The overexpressed HA-tagged MAGL showed a punctate expression (Figure 1D 1) similar to that of endogenous MAGL (Figure 1D 2). Western-blot analysis of hippocampal lysates against the HA-tag confirmed exclusive transgene expression in AAV-Glu-MAGL (Figure 1E). MAGL protein levels were highly elevated in AAV-Glu-MAGL compared with AAV-WT controls (P=.008; unpaired t test) (Figure 1F). To address a possible compensatory effect upon strong overexpression of MAGL, which may lead to an impairment of 2-AG signaling, we quantified the expression of the major 2-AG synthesizing enzyme DAGLα by Western-blot analysis (Figure 1G). No differences in DAGLα protein levels were observed between the 2 genotypes (P=.818; unpaired t test) (Figure 1H).
Figure 1.
Monoacylgycerol lipase (MAGL) overexpression in hippocampal pyramidal neurons. (A) Overview representation of a section of one brain hemisphere, showing hemagglutinin (HA) immunostaining and indicating strong MAGL transgene expression in hippocampal pyramidal neurons of adeno-associated virus (AAV)-Glu-MAGL mice. (1–3) Higher magnification of HA immunostaining of CA3 (1), CA1 (2), and basolateral amygdala (BLA) (3). (B) Schematic diagrams of the mouse brain depicting the approximate rostrocaudal extent of AAV-mediated MAGL expression (gray shading). Numbers indicate the distance from bregma according to Paxinos and Franklin (2001). (C) HA and MAGL immunostaining in the hippocampus of AAV-Glu-MAGL (1) and AAV-WT (2) mice, indicating strong MAGL overexpression in AAV-Glu-MAGL compared with endogenous MAGL expression in AAV-WT. (D) HA-tagged MAGL in AAV-Glu-MAGL (1) displayed similar punctate immunostaining as endogenous MAGL in AAV-WT (2). (E) Western-blot analysis of the HA tag revealed exclusive transgene expression in AAV-Glu-MAGL mice. MAGL immunoblot indicated the magnitude of MAGL overexpression. (F) Quantification of MAGL protein levels in hippocampal homogenates showed more than 20-fold increase in AAV-Glu-MAGL mice compared with AAV-WT controls (n=4); **P<.01. (G-H) Western-blot analysis revealed no alterations of diacylglycerol lipase-α (DAGLα) protein levels in AAV-Glu-MAGL mice compared with AAV-WT (n=8).
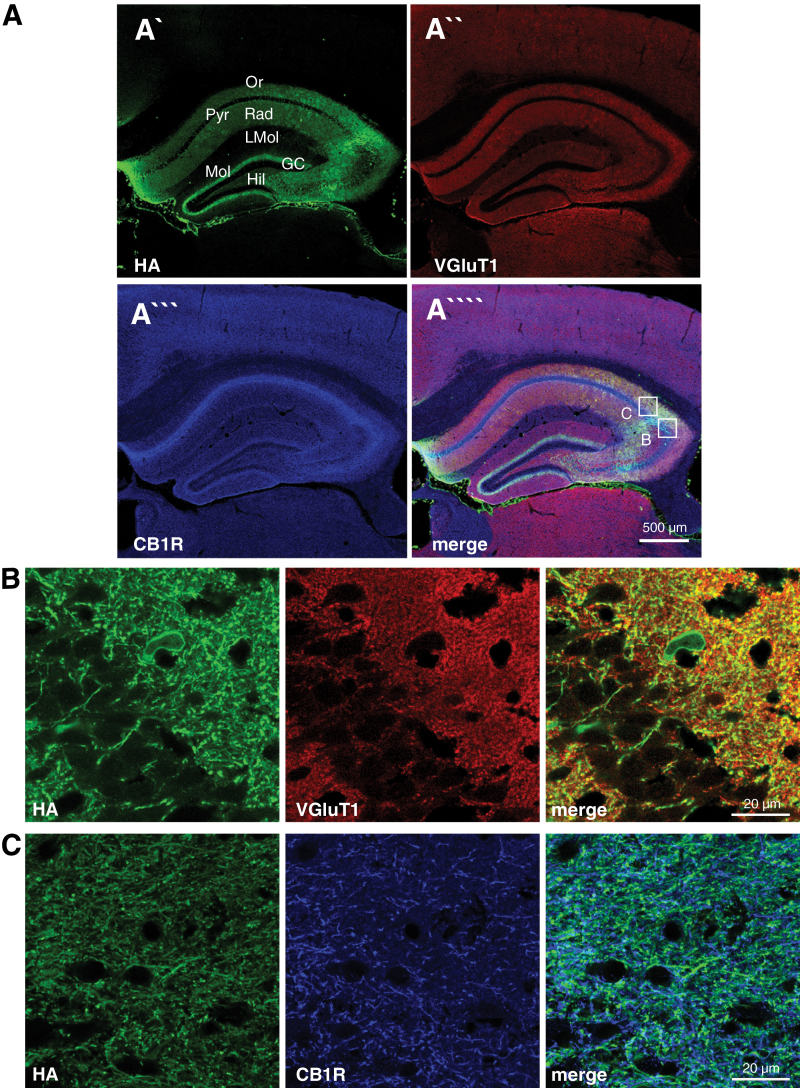
Next, we investigated the areas of overexpression of HA-tagged MAGL in AAV-Glu-MAGL mice (Figure 2). High levels of MAGL were found in the stratum radiatum and stratum oriens of CA1 and CA3 and in the hilar region (Figure 2A), while cell bodies were vastly spared, resembling the expression pattern of the endogenous MAGL protein (Gulyas et al., 2004). The expression of ectopic MAGL in the inner molecular layer of the dentate gyrus was derived from mossy cells (Figure 2A), which do not express MAGL protein endogenously (Uchigashima et al., 2011). In our overexpression system, however, Cre recombinase was present in mossy cells. As the Stop cassette was removed in this cell type, this finally leads to the presence of HA-tagged MAGL in the presynaptic terminals located in the inner molecular layer. Furthermore, HA-tagged MAGL was shown to be present at glutamatergic terminals as shown by colocalization with VGluT1, a marker for glutamatergic presynaptic terminals, for example, in CA3 (Figure 2B), and was similar to the endogenous MAGL expression. In addition, ectopic MAGL also colocalized with CB1R-positive terminals (Figure 2C). Thus, HA-tagged MAGL is expected to decrease 2-AG signaling at CB1R-containing glutamatergic synapses in the hippocampus, including the projections of mossy cells, which express very high levels of CB1R (Monory et al., 2006) and are essentially involved in epileptogenic circuits (Ratzliff et al., 2002).
Figure 2.
Hemagglutinin (HA)-tagged monoacylgycerol lipase (MAGL) colocalization with VGluT1 and cannabinoid type-1 receptor (CB1R) in glutamatergic terminals. (A) Overview of triple immunostaining in the hippocampus of adeno-associated virus (AAV)-Glu-MAGL mice to localize HA (A`), VGluT1 (A``), and CB1R (A```), respectively. Ectopic MAGL expression was found in the stratum oriens, stratum radiatum, and the inner third of the molecular layer, while cell somata of CA1 pyramidal neurons were spared. GC, granule cell layer; Hil, hilar region; LMol, stratum lacunosum-moleculare; Mol, stratum moleculare; Or, stratum oriens; Pyr, CA1/CA3 pyramidal cell layer; Rad, stratum radiatum. (B-C) Higher magnification micrographs as shown in A, indicating that HA-tagged MAGL colocalized with VGluT1 and CB1R.
AAV-Glu-MAGL mice exhibited significantly enhanced MAGL activity (F(1, 30)=94.31, P<.0001; 2-way ANOVA) (Figure 3A). The mean maximum substrate turnover rate in AAV-Glu-MAGL was 784.1±55.1 µmol/min/mg protein and highly increased as compared with AAV-WT, which reached 59.9±6.8 µmol/min/mg protein (P=.0004; unpaired t test) (Figure 3B).
Consistently, 2-AG levels were significantly lower in the hippocampus of AAV-Glu-MAGL compared with AAV-WT (P=.0003; unpaired t test) (Figure 3C). Importantly, levels of AEA (Figure 3D) and arachidonic acid (Figure 3E) were unchanged, indicating that MAGL overexpression exclusively affected 2-AG levels.
Furthermore, we asked whether overexpression of MAGL may lead to altered CB1R expression and signaling. Western-blot analysis revealed no genotype differences of CB1R protein levels (Figure 3F). Furthermore, [35S]-GTPγS binding assays showed that CB1R activity was similar in AAV-WT and AAV-Glu-MAGL mice (Figure 3G). Indeed, WIN 55,212-2–mediated activation of CB1R revealed a similar affinity in the 2 experimental groups analyzed (EC50 AAV-WT=0.36 µM, and EC50 AAV-Glu-MAGL=0.48 µM, respectively). Therefore, upregulation of MAGL in glutamatergic hippocampal neurons did not alter CB1R activity in the hippocampus. Basal [35S]-GTPγS binding in absence of WIN 55,212-2 was also similar in the 2 experimental groups examined (data not shown).
Elevated MAGL Levels at Glutamatergic Terminals Impair DSE but Not DSI
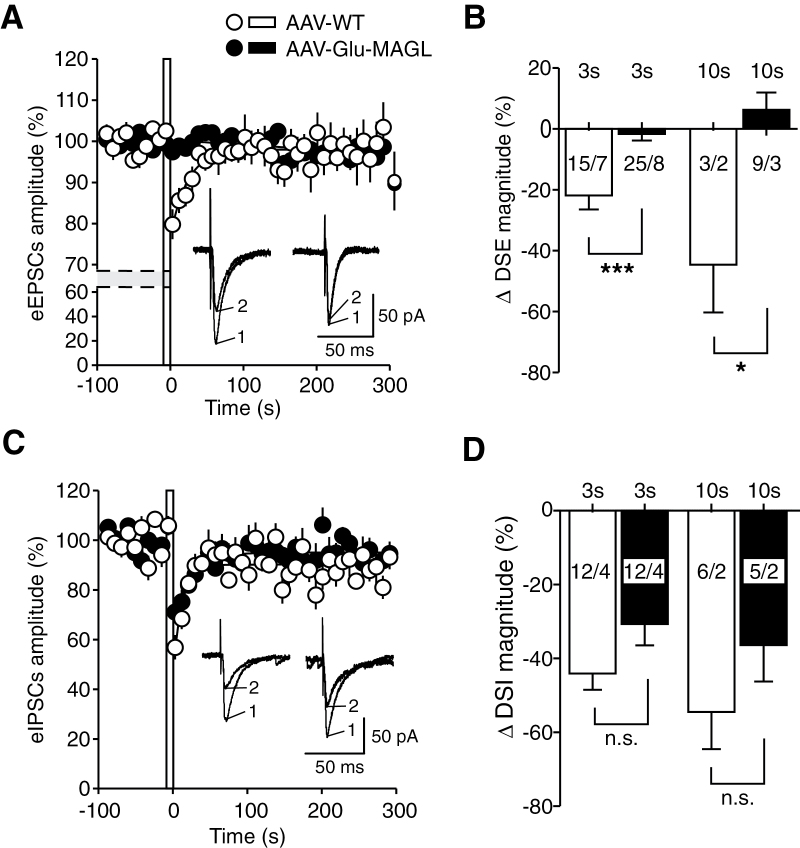
To investigate the functional effects of our experimental interference with 2-AG signalling at the synaptic level, we focused on DSE and DSI, 2 well-established and prototypical electrophysiological processes mediated by 2-AG and CB1R. An enhanced degradation of 2-AG at glutamatergic presynaptic sites is expected to compromise DSE, but not DSI. Thus, eEPSCs and eIPSCs were recorded in hippocampal CA1 pyramidal neurons upon local electrical stimulation in the stratum radiatum, and DSE and DSI protocols were applied in AAV-WT and AAV-Glu-MAGL mice. In AAV-WT controls, a 3-second postsynaptic depolarization reduced eEPSCs to 79.82±0.04% (Figure 4A), amounting to a ΔDSE magnitude of -21.77±4.02% (n=15) (Figure 4B). Increasing the duration of the postsynaptic depolarization to 10 seconds reduced eEPSCs to 57.94±15.17%, corresponding to a ΔDSE magnitude of -44.59±15.66% (n=3) (Figure 4B). By comparison, in AAV-Glu-MAGL mice, none of the tested neurons displayed a significant suppression of eEPSCs upon postsynaptic depolarization steps at both 3-second and 10-second duration (3 seconds: 98.10±1.56%, n=25; 10 seconds: 100.2±3.86%, n=9), indicating that DSE was abolished in this group (ΔDSE: AAV-WT 3s, -21.77±4.02%, n=15 vs AAV-Glu-MAGL 3 seconds, -1.63±2.18%, n=25; P<.0001, unpaired t test; AAV-WT 10 seconds, -44.59±15.66% n=3 vs AAV-Glu-MAGL 10 seconds, 6.27±5.65 n=9; P=.018, Mann-Whitney) (Figure 4B).
Figure 4.
Depolarization-induced suppression of excitation (DSE) (A, B) and of inhibition (DSI) (C-D) in hippocampal CA1 pyramidal neurons from adeno-associated virus (AAV)-WT (open circles/empty columns) and AAV-Glu-MAGL mice (closed circles/filled columns). (A) Averaged evoked excitatory postsynaptic current (eEPSC) amplitude before and after application of a postsynaptic depolarization step (indicated by an open bar at times zero; 3-second duration; -70 to 0 mV). Depression of eEPSCs (DSE) was found in AAV-WT, but not in AAV-Glu-MAGL mice after the postsynaptic depolarization. Inset: Original traces of eEPSCs recorded in the same cell, immediately before (1; n=5 responses averaged) and after (2; n=3) the 3-second depolarization step, recorded in AAV-WT (left) and AAV-Glu-MAGL (right) mice. (B) Summary bar graph showing the magnitude of ΔDSE obtained upon a 3- and 10-second depolarization step, respectively, in the 2 groups. Numbers close to or inside the columns indicate the number of recorded cells/animals. *P<.05, ***P<.001. (C) Averaged inhibitory postsynaptic inhibitory current (eIPSC) amplitude before and after application of a postsynaptic depolarization step (3-second duration; -70 to 0 mV). Depression of eIPSCs (DSI) was measured but no difference between the 2 groups after the postsynaptic depolarization was found. Inset: Original traces of eIPSCs recorded in the same cell, immediately before (1; n=5 responses averaged) and after (2; n=3) the 3-second depolarization step, recorded in AAV-WT (left) and AAV-Glu-MAGL (right) mice. (D) Summary bar graph showing the magnitude of ΔDSI obtained upon a 3- and 10-second depolarization step. Numbers indicate the number of recorded cells/animals.
Importantly, glutamatergic MAGL overexpression did not significantly influence DSI, as indicated by the finding that eIPSCs were significantly reduced after postsynaptic depolarization at both 3 and 10 seconds in AAV-WT (3 seconds: 56.91±4.91%, n=12; 10 seconds: 46.36±10.10%, n=6) (Figure 4C) and AAV-Glu-MAGL mice (3 seconds: 71.09±5.42%, n=12; 10 seconds: 60.55±11.57%, n=5) (Figure 4C). DSI magnitudes were not statistically different between the 2 groups at 3-second depolarization (AAV-WT: -44.08±4.42%, n=12; AAV-Glu-MAGL: -30.69±5.76%, n=12; P= .079, unpaired t test; Figure 4D) and at 10-second depolarization (AAV-WT: -54.49±10.07%, n=6; AAV-Glu-MAGL: -36.37±9.89%, n=5; P=.236, unpaired t test; Figure 4D).
These results demonstrate that overexpression of MAGL in glutamatergic neurons induces a selective functional interference by promoting a significant reduction of DSE, but not of DSI. This suggests that our genetic modulation of 2-AG signalling impacts glutamatergic synaptic transmission with no significant effect on GABAergic transmission.
AAV-Glu-MAGL Mice Exhibit an Increase in Anxiety-Like Behavior
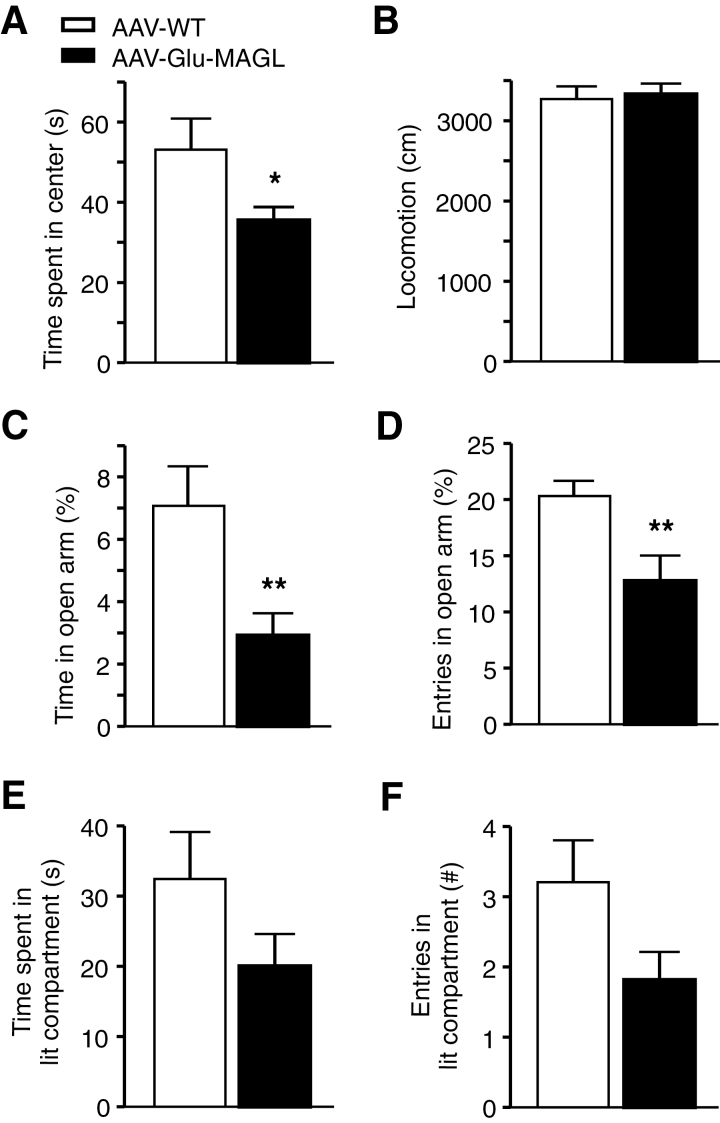
Next, AAV-WT and AAV-Glu-MAGL mice were subjected to a battery of tests to measure anxiety-like behavior. The open field test revealed a significant difference in the time spent in the center (P=.046, unpaired t test; Figure 5A), whereas locomotor activity was unchanged (Figure 5B), suggesting an increase in anxiety-like behavior in AAV-Glu-MAGL mice. This finding was confirmed in the EPM. Indeed, AAV-Glu-MAGL mice spent significantly less time in the open arm of the EPM (P=.003, unpaired t test; Figure 5C) and entered the open arm less frequently than AAV-WT controls (P=.007, unpaired t test; Figure 5D). An analogous behavior was found in the light/dark test, where the entries and the time spent in the aversive lit compartment showed a trend towards being reduced in AAV-Glu-MAGL, though without reaching statistical significance (time in lit compartment P=.133, unpaired t test; Figure 5E; entries in lit compartment P=.060, unpaired t test; Figure 5F). Altogether, these findings indicate that 2-AG signaling at glutamatergic hippocampal neurons is involved in regulating anxiety-like behavior.
Figure 5.
Tests of anxiety-like behavior. (A) Adeno-associated virus (AAV)-Glu-MAGL mice spent less time in the center in the open field test than AAV-WT controls. (B) Locomotor activity did not differ between the 2 groups in the open field. (C-D) In the elevated plus maze (EPM), time spent in the open arm and entries in the open arm were reduced in AAV-Glu-MAGL. (E-F) Entries and time spent in the aversive lit compartment were reduced in AAV-Glu-MAGL in the light/dark test without reaching statistical significance. n=20–24 mice/group. *P<.05, **P<.01.
Analysis in the passive avoidance test did not show any difference between AAV-WT and AAV-Glu-MAGL (P=.911, unpaired t test; Figure 6A), suggesting that impaired 2-AG signaling at glutamatergic synapses has no impact on the formation of aversive memory.
As glutamatergic CB1R activation in the hippocampus is important for the protection against epileptiform seizures (Monory et al., 2006), we evoked chemically induced seizures by intraperitoneal injection of KA (35mg/kg). Surprisingly, seizure severity was not altered in AAV-Glu-MAGL compared with AAV-WT controls (F(1, 145)=0.008, P=.931, repeated-measures ANOVA) (Figure 6B). Moreover, Kaplan-Meier survival curves depicted a similar survival rate during the course of the experiment (P=.447, log rank test) (Figure 6C).
To further analyze this unexpected finding, we measured endocannabinoid levels 30 minutes after KA injection. 2-AG levels did not change significantly in response to KA in AAV-WT animals (P=.247, 2-way ANOVA) but remained decreased in AAV-Glu-MAGL mice compared with AAV-WT controls (vehicle, P=.021; KA, P=.001, 2-way ANOVA) (Figure 6D). In contrast, KA application increased the levels of AEA (P<.0001, 2-way ANOVA; Figure 6E) and arachidonic acid (P<.0001, 2-way ANOVA; Figure 6F) in a similar magnitude in both groups. Importantly, no difference between the 2 genotypes was found in the levels of AEA and arachidonic acid. This finding suggests that 2-AG signaling at hippocampal glutamatergic synapses is not crucially involved in the protection against epileptiform seizures induced by acute KA treatment.
To control for confounding effects of the NEX-Cre line, which was generated by a knock-in approach, whereby the endogenous NEX allele was inactivated (Goebbels et al., 2006), we analyzed the behavior of heterozygous NEX-Cre mice compared with wild-type littermates. For all the behavioral parameters investigated in the present study (OF, EPM, LD, KA-induced seizures), no significant differences between NEX-Cre and wild-type littermates were found (supplementary Figures 1 and 2).
Discussion
2-AG is considered the major endocannabinoid mediating retrograde synaptic suppression of neurotransmitter release (Katona and Freund, 2008). To selectively investigate the functions of 2-AG in vivo, 2 mouse models were generated that lack either the 2-AG biosynthetic enzyme DAGLα or the 2-AG degrading enzyme MAGL. However, MAGL knockout mice exhibited compensatory effects (Schlosburg et al., 2010; Nomura et al., 2011), showing a strong desensitization of CB1R and tolerance behaviors due to prolonged excessive levels of 2-AG (Schlosburg et al., 2010). Furthermore, these global gene ablations do not allow addressing 2-AG signaling effects on a particular cell population. Several studies used conditional CB1R knockout mice to show the differential effect of CB1R activation on glutamatergic and GABAergic neurons, respectively (Monory et al., 2006; Häring et al., 2011; Dubreucq et al., 2012; Rey et al., 2012; Ruehle et al., 2013). To even further dissect endocannabinoid signaling and diminish 2-AG-mediated activation of CB1R exclusively on a selective cell population, elevating the activity of the presynaptic enzyme MAGL in the cell population of interest is the only feasible approach. A transgenic mouse line was recently established that overexpresses MAGL under control of the CaMKIIα promoter, targeting MAGL overexpression to a large variety of forebrain projecting neurons, including those in hippocampus, cerebral cortex, striatum, and hypothalamus (Jung et al., 2012). These mice display a series of metabolic changes, including leanness, elevated energy expenditure, and resistance to diet-induced obesity. However, this study did not investigate hippocampus-dependent behavior or electrophysiological alterations.
In the present report, we used a Cre-inducible AAV vector to overexpress MAGL exclusively in glutamatergic hippocampal neurons of adult mice, allowing cell type-specific interference and avoiding possible developmental compensatory effects. Levels of HA-MAGL protein in the hippocampus were very high (Figure 1A). As hippocampal glutamatergic neurons also project to extra-hippocampal areas, HA-MAGL was observed in such areas to a much lower extent as shown exemplary in the BLA (Figure 1A 3). It remains to be determined whether the low ectopic expression of HA-MAGL in the BLA, which is derived from hippocampal glutamatergic neurons transduced with the AAV-Stop-MAGL vector and projecting to the BLA, allows the impairment of DSE, and, at the behavioral level, whether this impairment may contribute to the increased anxiety level observed in AAV-Glu-MAGL mice. This has to be taken into consideration, as glutamatergic 2-AG signaling in the BLA can regulate anxiety-like behavior (Shonesy et al., 2014).
Interestingly, MAGL overexpression in hippocampal glutamatergic neurons led to a 25-fold increase in MAGL protein levels and a 10-fold increase in MAGL activity but to only a 2-fold decrease in 2-AG levels, suggesting that the enzyme activity state is not directly proportional to the amount of 2-AG degraded. For the interpretation of this observation, it has to be considered that the endocannabinoid synthesizing and degrading machinery is characterized by a distinct neuroanatomical layout. The overexpression of MAGL in the AAV-Glu-MAGL mouse model is only present at synaptic terminals of hippocampal glutamatergic neurons (except for dentate gyrus neurons), where MAGL degrades the 2-AG that is produced on-demand postsynaptically. When performing the enzyme activity measurements using an in vitro assay (Figure 3A-B), the substrate is available in excess. Thus, the MAGL activity measured in the assay does not reflect the in vivo situation, when an on-demand production of 2-AG takes place. Furthermore, in the AAV-Glu-MAGL mouse model, we did not interfere with MAGL on GABAergic terminals, where intensive and dominant endocannabinoid signaling is known to occur. Altogether, the enhanced enzymatic MAGL activity in hippocampal glutamatergic terminals of AAV-Glu-MAGL mice led to a 2-fold decrease in 2-AG levels but did not alter the levels of AEA and arachidonic acid.
CB1R was shown to be localized at perisynaptic sites (Kawamura et al., 2006), while MAGL is predominantly found in the central part of axon terminals in proximity to synaptic vesicles and active zone release sites (Ludanyi et al., 2011). The fact that MAGL and CB1R are differently localized on the presynaptic membrane facilitates the MAGL-dependent regulation of retrograde endocannabinoid signaling. By overexpressing MAGL, 2-AG molecules are presumably degraded even before 2-AG can bind to the CB1R. Indeed, MAGL overexpression in glutamatergic pyramidal neurons selectively attenuated 2-AG-mediated synaptic depression at glutamatergic terminals without significantly affecting 2-AG action on GABAergic transmission in CA1 pyramidal neurons (Figure 4). This finding also indicates that presynaptic MAGL can precisely control the activation of the CB1R located at the same synaptic site where MAGL is expressed. Given that 2-AG content was reduced, but AEA content was unchanged in AAV-Glu-MAGL mice, 2-AG can be considered as the main mediator of DSE, which is consistent with previous reports showing a lack of DSE in DAGLα-deficient mice (Gao et al., 2010; Shonesy et al., 2014). Of note, DSI also seems to be influenced in AAV-Glu-MAGL mice, although differences compared to controls did not reach statistical significance (P=.079) (Figure 4D). Recent studies showed that MAGL regulates 2-AG signaling through both homosynaptic and heterosynaptic pathways in cerebellar neurons (Tanimura et al., 2012), and polysynaptic influences might have contributed to the tendency towards a decreased DSI magnitude observed in CA1 neurons in our AAV-Glu-MAGL mice. Altogether, we generated a mouse model that promotes a cell type-selective impairment of 2-AG-mediated effects and that will prove useful to discriminate between the functions of, for example, DSE and DSI.
The exposure to aversive stimuli or stress is characterized by a pronounced increase in glutamate release (Millan, 2003), and the blockade of glutamatergic neurotransmission causes antidepressant- and anxiolytic-like responses (Simon and Gorman, 2006; Palucha and Pilc, 2007). Thus, hyperfunction of glutamatergic signaling is associated with the development of depression and anxiety disorders (Palucha and Pilc, 2007). Furthermore, it was shown that stress enhances glutamate release in the hippocampus (Bagley and Moghaddam, 1997). The endocannabinoid system counteracts stress-mediated enhancement of excitatory activity with compensatory mechanisms in the hippocampus, such as impairment of DSI (Hu et al., 2011) and elevation of 2-AG levels (Dubreucq et al., 2012; Wang et al., 2012). An increase in 2-AG levels was proposed to be important for adaptation to stress (Hill et al., 2010). The present work focuses on overexpressing MAGL at glutamatergic terminals in the hippocampus to attenuate 2-AG signaling selectively at glutamatergic CB1Rs. This alteration may result in an imbalance between excitatory and inhibitory transmission, precluding an adaptation to stressful and aversive stimuli and leading to an increase in anxiety. Consistent with this reasoning, it has been recently reported that DAGLα deletion leads to anxiety and depression-like behavior in mice (Shonesy et al., 2014; Jenniches et al., 2015), which is reversed by pharmacological blockade of MAGL by JZL184. Interestingly, DAGLα-KO mice showed impaired amygdalar endocannabinoid-modulated glutamatergic neurotransmission, which ultimately led to anxiety (Shonesy et al., 2014). Although these previous reports used a different genetic manipulation (DAGLα deletion rather than MAGL overexpression), they are consistent with our data showing that impairment in 2-AG signaling (by overexpressing MAGL selectively at hippocampal glutamatergic neurons) leads to anxiety.
Interestingly, control over a stressful event was found to correlate to changes in glutamatergic excitability in pyramidal neurons of the prefrontal cortex, particularly in the prelimbic area (Varela et al., 2012). Given the tight functional link between the prelimbic prefrontal cortex and the hippocampus in high fear situations (Sotres-Bayon et al., 2012), and the role of endocannabinoid signaling in behavioral adaptation and habituation (Kamprath et al., 2006; Sugaya et al., 2013), it is tempting to conclude that excessive glutamatergic activity in these pathways will initiate endocannabinoid synthesis. This, in turn, will lead to a suppression of glutamate release on demand, thereby contributing to successful coping with a stressful event.
The endocannabinoid system is considered a therapeutic target in epilepsy. Indeed, it was shown that CB1R on hippocampal glutamatergic but not GABAergic neurons is required for the protection against epileptiform seizures (Monory et al., 2006). Seizure activity is accompanied by increased AEA synthesis (Marsicano et al., 2003; Wettschureck et al., 2006), while an increase in 2-AG levels in the KA model of epilepsy was only observed by Wettschureck and colleagues (Wettschureck et al., 2006). Katona and Freund (2008) assigned retrograde 2-AG signaling as a protective mechanism against excessive presynaptic activity. We found that 2-AG levels did not change in response to KA-induced excessive glutamate release. In contrast, the content of AEA increased significantly under these conditions. In agreement with our data, a recent study showed that bath application of KA to hippocampal slices resulted in an increase in AEA, but no change in 2-AG levels (Lourenco et al., 2011). Accordingly, the KA-induced depression of inhibitory postsynaptic currents is prolonged by application of a FAAH inhibitor, whereas no alterations occur in presence of a MAGL inhibitor. Although it was reported that FAAH-deficient mice are more susceptible to KA-induced seizures (Clement et al., 2003), other studies revealed that injection of a FAAH inhibitor prior to the induction of seizures markedly reduced the seizure scores (Coomber at al., 2008; Naidoo et al., 2011). Moreover, AEA content was decreased in cerebrospinal fluid of patients suffering from temporal lobe epilepsy, while 2-AG levels were not affected (Romigi et al., 2010). Altogether, these findings point to AEA rather than 2-AG as potential initiator of endocannabinoid-mediated protection against KA-induced epileptiform seizures, and the results of the present study support this notion. However, the differential importance of AEA vs 2-AG in the regulation of epileptic seizures appears to depend on the model used (Naydenov et al., 2014) and the brain region studied (von Rüden et al., 2015).
In conclusion, we demonstrated that the specific impairment of hippocampal glutamatergic 2-AG signaling affects anxiety-like behavior without affecting aversive memory or seizure susceptibility in the acute KA model. These findings suggest that glutamatergic 2-AG signaling is an essential component of adaptation to aversive situations but is not required to form aversive memory or protect against KA-induced excitotoxic seizures.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft with grants SFB-TRR 58 (subproject A04 to B.L. and H.C.P) and FOR926 (subprojects SP3 and CP1 to B.L.), and by a Max-Planck-Research Award (2007, to H.C.P.). M.K. is an Australian Research Council Future Fellow. We thank Ruth Jelinek, Andrea Conrad, Claudia Schwitter, Martin Purrio (Mainz laboratory), Svetlana Kiesling, Petra Berenbock, Elke Naß, and Angelika Klinge (Muenster laboratory) for excellent technical assistance. NEX-Cre mice were kindly provided by Klaus-Armin Nave (Göttingen). The mouse MAGL cDNA was a gift from Giovanni Marsicano (Bordeaux), and the anti-MAGL and anti-DAGLα antibodies from Ken Mackie (Bloomington, IN).
References
- Alger BE (2012) Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J Physiol 590:2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. (1997) Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neurosci 77:65–73. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. (2012) Endocannabinoid signaling and synaptic function. Neuron 76:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. (2003) Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci 23:3916–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B, O’Donoghue MF, Mason R. (2008) Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse 62:746–755. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99:10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Matias I, Cardinal P, Haring M, Lutz B, Marsicano G, Chaouloff F. (2012) Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacol 37:1885–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, et al. (2010) Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. (2006) Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44:611–21. [DOI] [PubMed] [Google Scholar]
- Guggenhuber S, Monory K, Lutz B, Klugmann M. (2010) AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PloS One 5:e15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441–458. [DOI] [PubMed] [Google Scholar]
- Häring M, Kaiser N, Monory K, Lutz B. (2011) Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS One 6:e26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring M, Guggenhuber S, Lutz B. (2012) Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience 204:145–58. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. (2010) Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA 107:9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Zhang W, Flugge G. (2011) Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res Bull 85:374–379. [DOI] [PubMed] [Google Scholar]
- Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, Zimmer A. (2015) Anxiety, stress and fear response in mice with reduced endocannabinoid levels. Biol Psychiatry. doi:http://dx.doi.org/10.1016/j.biopsych.2015.03.033 [DOI] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, Diano S, Piomelli D. (2012) 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab 15:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. (2006) Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26:6677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF. (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nature Medicine 14:923–930. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz RA, Alger BE. (1999) Calcium dependence of depolarization-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiology 521 Pt 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U, Lutz B. (2015) Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 40:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, Matias I, Marsicano G, Mulle C. (2011) Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci 31:3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludányi A, Hu SS, Yamazaki M, Tanimura A, Piomelli D, Watanabe M, Kano M, Sakimura K, Maglóczky Z, Mackie K, Freund TF, Katona I (2011) Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience 174:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302:84–88. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2003) The neurobiology and control of anxious states. Progr Neurobiol 70:83–244. [DOI] [PubMed] [Google Scholar]
- Monory K, et al. (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V, Nikas SP, Karanian DA, Hwang J, Zhao J, Wood JT, Alapafuja SO, Vadivel SK, Butler D, Makriyannis A, Bahr BA. (2011) A new generation fatty acid amide hydrolase inhibitor protects against kainate-induced excitotoxicity. J Mol Neurosci 43:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov AV, Horne EA, Cheah CS, Swinney K, Hsu KL, Cao JK, Marrs WR, Blankman JL, Tu S, Cherry AE, Fung S, Wen A, Li W, Saporito MS, Selley DE, Cravatt BF, Oakley JC, Stella N. (2014) ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 Mice. Neuron 83:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Pilc A. (2007) Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Therapeutics 115:116–147. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. (2011) Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci 31:13420–13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff A, Santhakumar V, Howard A, Soltesz I. (2002) Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci 25:140–144. [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. (2012) Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacol 37:2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, Izzi F, Prosperetti C, Zannino S, Corte F, Chiaramonte C, Maccarrone M. (2010) Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia 51:768–772. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wortge S, Haring M, Kaiser N, Marsicano G, Pape HC, Lutz B. (2013) Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci 33:10264–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte K, Steingrüber N, Jergas B, Redmer A, Kurz CM, Buchalla R, Lutz B, Zimmer A, Schlicker E (2012) Cannabinoid CB1 receptor activation, pharmacological blockade, or genetic ablation affects the function of the muscarinic auto- and heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol 385:385–396. [DOI] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS, Báldi R, Hermanson DJ, Kingsley PJ, Marnett LJ, Winder DG, Colbran RJ, Patel S. (2014) Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep 9:1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AB, Gorman JM. (2006) Advances in the treatment of anxiety: targeting glutamate. NeuroRx 3:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gómez E, et al. (2014) The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci 17:407–15. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. (2012) Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya Y, Cagniard B, Yamazaki M, Sakimura K, Kano M. (2013) The endocannabinoid 2-arachidonoylglycerol negatively regulates habituation by suppressing excitatory recurrent network activity and reducing long-term potentiation in the dentate gyrus. J Neurosci 33:3588–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Uchigashima M, Yamazaki M, Uesaka N, Mikuni T, Abe M, Hashimoto K, Watanabe M, Sakimura K, Kano M. (2012) Synapse type-independent degradation of the endocannabinoid 2-arachidonoylglycerol after retrograde synaptic suppression. Proc Natl Acad Sci USA 109:12195–12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65:320–327. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, Kano M, Watanabe M. (2011) Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci 31:7700–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. (2012) Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J Neurosci 32:12848–12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rüden EL, Bogdanovic RM, Wotjak CT, Potschka H. (2015) Inhibition of monoacylglycerol lipase mediates a cannabinoid 1-receptor dependent delay of kindling progression in mice. Neurobiol Dis 77:238–45. [DOI] [PubMed] [Google Scholar]
- Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. (2012) Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol 26:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, van der Stelt M, Tsubokawa H, Krestel H, Moers A, Petrosino S, Schütz G, Di Marzo V, Offermanns S. (2006) Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol Cellular Biol 26:5888–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Pan B, Gao XP, Blankman JL, Cravatt BF, Liu QS. (2011) Genetic deletion of monoacylglycerol lipase alters endocannabinoid-mediated retrograde synaptic depression in the cerebellum. J Physiol 589:4847–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.